Abstract
A green and low-cost removal method for cyanate, a toxic byproduct from the treatment of cyanide, is still needed. Cyanase converts cyanate to CO2 and NH3, but its industrial practicality is limited because the reaction requires HCO3− as a substrate. In this study, we used carbonic anhydrase from Sulfurihydrogenibium azorense (SazCA) to provide HCO3− for cyanase from Thermomyces lanuginosus (TlCyn); both TlCyn and SazCA were purified by one-step heating without prior cell lysis. The heat treatment resulted in higher activities of both enzymes than the conventional two-step process. From a 50 mL-culture, the highest total activity of 147 U and 47,174 WAU was obtained from 5 min of heating at 60 and 80 °C for TlCyn and SazCA, respectively. The coupled enzymatic system was used to degrade cyanate in three different matrices: 50 mM Tris-HCl (pH 8), industrial wastewater, and artificial wastewater. In the industrial wastewater, with the addition of 0.75 WAU (Wilbur-Anderson unit) of SazCA, cyanate degradation using 0.5 mM NaHCO3 was similar to that using 3 mM NaHCO3, indicating an 83% reduction in NaHCO3. We have demonstrated that the dependence on HCO3− of cyanate degradation can be effectively alleviated by using low-cost heat-purified TlCyn and SazCA; the industrial practicality of the coupled enzymatic system is therefore improved.
1. Introduction
Cyanides are widely used in industries such as precious metal extraction and electroplating [1]. These compounds are extremely toxic and difficult for biodegradation. Cyanate, a less toxic derivative from cyanide oxidation, often accompanies cyanides in industrial waste. Cyanate accumulation in the environment is sometimes found because it can be used as a pesticide and fungicide. Chemical methods such as alkaline chlorination and ozone oxidation have been applied to the treatment of wastes containing cyanides and cyanate [2]. An activated sludge process has also been used in the removal of cyanide [3]. However, these conventional methods have certain disadvantages. Special equipment is often necessary for chemical methods, and toxic byproducts may be produced if organic compounds are present in the waste [4]. The activity of microorganisms in activated sludge could be inhibited by the cyanide-containing waste because of its complex matrix.
Cyanase (EC 4.2.1.104), which converts cyanate to CO2 and ammonia using bicarbonate as a substrate, can be used as a catalyst for green cyanate detoxification. Cyanase is involved in nitrogen assimilation, detoxification of cyanate, and metabolism regulation in organisms [5]. Detoxification of cyanate using cyanase has limited industrial practicality because the reaction requires a large amount of bicarbonate. The dependence on bicarbonate can be lowered by supplementing carbonic anhydrase into the reaction [6].
Carbonic anhydrase (EC 4.2.1.1) catalyzes the CO2 hydration: CO2 + H2O  HCO3− + H+ [7]. The bicarbonate necessary for cyanate degradation is supplied by carbonic anhydrase-catalyzed CO2 hydration, and CO2 is replenished by cyanase. The genes of cyanase and carbonic anhydrase often cluster together in the fungal genomes [8]. For the purpose of detoxifying cyanate under harsh conditions, cyanase from the thermophilic fungus Thermomyces lanuginosus (TlCyn) is an ideal choice because of its stability and heavy metal tolerance; complete degradation was achieved up to 10 mM cyanate [9]. Ranjan et al. reported an enzymatic system composed of recombinant TlCyn and carbonic anhydrase also from T. lanuginosus (TlCA) for cyanate degradation; a similar extent of degradation can be achieved with much less bicarbonate when compared with only TlCyn [6]. These enzymes were also immobilized on magnetic nanoparticles to facilitate recycling.
HCO3− + H+ [7]. The bicarbonate necessary for cyanate degradation is supplied by carbonic anhydrase-catalyzed CO2 hydration, and CO2 is replenished by cyanase. The genes of cyanase and carbonic anhydrase often cluster together in the fungal genomes [8]. For the purpose of detoxifying cyanate under harsh conditions, cyanase from the thermophilic fungus Thermomyces lanuginosus (TlCyn) is an ideal choice because of its stability and heavy metal tolerance; complete degradation was achieved up to 10 mM cyanate [9]. Ranjan et al. reported an enzymatic system composed of recombinant TlCyn and carbonic anhydrase also from T. lanuginosus (TlCA) for cyanate degradation; a similar extent of degradation can be achieved with much less bicarbonate when compared with only TlCyn [6]. These enzymes were also immobilized on magnetic nanoparticles to facilitate recycling.
 HCO3− + H+ [7]. The bicarbonate necessary for cyanate degradation is supplied by carbonic anhydrase-catalyzed CO2 hydration, and CO2 is replenished by cyanase. The genes of cyanase and carbonic anhydrase often cluster together in the fungal genomes [8]. For the purpose of detoxifying cyanate under harsh conditions, cyanase from the thermophilic fungus Thermomyces lanuginosus (TlCyn) is an ideal choice because of its stability and heavy metal tolerance; complete degradation was achieved up to 10 mM cyanate [9]. Ranjan et al. reported an enzymatic system composed of recombinant TlCyn and carbonic anhydrase also from T. lanuginosus (TlCA) for cyanate degradation; a similar extent of degradation can be achieved with much less bicarbonate when compared with only TlCyn [6]. These enzymes were also immobilized on magnetic nanoparticles to facilitate recycling.
HCO3− + H+ [7]. The bicarbonate necessary for cyanate degradation is supplied by carbonic anhydrase-catalyzed CO2 hydration, and CO2 is replenished by cyanase. The genes of cyanase and carbonic anhydrase often cluster together in the fungal genomes [8]. For the purpose of detoxifying cyanate under harsh conditions, cyanase from the thermophilic fungus Thermomyces lanuginosus (TlCyn) is an ideal choice because of its stability and heavy metal tolerance; complete degradation was achieved up to 10 mM cyanate [9]. Ranjan et al. reported an enzymatic system composed of recombinant TlCyn and carbonic anhydrase also from T. lanuginosus (TlCA) for cyanate degradation; a similar extent of degradation can be achieved with much less bicarbonate when compared with only TlCyn [6]. These enzymes were also immobilized on magnetic nanoparticles to facilitate recycling.For any large-scale enzymatic process, an economical purification process and/or recycling of the enzymes are often used to reduce the overall cost. Heating can be applied to the purification of thermostable proteins; compared with conventional protein purification processes such as precipitation, dialysis, ultrafiltration, and chromatography, purification by heating is less cumbersome, and the process does not require specialized reagents nor equipment. In heat purification, the host cells are often lysed first by other methods [10,11]. In this work, we performed cyanate degradation by combining TlCyn with thermostable carbonic anhydrase from Sulfurihydrogenibium azorense (SazCA), a carbonic anhydrase with a very high kcat of 4.4 × 106 s−1 [12]. SazCA purification by heating has been reported [13]; sonication was used for cell lysis, and only two heat treatment conditions were examined. TlCyn and SazCA, used herein for cyanate degradation, were partially purified by a simple one-step heating, in which cell lysis and precipitation of constituent proteins are combined. The temperature and incubation period of heat purification were examined and optimized; the results were compared with conventional two-step methods. In cyanides and cyanate-containing wastewater, other constituents such as metal ions are often present [14]. In order to gain more insight into the degradation efficiency of the enzymatic system, three different matrices, including buffer, industrial wastewater, and artificial wastewater, were used.
2. Results and Discussion
2.1. TlCyn Purified by Heating
The recombinant TlCyn has 181 amino acids and a theoretical molecular mass of 20,085 Da, which was confirmed with SDS-PAGE using enzyme purified by a Ni-NTA column. The results from heat-purification are listed in Table S1. As determined from SDS-PAGE using densitometry, the heating time did not affect the purity significantly. The highest purity of 53% was obtained from a heating time of 5 min at 60 °C. The heating time had little effect on the total activity at 60 °C, but the total activities at 70 and 80 °C decreased significantly as the heating time increased. The total activities obtained at 80 °C were much lower than those at 60 and 70 °C; we suspected that TlCyn suffered from significant thermal denaturation at 80 °C, and thus the thermal stability of TlCyn (Ni-NTA column purified) was examined. After a 30-min incubation, the residual activities of TlCyn at 60 and 70 °C were 1.5 and 1.3-fold higher than that at 80 °C; the thermal stability data were in line with our previous observation. The highest total activity of 147 U was obtained with a 5 min-heating at 60 °C. The total protein that resulted from 60 °C was higher than those from 70 and 80 °C; it is possible that more constituent protein was precipitated at higher temperatures. At 60 °C, the heating time also had little effect on specific activity, but the specific activities at 70 and 80 °C decreased with increasing heating time. The specific activities obtained from 80 °C were much lower than those from 60 and 70 °C. A heating time of 5 min at 60 °C was chosen for later purification of TlCyn due to its optimal total activity.
The performance of the one-step heating was compared with a Ni-NTA column and the two-step heating, to which a sonication step was incorporated (Table 1). As expected, the highest purification and lowest total protein were obtained with a Ni-NTA column because the purified TlCyn had almost no other proteins, as shown in SDS-PAGE. The purification fold and yield of the one-step heating were 1.5-fold higher than those of the two-step heating.

Table 1.
Comparison of different purification methods for TlCyn.
2.2. SazCA Purified by Heating
The recombinant SazCA has 257 amino acids and a theoretical mass of 29,553 Da, which was also confirmed with SDS-PAGE using column-purified enzyme. The results from heat-purification are listed in Table S2. The purities at 70 and 80 °C were similar irrespective of the heating time, but the purity at 90 °C decreased with increasing heating time. The purities obtained from 90 °C were lower than those from other temperatures; the highest purity of 60% was obtained by heating at 80 °C for 5 min. The total activities at 70 °C were similar; however, the total activity at 80 and 90 °C decreased with increasing heating time. The total activities obtained from 90 °C were much lower than those from 70 and 80 °C. The thermal stability of SazCA (Ni-NTA column purified) was reported in our previous work [15]; the residual activities of SazCA after a 30-min incubation at 70 and 80 °C were 18 and 5.6-fold higher than that at 90 °C, indicating that the low total activity obtained from 90 °C was most likely due to thermal denaturation. The highest total activity was obtained from heating at 80 °C for 5 min, followed by that from heating at 70 °C for 10 min. Regardless of the heating time, the total protein was in the range of 11–14 mg at 70 and 80 °C, but the total protein was only 5–6 mg when the temperature was increased to 90 °C. The specific activity at 70 °C varied little with heating time; nevertheless, the specific activity at 80 and 90 °C decreased with increasing heating time. The specific activity resulting from heating at 80 °C for 5 min was very close to those from 70 °C. A heating time of 5 min at 80 °C was selected for later purification of SazCA due to its optimal total activity and high specific activity.
The performance of the one-step heating is compared with other purification methods (Table 2). As observed for TlCyn, the Ni-NTA column also resulted in the highest purification and the lowest total protein because the purified SazCA had a purity of 99% as determined from SDS-PAGE. The yield of the one-step heating was 3.4-fold higher than that of the two-step heating; moreover, it was even slightly higher than that of the Ni-NTA column. The total activity and specific activity obtained from the one-step heating at 80 °C for 5 min were roughly two-fold higher than those from a two-step process performed at 70 °C for 30 min [13]. Higher yields obtained from the one-step process for both TlCyn and SazCA could be partly explained by the early heat-denaturation of proteases [16].

Table 2.
Comparison of different purification methods for SazCA.
2.3. Cyanate Degradation Using Only TlCyn
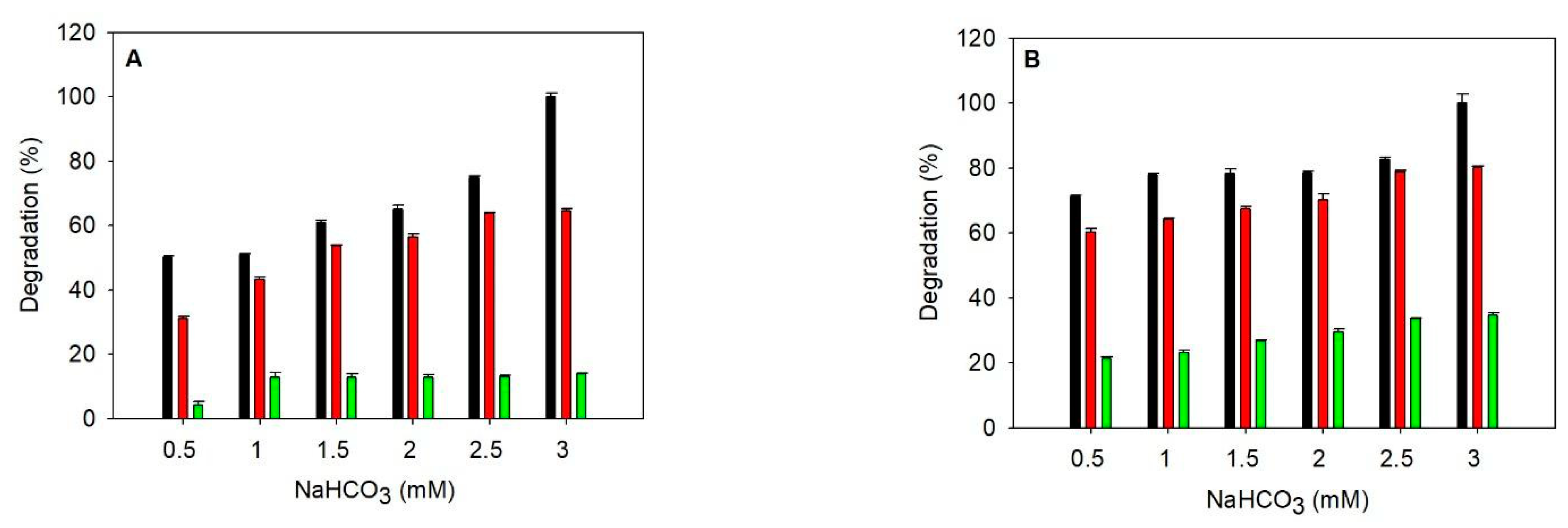
The data of cyanate degradation using only heat-purified TlCyn are shown in Figure 1A (control). In both 50 mM Tris-HCl, pH 8, and industrial wastewater, cyanate degradation increased with NaHCO3 concentration, showing a strong dependence on HCO3−. The dependence on HCO3− was also evident in a previous report [6] and the results obtained with column-purified TlCyn (Figure S1A). Cyanate degradation in industrial wastewater was lower than that in the buffer; the industrial wastewater contained 2 mM Na+, 0.2 µM Zn2+, 31.5 nM Cu2+, 44.3 nM Ni2+, 30.8 nM Cr2+, and 4.8 nM Pb2+ as determined with an ICP optical emission spectrometer. According to the metal ion inhibition on TlCyn, reported by others [9], components other than the metal ions in the industrial wastewater may be responsible for the lower degradation. In order to test the performance of TlCyn under high metal ion concentration, we prepared artificial wastewater containing 13.2 µM Cd2+, 0.24 mM Pb2+, 0.48 mM Cr6+, 0.86 mM Ni2+, and 1.2 µM Hg2+. The cyanate degradation in the artificial wastewater was independent of NaHCO3 concentration and much lower than those obtained from the other two matrices. Our data indicated that the TlCyn activity was inhibited by the artificial wastewater; a similar observation was reported by others, and such phenomena could be explained by a combined inhibition imposed by the metal ions [6].
Figure 1.
Degradation of cyanate in different matrices using heat-purified enzymes. The activity of SazCA added was 0 ((A) control), 0.5 (B), 0.75 (C), and 1 WAU (D)); the activity of TlCyn added was 0.03 U. Degradation was performed in 50 mM Tris-HCl, pH 8 (black), industrial wastewater (red), and artificial wastewater (green). The 100% was defined as the cyanate degradation observed with 3 mM NaHCO3 in the buffer.
2.4. Cyanate Degradation Using TlCyn and SazCA
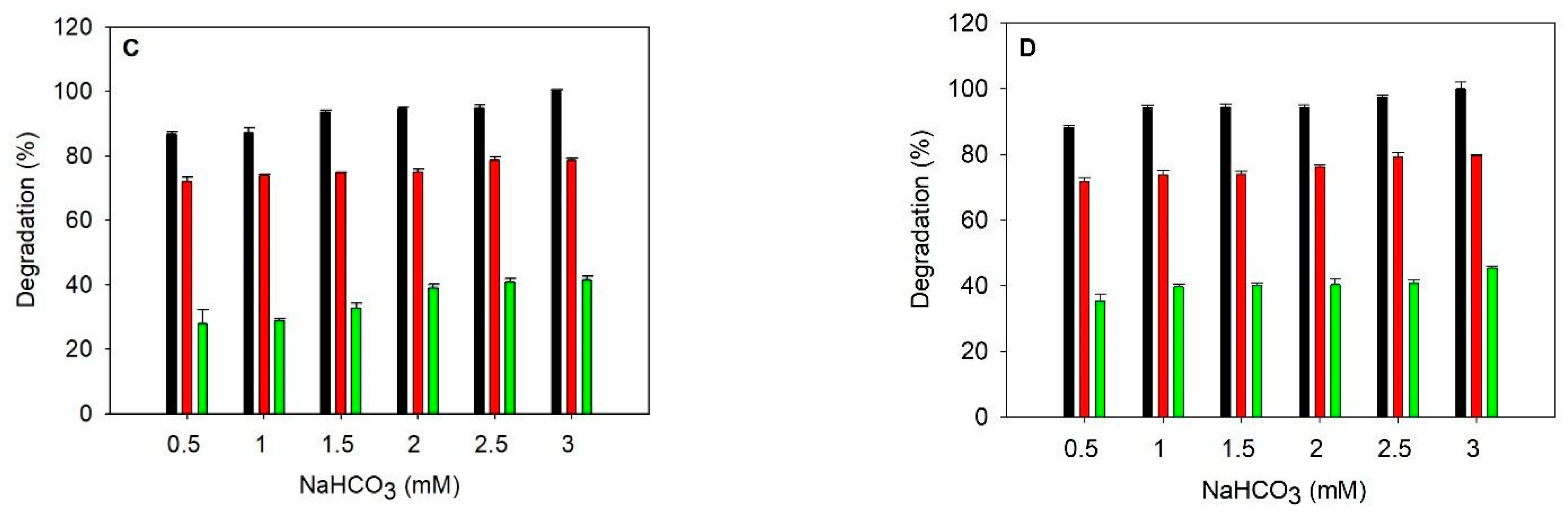
In Figure 1B–D, 0.5, 0.75, and 1 WAU (Wilbur-Anderson unit) of heat-purified SazCA were added to the reaction mixture in addition to heat-purified TlCyn, respectively. Difference in cyanate degradation (DCD, listed in Table 3), defined as the cyanate degradation in percentage using 0.5 mM NaHCO3 subtracted from that using 3 mM NaHCO3, is used to represent the dependence on HCO3−. A smaller DCD indicates that the system is less dependent on HCO3−. In the buffer, the addition of SazCA clearly improved the dependence on HCO3− because the DCD decreased as SazCA activity increased. In the industrial wastewater, the same trend as that in the buffer was observed up to 0.75 WAU; cyanate degradation using 0.5 mM NaHCO3 was similar to that using 3 mM NaHCO3, and 83% less NaHCO3 was required with the addition of 0.75 WAU of SazCA. The improvements on the HCO3− dependence using heat-purified enzymes are quite similar to those using column-purified ones (black and red bars in Figure S1B–D), suggesting that the impurities in heat-purified enzymes do not affect cyanate degradation. When TlCA was combined with TlCyn (both were column-purified) to alleviate its HCO3− dependence in the buffer, comparable results were reported [6]. However, the addition of SazCA did not lower the enzymatic system’s dependence on HCO3− in the artificial wastewater, and thus we examined the inhibition of SazCA by the metal ions in this matrix (Figure S2). These ions showed strong inhibition on SazCA, as less than half of the initial activity remained. The strongest inhibition was observed with Ni2+ and Hg2+; only 20 and 18% of the initial activities were retained, respectively. The combined inhibition by the metal ions in the artificial wastewater could explain why SazCA did not improve the dependence on HCO3−.

Table 3.
Difference in cyanate degradation under different reaction conditions.
3. Materials and Methods
3.1. Bacterial Strains and Vector Construction
DNA manipulation was carried out with E. coli DH5α, and recombinant enzyme expression was performed with E. coli BL21(DE3). For TlCyn cloning, the gene was degenerated from the amino acid sequence deposited in the Protein Data Bank (entry code 6XGT_A) [13]; the commercially synthesized gene was also inserted into a pET-28a(+) vector using the NdeI and XhoI restriction sites. The recombinant TlCyn has a N-terminal (His6)-tag because a stop codon was placed before the XhoI site. The construction of pET-28a(+)-SazCA plasmid was described in our previous work [15].
3.2. Expression and Purification of TlCyn and SazCA
The transformants carrying the pET-28a(+)-TlCyn were cultivated at 37 °C in LB medium containing 50 μg/mL kanamycin; protein expression was induced with 0.8 mM IPTG when OD600 reached 0.5–0.7. The cell pellet was collected after further incubation for 8 h at 25 °C. The BugBuster protein extraction reagent (from Merck, Darmstadt, Germany) was used for the disruption of the pellet; the Ni-NTA His-Bind Resin Chromatography kit (also from Merck) was used for purification. Purified TlCyn was desalted against 50 mM Tris-HCl, pH = 8. The protein concentration was determined with a Bradford assay using BSA standards. The expression and purification of SazCA were described elsewhere [15].
3.3. Purification by Heating
The cell pellet of TlCyn was resuspended in 50 mM Tris-HCl (pH 8, 5 mL buffer was added to every gram of pellet) followed by heating at 60, 70, and 80 °C for 5, 10, 15, and 20 min. The suspension was subject to centrifugation at 13,000× g for 5 min, and the supernatant was collected as partially purified TlCyn. As for SazCA, the differences were: (1) the pellet was suspended in 20 mM Tris-HCl, pH 8.3 and (2) the temperatures used were 70, 80, and 90 °C. For comparison, the cell suspension was subject to sonication (Q125 dismembrator from QSONICA, Newtown, CT, USA) for 20 min at 20% amplitude (3 s pulse on and 12 s pulse off) on ice before heating.
3.4. Activity Assays
A hydratase activity assay was used for SazCA according to reported procedure [17]. The activity was presented in WAU, which is defined as (T0 − T)/T. T0 and T are the time for the pH change from 8.3 to 6.3 in an uncatalyzed and catalyzed reaction, respectively. The cyanase activity assay was modified from a previous report [9]. Nessler reagent was prepared according to the procedure reported by Nxumalo et al. [18], except that KI was dissolved in approximately 7.5 mL of deionized water. The amount of ammonium produced was calculated from a calibration curve constructed with NH4Cl standards. One unit (U) of activity is defined as the production of 1 μmol of ammonium per minute under assay condition.
3.5. Cyanate Degradation by Coupled Enzymatic System
A reaction mixture was prepared by mixing 0.5 mL of 50 mM Tris-HCl, pH 8.0, 0.2 mL of 4 mM KOCN, and 0.2 mL of NaHCO3 in the range of 0.5 to 3 mM. To initiate cyanate degradation, 50 µL of TlCyn and 50 µL of SazCA (both diluted to appropriate concentration) were added to the mixture followed by incubation at 60 °C for 10 min in a shaking water bath at 100 rpm. The determination of ammonium released from cyanate degradation is the same as that described in Section 3.4. To assess the influence of the matrix on degradation, 50 mM Tris-HCl was replaced with industrial wastewater (obtained from an electric motor factory of the Tatung Company, Taipei, Taiwan) and artificial wastewater. The artificial wastewater contained 13.2 µM CdCl2, 0.24 mM Pb(NO3)2, 0.48 mM K2CrO4, 0.86 mM NiSO4, and 1.2 µM HgCl2. The composition of metal ions in industrial wastewater was determined with a Perkin Elmer OPTIMA 2000™ ICP optical emission spectrometer (Waltham, MA, USA).
4. Conclusions
We have demonstrated the feasibility of cyanate degradation using enzymes purified by one-step heating. Both enzymes can be partially purified in 5 min without a prior cell lysis step; to the best of our knowledge, this is the first systematic study of purifying TlCyn and SazCA using heat. The cost of heat purification is potentially much lower than that of conventional purification methods because the process is rapid and simple. In addition, as is evident from the results of the two enzymes we examined, more activity can be extracted with the one-step process. The heat purification process can be scaled up with ease, because heating is a common unit operation in industry. Such a process is also of interest to researchers who are seeking a simple approach to obtain these commercially unavailable enzymes. The reported enzymatic system composed of heat-purified TlCyn and SazCA is capable of detoxifying cyanate with low HCO3− dependence, and the overall performance is very close to those using highly purified enzymes. The overall cost of the whole process can be lowered even further if these heat-purified enzymes are immobilized and recycled. The degradation efficiency of the enzymatic system under extreme conditions, for instance, high concentrations of metal ions, could be improved by a variety of techniques such as rational design and directed evolution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal13010076/s1, Figure S1: Degradation of cyanate in different matrices using Ni-NTA column-purified enzymes; Figure S2: Inhibition of SazCA by metal ions; Table S1: Properties of TlCyn purified by heating; Table S2: Properties of SazCA purified by heating.
Author Contributions
Conceptualization, C.-Y.Y.; methodology, C.-Y.Y. and C.-J.H.; investigation, C.-J.H.; writing—original draft preparation, C.-Y.Y. and C.-J.H.; writing—review and editing, C.-Y.Y. and C.-J.H.; funding acquisition, C.-Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology, Taiwan, grant number MOST 110-2221-E-036-004-MY3.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, M.; Akhter, Y.; Chatterjee, S. A review on remediation of cyanide containing industrial wastes using biological systems with special reference to enzymatic degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef] [PubMed]
- Botz, M.M.; Mudder, T.I.; Akcil, A.U. Chapter 35—Cyanide treatment: Physical, chemical, and biological processes. In Gold Ore Processing, 2nd ed.; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 619–645. [Google Scholar]
- Papadimitriou, C.A.; Samaras, P.; Sakellaropoulos, G.P. Comparative study of phenol and cyanide containing wastewater in CSTR and SBR activated sludge reactors. Bioresour. Technol. 2009, 100, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.B.; Paknikar, K.M. Development of a process for biodetoxification of metal cyanides from waste waters. Process Biochem. 2000, 35, 1139–1151. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Huertas, M.J.; Sáez, L.P.; Luque-Romero, M.M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D.; Blasco, R. Characterization of the Pseudomonas pseudoalcaligenes CECT5344 cyanase, an enzyme that is not essential for cyanide assimilation. Appl. Environ. Microbiol. 2008, 74, 6280–6288. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. A novel strategy for the efficient removal of toxic cyanate by the combinatorial use of recombinant enzymes immobilized on aminosilane modified magnetic nanoparticles. Bioresour. Technol. 2018, 253, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, A.; Alterio, V.; Monti, S.M.; De Simone, G.; D’Ambrosio, K. Thermostable carbonic anhydrases in biotechnological applications. Int. J. Mol. Sci. 2015, 16, 15456–15480. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.H.; McGary, K.L.; Wisecaver, J.H.; Slot, J.C.; Geiser, D.M.; Sink, S.; O’Donnell, K.; Rokas, A. Clustering of two genes putatively involved in cyanate detoxification evolved recently and independently in multiple fungal lineages. Genome Biol. Evol. 2015, 7, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, B.; Pillai, S.; Permaul, K.; Singh, S. Expression of a novel recombinant cyanate hydratase (rTl-Cyn) in Pichia pastoris, characteristics and applicability in the detoxification of cyanate. Bioresour. Technol. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Olichon, A.; Schweizer, D.; Muyldermans, S.; de Marco, A. Heating as a rapid purification method for recovering correctly-folded thermotolerant VH and VHH domains. BMC Biotechnol. 2007, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Kirk, N.; Cowan, D. Optimising the recovery of recombinant thermostable proteins expressed in mesophilic hosts. J. Biotechnol. 1995, 42, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Luca, V.D.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. An α-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction. Bioorg. Med. Chem. 2013, 21, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, X.; Kaczmarek, M.B.; Chen, P.; Li, K.; Jin, P.; Liang, Y.; Daroch, M. Accelerated CO2 hydration with thermostable Sulfurihydrogenibium azorense carbonic anhydrase-chitin binding domain fusion protein immobilised on chitin support. Int. J. Mol. Sci. 2019, 20, 1494. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Blasco, R.; Martínez-Luque, M.; Moreno-Vivián, C.; Castillo, F.; Roldán, M.D. Bacterial cyanide degradation is under review: Pseudomonas pseudoalcaligenes CECT5344, a case of an alkaliphilic cyanotroph. Biochem. Soc. Trans. 2011, 39, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.-D.; Hsieh, C.-J.; Hu, C.-J.; Yu, C.-Y. Entrapment of carbonic anhydrase from Sulfurihydrogenibium azorense with polyallylamine-mediated biomimetic silica. Bioresour. Technol. Rep. 2022, 20, 101217. [Google Scholar] [CrossRef]
- Kalthoff, C. A novel strategy for the purification of recombinantly expressed unstructured protein domains. J. Chromatogr. B 2003, 786, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; De Luca, V.; Carginale, V.; Cannio, R.; Rossi, M. Biochemical properties of a novel and highly thermostable bacterial alpha-carbonic anhydrase from Sulfurihydrogenibium yellowstonense YO3AOP1. J. Enzym. Inhib. Med. Chem. 2012, 27, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, N.; Madikizela, L.; Kruger, G.; Onwubu, S.; Mdluli, P. Development of a paper-based microfluidic device for the quantification of ammonia in industrial wastewater. Water SA 2020, 46, 506–513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).