Catalytic Activation of Hydrogen Peroxide Using Highly Porous Hydrothermally Modified Manganese Catalysts for Removal of Azithromycin Antibiotic from Aqueous Solution
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Synthesis of the Catalysts
2.3. Characterization of the Catalysts
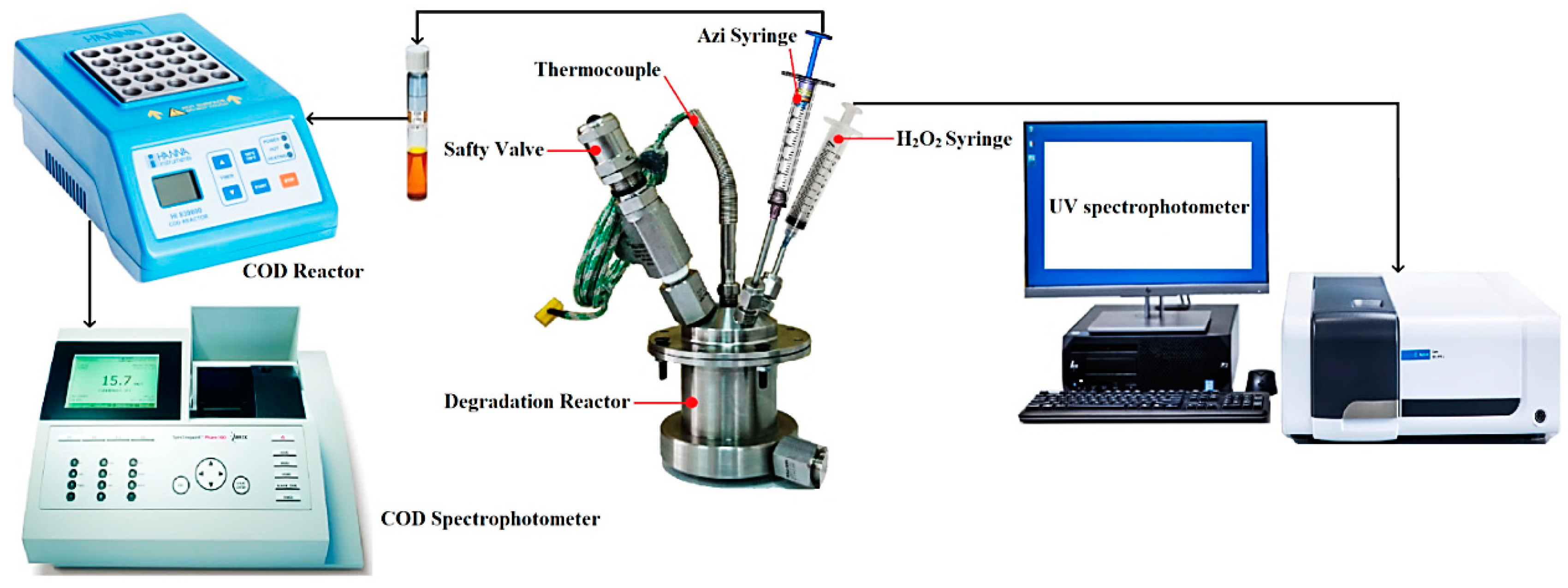
2.4. Experimental Set-Up
2.5. Experimental Procedure
3. Results and Discussion
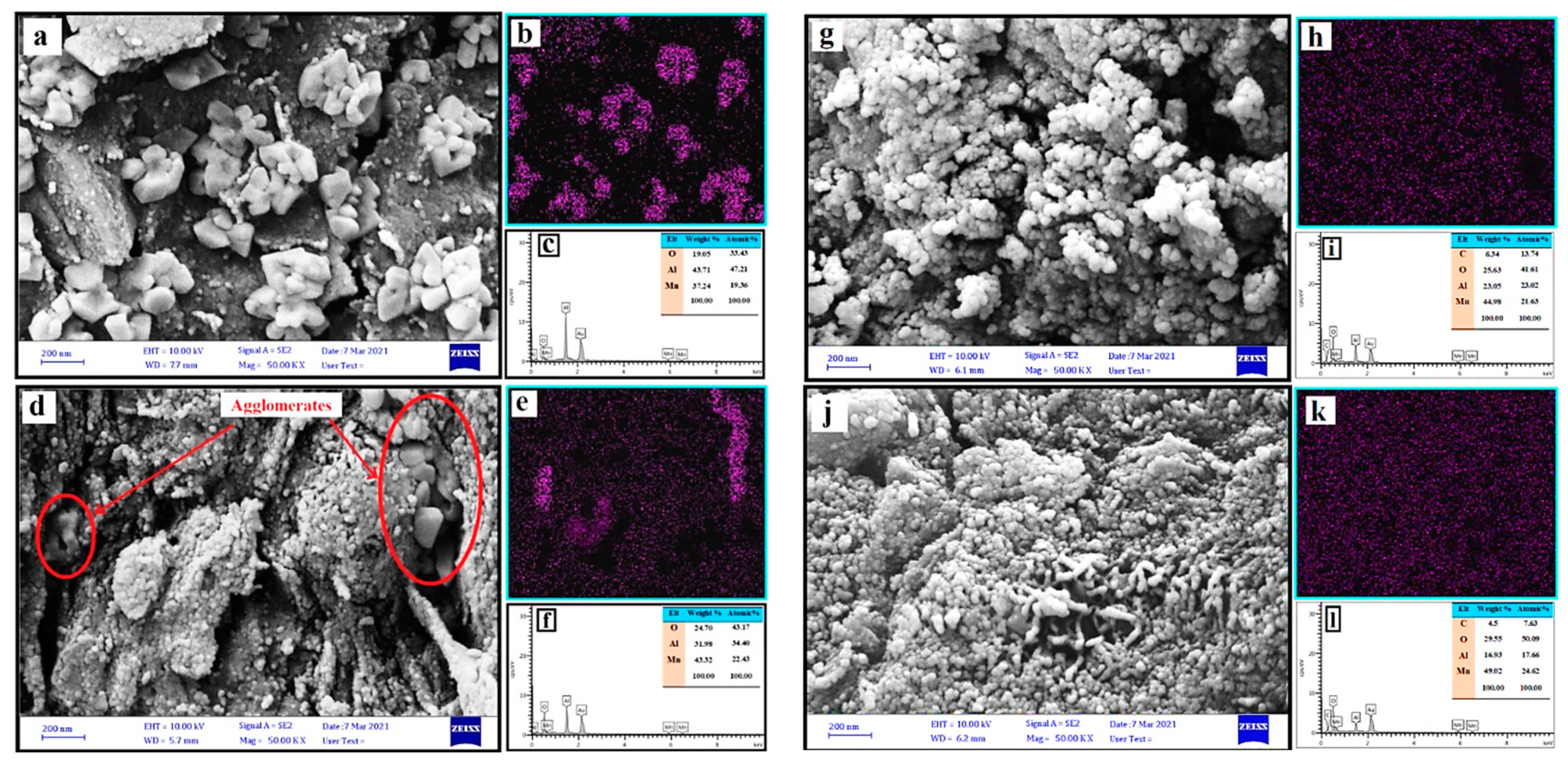
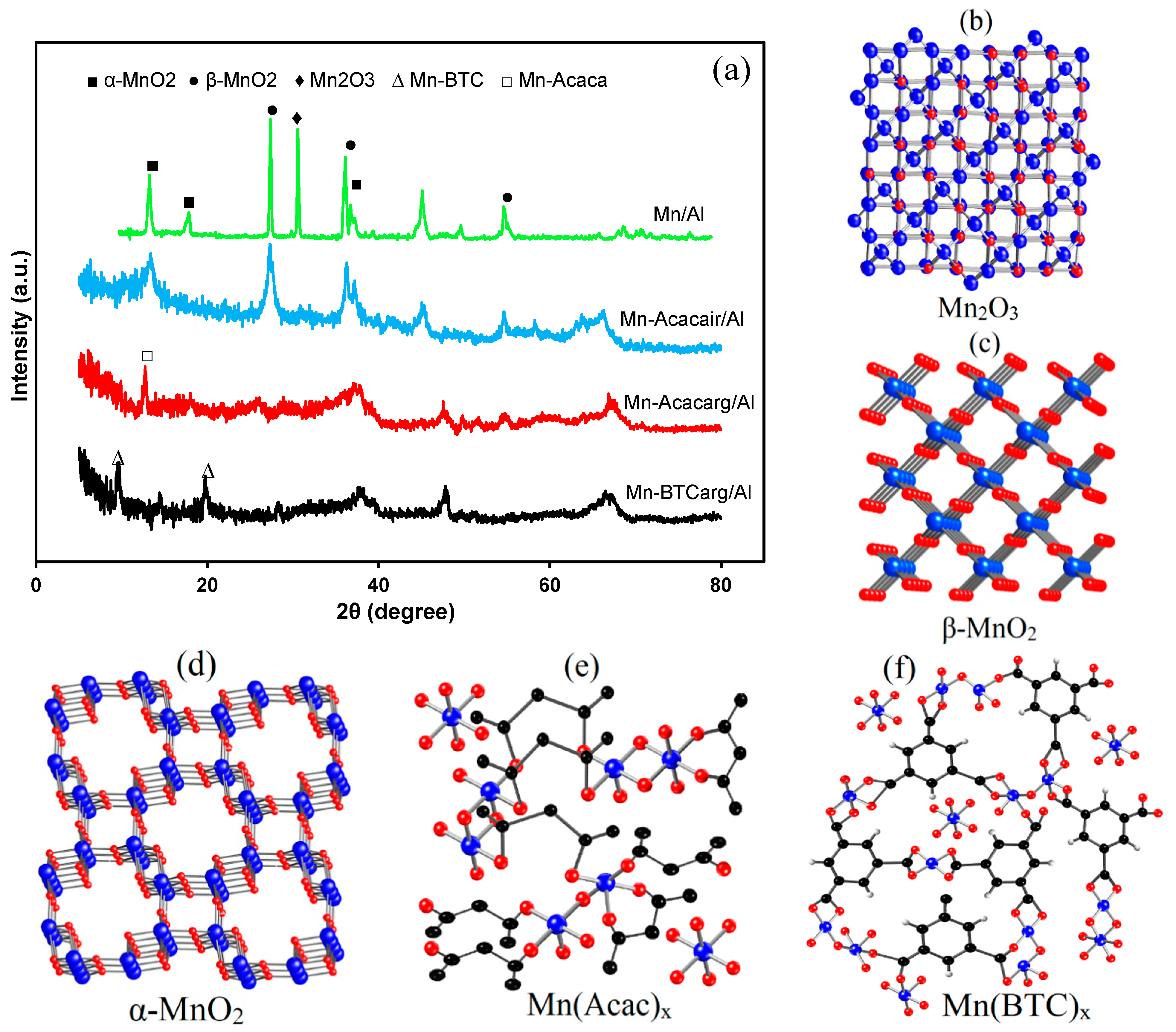
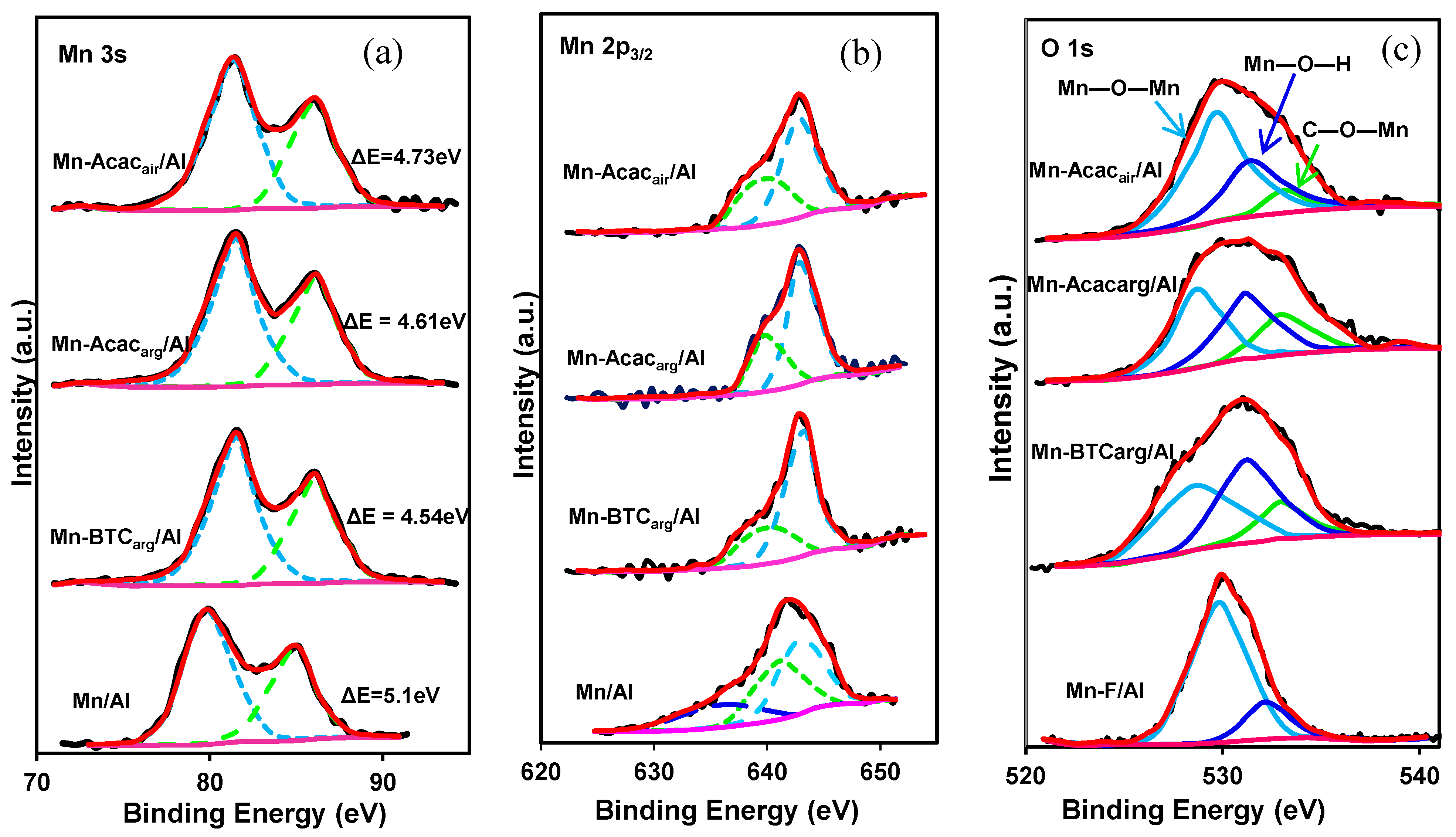
3.1. Characterization of the Catalysts
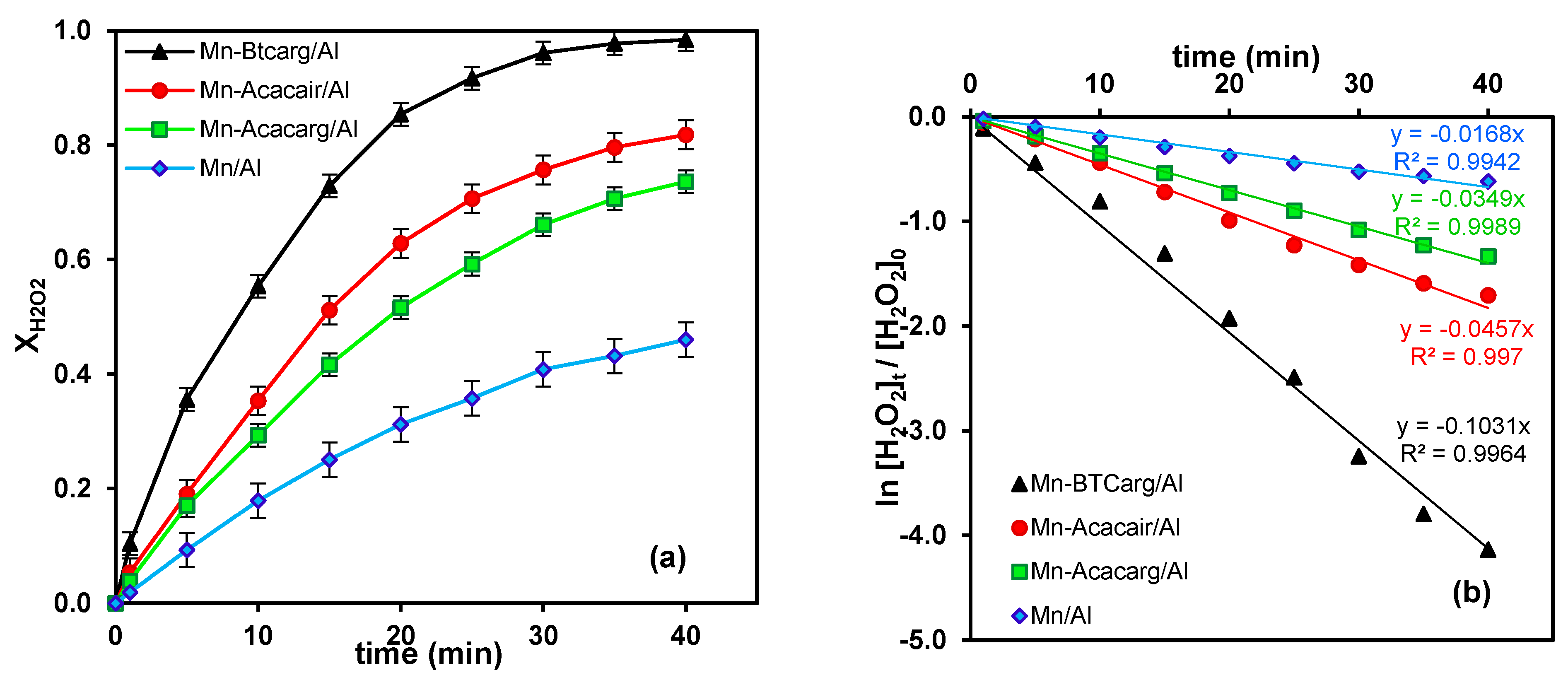
3.2. Performance of Catalysts for Hydrogen Peroxide Activation
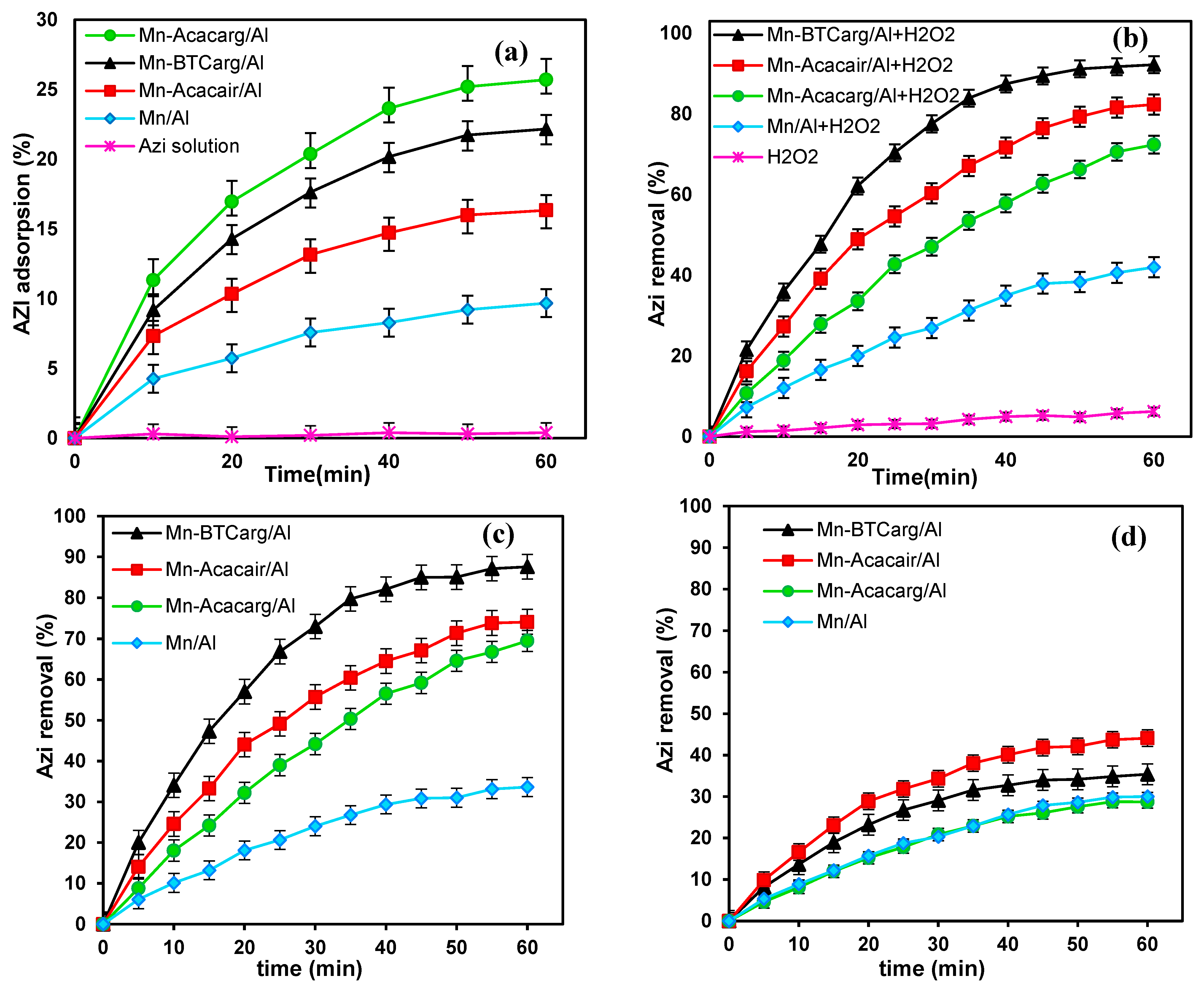
3.3. Performance of Catalysts for Azi Removal
3.4. Effects of the Solution pH and the Catalyst Dosage
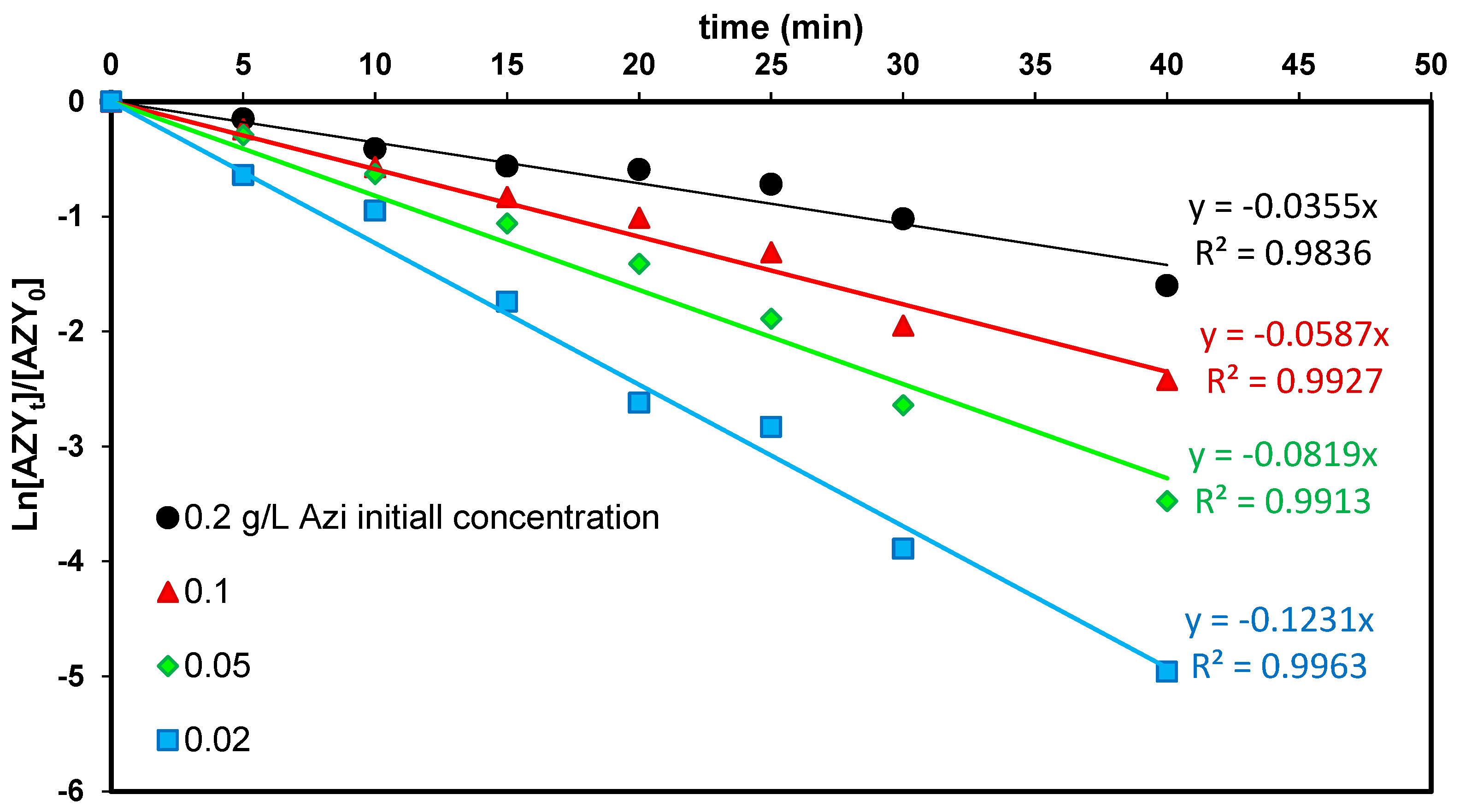
3.5. Effects of Azi and H2O2 Initial Concentrations
3.6. Kinetic of Azi Removal
3.7. Catalyst Stability and Reusability
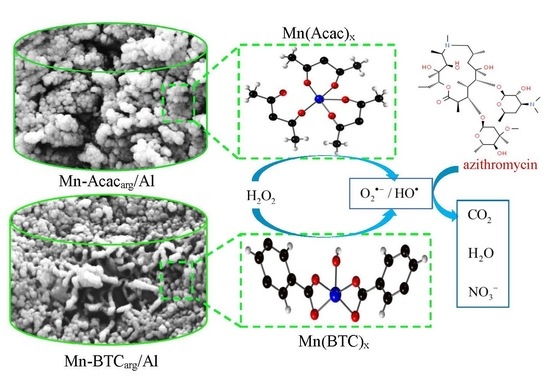
3.8. Azi Removal Mechanism
4. Conclusions and Future Works
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Zhao, X.; Huang, S.; Li, Y.; Zhang, X.; Zeng, G.; Niu, L.; Ling, Y.; Zhang, Y. Facile construction of 2D g-C3N4 supported nanoflower-like NaBiO3 with direct Z-scheme heterojunctions and insight into its photocatalytic degradation of tetracycline. J. Hazard. Mater. 2021, 414, 125547. [Google Scholar] [CrossRef] [PubMed]
- Ricky, R.; Shanthakumar, S. Phycoremediation integrated approach for the removal of pharmaceuticals and personal care products from wastewater–A review. J. Environ. Manag. 2022, 302, 113998. [Google Scholar] [CrossRef]
- Mowery, H.; Loganathan, B. Persistent organic compounds in wastewater: Azithromycin and urobilin concentrations in wastewater treatment plant samples from Murray, Kentucky, USA. Organohalogen Compd. 2007, 69, 483. [Google Scholar]
- Martínez-Polanco, M.P.; Martínez-Polanco, M.P.; Valderrama-Rincón, J.A.; Martínez-Rojas, A.J.; Luna-Wandurraga, H.J.; Díaz-Báez, M.C.; Bustos-López, M.C.; Valderrama-Rincon, J.D. Degradation of high concentrations of azithromycin when present in a high organic content wastewater by using a continuously fed laboratory-scale UASB bioreactor. Chemosphere 2022, 287, 132191. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Yang, Y.; Shi, W.; She, Y.; Wu, S. Heterogeneous Fenton degradation of azithromycin antibiotic in water catalyzed by amino/thiol-functionalized MnFe2O4 magnetic nanocatalysts. Environ. Chem. Engin. J. 2021, 9, 106184. [Google Scholar] [CrossRef]
- Voigt, M.; Jaeger, M. On the photodegradation of azithromycin, erythromycin and tylosin and their transformation products–A kinetic study. Sustain. Chem. Pharm. 2017, 5, 131–140. [Google Scholar] [CrossRef]
- Pan, K.; Yang, C.; Hu, J.; Yang, W.; Liu, B.; Yang, J.; Liang, S.; Xiao, K.; Hou, H. Oxygen vacancy mediated surface charge redistribution of Cu-substituted LaFeO3 for degradation of bisphenol A by efficient decomposition of H2O2. J. Hazard. Mater. 2020, 389, 122072. [Google Scholar] [CrossRef]
- Kholdeeva, O.; Maksimchuk, N. Metal-organic frameworks in oxidation catalysis with hydrogen peroxide. Catalysts 2021, 11, 283. [Google Scholar] [CrossRef]
- Schubert, J.S.; Popovic, J.; Haselmann, G.M.; Nandan, S.P.; Wang, J.; Giesriegl, A.; Cherevan, A.S.; Eder, D. Immobilization of Co, Mn, Ni and Fe oxide co-catalysts on TiO2 for photocatalytic water splitting reactions. J. Mater. Chem. A 2019, 7, 18568–18579. [Google Scholar] [CrossRef]
- Aunan, E.; Affolter, C.W.; Olsbye, U.; Lillerud, K.P. Modulation of the Thermochemical Stability and Adsorptive Properties of MOF-808 by the Selection of Non-structural Ligands. Chem. Mater. 2021, 33, 1471–1476. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Wu, Q.; Siddique, M.S.; Guo, Y.; Wu, M.; Yang, Y.; Yang, H. Low-crystalline bimetallic metal-organic frameworks as an excellent platform for photo-Fenton degradation of organic contaminants: Intensified synergism between hetero-metal nodes. Appl. Catal. B Environ. 2021, 286, 119950. [Google Scholar] [CrossRef]
- Reinsch, H.; Stock, N. Formation and characterisation of Mn-MIL-100. CrystEngComm 2013, 15, 544–550. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hui, K.N.; San Hui, K.; Peng, S.; Xu, Y. Recent progress in metal–organic framework/graphene-derived materials for energy storage and conversion: Design, preparation, and application. Chem. Sci. 2021, 12, 5737–5766. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Zhang, Q.; Liu, Z.; Fu, M.; Wu, J.; Hu, Y.; Ye, D. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template. Chem. Engin. J. 2019, 371, 78–87. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, F.; Li, H.; Yang, Y.; Wang, Y.; Liu, N.; Yang, Y. A strawsheave-like metal organic framework Ce-BTC derivative containing high specific surface area for improving the catalytic activity of CO oxidation reaction. Micropor. Mesopor. Mat. 2018, 259, 211–219. [Google Scholar] [CrossRef]
- Shalaby, M.; Abdallah, H. Preparation of manganese (III) acetylacetonate nanoparticles via an environmentally benign route. Front. Chem. Sci. Eng. 2013, 7, 329–337. [Google Scholar] [CrossRef]
- Hasanpour, F.; Saien, J. Incorporating Pb2+ templates into the crystalline structure of MnO2 catalyst supported on monolith: Applications in H2O2 decomposition. ACS Omega 2019, 4, 16638–16650. [Google Scholar] [CrossRef]
- Lee, E.; Lee, H.; Kim, Y.; Sohn, K.; Lee, K. Hydrogen peroxide interference in chemical oxygen demand during ozone based advanced oxidation of anaerobically digested livestock wastewater. Int. J. Environ. Sci. Technol. 2011, 8, 381–388. [Google Scholar] [CrossRef]
- Naraginti, S.; Yu, Y.-Y.; Fang, Z.; Yong, Y.-C. Visible light degradation of macrolide antibiotic azithromycin by novel ZrO2/Ag@TiO2 nanorod composite: Transformation pathways and toxicity evaluation. Process Safety Environ. Prot. 2019, 125, 39–49. [Google Scholar] [CrossRef]
- Sel, K.; Demirci, S.; Ozturk, O.F.; Aktas, N.; Sahiner, N. NH3 gas sensing applications of metal organic frameworks. Microelectron. Eng. 2015, 136, 71–76. [Google Scholar] [CrossRef]
- Niu, T.; Li, J.; Qi, Y.; Huang, X.; Ren, Y. Preparation and electrochemical properties of α-MnO2/rGO-PPy composite as cathode material for zinc-ion battery. J. Mater. Sci. 2021, 56, 16582–16590. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Pan, Y.; An, L.; Xu, K.; Wang, G.; Yu, Z.; Yu, L.; Hu, J. A facile synthesis of α-MnO2 used as a supercapacitor electrode material: The influence of the Mn-based precursor solutions on the electrochemical performance. Appl. Surf. Sci. 2015, 357, 1747–1752. [Google Scholar] [CrossRef]
- Huang, X.; Lv, D.; Yue, H.; Attia, A.; Yang, Y. Controllable synthesis of α-and β-MnO2: Cationic effect on hydrothermal crystallization. Nanotechnology 2008, 19, 225606. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142, 729–735. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, S.; Yang, J.; Wang, H.; Pollet, B.G.; Wang, R. Enhanced cycleability of amorphous MnO2 by covering on α-MnO2 needles in an electrochemical capacitor. Materials 2017, 10, 988. [Google Scholar] [CrossRef]
- Gou, L.; Mou, K.-L.; Fan, X.-Y.; Zhao, M.-J.; Wang, Y.; Xue, D.; Li, D.-L. Mn2O3/Al2O3 cathode material derived from a metal–organic framework with enhanced cycling performance for aqueous zinc-ion batteries. Dalton Trans. 2020, 49, 711–718. [Google Scholar] [CrossRef]
- Mahdavi, M.; Farrokhpour, H.; Tahriri, M. A Comparison of Catalytic Effect of Nano-Mn3O4 derived from MnC2O4.2H2O and Mn(acac)3 on Thermal Decomposition of Ammonium Perchlorate. Z. Anorg. Allgem. Chem. 2018, 644, 241–252. [Google Scholar] [CrossRef]
- Garakani, M.A.; Abouali, S.; Cui, J.; Kim, J.-K. In Situ TEM study of lithiation into a PPy coated α-MnO2/graphene foam freestanding electrode. Mater. Chem. Front. 2018, 2, 1481–1488. [Google Scholar] [CrossRef]
- Lee, J.W.; Hall, A.S.; Kim, J.-D.; Mallouk, T.E. A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem. Mater. 2012, 24, 1158–1164. [Google Scholar] [CrossRef]
- Rosen, A.S.; Mian, M.R.; Islamoglu, T.; Chen, H.; Farha, O.K.; Notestein, J.M.; Snurr, R.Q. Tuning the redox activity of metal–organic frameworks for enhanced, selective O2 binding: Design rules and ambient temperature O2 chemisorption in a cobalt–triazolate framework. J. Am. Chem. Soc. 2020, 142, 4317–4328. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Wen, H.; Qu, X.; Guo, Y.; Hu, L.; Liu, W.; Tian, S.; He, C.; Shu, D. Enhanced Fenton-like catalysis for pollutants removal via MOF-derived CoxFe3−xO4 membrane: Oxygen vacancy-mediated mechanism. Chemosphere 2022, 303, 135301. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.V.; Choudhury, B.; Madras, G.; Bose, S. ‘Trigger-free’self-healable electromagnetic shielding material assisted by co-doped graphene nanostructures. Chem. Eng. J. 2020, 382, 122816. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Estrela, F.N.; de Lima Rodrigues, A.S.; Chagas, T.Q.; Pereira, P.S.; Silva, F.G.; Malafaia, G. Nanopolystyrene particles at environmentally relevant concentrations causes behavioral and biochemical changes in juvenile grass carp (Ctenopharyngodon idella). J. Hazard. Mater. 2021, 403, 123864. [Google Scholar] [CrossRef]
- Jalali, S.; Ardjmand, M.; Ramavandi, B.; Nosratinia, F. Removal of amoxicillin from wastewater in the presence of H2O2 using modified zeolite Y-MgO catalyst: An optimization study. Chemosphere 2021, 274, 129844. [Google Scholar] [CrossRef]
- Jaramillo-Baquero, M.; Zúñiga-Benítez, H.; Peñuela, G.A. Use of photo-fenton for macrolide antibiotic azithromycin removal. Acta Period. Technol. 2020, 51, 29–37. [Google Scholar]
- Shokri, R.; Yengejeh, R.J.; Babaei, A.A.; Derikvand, E.; Almasi, A. UV activation of hydrogen peroxide for removal of azithromycin antibiotic from aqueous solution: Determination of optimum conditions by response surface methodology. Toxin Rev. 2019, 39, 284–291. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Sobhani, S.; Shekari, H. Photocatalytic degradation of azithromycin using GO@ Fe3O4/ZnO/SnO2 nanocomposites. J. Clean. Prod. 2019, 232, 127–136. [Google Scholar] [CrossRef]
- Tong, L.; Eichhorn, P.; Pérez, S.; Wang, Y.; Barceló, D. Photodegradation of azithromycin in various aqueous systems under simulated and natural solar radiation: Kinetics and identification of photoproducts. Chemosphere 2011, 83, 340–348. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Rožman, M.; Ašperger, D.; Ćurković, L.; Babić, S. Photocatalytic degradation of azithromycin by nanostructured TiO2 film: Kinetics, degradation products, and toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef]





| Catalyst/Support | Surface Area (BET) (m2.g−1) | Pore Volume (cm3.g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Alumina support | 259 | 0.52 | 4.72 |
| Mn/Al | 163 | 0.27 | 4.31 |
| Mn-Acacair/Al | 216 | 0.34 | 5.29 |
| Mn-Acacarg/Al | 251 | 0.48 | 6.13 |
| Mn-BTCarg/Al | 246 | 0.43 | 5.74 |
| Catalyst | ΔES (eV) | Mn4+/Mn3+ | Oxygen Distribution (%) | ||
|---|---|---|---|---|---|
| Oα | Oβ | Oβ/Oα | |||
| Mn/Al | 3.21 | 1.15 | 78.52 | 22.48 | 0.29 |
| Mn-Acacair/Al | 3.71 | 1.51 | 58.23 | 33.41 | 0.57 |
| Mn-Acacarg/Al | 3.77 | 2.15 | 42.61 | 37.94 | 0.89 |
| Mn-BTCarg/Al | 3.84 | 3.26 | 40.92 | 48.65 | 1.19 |
| Catalyst | H2 Desorption (µmol. gcat −1) | D (%) | Active Sites No., NAc, (×1020.gcat −1) | (TON) |
|---|---|---|---|---|
| Mn/Al | 821 | 22.46 | 3.52 | 1.53 |
| Mn-Acacair/Al | 1183 | 32.40 | 5.12 | 1.95 |
| Mn-Acacarg/Al | 916 | 25.06 | 3.97 | 2.17 |
| Mn-BTCarg/Al | 1275 | 34.91 | 5.61 | 2.32 |
| Process | Catalyst | Azi Initial Conc. (mg/L) | Reaction Time (min) | Azi Removal (%) | (min−1) | Ref. |
|---|---|---|---|---|---|---|
| Heterogeneous Fenton | MnFe2O4 | 1 | 240 | 92.6 | 0.01 | [5] |
| Photo-Fenton | FeSO4 | 1 | 30 | 83 | 0.06 | [37] |
| UV/H2O2 | - | 2 | 60 | 76 | 0.02 | [38] |
| Photocatalysis UVC | GO@Fe3O4/ZnO/SnO2 | 30 | 120 | 90 | 0.02 | [39] |
| Visible photocatalysis | 2D g-C3N4-NaBiO3 | 25 | 30 | 57.3 | 0.03 | [1] |
| H2O2 catalytic activation | Mn-BTCarg/Al | 20 | 40 | 99.3 | 0.12 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanpour, F.; Saien, J.; Norouzi, O. Catalytic Activation of Hydrogen Peroxide Using Highly Porous Hydrothermally Modified Manganese Catalysts for Removal of Azithromycin Antibiotic from Aqueous Solution. Catalysts 2023, 13, 77. https://doi.org/10.3390/catal13010077
Hasanpour F, Saien J, Norouzi O. Catalytic Activation of Hydrogen Peroxide Using Highly Porous Hydrothermally Modified Manganese Catalysts for Removal of Azithromycin Antibiotic from Aqueous Solution. Catalysts. 2023; 13(1):77. https://doi.org/10.3390/catal13010077
Chicago/Turabian StyleHasanpour, Fatemeh, Javad Saien, and Omid Norouzi. 2023. "Catalytic Activation of Hydrogen Peroxide Using Highly Porous Hydrothermally Modified Manganese Catalysts for Removal of Azithromycin Antibiotic from Aqueous Solution" Catalysts 13, no. 1: 77. https://doi.org/10.3390/catal13010077
APA StyleHasanpour, F., Saien, J., & Norouzi, O. (2023). Catalytic Activation of Hydrogen Peroxide Using Highly Porous Hydrothermally Modified Manganese Catalysts for Removal of Azithromycin Antibiotic from Aqueous Solution. Catalysts, 13(1), 77. https://doi.org/10.3390/catal13010077