High Photocatalytic Efficiency of Al2O3-TiO2 Coatings on 304 Stainless Steel for Methylene Blue and Wastewater Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Al2O3-TiO2 Coating Characterization
2.1.1. X-ray Diffraction
2.1.2. Scanning Electronic Microscopy
2.1.3. Atomic Force Microscopy
2.1.4. UV-Vis Spectroscopy
2.2. Photocatalytic Degradation
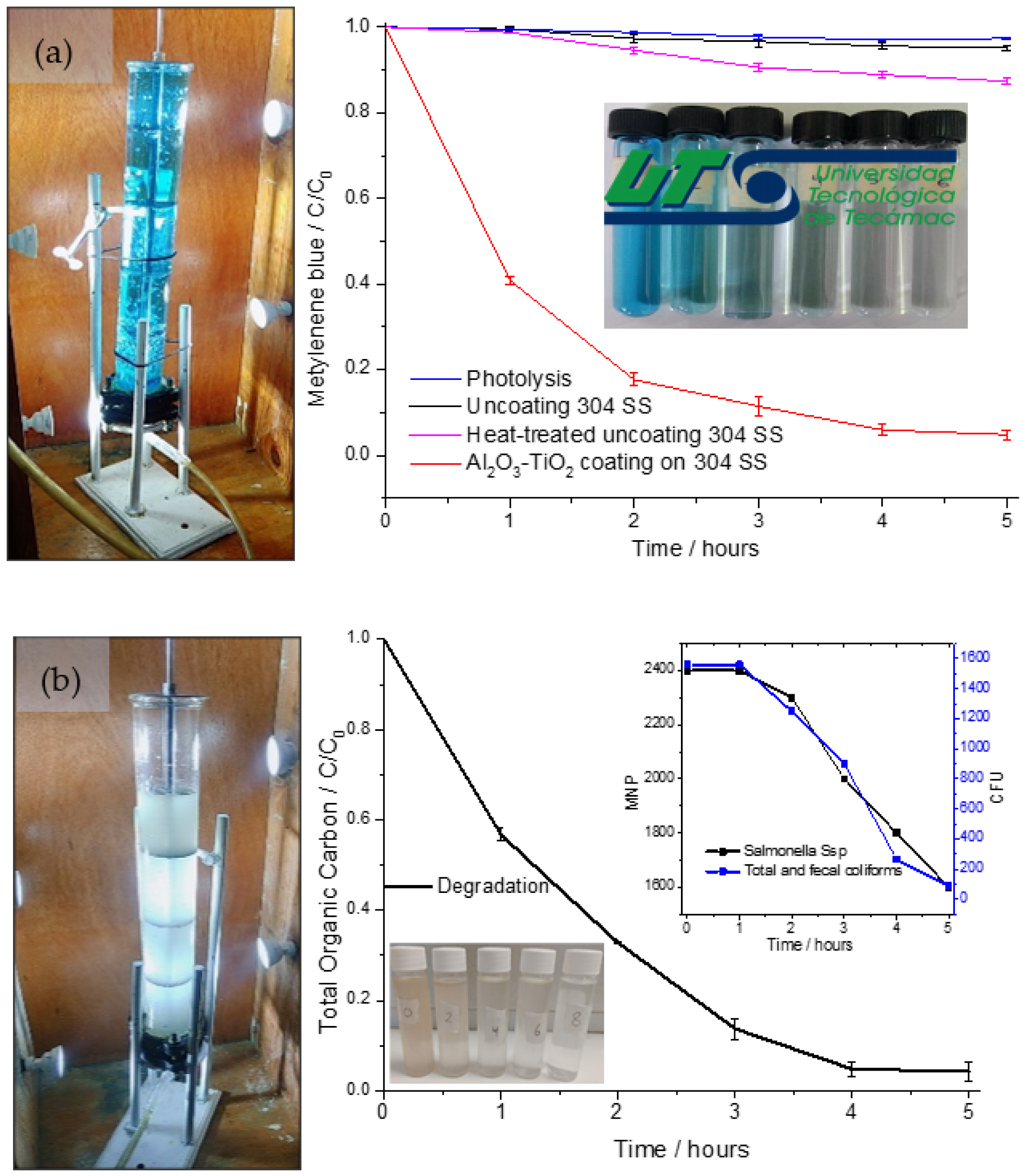
2.2.1. MB Degradation
2.2.2. TOC Quantification
2.2.3. Bactericidal Activity
3. Materials and Methods
3.1. Coating Preparation Using the Sol–Gel Route
3.2. Structural Characterization Measurements
3.3. Photocatalytic Degradation
3.3.1. MB Degradation
3.3.2. TOC Quantification
3.3.3. Bactericidal Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ONU-DAES. International Decade for Action Water for Life 2005–2015. 2021. Available online: https://www.un.org/waterforlifedecade/index.shtml (accessed on 15 January 2023).
- UNESCO. United Nations World Water Development Report 2020: Water and Climate Change; UNESCO: Paris, France, 2020; Available online: https://www.unesco.org/en/wwap/wwdr/2020 (accessed on 20 January 2023).
- Ismail, A.A.; Abdelfattah, I.; Atitar, M.F.; Robben, L.; Bouzid, H.; Al-Sayari, S.A.; Bahnemann, D.W. Photocatalytic degradation of imazapyr using mesoporous Al2O3–TiO2 nanocomposites. Sep. Purif. Technol. 2015, 145, 147–153. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández-Ibañez, P.; Sillanpää, M.A. Critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Švagelj, Z.; Mandić, V.; Ćurković, L.; Biošić, M.; Žmak, I.; Gaborardi, M. Titania-coated alumina foam photocatalyst for memantine degradation derived by replica method and sol-gel reaction. Materials 2020, 13, 227. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Li-Puma, G. Advanced oxidation processes for water and wastewater treatment. Water 2021, 13, 1309. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Abbas, G.; Qurashi, A.; Hussain, M. Efficient catalyst development for deep aerobic photocatalytic oxidative desulfurization: Recent advances, confines, and outlooks. Catal. Rev.-Sci. Eng. 2022, 64, 789–834. [Google Scholar] [CrossRef]
- Khatri, J.; Nidheesh, P.V.; Anantha Singh, T.S.; Kumar, M.S. Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem. Eng. J. 2018, 348, 67–73. [Google Scholar] [CrossRef]
- Khan, A.H.; Khan, N.A.; Ahmed, S.; Dhingra, A.; Singh, C.P.; Khan, S.U.; Mohammadi, A.A.; Changani, F.; Yousefi, M.; Alam, S.; et al. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020, 269, 122411. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for advanced oxidation processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef]
- Ahmed, S.; Raul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in heterogeneous photocatalytic degradation of Phenols and dyes in wastewater: A Review. Water Air Soil Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef]
- Wawrzyniak, B.; Morawski, A.W. Solar-light-induced photocatalytic decomposition of two azo dyes on new TiO2 photocatalyst containing nitrogen. Appl. Catal. B Environ. 2006, 62, 150–158. [Google Scholar] [CrossRef]
- Da Silva, S.W.; Klauck, C.R.; Siqueira, M.A.; Bernardes, A.M. Degradation of the commercial surfactant nonylphenol ethoxylate by advanced oxidation processes. J. Hazard. Mater. 2015, 282, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; García-Muñoz, P.; Hernández-Alonso, M.D.; Mena, E.; García-Rodríguez, S.; Beltrán, F.J. WO3–TiO2 based catalysts for the simulated solar radiation assisted photocatalytic ozonation of emerging contaminants in a municipal wastewater treatment plant effluent. Appl. Catal. B Environ. 2014, 154–155, 274–284. [Google Scholar] [CrossRef]
- Aguinaco, A.; Amaya, B.; Ramírez-Del-Solar, M. Facile fabrication of Fe-TiO2 thin film and its photocatalytic activity. Environ. Sci. Pollut. Res. 2022, 29, 23292–23302. [Google Scholar] [CrossRef] [PubMed]
- Chairungsri, W.; Subkomkaew, A.; Kijjanapanich, P.; Chimupala, Y. Direct dye wastewater photocatalysis using immobilized titanium dioxide on fixed substrate. Chemosphere 2022, 268, 131762. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Behpour, M.; Atouf, V. Study of the photocatalytic activity of nanocrystalline S, N-codoped TiO2 thin films and powders under visible and sun light irradiation. Appl. Surf. Sci. 2012, 258, 6595–6601. [Google Scholar] [CrossRef]
- Falk, G.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Neto, J.B.R.; Moreno, R. Microwave-assisted synthesis of Nb2O5 for photocatalytic application of nanopowders and thin films. J. Mater. Res. 2017, 32, 3271–3278. [Google Scholar] [CrossRef]
- Nisar, U.; Muralidharan, N.; Essehli, R.; Amin, R.; Belharouak, I. Valuation of surface coatings in high-energy density lithium-ion battery cathode materials. Energy Storage Mater. 2021, 38, 309–328. [Google Scholar] [CrossRef]
- Sahu, S.K.; Panthi, D.; Du, Y. Development of Multiple Ceramic Coatings on Porous Tubular Metal Support. ECS Trans. 2023, 6, 249. [Google Scholar] [CrossRef]
- Kim, S.G.; Yoon, S.P.; Nam, S.W.; Hyun, S.H.; Hong, S.A. Fabrication and characterization of a YSZ/YDC composite electrolyte by a sol–gel coating method. J. Power Source 2002, 110, 222–228. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Y.; Yan, M.; Tu, B.; Li, Y. Microstructure and mechanical properties of surface coating prepared on grade 2 titanium by Ni-B composite electroplating and laser cladding. Laser Technol. 2023, 164, 109498. [Google Scholar] [CrossRef]
- Hui, R.; Wang, Z.; Yick, S.; Maric, R.; Ghosh, D. Fabrication of ceramic films for solid oxide fuel cells via slurry spin coating technique. J. Power Source 2007, 172, 840–844. [Google Scholar] [CrossRef]
- Ogumi, Z.; Uchimoto, Y.; Tsuji, Y.; Takehara, Z.I. Preparation of thin yttria-stabilized zirconia films by vapor-phase electrolytic deposition. Solid State Ion. 1992, 58, 345–350. [Google Scholar] [CrossRef]
- Smeacetto, F.; Salvo, M.; Ajitdoss, L.C.; Perero, S.; Moskalewicz, T.; Boldrini, S.; Ferraris, M. Yttria-stabilized zirconia thin film electrolyte produced by RF sputtering for solid oxide fuel cell applications. Mater. Lett. 2010, 64, 2450–2453. [Google Scholar] [CrossRef]
- García-Sánchez, F.; Peña, J.; Ortiz, A.; Santana, G.; Fandiño, J.; Bizarro, m.; Cruz-Gandarilla, F.; Alonso, J.C. Nanostructured YSZ thin films for solid oxide fuel cells deposited by ultrasonic spray pyrolysis. Solid State Ionics 2008, 179, 243–249. [Google Scholar] [CrossRef]
- Gołąbiewska, A.; Kobylański, M.P.; Zaleska-Medynska, A. Fundamentals of Metal Oxide-Based Photocatalysis in Book Metal Oxide-Based Photocatalysis Fundamentals and Prospects for Application; Zaleska-Medynska, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–50. [Google Scholar] [CrossRef]
- Tian, L.; Qian, L.; Yao, X. Preparation of Sn4+-doped titanium dioxide photocatalytic films on 304 stainless steel by duplex treatment. J. Mater. Eng. Perform. 2014, 23, 1790–1798. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, J.; Son, J.Y.; Maeng, W.J.; Jo, D.H.; Choi, W.; Kim, H. Plasma-enhanced ALD of TiO2 thin films on SUS 304 stainless steel for photocatalytic application. J. Electrochem. Soc. 2009, 156, D188–D192. [Google Scholar] [CrossRef]
- Soylu, A.M.; Polat, M.; Erdogan, D.A.; Say, Z.; Yildiri, C.; Birer, Ö.; Ozensoy, E. TiO2–Al2O3 binary mixed oxide surfaces for photocatalytic NOx abatement. Appl. Surf. Sci. 2014, 318, 142–149. [Google Scholar] [CrossRef]
- Akkaya Arıer, U.O.; Tepehan, F.Z. Influence of Al2O3:TiO2 ratio on the structural and optical properties of TiO2–Al2O3 nano-composite films produced by sol gel method. Compos. B Eng. 2014, 58, 147–151. [Google Scholar] [CrossRef]
- Montalvo, D.; Corro, G.; Bañuelos, F.; Olivares-Xometl, O.; Arellanes, P.; Pal, U. Selective alcohols production through CO2 photoreduction using Co3O4/TiO2 photocatalyst exploiting synergetic interactions between Ti3+, Co2+ and Co3+. Appl. Catal. B Environ. 2023, 330, 122652. [Google Scholar] [CrossRef]
- Parnicka, P.; Lisowski, W.; Klimczuk, T.; Łuczak, J.; Żak, A.; Zaleska-Medynska, A. Visible-light-driven lanthanide-organic-frameworks modified TiO2 photocatalysts utilizing up-conversion effect. Appl. Catal. B Environ. 2021, 291, 120056. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, R.; Li, Z.; Li, A.; Wang, S.; Liang, Z.; Liao, S.; Li, C. The dependence of photocatalytic activity on the selective and nonselective deposition of noble metal co-catalysts on the facets of rutile TiO2. J. Catal. 2016, 337, 36–44. [Google Scholar] [CrossRef]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S. Effectiveness assessment of TiO2-Al2O3 nano-mixture as a filler material for improvement of packaging performance of PLA nanocomposite films. J. Polym. Eng. 2020, 40, 848–858. [Google Scholar] [CrossRef]
- Karunakaran, C.; Magesan, P.; Gomathisankar, P.; Vinayagamoorthy, P. Absorption, emission, charge transfer resistance and photocatalytic activity of Al2O3/TiO2 core/shell nanoparticles. Superlattices Microstruct. 2015, 83, 659–667. [Google Scholar] [CrossRef]
- Magnone, E.; Kim, K.M.; Lee, H.J.; Park, J.H. Facile synthesis of TiO2-supported Al2O3 ceramic hollow fiber substrates with extremely high photocatalytic activity and reusability. Ceram. Int. 2021, 47, 7764–7775. [Google Scholar] [CrossRef]
- Phattepur, H.; Hiremath, P.G. Fabrication of Al2O3 supported TiO2 membranes for photocatalytic applications. Mater. Today Proc. 2022, 65, 3694–3699. [Google Scholar] [CrossRef]
- Martinez-Gómez, C.; Rangel-Vázquez, I.; Zarraga, R.; Del Ángel, G.; Ruíz-Camacho, B.; Tzompantzi, F.; Vidal-Robles, E.; Perez-Larios, A. Photodegradation and Mineralization of Phenol Using TiO2 Coated γ-Al2O3: Effect of Thermic Treatment. Processes 2022, 10, 1186. [Google Scholar] [CrossRef]
- Anderson, C.A.; Bard, A.J. Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. B 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Lee, S.; Huang, E.-W.; Woo, W.; Yoon, C.; Chae, H.; Yoon, S.-G. Dynamic Strain Evolution around a Crack Tip under Steady- and Overloaded-Fatigue Conditions. Metals 2015, 5, 2109–2118. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. Facile synthesis of nano-crystalline alpha-alumina at low temperature via an absolute ethanol sol–gel strategy. Mater. Chem. Phys. 2012, 132, 1071–1076. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Sadeq, Z.S.; Mahdi, Z.F.; Hamza, A.M. Low cost, fast and powerful performance interfacial charge transfer nanostructured Al2O3 capturing of light photocatalyst eco-friendly system using hydrothermal method. Mater. Lett. 2019, 254, 120–124. [Google Scholar] [CrossRef]
- Miszczak, S.; Pietrzyk, B. Anatase–rutile transformation of TiO2 sol–gel coatings deposited on different substrates. Ceram. Int. 2015, 41, 7461–7465. [Google Scholar] [CrossRef]
- Marques, V.E.C.; Manfroi, L.A.; Vieira, A.A.; de Jesús Pereira, A.L.; das Chagas Marques, F.; Vieira, L. Crystalline Structure, Morphology, and Adherence of Thick TiO2 Films Grown on 304 and 316L Stainless Steels by Atomic Layer Deposition. Coatings 2023, 13, 757. [Google Scholar] [CrossRef]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Kim, H.T. Sol–gel synthesis of TiO2-Fe2O3 systems: Effects of Fe2O3 content and their photocatalytic properties. J. Ind. Eng. Chem 2016, 39, 112–120. [Google Scholar] [CrossRef]
- Da Trindade, C.; Da Silva, S.W.; Bortolozzi, J.P.; Banús, E.D.; Bernardes, A.M.; Ulla, M.A. Synthesis and characterization of TiO2 films onto AISI 304 metallic meshes and their application in the decomposition of the endocrine-disrupting alkylphenolic chemicals. Appl. Surf. Sci. 2018, 457, 644–654. [Google Scholar] [CrossRef]
- Sridevi, A.; Krishnamohan, S.; Thairiyaraja, M.; Prakash, B.; Yokeshwaran, R. Visible-light driven γ-Al2O3, CuO and γ-Al2O3/CuO nanocatalysts: Synthesis and enhanced photocatalytic activity. Inorg. Chem. Commun. 2022, 138, 109311. [Google Scholar] [CrossRef]
- Oladijo, O.P.; Popoola, A.P.I.; Booi, M.; Fayomi, J.; Collieus, L.L. Corrosion and mechanical behaviour of Al2O3-TiO2 composites produced by spark plasma sintering. S. Afr. J. Chem. Eng. 2020, 33, 58–66. [Google Scholar] [CrossRef]
- Mwema, F.M.; Oladijo, O.P.; Sathiaraj, T.S.; Akinlabi, E.T. Atomic force microscopy analysis of surface topography of pure thin aluminum films. Mater. Res. Express 2018, 5, 046416. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Tian, T.; Pan, X. An AFM-IR study on surface properties of nano-TiO2 coated polyethylene (PE) thin film as influenced by photocatalytic aging process. Sci. Total Environ. 2021, 757, 143900. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Dhawale, V.P.; Late, D.J.; Kulkarni, S.D. Synthesis, characterization of α-Al2O3 nanoparticles and its application in decolorization of methyl orange azo dye in the presence of UV light. J. Nanosci. Nanotechnol. 2019, 5, 580–583. [Google Scholar] [CrossRef]
- Prashanth, P.A.; Raveendra, R.S.; Krishna, R.H.; Ananda, S.; Bhagya, N.P.; Nagabhushana, B.M.; Lingaraju, K. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticle. J. Ceram. Process. Res. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, S.; Yu, Y. Experimental method to explore the adaptation degree of type-II and all-solid-state Z-scheme heterojunction structures in the same degradation system. Chin. J. Catal. 2020, 41, 1522–1534. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Komaraiah, D.; Madhukar Vijayakumar, Y.; Ramana Reddy, M.V.; Sayanna, R. Photocatalytic degradation study of methylene blue by brookite TiO2 thin film under visible light irradiation. Mater. Today Proc. 2016, 3, 3770–3778. [Google Scholar] [CrossRef]
- Yu, X.; Kou, S.; Nie, J.; Zhang, J.; Wei, Y.; Niu, N.; Yao, B. Preparation and performance of Cu2O/TiO2 nanocomposite thin film and photocatalytic degradation of Rhodamine B. Water Sci. Technol. 2018, 78, 913–924. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Zhurenok, A.V.; Kurenkova, A.Y.; Kremneva, A.M.; Saraev, A.A.; Zharkov, S.M.; Kozlova, E.A.; Kaichev, V.V. New titania-based photocatalysts for hydrogen production from aqueous-alcoholic solutions of methylene blue. RSC Adv. 2020, 10, 34137–34148. [Google Scholar] [CrossRef]
- Reda, S.M.; Khairy, M.; Mousa, M.A. Photocatalytic activity of nitrogen and copper doped TiO2 nanoparticles prepared by microwave-assisted sol-gel process. Arab. J. Chem. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Ni-doped TiO2 materials for photocurrent and photocatalytic applications. Sci. World J. 2012, 2012, 127326. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhai, J.; Li, H.; Zhao, Y.; Yang, H.; Yu, H. Study of homologous elements: Fe, Co, and Ni dopant effects on the photoreactivity of TiO2 nanosheets. ChemCatChem 2014, 6, 339–347. [Google Scholar] [CrossRef]
- Xu, J.; Wang, F.; Liu, W.; Cao, W. Nanocrystalline N-doped TiO2 powders: Mild hydrothermal synthesis and photocatalytic degradation of phenol under visible light irradiation. Int. J. Photoenergy 2013, 2013, 616139. [Google Scholar] [CrossRef]
- Sánchez Mora, E.; Gómez Barajas, E.; Rojas Rojas, E.; Silva González, R. Morphological, optical and photocatalytic properties of TiO2–Fe2O3 multilayers. Sol. Energy Mater. Sol. Cells 2007, 91, 1412–1415. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.; Wang, Y.; Ma, W.; Zhao, J.; Rajh, T.; Zang, L. Enhanced photocatalytic degradation of dye pollutants under visible irradiation on Al(III)-modified TiO2: Structure, interaction, and interfacial electron transfer. Environ. Sci. Technol. 2008, 42, 308–314. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Bizarro, M.; Gadhi, T.A. Good practices for reporting the photocatalytic evaluation of a visible-light active semiconductor: Bi2O3, a case study. Catal. Sci. Technol. 2019, 9, 1476–1496. [Google Scholar] [CrossRef]
- NOM-001-SEMARNAT-2021, DOF. Available online: https://www.gob.mx/semarnat/prensa/se-publica-nom-001-semarnat-2021-que-establece-limites-de-contaminantes-en-descargas-de-aguas-residuales (accessed on 19 September 2022).
- Dubber, D.; Gray, N.F. Replacement of chemical oxygen demand (COD) with total organic carbon (TOC) for monitoring wastewater treatment performance to minimize disposal of toxic analytical waste. J. Environ. Sci. Health A 2010, 45, 1595–1600. [Google Scholar] [CrossRef]
- Netto, G.C.; Sauer, T.; José, H.J.; Peralta Muniz Moreira, R.F.; Humeres, E. Evaluation of relative photonic efficiency in heterogeneous photocatalytic reactors. J. Air Waste Manag. Assoc. 2012, 54, 77–82. [Google Scholar] [CrossRef]
- Cao, C.; Hu, C.; Wang, X.; Wang, S.; Tian, Y.; Zhang, H. UV sensor based on TiO2 nanorod arrays on FTO thin film. Sens. Actuators B Chem. 2011, 156, 114–119. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Alsafar, H.; Yousef, A.F.; Banat, F.; Hasan, S.W. Bacterial nanotechnology: The intersection impact of bacteriology and nanotechnology on the wastewater treatment sector. J. Environ. Chem. Eng. 2023, 11, 109212. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Bohara, R.A.; Mali, S.S.; Pawar, S.H.; Delekar, S.D. Synthesis and visible light photocatalytic antibacterial activity of nickel-doped TiO2 nanoparticles against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A 2014, 294, 130–136. [Google Scholar] [CrossRef]
- Guillard, C.; Bui, T.H.; Felix, C.; Moules, V.; Lina, B.; Lejeune, P. Microbiological disinfection of water and air by photocatalysis. C. R. Chim. 2008, 11, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Nadtochenko, V.A.; Rincon, A.G.; Stanca, S.E.; Kiwi, J. Dynamics of E. coli membrane cell peroxidation during TiO2 photocatalysis studied by ATR-FTIR spectroscopy and AFM microscopy. J. Photochem. Photobiol. A 2005, 169, 131–137. [Google Scholar] [CrossRef]
- Thakur, I.; Verma, A.; Örmeci, B. Visibly active Fe-TiO2 composite: A stable and efficient catalyst for the catalytic disinfection of water using a once-through reactor. J. Environ. Chem. Eng. 2021, 9, 106322. [Google Scholar] [CrossRef]
- Barajas-Ledesma, E.; García-Benjume, M.L.; Espitia-Cabrera, I.; Ortiz-Gutiérrez, M.; Espinoza-Beltrán, F.J.; Mostaghimi, J.; Contreras-García, M.E. Determination of the band gap of TiO2–Al2O3 films as a function of processing parameters. Mater. Sci. Eng. B 2010, 174, 71–73. [Google Scholar] [CrossRef]
- ISO 8245:1999; Water Quality—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). International Organization for Standardization: Geneva, Switzerland, 1999.
- NOM-210-SSA1-2014, DOF. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5398468&fecha=26/06/2015#gsc.tab=0 (accessed on 28 September 2022).
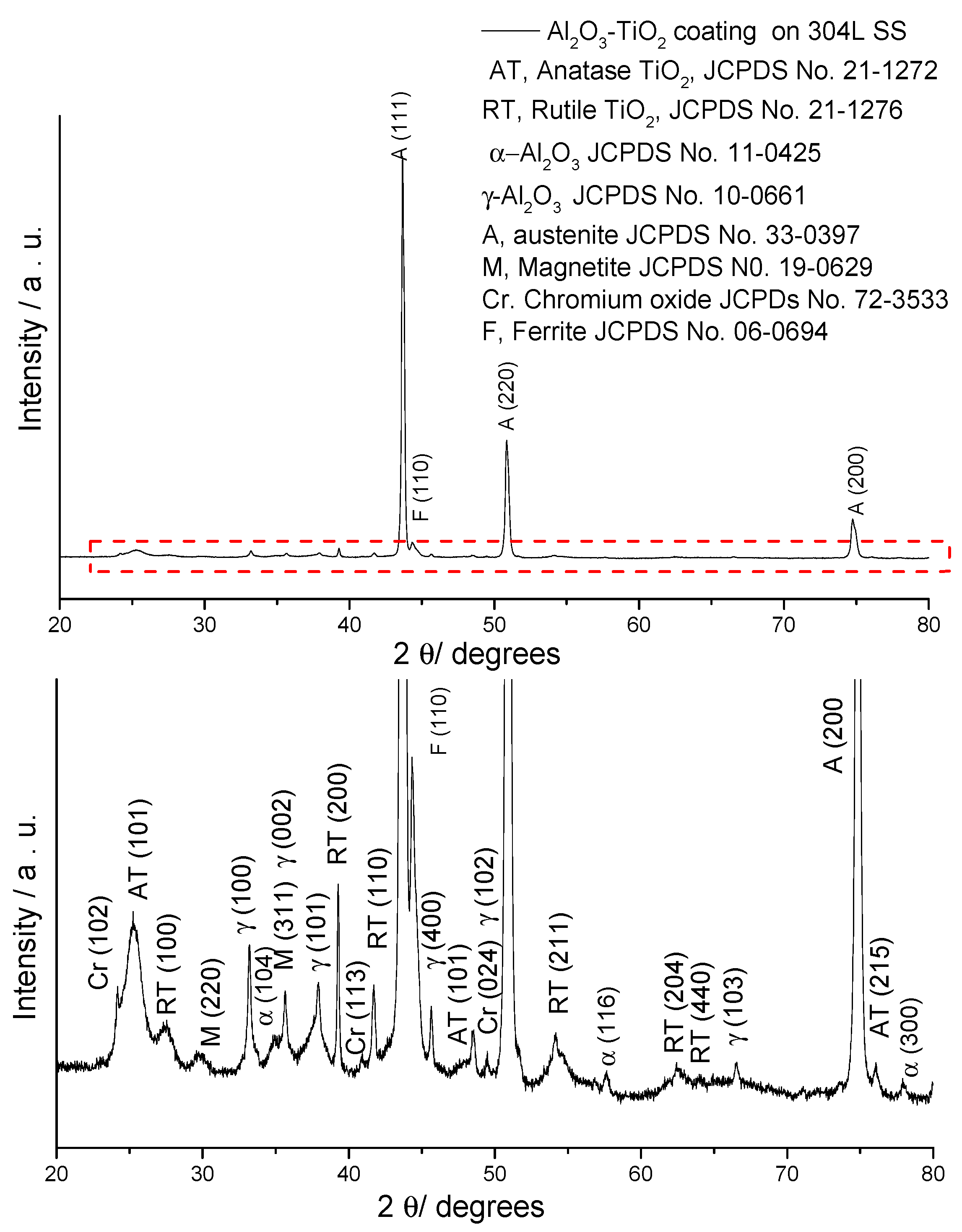
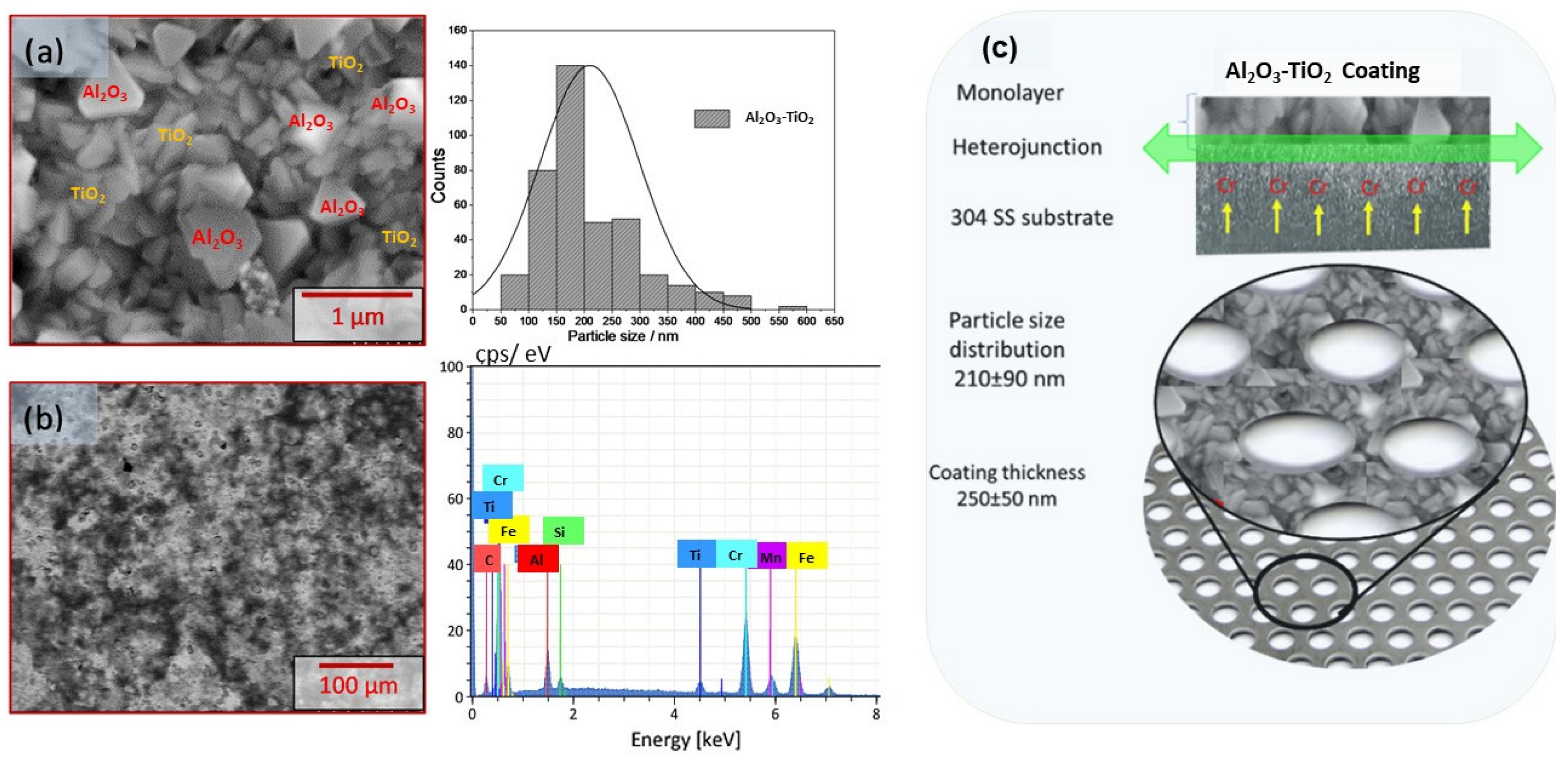
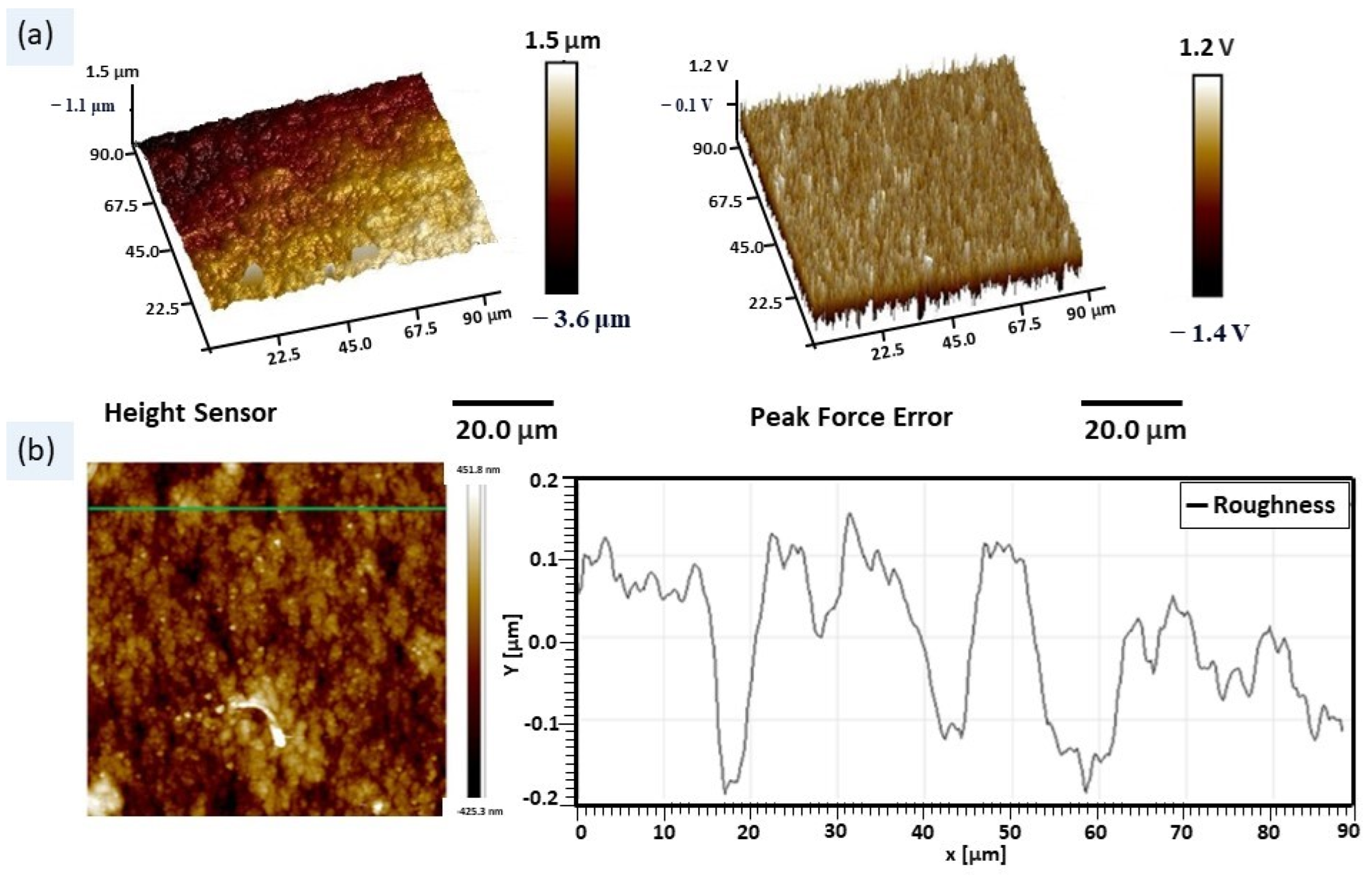
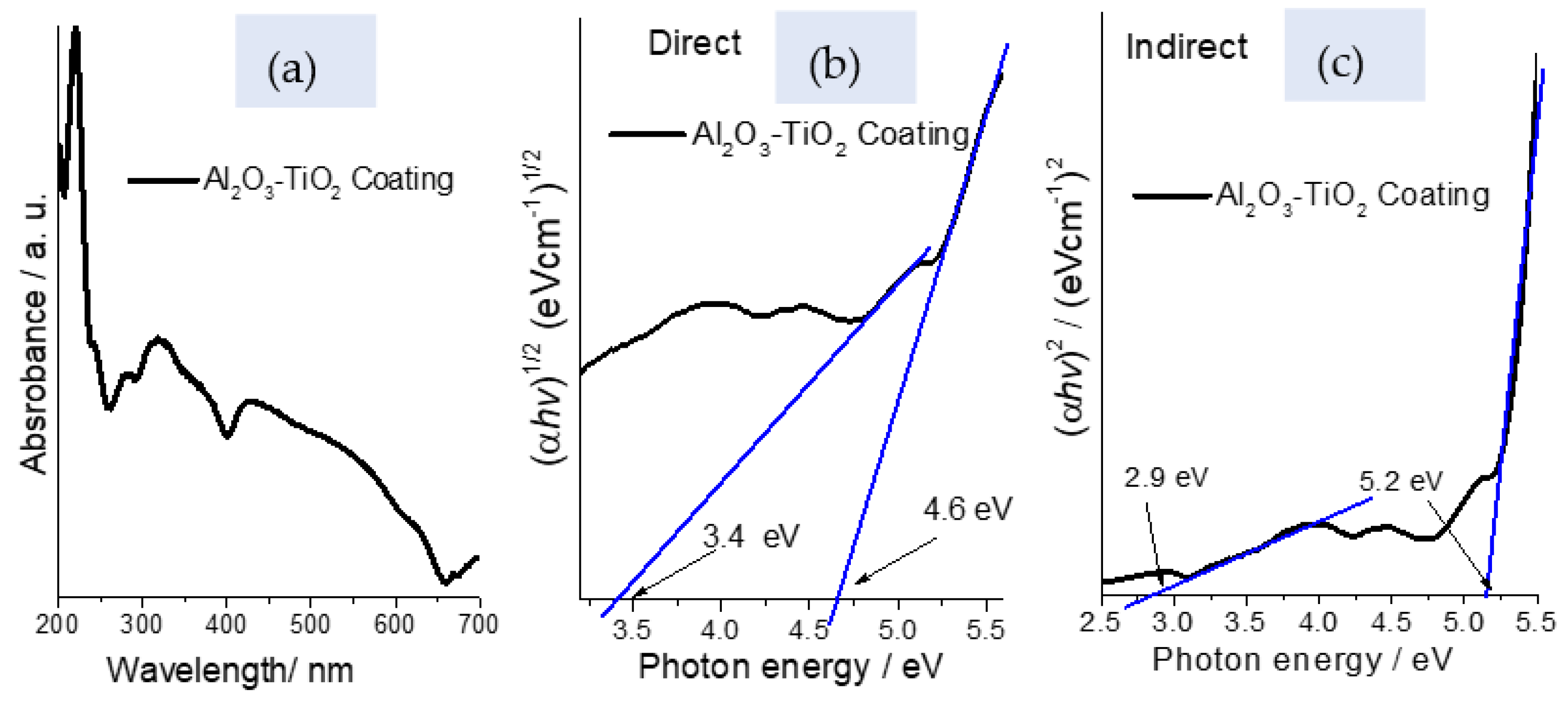


| Phases | Quantity (%) | Lattice Constants (nm) | ||
|---|---|---|---|---|
| a | b | c | ||
| α-Al2O3 | 7.4 | 0.4748 | - | 1.294 |
| γ-Al2O3 | 3.0 | 0.8017 | - | - |
| Anatase-TiO2 | 6.9 | 0.3801 | - | 0.9595 |
| Rutile-TiO2 | 3.7 | 0.4620 | - | 0.2958 |
| Fe Austenite | 72 | 0.35901 | - | - |
| Ferrite | 4 | 0.558 | 1.465 | 0.542 |
| Magnetite | 1 | 0.8353 | - | - |
| Cr2O3 | 2 | - | 0.5009 | 1.3734 |
| Photocatalyst | Conditions of Photocatalysis | Degradation Efficiency (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Doping | Presentation | Loading | Pollutant | Concentration | Light Source | Time (h) | Volume (L) | Ref. | |
| TiO2 | -- | Spherical, 70 nm | Films (glass substrate) --- | MB | 1 × 10−6 M | 200 W Tungsten filament bulb. UV light (λ = 386 nm) | 4 | 0.015 | 92.00 | [62] |
| CuNTiO2 | 1:1:1 atomic ratio | Spherical, ~20 nm. | 0.1 g | MB | 3 × 10−5 M | 500 W Xenon lamp (upon blocking UV light by a 400 nm glass filter) | 6 | 0.5 | 91.00 | [63] |
| CuO/TiO2 | 1% wt | nanorods | 0.05 g | MB | 1.8 to 18 mg L−1 | 386-LED or 450-LED light emitting diode | 2 | 0.10 | 92.00 | [60] |
| Ni_TiO2 | 0.5 Ni/Ti | Spherical, 50 nm | 0.24 gL−1 | MB | 3.2 mg L−1 | Osram 1000 W Xenon short arc display/optic lamp, XBO, | 4 | 0.20 | 99.00 | [64] |
| Ni_TiO2 | 0.75 Ni/Ti | Nanosheets 90–100 nm, thickness: 15–20 nm | 0.03 g | MB | 3 mg L−1 | 500 W power Xenon lamp (LSXS500) with a visible cut-off filter (l < 400 nm) | 3 | 0.03 | 96.00 | [65] |
| N_TiO2 | 1.6 N/Ti | Spherical, 10 nm | 0.25 g | Phe | 25 mg L−1 | luminous intensity 15.54 mWcm−2 (400 < λ < 650 nm) | 10 | 0.10 | 81.00 | [66] |
| TiO2-Fe2O3 | 1:1 | porous diameter < 1 µm | Coating (glass substrates) | MB | 3 mg L−1 | 150 W, halogen lamp | 10 | 0.003 | 67.00 | [48] |
| TiO2-Fe2O3 | 5 Fe/Ti | Granular, 9–17 nm | 0.4 g | MB | 1 × 10−5 M | 100 W Halogen Lamp, 100 PAR30/FL 240 V (350 < λ < 1050 nm) INTENSITY: 85 Mw cm−2 | 2 | 0.02 | 87.00 | [67] |
| Al2O3-TiO2 | 0.1 Al/Ti | Granular, 10–20 nm | 0.025 g | Rh(B) | 2.0 × 10−5 M | 500 W halogen lamp, cut-off filter (λ < 450 nm) | 0.16 | 0.05 | 89.25 | [68] |
| Al2O3-TiO2 | 1.5 Al/Ti | Tetrahedral, and rice-like, 210 ± 90 nm | Coating (304SS substrates) | MB | 6 mg L−1 | 4 LED lamps (4.5 W, 400 Lumens, MR16 Philips) 410 < λ < 760 nm | 6 | 0.98 | 97.43 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camacho-González, M.A.; Lijanova, I.V.; Reyes-Miranda, J.; Sarmiento-Bustos, E.; Quezada-Cruz, M.; Vera-Serna, P.; Barrón-Meza, M.Á.; Garrido-Hernández, A. High Photocatalytic Efficiency of Al2O3-TiO2 Coatings on 304 Stainless Steel for Methylene Blue and Wastewater Degradation. Catalysts 2023, 13, 1351. https://doi.org/10.3390/catal13101351
Camacho-González MA, Lijanova IV, Reyes-Miranda J, Sarmiento-Bustos E, Quezada-Cruz M, Vera-Serna P, Barrón-Meza MÁ, Garrido-Hernández A. High Photocatalytic Efficiency of Al2O3-TiO2 Coatings on 304 Stainless Steel for Methylene Blue and Wastewater Degradation. Catalysts. 2023; 13(10):1351. https://doi.org/10.3390/catal13101351
Chicago/Turabian StyleCamacho-González, Mónica Araceli, Irina Victorovna Lijanova, Joan Reyes-Miranda, Estela Sarmiento-Bustos, Maribel Quezada-Cruz, Pedro Vera-Serna, Miguel Ángel Barrón-Meza, and Aristeo Garrido-Hernández. 2023. "High Photocatalytic Efficiency of Al2O3-TiO2 Coatings on 304 Stainless Steel for Methylene Blue and Wastewater Degradation" Catalysts 13, no. 10: 1351. https://doi.org/10.3390/catal13101351









