Continuous Production of DHA and EPA Ethyl Esters via Lipase-Catalyzed Transesterification in an Ultrasonic Packed-Bed Bioreactor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Ultrasonication on DHA and EPA Ethyl Ester Synthesized Using Lipase Packed-Bed Bioreactor
2.2. Mass Transfer Kinetic Model of an Ultrasonic Packed-Bed Reactor
2.3. Continuous Synthesis of DHA + EPA Ethyl Ester by Packed-Bed Bioreactor with Long-Term Operation
2.4. Recovery of DHA/EPA Ethyl Ester
2.5. Evaluate the Ultrasonication Effect by the Kinetic Model in the Batch Reaction
3. Materials and Methods
3.1. Materials
3.2. Continuous Synthesis of DHA and EPA Ethyl Ester in Packed-Bed Bioreactor
3.3. Experimental Design
3.4. Measure Initial Rate of DHA and EPA Ethyl Ester Synthesized in the Batch Reaction
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing omega-3 polyunsaturated fatty acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Sankar, T.V.; Anandan, R.; Chakraborty, K.; Paul, B.N.; Sarma, D.; Syama Dayal, J.; Venkateshwarlu, G.; et al. DHA and EPA Content and Fatty Acid Profile of 39 Food Fishes from India. BioMed Res. Int. 2016, 2016, 4027437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innes, J.K.; Calder, P.C. Marine omega-3 (N-3) fatty acids for cardiovascular health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovale-Rosabal, G.; Rodríguez, A.; Contreras, E.; Ortiz-Viedma, J.; Muñoz, M.; Trigo, M.; Aubourg, S.P.; Espinosa, A. Concentration of EPA and DHA from refined salmon oil by optimizing the urea–fatty acid adduction reaction conditions using response surface methodology. Molecules 2019, 24, 1642. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Wu, F.; Yue, L.; Du, Q.; Tian, L.; Wang, Z. Combination of urea complexation and molecular distillation to purify DHA and EPA from sardine oil ethyl esters. J. Am. Oil Chem. Soc. 2014, 91, 687–695. [Google Scholar] [CrossRef]
- Montañés, F.; Tallon, S. Supercritical fluid chromatography as a technique to fractionate high-valued compounds from lipids. Separations 2018, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Oh, C.-E.; Kim, G.-J.; Park, S.-J.; Choi, S.; Park, M.-J.; Lee, O.-M.; Seo, J.-W.; Son, H.-J. Purification of high purity docosahexaenoic acid from Schizochytrium sp. SH103 using preparative-scale HPLC. Appl. Biol. Chem. 2020, 63, 1–8. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Chen, J.-W.; Wang, H.-M.D.; Shieh, C.-J. Concentration of Docosahexaenoic and Eicosapentaenoic Acid from Cobia Liver Oil by Acetone Fractionation of Fatty Acid Salts. Appl. Biochem. Biotechnol. 2020, 192, 517–529. [Google Scholar] [CrossRef]
- Kralovec, J.A.; Zhang, S.; Zhang, W.; Barrow, C.J. A review of the progress in enzymatic concentration and microencapsulation of omega-3 rich oil from fish and microbial sources. Food Chem. 2012, 131, 639–644. [Google Scholar] [CrossRef]
- Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and bioactive components from fish for dyslipidemia and cardiovascular risk reduction. Mar. Drugs 2016, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Katanaev, V.L.; Di Falco, S.; Khotimchenko, Y. The anticancer drug discovery potential of marine invertebrates from Russian Pacific. Mar. Drugs 2019, 17, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, A.; Gładkowski, W.; Grudniewska, A. Lipase-catalyzed transesterification of egg-yolk phophatidylcholine with concentrate of n-3 polyunsaturated fatty acids from cod liver oil. Molecules 2017, 22, 1771. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Huang, C.-Y.; Lee, C.-L.; Kuo, W.-C.; Hsieh, S.-L.; Shieh, C.-J. Synthesis of DHA/EPA ethyl esters via lipase-catalyzed acidolysis using Novozym® 435: A kinetic study. Catalysts 2020, 10, 565. [Google Scholar] [CrossRef]
- Shimada, Y.; Sugihara, A.; Nakano, H.; Kuramoto, T.; Nagao, T.; Gemba, M.; Tominaga, Y. Purification of docosahexaenoic acid by selective esterification of fatty acids from tuna oil with Rhizopus delemar lipase. J. Am. Oil Chem. Soc. 1997, 74, 97–101. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Baba, T.; Ooguri, T.; Moriyama, S.; Terai, T.; Tominaga, Y. Ethyl esterification of docosahexaenoic acid in an organic solvent-free system with immobilized Candida antarctica lipase. J. Biosci. Bioeng. 2001, 92, 19–23. [Google Scholar] [CrossRef]
- Bispo, P.; Batista, I.; Bernardino, R.J.; Bandarra, N.M. Preparation of triacylglycerols rich in omega-3 fatty acids from sardine oil using a Rhizomucor miehei lipase: Focus in the EPA/DHA Ratio. Appl. Biochem. Biotechnol. 2014, 172, 1866–1881. [Google Scholar] [CrossRef]
- Chen, H.C.; Kuo, C.H.; Twu, Y.K.; Chen, J.H.; Chang, C.M.J.; Liu, Y.C.; Shieh, C.J. A continuous ultrasound-assisted packed-bed bioreactor for the lipase-catalyzed synthesis of caffeic acid phenethyl ester. J. Chem. Technol. Biotechnol. 2011, 86, 1289–1294. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Shieh, C.-J. Biocatalytic Process Optimization. Catalysts 2020, 10, 1303. [Google Scholar] [CrossRef]
- Huang, S.-M.; Huang, H.-Y.; Chen, Y.-M.; Kuo, C.-H.; Shieh, C.-J. Continuous production of 2-phenylethyl acetate in a solvent-free system using a packed-bed reactor with Novozym® 435. Catalysts 2020, 10, 714. [Google Scholar] [CrossRef]
- Zhou, N.; Shen, L.; Dong, Z.; Shen, J.; Du, L.; Luo, X. Enzymatic synthesis of thioesters from thiols and vinyl esters in a continuous-flow microreactor. Catalysts 2018, 8, 249. [Google Scholar] [CrossRef] [Green Version]
- Lindeque, R.M.; Woodley, J.M. Reactor selection for effective continuous biocatalytic production of pharmaceuticals. Catalysts 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.-R.; Tsai, M.-F.; Shieh, C.-J.; Arakawa, O.; Dong, C.-D.; Huang, C.-Y.; Kuo, C.-H. Ultrasonic-Assisted Extraction and Structural Characterization of Chondroitin Sulfate Derived from Jumbo Squid Cartilage. Foods 2021, 10, 2363. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-A.; Kuo, C.-H.; Chen, B.-Y.; Li, Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Murillo, G.; He, Y.; Yan, Y.; Sun, J.; Bartocci, P.; Ali, S.S.; Fantozzi, F. Scaled-up biodiesel synthesis from Chinese Tallow Kernel oil catalyzed by Burkholderia cepacia lipase through ultrasonic assisted technology: A non-edible and alternative source of bio energy. Ultrason. Sonochem. 2019, 58, 104658. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.S.; Garcia-Galan, C.; Schein, M.F.; Silva, A.M.; Barbosa, O.; Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combined effects of ultrasound and immobilization protocol on butyl acetate synthesis catalyzed by CALB. Molecules 2014, 19, 9562–9576. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-H.; Hsiao, F.-W.; Chen, J.-H.; Hsieh, C.-W.; Liu, Y.-C.; Shieh, C.-J. Kinetic aspects of ultrasound-accelerated lipase catalyzed acetylation and optimal synthesis of 4′-acetoxyresveratrol. Ultrason. Sonochem. 2013, 20, 546–552. [Google Scholar] [CrossRef]
- Onoja, E.; Chandren, S.; Razak, F.I.A.; Wahab, R.A. Enzymatic synthesis of butyl butyrate by Candida rugosa lipase supported on magnetized-nanosilica from oil palm leaves: Process Optimization, Kinetic and Thermodynamic Study. J. Taiwan Inst. Chem. Eng. 2018, 91, 105–118. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, G.-J.; Chen, C.-I.; Liu, Y.-C.; Shieh, C.-J. Kinetics and optimization of lipase-catalyzed synthesis of rose fragrance 2-phenylethyl acetate through transesterification. Process Biochem. 2014, 49, 437–444. [Google Scholar] [CrossRef]
- Sousa, R.R.; Silva, A.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-free esterifications mediated by immobilized lipases: A Review from Thermodynamic and Kinetic Perspectives. Catal Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Yadav, G.D. Synthesis of geranyl acetate by transesterification of geraniol with ethyl acetate over Candida antarctica lipase as catalyst in solvent-free system. Flavour Fragr. J. 2019, 34, 288–293. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Liu, T.-A.; Chen, J.-H.; Chang, C.-M.J.; Shieh, C.-J. Response surface methodology and artificial neural network optimized synthesis of enzymatic 2-phenylethyl acetate in a solvent-free system. Biocatal. Agric. Biotechnol. 2014, 3, 1–6. [Google Scholar] [CrossRef]
- Zanwar, S.; Pangarkar, V. Solid-liquid mass transfer in packed beds: Enhancement Due to Ultrasound. Chem. Eng. Commun. 1988, 68, 133–142. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Fatty acids methyl esters from vegetable oil by means of ultrasonic energy. Ultrason. Sonochem. 2005, 12, 367–372. [Google Scholar] [CrossRef]
- Ji, J.; Wang, J.; Li, Y.; Yu, Y.; Xu, Z. Preparation of biodiesel with the help of ultrasonic and hydrodynamic cavitation. Ultrasonics 2006, 44, e411–e414. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, Q.; Shan, L.; Liu, Y.; Shen, W.; Wang, X. The effect of ultrasound on lipase-catalyzed hydrolysis of soy oil in solvent-free system. Ultrason. Sonochem. 2008, 15, 402–407. [Google Scholar] [CrossRef]
- Yu, D.; Tian, L.; Wu, H.; Wang, S.; Wang, Y.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]
- Patchimpet, J.; Zhang, Y.; Simpson, B.K.; Rui, X.; Sangkharak, K.; Eiad-ua, A.; Klomklao, S. Ultrasonic enhancement of lipase-catalyzed transesterification for biodiesel production from used cooking oil. Biomass Convers. Biorefinery 2021, 1–10. [Google Scholar] [CrossRef]
- Todero, L.M.; Bassi, J.J.; Lage, F.A.; Corradini, M.C.C.; Barboza, J.; Hirata, D.B.; Mendes, A.A. Enzymatic synthesis of isoamyl butyrate catalyzed by immobilized lipase on poly-methacrylate particles: Optimization, Reusability and Mass Transfer Studies. Bioprocess Biosyst. Eng. 2015, 38, 1601–1613. [Google Scholar] [CrossRef]
- Cavallaro, V.; Tonetto, G.; Ferreira, M.L. Optimization of the enzymatic synthesis of pentyl oleate with lipase immobilized onto novel structured support. Fermentation 2019, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Claon, P.A.; Akoh, C.C. Effect of reaction parameters on SP435 lipase-catalyzed synthesis of citronellyl acetate in organic solvent. Enzym. Microb. Technol. 1994, 16, 835–838. [Google Scholar] [CrossRef]
- Kuo, C.; Chen, C.; Chiang, B. Process characteristics of hydrolysis of chitosan in a continuous enzymatic membrane reactor. J. Food Sci. 2004, 69, 332–337. [Google Scholar] [CrossRef]
- Zenevicz, M.C.P.; Jacques, A.; Silva, M.J.A.; Furigo, A.; Oliveira, V.; de Oliveira, D. Study of a reactor model for enzymatic reactions in continuous mode coupled to an ultrasound bath for esters production. Bioprocess. Biosyst. Eng. 2018, 41, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Continuous biodiesel conversion via enzymatic transesterification catalyzed by immobilized Burkholderia lipase in a packed-bed bioreactor. Appl. Energy 2016, 168, 340–350. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Zhao, C.; Ding, Y.; Xu, P. Biodiesel production in packed-bed reactors using lipase–nanoparticle biocomposite. Bioresour. Technol. 2011, 102, 6352–6355. [Google Scholar] [CrossRef]
- Aljawish, A.; Heuson, E.; Bigan, M.; Froidevaux, R. Lipase catalyzed esterification of formic acid in solvent and solvent-free systems. Biocatal. Agric. Biotechnol. 2019, 20, 101221. [Google Scholar] [CrossRef]
- Jaiswal, K.S.; Rathod, V.K. Green synthesis of amyl levulinate using lipase in the solvent free system: Optimization, Mechanism and Thermodynamics Studies. Catal. Today 2021, 375, 120–131. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Shi, J.; Zheng, M.; Xiang, X.; Huang, F.; Xiao, J. Ultrasound irradiation promoted enzymatic alcoholysis for synthesis of monoglyceryl phenolic acids in a solvent-free system. Ultrason. Sonochem. 2018, 41, 120–126. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Hazer, B.; Altıntaş, B.; Arıca, M.Y. Covalent immobilization of lipase onto amine functionalized polypropylene membrane and its application in green apple flavor (ethyl valerate) synthesis. Process Biochem. 2011, 46, 372–378. [Google Scholar] [CrossRef]
- Mahapatra, P.; Kumari, A.; Kumar, G.V.; Banerjee, R.; Nag, A. Kinetics of solvent-free geranyl acetate synthesis by Rhizopus oligosporus NRRL 5905 lipase immobilized on to cross-linked silica. Biocatal. Biotransform. 2009, 27, 124–130. [Google Scholar] [CrossRef]
- Rodriguez, J.-M.G.; Hux, N.P.; Philips, S.J.; Towns, M.H. Michaelis–Menten graphs, Lineweaver–Burk plots, and reaction schemes: Investigating Introductory Biochemistry Students’ Conceptions of Representations in Enzyme Kinetics. J. Chem. Educ. 2019, 96, 1833–1845. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Shieh, C.-J.; Huang, S.-M.; Wang, H.-M.D.; Huang, C.-Y. The effect of extrusion puffing on the physicochemical properties of brown rice used for saccharification and Chinese rice wine fermentation. Food Hydrocoll. 2019, 94, 363–370. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, Z.; Tian, Y.; Fan, H.; Huang, S.; Lu, Y.; Jin, Z. Highly efficient regioselective decanoylation of hyperoside using nanobiocatalyst of Fe3O4@ PDA-thermomyces lanuginosus lipase: Insights of Kinetics and Stability Evaluation. Front. Bioeng. Biotechnol. 2020, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Jadhav, S.V.; Rathod, V.K. Lipase catalysed synthesis of cetyl oleate using ultrasound: Optimisation and Kinetic Studies. Ultrason. Sonochem. 2015, 27, 522–529. [Google Scholar] [CrossRef]
- Tomke, P.D.; Rathod, V.K. Ultrasound assisted lipase catalyzed synthesis of cinnamyl acetate via transesterification reaction in a solvent free medium. Ultrason. Sonochem. 2015, 27, 241–246. [Google Scholar] [CrossRef]
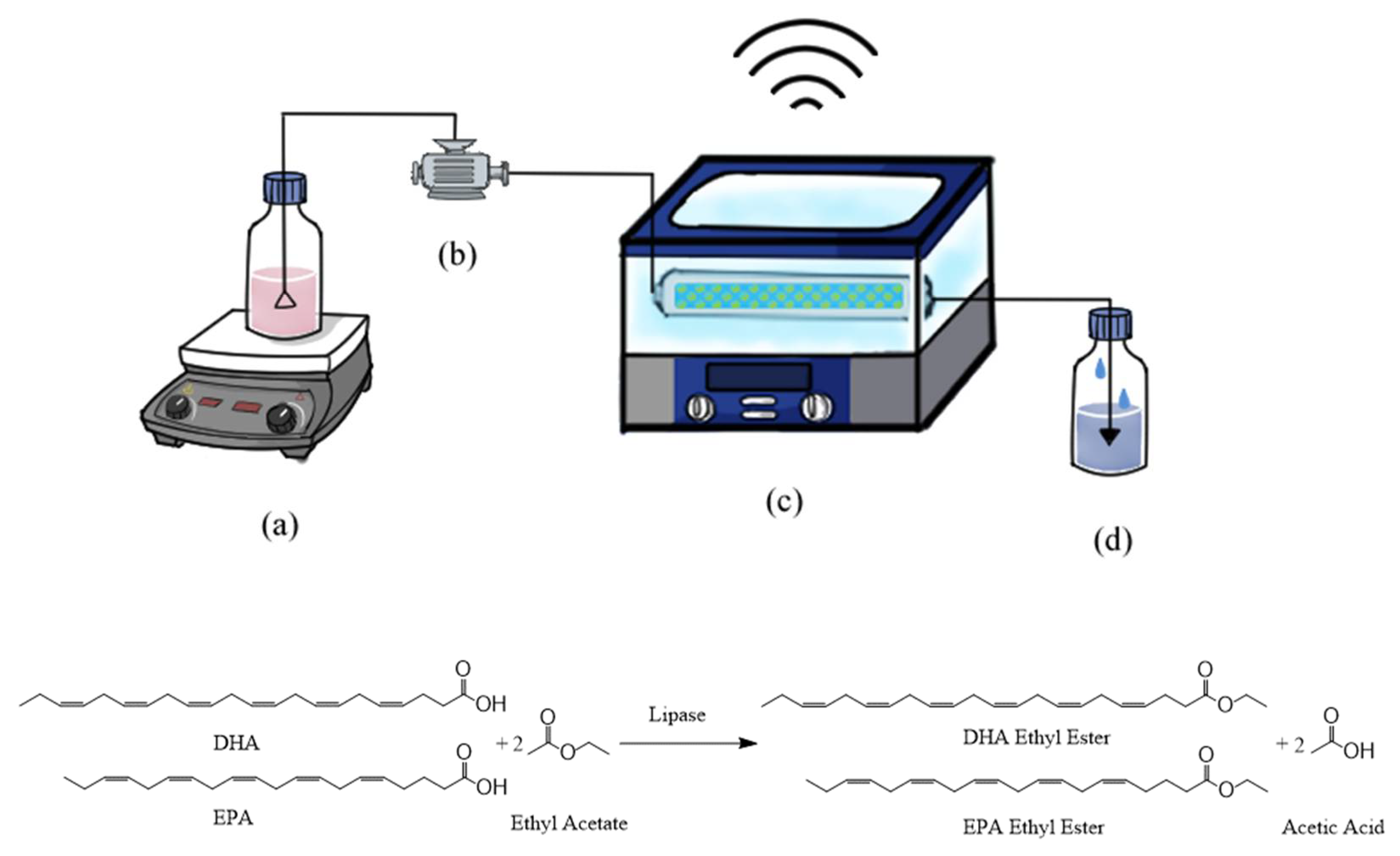
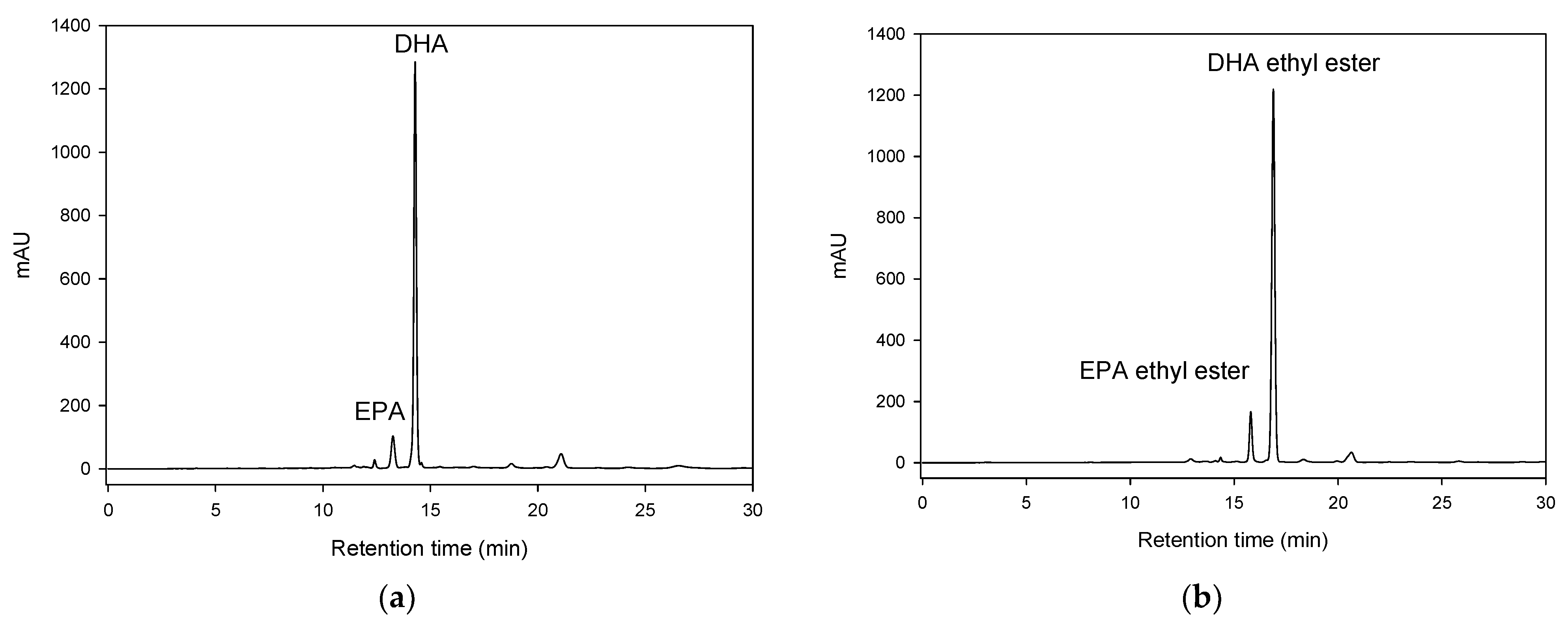


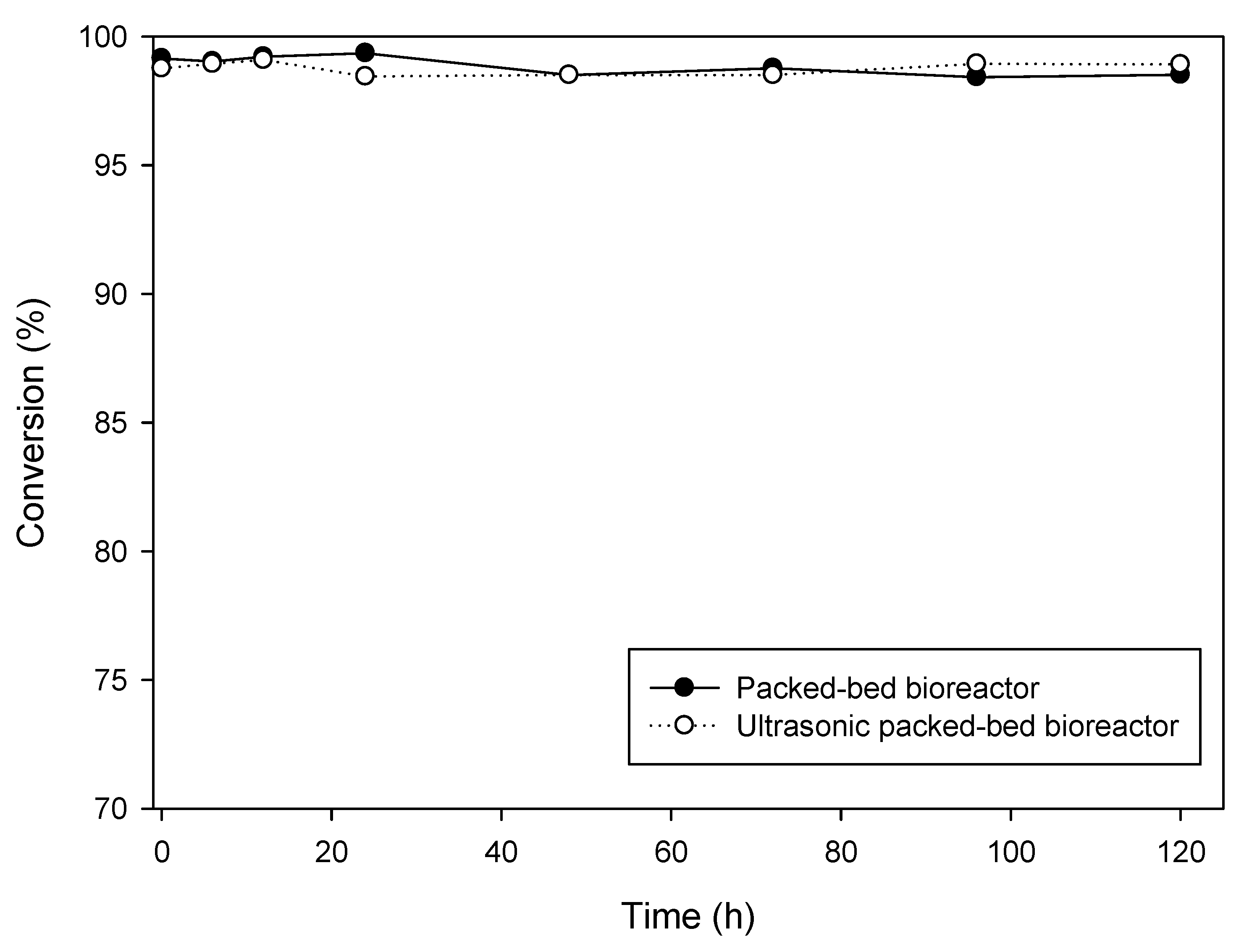
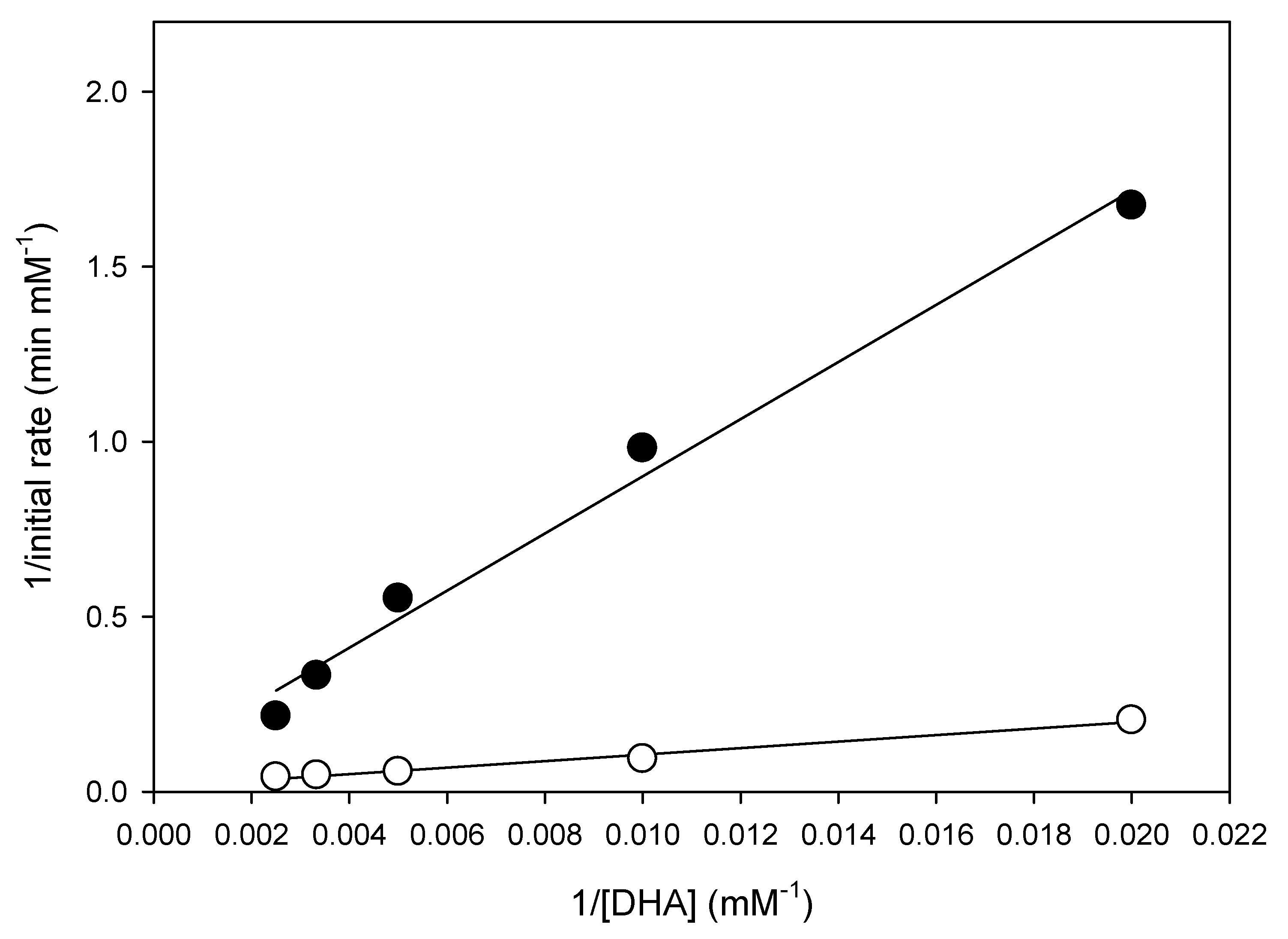
| Treatment No. | Factor | Conversion (%) | ||
|---|---|---|---|---|
| x1 Flow Rate (mL min−1) | x2 DHA + EPA (mM) | Packed-Bed Bioreactor | Ultrasonic Packed-Bed Bioreactor | |
| 1 | −1 a (1) | −1 (100) | 98.84 ± 0.05 | 99.21 ± 0.00 |
| 2 | −1 (1) | 0 (300) | 97.01 ± 1.30 | 97.61 ± 0.03 |
| 3 | −1 (1) | 1 (500) | 95.49 ± 0.42 | 97.34 ± 0.10 |
| 4 | 0 (3) | −1 (100) | 96.59 ± 0.91 | 98.82 ± 0.01 |
| 5 | 0 (3) | 0 (300) | 95.48 ± 0.94 | 96.34 ± 0.19 |
| 6 | 0 (3) | 0 (300) | 95.13 ± 0.14 | 96.00 ± 0.05 |
| 7 | 0 (3) | 1 (500) | 91.79 ± 1.33 | 95.69 ± 0.32 |
| 8 | 1 (5) | −1 (100) | 95.51 ± 0.35 | 96.74 ± 0.61 |
| 9 | 1 (5) | 0 (300) | 92.41 ± 1.03 | 94.50 ± 0.66 |
| 10 | 1 (5) | 1 (500) | 86.61 ± 1.28 | 93.03 ± 1.55 |
| Factor a | Packed-Bed Bioreactor (Y1) | Ultrasonic Packed-Bed Bioreactor (Y2) | ||||
|---|---|---|---|---|---|---|
| Degree of Freedom | Sum of Squares | Prob > F | Degree of Freedom | Sum of Squares | Prob > F | |
| Model | 5 | 105.27 | 0.0009 * | 5 | 31.35 | 0.0004 * |
| Linear term | ||||||
| x1 | 1 | 47.12 | 0.0004 * | 1 | 16.31 | 0.0001 * |
| x2 | 1 | 48.40 | 0.0004 * | 1 | 12.61 | 0.0002 * |
| Quadratic | ||||||
| x11 | 1 | 0.21 | 0.5053 | 1 | 0.44 | 0.0735 |
| x22 | 1 | 1.57 | 0.1147 | 1 | 1.36 | 0.0133 * |
| Interaction | ||||||
| x2x1 | 1 | 7.72 | 0.0112 * | 1 | 0.85 | 0.0284 * |
| R2 = 0.99 | R2 = 0.99 | |||||
| DHA + EPA Conc. (mM) | Packed-Bed Reactor | Ultrasonic Packed-Bed Reactor | ||||
|---|---|---|---|---|---|---|
| km (cm min−1) | Si (mM) | R2 | km (cm min−1) | Si (mM) | R2 | |
| 100 | 0.0138 | 1.66 | 0.99 | 0.0163 | 0.68 | 0.99 |
| 300 | 0.0123 | 6.60 | 0.99 | 0.0140 | 7.20 | 0.99 |
| 500 | 0.0112 | 21.50 | 0.99 | 0.0133 | 13.00 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-H.; Tsai, M.-L.; Wang, H.-M.D.; Liu, Y.-C.; Hsieh, C.; Tsai, Y.-H.; Dong, C.-D.; Huang, C.-Y.; Shieh, C.-J. Continuous Production of DHA and EPA Ethyl Esters via Lipase-Catalyzed Transesterification in an Ultrasonic Packed-Bed Bioreactor. Catalysts 2022, 12, 404. https://doi.org/10.3390/catal12040404
Kuo C-H, Tsai M-L, Wang H-MD, Liu Y-C, Hsieh C, Tsai Y-H, Dong C-D, Huang C-Y, Shieh C-J. Continuous Production of DHA and EPA Ethyl Esters via Lipase-Catalyzed Transesterification in an Ultrasonic Packed-Bed Bioreactor. Catalysts. 2022; 12(4):404. https://doi.org/10.3390/catal12040404
Chicago/Turabian StyleKuo, Chia-Hung, Mei-Ling Tsai, Hui-Min David Wang, Yung-Chuan Liu, Chienyan Hsieh, Yung-Hsiang Tsai, Cheng-Di Dong, Chun-Yung Huang, and Chwen-Jen Shieh. 2022. "Continuous Production of DHA and EPA Ethyl Esters via Lipase-Catalyzed Transesterification in an Ultrasonic Packed-Bed Bioreactor" Catalysts 12, no. 4: 404. https://doi.org/10.3390/catal12040404








