Abstract
The development of non-natural enzymatic catalysis is important for multicomponent tandem organic transformations. However, the delicate acting environments of biological enzymes still present some challenges in the synthesis of spirooxindole skeleton via enzymatic catalysis. To address these issues, a lipase-catalyzed method was developed for the synthesis of spirooxindole frameworks. Using easily available isatins, cycloketones, and malononitriles as substrates, mild reaction conditions, and a reasonable reaction time, moderate to good yields (67–92%) and excellent functional group tolerance were accomplished via this protocol. The related mechanism explanation is also speculated in this paper.
1. Introduction
Compared with traditional stepwise processes, one-pot tandem processes represent a greener and more versatile synthesis approach, given their inherent simplicity. The multi-step reaction in one-pot processes can start from relatively simple and easily available raw materials without the separation of intermediates to directly obtain complex molecules, which is evidently economical and environmentally friendly [1]. The one-pot tandem catalytic system combined with chemical catalysis has been widely reported, whereas systems using biocatalysis still have development space and potential [2,3]. As Nicholas Harmer mentioned, sets of enzymes or multiple substrates working together—a “cascade”—to drive desirable reactions in one-pot systems will help deliver the target product on a large scale using fewer resources and generating less waste [4,5]. Large-scale demand for target products, such as fine chemicals, medicine, and food, are present in many fields. The starting reagents used in the cascade process can be derived from low-cost, high-yield isolates, and we can create maximum production value based on the convenience of initial materials.
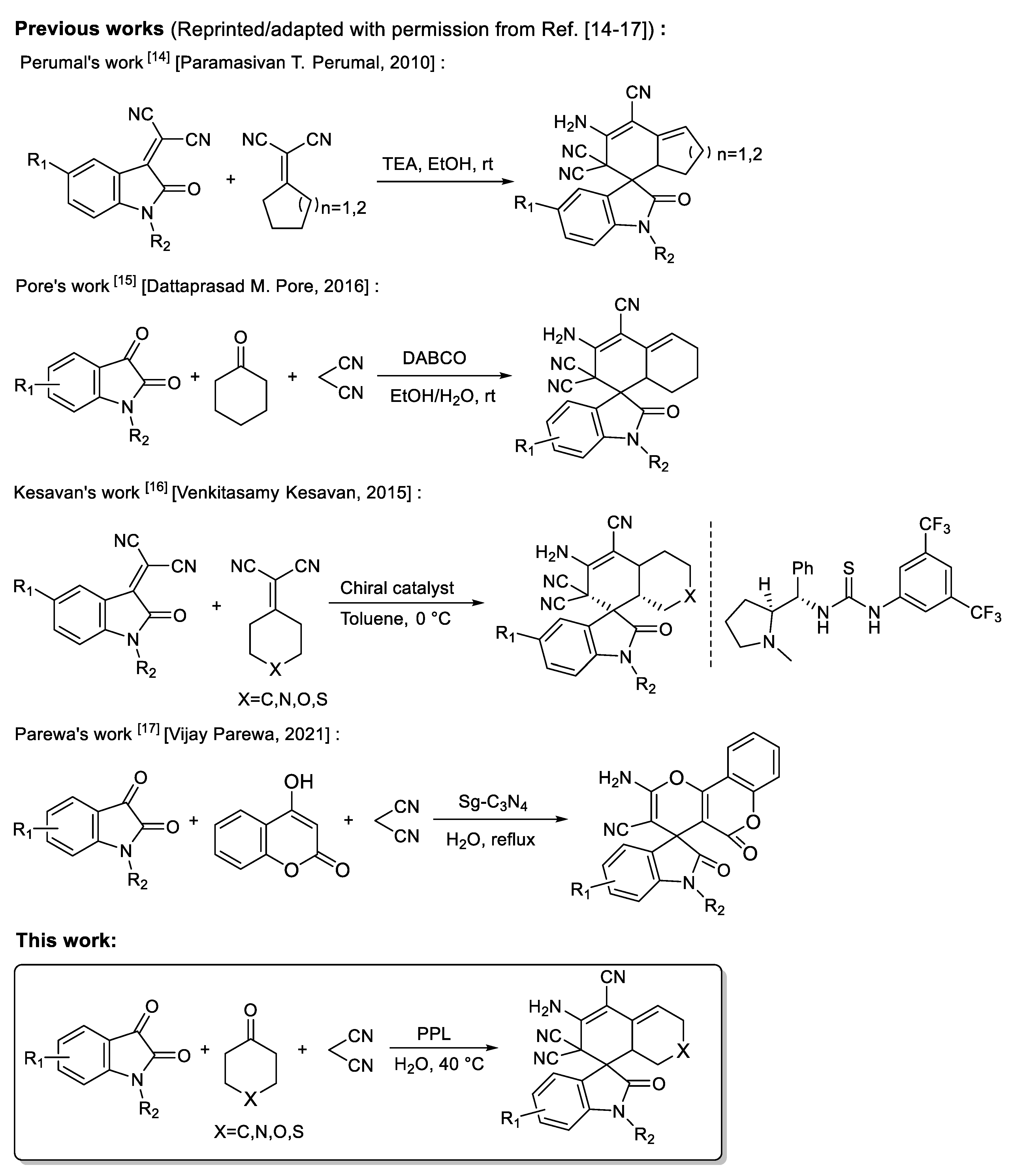
Spirocytic structures have attracted attention due to their wide range of biological and pharmacological activities [6,7,8]. Highly functional spirooxindoles have become a research hotspot due to their remarkable and diverse biological activities [9,10]. These oxindole skeleton compounds with various spiral–ring structures have been shown to be used as anti-infective, anti-tumor, and antibacterial materials, and as molecular probes [11,12,13]. Therefore, spiroxide indole compounds with different structures should be designed and synthesized. At present, various organocatalyzed schemes have been developed to obtain spiroindole structures. In 2010, Perumal’s group reported a method for the synthesis of functionalized spirocyclic oxindoles catalyzed by triethylamine [14]. In 2016, Pore’s group reported the use of the strong base-DABCO to catalyze the synthesis of functionalized spirooxindoles [15]. In 2015, Kesavan et al. developed an enantioselective synthesis protocol in toluene, obtaining carbocyclic spirooxindoles with good yield and enantioselectivity using L-proline derived thiourea organocatalyst [16]. In 2021, Parewa et al. manufactured functionalized graphitic carbon nitride (Sg-C3N4) and utilized a reusable catalyst for the one-pot production of various spiro-pyrano chromenes and spiro indole-3,10-naphthalene tetracyclic systems in aqueous media [17]. Although these methods can obtain spirooxindoles with good yield in a relatively short time, the reactions need to be catalyzed by weak or strong organic base catalysts, and some catalysts require complex synthesis procedures. For example, thiourea catalysts reported in Kesavan’s work require chiral prolines for derivational synthesis [16], and the catalyst reported by Parewa requires high temperature, a strong acid solution and tedious characterization [17]. In addition, most of the methods reported need to be carried out in organic solvents, such as toluene or ethylene glycol; these organic solvents do not conform to the concept of green chemistry to some extent (Figure 1).
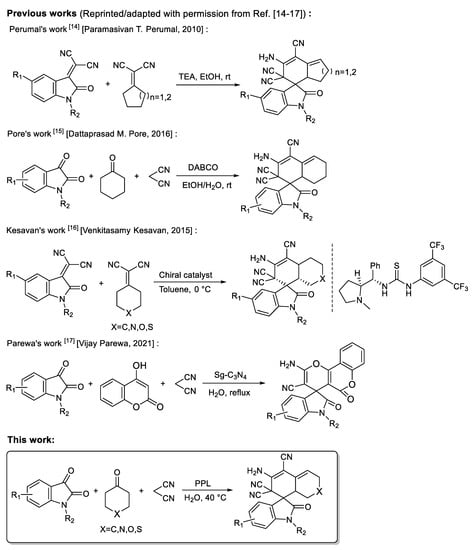
Figure 1.
Overview of methods for synthesizing spiroxide indole compounds.
With the introduction of the concept of green chemistry and the emergence of new technologies, biocatalysts are gradually becoming an important part of the field of chemical synthesis. In many cases, biocatalysts have replaced traditional chemical catalysts and have significant application prospects in the future [18,19,20,21,22,23]. Through enzymatic reactions, we can construct drug modules and biologic therapeutics effectively, and reduce the use of polluting chemicals and solvents [24,25,26,27]. The synthesis of spirooxindole compounds catalyzed by enzymes has been partially studied. In 2011, Zhang reported the synthesis of spirooxindole catalyzed via lipase (porcine pancreatic lipase), but the process was carried out in a mixed solvent of ethanol/water, and the ratio of water significantly affected the yield [28]. In 2014, Lin’s group used isatin derivatives and 1,3-dicarbonyl as starting materials; spirooxindole derivatives were obtained via a two-component enzymatic (Acylase Amano from Aspergillus oryzae) catalytic process in ethylene glycol [29]. However, the enzymatic synthesis of spirooxindole is still a hot spot in enzyme catalysis. Lipase, as an efficient and green biocatalyst, has been widely studied by researchers due to its good applicability of reaction types, stable temperature, solvent compatibility and commercial simplicity [30,31,32].
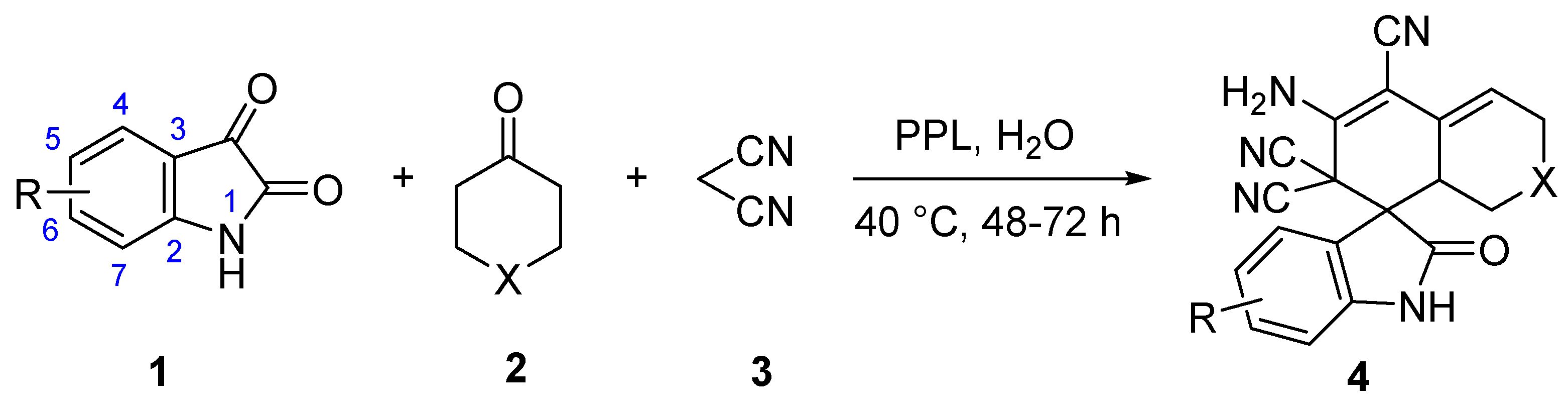
In this study, we investigated the synthesis of spirooxindoles from isatin, malononitrile and cycloketone substrates with lipase from porcine pancreatic lipase (PPL) in the presence of water to expand the study of non-natural enzymatic reactions and explore favorable biocatalytic synthesis. To the best of our knowledge, the synthesis of spirooxindole using a one-pot tandem process catalyzed via lipase in aqueous media has not been reported before (Figure 1).
2. Results and Discussion
2.1. Optimization of Reaction Conditions for the One-Pot Tandem Process
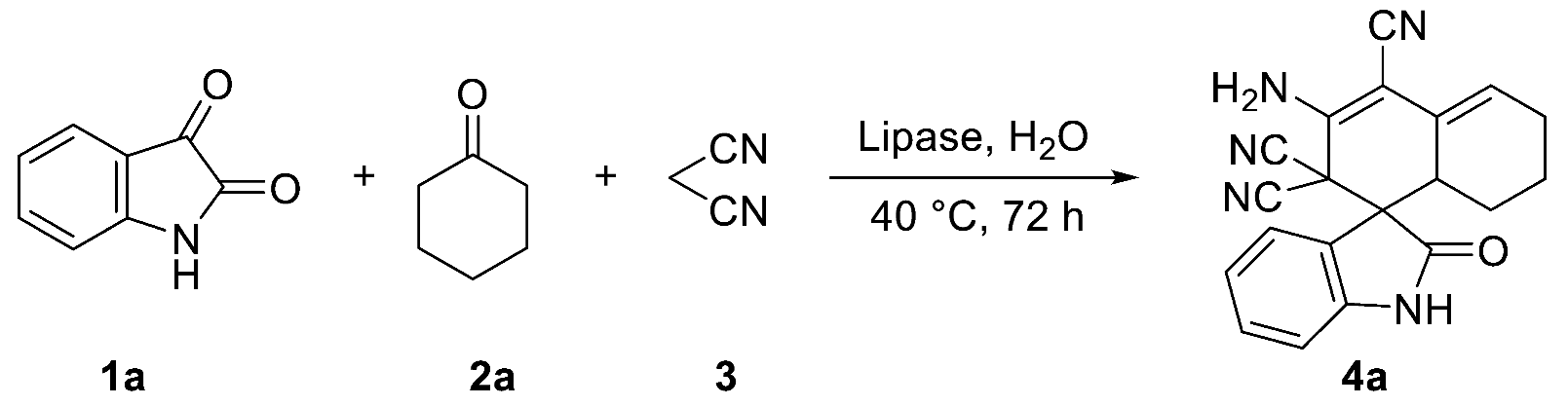
1a, 2a, and 3 were selected as model substrates to optimize a series of reaction conditions. We discovered the catalytic effects of different lipases at a temperature of 40 °C (Table 1), and selected several lipases that showed different catalytic activities. Among these lipases, porcine pancreatic lipase (PPL) showed the highest reactivity (Table 1, entry 1 versus entries 2–5). Although PSL, BSA, CALB, and Novozym 435 had certain catalytic effects, the target product was obtained with low yield (Table 1, entries 2–5), whereas 4a was not obtained when denatured PPL was replaced with PPL (Table 1, entry 6); similar results occurred in the controlled experiment (Table 1, entry 7). The above results indicated that the difference in the lipase active site was crucial for the catalytic synthesis of spirooxindoles. Based on the highest lipase activity temperature range reported in the relevant literature [33], analyses were conducted regarding experimental temperature (see Supplementary Materials, Table S1).

Table 1.
Optimization of lipase types for the one-pot tandem synthesis of 4a.
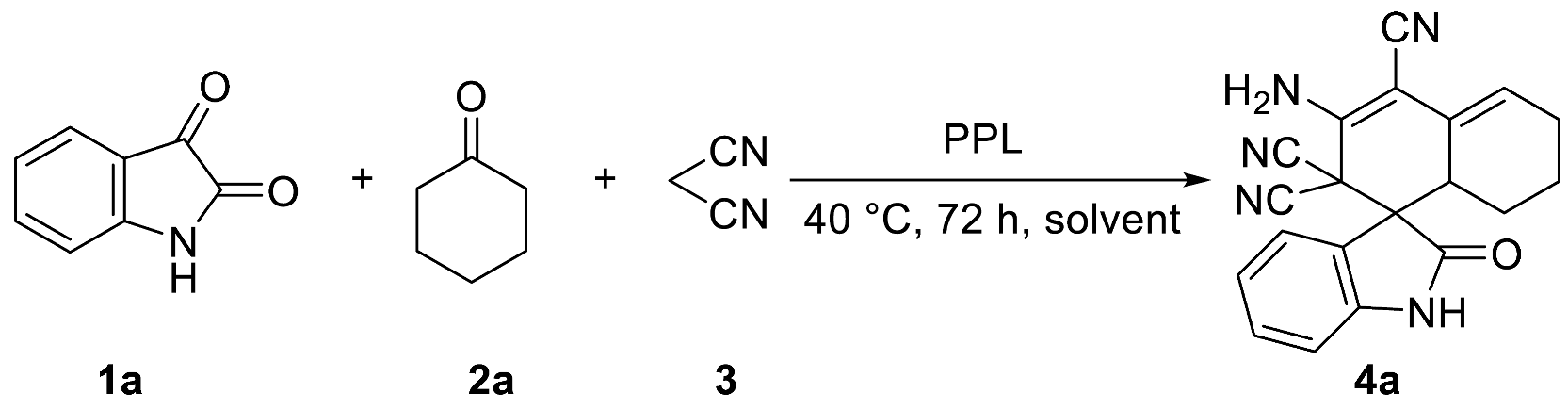
Solvents could significantly affect the activity of enzymes in the catalytic enzymatic reaction. Therefore, we proceeded to examine the effects of solvents. The model reaction showed excellent reactivity in the presence of polar solvents, such as EtOH, DMF, DMSO, and water (Table 2, entries 1–4). However, when THF, toluene, EA, and DCM were used as reaction solvents separately, the catalytic effect of PPL was extremely poor or even non-reactive (Table 2, entries 5–8). Although PPL in DMF or DMSO could make the model reaction achieve the highest yield in a relatively short time (Table 2, entries 2–3), water, as the best medium in enzymatic chemical reactions, has a series of advantages, including its safety and non-toxicity, and that it is a green solvent [34]. In addition, in subsequent separation and purification processes, due to the low solubility of the target product 4a in the water phase, we could easily obtain the target product via simple filtration, washing, and drying, rather than using a complicated column chromatography separation. Even though the reaction takes longer in the aqueous phase, we still chose water as the best solvent for an almost identical yield.

Table 2.
Optimization of solvent for the one-pot tandem synthesis of 4a.
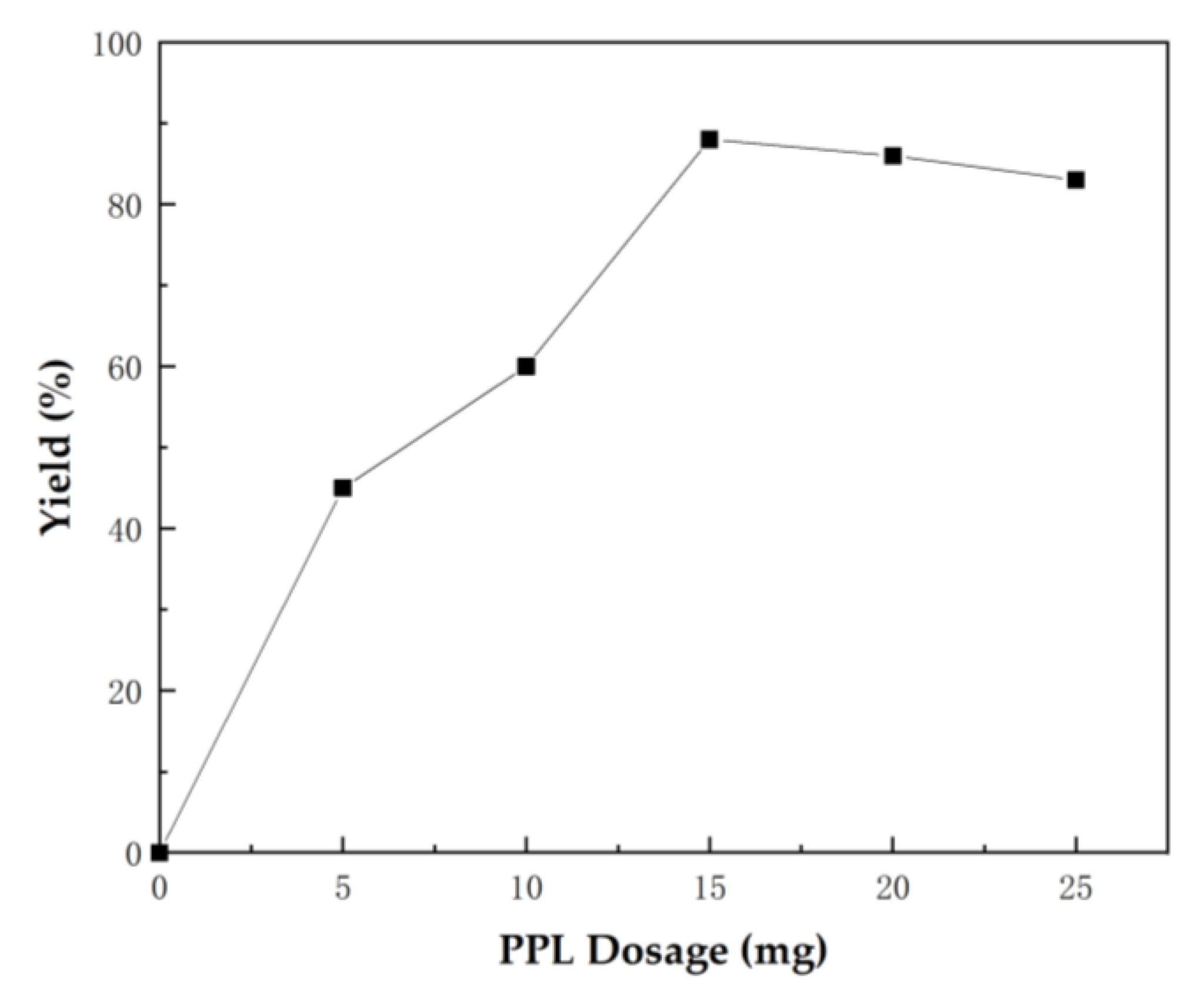
The enzyme dosage also has an evident effect on the reaction. Insufficient enzyme reduces catalytic efficiency, whereas excessive enzyme increases production cost, and even leads to negative effects to some extent. Therefore, we explored the influence of enzyme dosage. Figure 2 shows that the yield of spirooxindole 4a increased as the lipase dosage increased from 5 mg to 15 mg, but the yield of 4a decreased as the lipase dosage increased from 20 mg to 25 mg. This phenomenon might have been caused by excessive enzyme aggregation in a quantitative solvent that was not conducive to the contact between substrates and enzyme active centers. Thus, 15 mg PPL was the optimal reaction dosage.
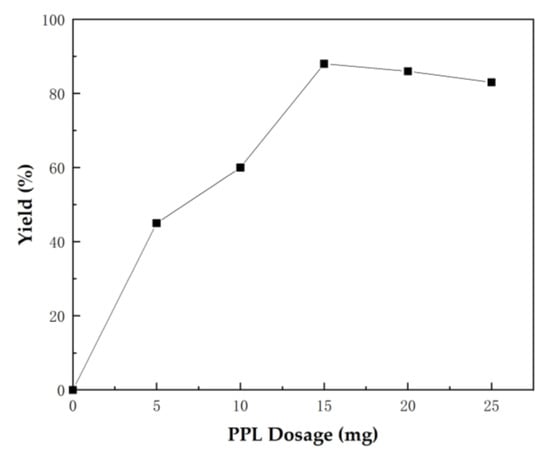
Figure 2.
Optimization of PPL dosage for the one-pot tandem synthesis of 4a. Reaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), 3 (0.6 mmol), H2O (3 mL), 40 °C for 72 h.
2.2. Substrate Scopes for the One-Pot Tandem Process
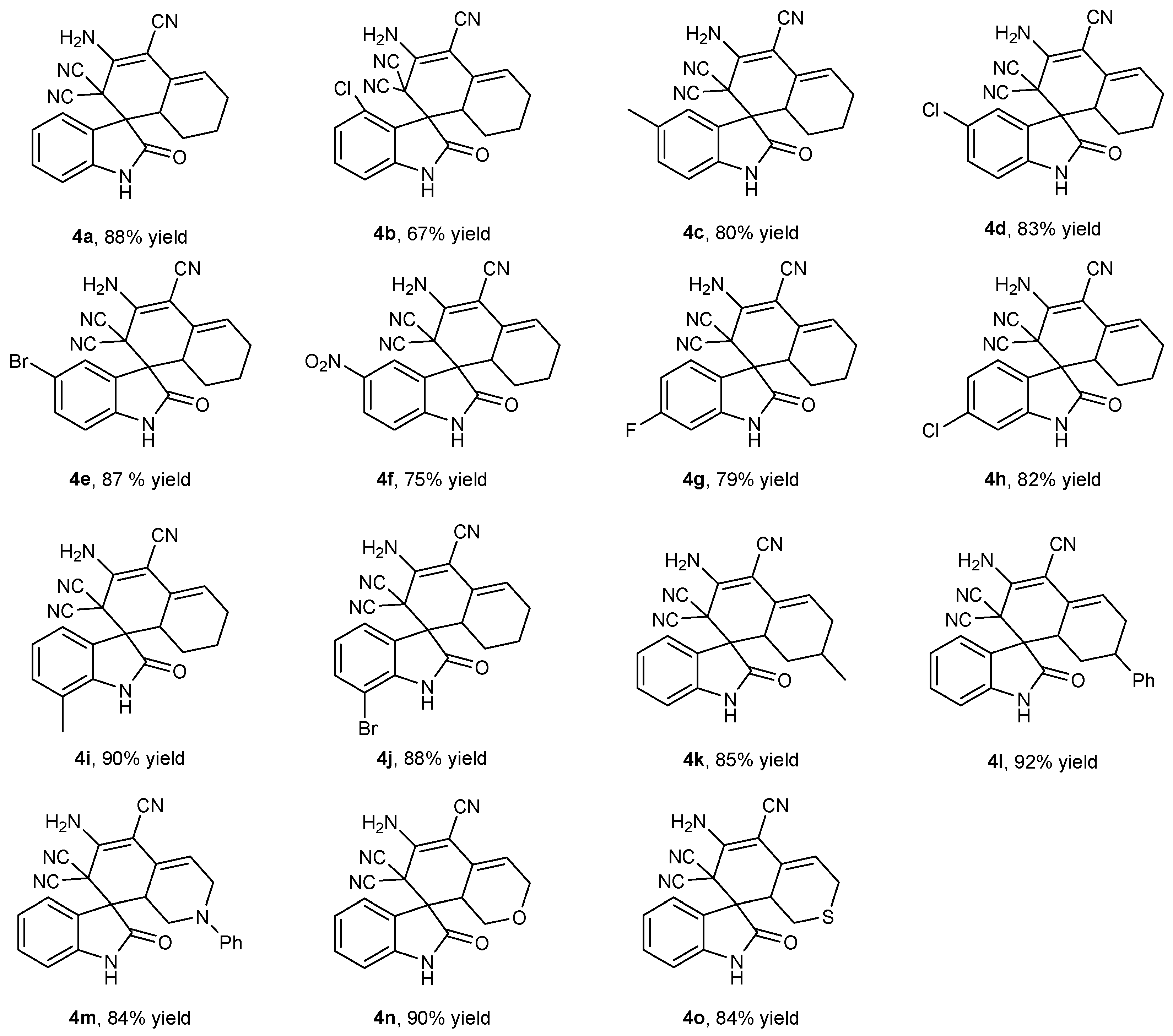
With optimized conditions in hand, respective series of isatins 1 and cyclic ketones 2 were examined using this one-pot tandem process; results are summarized in Figure 3. Cyclohexanone 2a easily reacted with isatins at different substitution sites and afforded the final products 4a–4m with 67–90% isolated yields. It was notable that their electronic properties did not significantly affect their yields. One-pot tandem reactions with substrates (2a–2m) had electron-withdrawing and electron-donating substituents at 2- to 7-positions. To be clear, all products except 4c (67% isolated yield) achieved excellent yield, which might have been caused by the high steric hindrance of the 4-substituted site. In addition, 6-membered cycloketone substrates were tested for this transformation. Similarly, 1a smoothly reacted with 2n–2r, producing desired products 4n–4r in high isolated yields of 84–92%.
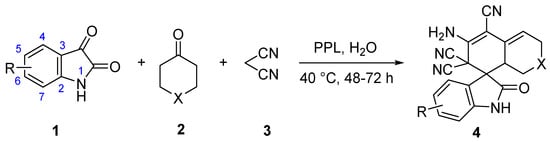
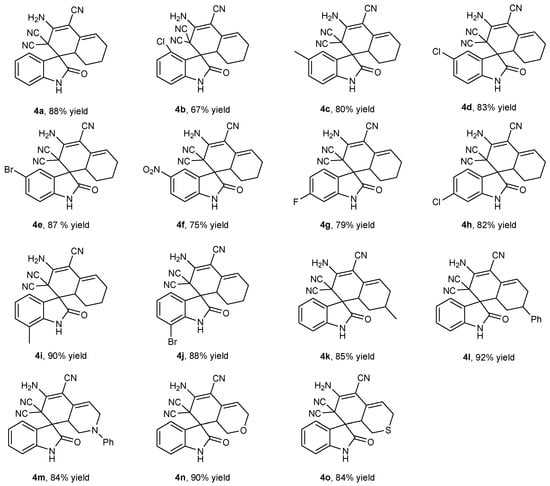
Figure 3.
Substrate scope for the one-pot tandem synthesis of 4. Reaction conditions: 1 (0.2 mmol), 2 (0.2 mmol), 3 (0.6 mmol), PPL(15 mg), H2O (3 mL), 40 °C; 4a–4l for 72 h, and 4m–4o for 48 h. Yields are the isolated yield.
2.3. Mechanism of the One-Pot Tandem Process
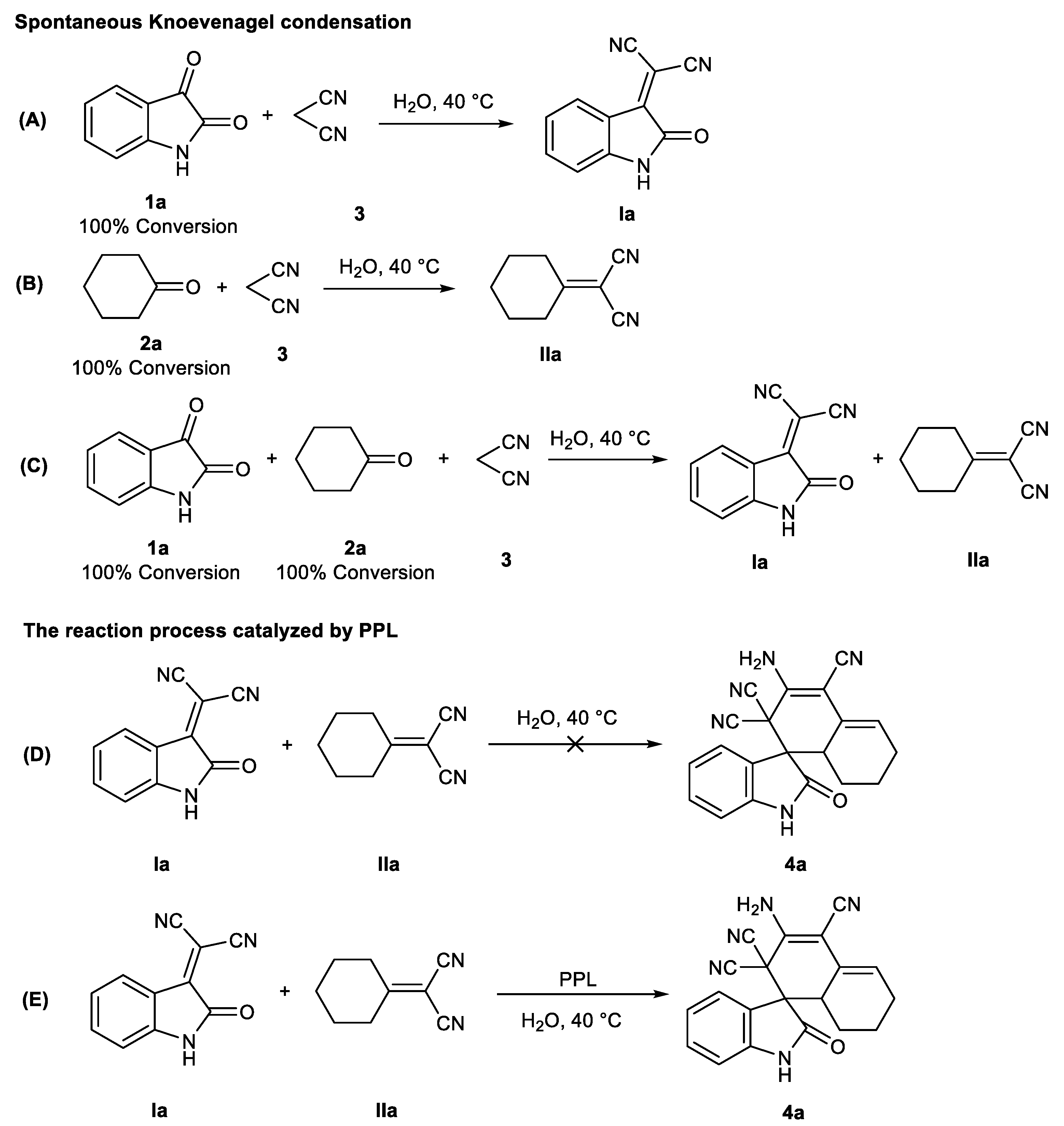
To understand the mechanism better, some control experiments were performed. We found that both isatin 1 and cyclohexanone 2 could form corresponding intermediates Ia and IIa with malonitrile 3 in either a single system or a mixed system, indicating that the formation of intermediates Ia and IIa in a one-pot tandem system was a spontaneous Knoevenagel condensation process (Figure 4A,B versus Figure 4C). Subsequently, it was found that the reaction between intermediate Ia and IIa could not form the target product 4a without the action of PPL. On the contrary, 4a could be effectively generated under the catalysis of PPL (Figure 4D versus Figure 4E).
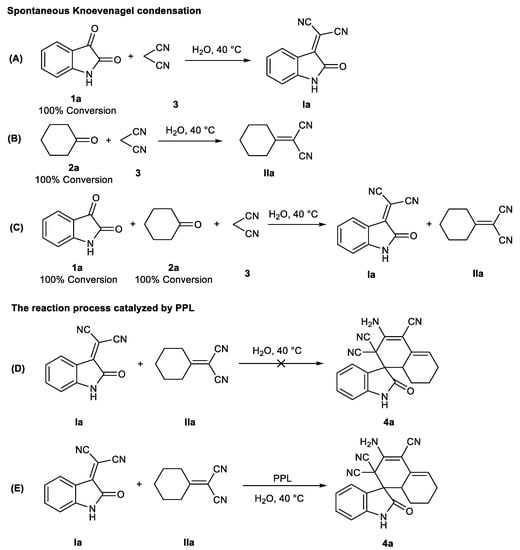
Figure 4.
Control experiments. (A) and (B): spontaneous Knoevenagle condensation in single system; (C): spontaneous Knoevenagle condensation in mixed system; (D) and (E): Investigation on the catalytic effect of PPL. 1H NMR of intermediates Ia (400 MHz, DMSO-d6) δ 11.23 (s, 1H), 7.90 (d, J = 7.8 Hz, 1H), 7.59 (td, J = 7.8, 1.2 Hz, 1H), 7.16 (td, J = 7.7, 1.0 Hz, 1H), 6.96 (d, J = 7.9 Hz, 1H). 1H NMR of intermediates IIa (400 MHz, DMSO-d6) δ 2.09–2.21 (m, 4H), 1.66–1.49 (m, 6H).
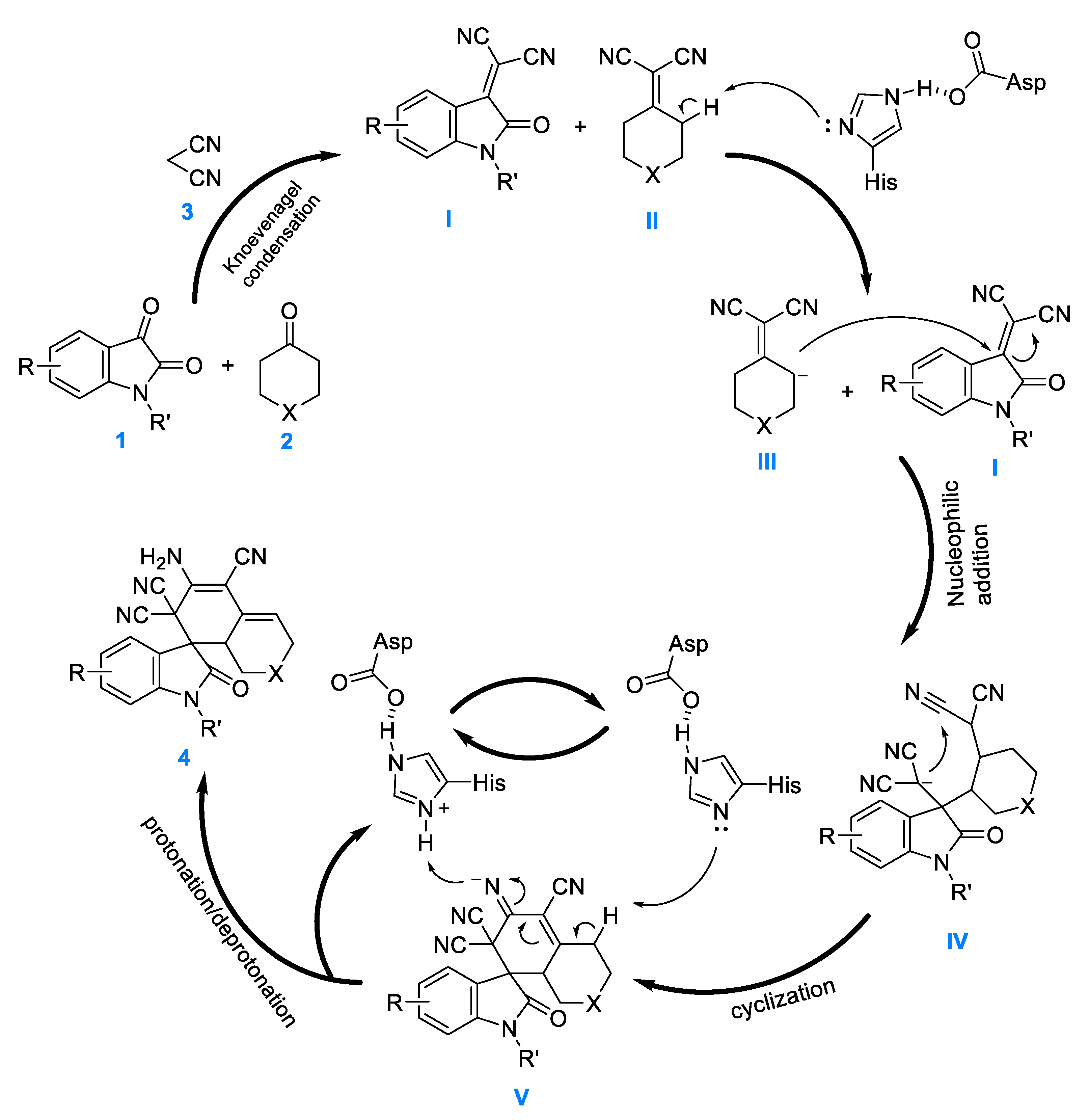
On the basis of our experimental results, we speculated regarding the plausible mechanism of this PPL-catalyzed one-pot tandem reaction (Figure 5). Given the strong electron-withdrawing ability of –CN, first, using this one-pot tandem process, adducts alkenyl dinitrile I and II were obtained via Knoevenagel condensation of isatin 1 and cycloketone 2, respectively, using malonitrile 3. Next, the adduct II was deprotonated using Asp-His dyad to produce intermediate III. Immediately after that, the carboanion of the intermediate III nucleophile attacked the C=C of the isatilidenemalononitrile I and formed IV, which further attacked the cyanogroup and cyclized to obtain the anionic intermediate V. Finally, V was protonated/deprotonated to provide target product 4; the regeneration of PPL maintained the catalytic cycle.
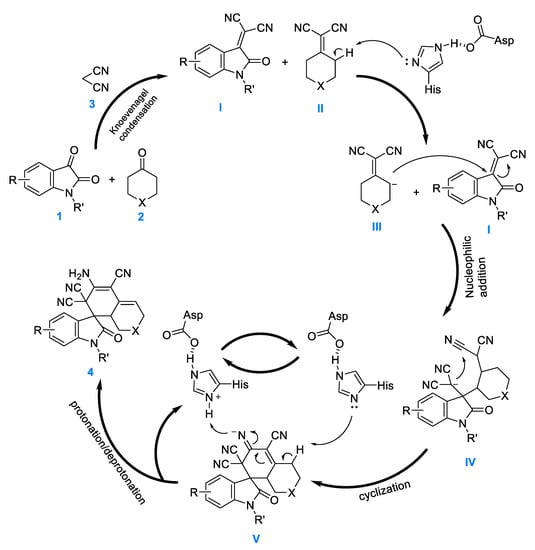
Figure 5.
Proposed reaction mechanism for the synthesis of spirooxindoles. I and II: alkenyl dinitrile adducts; III: vinyl dinitrile carboanion; IV: nucleophilic addition intermediate; V: cyclization intermediate of carbanion.
3. Materials and Methods
3.1. General Information
PPL (porcine pancreas lipase), PSL (Pseudomonas sp. lipase), and CalB (lipase B from Candida antarctica) were purchased from Shanghai Yuan Ye Biological Technology Company (Shanghai, China), and Novozym 435 was purchased from Sigma–Aldrich China Co. (Beijing, China). All other chemical reagents were purchased from commercial suppliers (Bide Pharmatech (Shanghai, China), Aladdin (Shanghai, China), and Energy Chemical (Beijing, China)). All commercially available reagents and solvents were used without further purification. Proton nuclear magnetic resonance (1H NMR) spectra were recorded using a 400 MHz spectrometer in DMSO. Chemical shifts of protons were reported in parts per million downfield from tetramethyl silane (TMS) and referenced to residual protium in the NMR solvent (DMSO = δ 2.50 ppm). NMR data are presented as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, and br = broad), coupling constant in Hertz (Hz), and integration. A 100 MHz spectrometer in DMSO (δ 39.52 ppm) was used to report 13C NMR spectra. Experiments were performed in triplicate, and all data were obtained based on the average values.
3.2. General Procedure for Lipase-Catalyzed Synthesis of 4
PPL (15 mg) was added into a mixture of isatins (1, 0.2 mmol), cycloketones (2, 0.2 mmol), and malononitrile (3, 0.6 mmol) in water (3 mL) at 40 °C for 48–72 h. After the reaction was complete, as indicated by TLC, the precipitate was filtered and washed using 20% ethanol/petroleum ether, then dried in a vacuum to afford pure and solid product (4). All the isolated products were well characterized by their NMR.
4. Conclusions
In conclusion, we have successfully established a novel lipase catalyzed one-pot tandem reaction to synthesize a series of spirooxindoles. This method used lipase to catalyze the reaction of three components in aqueous solution. The developed three-component enzymatic process requires only common equipment and obtainable raw materials. Simple filtration and washing operations result in product with an excellent yield, without any further purification steps. Combined with the green chemistry concept that is now being advocated, this non-natural enzymatic method for synthesizing spirooxindole structures is expected to be widely used in the future. Furthermore, immobilization is an efficient tool for improving enzyme features in the biotechnology toolbox. Through enzyme immobilization, many enzyme limitations can be addressed; for example, enzyme stability can be improved; enzyme selectivity, specificity, and activity can be altered and inhibitions reduced; and the operation window and resistance to chemicals can be enlarged, and even be coupled to enzyme purification [35,36,37,38,39]. Therefore, to improve the feasibility and efficiency of this synthetic method, related PPL immobilization methods are being studied and will be reported in due course.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal13010143/s1, Figure S1: Data of Products; Figure S2: Spectra of Products; Table S1: Optimization of temperature for the one-pot tandem synthesis of 4a.
Author Contributions
Investigation, methodology, visualization, writing—original draft, and formal analysis, Y.T.; methodology, C.W. (Ciduo Wang); visualization, H.X.; formal analysis, Y.T., C.W. (Chunyu Wang); H.X., Y.X. and C.D.; supervision, conceptualization, funding acquisition, and writing review and editing, Z.W. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Development Program of Jilin Province (No. 20200301029RQ and No. 20200201396JC) and the Key Research Development Program of Jilin Province (No. 20200402067NC).
Data Availability Statement
Data presented in this study are available in the Supplementary Materials.
Acknowledgments
We gratefully acknowledge the Science and Technology Development Program of Jilin Province (No. 20200301029RQ and No. 20200201396JC) and the Key Research Development Program of Jilin Province (No. 20200402067NC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calmanti, R.; Selva, M.; Perosa, A. Tandem catalysis: One–pot synthesis of cyclic organic carbonates from olefins and carbon dioxide. Green Chem. 2021, 23, 1921–1941. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H. Tandem Reactions Combining Biocatalysts and Chemical Catalysts for Asymmetric Synthesis. Catalysts 2016, 6, 194. [Google Scholar] [CrossRef]
- Diego, C.; Roberto, M.S.; Roberto, F.L. Design of Artificial Enzymes Bearing Several Active Centers: New Trends, Opportunities and Problems. Int. J. Mol. Sci. 2022, 23, 5304. [Google Scholar]
- Finnigan, W.; Cutlan, R.; Snajdrova, R.; Adams, J.P.; Littlechild, J.A.; Harmer, N.J. Engineering a Seven Enzyme Biotransformation using Mathematical Modelling and Characterized Enzyme Parts. ChemCatChem 2019, 11, 3474–3489. [Google Scholar] [CrossRef]
- Cutlan, R.; De Rose, S.; Isupov, M.N.; Littlechild, J.A.; Harmer, N.J. Using enzyme cascades in biocatalysis: Highlight on transaminases and carboxylic acid reductases. BBA—Proteins Proteom. 2020, 1868, 140322. [Google Scholar] [CrossRef]
- Rios, R. Enantioselective methodologies for the synthesis of spiro compounds. Chem. Soc. Rev. 2012, 41, 1060–1074. [Google Scholar] [CrossRef]
- Carreira, E.M.; Fessard, T.C. Four–Membered Ring–Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. [Google Scholar] [CrossRef]
- Ding, A.; Meazza, M.; Guo, H.; Yang, J.W.; Rios, R. New development in the enantioselective synthesis of spiro compounds. Chem. Soc. Rev. 2018, 47, 5946–5996. [Google Scholar] [CrossRef]
- Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C.F. Organocatalytic Asymmetric Assembly Reactions: Synthesis of Spirooxindoles via Organocascade Strategies. ACS Catal. 2014, 4, 743–762. [Google Scholar] [CrossRef]
- Pavlovska, T.L.; Redkin, R.G.; Lipson, V.V.; Atamanuk, D.V. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol. Divers 2016, 20, 299–344. [Google Scholar] [CrossRef]
- Kathirvelan, D.; Haribabu, J.; Reddy, B.S.; Balachandran, C.; Duraipandiyan, V. Facile and diastereoselective synthesis of 3,2’–spiropyrrolidine–oxindoles derivatives, their molecular docking and antiproliferative activities. Bioorg. Med. Chem. Lett. 2015, 25, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Vintonyak, V.V.; Warburg, K.; Kruse, H.; Grimme, S.; Hubel, K.; Rauh, D.; Waldmann, H. Identification of Thiazolidinones Spiro–Fused to Indolin–2–ones as Potent and Selective Inhibitors of the Mycobacterium tuberculosis Protein Tyrosine Phosphatase B. Angew. Chem. Int. Ed. 2010, 49, 5902–5905. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Marvin, C.C.; Pettersson, M.; Martin, S.F. Enantioselective Total Syntheses of Citrinadins A and B. Stereochemical Revision of Their Assigned Structures. J. Am. Chem. Soc. 2014, 136, 14184–14192. [Google Scholar] [CrossRef] [PubMed]
- Hari Babu, T.; Abragam Joseph, A.; Muralidharan, D.; Perumal, P.T. A novel method for the synthesis of functionalized spirocyclic oxindoles by one–pot tandem reaction of vinyl malononitriles with isatylidene malononitriles. Tetrahedron Lett. 2010, 51, 994–996. [Google Scholar] [CrossRef]
- Hegade, P.G.; Chinchkar, S.D.; Pore, D.M. DABCO catalyzed pseudo multi–component synthesis of functionalized spirooxindoles. Monatsh. Chem. 2016, 147, 1243–1249. [Google Scholar] [CrossRef]
- Reddy Gajulapalli, V.P.; Vinayagam, P.; Kesavan, V. Enantioselective assembly of functionalized carbocyclic spirooxindoles using anl–proline derived thiourea organocatalyst. RSC Adv. 2015, 5, 7370–7379. [Google Scholar] [CrossRef]
- Dandia, A.; Mahawar, D.K.; Saini, P.; Saini, S.; Gupta, S.L.; Rathore, K.S.; Parewa, V. Site–specific role of bifunctional graphitic carbon nitride catalyst for the sustainable synthesis of 3,3–spirocyclic oxindoles in aqueous media. RSC Adv. 2021, 11, 28452–28465. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Tang, X.; Cao, X.; Wang, C.; Li, J.; Wang, L. Hemoglobin: A New Biocatalyst for the Synthesis of 2–substituted BenzoxazolesviaOxidative Cyclization. ChemCatChem 2019, 11, 1192–1195. [Google Scholar] [CrossRef]
- Li, F.; Tang, X.; Xu, Y.; Wang, C.; Zhang, L.; Zhang, J.; Liu, J.; Li, Z.; Wang, L. Hemoglobin–Catalyzed Synthesis of Indolizines Under Mild Conditions. Eur. J. Org. Chem. 2019, 2019, 7720–7724. [Google Scholar] [CrossRef]
- Li, F.; Tang, X.; Xu, Y.; Wang, C.; Wang, Z.; Li, Z.; Wang, L. A Dual–Protein Cascade Reaction for the Regioselective Synthesis of Quinoxalines. Org. Lett. 2020, 22, 3900–3904. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zhao, N.; Su, J.; Wang, C.; Wang, C.; Li, Z.; Wang, L. Environment–friendly and efficient synthesis of 2–aminobenzo–xazoles and 2–aminobenzothiazoles catalyzed by Vitreoscilla hemoglobin incorporating a cobalt porphyrin cofactor. Green Chem. 2021, 23, 8047–8052. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Wang, C.; Wang, C.; Zhao, R.; Wang, L. Efficient synthesis of cyano–containing multi–substituted indoles catalyzed by lipase. Bioorg. Chem. 2021, 107, 104583. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Goel, V.; Garg, N.; Choudhury, D.; Mallick, D.; Tyagi, V. Biocatalytic Aza-Michael Addition of Aromatic Amines to Enone Using α-Amylase in Water. Adv. Synth. Catal. 2020, 362, 858–866. [Google Scholar] [CrossRef]
- Xu, Y.; Smith, R.; Vivoli, M.; Ema, M.; Goos, N.; Gehrke, S.; Harmer, N.J.; Wagner, G.K. Covalent inhibitors of LgtC: A blueprint for the discovery of non–substrate–like inhibitors for bacterial glycosyltransferases. Bioorg. Med. Chem. 2017, 25, 3182–3194. [Google Scholar] [CrossRef]
- Finnigan, W.; Thomas, A.; Cromar, H.; Gough, B.; Snajdrova, R.; Adams, J.P.; Littlechild, J.A.; Harmer, N.J. Characterization of Carboxylic Acid Reductases as Enzymes in the Toolbox for Synthetic Chemistry. ChemCatChem 2017, 9, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Vivoli, M.; Pang, J.; Harmer, N.J. A half–site multimeric enzyme achieves its cooperativity without conformational changes. Sci. Rep. 2017, 7, 16529. [Google Scholar] [CrossRef]
- Iwasaki, J.; Lorimer, D.D.; Vivoli-Vega, M.; Kibble, E.A.; Peacock, C.S.; Abendroth, J.; Mayclin, S.J.; Dranow, D.M.; Pierce, P.G.; Fox, D.; et al. Broad–spectrum in vitro activity of macrophage infectivity potentiator inhibitors against Gram–negative bacteria and Leishmania major. J. Antimicrob. Chemother. 2022, 77, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Lai, Y.; Xu, J.; Zheng, H.; Zhu, Q.; Zhang, P. One-Pot Synthesis of Spirooxindole Derivatives Catalyzed by Lipase in the Presence of Water. Adv. Synth. Catal. 2011, 353, 371–375. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Wu, Q.; Lin, X. Diastereoselective synthesis of spirooxindole derivatives via biocatalytic domino reaction. Tetrahedron 2015, 71, 616–621. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, Z.; Fang, K.; He, X.; Huang, H.; Hu, Y. Promiscuous enzyme–catalyzed cascade reaction in water: Synthesis of dicoumarol derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1236–1240. [Google Scholar] [CrossRef]
- Ding, X.; Dong, C.L.; Guan, Z.; He, Y.H. Concurrent Asymmetric Reactions Combining Photocatalysis and Enzyme Catalysis: Direct Enantioselective Synthesis of 2,2–Disubstituted Indol–3–ones from 2–Arylindoles. Angew. Chem. Int. Ed. 2019, 58, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bavandi, H.; Habibi, Z.; Yousefi, M. Porcine pancreas lipase as a green catalyst for synthesis of bis–4–hydroxy coumarins. Bioorg. Chem. 2020, 103, 104139. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, P.K.; Prabhu, N.P. Stability and Activity of Porcine Lipase Against Temperature and Chemical Denaturants. Appl. Biochem. Biotechnol. 2014, 174, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou–Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical– and less classical–solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernández-Lafuente, R. Use of polyethylenimine to produce immobilized lipase multilayers biocatalysts with very high volumetric activity using octyl-agarose beads: Avoiding enzyme release during multilayer production. Enzyme Microb. Technol. 2020, 137, 109535. [Google Scholar] [CrossRef]
- Arana-Pena, S.; Carballares, D.; Morellon-Sterling, R.; Rocha-Martin, J.; Fernandez-Lafuente, R. The combination of covalent and ionic exchange immobilizations enables the coimmobilization on vinyl sulfone activated supports and the reuse of the most stable immobilized enzyme. Int. J. Biol. Macromol. 2022, 199, 51–60. [Google Scholar] [CrossRef]
- Xiang, X.; Ding, S.; Suo, H.; Xu, C.; Gao, Z.; Hu, Y. Fabrication of chitosan-mesoporous silica SBA-15 nanocomposites via functional ionic liquid as the bridging agent for PPL immobilization. Carbohydr. Polym. 2018, 182, 245–253. [Google Scholar] [CrossRef]
- Remonatto, D.; Miotti Jr, R.H.; Monti, R.; Bassan, J.C.; de Paula, A.V. Applications of immobilized lipases in enzymatic reactors: A review. Process Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Hu, R.; Niu, Z.; Lu, Y.; Zhu, H.; Mao, Z.; Yan, K.; Hu, X.; Chen, H. Immobilization for Lipase: Enhanced Activity and Stability by Flexible Combination and Solid Support. Appl. Biochem. Biotechnol. 2022, 194, 5963–5976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).