Oxidative Dehydrogenation of Ethane with CO2 over Mo/LDO Catalyst: The Active Species of Mo Controlled by LDO
Abstract
:1. Introduction
2. Results
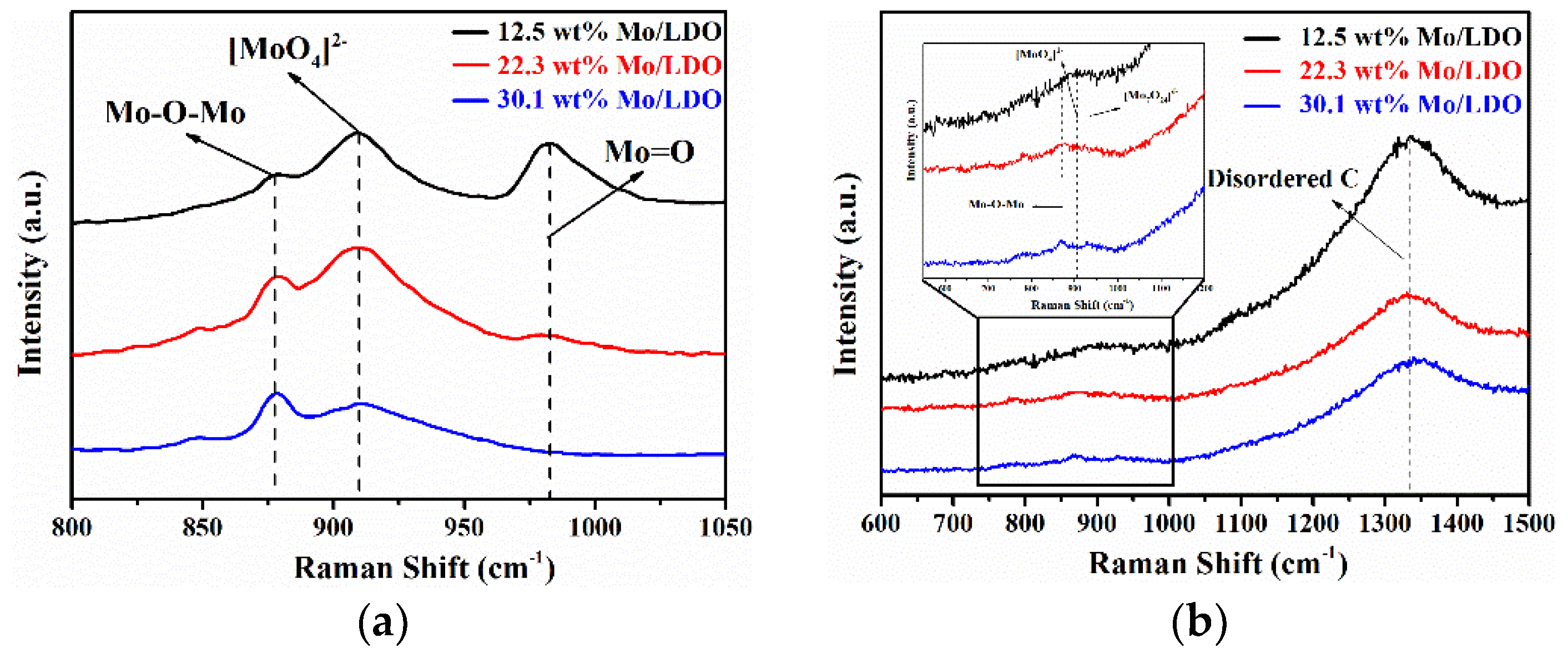
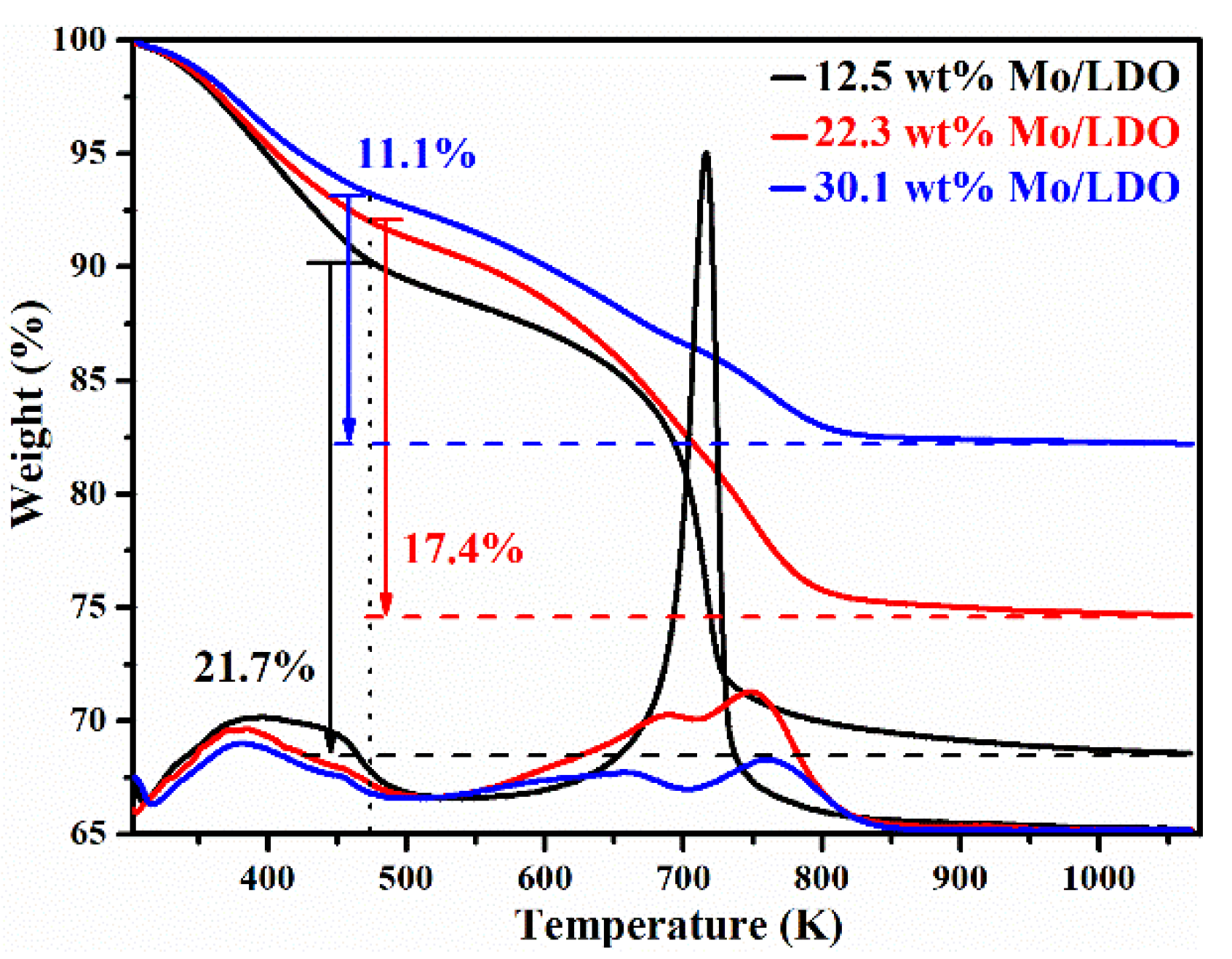
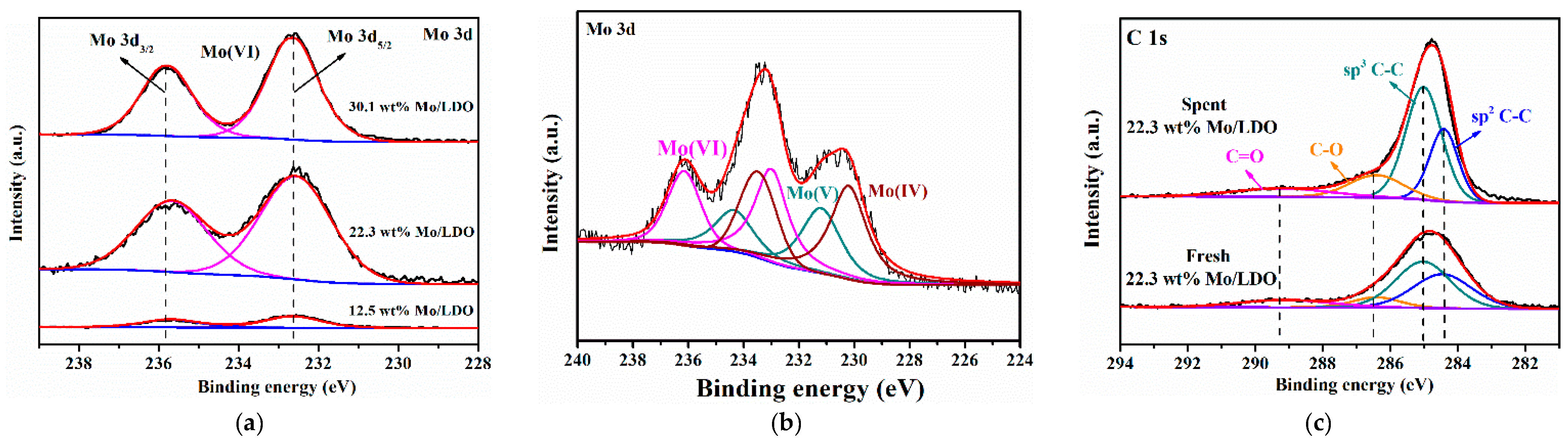
2.1. Morphological Characteristics and Structural Properties
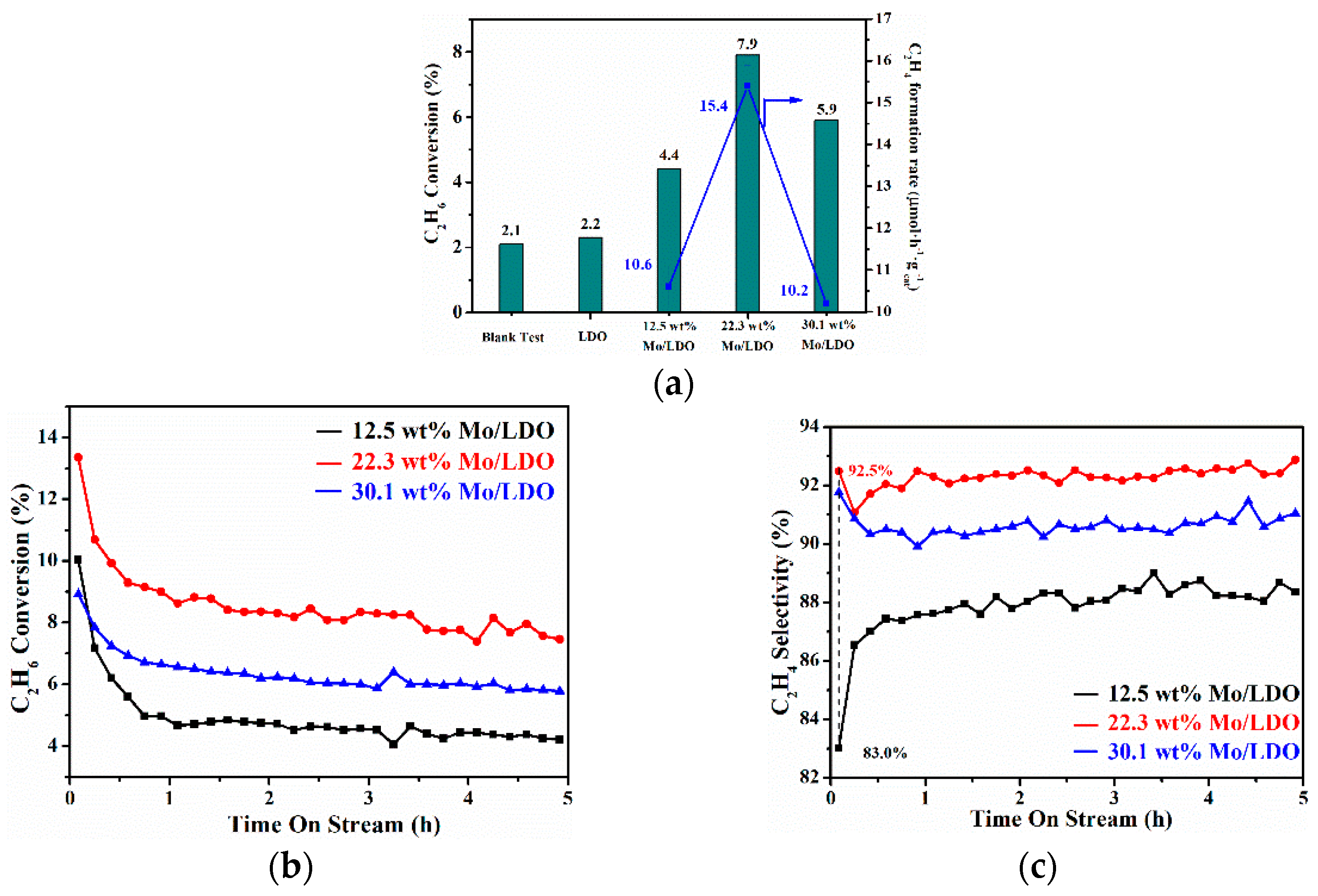
2.2. Reaction Mechanism Analysis
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.1.1. Preparation of LDH
4.1.2. Preparation of Mo/LDO
4.2. Catalyst Characterizations
4.3. Catalyst Test
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Bhowmick, A.; Wu, W.; Zhang, Y.; Diao, Y.; Zheng, A.; Zhang, C.; Xie, R.; Liu, Z.; Meng, J. Titanium Silicalite-1 Nanosheet-Supported Platinum for Non-oxidative Ethane Dehydrogenation. ACS Catal. 2021, 11, 9970–9985. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, B.; Zhao, Y.; Zhang, L. Review of Formation and Gas Characteristics in Shale Gas Reservoirs. Energies 2020, 13, 5427. [Google Scholar] [CrossRef]
- Dar, H.J.; Jakobsen, H.A.; Rout, K.R.; Jens, K.J.; Chen, D. Autothermal Gas-Phase Oxidative Dehydrogenation of Ethane to Ethylene at Atmospheric Pressure. Ind. Eng. Chem. Res. 2021, 60, 6784–6802. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, J.; Li, L.; Tian, M.; Li, X.; Pan, X.; Chen, Y.; Wang, X. Improving the selectivity of Ni-Al mixed oxides with isolated oxygen species for oxidative dehydrogenation of ethane with nitrous oxide. J. Catal. 2019, 377, 438–448. [Google Scholar] [CrossRef]
- Xie, Z.; Tian, D.; Xie, M.; Yang, S.-Z.; Xu, Y.; Rui, N.; Lee, J.H.; Senanayake, S.D.; Li, K.; Wang, H. Interfacial Active Sites for CO2 Assisted Selective Cleavage of C–C/C–H Bonds in Ethane. Chem 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Yao, R.; Herrera, J.E.; Chen, L.; Chin, Y.-H.C. Generalized Mechanistic Framework for Ethane Dehydrogenation and Oxidative Dehydrogenation on Molybdenum Oxide Catalysts. ACS Catal. 2020, 10, 6952–6968. [Google Scholar] [CrossRef]
- Abello, M.C.; Gomez, M.F.; Ferretti, O. Mo/γ-Al2O3 catalysts for the oxidative dehydrogenation of propane.: Effect of Mo loading. Appl. Catal. A 2001, 207, 421–431. [Google Scholar] [CrossRef]
- Woods, M.P.; Mirkelamoglu, B.; Ozkan, U.S. Oxygen and Nitrous Oxide as Oxidants: Implications for Ethane Oxidative Dehydrogenation over SilicaTitania-Supported Molybdenum. J. Phys. Chem. C 2009, 113, 10112–10119. [Google Scholar] [CrossRef]
- Christodoulakis, A.; Heracleous, E.; Lemonidou, A.; Boghosian, S. An operando Raman study of structure and reactivity of alumina-supported molybdenum oxide catalysts for the oxidative dehydrogenation of ethane. J. Catal. 2006, 242, 16–25. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Boghosian, S. An operando Raman study of molecular structure and reactivity of molybdenum(VI) oxide supported on anatase for the oxidative dehydrogenation of ethane. Phys. Chem. Chem. Phys. 2012, 14, 2216–2228. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Behzadi Nia, S.; Namazi, H. Green encapsulation of LDH(Zn/Al)-5-Fu with carboxymethyl cellulose biopolymer; new nanovehicle for oral colorectal cancer treatment. Int. J. Biol. Macromol. 2019, 139, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Kashani, M.; Dehghani, H.; Zarrabi, A. A biocompatible nanoplatform formed by MgAl-layered double hydroxide modified Mn3O4/N-graphene quantum dot conjugated-polyaniline for pH-triggered release of doxorubicin. Mat. Sci. Eng. C-Mater. 2020, 114, 111055. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Z.; Xu, Y.; Liu, Q.; Qian, G. CaFeAl mixed oxide derived heterogeneous catalysts for transesterification of soybean oil to biodiesel. Bioresour. Technol. 2015, 190, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Du, Y.; Liu, X.; Ci, C.; Wu, X.; Xie, X. Tunable preparation of highly dispersed NixMn-LDO catalysts derived from NixMn-LDHs precursors and application in low-temperature NH3-SCR reactions. RSC Adv. 2019, 9, 24377–24385. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yu, Y.; Huang, P.-P.; Liu, H.; Cao, C.-Y.; Song, W.-G. Core–shell structured MgAl-LDO@Al-MS hexagonal nanocomposite: An all inorganic acid–base bifunctional nanoreactor for one-pot cascade reactions. J. Mater. Chem. A 2014, 2, 339–344. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Zhang, S.; Liu, Q.; Chen, L.; Li, X.; Wang, C.; Ma, L. Ga3+-stabilized Pt in PtSn-Mg(Ga)(Al)O catalyst for promoting ethane dehydrogenation. J. Catal. 2018, 368, 79–88. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Li, F. Formation of Carbon Nanofibers from Supported Pt Catalysts through Catalytic Chemical Vapor Deposition from Acetylene. Ind. Eng. Chem. Res. 2011, 50, 9034–9042. [Google Scholar] [CrossRef]
- Ansari, A.A.; Parchur, A.K.; Alam, M.; Azzeer, A. Structural and photoluminescence properties of Tb-doped CaMoO4 nanoparticles with sequential surface coatings. Mater. Chem. Phys. 2014, 147, 715–721. [Google Scholar] [CrossRef]
- Ramakrishna, P.V. Cathodoluminescence properties of gadolinium-doped CaMoO4:Eu nanoparticles. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 149, 312–316. [Google Scholar] [CrossRef]
- Liu, X.; Fan, B.; Gao, S.; Li, R. Transesterification of tributyrin with methanol over MgAl mixed oxides derived from MgAl hydrotalcites synthesized in the presence of glucose. Fuel Process. Technol. 2013, 106, 761–768. [Google Scholar] [CrossRef]
- Yuan, X.; Niu, J.; Lv, Y.; Jing, Q.; Li, L. Ultrahigh-capacity and fast-rate removal of graphene oxide by calcined MgAl layered double hydroxide. Appl. Clay Sci. 2018, 156, 61–68. [Google Scholar] [CrossRef]
- Deng, S.; Li, S.; Li, H.; Zhang, Y.; Deng, S. Oxidative Dehydrogenation of Ethane to Ethylene with CO2 over Fe-Cr/ZrO2 Catalysts. Ind. Eng. Chem. Res. 2009, 16, 7561–7566. [Google Scholar] [CrossRef]
- Solsona, B.; Dejoz, A.; Garcia, T.; Concepción, P.; Nieto, J.; Vázquez, M.; Navarro, M.T. Molybdenum–vanadium supported on mesoporous alumina catalysts for the oxidative dehydrogenation of ethane. Catal. Today 2006, 117, 228–233. [Google Scholar] [CrossRef]
- Wu, X.; Feng, Y.; Du, Y.; Liu, X.; Zou, C.; Li, Z. Enhancing DeNOx performance of CoMnAl mixed metal oxides in low-temperature NH3-SCR by optimizing layered double hydroxides (LDHs) precursor template. Appl. Surf. Sci. 2019, 467–468, 802–810. [Google Scholar] [CrossRef]
- Mohd Sidek, H.B.; Jo, Y.K.; Kim, I.Y.; Hwang, S.-J. Stabilization of Layered Double Oxide in Hybrid Matrix of Graphene and Layered Metal Oxide Nanosheets: An Effective Way To Explore Efficient CO2 Adsorbent. J. Phys. Chem. C 2016, 120, 23421–23429. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Liu, H.; Hu, X.; Zhang, J.; Xia, C.; Chen, J.; Liu, H.; Huang, Z. Efficient Synthesis of 5-Amino-1-pentanol from Biomass-Derived Dihydropyran over Hydrotalcite-Based Ni–Mg3AlOx Catalysts. ACS Sust. Chem. Eng. 2020, 8, 6352–6362. [Google Scholar] [CrossRef]
- Tao, Q.; Zhu, J.; Frost, R.L.; Bostrom, T.E.; Wellard, R.M.; Wei, J.; Yuan, P.; He, H. Silylation of layered double hydroxides via a calcination-rehydration route. Langmuir 2010, 26, 2769–2773. [Google Scholar] [CrossRef]
- Cao, X.; Zhou, J.; Zhai, Z.; Li, S.; Yuan, G.; Qin, G. Synchronous Growth of Porous MgO and Half-Embedded Nano-Ru on a Mg Plate: A Monolithic Catalyst for Fast Hydrogen Production. ACS Sust. Chem. Eng. 2021, 9, 3616–3623. [Google Scholar] [CrossRef]
- Bhagwan, J.; Hussain, S.K.; Yu, J.S. Facile Hydrothermal Synthesis and Electrochemical Properties of CaMoO4 Nanoparticles for Aqueous Asymmetric Supercapacitors. ACS Sust. Chem. Eng. 2019, 14, 12340–12350. [Google Scholar] [CrossRef]
- Liu, L.-J.; Wang, Z.-M.; Lyu, Y.-J.; Zhang, J.-F.; Huang, Z.; Qi, T.; Si, Z.-B.; Yang, H.-Q.; Hu, C.-W. Catalytic mechanisms of oxygen-containing groups over vanadium active sites in an Al-MCM-41 framework for production of 2,5-diformylfuran from 5-hydroxymethylfurfural. Catal. Sci. Technol. 2020, 10, 278–290. [Google Scholar] [CrossRef]
- Xiong, G.; Feng, Z.; Li, J.; Yang, Q.; Ying, P.; Xin, Q.; Li, C. UV Resonance Raman Spectroscopic Studies on the Genesis of Highly Dispersed Surface Molybdate Species on γ-Alumina. J. Phys. Chem. B 2000, 104, 3581–3588. [Google Scholar] [CrossRef]
- Ji, Z.; Lv, H.; Pan, X.; Bao, X. Enhanced ethylene selectivity and stability of Mo/ZSM-5 upon modification with phosphorus in ethane dehydrogenation. J. Catal. 2018, 361, 94–104. [Google Scholar] [CrossRef]
- Che, S.; Sakamoto, Y.; Yoshitake, H.; Terasaki, O.; Tatsumi, T. Synthesis and Characterization of Mo/SBA-1 Cubic Mesoporous Molecular Sieves. J. Phys. Chem. B 2001, 14, 2433. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Zheng, S.; Cai, G.; Fu, W.; Tang, T.; He, M. Effect of the Co/Mo Ratio on the Morphology and Activity of the CoMo Catalyst Supported on MgO Nanosheets in Dibenzothiophene Hydrodesulfurization. Ind. Eng. Chem. Res. 2020, 59, 12338–12351. [Google Scholar] [CrossRef]
- Kong, L.; Li, J.; Zhao, Z.; Liu, Q.; Sun, Q.; Liu, J.; Wei, Y. Oxidative dehydrogenation of ethane to ethylene over Mo-incorporated mesoporous SBA-16 catalysts: The effect of MoO dispersion. Appl. Catal. A 2016, 510, 84–97. [Google Scholar] [CrossRef]
- Weber, R.S. Effect of Local Structure on the UV-Visible Absorption Edges of Molybdenum Oxide Clusters and Supported Molybdenum Oxides. J. Catal. 1995, 151, 470–474. [Google Scholar] [CrossRef]
- Nair, H.; Liszka, M.J.; Gatt, J.E.; Baertsch, C.D. Effects of Metal Oxide Domain Size, Dispersion, and Interaction in Mixed WOx/MoOx Catalysts Supported on Al2O3 for the Partial Oxidation of Ethanol to Acetaldehyde. J. Phys. Chem. C 2008, 112, 1612–1620. [Google Scholar] [CrossRef]
- Lee, S.; Zoican Loebick, C.; Pfefferle, L.D.; Haller, G.L. High-Temperature Stability of Cobalt Grafted on Low-Loading Incorporated Mo/MCM-41 Catalyst for Synthesis of Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2011, 115, 1014–1024. [Google Scholar] [CrossRef]
- Tian, H.; Roberts, C.A.; Wachs, I.E. Molecular Structural Determination of Molybdena in Different Environments: Aqueous Solutions, Bulk Mixed Oxides, and Supported MoO3 Catalysts. J. Phys. Chem. C 2010, 114, 14110–14120. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Villain, F.; Appel, L.G. Characterization of Mo–Sn–O system by means of Raman spectroscopy and electrical conductivity measurements. Appl. Catal. A 2003, 240, 177–182. [Google Scholar] [CrossRef]
- Wachs, I.E.; Roberts, C.A. Monitoring surface metal oxide catalytic active sites with Raman spectroscopy. Chem. Soc. Rev. 2010, 39, 5002–5017. [Google Scholar] [CrossRef]
- Savenko, D.Y.; Velieva, N.Y.; Svetlichnyi, V.A.; Vodyankina, O.V. The influence of the preparation method on catalytic properties of Mo–Fe–O/SiO2 catalysts in selective oxidation of 1,2-propanediol. Catal. Today 2020, 357, 399–408. [Google Scholar] [CrossRef]
- Gibson, E.K.; Zandbergen, M.W.; Jacques, S.D.M.; Biao, C.; Cernik, R.J.; O’Brien, M.G.; Di Michiel, M.; Weckhuysen, B.M.; Beale, A.M. Noninvasive Spatiotemporal Profiling of the Processes of Impregnation and Drying within Mo/Al2O3 Catalyst Bodies by a Combination of X-ray Absorption Tomography and Diagonal Offset Raman Spectroscopy. ACS Catal. 2013, 3, 339–347. [Google Scholar] [CrossRef]
- Bugrova, T.A.; Dutov, V.V.; Svetlichnyi, V.A.; Cortés Corberán, V.; Mamontov, G.V. Oxidative dehydrogenation of ethane with CO2 over CrOx catalysts supported on Al2O3, ZrO2, CeO2 and CexZr1-xO2. Catal. Today 2019, 333, 71–80. [Google Scholar] [CrossRef]
- Ou, J.Z.; Campbell, J.L.; Yao, D.; Wlodarski, W.; Kalantar-zadeh, K. In Situ Raman Spectroscopy of H2 Gas Interaction with Layered MoO3. J. Phys. Chem. C 2011, 115, 10757–10763. [Google Scholar] [CrossRef]
- Xi, Y.; Xiao, J.; Lin, X.; Yan, W.; Wang, C.; Liu, C. SiO2-Modified Pt/Al2O3 for Oxidative Dehydrogenation of Ethane: A Preparation Method for Improved Catalytic Stability, Ethylene Selectivity, and Coking Resistance. Ind. Eng. Chem. Res. 2018, 57, 10137–10147. [Google Scholar] [CrossRef]
- Fu, T.; Wang, Y.; Li, Z. Surface-Protection-Induced Controllable Restructuring of Pores and Acid Sites of the Nano-ZSM-5 Catalyst and Its Influence on the Catalytic Conversion of Methanol to Hydrocarbons. Langmuir 2020, 36, 3737–3749. [Google Scholar] [CrossRef]
- Shah, M.; Bordoloi, A.; Nayak, A.K.; Mondal, P. Experimental and Kinetic Studies of Methane Reforming with CO2 over a La-Doped Ni/Al2O3 Bimodal Catalyst. Ind. Eng. Chem. Res. 2021, 60, 18243–18255. [Google Scholar] [CrossRef]
- Liu, C.; He, Y.; Wei, L.; Zhang, Y.; Zhao, Y.; Hong, J.; Chen, S.; Wang, L.; Li, J. Hydrothermal Carbon-Coated TiO2 as Support for Co-Based Catalyst in Fischer–Tropsch Synthesis. ACS Catal. 2018, 8, 1591–1600. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, F.; Zhang, J.; Zheng, Q.; Xu, J.; Pan, L.; Wang, L.; Zou, J.; Zhang, X.; Li, G. Methane Dry Reforming by Ni-Cu Nanoalloys Anchored on Periclase-Phase MgAlOx Nanosheets for Enhanced Syngas Production. ACS Appl. Mater. Inter. 2021, 13, 48838–48854. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L.; Yu, X.; Qian, W.; Chu, W. Facile Route for Synthesizing Ordered Mesoporous Ni–Ce–Al Oxide Materials and Their Catalytic Performance for Methane Dry Reforming to Hydrogen and Syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Vo, T.K.; Kim, W.-S.; Kim, S.-S.; Yoo, K.S.; Kim, J. Facile synthesis of Mo/Al2O3–TiO2 catalysts using spray pyrolysis and their catalytic activity for hydrodeoxygenation. Energ Convers. Manag. 2018, 158, 92–102. [Google Scholar] [CrossRef]
- Lede, E.J.; Requejo, F.G.; Pawelec, B.; Fierro, J. XANES Mo L-edges and XPS study of Mo loaded in HY zeolite. J. Phys. Chem. B 2002, 106, 7824–7831. [Google Scholar] [CrossRef]
- Song, Y.; Sun, C.; Shen, W.; Lin, L. Hydrothermal post-synthesis of HZSM-5 zeolite to enhance the coke-resistance of Mo/HZSM-5 catalyst for methane dehydroaromatization reaction: Reconstruction of pore structure and modification of acidity. Appl. Catal. A 2007, 317, 266–274. [Google Scholar] [CrossRef]
- Julian, I.; Roedern, M.B.; Hueso, J.L.; Irusta, S.; Baden, A.K.; Mallada, R.; Davis, Z.; Santamaria, J. Supercritical solvothermal synthesis under reducing conditions to increase stability and durability of Mo/ZSM-5 catalysts in methane dehydroaromatization. Appl. Catal. B 2020, 263, 118360. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, W.; Chen, K.; Peng, Z.; Chen, W. Enhanced Photothermocatalytic Synergetic Activity Toward Gaseous Benzene for Mo+C-Codoped Titanate Nanobelts. ACS Catal. 2012, 2, 2557–2565. [Google Scholar] [CrossRef]
- Liu, B.S.; Chang, R.Z.; Jiang, L.; Liu, W.; Au, C.T. Preparation and High Performance of La2O3−V2O5/MCM-41 Catalysts for Ethylbenzene Dehydrogenation in the Presence of CO2. J. Phys. Chem. C 2008, 112, 15490–15501. [Google Scholar] [CrossRef]
- Elkins, T.W.; Neumann, B.; Bäumer, M.; Hagelin-Weaver, H.E. Effects of Li Doping on MgO-Supported Sm2O3 and TbOx Catalysts in the Oxidative Coupling of Methane. ACS Catal. 2014, 4, 1972–1990. [Google Scholar] [CrossRef]
- Shu, J.; Adnot, A.; Grandjean, B. Bifunctional Behavior of Mo/HZSM-5 Catalysts in Methane Aromatization. Ind. Eng. Chem. Res. 1999, 38, 3860–3867. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Chang, L.; Zhang, J.; Zheng, C. Mercury Adsorption and Oxidation over Cobalt Oxide Loaded Magnetospheres Catalyst from Fly Ash in Oxyfuel Combustion Flue Gas. Environ. Sci. Technol. 2015, 49, 8210–8218. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Gil-Calvo, M.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidative Steam Reforming and Steam Reforming of Methane, Isooctane, and N-Tetradecane over an Alumina Supported Spinel-Derived Nickel Catalyst. Ind. Eng. Chem. Res. 2016, 55, 3920–3929. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, X.; Zhang, T.; Han, Z.; Jiang, H. The deactivation of a ZnO doped ZrO2–SiO2 catalyst in the conversion of ethanol/acetaldehyde to 1,3-butadiene. RSC Adv. 2018, 8, 34069–34077. [Google Scholar] [CrossRef] [Green Version]
- Koirala, R.; Safonova, O.V.; Pratsinis, S.E.; Baiker, A. Effect of cobalt loading on structure and catalytic behavior of CoOx/SiO2 in CO2-assisted dehydrogenation of ethane. Appl. Catal. A 2018, 552, 77–85. [Google Scholar] [CrossRef]
- Heracleous, E.; Vakros, J.; Lemonidou, A.A.; Kordulis, C. Role of preparation parameters on the structure–selectivity properties of MoO3/Al2O3 catalysts for the oxidative dehydrogenation of ethane. Catal. Today 2004, 91–92, 289–292. [Google Scholar] [CrossRef]

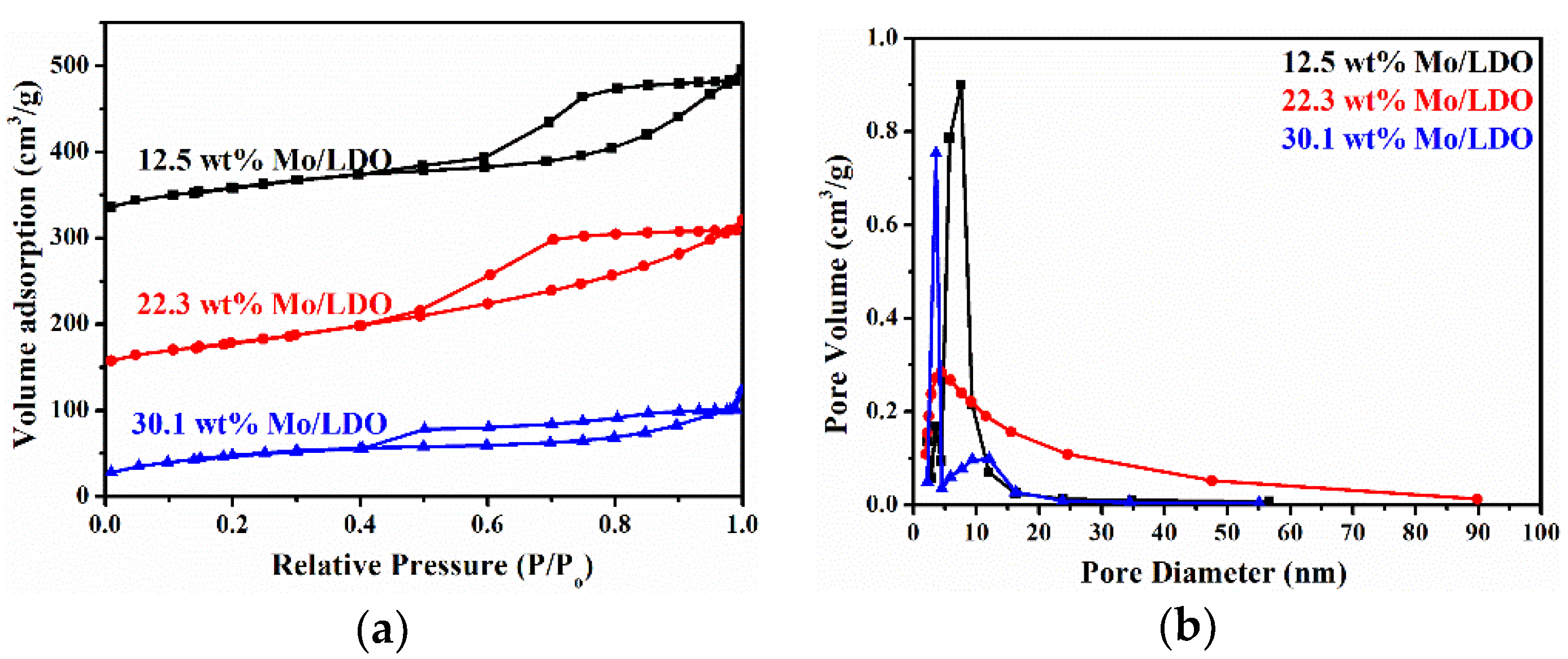
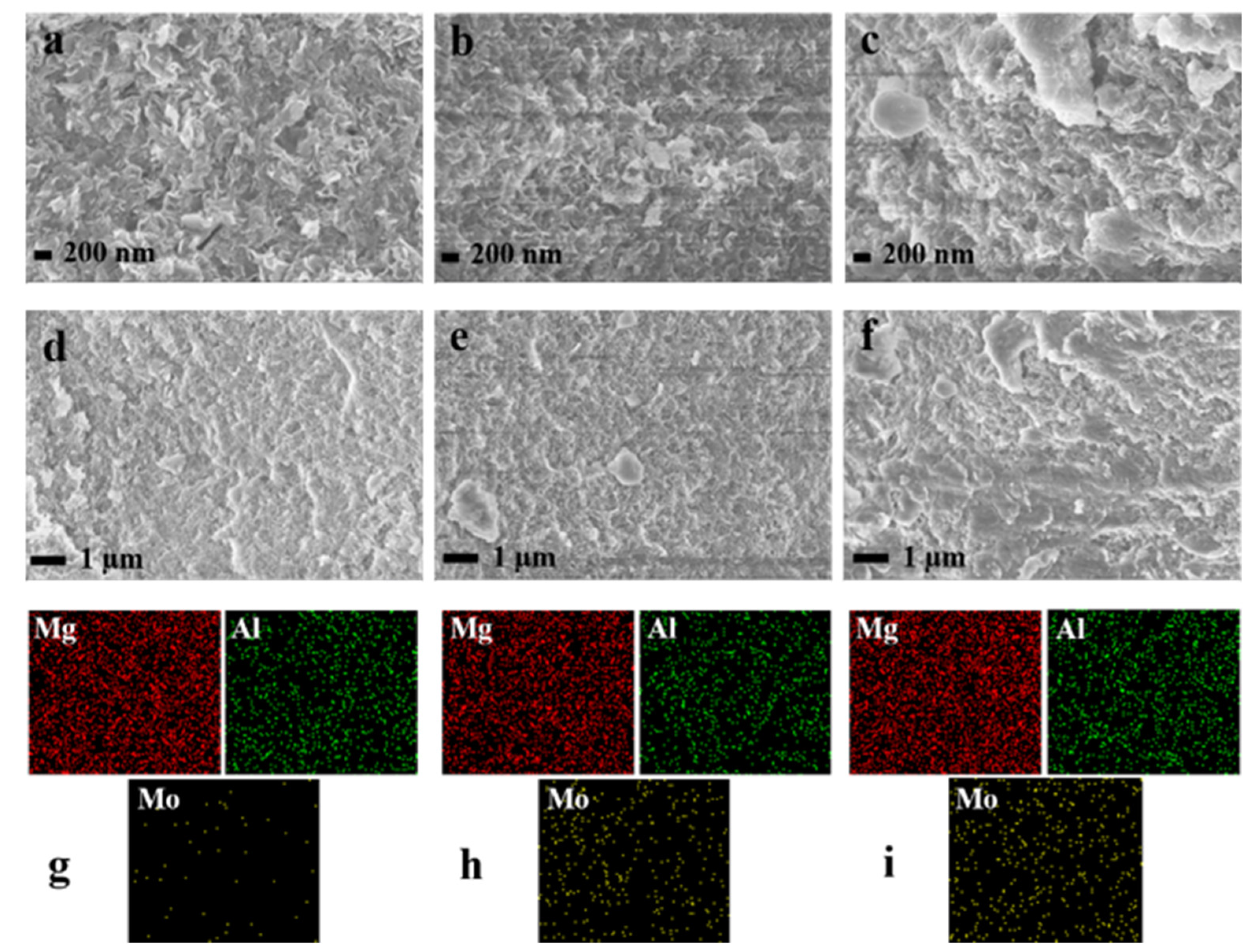
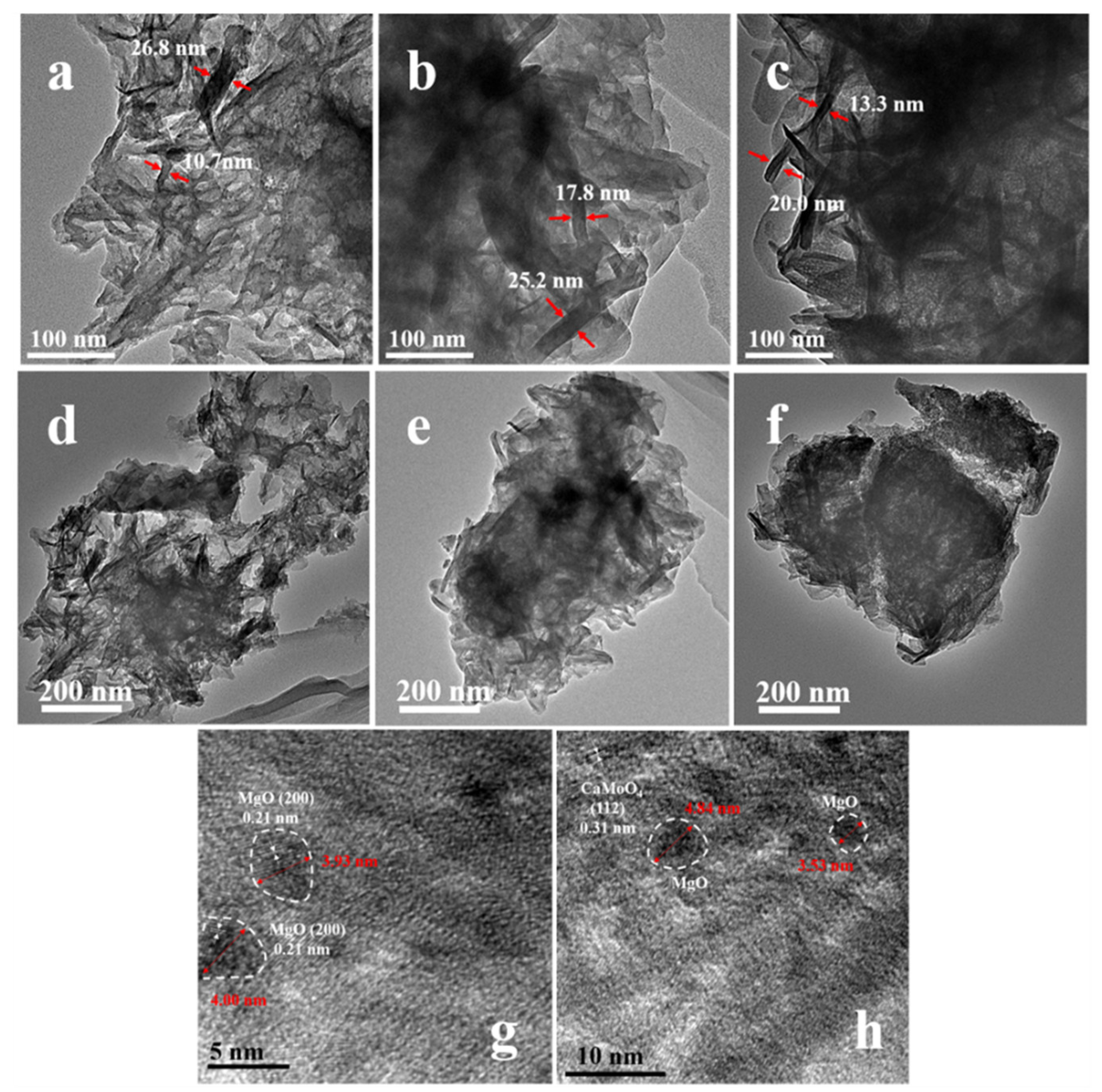
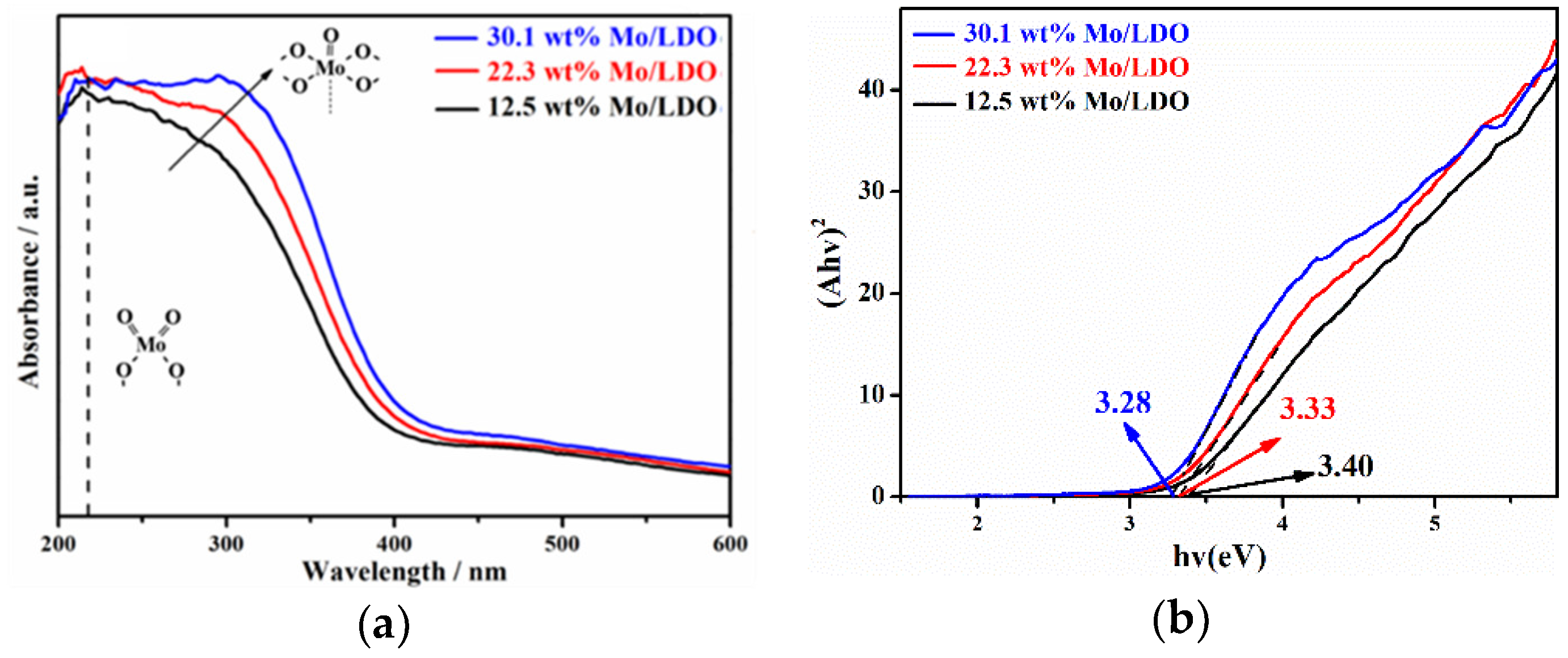

| Sample | SBET (m2·g−1) | Average Pore Diameter (nm) | Total Pore Volume (cm3·g−1) |
|---|---|---|---|
| 12.5 wt% Mo/LDO | 203.8 | 5.6 | 0.29 |
| 22.3 wt% Mo/LDO | 194.0 | 5.9 | 0.29 |
| 30.1 wt% Mo/LDO | 171.0 | 4.0 | 0.17 |
| Sample | Mo 3d5/2 | Mo 3d3/2 | Mo/(Mg + Al) (%) |
|---|---|---|---|
| 12.5 wt% Mo/LDO | 232.6 | 235.9 | 8.0 |
| 22.3 wt% Mo/LDO | 232.6 | 235.9 | 13.8 |
| 30.1 wt% Mo/LDO | 232.6 | 235.9 | 18.3 |
| Sample | Conversion (%) | Selectivity (%) | Yield (%) | C2H4 Formation Rate, (µmol·h−1·g−1cat) |
|---|---|---|---|---|
| 12.5 wt% Mo/LDO | 4.4 | 90.5 | 4.0 | 10.6 |
| 22.3 wt% Mo/LDO | 7.9 | 92.3 | 7.3 | 15.4 |
| 30.1 wt% Mo/LDO | 5.9 | 88.9 | 5.2 | 10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Wang, Q.; Yang, L.; Liao, D.; Li, S. Oxidative Dehydrogenation of Ethane with CO2 over Mo/LDO Catalyst: The Active Species of Mo Controlled by LDO. Catalysts 2022, 12, 493. https://doi.org/10.3390/catal12050493
Song G, Wang Q, Yang L, Liao D, Li S. Oxidative Dehydrogenation of Ethane with CO2 over Mo/LDO Catalyst: The Active Species of Mo Controlled by LDO. Catalysts. 2022; 12(5):493. https://doi.org/10.3390/catal12050493
Chicago/Turabian StyleSong, Gengzhe, Qi Wang, Liang Yang, Duohua Liao, and Shuang Li. 2022. "Oxidative Dehydrogenation of Ethane with CO2 over Mo/LDO Catalyst: The Active Species of Mo Controlled by LDO" Catalysts 12, no. 5: 493. https://doi.org/10.3390/catal12050493





