Abstract
C–H bonds are common in organic molecules, and the functionalization of these inactive C–H bonds has become one of the most powerful methods used to assemble complicated bioactive molecules from readily available starting materials. However, a central challenge in these reactions is controlling their stereoselectivity. Recently, significant progress has been made in the development of enantioselective C–H activation enabled by different chiral ligands for the formation of C–C and C–X bonds bearing a chiral center. In this paper, we focus on some archetypal chiral ligands for enantioselective C–H functionalization developed in recent years and analyze the mechanism of these methods, aiming to accelerate related research and to search for more efficient strategies.
1. Introduction
Chemists, similarly to construction workers, usually use different chemical bonds as “bricks and mortar” to build chemicals for several purposes, such as pharmaceuticals, agrochemicals, and materials. Therefore, looking for more efficient methods is the basic driving force for chemists. The development of C–H activation is a classic example in synthetic chemistry that can directly functionalize the most abundant C–H bonds of organic molecules. The excellent atomic economy makes it one of the most powerful methods for the construction of complicated molecules [1,2]. Over the past few decades, C(sp2)–H activation has been well developed, and it can be converted to a C–C [3] bond, C–N [4,5,6] bond, C–O [7,8,9] bond, or C–X [10,11] bond catalyzed by different transition-metal catalysts. However, the development of methods to functionalize the C(sp3)–H bond, another abundant bond in organic molecules, has faced tremendous difficulties. Compared with C(sp2)-H bonds, their high bond energies (typically 90 ~ 100 kcal/mol), low acidity (estimated pKa = 45 ~ 60), and unreactive molecular orbital profile all lead to the poor reactivity of C(sp3)–H bonds [12]. Although recent achievements have been made by chemists in this field, there are still many challenges remaining to be solved, such as stereoselectivity and regioselectivity. Therefore, C–H activation still requires in-depth study (Figure 1).
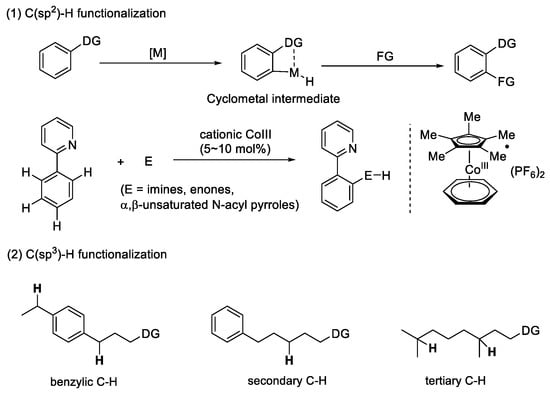
Figure 1.
Transition-metal-catalyzed C–H functionalization.
Chirality is a basic characteristic in life processes, and most of the organic molecules that make up a living body are chiral molecules. The research of chiral organic molecules has greatly improved our quality of life, particularly in the field of drug discovery [13]. Recently, strategies to easily access chiral molecules have attracted the widespread attention of chemists. At present, the development of transition-metal-catalyzed enantioselective C–H activation is undoubtedly the most effective method [14]. The binding of chiral ligands to transition metals usually creates a chiral environment for the stereo-induction of C–H functionalization. Thus, determining how to design a prominent chiral ligand is a major key to the development of this field.
In past decades, transition-metal-catalyzed asymmetric C–H functionalization has been rapidly developed, and many novel types of chiral ligands have been invented [15]. Thus, in this paper, we summarize some archetypal chiral ligands applied in the enantioselective functionalization of different types of C–H bonds and analyze the mechanism of these methods, aiming to accelerate related research and search for more efficient strategies.
2. Chiral Ligands for Enantioselective C–H Functionalization
2.1. Chiral Phosphoric Acid (CPA) Derivatives as Chiral Ligands
The arylation of the benzylic C(sp3)–H bond (as shown in Scheme 1): Chiral phosphoric acids with BINOL motifs are commonly used in asymmetric catalysis as chiral Brønsted acids or hydrogen-bond donors [16,17]. In 2015, Duan et al. reported the first example of a palladium(II)-catalyzed enantioselective arylation of the benzylic C(sp3)–H bond, using chiral phosphoric acids and amides as ligands [18]. The author hypothesized that the carbonyl group of the P=O function will facilitate the cleavage of benzyl C(sp3)–H and that the chiral environment induced by the binaphthyl skeleton will control the stereochemistry. However, moderate enantiomeric ratios (74:26 to 91:9) were obtained. In addition, the yields and the enantioselectivies dropped dramatically when this procedure was used for unbiased methylene C–H bonds. The reason for this poor enantioselectivity is the strongly coordinating bidentate auxiliary 8-aminoquinoline (AQ), which can promote the cleavage of the C–H bond in the absence of chiral ligands. Moreover, chiral phosphoric acid is relatively free in the pre-transition state. All of these factors lead to the erosion of enantioselectivity in this reaction [19,20]. Subsequently, Chen et al. developed a protocol for Pd(0)-catalyzed enantioselective benzylic C(sp3)–H arylation, mediated by the combination of AQ with the BINOL-phosphoramidite (PIII) ligand. The Pd(0/II) catalytic cycle is an unprecedented example of AQ-directed C(sp3)–H functionalization, which can improve the enantioselectivity by up to 95%. The association of chiral phosphoramidite with cesium carbonate plays an important role in the process of enantiodetermination [21].
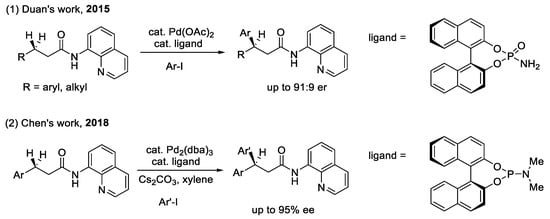
Scheme 1.
CPAs for enantioselective functionalization of benzylic C(sp3)–H bond.
The arylation of C(sp3)–H bonds adjacent to a heteroatom (as shown in Scheme 2): Saturated-aza-heterocycles are important motifs in bioactive compounds, and most could be approved as therapeutic agents. As shown in Scheme 2, many drug molecules contain a chiral aryl group at the α-position of the N-heteroatom. However, the synthesis of such molecules usually requires cumbersome synthetic routes. In 2017, Yu et al. developed a palladium-catalyzed enantioselective arylation of the α-methylene C–H bonds of pyrrolidines, piperidines, and azepanes, using thioamide as the directing group and arylboronic acids as the coupling partner [22]. Considering the significance of anionic ligands in improving the reactivity of a palladium catalyst, the authors utilized the combination of Pd2(dba)3 with CPAs to successfully control the stereochemistry. The key to this enantioselective reaction is the bulky 9-anthracenyl at the 3,3′-postion of CPAs. Although only few methods employing stoichiometric metalation at the α-position of amines to access enantioenriched α-arylation products have been developed [23,24], this methodology still exhibits excellent practicality.
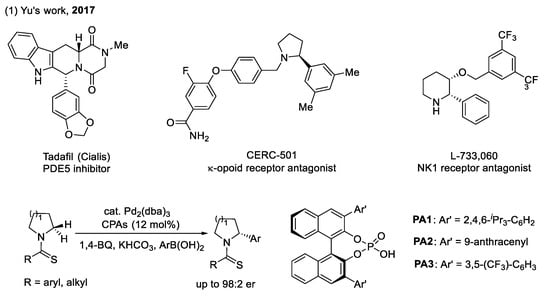
Scheme 2.
CPAs for enantioselective functionalization of the C(sp3)–H bond adjacent to the heteroatom.
The amination of the methyl C(sp3)–H bond (as shown in Scheme 3): The energy of methyl C(sp3)–H bonds is comparably weak, which means that they can be more readily functionalized. The developing activation of the methyl C(sp3)–H bond has been well studied over the past five years [25]. Among the strategies studied, some are distinct and show excellent performance, which is an unprecedent reformation of traditional synthesis. For instance, in 2014, Gaunt et al. found that secondary aliphatic amines can undergo methyl C–H bond activation, producing strained nitrogen heterocycles through a distinct four-membered-ring cyclopalladation [26]. Moreover, they also used a CPA to enable the conversion of readily available amines into synthetically valuable aziridines in high enantiomeric ratios. The oxidant additive (PIDA, diacetoxyliodo-benzene) beneficially modulates the concentration of AcO−/AcOH to avoid the erosion of enantioselectivity caused说 by the coordination of AcO− with Pd(II). A range of amines bearing prochiral methyl groups undergo enantioselective desymmetrizing methyl C–H activation to produce highly substituted and functionalized products [27].
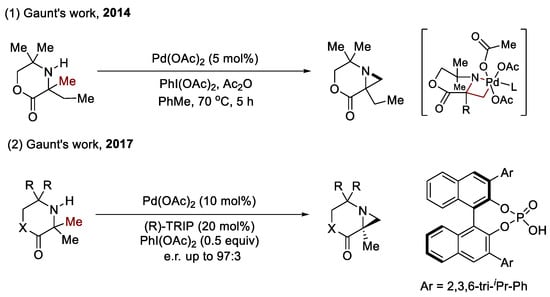
Scheme 3.
CPAs for the enantioselective functionalization of the methyl C(sp3)–H bond.
The arylation of the methylene C(sp3)–H bond (as shown in Scheme 4): Achieving high levels of enantiocontrol remains challenging for transformations via methylene C(sp3)–H bond activation due to their high bond energy, bulky steric hindrance, and ease of β-hydride elimination. However, significant progress has recently been made.
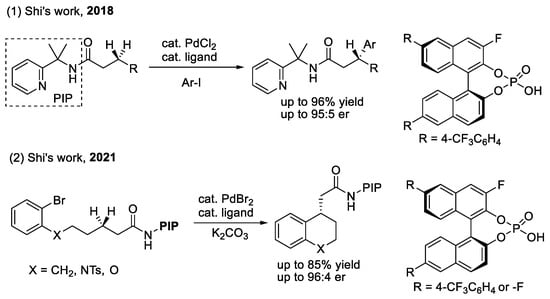
Scheme 4.
CPAs for enantioselective functionalization of methylene C(sp3)–H bond.
In 2018, Shi’s group achieved a palladium (II)-catalyzed desymmetrization of the prochiral methylene C(sp3)–H bond of linear systems using 2-pyridinylisoprpyl (PIP) as the directing group, combined with a 3-fluorine-substituted non-C2-symmetric CPA as a chiral ligand, and less reactive and cost-effective aryl bromide as the arylating reagent [28]. The key to the success of this method was the steric communication between the gem-dimethyl group of PIP and the backbone of the monodentate CPA ligand, which can restrict the rotation and, therefore, induce good stereocontrol. Based on this result, their group also successfully applied the method to the intramolecular arylation of the methylene C(sp3)–H bond to construct chiral benzo-ring compounds [29].
2.2. 3,3’-Dihalogen-BINOL as a Chiral Ligand
BINOL was first synthesized in 1926, but its potential as a ligand for metal-mediated catalysis was first recognized in 1979 by Noyori in the reduction of aromatic ketones and aldehydes [30]. With the development of asymmetric synthesis in recent years, BINOL derivatives have become some of the most commonly used chiral ligands in a wide range of reactions. However, their application in transition-metal-catalyzed C–H functionalization is still rare. Recently, Shi’s group made an outstanding achievement in the palladium-catalyzed enantioselective functionalization of C(sp3)–H bonds by using 3,3′-dihalogen-BINOL as a chiral ligand.
The intramolecular amidation of the benzyl C(sp3)–H bond and methylene C(sp3)–H bond for chiral β-lactams (as shown in Scheme 5): The challenge in constructing β-lactams is great due to the inherently higher ring tension of the four-membered ring, the lower reactivity of the C(sp3)–H bond, and the difficulty to control chemoselective intramolecular C–N reductive elimination competing with potential intermolecuar counterparts [31,32]. In 2019, Shi’s group developed the first example of the Pd(II)-catalyzed enantioselective intramolecular amidation of benzyl and the methylene C(sp3)–H bond to access β-lactams, enabled by PIP auxiliary and 3,3’-dichloro-BINOL ligands, and used 2-fluoro-1-iodo-4-nitrobenzene as an oxidant to control the desired chemoselective elimination [33]. Next, Chen’s group reported another asymmetric synthesis of β-lactams via Pd(II)-catalyzed enantioselective intramolecular C(sp3)–H amidation using the combination of 8-amino-quinoline (AQ) as the directing group and 3,3′-difluoro-BINOL as a chiral ligand [34].
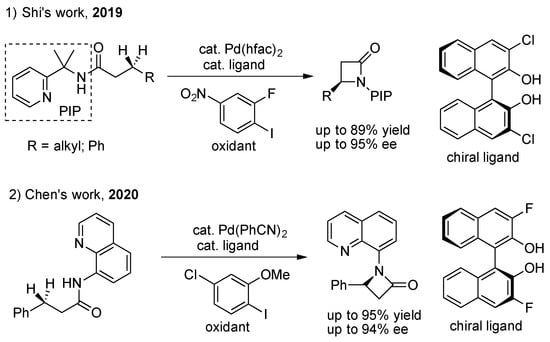
Scheme 5.
3,3′-dihalogen-BINOL for enantioselective C(sp3)–H amidation.
The alkynylation and arylation of the methylene C(sp3)–H bond (as shown in Scheme 6): Shi’s group also used 3,3′-difluoro-BINOL as a chiral ligand combined with PIP to achieve the first example of the Pd(II)-catalyzed enantioselective alkynylation of the methylene C(sp3)–H bond [35]. The palladium-catalyzed alkynylation of methylene C(sp3)–H bonds using AQ as the directing group was previously reported by Chatani and co-workers [36]. As a result, the key to the strong coordination of the auxiliary attended enantioselective version is to overcome the background reaction, which leads to the erosion of stereoselectivity. However, mechanistic studies suggest that a BINOL-derived chiral ligand can largely enhance both the reactivity and enantioselectivity. In addition, they also used the same chiral ligand to realize the Pd(II)-catalyzed intramolecular [37] and intermolecular [38] enantioselective arylation of the methylene C(sp3)–H bond.
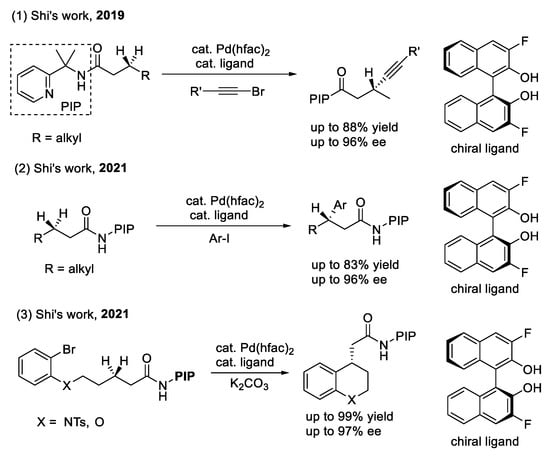
Scheme 6.
3,3′-dihalogen-BINOL for enantioselective C(sp3)–H alkynylation and arylation.
2.3. Amino Acids and Their Derivatives as Chiral Ligands
Given their easy preparation from commercially available and naturally occurring amino acids, as well as their ability to coordinate with transition metals, amino acid derivatives have been investigated in the desymmetrization of different types of C–H bonds [39].
The alkylation and amidation of the C(sp2)–H bond (as shown in Scheme 7): The first example of enantioselective C–H functionalization using an amino acid as a chiral ligand was developed by Shi and Yu et al. in 2008 [40]. They used the most traditional pyridine as the directing group to direct the asymmetric palladation of the C(sp2)–H bond. The two coordinating sites of amino acid ligands (the carboxylate and the mono-protected amine) are crucial for enantiocontrol. The steric repulsion between the bulky chain of N-Boc-isoleucine and the large aryl group of substrate led to excellent enantioselectivity. Recently, He’s group disclosed an Ir(III)-catalyzed enantioselective C(sp2)–H amidation of biphenyl sulfoxides [41]. An Ir(III) complex equipped with a t-butyl cyclopentadienyl ligand and combined with N-Piv-2-methyl-proline enabled the highly stereoselective sulfoxide derivatives. The DFT calculation results demonstrated that the combination of the Cp*tBu ligand with the (Piv)-protected methyl-substituted tertiary proline ligand created a chiral transition state.
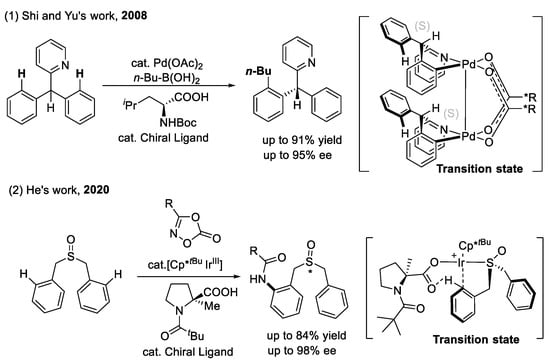
Scheme 7.
Amino acids as chiral ligands for enantioselective C(sp2)–H functionalization.
The alkylation and arylation of the methyl C(sp3)–H bond (as shown in Scheme 8): Amino acids as chiral ligands were first applied in the enantioselective methyl C(sp3)–H alkylation of 2-isopropyl pyridine and were found to be less effective, giving only moderate yield and enantioselectivity [40]. One reason for this poor result may be that the catalyst has to differentiate between a hydrogen atom and a methyl group, which are relatively close in size compared with the differentiation between an aryl group and a hydrogen atom (as shown in Scheme 8). Another prominent example of enantioselective methyl C(sp3)–H arylation, using a bidentated N-acetyl-protected aminomethyl chiral oxazoline (APAO) as a chiral ligand, was developed by Yu et al. in 2016 [42]. The ligand showed excellent compatibility and was capable of the enantioselective arylation, alkenylation, and alkynylation of isobutylamides in moderate yields with high enantioselectivities. The steric clashing between the bulky benzyl group on the oxazoline and the tert-butyl group on the side chain form a sterically chiral environment for stereoinduction. In addition, avoiding the repulsion between the methyl group of the substrate and the benzyl group of the APAO ligand is also important for enantiocontrol.
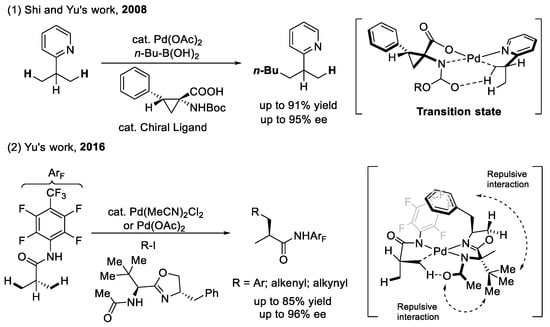
Scheme 8.
Amino acids as chiral ligands for enantioselective methyl C(sp3)–H functionalization.
The arylation of the methylene C(sp3)–H bond (as shown in Scheme 9): In 2016, Yu et al. reported the first example of a palladium (II)-catalyzed enantioselective methylene C(sp3)–H arylation via the combination of a weakly coordinating monodentate directing group with a bidentate acetyl-protected aminoethyl quinolone ligand [43]. The quinolinyl and the protected amino group chelated from the palladium (II) center to form a six-membered palladacycle, which could accelerate methylene C–H bond activation and control the stereoselectivity. The authors believed that the enantioselectivity was caused by the sterically hindered 3,5-di-tert-butyl phenyl of the ligand and the bulky directing group (-CONHArF) of the substrate, forcing the quinoline moiety of the APAQ ligand to be perpendicular to the square plane of Pd(II), which, in turn, forced the bulky R-group of the substrate to orient itself trans to the quinoline. Since this finding, transition-metal-catalyzed enantioselective methylene C(sp3)–H bonds (especially the acyclic methylene C(sp3)–H bond) have attained widespread attention. Amino acids as alternative chiral ligands have also been successfully applied in the desymmetrization of methylene C(sp3)–H and the functionalization of cyclopropyl [44,45] and cyclobutyl [46].
Scheme 9.
Amino acids as chiral ligands for enantioselective methylene C(sp3)–H functionalization.
However, they were found to be ineffective in promoting the insertion of palladium (II) into the acyclic methylene C(sp3)–H bond [42]. Recently, our group reported a rare example of the amino-acid-controlled enantioselective arylation of an acyclic methylene C(sp3)–H bond directed by a pyridyl or isoquinolinyl group [47]. We speculated that the steric repulsion between the methyl group of the substrate and the pentyl group of N-Boc-2-pentyl proline is key to promoting the enantioselectivity of this reaction.
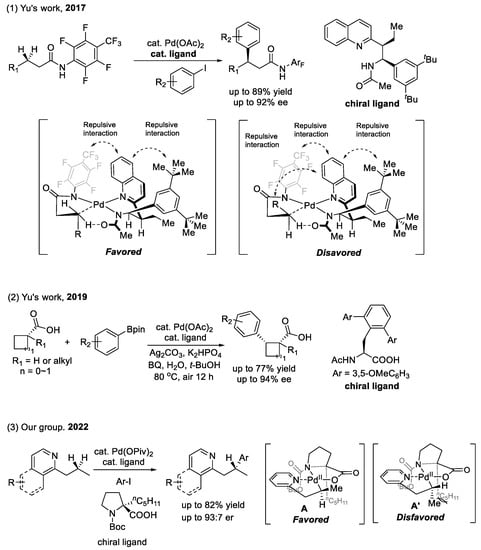
2.4. Cyclopentadiene (Cpx) and Carboxylic Acid as Chiral Ligands
Chiral Cp×Rh(I) for the functionalization of C(sp2)–H bonds (as shown in Scheme 10): Chiral cyclopentadienyl ligands as a novel auxiliary have been successfully employed in realizing the enantiocontrol of C–H functionalization. In this field, the most typical method is to use a chiral CpxRh(I) complex to achieve enantioselective C(sp2)–H activation-ring closure, developed by Cramer and co-workers in 2012 [48]. The chiral Cpx ligands do not directly participate in bond cleavage or formation and simply construct a chiral environment around the metal center. For example, the bulky substituent of the chiral Cpx ligand forces the alkene to approach from the less-hindered side, reacting with the phenyl group of substrates in the following scheme. Taking advantage of this strategy, the group of You [49], Antochick [50], and Waldman [51] developed a series of chiral Cpx ligands applied in catalytic asymmetric C–H functionalization reactions. However, the design and synthesis of these ligands usually require cumbersome synthetic routes. Therefore, developing another easily accessible method for CpxM-catalyzed stereoselective C–H functionalization is desirable.
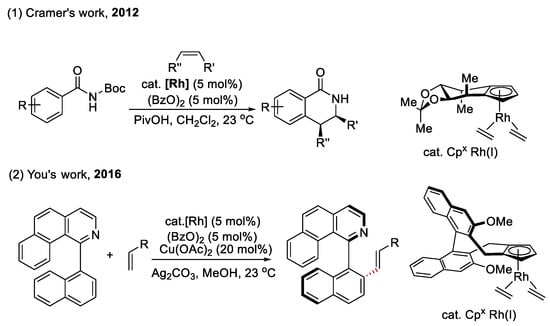
Scheme 10.
Chiral Cpx ligands applied in enantioselective C(sp2)–H functionalization.
Chiral carboxylic acid (CCA) combined with CpxM(III) for the functionalization of C(sp2)–H bonds (as shown in Scheme 11): An alternative method of CpxM-catalyzed C–H functionalization is to introduce an external chiral source in the catalytic system. Trivalent group 9 metals with a CpxM(III) catalyst and a pentadiene (Cp = Cyclopentadienyl, M = Co, Ir, Rh) are the most suitable candidates for this strategy [52,53]. Considering that CpxM(III) complexes have only one coordination site for chiral ligands (the other two for the directing group and the cleavage of the C–H bond), mono-dentated chiral carboxylic acid can be considered as a chiral auxiliary for the CpxM(III) catalyst. In this area, Matsunaga and co-workers have performed many elegant studies [54]. They developed a series of chiral mono-dentated carboxylic acid ligands and applied them in combination with different CpxM(III) catalysts to develop a variety of enantioselective C–H functionalization reactions. In 2018, they used a new binaphthyl-based chiral mono-carboxylic acid combined with CpxRh (III) to achieve the enantioselective C(sp2)–H bond functionalization of diarylmethanamines, affording potentially bioactive 1,4-dihydroisoquinolin-3(2H)-ones [55]. The sterically hindered phosphine oxide and the bulky aryl substituent at the 2’- and 3-positions on the C1-asymmetric binaphthyl-based ligand were key to the control of the enantioselectivity.
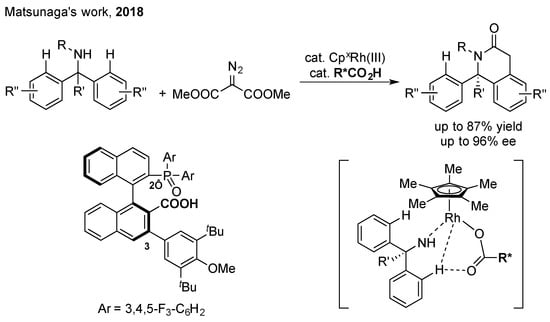
Scheme 11.
Chiral carboxylic acid combined with CpxM(III) for functionalization of C(sp2)–H bonds.
CCA combined with CpxM(III) for the functionalization of C(sp3)–H bonds (as shown in Scheme 12): Matsunaga’s group also achieved the more challenging enantioselective methyl C(sp3)–H amidation of thioamides using a CpxCo(III)/CCA catalytic system to afford chiral β-amino thiocarbonyl building blocks bearing a quaternary carbon stereocenter with high enantioselectivity and good functional group tolerance [56]. In this reaction, the N-protected tert-leucine derivative as an external chiral source coordinates with CpxCo(III) to control the enantioselectivity through an enantioselective carboxylate-assisted concerted metalation-deprotonation process. Fixing the confirmation of the CCA to form an appropriate chiral environment is the most challenging aspect of this work, since the CCA ligand is bound to the metal center by only a single σ-bond and the flexibility of the C(sp3)–H bond. The author solved this problem by modifying the steric hindrance of the protecting group on the N-atom of tert-leucine.
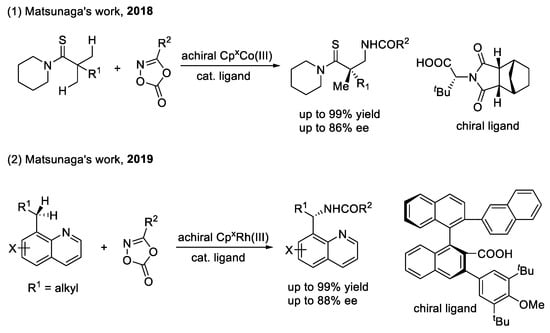
Scheme 12.
Chiral carboxylic acid combined with CpxM(III) for the functionalization of C(sp3)–H bonds.
Afterwards, they applied an achiral CpxRh(III) combined with a binaphthyl-based CCA as an external chiral source for the enantioselective amidation of methylene C(sp3)–H bonds [57], which are more attractive from a synthetic point of view. The steric hindrance of binaphthyl-based CCA created a chiral environment for the enantioselective reaction.
2.5. Other Chiral Catalysts: Chiral Sulfoxide; Chiral Phosphate Ligand (CPL); Chiral Boryl Ligand (CBL)
Chiral sulfoxide applied in enantioselective C–H functionalization (as shown in Scheme 13): Due to their similar coordinating ability to metal catalysts and close resemblance to phosphines in terms of chemical properties, sulfoxides were also proved to be an excellent chiral auxiliary in stereoselective C–H functionalization [58]. Generally, there are two main approaches when using sulfoxides as chiral reagents: chiral induction and chiral catalysis.
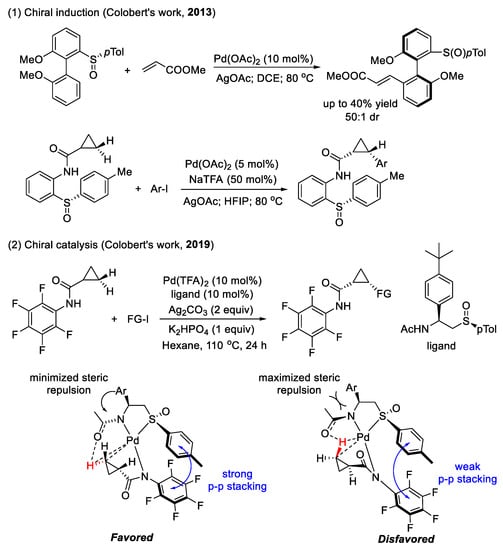
Scheme 13.
Chiral sulfoxide as a chiral ligand for enantioselective C(sp3)–H functionalization.
In 2013, Colobert and co-workers used a chiral phenyl sulfoxide as the directing group to realize the diastereoselective olefination of the biaryl C(sp2)–H bond to form an axial chirality [59]. The steric repulsion between the acrylate scaffold and the p-Tol of the chiral sulfoxide center is the key to stereoselectivity. In addition, Chahdoura and co-workers also developed an original chiral bidentate directing group to facilitate the C(sp3)–H functionalization of cyclopropanes [60]. The sulfinylaniline directing group was able to provide mono-cis-arylated cyclopropanes with good yield and moderate diastereoselectivity.
The first example of a chiral sulfoxide ligand used in the transition-metal-catalyzed enantioselective functionalization of C(sp3)–H bonds was reported by the Colobert group in 2019 [61]. They disclosed a series of enantiopure N/S ligands: N-protected-aminosulfoxides, which were applied in the enantioselective arylation and alkynylation of methylene C(sp3)–H bonds of cyclopropanes, with a good enantiomeric ratio. The stereodiscrimination proceeded via chirality relay, including (1) the repulsion between the chiral aryl group on the ethane linkage and the N-acetyl-protecting moiety, which could act as an internal base to facilitate deprotonation, and (2) the strong π–π stacking between p-Tol on the chiral sulfoxide center and the Ar5F on the directing group.
A chiral phosphate ligand (CPL) and chiral boryl ligand (CBL) applied in enantioselective C–H borylation (as shown in Scheme 14): The iridium-catalyzed transformation of C–H bonds to C–B bonds is one of the most useful methods, because borylated compounds can be converted to more complex molecules by further transformations, such as Suzuki-Miyaura cross-coupling [62]. The recent development of the enantioselective borylation of C–H bonds by Sawamura’s group and Xu’s group is of great significance.
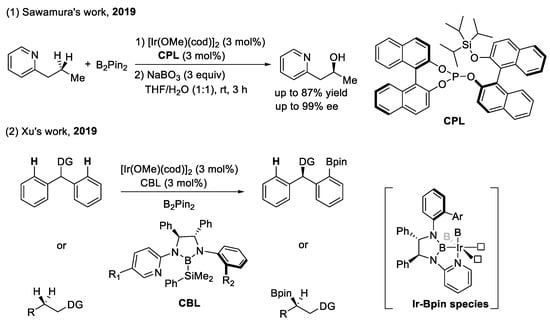
Scheme 14.
Chiral phosphate applied in enantioselective C(sp3)–H borylation.
In 2019, Sawamura and co-workers developed the highly enantioselective borylation of an unbiased methylene C(sp3)–H bond using a combination of pyridyl and 1,3-azole derivatives as the directing group, with a BINOL-based chiral monophosphate catalyst [63]. The two ligand naphthalene rings and the Ir-Bpin catalytic moiety produce a chiral environment for C(sp3)–H borylation. In addition, the weak attractive interactions, such as the π–π interaction between the heteroaromatic ring of the substrate and the naphthalene ring of the ligand, are also beneficial for chiral control.
At the same time, Xu and co-workers reported another chiral bidentate boryl ligand, which has been successfully applied to enantioselective C–H borylation [64]. The ligand coordinates with the iridium center via the pyridyl group and boryl moiety by removing PhMe2Si. The chiral scaffold of (S,S)-1,2-diphenyl-1,2-ethanediamine is responsible for the control of stereoselectivity. In the Ir(III) catalytic species, another two vacant coordinating sites (one for the directing group and another for C–H cleavage) control the regioselectivity (at the β-position of the directing group) of this enantioselective borylation.
3. Conclusions
Transition-metal-catalyzed enantioselective C–H activation has been well developed over the past few decades. As a result, numerous novel chiral ligands have emerged and have revolutionized the traditional asymmetric synthetic method. In this review, we summarized some archetypal chiral ligands and examples of their applications in the past ten years, with the aim to provide a reference for the development of new ligands. However, many challenges still exist in this research field, such as the stereoselective functionalization of any desired C–H bond at a remote position of the directing group. We are confident that the continuous development of this field will stimulate more exciting advances in the future.
Author Contributions
Writing—original draft preparation, J.-Z.L. and Y.-T.W.; writing—review and editing, D.-F.Y. and H.-L.L. We are grateful for L.-Y.Y.’s help in the explanation of the mechanism. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of China (22101058 for H.-L.L.); Guangxi Natural Science Foundation (2021GXNSFBA075060 for H.-L.L.; 2020GXNSFAA159070 for J.-Z.L.); Guangxi Science and Technology Base and Talent Project (AD21220066 for H.-L.L.); Funded Project of Guangxi Academy of Sciences (2021YBJ701 for H.-L.L.); Talent Highland Project of the Guangxi Academy of Science (for H.-L.L.); Guangxi Collaborative Innovation Center for Scientific Achievements Transformation and Application on Traditional Chinese Medicine (05020058 for J.-Z.L.); and the China-ASEAN International Innovative Center for Health Industry of Traditional Chinese Medicine (AD20297142 for J.-Z.L.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, C.; You, S.-L. Recent Develoment of Direct Asymmetric Functionalization of Inert C-H Bonds. RSC. Adv. 2014, 4, 6173–6214. [Google Scholar] [CrossRef]
- Dalton, T.; Faber, T.; Glorius, F. C-H Activation: Toward Sustainability and Applications. ACS Cent. Sci. 2021, 7, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.Y.; Wu, K.; Yu, J.-Q. Advancing the Logic of Chemical Synthesis: C-H Activation as Strategic and Tactical Disconnections for C-C Bond Construction. Angew. Chem. Int. Ed. 2021, 60, 15767–15790. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Grebies, S.; Boultadakis-Arapinis, M.; Daniliuc, C.; Glorius, F. Rh(I)/NHC*-Catalyzed Site- and Enantioselective Functionalization of C(sp3)-H Bonds Toward Chiral Triarylmethanes. ACS Catal. 2016, 6, 7652–7656. [Google Scholar] [CrossRef]
- Nasrallah, A.; Boquet, V.; Hecker, A.; Retailleau, P.; Darses, B.; Dauban, P. Catalytic Enantioselective Intermolecular Benzylic C(sp3)-H Amination. Angew. Chem. Int. Ed. 2019, 58, 8192–8196. [Google Scholar] [CrossRef]
- Qiu, M.; Fu, X.; Fu, P.; Huang, J.-H. Construction of Aziridine, Azetiding, Indole and Quinoline-like Heterocycles via Pd-mediate C-H Activation/Annulation Strategies. Org. Biomol. Chem. 2022, 20, 1339–1359. [Google Scholar] [CrossRef]
- Desai, L.-V.; Hull, K.-L.; Sanford, M.-S. Palladium-Catalyzed Oxygenation of Unactivated C(sp3)-H Bonds. J. Am. Chem. Soc. 2004, 126, 9542–9543. [Google Scholar] [CrossRef]
- Ren, Z.; Mo, F.; Dong, G. Catalytic Functionalization of Unactivated C(sp3)-H Bonds via exo-Directing Groups: Synthesis of Chemically Differnetiated 1,2-Diols. J. Am. Chem. Soc. 2012, 134, 16991–16994. [Google Scholar] [CrossRef]
- Moghimi, S.; Mahdavi, M.; Shafiee, A.; Foroumadi, A. Transition-Metal-Catalyzed Acyloxylation: Activation of C(sp2)-H and C(sp3)-H Bonds. Eur. J. Org. Chem. 2016, 2016, 3282–3299. [Google Scholar] [CrossRef]
- Giri, R.; Chen, X.; Yu, J.-Q. Palladium-Catalyzed Asymmetric Iodination of Unactivated C-H Bonds under Mild Conditions. Angew. Chem. Int. Ed. 2005, 44, 2112–2115. [Google Scholar] [CrossRef]
- Sahoo, S.-R.; Dutta, S.; Al-Thabaiti, S.; Mokhtar, M.; Maiti, D. Transition-Metal Catalyzed C-H Bond Activation by exo-metallacyle Intermediates. Chem. Commun. 2021, 57, 11885–11903. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhu, L.; Luo, S. Organocatalysis in Inert C-H Bond Functionalization. Chem. Rev. 2017, 117, 9433–9520. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.-S.; Laffan, D.; Thomson, C.; Williams, M.-T. Analysis of the Reactions used for the Preparation of Drug Candiate Molecules. Org. Biomol. Chem. 2006, 4, 2337–2347. [Google Scholar] [CrossRef]
- Karimov, R.; Hartwig, J.-F. Transition-Metal-Catalyzed Selective Functionalization of C(sp3)-H Bonds in Natural Products. Angew. Chem. Int. Ed. 2018, 57, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Saint-Denis, T.-G.; Zhu, R.-Y.; Chen, G.; Wu, Q.-F.; Yu, J.-Q. Enantioselective C(sp3)-H Bond Activation by Chiral Transition Metal Catalysis. Science 2018, 359, 759–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamfir, A.; Schenker, S.; Tsogoeva, S.-B. Chiral BINOL-Derived Phosphoric Acids: Privileged Brϕnsted Acid Organocatalysts for C-C Bond Fromation Reactions. Org. Biomol. Chem. 2010, 8, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brϕnsted Acid and and Metal Catalysis: History and Classification by Mode of Activation: Brϕnsted Acidity, Hydorgen Bonding, Ion Paring and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar] [CrossRef]
- Yan, S.-B.; Zhang, S.; Duan, W.-L. Palladium-Catalyzed Asymmetric Arylation of C(sp3)-H Bonds of Aliphatic Amides: Controlling Enantioselectivity Using Chiral Phosphoric Amides/Acids. Org. Lett. 2015, 17, 2458–2461. [Google Scholar] [CrossRef]
- Shabashov, D.; Daugulis, O. Auxilliary-Assisted Palladium-Catalyzed Arylation and Akylation of sp2 and sp3 Carborn-Hydrogen Bonds. J. Am. Chem. Soc. 2010, 132, 3965–3972. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Origins of the Enantioselectivity of a Palladium Catalyst with BINOL-Phosphoric Acid Ligands. Org. Biomol. Chem. 2018, 16, 8064–8071. [Google Scholar] [CrossRef]
- Tong, H.-R.; Zheng, S.; Li, X.; Deng, Z.; Wang, H.; He, G.; Peng, Q.; Chen, G. Pd(0)-Catalyzed Bidentate Auxiliary Directed Enantioselective Benzylic C-H Arylation of 3-Arylpropanamides Using the BINOL Phosphoramidite Ligand. ACS Catal. 2018, 8, 11502–11512. [Google Scholar] [CrossRef]
- Jain, P.; Verma, P.; Xia, G.; Yu, J.-Q. Enantioselective Amine α-Functionalization via Palladium-Catalyzed C-H Arylation of Thioamides. Nat. Chem. 2017, 9, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.-R.; Klapars, A.; Waldman, J.-H.; Dormer, P.-G.; Chen, C.-Y. Enantioselective, Palladium-Catalyzed α-arylation of N-Boc-Pyrrolidine. J. Am. Chem. Soc. 2006, 128, 3538–3539. [Google Scholar] [CrossRef] [PubMed]
- Beng, T.-K.; Gawley, R.-E. Application of Catalytic Dynamic Resolution of N-Boc-2-Lithiopiperidine to the Asymmetric Synthesis of 2-Aryl and 2-Vinyl Piperidines. Org. Lett. 2011, 13, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Romine, A.-M.; Rubel, C.-Z.; Engle, K.-M.; Shi, B.-F. Transition-Metal-Catalyzed, Coordination-Assisted Functionalization of Nonactivated C(sp3)-H Bonds. Chem. Rev. 2021, 121, 14957–15074. [Google Scholar] [CrossRef]
- He, C.; Gaunt, M.-J. Ligand-Enabled Catalytic C-H Arylation of Aliphatic Amines by a Four-membered-Ring Cyclopalladation Pathway. Angew. Chem. 2015, 127, 16066–16070. [Google Scholar] [CrossRef]
- Smalley, A.-P.; Cuthbertson, J.-D.; Gaunt, M.-J. Palladium-Catalyzed Enantioselective C-H Activation of Aliphatic Amines Using Chiral Anionic BINOL-Phosphoric Acid Ligands. J. Am. Chem. Soc. 2017, 139, 1412–1415. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.-Y.; Han, Y.-Q.; Yao, Q.-J.; Nie, X.-L.; Liu, L.; Shi, B.-F. Palladium(II)-Catalyzed Enantioselective Arylation of Unbiased Methylene C(sp3)-H Bonds Enabled by a 2-Pyridinylisopropyl Auxiliary and Chiral Phosphoric Acids. Angew. Chem. Int. Ed. 2018, 57, 9093–9097. [Google Scholar] [CrossRef]
- Han, Y.-Q.; Zhang, Q.; Yang, X.; Jiang, M.-X.; Ding, Y.; Shi, B.-F. Pd(II)-Catalyzed Enantioselective Intramolecular Arylation of Unbiased C(sp3)−H Bonds to Construct Chiral Benzo-ring Compounds. Org. Lett. 2021, 23, 97–101. [Google Scholar] [CrossRef]
- Chen, Y.; Yekta, S.; Yudin, A.-K. Modified BINOL Ligands in Asymmetric Catalysis. Chem. Rev. 2003, 103, 3155–3212. [Google Scholar] [CrossRef]
- Melander, C.; Worthington, R.-J. Overcoming Resistance to b-Lactam Antibiotics. J. Org. Chem. 2013, 78, 4207–4213. [Google Scholar]
- Mimieux Vaske, Y.-S.; Mahoney, M.-E.; Konopelski, J.-P.; Rogow, D.-L.; McDonald, W.-J. Enantiomerically Pure trans-β-Lactams from α-Amino Acids via Compact Flourescent Light (CFL) Continuous-Flow Photolysis. J. Am. Chem. Soc. 2010, 132, 11379–11385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Jiang, M.-X.; Yang, X.; Yue, Q.; Han, Y.-Q.; Ding, Y.; Shi, B.-F. Synthesis of Chiral b-Lactams by Pd-Catalyzed Enantioselective Amidation of Methylene C(sp3)-H Bonds. Chin. J. Chem. 2020, 38, 242–246. [Google Scholar] [CrossRef]
- Tong, H.-R.; Zheng, W.; Lv, X.; He, G.; Liu, P.; Chen, G. Asymmetric Synthesis of beta-Lactam via Palladium-Catalyzed Enantioselective Intramolecular C(sp3)-H Amidation. ACS Catal. 2020, 10, 114–120. [Google Scholar] [CrossRef]
- Han, Y.-Q.; Ding, Y.; Zhou, T.; Yan, S.-Y.; Song, H.; Shi, B.-F. Pd(II)-Catalyzed Enantioselective Alkynylation of Unbiased Methylene C(sp3)-H Bonds Using 3,3′-Fluorinated-BINOL as a Chiral Ligand. J. Am. Chem. Soc. 2019, 141, 4558–4563. [Google Scholar] [CrossRef]
- Ano, Y.; Tobisu, M.; Chatani, N. Palladium-Catalyzed Direct Ethynylation of C(sp3)-H Bonds in Aliphatic Carboxylic Acid Derivatives. J. Am. Chem. Soc. 2011, 133, 12984–12986. [Google Scholar] [CrossRef]
- Jiang, M.-X.; Yang, X.; Han, Q.-Y.; Zhou, T.; Xu, X.-T.; Zhang, K.; Shi, B.-F. Pd(II)-Catalyzed Asymmetric Intramolecular Arylation of Unbaised Methylene C(sp3)-H Bonds Using Readily Accessible 3,3′-F2-BINOL as Chiral Ligand. Org. Chem. Front. 2021, 8, 2903–2908. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, M.-X.; Zhou, T.; Han, Q.-Y.; Xu, X.-T.; Zhang, K.; Shi, B.-F. Pd(II)-Catalyzed Enantioselective Arylation of Unbasied Methylene C(sp3)-H Bonds Enabled by a 3,3′-F2-BINOL Ligand. Chem. Commun. 2021, 57, 5562–5565. [Google Scholar] [CrossRef]
- Shao, Q.; Wu, K.; Zhuang, Z.; Qian, S.; Yu, J.-Q. From Pd(OAc)2 to Chiral Catalysts: The Discovery and Development of Bifunctional Mono-N-Protected Amino Acid Ligands for Diverse C-H Functionalization Reactions. Acc. Chem. Res. 2020, 53, 833–851. [Google Scholar] [CrossRef]
- Shi, B.-F.; Maugel, N.; Zhang, Y.-H.; Yu, J.-Q. PdII-Catalyzed Enantioselective Activation of sp2 and sp3 C-H Bonds Using mono Protected Amino Acids as Chiral Ligands. Angew. Chem. Int. Ed. 2008, 47, 4882–4886. [Google Scholar] [CrossRef]
- Liu, W.; Yang, W.; Zhu, J.; Guo, Y.; Wang, N.; Ke, J.; Yu, P.; He, C. Dual-Ligand-Enabled Ir(III)-Catalyzed Enantioselective C-H Amidation for the Synthesis of Chiral Sulfoxides. ACS Catal. 2020, 10, 7207–7215. [Google Scholar] [CrossRef]
- Chen, G.; Gong, W.; Zhuang, Z.; Andra, M.-S.; Chen, Y.-Q.; Hong, X.; Yang, Y.-F.; Liu, T.; Houk, K.-N.; Yu, J.-Q. Ligand-Accelerated Enantioselective Methylene C(sp3)-H Bond Activation. Science 2016, 353, 1023–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.-F.; Shen, P.-X.; He, J.; Wang, X.-B.; Zhang, F.; Shao, Q.; Zhu, R.-Y.; Mapelli, C.; Qiao, J.-X.; Poss, M.-A.; et al. Fromation of a-chiral centers by asymmetric β-C(sp3)-H Arylation, Alkenylation and Alkynylation. Science 2017, 355, 499–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Shen, P.-X.; Shao, Q.; Hong, K.; Qiao, T.-X.; Yu, J.-Q. PdII-Catalyzed Enantioselective C(sp3)-H Activation/Cross-Coupling Reactions of Free Carboxylic Acids. Angew. Chem. Int. Ed. 2019, 58, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Yu, J.-Q. Pd(II)-Catalyzed Enantioselective γ-C(sp3)-H Functionalization of Free Cyclopropylmethyl Amines. J. Am. Chem. Soc. 2020, 142, 12015–12019. [Google Scholar] [CrossRef]
- Xiao, L.-J.; Hong, K.; Luo, F.; Hu, L.; Ewing, W.-R.; Yeung, K.-S.; Yu, J.-Q. PdII-Catalyzed Enantioselective C(sp3)-H Arylation of Cyclobutyl Ketones Using a Chiral Transient Directing Group. Angew. Chem. Int. Ed. 2020, 59, 9594–9600. [Google Scholar] [CrossRef]
- Li, H.-L.; Yang, D.-F.; Jing, H.-Q.; Antilla, J.-C.; Kuninobu, Y. Palladium-Catalyzed Enantioselective C(sp3)-H Arylation of 2-Propyl Azaaryls Enabled by an Amino Acid Ligand. Org. Lett. 2022, 24, 1286–1291. [Google Scholar] [CrossRef]
- Ye, B.; Cramer, N. Chiral Cyclopenyadienyl Ligands as Stereocontrolling Element in Asymmetric C-H Functionalization. Science 2012, 338, 504–506. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; You, S.-L. Construction of Axial Chirality by Rhodium-Catalyzed Asymmetric Dehydrogenative Heck Coupling of Biaryl Compounds with Alkenes. Angew. Chem. Int. Ed. 2014, 53, 13244–13247. [Google Scholar] [CrossRef]
- Li, H.; Gontla, R.; Flegel, J.; Merten, C.; Ziegler, S.; Antonchick, A.-P.; Waldmann, H. Enantioselective Formal C(sp3)-H Bond Activation in the Synthesis of Bioactive Spiropyrazolone Derivatives. Angew. Chem. Int. Ed. 2019, 58, 307–311. [Google Scholar] [CrossRef]
- Shan, G.; Flegel, J.; Li, H.; Merten, C.; Ziegler, S.; Antonchick, A.-P.; Waldmann, H. C-H Bond Activation for the Synthesis of Heterocyclic Atropisomers Yields Hedgehog Pathway Inhibitors. Angew. Chem. Int. Ed. 2018, 57, 14250–14254. [Google Scholar] [CrossRef] [PubMed]
- Ueura, K.; Satoh, T.; Miura, M. An Efficient Waste-Free Oxidative Coupling via Regioselective C-H Bond Cleavage: Rh/Cu-Catalyzed Reaction of Benzoic Acids with Alkynes and Acrylates under Air. Org. Lett. 2007, 9, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Ikemoto, H.; Matsunaga, S.; Kanai, M. A Cationic High-Valent Cp*CoIII Complex for the Catalytic Generation of Nucleophilic Organometallic Species: Directed C-H Bond Activation. Angew. Chem. Int. Ed. 2013, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Matsnaga, S. Chiral Carboxylic Acid Assisted Enantioselective C-H Activation with Achiral CpxMIII (M = Co, Rh, Ir) Catalysts. ACS Catal. 2021, 11, 6455–6466. [Google Scholar] [CrossRef]
- Lin, L.; Fukagawa, S.; Sekine, D.; Tomita, E.; Yoshino, T.; Matsunaga, S. Chiral Carboxylic Acid-Enabled Achiral Rhodium(III)-Catalyzed Enantioselective C-H Functionalization. Angew. Chem. Int. Ed. 2018, 57, 12048–12052. [Google Scholar] [CrossRef] [Green Version]
- Fukagawa, S.; Kato, Y.; Tanaka, R.; Kojima, M.; Yoshino, T.; Matsunaga, S. Enantioselective C(sp3)-H Amidation of Thioamides Catalyzed by a CobaltIII/Chiral Carboxylic Acid Hybrid System. Angew. Chem. Int. Ed. 2018, 58, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, S.; Kojima, M.; Yoshino, T.; Matsunaga, S. Catalytic Enantioselective Methylene C(sp3)-H Amidation of 8-Alkylquinolines Using Cp*RhIII/Chiral Carboxylic Acid System. Angew. Chem. Int. Ed. 2019, 58, 18154–18158. [Google Scholar] [CrossRef]
- Jerhaoui, S. Sulfoxydes: Novel Stratefy for the Asymmetric C(sp3)-H Activation. Organic Chemistry. Ph.D. Thesis, Université de Strasbourg, Strasbourg, France, 2018. [Google Scholar]
- Wesch, T.; Leroux, F.-R.; Colobert, F. Atropodiastereoselective C-H Olefination of Biphenyl p-Tolyl Sulfoxides with Acrylates. Adv. Synth. Catal. 2013, 355, 2139–2144. [Google Scholar] [CrossRef]
- Jerhaoui, S.; Chahdoura, F.; Rose, C.; Djukic, J.-P.; Wencel-Delord, J.; Colobert, F. Enantiopure Sulfinyl Aniline as a Removable and Recyclable Chiral Auxiliary for Asymmetric C(sp3)-H Bond Activation. Chem. Eur. J. 2016, 22, 17397–17406. [Google Scholar] [CrossRef]
- Jerhaoui, S.; Djukic, J.-P.; Wencel-Delord, J.; Colobert, F. Asymmetric, Nearly Barrierless C(sp3)-H Activation Promoted by Easily-Accessible N-Protected Aminosulfoxides as New Chiral Ligands. ACS Catal. 2019, 9, 2532–2542. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.-L. Palladium-Catalyzed Suzuki-Miyaura Cross-Coupline Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, R.-L.; Iwai, T.; Maeda, S.; Sawamura, M. Iridium-catalyzed Asymmetric Borylation of Unactivated Methylene C(sp3)-H Bonds. J. Am. Chem. Soc. 2019, 141, 6817–6821. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhao, H.; Li, Y.; Gao, Q.; Ke, Z.; Xu, S. Chiral Bidentate Boryl Ligand Enabled Iridium-Catalyzed Asymmetric C(sp2)-H Borylation of Diarylmethylamines. J. Am. Chem. Soc. 2019, 141, 5334–5342. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).