Abstract
The environmental impact of corrosion is very dangerous and consumes much of world’s efforts and funds. This work discusses the safeguarding of the environment, metals, and metal-infra structures by efficient Schiff’s base inhibitors. The corrosion inhibitors [(1E,3E)-N1,N3-dibutyl-1-(thiophen-2-yl)butane-1,3-diimine] (GSB-I) and [(1Z,3Z)-N1,N3-bis(4-methylhexan-2-yl)-1-(thiophen-2-yl)butane-1,3-diimine] (GSB-II) were successfully synthesized and evaluated for the protection of API 5L X65 steel (CS) in 1 M HCl media using electrochemical techniques, SEM/EDS, and quantum chemical calculations. GSB-I and GSB-inhibitory I’s efficiency is proportional to the concentration of the test. In the presence of 1 mM GSB-I and GSB-II, the maximum inhibitory efficiency was determined to be 90.6 and 93.8 percent, respectively. According to potentiodynamic polarization tests, the two compounds are effective inhibitors of mixed-type corrosion. The physisorption and chemisorption of both inhibitors followed the Langmuir adsorption isotherm on CS surfaces. The biological reactivity of both GSB has been examined, and encouraging results have been obtained as antifungal, antibacterial, and biocidal agents against sulfate-reducing bacteria (SRB). In addition, using DFT calculations and molecular dynamic (MD) simulation, the effect of GSB-I and GSB-II molecular configuration on corrosion inhibition behavior in acidic environments was investigated.
1. Introduction
The depletion of natural resources and the loss of energy by the destruction of energy plants or infra-structures are common problems caused by corrosion. Serious environmental crises to marine life, human, animals, and plants can originate from the flow of energy sources to their surroundings [1]. The sudden release of toxic substances into the atmosphere by the cracks in pipelines and energy plants threatens innocent lives and safety as a whole. Environmental pollution from the escaped products due to unexpected corrosion mechanism is notable; more than 2.5 trillion US dollars were consumed for corrosion fighting in 2006. Generally, corrosion has a dangerous impact on the environment and increasing worldwide attention is directed towards this problem [2]. In this work, we introduce an effective methodology to protect the environment through the effective protection of common types of steel, which is the backbone of many industrial plants. Due to its high mechanical force, strong conductivity, low cost, and ease of assembly, carbon steel (CS) is used in several industries, including chemical industrial units, petroleum and gas production, maritime manufacturing, and building parts [3,4,5]. Acidic solutions, particularly HNO3, H2SO4, and HCl, are commonly used for acetification, scaling removal, boiler scrub, and petroleum well acidization [6,7]. Because acids are corrosive, pickling would damage the CS substrate surface much more when removing corrosion crops. In an acidic environment, the effectiveness and lifetime of CS instruments or equipment are compromised [8,9,10,11]. To protect metallic materials from acid-induced corrosion, a variety of techniques and strategies have been developed. Organic corrosion inhibitors have been proven to be one of the most successful technological methods for avoiding metal corrosion in harsh environments [12,13,14]. Organic corrosion inhibitors are often made up of heteroatom donors, including O, N, S, and P, as well as organic compounds with a high cloud of electronic density as π-electrons at double bonds [15,16]. The formation of protective layers on metal surfaces to protect against corrosive environments has increased their physicochemical adsorption bulks on the metal substrate [17,18,19]. Schiff bases are compounds that are valuable in drug development, photochromic applications, and corrosion prevention [20,21]. Schiff bases, particularly those used in the corrosion industry, provide corrosion protection for a wide range of metals and alloys, including CS, Al, and Cu [22]. Because of their availability, ease of synthesis, high purity, low toxicity, and environmental friendliness, Schiff bases are more common than other organic compounds as anticorrosive inhibitors [23]. A Schiff base based on phenylene pyridinyl methenamine as a novel inhibitor for CS corrosion in 1 M HCl has been explored.
Soraya et al. [24] prepared and characterized a novel Schiff base 2,4-Bis (2-hydroxy naphthaldehyde). The corrosion inhibition of carbon steel in 1 M hydrochloric acid solutions using a Schiff base was investigated. The inhibition efficiency obtained by this compound increased by increasing its concentration. The polarization studies showed that this compound acts as mixed type inhibitor through adsorption following the Langmuir isotherm. Xiao-Long Li et al. claimed that at 30 °C, the exitance 800 ppm of this Schiff base reached a 94 percent efficiency [25]. Abdelmalik et al. also examined novel Schiff bases based on imidazole pyridine in a 1 M hydrochloric acid solution. They hypothesized that these inhibitors merely reduce the cathode area without changing the cathodic reaction mechanism and that the efficiency of the inhibition improves as the inhibitor concentration rises [26]. To investigate the corrosion inhibition efficacy of GSB-I and GSB-II in 1 M HCl, we used open circuit potential (OCP), electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization (PDP). GSB compounds were also tested for their antifungal, anti-candida, antibacterial, and biocide properties against sulfate-reducing bacteria (SRB). DFT has become a popular method for determining how molecule prearrangement in planetary and molecular assets affects the effectiveness of inhibitor corrosion reserves. The importance of quantum chemical calculations in establishing the link between molecular composition, chemical reactivity, and inhibitory selectivity is well recognized [27]. DFT was used to explore the regular optimization assembly and e-mass spreading of the inhibitor molecule’s frontier molecules orbitals, as well as how quantum chemical factors influence the inhibitor’s corrosion inhibition property. As a result, the GSB-I and GSB-II reaction sites have been identified as being sensitive to electron donation and receipt at the local level. The adsorption of GSB-I and GSB-II on the CS surface was also simulated using molecular dynamic (MD) simulations.
2. Materials and Methods
2.1. Materials
Analytical mark chemicals and solvents were used without additional purification for all synthesis applications. All of the chemicals required were provided by Sigma-Aldrich (St. Louis, MO, USA), including 1-(thiophen-2-yl)butane-1,3-dione, butan-1-amine, and 4-methylhexan-2-amine. Egypt’s Al-Nasr Co. (Giza, Egypt) contributed CH3OH, C2H5OH, (CH3)2CO, petroleum ether, and 37% hydrochloric acid.
2.2. Synthesis of Gemini Schiff Bases
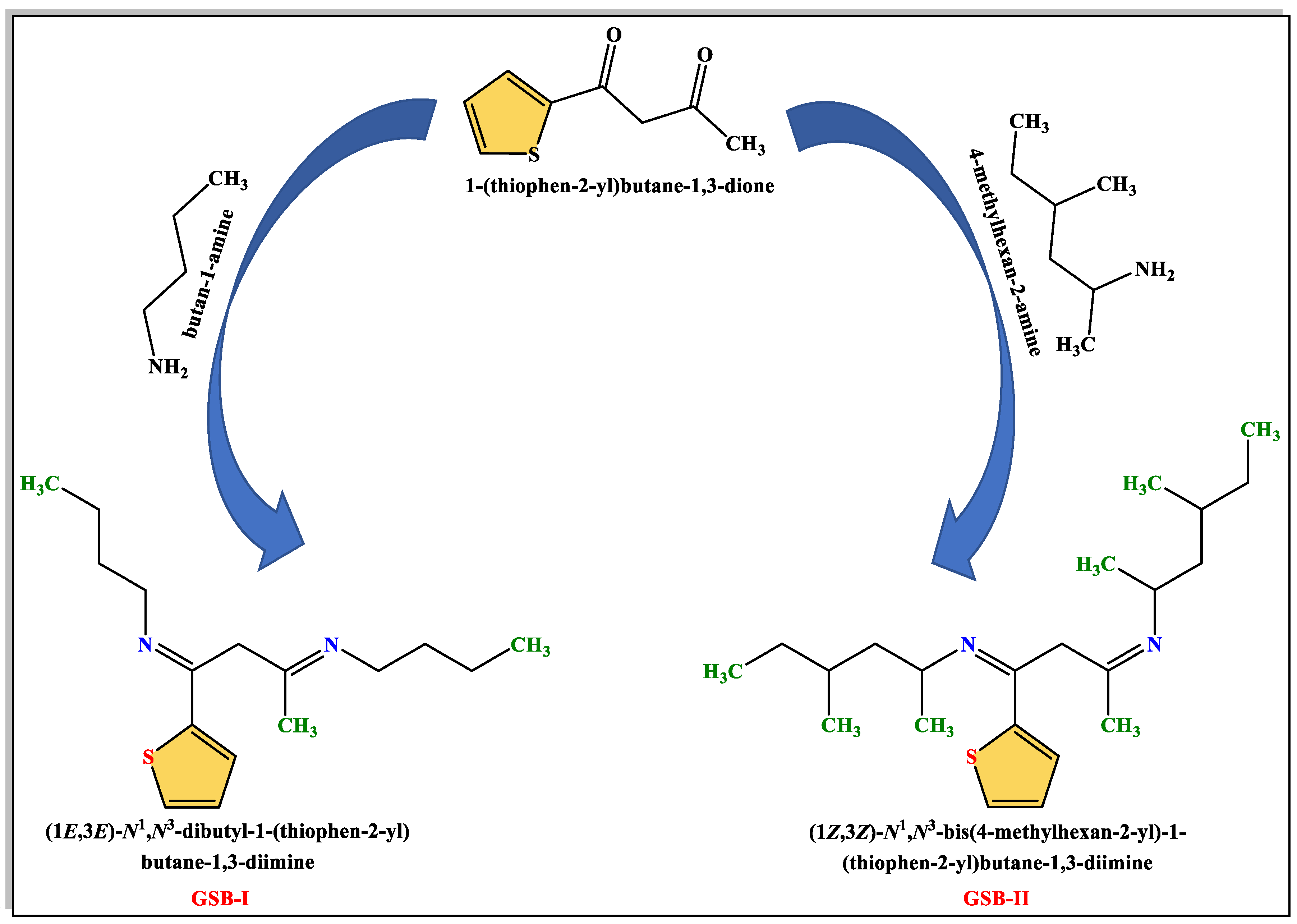
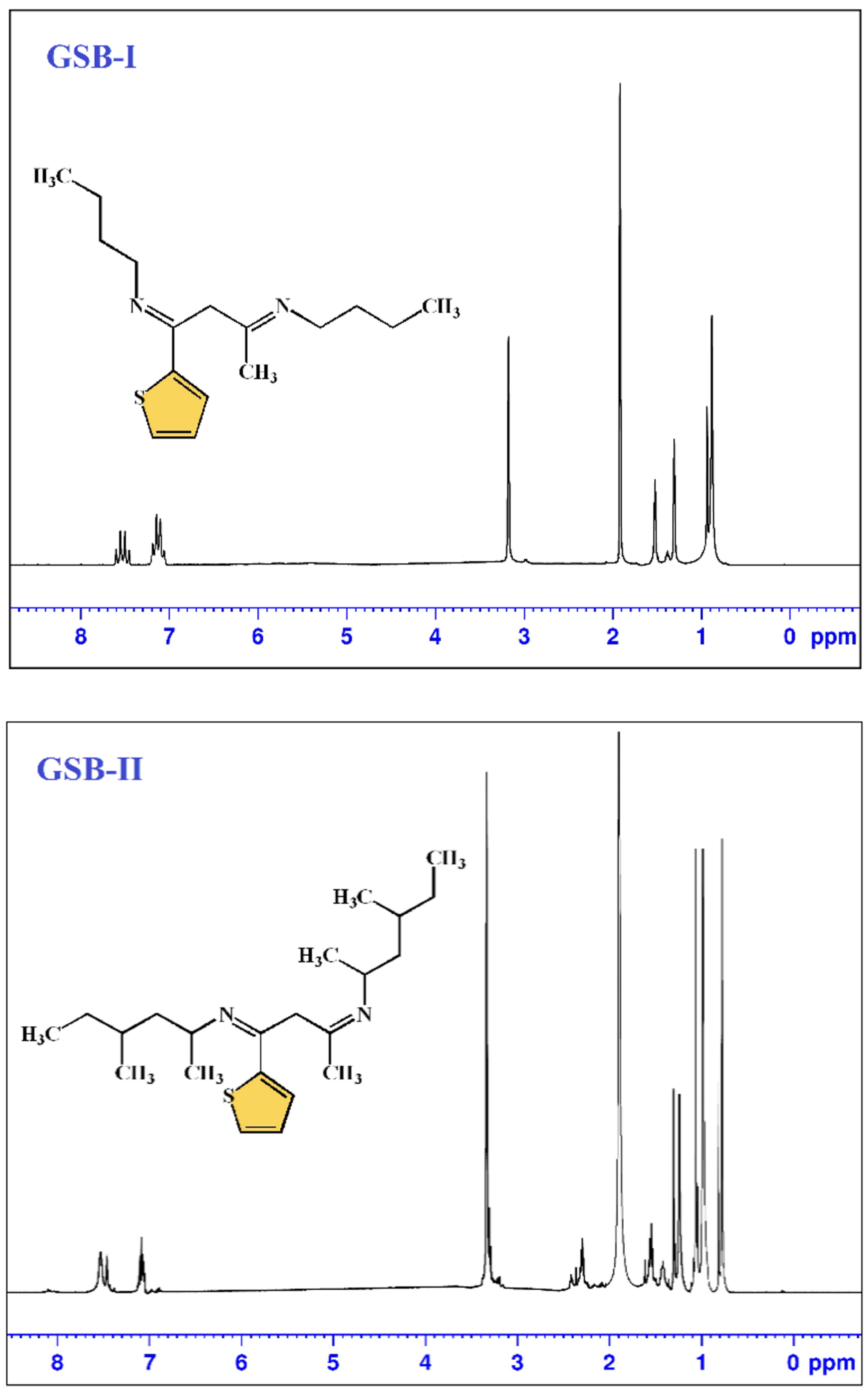
Gemini Schiff bases [(1E,3E)-N1,N3-dibutyl-1-(thiophen-2-yl)butane-1,3-diimine] (GSB-I) and [(1Z,3Z)-N1,N3-bis(4-methylhexan-2-yl)-1-(thiophen-2-yl)butane-1,3-diimine] (GSB-II) were prepared through the addition of a solution of 1-(thiophen-2-yl)butane-1,3-dione 0.01 mol in 10 mL methanol to a solution of butan-1-amine 0.02 mol in 10 mL methanol or 4-methylhexan-2-amine 0.02 mol in 10 mL methanol and the reaction mixture refluxed for 1 h [28]. The precipitates product of GSB-I and GSB-II were successively re-crystallized by washing many times with C2H5OH, (CH3)2CO, and finally dried (Figure 1). 1H NMR of GSB-I (400 MHz, d6-DMSO, 25 °C) δppm: 0.9 (6H, two terminal CH3), 1.3 (8H, 2(CH2CH2)–CH3), 1.5 (4H, 2N–CH2), 1.9 (3H, CH3–C=N), 3.2 (2H, N=C–CH2–C=N), and 7.1–7.6 (3H, 3CH of thiophene group). 1H NMR of GSB-II (400 MHz, d6-DMSO, 25 °C) δppm: 0.9–1.1 (18H, two terminal, and four substituted CH3), 1.2 (4H, 2(CH3CHCH2CHCH3)), 1.4 (2H), 1.6 (4H, 2CH2 before two terminal methyl groups), 1.9 (3H, CH3–C=N), 2.4 (2H, 2CH–N), 3.2 (2H, N=C–CH2–C=N), and 7.1–7.6 (4H, 4CH of thiophene ring) (Figure 2).
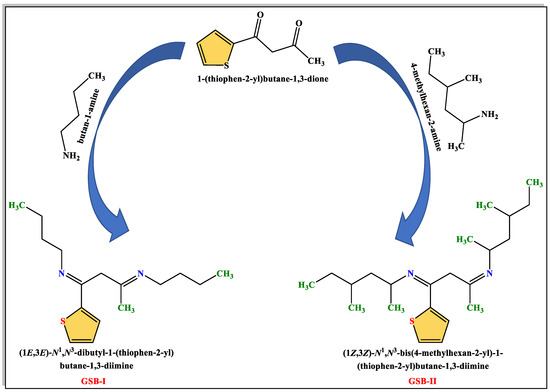
Figure 1.
The synthesis route of GSB-I and GSB-II.
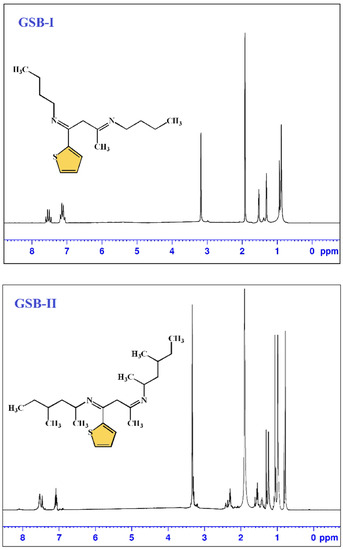
Figure 2.
H1 NMR spectrum of GSB compounds.
2.3. Specimens
The API 5L X65 steel (CS) used was of a standard grade and its composition complied with NACE MR 0175. It consisted of a carbon content of 0.28 percent, 1.4 percent manganese, 0.03 percent phosphorus, and 0.03 percent sulfur, with the rest being elemental iron. For electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization investigations, a rod-shaped CS with a cross-exposed section of 0.5 cm2 was utilized. The surface of each CS sample was polished with emery sheets ranging in grade from 400 to 2000. The samples were then thoroughly ultrasonically cleaned, degreased using a suitable solvent, and dried [29].
2.4. PDP and EIS Experiments
The studies on potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS) were carried out using a three-electrode system. For the electrochemical cell, reference (saturated-calomel-electrode, SCE), auxiliary (Pt-electrode), and working electrodes (WE, CS-electrode) were utilized. Before the measurements, the WE were immersed in the electrochemical cell containing the acidic or inhibitory solution for 1800 s to achieve a steady state. The EIS was then performed with a frequency range of 0.01 to 100 kHz and a 5-mV amplitude. The PDP was performed using a scan speed of 0.5 mV s−1 and a voltage range of −0.25 to +0.25 V.
2.5. Biological Examination
The biological activity against bacteria, fungi, and SRB using GSB compounds has been explored through a serialized dilution technique based on standard test methods for sulfate-reducing bacteria in water and water-formed deposits D 4412–84 (reapproved 2002).
2.6. DFT Calculation Details
The data on the regular optimization assembly and electronic mass spreading of the inhibitor molecules were obtained using the Gaussian-9 software. Theoretical computations were performed using density functional theory (DFT) at B3LYP and the 6-311G (d, p) approach [30], with atomic orbitals as the basis set. The energy of the Frontier (EHOMO and ELUMO), the gap energy (ΔE = ELUMO − EHOMO), the segment of electrons-transferred (N), natural-bond-orbital (NBO) charges, Mulliken scale, and molecular electrostatic potential were obtained from theoretical calculations to further describe the inhibition properties (MEP).
2.7. Simulation Studies
For the MD simulations, which were largely built on the Forcite module, Material Studio 2017 software (Accelrys Inc., San Diego, CA, USA) was used. Inhibitor molecules were tested in a simulated box with periodic border settings, ensuring that a portion of the restrictive substrate was free of random border impact. To confirm a large enough surface for the molecules to communicate, Fe (110) with body-centered cubic (BCC) was split lengthwise in a 5 Å sheet [31] and expanded into a (10 × 10) supercell. Furthermore, in 1 M HCl, the H2O:HCl ratio is roughly 167/3 (167 H2O and 1 inhibitor molecule) [32].
3. Results and Discussion
3.1. OCP Details
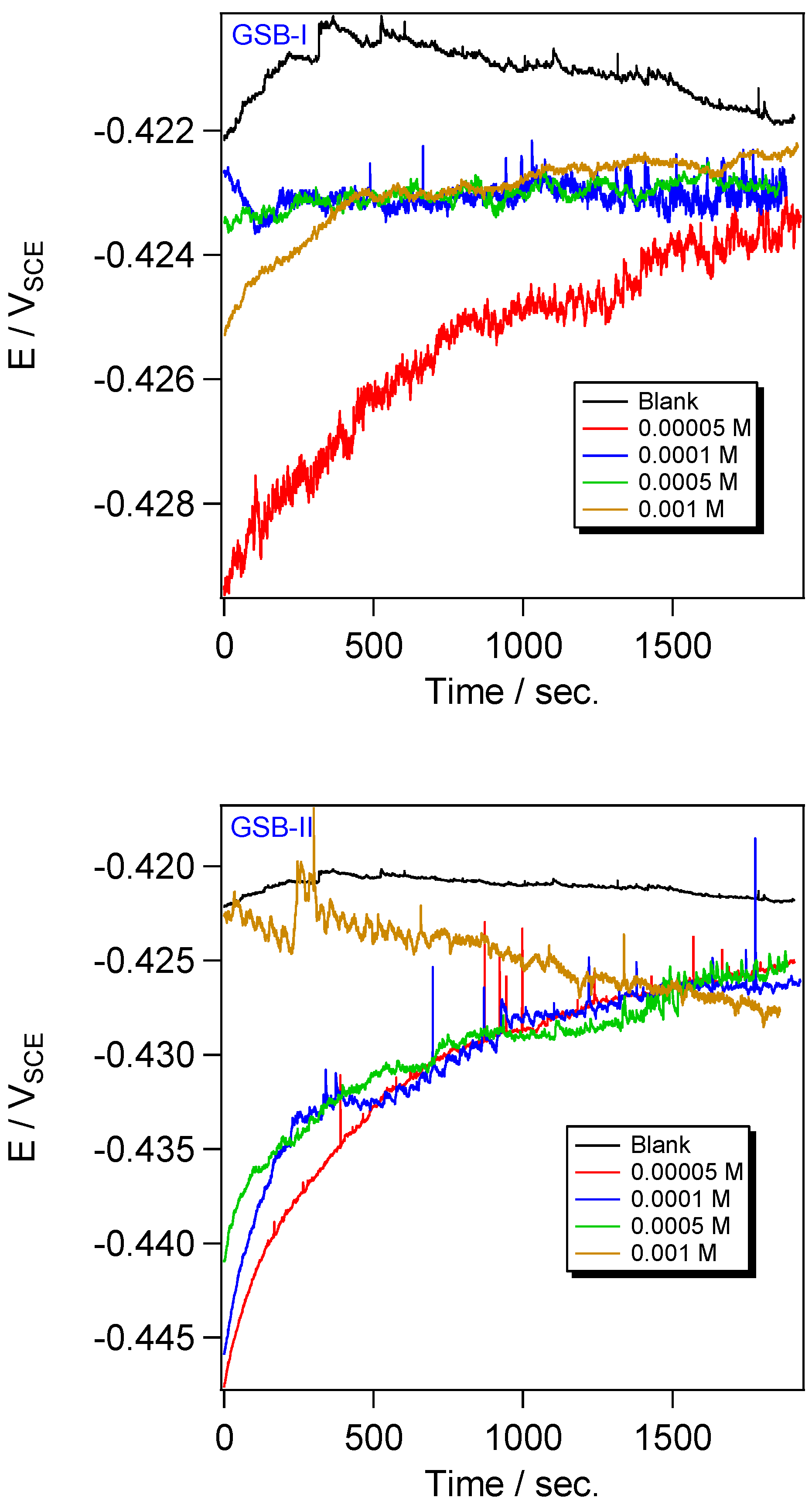
Before performing the PDP and EIS experiments, the stabilization of the open circuit potential (OCP) needs to be preserved. Figure 3 presents the shift of the OCP of the CS electrode against time in 1 M HCl media and the presence of various concentrations of GSB-I and GSB-II. The OCP steady-state range of the CS in the blank solution is more positive, signifying that EOCP is related to the electrochemical interactions at the metal substrate, which the EOCP shifted to be more positive when the metal dissolved. However, the EOCP shifted to a more negative value of potential when the inhibitor molecules of GSB added the blank solution of HCl. These suggested the metal substrate was covered by a settled passivation film of the GSB inhibitors molecules [33]. On the other hand, the amount of EOCP shift in the inhibited solution compared to the blank one is not enough to decide whether the type of inhibitor is the anodic or cathodic type. Therefore, the studied GSB inhibitors are considered mixed-type inhibitors [34].
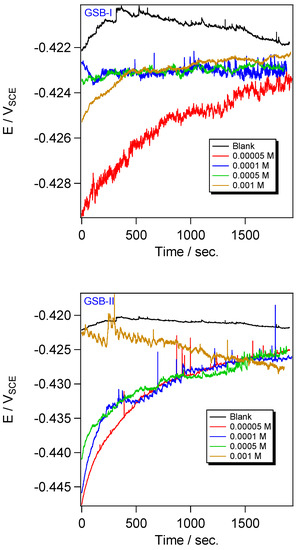
Figure 3.
OCP plots for the API 5L X65 steel in 1 M HCl medium with different concentrations of the studied inhibitors at 298 K.
3.2. PDP Details
The CS is subjected to two electrochemical interactions in an acidic solution. Oxidation and reduction cause these two reactions. The reduction occurs by the proton accepting the electrons in cathodic parts, while the oxidation occurs at the anodic section of CS, which is related to ferrous iron dissolution:
Fe → Fe2+ + 2e−
2H+ + 2e− → H2
Another reduction reaction, however, could occur in the cathodic regions. In an acidic solution, O2 can be decreased in the following way:
O2 + 4H+ + 2e− → 2H2O
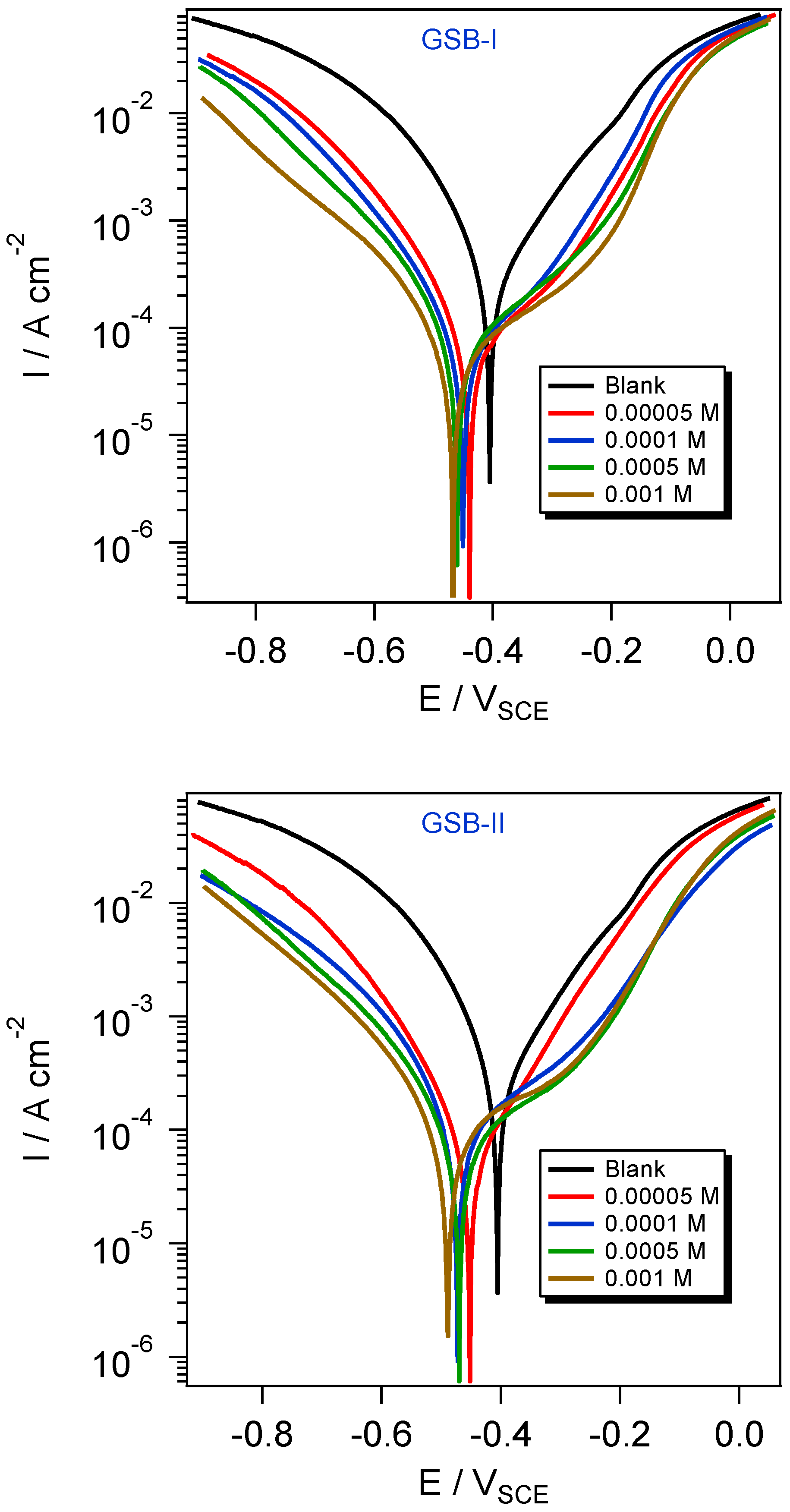
The reduction interaction was modified by introducing a corrosion inhibitor to the acidic solution through the active adsorption centers of inhibitor molecules with the CS surface. Figure 4 shows a simulation of iron corrosion in HCl solution. At room temperature, Figure 4 shows the potentiodynamic polarization and corrosion current density behavior of the CS substrate in 1 M HCl solution (blank) without and with GSB-I and GSB-II inhibitors. Because of the direct contact with an acidic solution, the CS displayed a notable high corrosion current density in the blank solution, resulting in extensive electrochemical oxidation-reduction processes. When GSB-I and GSB-II inhibitor molecules were introduced to the acidic blank solution, the electrochemical oxidation–reduction reactions were brought under control [35]. The GSB-I and GSB-II inhibitor molecules are adsorbed at the interface between the CS substrate and the acidic environment, as shown by potentiodynamic polarization curves that are virtually in parallel forms [36]. In comparison to the blank unrestricted potential curve, the trend of corrosion potentials (Ecorr) curves of GSB-I and GSB-II, as shown in Figure 4, showed no significant difference toward the negative direction of potential. Against a saturated calomel reference electrode, however, this shift in Ecorr is less than 85 mV. As a result, the behavior of GSB-I and GSB-II as corrosion inhibitors for CS in acidic HCl solution can control both anodic and cathodic interactions, classifying them as mixed-type inhibitors [37]. Table 1 summarizes all of the potentiodynamic polarization data, including inhibition percentages (ηPDP), Ecorr, the current density of corrosion (icorr), and Tafel slopes (βa and βc). The percentages of inhibition can be calculated using the equation below [38]:
where and denotes the densities of corrosion current in the uninhibited and inhibited solution, respectively. The PDP was observed to improve as the inhibitor concentrations in the acidic HCl solution increased, with the inhibition order GSB-II > GSB-I. Table 1 shows that the extreme PDP values for GSB-I and GSB-II at 1 mM are 89.9% and 93.3 percent, respectively. The parallel cathodic curves in Figure 4 suggested that the cathodic hydrogen reduction mechanism (2H+ + 2e− → H2) had not changed. The anodic curves, on the other hand, showed considerable changes due to a shift in the anodic dissolution reaction mechanism (Fe → Fe2+ + 2e−). After this part, we saw a substantial difference in the slope between 0.3 and 0.1 V (SCE) of Ecorr, as well as an increase in corrosion current density. This phenomenon could be linked to the disruption of inhibitor molecules at the CS electrode’s substrate [39,40]. In addition to the greater e−-releasing methyl groups, the higher ηPDP for GSB compounds is due to their numerous adsorption centers on the CS surface, such as the free lone e− pairs of thiophene rings that bonded to the two imine (C=N) groups. Because of the differences in their chemical structures, the ηPDP values revealed that GSB-II may effectively protect CS from aggressive solution corrosion better than GSB-I. The extra higher e−-releasing methyl groups in GSB-II increase not only the electronic cloudy GSB structure but also the surface coverage on the CS substrate, resulting in a higher electronic cloud. As a result, the GSB-II inhibitor is more adsorbed on the CS surface, providing greater protection than the GSB-I inhibitor.
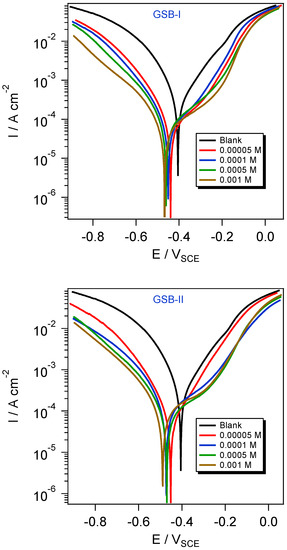
Figure 4.
Polarization curves for the API 5L X65 steel in 1 M HCl medium with different concentrations of the studied inhibitors at 298 K.

Table 1.
Tafel factors for the API 5L X65 steel corrosion in 1 M HCl without and with different concentrations of studied inhibitors at 298 K.
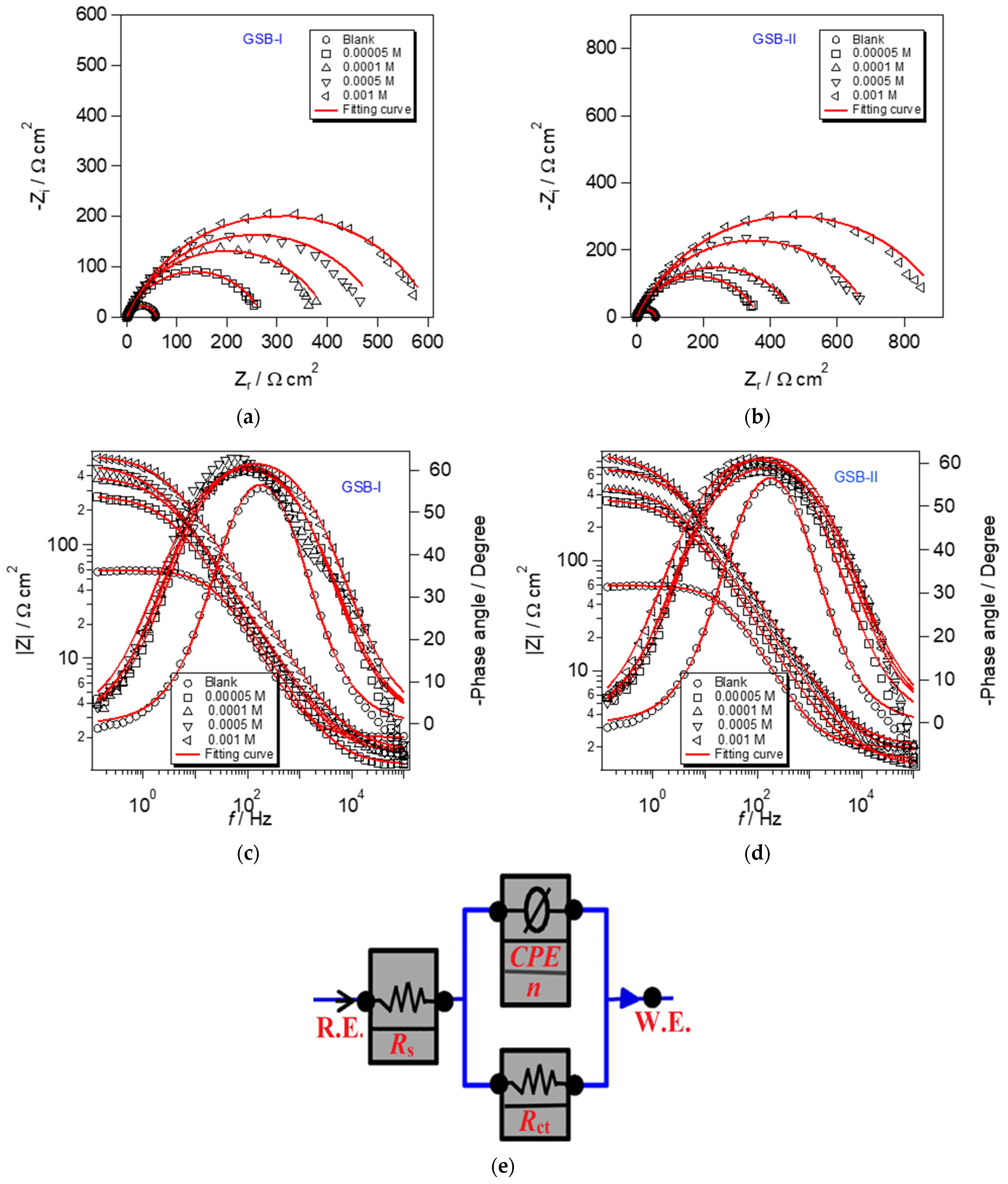
3.3. EIS Details
The EIS experiments can obtain the specifics of the interface assembly created on metal surfaces promptly [41]. The obtained EIS data provide resistance and capacitance, which are commonly used to assess corrosion advancement. As a result, it is frequently used in the metal safety industry. Figure 5 shows EIS curves of CS corrosion in 1 M HCl without and with GSB-I and GSB-II at various concentrations in 1 M HCl. The presence of a single loop in all Nyquist curves (Figure 5a,b) is clear, indicating that the corrosion process is mostly driven by charge transfer [42]. The capacitive circle diameter increased as the GSB-I and GSB-II concentrations increased, forming a corrosion fence coating on the CS substrate. Furthermore, the forms of the Nyquist curves do not vary with increasing GSB-I (Figure 5a) and GSB-II (Figure 5b) concentrations over the entire frequency range, indicating that the inserted inhibitor’s molecules arrange a tiny adsorption layer without modifying the features of the corrosion mechanism [43]. This result revealed that the GSB-I and GSB-II controller effects were joined by their concentration in the layer created [44]. Capacitance quality and corrosion defense capabilities, as indicated by resistance quality, reproduce the value of the layer created by inhibitor molecules (permeable/compacted, sullied/complete). Lower capacitance and higher resistance indicate the formation of a dense and important protective layer [45]. Figure 5c,d also showed the Bode curves, which grew in size when the concentrations of GSB-I and GSB-II rose. This indicates that the defensive capability has grown in comparison to the layer capacity adsorbed of GSB-I and GSB-II at the CS substrate. The equivalent chemical circuit shown in Figure 5e was created using ZSimpWin software to calculate and adjust the impedance profile at the CS/media interface with various concentrations. The model (R (QR)) circuit is the most suitable for the data of the researched system. In this model circuit, (Rs) denotes solution resistance, (Rct) denotes charge transfer resistance, and (CPE) is the constant phase element that should be included in the impedance profile. Equation can be used to compute the percentage of inhibition (ηz) [46]:
where and are the charge transfer resistance of CS in the 1 M HCl solution without and with the inhibitor, respectively. Table 2 shows the EIS parameters exported for CS contact to the corrosive acidic environment, which was calculated using a semicircle approach. The constant phase element, CPE, was discovered to reduce errors caused by nonideal capacitance, which may be calculated using the following equation [47,48]:
where Q is the proportionality factor for CPE degree, j is the number of unreal (j2 = −1), ω is the frequency of angular (ω = 2πf), and n represents phase shift, which is associated with the surface morphology (−1 ≤ n ≤ +1). According to Table 2, the value of Rct increases with the addition of the GSB-I and GSB-II molecules, indicating that they adsorb at the CS surface and impede charge transfer, making the corrosion operation more difficult. Furthermore, the higher the concentration of GSB-I and GSB-II, the greater the percentage of inhibition, which is 90.6 percent and 93.8 percent at 1 mM, respectively [49]. This is sympathy with potentiodynamic polarization, and it occurred in the order GSB-II > GSB-I.
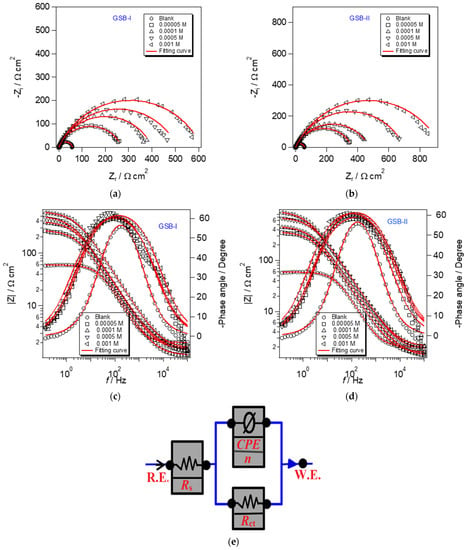
Figure 5.
(a,b) Nyquist plot and (c,d) Bode modulus plot of EIS and (e) its equivalent circuit model for the API 5L X65 steel in 1 M HCl medium with different concentrations of the studied inhibitors at 298 K.

Table 2.
EIS factors for the API 5L X65 steel corrosion in 1 M HCl without and with different concentrations of studied inhibitors at 298 K.
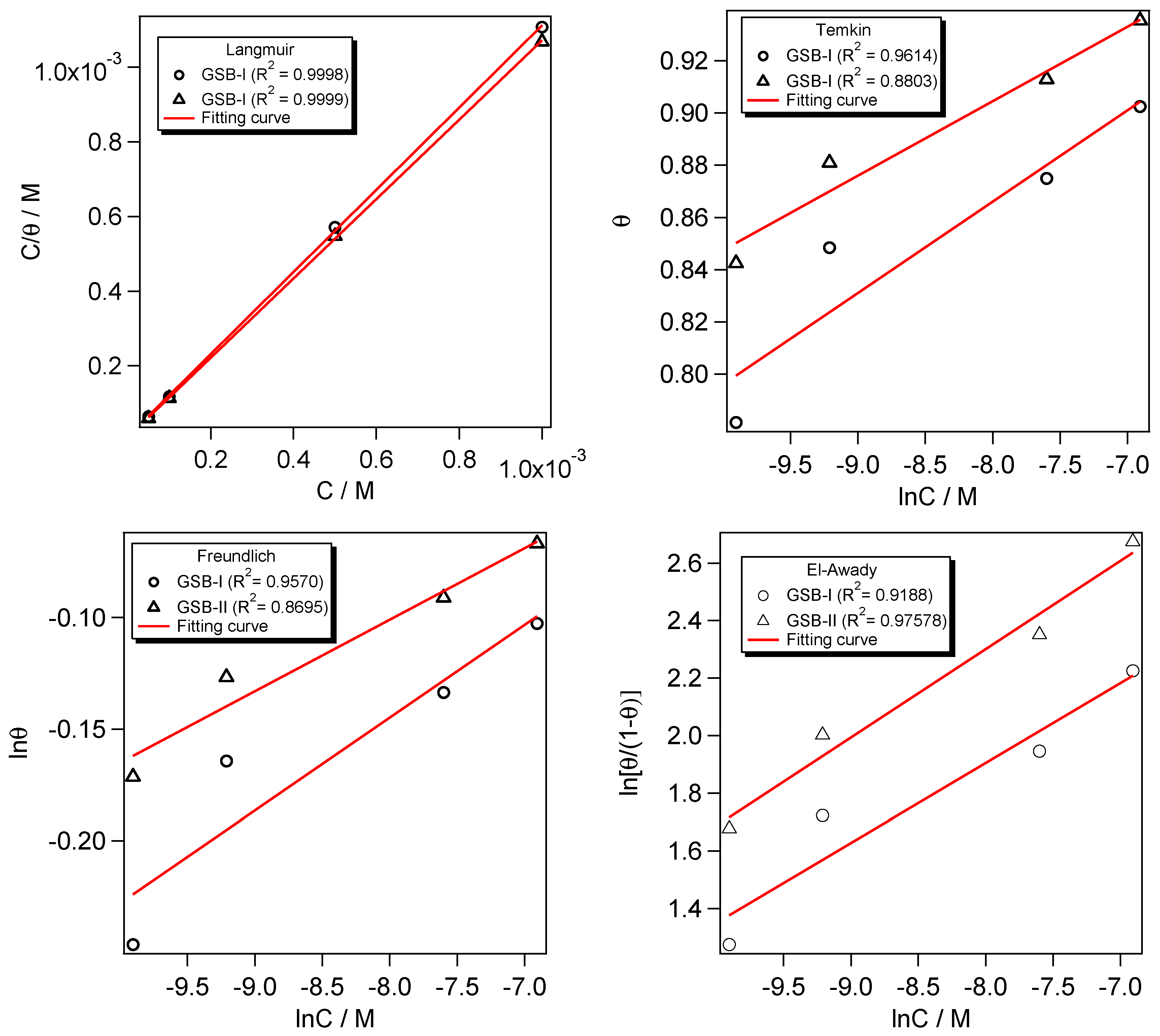
3.4. Adsorption Isotherm Details
It is critical to investigate an inhibitor molecule’s adsorption efficiency at a metal substrate in an acidic environment [50]. The CS surface coverage (θ = η/100) is commonly used to denote inhibitory behavior. The Langmuir, Temkin, Freundlich, and El-Awady isotherms were used to fit the adsorption process of GSB inhibitors at the CS substrate (Figure 6).
where C denotes the GSB-I and GSB-II concentration, and denotes the constant adsorption equipoise. Langmuir isotherm can provide a fair fitting with adsorption performance, according to the average determined from PDP and EIS data [51]. The Langmuir isotherm’s lined regression (R2) factor was determined to be near unity [52]. For both GSB-I and GSB-II inhibitor compounds, the relationship between C/θ and C in Figure 6 shows a straight curve with regression factors (R2) for slopes that are almost identical and close to unity. These findings show that the GSB-I and GSB-II molecules were adsorbed on the CS substrate following the Langmuir model [53,54,55]. As shown in Figure 6, the constant of adsorptive equipoise () was calculated from the intercepts of the C/θ and C curves to replicate the contact adsorption strength of GSB-I and GSB-II to the CS surface, which can be used to calculate the standard Gibbs’ free energy () of adsorption using the following equation [56,57]:
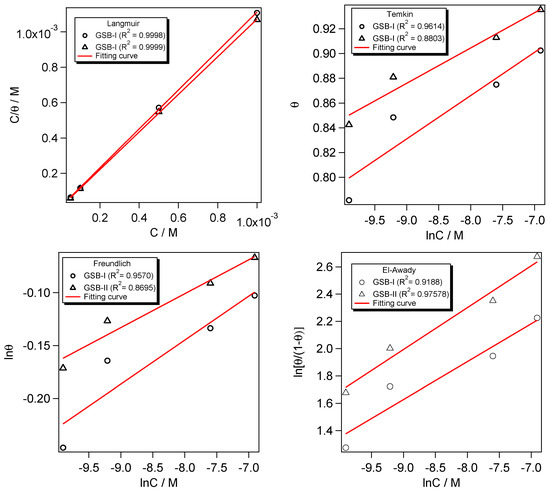
Figure 6.
Plots of Langmuir, Temkin, Freundlich, and El-Awady isotherms for studied inhibitors at different concentrations.
The number of 55.5 is related to the molarity of the aqueous media, the factor R denotes the constant of universal gas, and factor T denotes the temperature in Kelvin units. As seen in Figure 6, the value of GSB-II is more than GSB-I, which confirms the strongest binding of GSB-II at the CS substrate than GSB-I molecules.
The minus values of standard Gibbs’ free energy are obvious in the spontaneous adsorption process for GSB-I and GSB-II at the CS substrate. As it is widely accepted that when the values of standard Gibbs’ free energy are lesser than −20 kJ mol−1, it could be decided that the adsorption of organic molecules at the solid surface is the physisorption type, including electrostatic contact among the organic molecules at the metal substrate. However, if the values of standard Gibbs’ free energy are bigger than −40 kJ mol−1, it could be decided that the adsorption of organic molecules at the solid surface is chemisorption type, including electronic distribution between the lone pair of organic molecules with the vacant d-orbital at the metal substrate.
The negative values of standard Gibbs’ free energy show that the GSB-I and GSB-II adsorption processes occur spontaneously on the CS substrate. Given that the values of standard Gibbs’ free energy are less than −20 kJ mol−1, it is possible to conclude that the adsorption of an organic molecule at a solid surface is of the physisorption type, which includes electrostatic interaction between the organic molecules and the metal substrate. However, if the values of the standard Gibbs’ free energy are greater than −40 kJ mol−1, it is possible to conclude that the adsorption of an organic molecule at a solid surface is chemisorption, with the electronic distribution between the lone pair of organic molecules and the vacant d-orbital at the metal substrate [58]. The standard Gibbs’ free energy measurements acquired in our work show that the GSB-I and GSB-II with 38.5 and 38.7 kJ mol−1 adsorbed at the CS surface via a mixed-type process [59,60,61,62,63].
3.5. Biological Activity
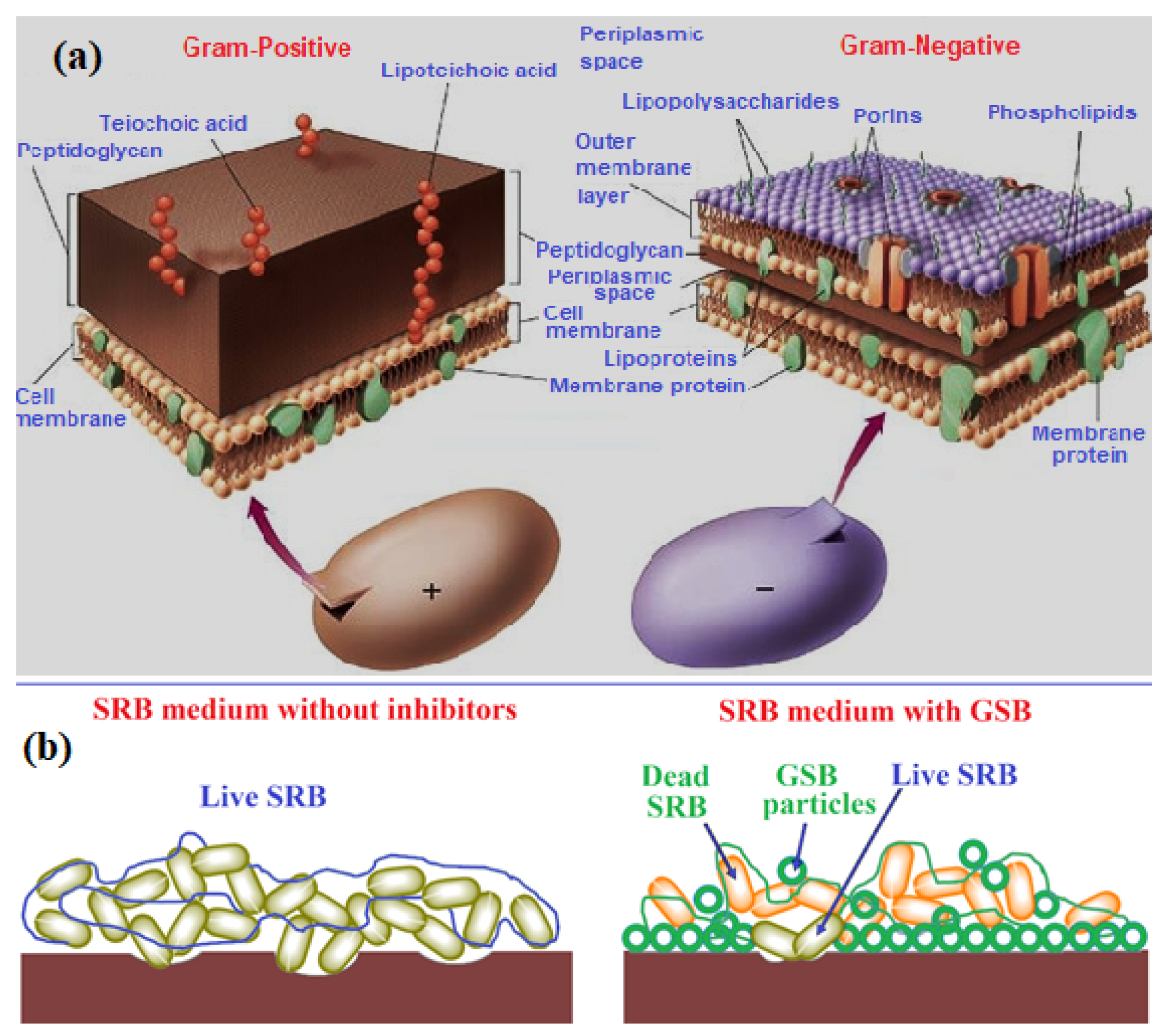
The synthesized GSB compounds have an outstanding activity to all assessed microbial straining. The obtained statistics listed in Table 3 replicate a notable antimicrobial achievement for both investigated GSB-I and GSB-II inhibitors with inhibition zones ranging from 17–29 mm and 17–23 mm for all examined bacteria and fungi, respectively. The antibacterial effect of GSB-II was found more than GSB-I, which is related to changes in their chemical structures. Furthermore, both compounds exhibited better antimicrobial action against Gram-positive than Gram-negative bacteria, which may be due to variances of the cell wall of positive and negative Gram bacteria [64]. Figure 7a showed an explanation for the Gram-positive and Gram-negative bacterial cell wall structure.

Table 3.
Anti-bacterial and anti-fungi activities of a synthesized gemini Schiff base.
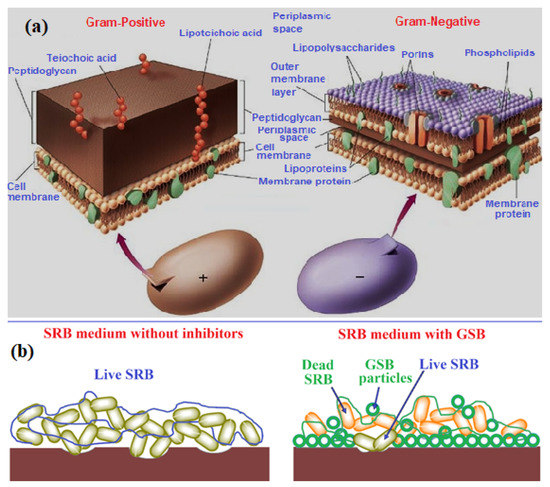
Figure 7.
(a) Gram-positive and Gram-negative bacterial cell wall structure, (b) representative scheme for SRB inhibition process through GSB particles [64].
SRB is known as anaerobic microorganisms accomplished by reducing to hydrogen H2S [65]. The presence of H2S causes noteworthy difficulties in most industries, particularly in the oil and gas field due to its toxicity and corrosivity to metallic structures [66]. The contacted H2S-containing solution with the iron surfaces produces FeS associated with corrosion damage. Therefore, the inhibition of SRB is vital in defending the metallic structure against corrosion effects. The biocidal action of GSB-I and GSB-II against SRB-increase was assessed through sequential dilution (10–50 ppm) with the blank solution of 107 cell/mL, as listed in Table 4. When examining Table 4, it can be established that the studied GSB-I and GSB-II have an anti-microbial action on the inspected SRB cells. Figure 7b showed a representative scheme for the SRB inhibition process by GSB particles. SRB is categorized as a dense cell wall that is decidedly resistant to biocides compounds. The resemblance between the hydrophobic parts of GSB inhibitors and the lipid films of the cell wall of SRB eases the adsorption and diffusion of GSB molecules into the cell wall. In addition to the thiophene group, two imine groups in the GSB molecules affect the growth of the SRB cell by disturbing their selectivity that cause cell death [67]. Therefore, the synthesis of gemini Schiff bases (GSB) is considered a promising biocidal against bacteria and SRB.

Table 4.
Biocidal effect of the GSB inhibitors toward SRB.
3.6. Quantum Chemical Calculations
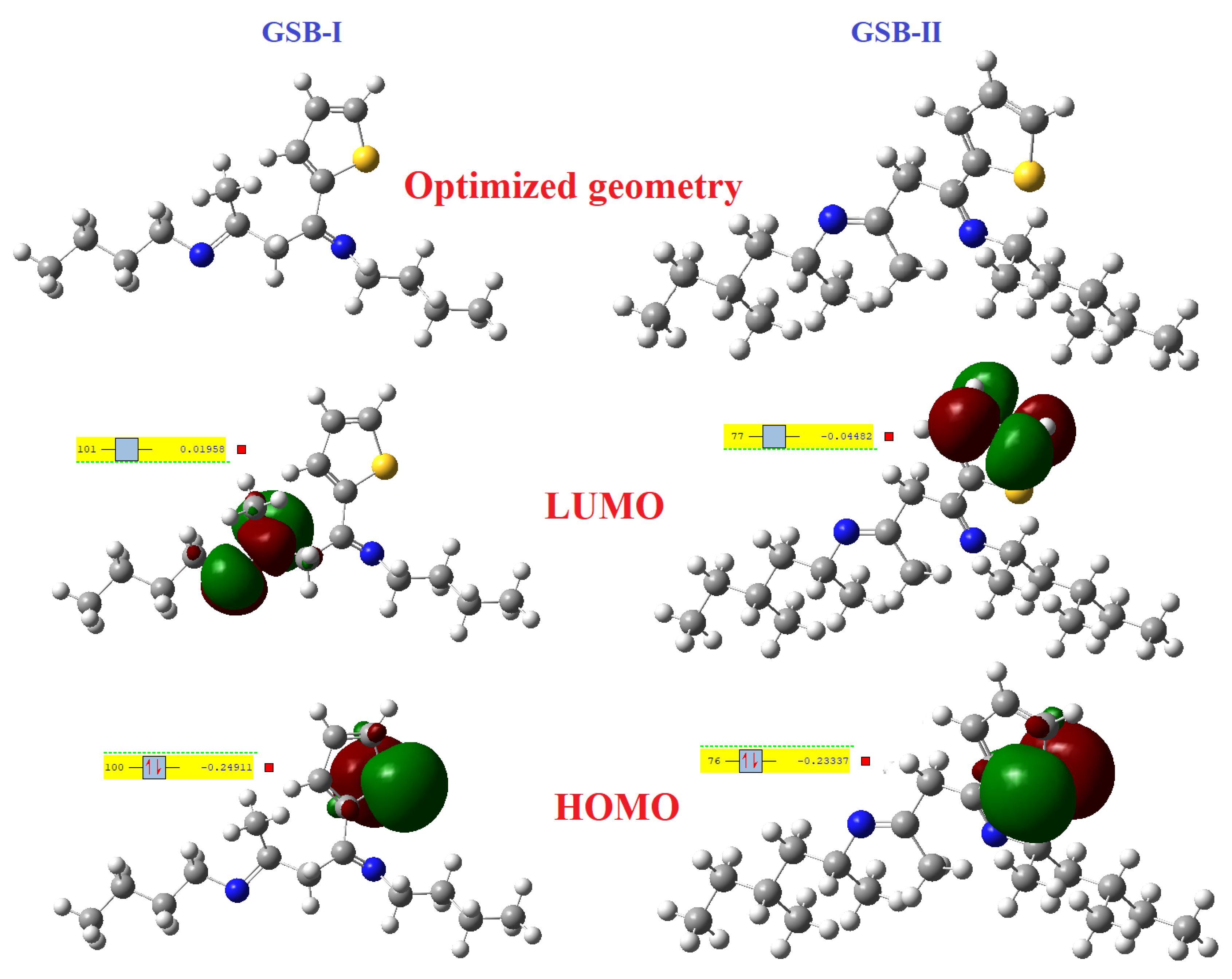
3.6.1. Frontier Molecular Orbitals (FMOs) and Energy Details
The Gaussian-9 package is used to alter the structure of the GSB-I and GSB-II molecules, which is helpful in further uncovering the electronic assets of the particle and their active sites that assist the process of CS corrosion reserve. Figure 8 shows the optimization building of GSB-I and GSB-II. The molecules could be classified as highest occupied molecular orbital (HOMO) or lowest unoccupied molecular orbital (LUMO) using frontier molecular orbital theory (LUMO). Because of the characteristics of HOMO and LUMO, they play an important role in the corrosion inhibition process. The HOMO component of a molecule measures its ability to release electrons, whereas the LUMO part of a molecule measures its ability to accept electrons [68]. In general, the metal surface with the lowest empty orbital has a greater capacity to gain electrons from the electron release chemical as inhibitor particles [69]. In contrast, the metal might backlink its HOMO electrons to the LUMO with an appropriate vacant orbital of the inhibitor particles [70]. The main clarification concerning the adsorption process of inhibitor particles at metallic substrates is the donor/acceptor relationship between the inhibitor particles and the empty orbitals of the metallic surface [71]. Adsorption occurs after the HOMO/LUMO contact via the link between the iron atoms and the CS substrate. The quantum computation parameters EHOMO, ELUMO, energy gap ΔE (ELUMO − EHOMO), electronegativity (χ), hardness (η), electron affinity (A), ionization potential (I), and electron-transfer-fraction (ΔN) were computed from the HOMO and LUMO vitalities of GSB-I and GSB-II, as recorded in Table 5. Demonstrations are shown in Figure 8. In both GSB particles, the electronic mass of the HOMO component is primarily scattered in the S-atoms of thiophene rings, indicating their ability to release electrons. However, in GSB-I and GSB-II, the electronic mass of the LUMO component is largely established around a single imine group and carbon atoms attached to the thiophene ring, which is responsible for electron-taking in the back-donation process. The back-donation procedure had a synergetic effect on the inhibitors’ adsorptive particles on the metal substrate, making them stronger. The bigger the EHOMO, the more difficult it is to donate electrons and the more solid the contact with the metal substrate. Similarly, the smaller the ELUMO, the more difficult it is to accept electron-taking and the firmer the adsorption at the metal substrate. As a result, a small energy gap ΔE corresponds to a larger adsorption volume, which is beneficial for enhancing corrosion inhibition [72,73]. Table 5 shows that the E value of GSB-I is 0.2295 eV, which is close to the value of GSB-II (0.1886 eV), indicating that their activity is not considerably different [74]. Though the inhibitory result of GSB-II is better than that of GSB-I, this is mostly due to GSB-seamless II’s planar construction and higher surface covering rate during the adsorption process. The electron-transfer-fraction (ΔN) of inhibitor particles and the CS substrate was determined based on Pearson’s notion of determining the electron contribution throughout the corrosion reserve [75,76]:
where the factors χ and η denote the values of electro-negativity and hardness, respectively. The factors of χFe and ηFe were implemented with the theoretic values of 4.82 and zero, respectively, which could be computed as the following [77,78,79]:
where I (−EHOMO) and A (−ELUMO) denote the ionization potentials and electron-affinities, respectively. The analysis shows that when the metal substrates and inhibitor particles are close together, the electrons will move from the lower electronegativity site to the higher electronegativity region until equilibrium is reached. In general, electrons move from the inhibitor particles into the Fe atoms at the CS substrate when the value of electron-transfer-fraction (ΔN) is positive, whereas electrons move from the Fe atoms at the CS substrate into the inhibitor particles when the value of electron-transfer-fraction (ΔN) is negative, which is known as back-donation [80]. Table 5 shows that the electron-transfer-fraction (ΔN) values of GSB-I and GSB-II are 57.4082 and 62.1786, respectively, demonstrating their exceptional ability to release electrons from inhibitor particles to the Fe atom at the CS surface via co-ordination bonds, thereby producing an effective protective film and preventing metal dissolution. The hard-soft-acid-base (HSAB) approach is one of the needs of theoretical fundamentals in corrosion investigation. CS is a soft acid, while GSB-I and GSB-II are soft alkalis [81]. The reasonably significant global softness (σ = 1/η) of inhibitors, as shown in Table 5, indicates further information about the outstanding corrosion inhibitor, suggesting that the combination of GSB-I and GSB-II and the CS surface adheres to the HSAB concept.
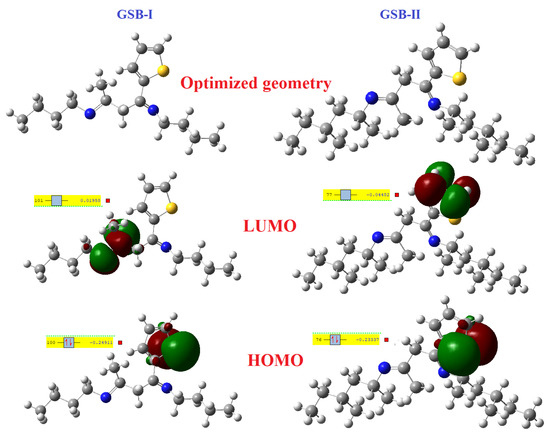
Figure 8.
Optimized structures, LUMO and HOMO plots of the studied inhibitors.

Table 5.
The calculated quantum chemical parameters for GSB inhibitors.
3.6.2. Mulliken Scale, NBO Charge, and 3D MEP Details
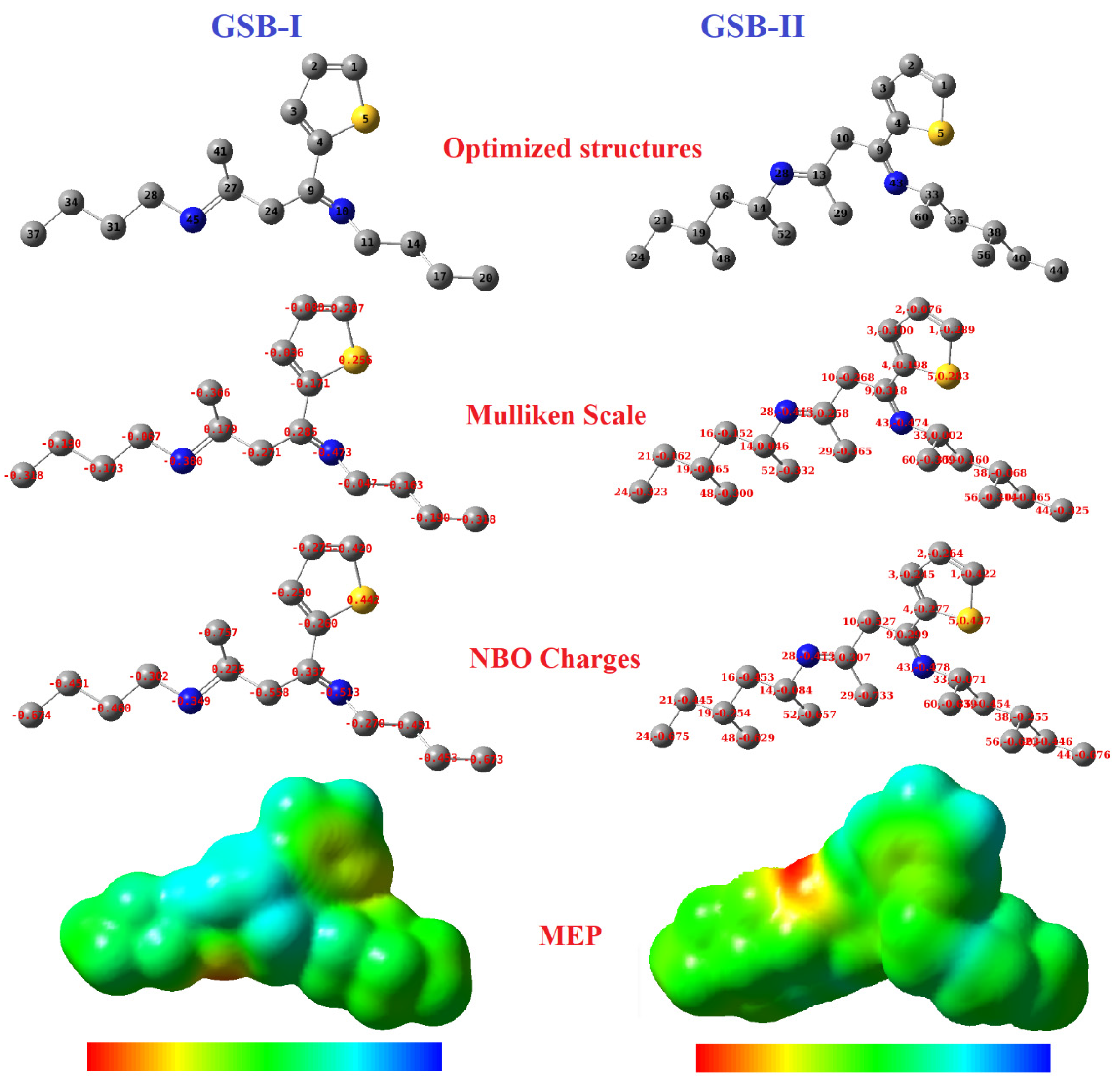
The Mulliken scale was generally utilized to explore the active locations, donor, and acceptor electrons communications among the inhibitor particles and the Fe-atoms [82,83]. The Mulliken scale, NBO charges, and the 3D-molecular-electrostatic-potential (MEP) of GSB-I and GSB-II were presented in Figure 9. According to calculated Mulliken scales it can be seen that the atoms with the higher negative charges in GSB-I were found at C1 and C4 that linked with the sulfur atom of the thiophene ring. In addition to the N10, N45 (of imine groups), C17, C20, and C37 (of terminal-releasing methyl groups). On the other hand, the higher negative charges in GSB-II were also found at C1 and C4, linked with the sulfur atom of the thiophene ring. Besides the N28, N43 (of imine groups), C24, C44, C48, C52, and C56 (of terminal releasing methyl groups), these atoms are considered the main reactive sites that are accomplished to establish co-ordinate links via the lone-pair e− and the vacant d-orbital of Fe-atoms. Therefore, the thiophene ring, imine group, and terminal CH3 groups are the main adsorption anchors of the inhibitor particles onto the steel substrate. The NBO charges map has also been represented in Figure 9, which is helpful to predict the adsorption reactivity sites of inhibitor particles. The NBO map refers to the larger negative charge focused on the heteroatoms and the terminal CH3 groups in both GSB-I and GSB-II. Furthermore, the MEP could afford a photographic system to describe the comparative polarity, which helps in understanding the adsorption process. The blue region is a sign for the electrostatic-potential negative charges (electrophilic-attack), while the red region is a mark for the positive-potential charges (nucleophilic-attack). Consequently, these findings results are compatible with the obtained experimental data of OCP, PDP, and EIS techniques. Additionally, matching conclusions from the frontier molecular orbitals (EHOMO and ELUMO) and ΔN parameters. Therefore, the GSB particles exhibited a higher inhibition percentage for the CS corrosion in 1 M HCl with the superiority of GSB-II over GSB-I.
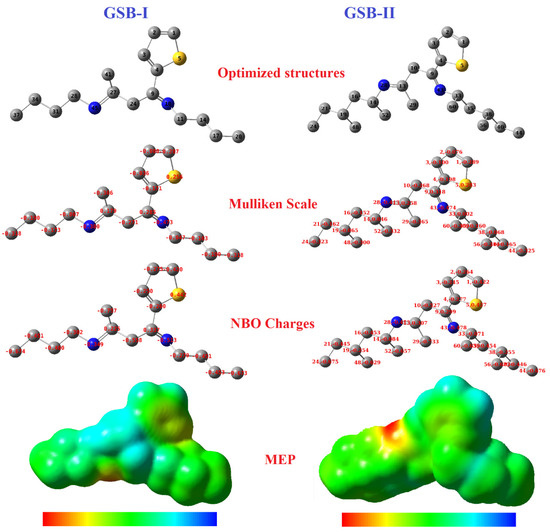
Figure 9.
Optimized structures, Mulliken, NBO, and ESP plots of the studied inhibitors.
3.7. MD Simulation Details
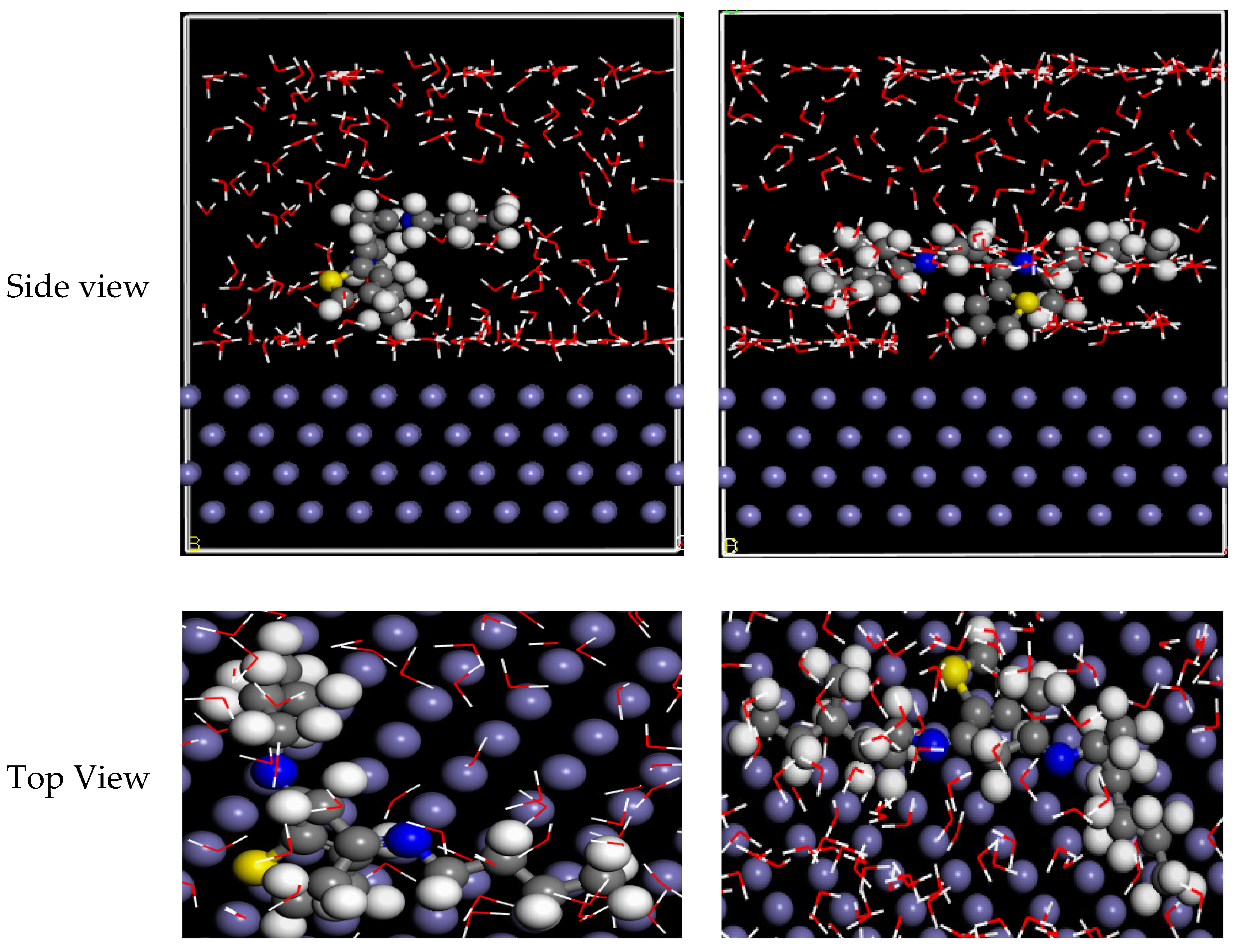
MD simulations on Fe(110) substrates were performed to better understand the communication between the examined inhibitor particles and the CS substrate [84]. The side and top views in Figure 10 show the equipoise outlines of GSB-I and GSB-II inhibitors’ particles at the CS. Similarly, both GSB-I and GSB-II conformers were securely absorbed at the Fe(110) substrate. To suspend metallic deterioration, it is suggested that the thoroughgoing handling created by the parallel approach is matched to further adsorption directions [85]. This stimulates electrons at the inhibitor particles to migrate to the unoccupied d-orbitals of iron atoms at the CS surface generating a defensive layer, according to the examination of contacting mechanism [86]. As shown in Table 6, the adsorptive energy might predict the inhibitor particle–CS substrate binding permanence. The interaction-energies (Einteraction) and binding-energies (Ebinding) computed be useful to clarify the interactions between the considered inhibitor particles at the CS substrate in the reproduction system, which can be defined as the following Equations:
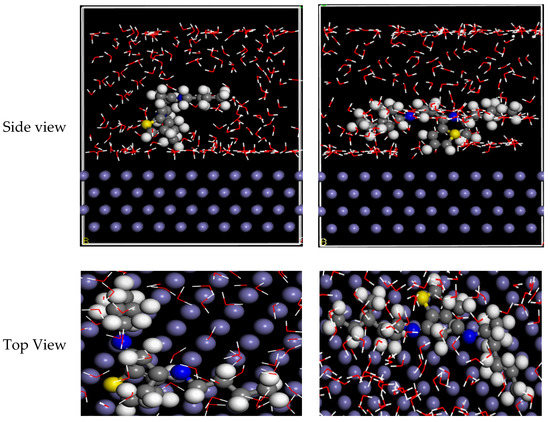
Figure 10.
Side and top view for equilibrium adsorption configuration of GSB inhibitors at the Fe substrate.

Table 6.
Interaction energy and binding energy values between the inhibitors and Fe(110) surface.
The total energy (Etotal) denotes the totality energy of the H2O particles, the considered inhibitor particles, and the CS surface. In addition, the value of correlates to the energy of CS surface and acid solution. In balanced situations, the Ebinding ensures that the GSB-II (−990.275 kcal/mol) > GSB-I (−492.743 kcal/mol). Therefore, the maximum value of Ebinding was obtained for GSB-II particles, signifying that they have a tougher and more impulsive adsorptive behavior at the CS substrate than the GSB-I particles and hence have the advanced percentage inhibition efficiency regarding corrosion.
3.8. Mechanism of Inhibition
The inhibitory mechanism of corrosion is performed constructively through mixed physical and chemical adsorption via the adsorptive of inhibitors molecules at the CS substrate, according to experimental and theoretical findings. Through the adsorptive development of chemical adsorption, heteroatom lone pairs of N and S, imine (C=N) links, and π-electrons in the aromatic-ring performed a major feature. The protonated inhibitor particles in the acidic HCl solution, on the other hand, can be physically adsorbed at the CS substrate’s negative ions. However, using electrons through the iron atom at the CS surface may result in the formation of more negative ions, which may affect the movement of electrons back to the GSB-I and GSB-II particles. As a result, the releasing and back-releasing of elections have a synergetic effect, enhancing the adsorptive properties of GSB-I and GSB-II particles on the CS substrate. As a result of these notions, the GSB-I and GSB-II inhibitors examined are good candidates for inhibiting CS corrosion in the HCl solution at one molar concentration. Table 7 compares the effectiveness of GSB-II’s suppression of other recently synthesized Schiff bases on the steel substrate. Although inhibitor doses vary, our results are comparable to those of other inhibitors.

Table 7.
Comparison of the inhibition efficiencies (η %) of GSB-II with other Schiff bases.
4. Conclusions
An effective pathway to safeguard the environment by the inhibition of corrosion was performed in this work through formulated Schiff base inhibitors. The corrosion inhibitors GSB-I and GSB-II are newly created compounds that have been tested for the protection of carbon steel in severe conditions: 1 M HCl solution. Using potentiodynamic polarization, EIS, and OCP test techniques, the inhibitory efficiency percentages of GSB-I and GSB-II at 1 mM were 90.6 percent and 93.8 percent, respectively. These chemicals can postpone anodic and cathodic processes, according to Tafel data. The creation of an adsorbed layer from GSB-I and GSB-II inhibitors increased CS dissolving resistance in HCl solution, according to EIS data. Both inhibitors adhered to the Langmuir adsorption isotherm model. Theoretical data were compared to experimental corrosion test findings using DFT and MD simulation. Both GSB-I and GSB-II showed activity as antifungal, antibacterial, and biocidal agents against sulfate-reducing bacteria (SRB).
Author Contributions
Investigation: A.A.F.; Methodology: A.A.F. and M.S.M.; Software: A.A.F. and A.T.; Supervision: C.L. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors gratefully thank the Egyptian Petroleum Research Institute (EPRI) and the National Center for Nanoscience and Technology (NCNST, China) for their kind support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Javaherdashti, R. Environmental Impacts of Corrosion and Assessment Strategies. Corros. Policy Decis. Mak. 2022, 349–367. [Google Scholar]
- Vasant, P.C.; Bansal, C.K. An Investigation Into the Environmental Impacts of Atmospheric Corrosion of Building Materials. Int. J. Chem. Sci. Appl. 2013, 4, 1–6. [Google Scholar]
- Olasunkanmi, L.O.; Aniki, N.I.; Adekunle, A.S.; Durosinmi, L.M.; Durodola, S.S.; Wahab, O.O.; Ebenso, E.E. Investigating the Synergism of Some Hydrazinecarboxamides and Iodide Ions as Corrosion Inhibitor Formulations for Mild Steel in Hydrochloric Acid: Experimental and Computational Studies. J. Mol. Liq. 2021, 343, 117600. [Google Scholar] [CrossRef]
- Karki, N.; Neupane, S.; Gupta, D.K.; Das, A.K.; Singh, S.; Koju, G.M.; Chaudhary, Y.; Yadav, A.P. Berberine Isolated from Mahonia Nepalensis as an Eco-Friendly and Thermally Stable Corrosion Inhibitor for Mild Steel in Acid Medium. Arab. J. Chem. 2021, 14, 103423. [Google Scholar] [CrossRef]
- Elaryian, H.M.; Bedair, M.A.; Bedair, A.H.; Aboushahba, R.M.; Fouda, A.E.-A.S. Synthesis, Characterization of Novel Coumarin Dyes as Corrosion Inhibitors for Mild Steel in Acidic Environment: Experimental, Theoretical, and Biological Studies. J. Mol. Liq. 2022, 346, 118310. [Google Scholar] [CrossRef]
- Farag, A.A.; Badr, E.A. Non-Ionic Surfactant Loaded on Gel Capsules to Protect Downhole Tubes from Produced Water in Acidizing Oil Wells. Corros. Rev. 2020, 38, 151–164. [Google Scholar] [CrossRef]
- Khadom, A.A.; Jassim, S.A.; Kadhim, M.M.; Ali, N.B. Influence of Apricot Constituents as Eco-Friendly Corrosion Inhibitor for Mild Steel in Acidic Medium: A Theoretical Approach. J. Mol. Liq. 2022, 347, 117984. [Google Scholar] [CrossRef]
- Konno, Y.; Farag, A.A.; Tsuji, E.; Aoki, Y.; Habazaki, H. Formation of Porous Anodic Films on Carbon Steels and Their Application to Corrosion Protection Composite Coatings Formed with Polypyrrole. J. Electrochem. Soc. 2016, 163, C386. [Google Scholar] [CrossRef]
- Al-Gamal, A.G.; Farag, A.A.; Elnaggar, E.M.; Kabel, K.I. Comparative Impact of Doping Nano-Conducting Polymer with Carbon and Carbon Oxide Composites in Alkyd Binder as Anti-Corrosive Coatings. Compos. Interfaces 2018, 25, 959–980. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Abdou, M.I.; Migahed, M.A.; Fadl, A.M.; Farag, A.A.; Mohammedy, M.M.; Abd-Elwanees, S.; Deiab, A. Influence of Ilmenite Ore Particles as Pigment on the Anticorrosion and Mechanical Performance Properties of Polyamine Cured Epoxy for Internal Coating of Gas Transmission Pipelines. Egypt. J. Pet. 2018, 27, 427–436. [Google Scholar] [CrossRef]
- Oubaaqa, M.; Ouakki, M.; Rbaa, M.; Abousalem, A.S.; Maatallah, M.; Benhiba, F.; Jarid, A.; Ebn Touhami, M.; Zarrouk, A. Insight into the Corrosion Inhibition of New Amino-Acids as Efficient Inhibitors for Mild Steel in HCl Solution: Experimental Studies and Theoretical Calculations. J. Mol. Liq. 2021, 334, 116520. [Google Scholar] [CrossRef]
- Farag, A.A.; Mohamed, E.A.; Sayed, G.H.; Anwer, K.E. Experimental/Computational Assessments of API Steel in 6 M H2SO4 Medium Containing Novel Pyridine Derivatives as Corrosion Inhibitors. J. Mol. Liq. 2021, 330, 115705. [Google Scholar] [CrossRef]
- Belghiti, E.; Benhiba, F.; Benzbiria, N.; Lai, C.-H.; Echihi, S.; Salah, M.; Zeroual, A.; Karzazi, Y.; Tounsi, A.; Abbiche, K.; et al. Performance of Triazole Derivatives as Potential Corrosion Inhibitors for Mild Steel in a Strong Phosphoric Acid Medium: Combining Experimental and Computational (DFT, MDs & QSAR) Approaches. J. Mol. Struct. 2022, 1256, 132515. [Google Scholar] [CrossRef]
- Saraswat, V.; Yadav, M. Improved Corrosion Resistant Performance of Mild Steel under Acid Environment by Novel Carbon Dots as Green Corrosion Inhibitor. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127172. [Google Scholar] [CrossRef]
- Farag, A.A. Oil-in-Water Emulsion of a Heterocyclic Adduct as a Novel Inhibitor of API X52 Steel Corrosion in Acidic Solution. Corros. Rev. 2018, 36, 575–588. [Google Scholar] [CrossRef]
- Guruprasad, A.M.; Sachin, H.P. Novel Cost-Effective Aqueous Amorphophallus Paeoniifolius Leaves Extract as a Green Corrosion Inhibitor for Mild Steel Corrosion in Hydrochloric Acid Medium: A Detailed Experimental and Surface Characterization Studies. Chem. Data Collect. 2021, 34, 100734. [Google Scholar] [CrossRef]
- Kabel, K.I.; Farag, A.A.; Elnaggar, E.M.; Al-Gamal, A.G. Improvement of Graphene Oxide Characteristics Depending on Base Washing. J. Superhard Mater. 2015, 37, 327–334. [Google Scholar] [CrossRef]
- Kabel, K.I.; Farag, A.A.; Elnaggar, E.M.; Al-Gamal, A.G. Removal of Oxidation Fragments from Multi-Walled Carbon Nanotubes Oxide Using High and Low Concentrations of Sodium Hydroxide. Arab. J. Sci. Eng. 2016, 41, 2211–2220. [Google Scholar] [CrossRef]
- Arifa Farzana, B.; Mushira Banu, A.; Riaz Ahamed, K. Andrographis Echioides Leaves Extract as an Eco-Friendly Corrosion Inhibitor for Mild Steel in Acid Medium. Mater. Today Proc. 2021, 47, 2080–2090. [Google Scholar] [CrossRef]
- Guo, W.; Talha, M.; Lin, Y.; Kong, X. Schiff’s Base with Center of Symmetry as an Effective Corrosion Inhibitor for Mild Steel in Acid Medium: Electrochemical & Simulation Studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126234. [Google Scholar] [CrossRef]
- Messali, M.; Larouj, M.; Lgaz, H.; Rezki, N.; Al-Blewi, F.F.; Aouad, M.R.; Chaouiki, A.; Salghi, R.; Chung, I.-M. A New Schiff Base Derivative as an Effective Corrosion Inhibitor for Mild Steel in Acidic Media: Experimental and Computer Simulations Studies. J. Mol. Struct. 2018, 1168, 39–48. [Google Scholar] [CrossRef]
- Migahed, M.A.; Farag, A.A.; Elsaed, S.M.; Kamal, R.; Abd El-Bary, H. Corrosion Inhibition of Carbon Steel in Formation Water of Oil Wells by Some Schiff Base Non Ionic Surfactants. In Proceedings of the European Corrosion Congress 2009, EUROCORR, Nice, France, 6–10 September 2009. [Google Scholar]
- Rugmini Ammal, P.; Prajila, M.; Joseph, A. Physicochemical Studies on the Inhibitive Properties of a 1,2,4-Triazole Schiff’s Base, HMATD, on the Corrosion of Mild Steel in Hydrochloric Acid. Egypt. J. Pet. 2018, 27, 307–317. [Google Scholar] [CrossRef]
- Boukazoula, S.; Haffar, D.; Bourzami, R.; Toukal, L.; Dorcet, V. Synthesis, Characterizations, Crystal Structure, Inhibition Effects and Theoretical Study of Novel Schiff Base on the Corrosion of Carbon Steel in 1 M HCl. J. Mol. Struct. 2022, 1261, 132852. [Google Scholar] [CrossRef]
- Li, X.-L.; Xie, B.; Feng, J.-S.; Lai, C.; Bai, X.-X.; Li, T.; Zhang, D.-L.; Mou, W.-Y.; Wen, L.; Gu, Y.-T. 2-Pyridinecarboxaldehyde-Based Schiff Base as an Effective Corrosion Inhibitor for Mild Steel in HCl Medium: Experimental and Computational Studies. J. Mol. Liq. 2022, 345, 117032. [Google Scholar] [CrossRef]
- El Aatiaoui, A.; Koudad, M.; Chelfi, T.; Erkan, S.; Azzouzi, M.; Aouniti, A.; Savaş, K.; Kaddouri, M.; Benchat, N.; Oussaid, A. Experimental and Theoretical Study of New Schiff Bases Based on Imidazo(1,2-a)Pyridine as Corrosion Inhibitor of Mild Steel in 1M HCl. J. Mol. Struct. 2021, 1226, 129372. [Google Scholar] [CrossRef]
- Sengupta, S.; Murmu, M.; Murmu, N.C.; Banerjee, P. Adsorption of Redox-Active Schiff Bases and Corrosion Inhibiting Property for Mild Steel in 1 MolL−1 H2SO4: Experimental Analysis Supported by Ab Initio DFT, DFTB and Molecular Dynamics Simulation Approach. J. Mol. Liq. 2021, 326, 115215. [Google Scholar] [CrossRef]
- Saha, S.K.; Murmu, M.; Murmu, N.C.; Banerjee, P. Synthesis, Characterization and Theoretical Exploration of Pyrene Based Schiff Base Molecules as Corrosion Inhibitor. J. Mol. Struct. 2021, 1245, 131098. [Google Scholar] [CrossRef]
- El Azzouzi, M.; Azzaoui, K.; Warad, I.; Hammouti, B.; Shityakov, S.; Sabbahi, R.; Saoiabi, S.; Youssoufi, M.H.; Akartasse, N.; Jodeh, S.; et al. Moroccan, Mauritania, and Senegalese Gum Arabic Variants as Green Corrosion Inhibitors for Mild Steel in HCl: Weight Loss, Electrochemical, AFM and XPS Studies. J. Mol. Liq. 2022, 347, 118354. [Google Scholar] [CrossRef]
- Berrissoul, A.; Ouarhach, A.; Benhiba, F.; Romane, A.; Guenbour, A.; Outada, H.; Dafali, A.; Zarrouk, A. Exploitation of a New Green Inhibitor against Mild Steel Corrosion in HCl: Experimental, DFT and MD Simulation Approach. J. Mol. Liq. 2022, 349, 118102. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, B.; Yang, W.; Yin, X.; Liu, Y.; Chen, Y. Halogen-Substituted Imidazoline Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2015, 90, 284–295. [Google Scholar] [CrossRef]
- Mashuga, M.E.; Olasunkanmi, L.O.; Lgaz, H.; Sherif, E.-S.M.; Ebenso, E.E. Aminomethylpyridazine Isomers as Corrosion Inhibitors for Mild Steel in 1 M HCl: Electrochemical, DFT and Monte Carlo Simulation Studies. J. Mol. Liq. 2021, 344, 117882. [Google Scholar] [CrossRef]
- Strachan, A.; Çağin, T.; Goddard, W.A. Phase Diagram of MgO from Density-Functional Theory and Molecular-Dynamics Simulations. Phys. Rev. B 1999, 60, 15084–15093. [Google Scholar] [CrossRef]
- Riggs, O.L., Jr.; Nathan, C.C. Corrosion Inhibitors; CC Nathan: Houston, TX, USA, 1973; Available online: https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=209587 (accessed on 8 June 2022).
- Zhang, W.; Nie, B.; Li, H.-J.; Li, Q.; Li, C.; Wu, Y.-C. Inhibition of Mild Steel Corrosion in 1 M HCl by Chondroitin Sulfate and Its Synergistic Effect with Sodium Alginate. Carbohydr. Polym. 2021, 260, 117842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, L.; Cao, L.; Yang, N.; Huang, C. Studies of Corrosion Inhibitors for Zinc–Manganese Batteries: Quinoline Quaternary Ammonium Phenolates. Corros. Sci. 2001, 43, 1627–1636. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giacomelli, C.; Giacomelli, F.C.; Spinelli, A. Evaluation of the Inhibitor Effect of L-Ascorbic Acid on the Corrosion of Mild Steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Shaban, S.M.; Badr, E.a.; Shenashen, M.A.; Farag, A.A. Fabrication and Characterization of Encapsulated Gemini Cationic Surfactant as Anticorrosion Material for Carbon Steel Protection in Down-Hole Pipelines. Environ. Technol. Innov. 2021, 23, 101603. [Google Scholar] [CrossRef]
- Abbout, S.; Chebabe, D.; Zouarhi, M.; Rehioui, M.; Lakbaibi, Z.; Hajjaji, N. Ceratonia Siliqua L Seeds Extract as Eco-Friendly Corrosion Inhibitor for Carbon Steel in 1 M HCl: Characterization, Electrochemical, Surface Analysis, and Theoretical Studies. J. Mol. Struct. 2021, 1240, 130611. [Google Scholar] [CrossRef]
- Haruna, K.; Obot, I.B.; Ankah, N.K.; Sorour, A.A.; Saleh, T.A. Gelatin: A Green Corrosion Inhibitor for Carbon Steel in Oil Well Acidizing Environment. J. Mol. Liq. 2018, 264, 515–525. [Google Scholar] [CrossRef]
- Al-Gamal, A.G.; Chowdhury, T.H.; Kabel, K.I.; Farag, A.A.; Abd El-Sattar, N.E.A.; Rabie, A.M.; Islam, A. N-Functionalized Graphene Derivatives as Hole Transport Layers for Stable Perovskite Solar Cell. Sol. Energy 2021, 228, 670–677. [Google Scholar] [CrossRef]
- Kumar, B.; Vashisht, H.; Goyal, M.; Kumar, A.; Benhiba, F.; Prasad, A.K.; Kumar, S.; Bahadur, I.; Zarrouk, A. Study of Adsorption Mechanism of Chalcone Derivatives on Mild Steel-Sulfuric Acid Interface. J. Mol. Liq. 2020, 318, 113890. [Google Scholar] [CrossRef]
- Wang, C.; Zou, C.; Cao, Y. Electrochemical and Isothermal Adsorption Studies on Corrosion Inhibition Performance of β-Cyclodextrin Grafted Polyacrylamide for X80 Steel in Oil and Gas Production. J. Mol. Struct. 2021, 1228, 129737. [Google Scholar] [CrossRef]
- Bentiss, F.; Lebrini, M.; Lagrenée, M. Thermodynamic Characterization of Metal Dissolution and Inhibitor Adsorption Processes in Mild Steel/2,5-Bis(n-Thienyl)-1,3,4-Thiadiazoles/Hydrochloric Acid System. Corros. Sci. 2005, 47, 2915–2931. [Google Scholar] [CrossRef]
- Bayol, E.; Gürten, T.; Gürten, A.A.; Erbil, M. Interactions of Some Schiff Base Compounds with Mild Steel Surface in Hydrochloric Acid Solution. Mater. Chem. Phys. 2008, 112, 624–630. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo Leaf Extract as an Eco-Friendly Corrosion Inhibitor of X70 Steel in HCl Solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Farag, A.A.; Abdallah, H.E.; Badr, E.A.; Mohamed, E.A.; Ali, A.I.; El-Etre, A.Y. The Inhibition Performance of Morpholinium Derivatives on Corrosion Behavior of Carbon Steel in the Acidized Formation Water: Theoretical, Experimental and Biocidal Evaluations. J. Mol. Liq. 2021, 341, 117348. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Hashem, H.E.; Azmy, E.M.; Negm, N.A.; Farag, A.A. Synthesis, Structural Analysis, and Inhibition Approach of Novel Eco-Friendly Chalcone Derivatives on API X65 Steel Corrosion in Acidic Media Assessment with DFT & MD Studies. Environ. Technol. Innov. 2021, 24, 101966. [Google Scholar] [CrossRef]
- Hamani, H.; Douadi, T.; Daoud, D.; Al-Noaimi, M.; Rikkouh, R.A.; Chafaa, S. 1-(4-Nitrophenylo-Imino)-1-(Phenylhydrazono)-Propan-2-One as Corrosion Inhibitor for Mild Steel in 1M HCl Solution: Weight Loss, Electrochemical, Thermodynamic and Quantum Chemical Studies. J. Electroanal. Chem. 2017, 801, 425–438. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardaş, G.; Çulha, M.; Yazıcı, B.; Erbil, M. Investigation of Adsorption and Inhibitive Effect of 2-Mercaptothiazoline on Corrosion of Mild Steel in Hydrochloric Acid Media. Electrochim. Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Shaabani, B.; Seifzadeh, D. Corrosion Inhibition of Mild Steel by Some Schiff Base Compounds in Hydrochloric Acid. Appl. Surf. Sci. 2005, 239, 154–164. [Google Scholar] [CrossRef]
- Lagrenée, M.; Mernari, B.; Bouanis, M.; Traisnel, M.; Bentiss, F. Study of the Mechanism and Inhibiting Efficiency of 3,5-Bis (4-Methylthiophenyl)-4H-1,2,4-Triazole on Mild Steel Corrosion in Acidic Media. Corros. Sci. 2002, 44, 573–588. [Google Scholar] [CrossRef]
- Amer, A.; Sayed, G.H.; Ramadan, R.M.; Rabie, A.M.; Negm, N.A.; Farag, A.A.; Mohammed, E.A. Assessment of 3-Amino-1H-1,2,4-Triazole Modified Layered Double Hydroxide in Effective Remediation of Heavy Metal Ions from Aqueous Environment. J. Mol. Liq. 2021, 314, 116935. [Google Scholar] [CrossRef]
- Altalhi, A.A.; Mohammed, E.A.; Morsy, S.S.M.; Negm, N.A.; Farag, A.A. Catalyzed Production of Different Grade Biofuels Using Metal Ions Modified Activated Carbon of Cellulosic Wastes. Fuel 2021, 295, 120646. [Google Scholar] [CrossRef]
- Abubshait, H.A.; Farag, A.A.; El-Raouf, M.A.; Negm, N.A.; Mohamed, E.A. Graphene Oxide Modified Thiosemicarbazide Nanocomposite as an Effective Eliminator for Heavy Metal Ions. J. Mol. Liq. 2021, 327, 114790. [Google Scholar] [CrossRef]
- Martinez, S.; Stern, I. Thermodynamic Characterization of Metal Dissolution and Inhibitor Adsorption Processes in the Low Carbon Steel/Mimosa Tannin/Sulfuric Acid System. Appl. Surf. Sci. 2002, 199, 83–89. [Google Scholar] [CrossRef]
- Dutta, A.; Saha, S.K.; Adhikari, U.; Banerjee, P.; Sukul, D. Effect of Substitution on Corrosion Inhibition Properties of 2-(Substituted Phenyl) Benzimidazole Derivatives on Mild Steel in 1M HCl Solution: A Combined Experimental and Theoretical Approach. Corros. Sci. 2017, 123, 256–266. [Google Scholar] [CrossRef]
- Galai, M.; Rbaa, M.; Ouakki, M.; Abousalem, A.S.; Ech-chihbi, E.; Dahmani, K.; Dkhireche, N.; Lakhrissi, B.; EbnTouhami, M. Chemically Functionalized of 8-Hydroxyquinoline Derivatives as Efficient Corrosion Inhibition for Steel in 1.0 M HCl Solution: Experimental and Theoretical Studies. Surf. Interfaces 2020, 21, 100695. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Nie, B.-L.; Li, H.-J.; Zhang, W.-W.; Wu, Y.-C. Anticorrosion Performance of Grape Seed Proanthocyanidins Extract and Tween-80 for Mild Steel in Hydrochloric Acid Medium. J. Mol. Liq. 2021, 331, 115799. [Google Scholar] [CrossRef]
- Khaled, K.F. Molecular Simulation, Quantum Chemical Calculations and Electrochemical Studies for Inhibition of Mild Steel by Triazoles. Electrochim. Acta 2008, 53, 3484–3492. [Google Scholar] [CrossRef]
- Torres, V.V.; Rayol, V.A.; Magalhães, M.; Viana, G.M.; Aguiar, L.C.S.; Machado, S.P.; Orofino, H.; D’Elia, E. Study of Thioureas Derivatives Synthesized from a Green Route as Corrosion Inhibitors for Mild Steel in HCl Solution. Corros. Sci. 2014, 79, 108–118. [Google Scholar] [CrossRef]
- Solmaz, R. Investigation of the Inhibition Effect of 5-((E)-4-Phenylbuta-1,3-Dienylideneamino)-1,3,4-Thiadiazole-2-Thiol Schiff Base on Mild Steel Corrosion in Hydrochloric Acid. Corros. Sci. 2010, 52, 3321–3330. [Google Scholar] [CrossRef]
- Saranya, J.; Benhiba, F.; Anusuya, N.; Subbiah, R.; Zarrouk, A.; Chitra, S. Experimental and Computational Approaches on the Pyran Derivatives for Acid Corrosion. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125231. [Google Scholar] [CrossRef]
- Palanimurugan, A.; Kulandaisamy, A. DNA, in Vitro Antimicrobial/Anticancer Activities and Biocidal Based Statistical Analysis of Schiff Base Metal Complexes Derived from Salicylalidene-4-Imino-2,3-Dimethyl-1-Phenyl-3-Pyrazolin-5-One and 2-Aminothiazole. J. Organomet. Chem. 2018, 861, 263–274. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.; Sayed, M.A. Some Novel Rare Earth Metal Ions Complexes: Synthesis, Characterization, Luminescence and Biocidal Efficiency. Anal. Biochem. 2020, 598, 113645. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.M.; Negm, N.A.; Farag, R.K.; Fadda, A.A.; Gomaa, A.E.; Farag, A.A.; Migahed, M.A. Anti-Corrosion, Antiscalant and Anti-Microbial Performance of Some Synthesized Trimeric Cationic Imidazolium Salts in Oilfield Applications. J. Mol. Liq. 2022, 351, 118610. [Google Scholar] [CrossRef]
- Sainis, I.; Banti, C.N.; Owczarzak, A.M.; Kyros, L.; Kourkoumelis, N.; Kubicki, M.; Hadjikakou, S.K. New Antibacterial, Non-Genotoxic Materials, Derived from the Functionalization of the Anti-Thyroid Drug Methimazole with Silver Ions. J. Inorg. Biochem. 2016, 160, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.E.; Mohamed, E.A.; Farag, A.A.; Negm, N.A.; Azmy, E.A.M. New Heterocyclic Schiff Base-Metal Complex: Synthesis, Characterization, Density Functional Theory Study, and Antimicrobial Evaluation. Appl. Organomet. Chem. 2021, 35, e6322. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of Stereochemical Conformation into the Corrosion Inhibitive Behaviour of Double Azomethine Based Schiff Bases on Mild Steel Surface in 1 Mol L−1 HCl Medium: An Experimental, Density Functional Theory and Molecular Dynamics Simulation Study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Farag, A.A.; Migahed, M.A.; Badr, E.A. Thiazole Ionic Liquid as Corrosion Inhibitor of Steel in 1 M HCl Solution: Gravimetrical, Electrochemical, and Theoretical Studies. J. Bio-Tribo-Corros. 2019, 5, 53. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R. Investigation of Corrosion Inhibition Effect and Adsorption Activities of Cuscuta Reflexa Extract for Mild Steel in 0.5 M H2SO4. Bioelectrochemistry 2018, 124, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Lgaz, H.; Subrahmanya Bhat, K.; Salghi, R.; Shubhalaxmi; Jodeh, S.; Algarra, M.; Hammouti, B.; Ali, I.H.; Essamri, A. Insights into Corrosion Inhibition Behavior of Three Chalcone Derivatives for Mild Steel in Hydrochloric Acid Solution. J. Mol. Liq. 2017, 238, 71–83. [Google Scholar] [CrossRef]
- Li, W.; He, Q.; Pei, C.; Hou, B. Experimental and Theoretical Investigation of the Adsorption Behaviour of New Triazole Derivatives as Inhibitors for Mild Steel Corrosion in Acid Media. Electrochim. Acta 2007, 52, 6386–6394. [Google Scholar] [CrossRef]
- Singh, D.K.; Ebenso, E.E.; Singh, M.K.; Behera, D.; Udayabhanu, G.; John, R.P. Non-Toxic Schiff Bases as Efficient Corrosion Inhibitors for Mild Steel in 1M HCl: Electrochemical, AFM, FE-SEM and Theoretical Studies. J. Mol. Liq. 2018, 250, 88–99. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Sharma, P.; Guo, L.; Dagdag, O.; Kumar, V. Molecular Dynamic Simulation, Quantum Chemical Calculation and Electrochemical Behaviour of Punica Granatum Peel Extract as Eco-Friendly Corrosion Inhibitor for Stainless Steel (SS-410) in Acidic Medium. J. Mol. Liq. 2022, 346, 118237. [Google Scholar] [CrossRef]
- Momeni, M.J.; Behzadi, H.; Roonasi, P.; Sadjadi, S.A.S.; Mousavi-Khoshdel, S.M.; Mousavi, S.V. Ab Initio Study of Two Quinoline Derivatives as Corrosion Inhibitor in Acidic Media: Electronic Structure, Inhibitor–Metal Interaction, and Nuclear Quadrupole Resonance Parameters. Res. Chem. Intermed. 2015, 41, 6789–6802. [Google Scholar] [CrossRef]
- Srivastava, V.; Haque, J.; Verma, C.; Singh, P.; Lgaz, H.; Salghi, R.; Quraishi, M.A. Amino Acid Based Imidazolium Zwitterions as Novel and Green Corrosion Inhibitors for Mild Steel: Experimental, DFT and MD Studies. J. Mol. Liq. 2017, 244, 340–352. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, D.; Kumar, S.; Yadav, P. Experimental and Quantum Chemical Studies on Corrosion Inhibition Performance of Thiazolidinedione Derivatives for Mild Steel in Hydrochloric Acid Solution. Chem. Eng. Commun. 2015, 202, 303–315. [Google Scholar] [CrossRef]
- Zhan, C.-G.; Nichols, J.A.; Dixon, D.A. Ionization Potential, Electron Affinity, Electronegativity, Hardness, and Electron Excitation Energy: Molecular Properties from Density Functional Theory Orbital Energies. J. Phys. Chem. A 2003, 107, 4184–4195. [Google Scholar] [CrossRef]
- Hashem, H.E.; Farag, A.A.; Mohamed, E.A.; Azmy, E.M. Experimental and Theoretical Assessment of Benzopyran Compounds as Inhibitors to Steel Corrosion in Aggressive Acid Solution. J. Mol. Struct. 2022, 1249, 131641. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Banerjee, P.; Murmu, M.; Quraishi, M.A.; Lin, Y. Corrosion Inhibition Behavior of Piperidinium Based Ionic Liquids on Q235 Steel in Hydrochloric Acid Solution: Experimental, Density Functional Theory and Molecular Dynamics Study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126708. [Google Scholar] [CrossRef]
- Yang, X.; Li, F.; Zhang, W. 4-(Pyridin-4-Yl)Thiazol-2-Amine as an Efficient Non-Toxic Inhibitor for Mild Steel in Hydrochloric Acid Solutions. RSC Adv. 2019, 9, 10454–10464. [Google Scholar] [CrossRef]
- Layla Mehdi, B.; Gu, M.; Parent, L.R.; Xu, W.; Nasybulin, E.N.; Chen, X.; Unocic, R.R.; Xu, P.; Welch, D.A.; Abellan, P.; et al. In-Situ Electrochemical Transmission Electron Microscopy for Battery Research. Microsc. Microanal. 2014, 20, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.A.; Eid, A.M.; Shaban, M.M.; Mohamed, E.A.; Raju, G. Integrated Modeling, Surface, Electrochemical, and Biocidal Investigations of Novel Benzothiazoles as Corrosion Inhibitors for Shale Formation Well Stimulation. J. Mol. Liq. 2021, 336, 116315. [Google Scholar] [CrossRef]
- Anwer, K.E.; Farag, A.A.; Mohamed, E.A.; Azmy, E.M.; Sayed, G.H. Corrosion Inhibition Performance and Computational Studies of Pyridine and Pyran Derivatives for API X-65 Steel in 6M H2SO4. J. Ind. Eng. Chem. 2021, 97, 523–538. [Google Scholar] [CrossRef]
- Farag, A.A.; Ismail, A.S.; Migahed, M.A. Squid By-Product Gelatin Polymer as an Eco-Friendly Corrosion Inhibitor for Carbon Steel in 0.5 M H2SO4 Solution: Experimental, Theoretical, and Monte Carlo Simulation Studies. J. Bio- Tribo-Corros. 2020, 6, 16. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Z.; Liang, Q.; Han, G.-C.; Han, J.; Zhang, S.; Feng, X.-Z. Synthesis of 2-Aminofluorene Bis-Schiff Base and Corrosion Inhibition Performance for Carbon Steel in HCl. J. Mol. Liq. 2019, 277, 330–340. [Google Scholar] [CrossRef]
- Abdallah, M.; Alfakeer, M.; Altass, H.M.; Alharbi, A.M.; Althagafi, I.; Hasan, N.F.; Mabrouk, E.M. The Polarographic and Corrosion Inhibition Performance of Some Schiff Base Compounds Derived from 2-Amino-3-Hydroxypyridine in Aqueous Media. Egypt. J. Pet. 2019, 28, 393–399. [Google Scholar] [CrossRef]
- Bedair, M.A.; Soliman, S.A.; Bakr, M.F.; Gad, E.S.; Lgaz, H.; Chung, I.-M.; Salama, M.; Alqahtany, F.Z. Benzidine-Based Schiff Base Compounds for Employing as Corrosion Inhibitors for Carbon Steel in 1.0 M HCl Aqueous Media by Chemical, Electrochemical and Computational Methods. J. Mol. Liq. 2020, 317, 114015. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).