Sonophotocatalysis—Limits and Possibilities for Synergistic Effects
Abstract
:1. Introduction
2. Results and Discussion
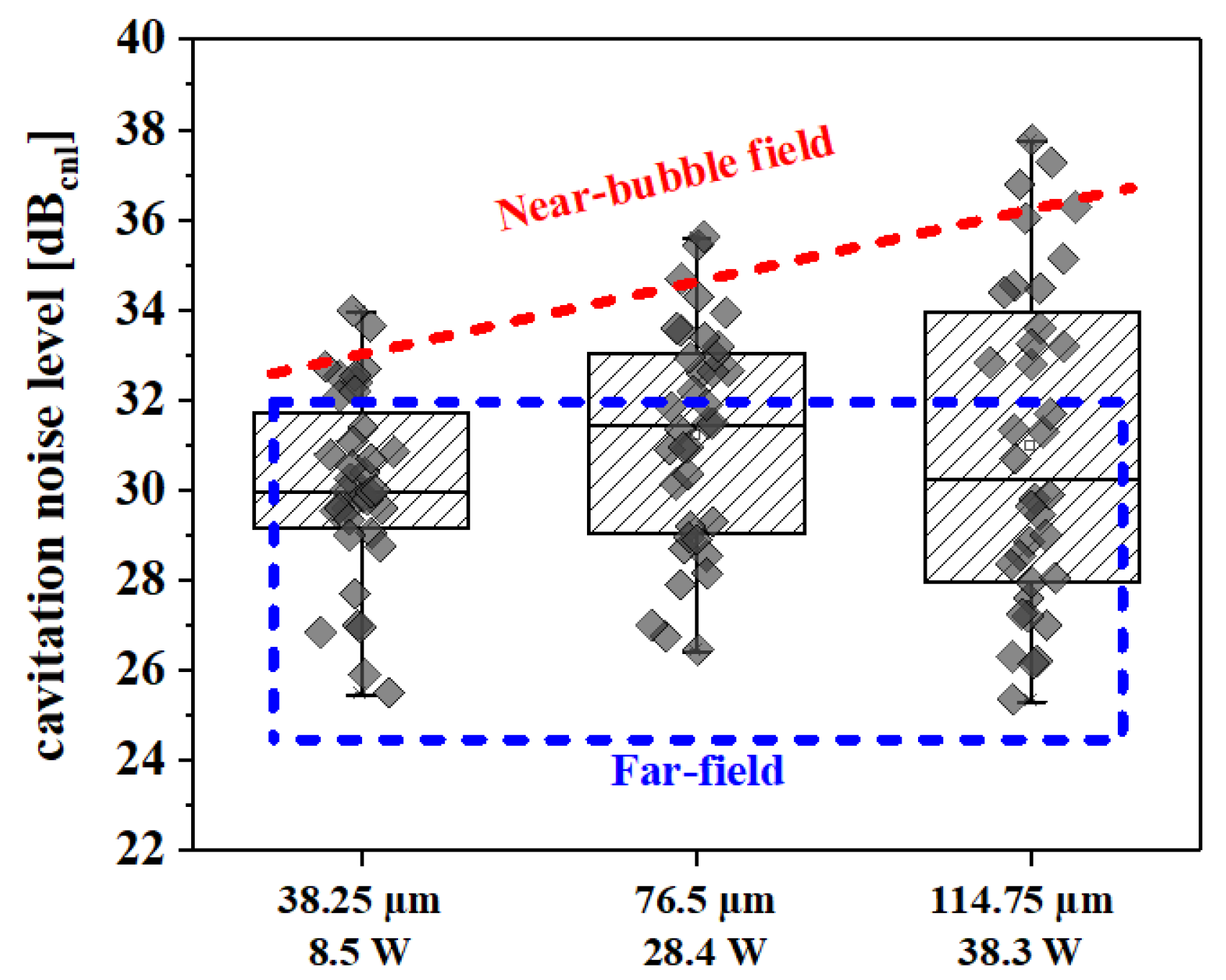
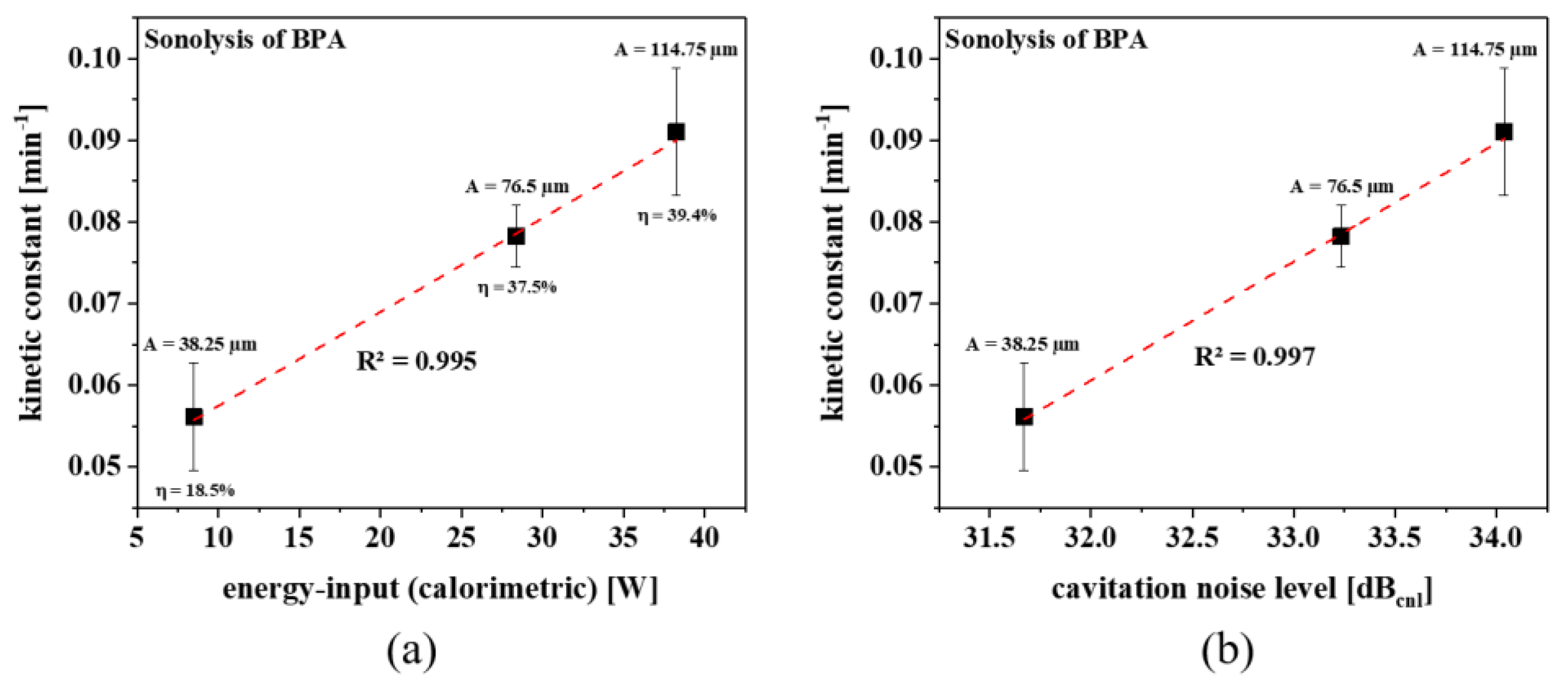
2.1. Sonolysis
2.2. Photolysis and Photocatalysis
2.2.1. Photolysis
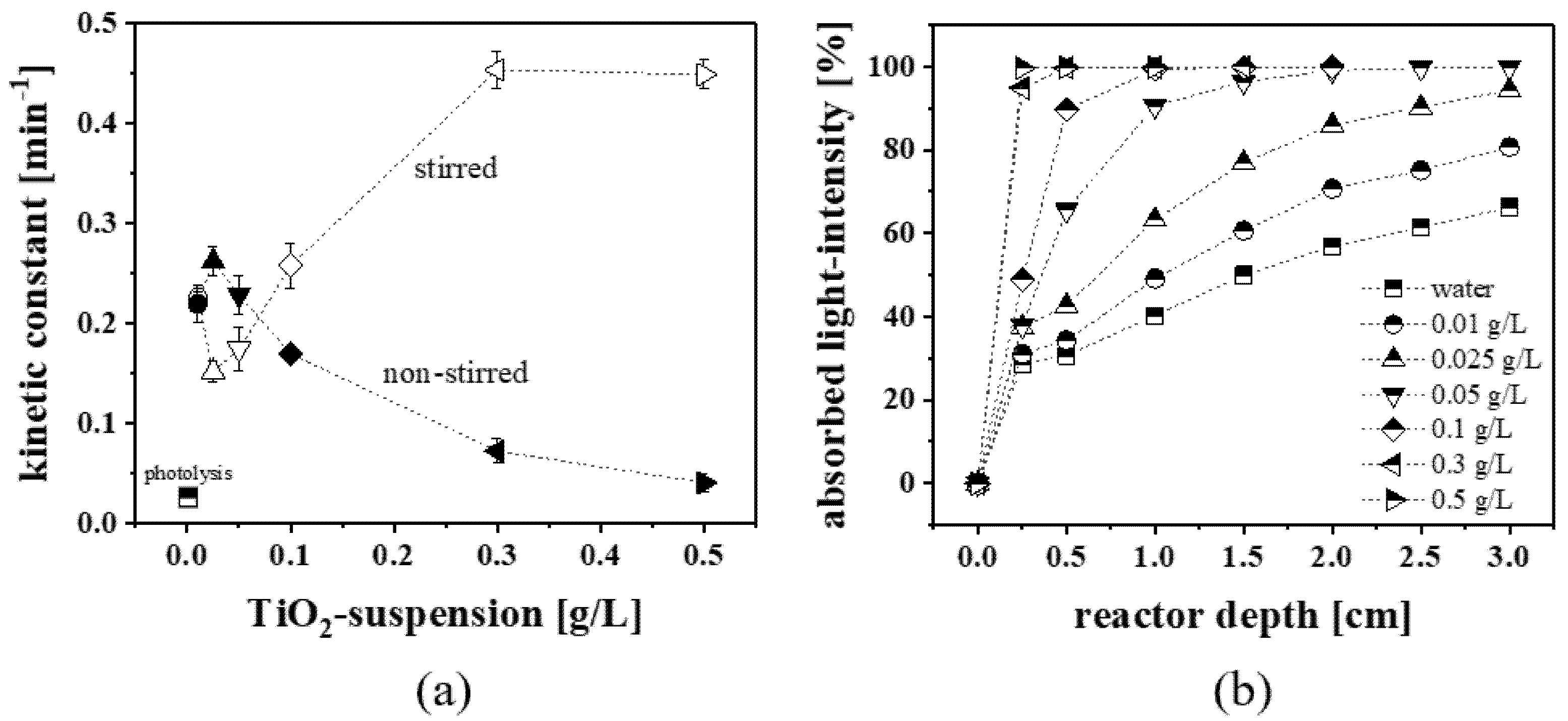
2.2.2. Stationary Photocatalysis (Non-Stirred)
2.2.3. Instationary Photocatalysis (Mechanical Stirred)
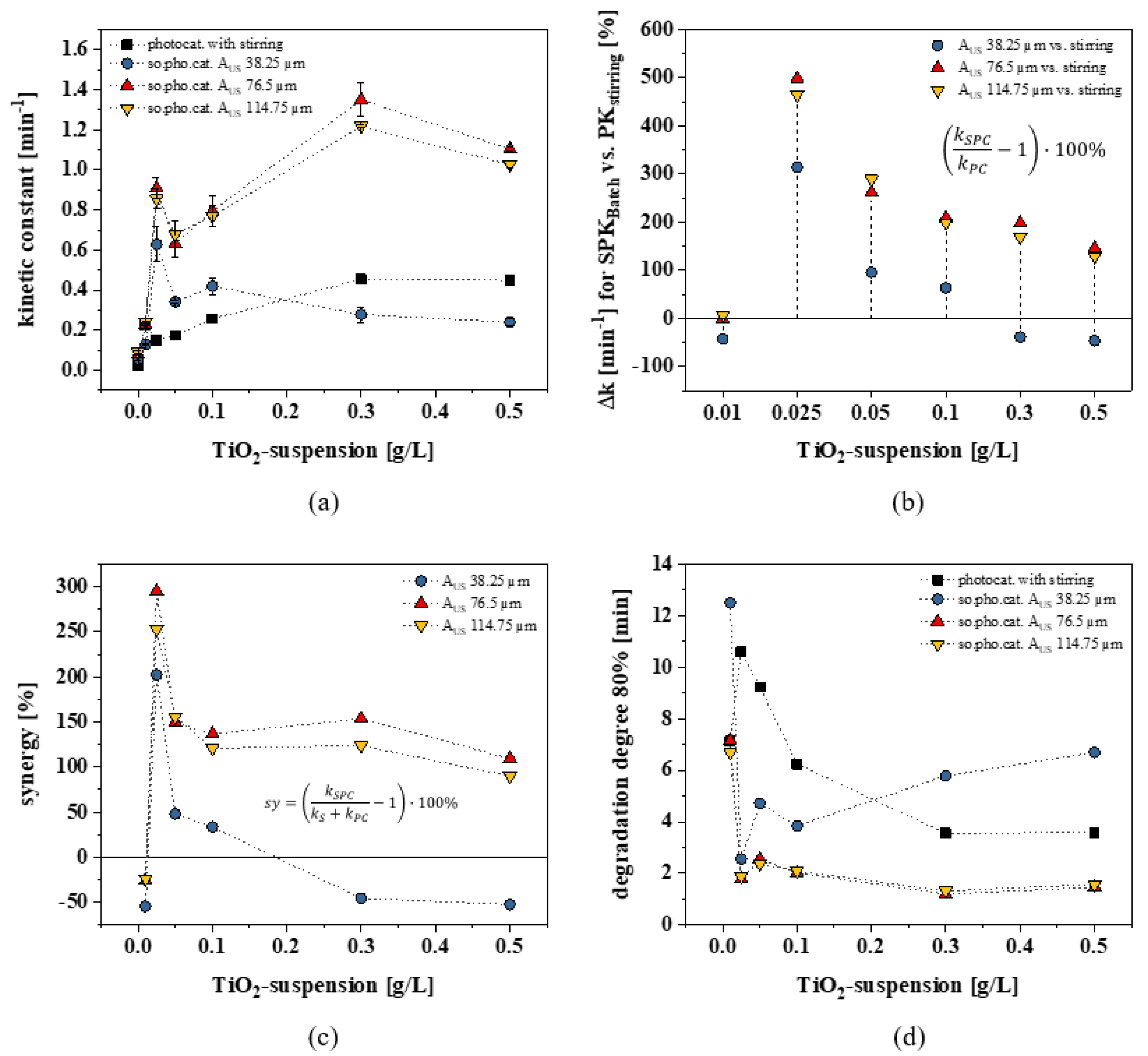
2.3. Sonophotocatalysis
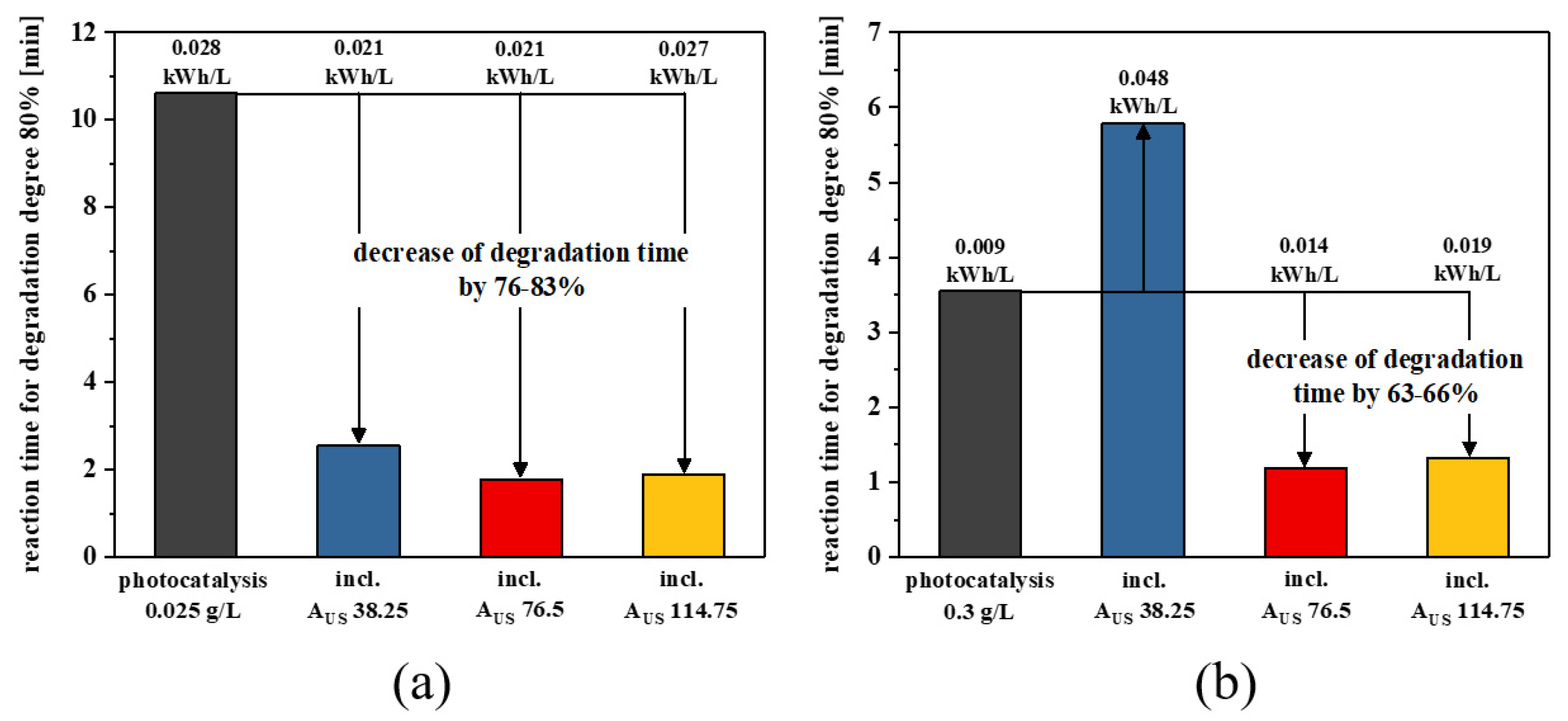
2.3.1. Low-Dosage Sonophotocatalysis
2.3.2. High-Dosage Sonophotocatalysis
2.3.3. Energy Assessment
3. Materials and Methods
3.1. Materials
3.2. Setup and Reactor Concept
3.3. Mapping of Ultrasound-Induced Bubble Fields
3.4. Mapping of Oxidative Species by Chemiluminescence
3.5. Measuring the Cavitation Noise via Hydrophone
3.6. Estimation of Light Absorption by TiO2 Nanoparticles
3.7. Sono(photo)catalytic Degradation Experiments of Bisphenol A
3.8. Quantification of Bisphenol A with HPLC/FD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Chaminda, G.G.T.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef]
- Yap, H.C.; Pang, Y.L.; Lim, S.; Abdullah, A.Z.; Ong, H.C.; Wu, C.H. A comprehensive review on state-of-the-art photo-, sono-, and sonophotocatalytic treatments to degrade emerging contaminants. Int. J. Environ. Sci. Technol. 2018, 16, 601–628. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Anandan, S. Review on the recent improvements in sonochemical and combined sonochemical oxidation processes—A powerful tool for destruction of environmental contaminants. Renew. Sustain. Energy Rev. 2016, 55, 426–454. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemoller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Selli, E. Synergistic effects of sonolysis combined with photocatalysis in the degradation of an azo dye. Phys. Chem. Chem. Phys. 2002, 4, 6123–6128. [Google Scholar] [CrossRef]
- Verma, A.; Chhikara, I.; Dixit, D. Photocatalytic treatment of pharmaceutical industry wastewater over TiO2 using immersion well reactor: Synergistic effect coupling with ultrasound. Desalination Water Treat. 2014, 52, 6591–6597. [Google Scholar] [CrossRef]
- Joseph, C.G.; Puma, G.L.; Bono, A.; Krishnaiah, D. Sonophotocatalysis in advanced oxidation process: A short review. Ultrason. Sonochem. 2009, 16, 583–589. [Google Scholar] [CrossRef]
- Adewuyi, Y.G. Sonochemistry in Environmental Remediation. 2. Heterogeneous Sonophotocatalytic Oxidation Processes for the Treatment of Pollutants in Water. Environ. Sci. Technol. 2005, 39, 8557–8570. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.S.; Babgi, B.; Alghamdi, Y.; Aksu, M.; Madhavan, J.; Ashokkumar, M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason. Sonochem. 2016, 29, 568–576. [Google Scholar] [CrossRef] [PubMed]
- van Wijngaarden, L. Mechanics of collapsing cavitation bubbles. Ultrason. Sonochem. 2016, 29, 524–527. [Google Scholar] [CrossRef] [Green Version]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E., Jr. The Sonochemical Hot-Spot. J. Am. Chem. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, N.; Deng, Y.; Lin, T.F.; Ma, Y.; Li, L.; Sui, M. Degradation of bisphenol-A using ultrasonic irradiation assisted by low-concentration hydrogen peroxide. J. Environ. Sci. 2011, 23, 31–36. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Tabrizi, S.B.; Molanee, S. Ultrasonic degradation of Rhodamine B in aqueous solution: Influence of operational parameters. J. Hazard. Mater. 2008, 152, 381–386. [Google Scholar] [CrossRef]
- Zeng, Q.; Gonzalez-Avila, S.R.; Dijkink, R.; Koukouvinis, P.; Gavaises, M.; Ohl, C.-D. Wall shear stress from jetting cavitation bubbles. J. Fluid Mech. 2018, 846, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Vinesh, V.; Shaheer, A.R.M.; Neppolian, B. Reduced graphene oxide (rGO) supported electron deficient B-doped TiO2 (Au/B-TiO2/rGO) nanocomposite: An efficient visible light sonophotocatalyst for the degradation of Tetracycline (TC). Ultrason. Sonochem. 2019, 50, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Sonophotocatalytic treatment of rhodamine B using visible-light-driven CeO2/Ag2CrO4 composite in a batch mode based on ribbon-like CeO2 nanofibers via electrospinning. Environ. Sci. Pollut. Res. Int. 2019, 26, 8050–8068. [Google Scholar] [CrossRef] [PubMed]
- Kakavandi, B.; Bahari, N.; Kalantary, R.R.; Fard, E.D. Enhanced sono-photocatalysis of tetracycline antibiotic using TiO2 decorated on magnetic activated carbon (MAC@T) coupled with US and UV: A new hybrid system. Ultrason. Sonochem. 2019, 55, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.G.; Karthik, P.; John, M.C.; Lakhera, S.K.; Ashokkumar, M.; Khim, J.; Neppolian, B. Synergistic effect of sono-photocatalytic process for the degradation of organic pollutants using CuO-TiO2/rGO. Ultrason. Sonochem. 2019, 50, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Vinoth, R.; Karthik, P.; Devan, K.; Neppolian, B.; Ashokkumar, M. TiO2-NiO p-n nanocomposite with enhanced sonophotocatalytic activity under diffused sunlight. Ultrason. Sonochem. 2017, 35, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Tabasideh, S.; Maleki, A.; Shahmoradi, B.; Ghahremani, E.; McKay, G. Sonophotocatalytic degradation of diazinon in aqueous solution using iron-doped TiO2 nanoparticles. Sep. Purif. Technol. 2017, 189, 186–192. [Google Scholar] [CrossRef]
- Sunasee, S.; Wong, K.T.; Lee, G.; Pichiah, S.; Ibrahim, S.; Park, C.; Kim, N.C.; Yoon, Y.; Jang, M. Titanium dioxide-based sonophotocatalytic mineralization of bisphenol A and its intermediates. Environ. Sci. Pollut. Res. Int. 2017, 24, 15488–15499. [Google Scholar] [CrossRef]
- May-Lozano, M.; Mendoza-Escamilla, V.; Rojas-García, E.; López-Medina, R.; Rivadeneyra-Romero, G.; Martinez-Delgadillo, S.A. Sonophotocatalytic degradation of Orange II dye using low cost photocatalyst. J. Clean. Prod. 2017, 148, 836–844. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Recent advancements in the sonophotocatalysis (SPC) and doped-sonophotocatalysis (DSPC) for the treatment of recalcitrant hazardous organic water pollutants. Ultrason. Sonochem. 2017, 36, 481–496. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Sonophotocatalytic reactors for wastewater treatment: A critical review. AIChE J. 2004, 50, 1051–1079. [Google Scholar] [CrossRef]
- Selli, E.; Bianchi, C.L.; Pirola, C.; Cappelletti, G.; Ragaini, V. Efficiency of 1,4-dichlorobenzene degradation in water under photolysis, photocatalysis on TiO2 and sonolysis. J. Hazard. Mater. 2008, 153, 1136–1141. [Google Scholar] [CrossRef]
- Ryu, B.; Wong, K.T.; Choong, C.E.; Kim, J.R.; Kim, H.; Kim, S.H.; Jeon, B.H.; Yoon, Y.; Snyder, S.A.; Jang, M. Degradation synergism between sonolysis and photocatalysis for organic pollutants with different hydrophobicity: A perspective of mechanism and application for high mineralization efficiency. J. Hazard. Mater. 2021, 416, 125787. [Google Scholar] [CrossRef]
- Mrowetz, M.; Pirola, C.; Selli, E. Degradation of organic water pollutants through sonophotocatalysis in the presence of TiO2. Ultrason. Sonochem. 2003, 10, 247–254. [Google Scholar] [CrossRef]
- Gonzalez, A.S.; Martinez, S.S. Study of the sonophotocatalytic degradation of basic blue 9 industrial textile dye over slurry titanium dioxide and influencing factors. Ultrason. Sonochem. 2008, 15, 1038–1042. [Google Scholar] [CrossRef]
- Hapeshi, E.; Fotiou, I.; Fatta-Kassinos, D. Sonophotocatalytic treatment of ofloxacin in secondary treated effluent and elucidation of its transformation products. Chem. Eng. J. 2013, 224, 96–105. [Google Scholar] [CrossRef]
- Jelic, A.; Michael, I.; Achilleos, A.; Hapeshi, E.; Lamnropoulou, D.; Perez, S.; Petrovic, M.; Fatta-Kassinos, D.; Barcelo, D. Transformation products and reaction pathways of carbamazepine during photocatalytic and sonophotocatalytic treatment. J. Hazard. Mater. 2013, 263P, 177–186. [Google Scholar] [CrossRef]
- Yetim, T.; Tekin, T. Synergistic effect of sonolysis and photocatalysis in the degradation kinetics of effluent solution. Kinet. Catal. 2016, 57, 578–585. [Google Scholar] [CrossRef]
- Ghasemi, M.; Ghoreishian, S.M.; Norouzi, M.; Badii, K.; Ozaee, K.; Kwak, C.H.; Huh, Y.S. Optimization of sonophotocatalytic decolorization of Begazol Black B by loaded, double-sided nanophotocatalysts on porous substrate: A central composite design approach. J. Taiwan Inst. Chem. Eng. 2018, 93, 166–175. [Google Scholar] [CrossRef]
- Eshaq, G.; ElMetwally, A.E. Bmim[OAc]-Cu2O/g-C3N4 as a multi-function catalyst for sonophotocatalytic degradation of methylene blue. Ultrason. Sonochem. 2019, 53, 99–109. [Google Scholar] [CrossRef]
- Zargazi, M.; Entezari, M.H. Sonochemical versus hydrothermal synthesis of bismuth tungstate nanostructures: Photocatalytic, sonocatalytic and sonophotocatalytic activities. Ultrason. Sonochem. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Abo, R.; Kummer, N.-A.; Merkel, B.J. Optimized photodegradation of Bisphenol A in water using ZnO, TiO2 and SnO2 photocatalysts under UV radiation as a decontamination procedure. Drink. Water Eng. Sci. 2016, 9, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Estácio, S.G.; Couto, P.C.d.; Guedes, R.C.; Cabral, B.J.C.; Simoes, J.A.M. Homolytic dissociation in hydrogen-bonding liquids: Energetics of the phenol O-H bond in methanol and the water O-H bond in water. Theor. Chem. Acc. 2004, 112, 282–289. [Google Scholar] [CrossRef]
- Jamali, A.; Vanraes, R.; Hanselaer, P.; van Gerven, T. A batch LED reactor for the photocatalytic degradation of phenol. Chem. Eng. Process. 2013, 71, 43–50. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on UV-LED/TiO2 process for degradation of Rhodamine B dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Swiss Federal Council, Water Protection Regulation (GSchV): Amendment of the Law (AS 2015 4791) from 4 November 2015. pp. 4791–4808. Available online: https://www.newsd.admin.ch/newsd/message/attachments/42169.pdf (accessed on 10 June 2022).


| m(TiO2) [g/L] | Reactor | 0.01 | 0.025 | 0.05 | 0.1 | 0.3 | 0.5 |
|---|---|---|---|---|---|---|---|
| Sorption [%] | 4.51 ± 0.79 | 4.90 ± 0.94 | 4.52 ± 1.69 | 5.69 ± 0.66 | 5.34 ± 2.05 | 5.67 ± 1.89 | 4.01 ± 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paustian, D.; Franke, M.; Stelter, M.; Braeutigam, P. Sonophotocatalysis—Limits and Possibilities for Synergistic Effects. Catalysts 2022, 12, 754. https://doi.org/10.3390/catal12070754
Paustian D, Franke M, Stelter M, Braeutigam P. Sonophotocatalysis—Limits and Possibilities for Synergistic Effects. Catalysts. 2022; 12(7):754. https://doi.org/10.3390/catal12070754
Chicago/Turabian StylePaustian, Dirk, Marcus Franke, Michael Stelter, and Patrick Braeutigam. 2022. "Sonophotocatalysis—Limits and Possibilities for Synergistic Effects" Catalysts 12, no. 7: 754. https://doi.org/10.3390/catal12070754
APA StylePaustian, D., Franke, M., Stelter, M., & Braeutigam, P. (2022). Sonophotocatalysis—Limits and Possibilities for Synergistic Effects. Catalysts, 12(7), 754. https://doi.org/10.3390/catal12070754





