A Novel Organic/Inorganic Dual Z-Scheme Photocatalyst with Visible-Light Response for Organic Pollutants Degradation
Abstract
:1. Introduction
2. Results and Discussion
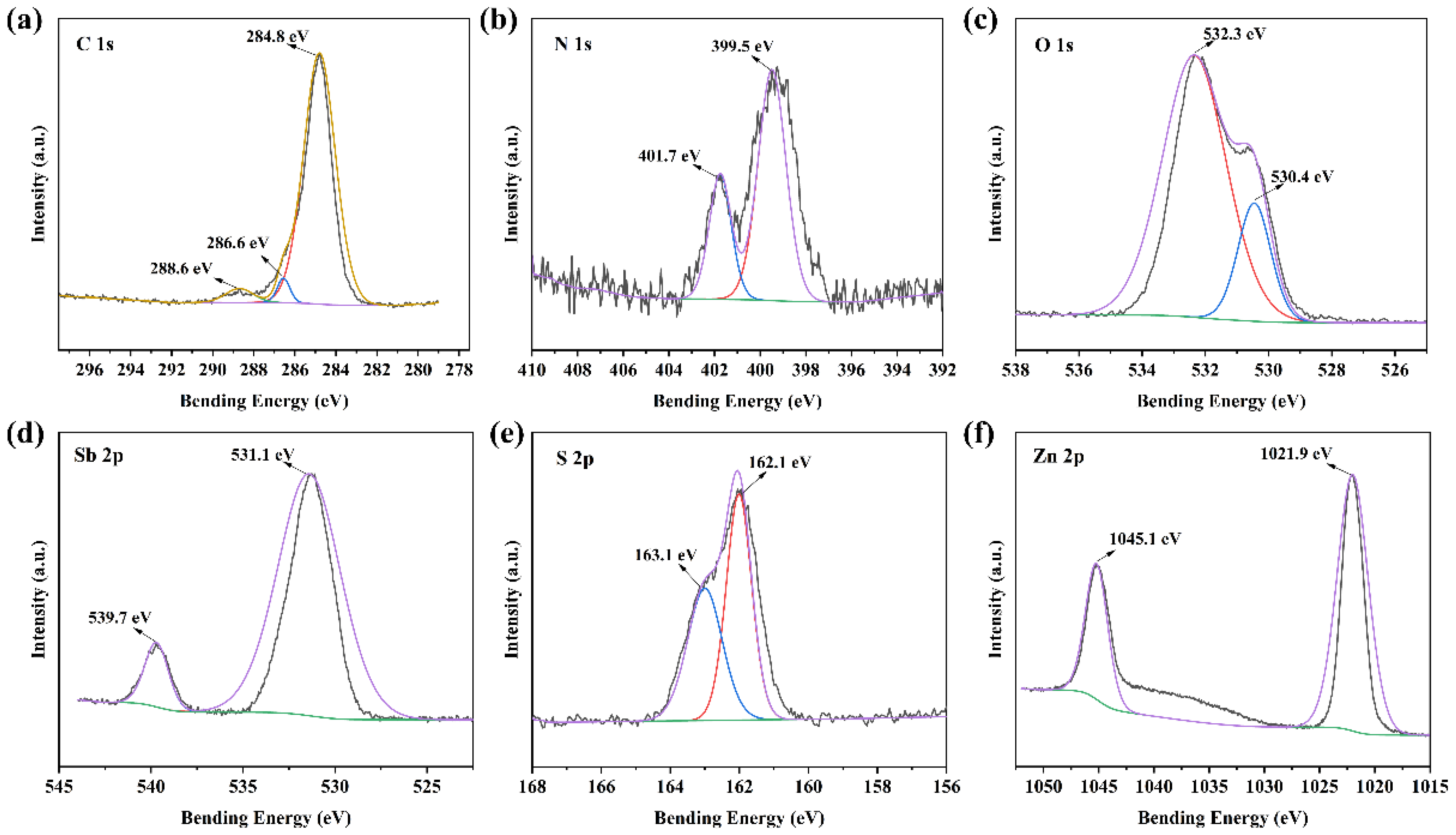
2.1. The Structure of the Composite Membranes
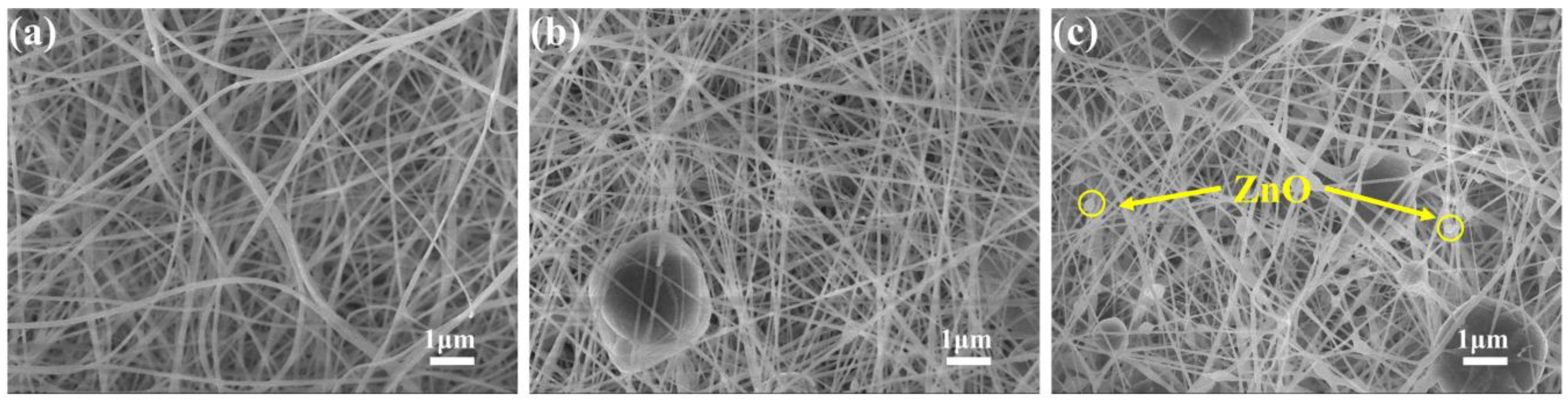
2.2. The Morphologies of the Composite Membrane
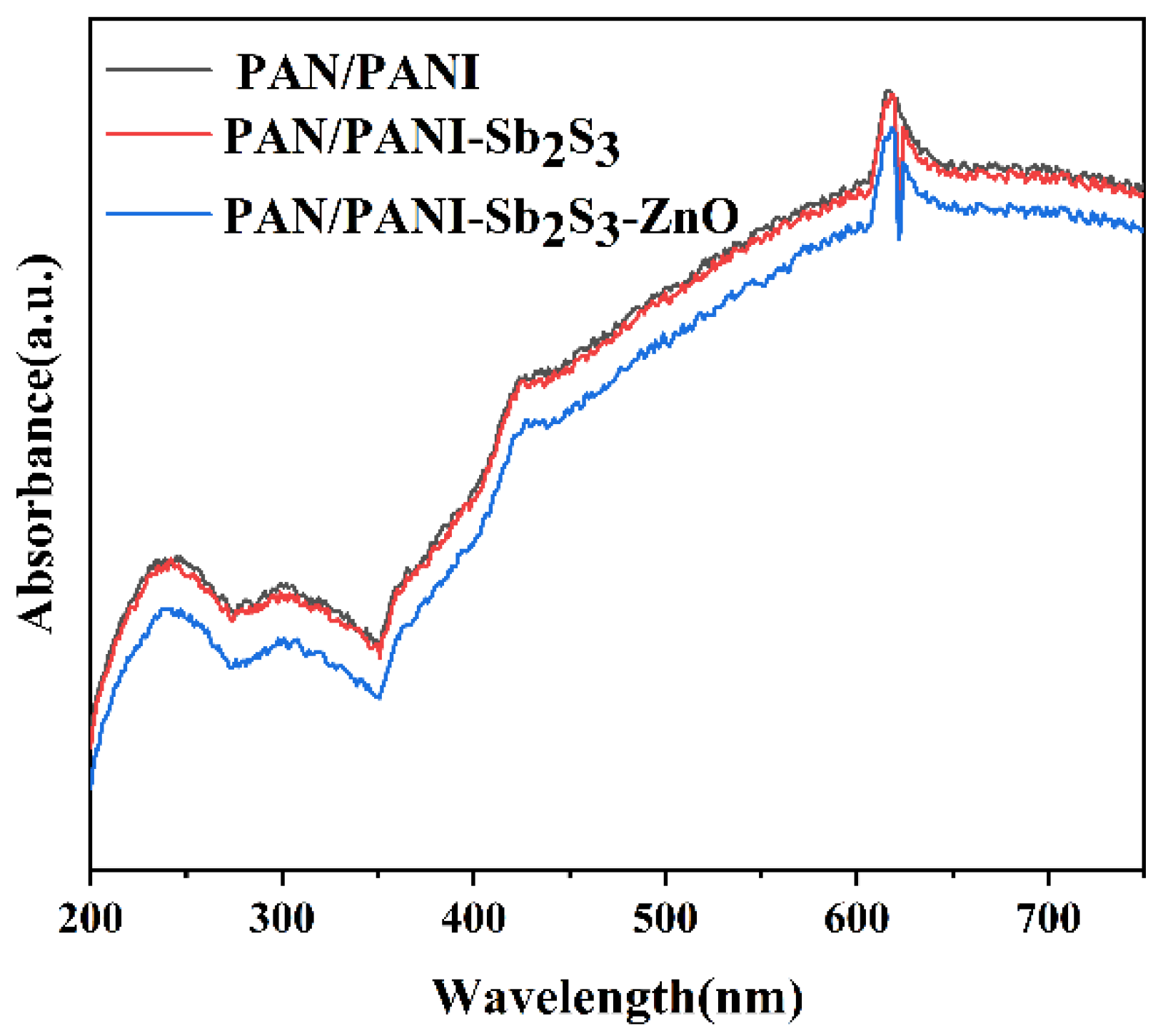
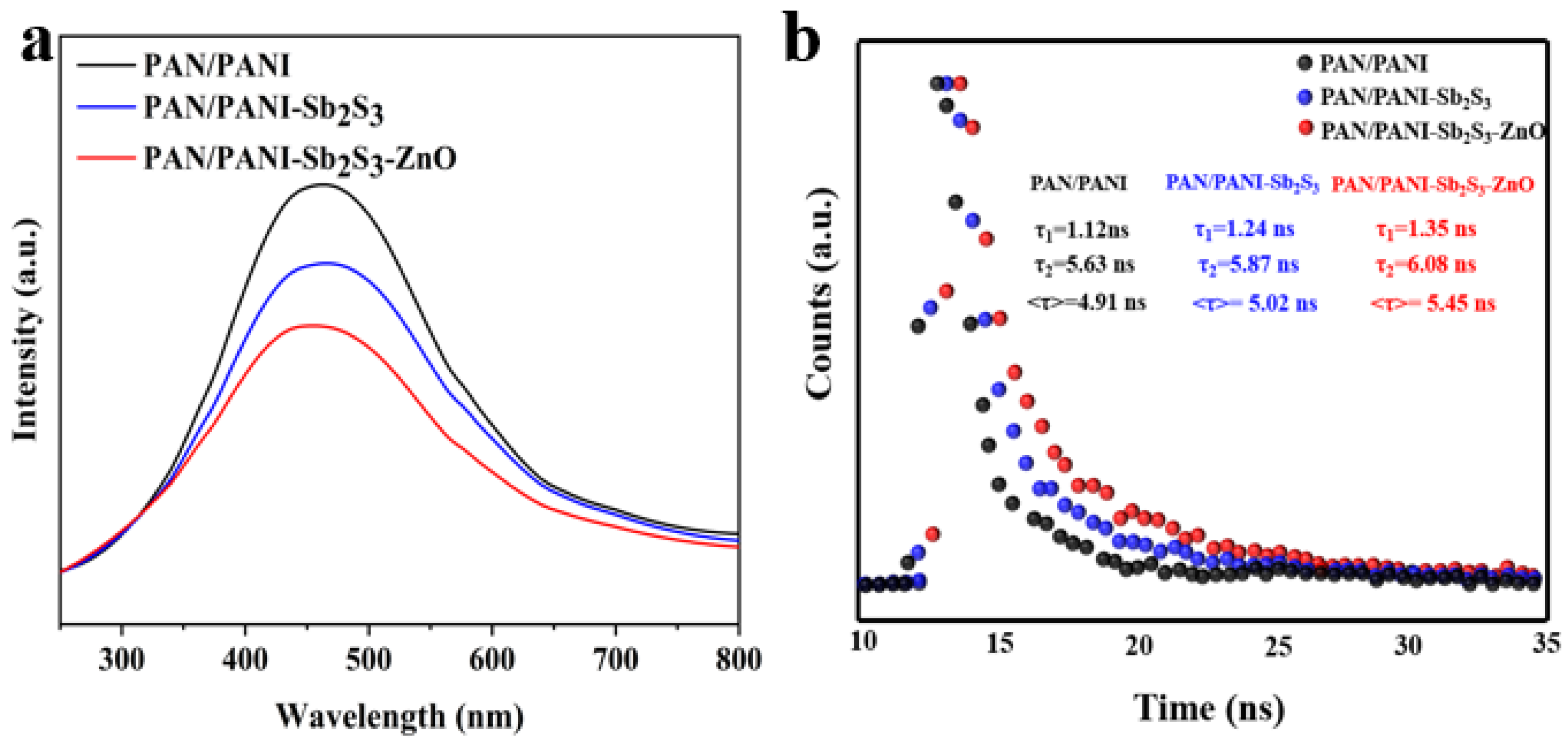
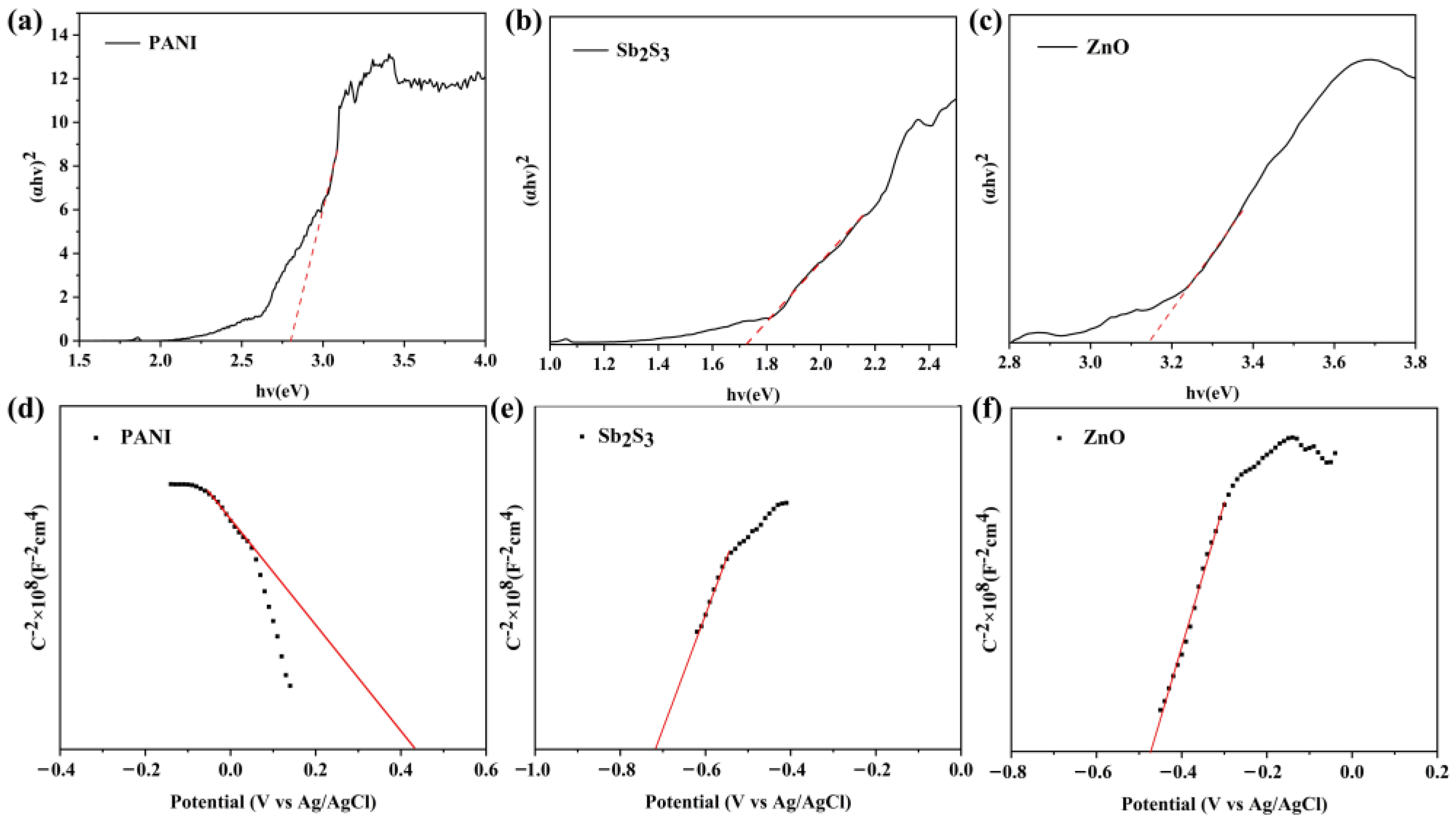
2.3. The Optical and Electrochemical Properties of Composite Membranes
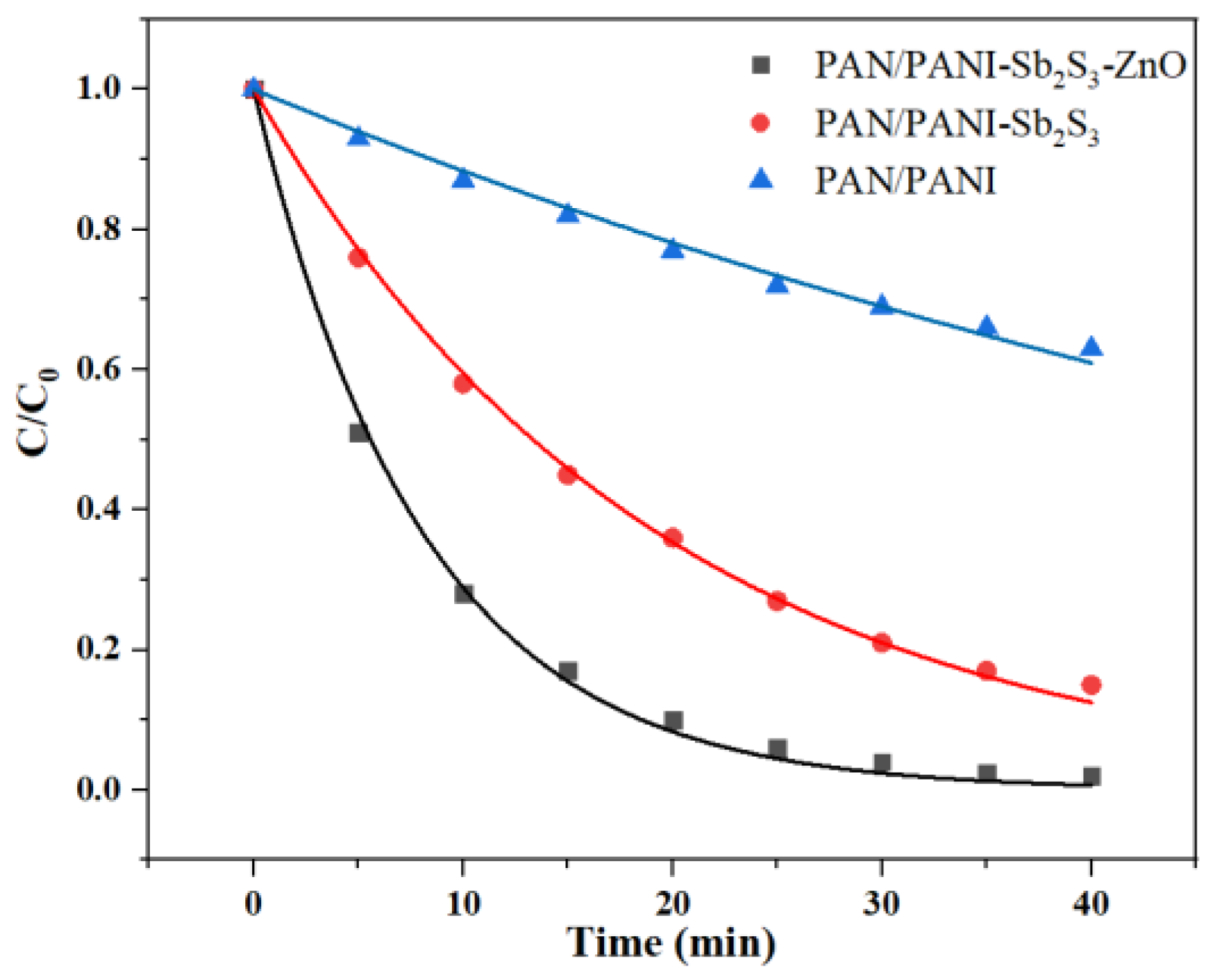
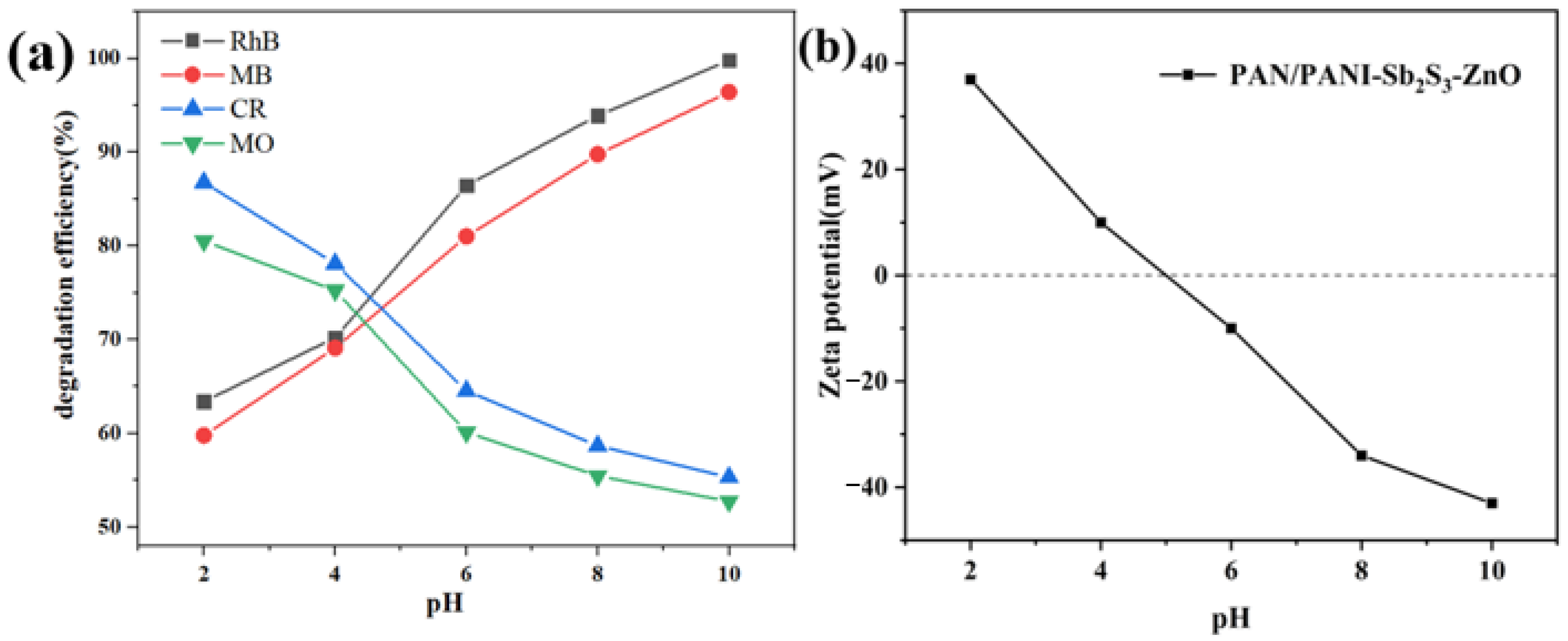
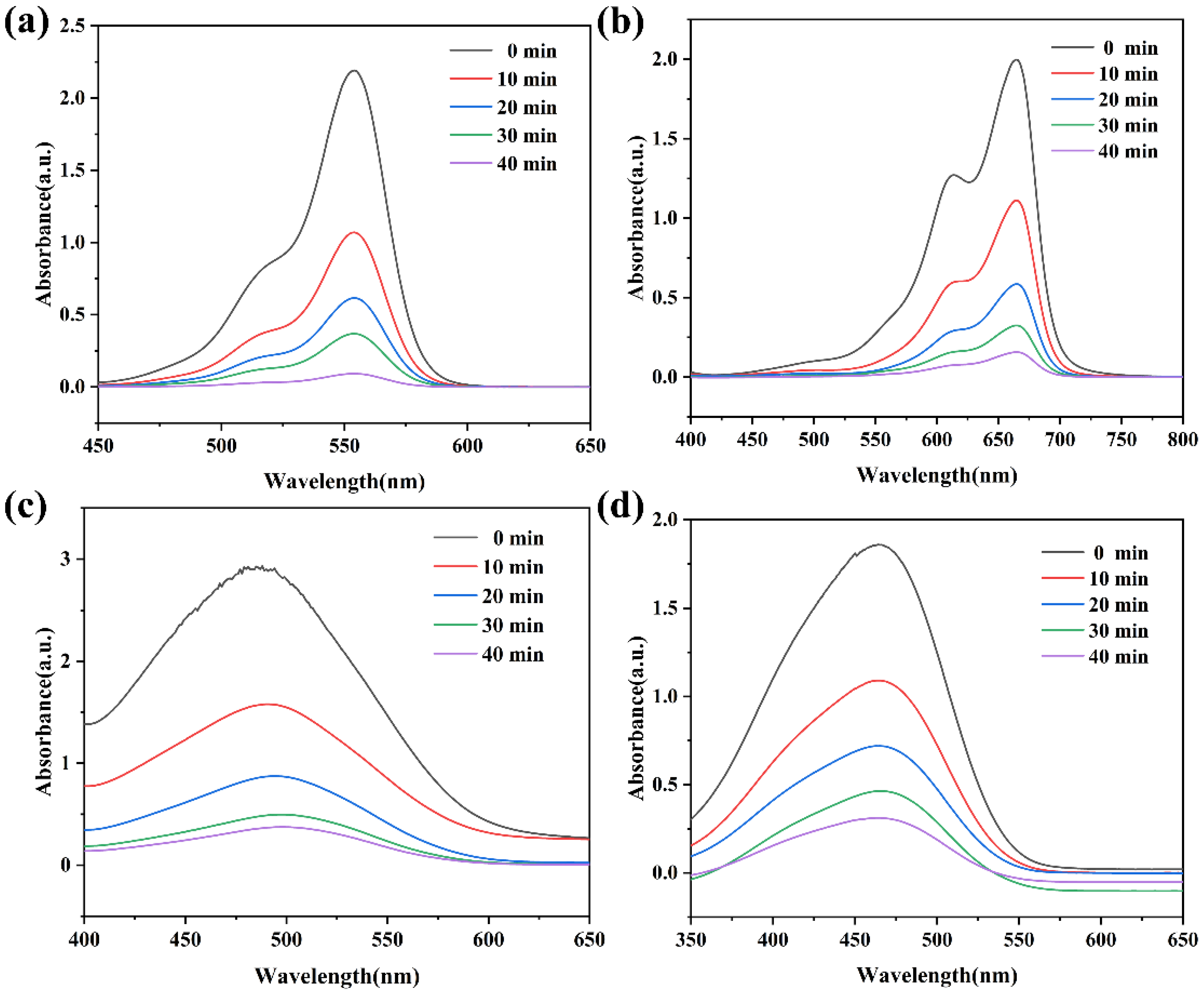
2.4. Evaluation of Removal Performance of Organic Dyes
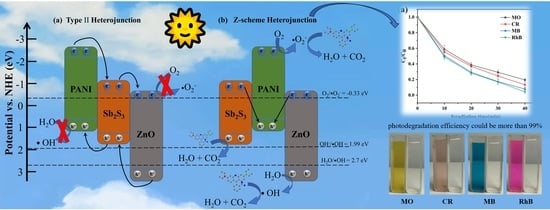
2.5. The Photocatalytic Mechanism of PAN/PANI−Sb2S3−ZnO Membrane
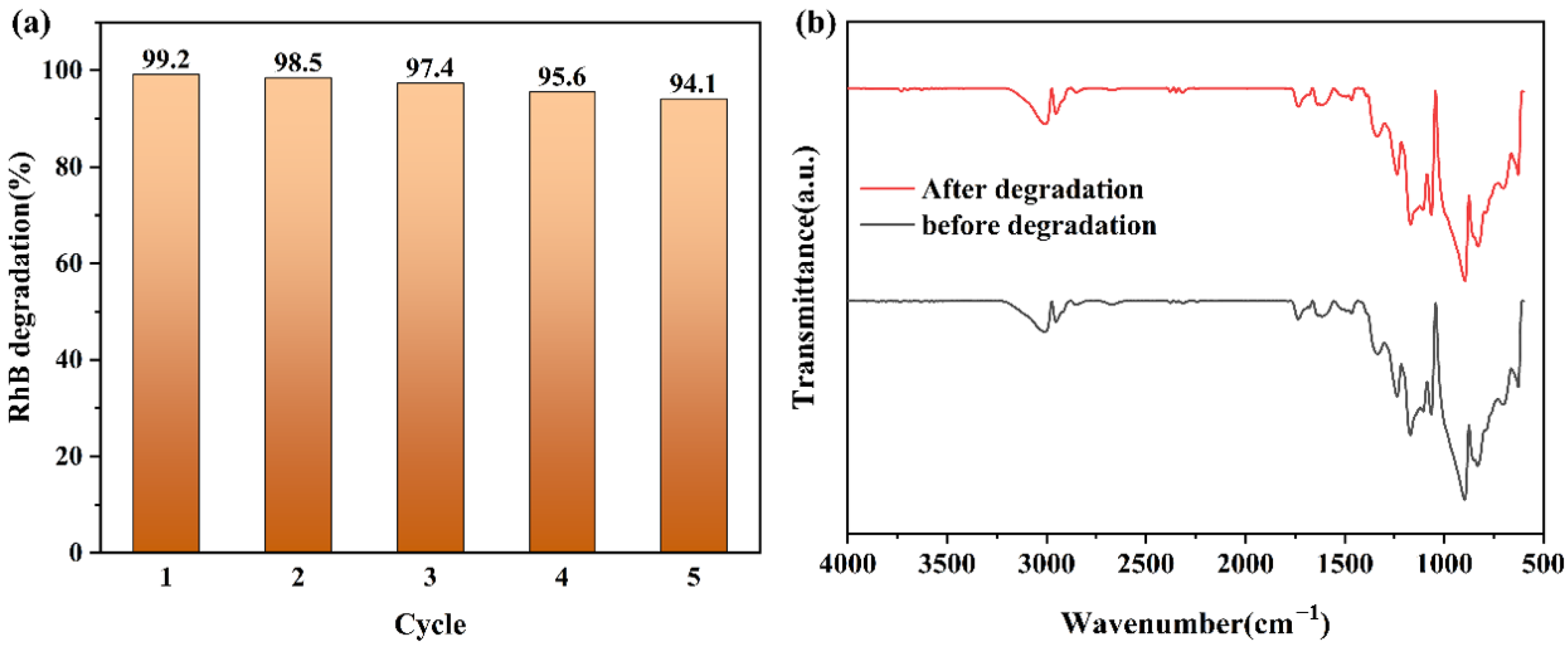
2.6. Reusability and Stability of PAN/PANI–Sb2S3–ZnO
3. Experimental
3.1. Materials
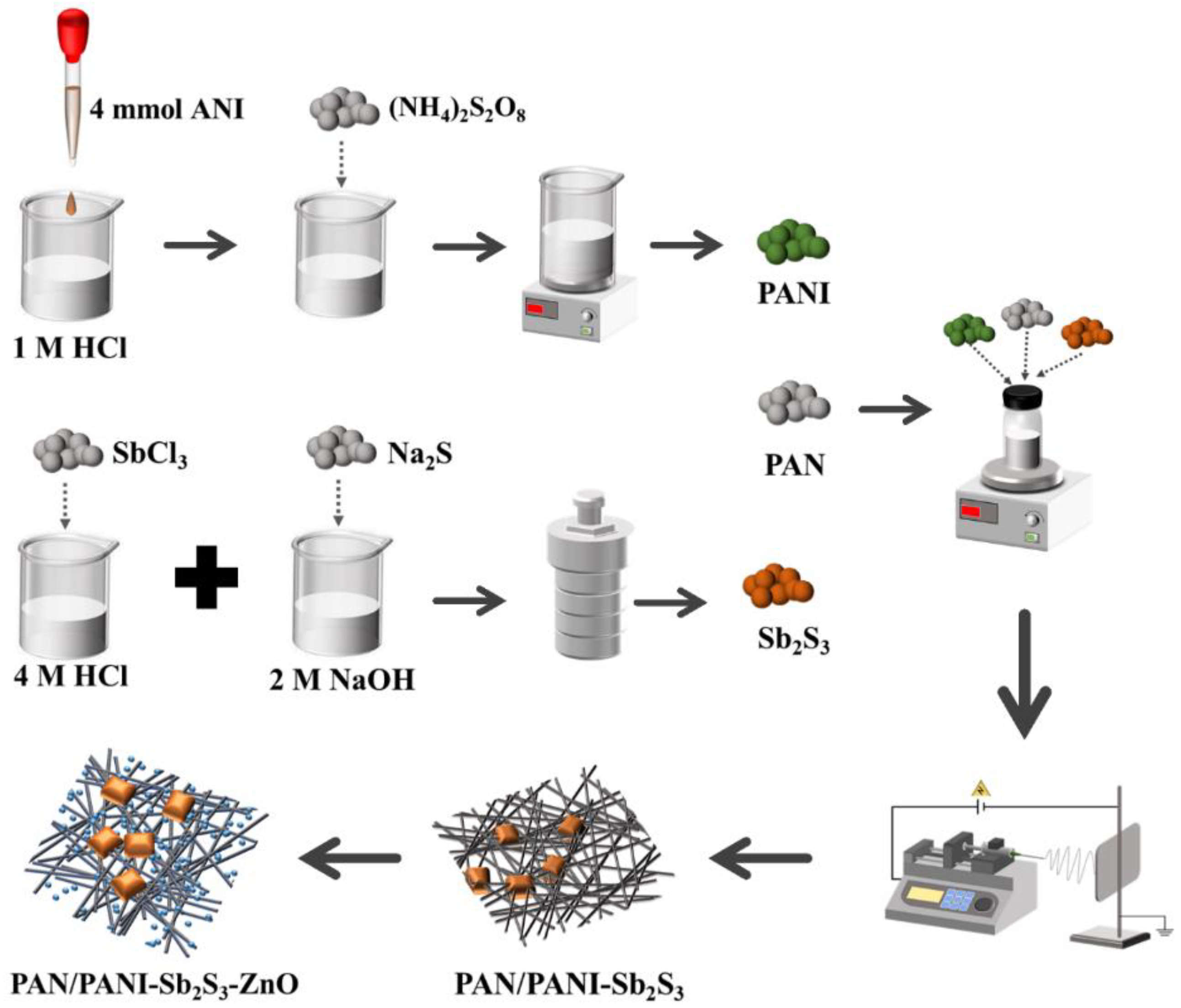
3.2. The Preparation of Composite Membranes
3.2.1. The Preparation of PANI
3.2.2. The Preparation of Sb2S3
3.2.3. The Preparation of PAN/PANI-Sb2S3 Fiber Membrane
3.2.4. The Preparation of PAN/PANI–Sb2S3–ZnO Membrane
3.3. Characterizations
3.4. Photocatalytic Degradation of Dyes
3.5. Kinetic Model Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.; Xiao, G.Q.; He, Y.; Wu, J.C.; Shi, H.; Yin, X.Y.; He, T.; Li, Z.Y.; Chen, J.Y. Multi-Functional Composite Membrane with Strong Photocatalysis to Effectively Separate Emulsified-Oil/Dyes from Complex Oily Sewage. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 643, 13. [Google Scholar] [CrossRef]
- Du, F.Q.; Yang, D.M.; Kang, T.X.; Ren, Y.X.; Hu, P.; Song, J.M.; Teng, F.; Fan, H.B. SiO2/Ga2O3 Nanocomposite for Highly Efficient Selective Removal of Cationic Organic Pollutant Via Synergistic Electrostatic Adsorption and Photocatalysis. Sep. Purif. Technol. 2022, 295, 10. [Google Scholar] [CrossRef]
- Cionti, C.; Pargoletti, E.; Falletta, E.; Bianchi, C.L.; Meroni, D.; Cappelletti, G. Combining Ph-Triggered Adsorption and Photocatalysis for the Remediation of Complex Water Matrices. J. Environ. Chem. Eng. 2022, 10, 9. [Google Scholar] [CrossRef]
- Huang, X.F.; Xu, X.Y.; Yang, R.Y.; Fu, X.H. Synergetic adsorption and photocatalysis performance of g-C3N4/Ce-doped MgAl-LDH in degradation of organic dye under LED visible light. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 643, 13. [Google Scholar] [CrossRef]
- Lan, F.; Liu, C.S.; Zhou, C.; Huang, X.Z.; Wu, J.Y.; Zhang, X. Developing highly reducing conjugated porous polymer: A metal-free and recyclable approach with superior performance for pinacol C-C coupling under visible light. J. Mater. Chem. A 2022, 10, 16578–16584. [Google Scholar] [CrossRef]
- Yuan, X.J.; Remita, H. Conjugated Polymer Polypyrrole Nanostructures: Synthesis and Photocatalytic Applications. Top. Curr. Chem. 2022, 380, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, Y.; Zhang, Y.X.; Fan, L.; Li, Q.Y.; Yu, Z.Y.; Zhao, X.S.; Zheng, Y.C.; Wang, X.J. Molecular engineering of covalent triazine frameworks for highly enhanced photocatalytic aerobic oxidation of sulfides. J. Mater. Chem. A 2022, 10, 12489–12496. [Google Scholar] [CrossRef]
- Li, Z.L.; Fang, H.; Chen, Z.P.; Zou, W.X.; Zhao, C.X.; Yang, X.F. Regulating donor-acceptor interactions in triazine-based conjugated polymers for boosted photocatalytic hydrogen production. Appl. Catal. B-Environ. 2022, 312, 9. [Google Scholar] [CrossRef]
- Kumar, H.; Luthra, M.; Punia, M.; Singh, D. Ag2O@PANI nanocomposites for advanced functional applications: A sustainable experimental and theoretical approach. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 640, 12. [Google Scholar] [CrossRef]
- Celebi, N.; Soysal, F.; Salimi, K. Dual-functional PANI/MIL-125(Ti) photoanodes for enhanced photoelectrochemical H-2 generation and photothermal heating. Mater. Chem. Phys. 2022, 291, 9. [Google Scholar] [CrossRef]
- Lin, A.J.; Ren, M.; Tan, X.; Ma, J.; Zhang, Y.; Yang, T.X.; Pei, Y.S.; Cui, J. Visible-light-driven melamine foam/PANI/N,O,P containing covalent organic polymer for achieving H2O2 production and boosting Fe(II)/Fe(III) cycle. J. Clean. Prod. 2022, 345, 12. [Google Scholar] [CrossRef]
- Sboui, M.; Niu, W.K.; Li, D.Z.; Lu, G.; Zhou, N.; Zhang, K.; Pan, J.H. Fabrication of electrically conductive TiO2/PANI/PVDF composite membranes for simultaneous photoelectrocatalysis and microfiltration of azo dye from wastewater. Appl. Catal. A-Gen. 2022, 644, 10. [Google Scholar] [CrossRef]
- Xu, D.; Huang, Y.; Ma, Q.; Qiao, J.Z.; Guo, X.; Wu, Y.Q. A 3D porous structured cellulose nanofibrils-based hydrogel with carbon dots-enhanced synergetic effects of adsorption and photocatalysis for effective Cr(VI) removal. Chem. Eng. J. 2023, 456, 11. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Wang, Z.P.; Lou, Y.; Pan, C.S.; Zhu, Y.F. Unprecedentedly efficient mineralization performance of photocatalysis-self-Fenton system towards organic pollutants over oxygen-doped porous g-C3N4 nanosheets. Appl. Catal. B-Environ. 2022, 312, 9. [Google Scholar] [CrossRef]
- Chen, Q.S.; Zhou, H.Q.; Wang, J.C.; Bi, J.H.; Dong, F. Activating earth-abundant insulator BaSO4 for visible-light induced degradation of tetracycline. Appl. Catal. B-Environ. 2022, 307, 12. [Google Scholar] [CrossRef]
- Xia, L.H.; Sun, Z.L.; Wu, Y.N.; Yu, X.F.; Cheng, J.B.; Zhang, K.S.; Sarina, S.; Zhu, H.Y.; Weerathunga, H.; Zhang, L.X.; et al. Leveraging doping and defect engineering to modulate exciton dissociation in graphitic carbon nitride for photocatalytic elimination of marine oil spill. Chem. Eng. J. 2022, 439, 15. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Zhu, X.W.; Yang, J.M.; Zhong, K.; Jiang, Z.F.; Yu, Q.; Song, Y.H.; Hua, Y.J.; Li, H.M.; Xu, H. Inherent Facet-Dominant effect for cobalt oxide nanosheets to enhance photocatalytic CO2 reduction. Appl. Surf. Sci. 2022, 578, 9. [Google Scholar] [CrossRef]
- Chen, Y.N.; Yang, L.; Sun, Y.N.; Guan, R.Q.; Liu, D.; Zhao, J.; Shang, Q.K. A high-performance composite CDs@Cu-HQCA/TiO2 flower photocatalyst: Synergy of complex-sensitization, TiO2-morphology control and carbon dot-surface modification. Chem. Eng. J. 2022, 436, 17. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhang, T.; Jia, J.; Lin, G.; Li, K.K.; Zheng, L.S.; Li, X.; Kong, Z.H. Construction of Zn0.2Cd0.8S/g-C3N4 nanosheet array heterojunctions toward enhanced photocatalytic reduction of CO2 in visible light. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 655, 10. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, X.K.; Liu, K.; Zhong, W.L.; Zhang, Y.J.; Xing, J.J. Synthesis of silica nanofibers-supported BiOCl/TiO2 heterojunction composites with enhanced visible-light photocatalytic performance. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 652, 11. [Google Scholar] [CrossRef]
- Yang, H.; Hao, H.S.; Zhao, Y.R.; Hu, Y.T.; Min, J.K.; Zhang, G.L.; Bi, J.R.; Yan, S.; Hou, H.M. An efficient construction method of S-scheme Ag2CrO4/ZnFe2O4 nanofibers heterojunction toward enhanced photocatalytic and antibacterial activity. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 641, 10. [Google Scholar] [CrossRef]
- Xu, F.; Chai, B.; Liu, Y.Y.; Liu, Y.L.; Fan, G.Z.; Song, G.S. Superior photo-Fenton activity toward tetracycline degradation by 2D?-Fe2O3 anchored on 2D g-C3N4: S-scheme heterojunction mechanism and accelerated Fe3+/Fe2+ cycle. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 652, 14. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.C.; Liu, J. In situ synthesis and enhanced visible light photocatalytic activities of novel PANI-g-C3N4 composite photocatalysts. J. Mater. Chem. 2012, 22, 11843–11850. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Hui, J.; Li, J.J.; Cai, Y.X.; Yin, S.Q.; Wang, F.P.; Su, B.T. Photodegradation of methyl orange with PANI-modified BiOCl photocatalyst under visible light irradiation. Appl. Surf. Sci. 2013, 283, 577–583. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Cheng, Y.F.; Zhao, J.F.; Zhang, W.W.; Gao, J.H.; Miao, H.; Hu, X.Y. Construction of Sb2S3/CdS/CdIn2S4 cascaded S-scheme heterojunction for improving photoelectrochemical performance. J. Colloid Interface Sci. 2022, 627, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, C.L.; Wang, M.S.; Ma, X.G. Photocatalytic hydrogen evolution reaction with high solar-to-hydrogen efficiency driven by the Sb2S3 monolayer and RuI2/Sb2S3 heterostructure with solar light. J. Power Sources 2022, 532, 10. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Gong, M.; Xu, T.T.; Liu, E.Z.; Fan, J.; Miao, H.; Hu, X.Y. Epitaxial Grown Sb2Se3@Sb2S3 Core-Shell Nanorod Radial-Axial Hierarchical Heterostructure with Enhanced Photoelectrochemical Water Splitting Performance. ACS Appl. Mater. Interfaces 2022, 14, 23785–23796. [Google Scholar] [CrossRef]
- Xu, W.; Xu, L.H.; Pan, H.; Wang, L.M.; Shen, Y. Superamphiphobic cotton fabric with photocatalysis and ultraviolet shielding property based on hierarchical ZnO/halloysite nanotubes hybrid particles. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 654, 11. [Google Scholar] [CrossRef]
- Meenakshi, G.; Sivasamy, A. Enhanced photocatalytic activities of CeO2@ZnO core-shell nanostar particles through delayed electron hole recombination process. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 645, 13. [Google Scholar] [CrossRef]
- Ramos, P.G.; Sanchez, L.A.; Rodriguez, J.M. A review on improving the efficiency of photocatalytic water decontamination using ZnO nanorods. J. Sol-Gel Sci. Technol. 2022, 102, 105–124. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, N.; Jiang, C.; Xu, C.; Yu, S.; Liang, P.; Zhang, X.; Liang, S.; Huang, X. Filtration-enhanced highly efficient photocatalytic degradation with a novel electrospun rGO@TiO2 nanofibrous membrane: Implication for improving photocatalytic efficiency. Appl. Catal. B Environ. 2020, 268, 118737. [Google Scholar] [CrossRef]
- Selvinsimpson, S.; Gnanamozhi, P.; Pandiyan, V.; Govindasamy, M.; Habila, M.A.; AlMasoud, N.; Chen, Y. Synergetic effect of Sn doped ZnO nanoparticles synthesized via ultrasonication technique and its photocatalytic and antibacterial activity. Environ. Res. 2021, 197, 111115. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Zhao, P.; Wu, C.D.; Zhuang, Y.; Liu, J.M.; Li, W.H.; Liu, Z.; Liu, J.H. Electrospun PAN/PANI fiber film with abundant active sites for ultrasensitive trimethylamine detection. Sens. Actuator B-Chem. 2021, 338, 9. [Google Scholar] [CrossRef]
- Dashairya, L.; Sharma, M.; Basu, S.; Saha, P. Enhanced dye degradation using hydrothermally synthesized nanostructured Sb2S3/rGO under visible light irradiation. J. Alloys Compd. 2018, 735, 234–245. [Google Scholar] [CrossRef]
- Lopez-Lopez, J.; Tejeda-Ochoa, A.; Lopez-Beltran, A.; Herrera-Ramirez, J.; Mendez-Herrera, P. Sunlight Photocatalytic Performance of ZnO Nanoparticles Synthesized by Green Chemistry Using Different Botanical Extracts and Zinc Acetate as a Precursor. Molecules 2022, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Y.; Zhang, M.Y.; Feng, J.; Wei, T.; Ren, Y.M.; Ma, J. Electrostatic self-assembled layered polymers form supramolecular heterojunction catalyst for photocatalytic reduction of high-stability nitrate in water. J. Colloid Interface Sci. 2022, 622, 828–839. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; Eltaweil, A.S. Green synthesis of bimetallic Ag/ZnO@Biohar nanocomposite for photocatalytic degradation of tetracycline, antibacterial and antioxidant activities. Sci. Rep. 2022, 12, 17. [Google Scholar] [CrossRef]
- Zhang, H.L.; Hu, C.G.; Ding, Y.; Lin, Y. Synthesis of 1D Sb2S3 nanostructures and its application in visible-light-driven photodegradation for MO. J. Alloys Compd. 2015, 625, 90–94. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; ul Haq, A.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 12. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Jin, J.; Liu, J.; Li, Y.; Wu, M.; Chen, L.H.; Wang, B.J.; Yang, X.Y.; Su, B.L. Probing effective photocorrosion inhibition and highly improved photocatalytic hydrogen production on monodisperse PANI@CdS core-shell nanospheres. Appl. Catal. B-Environ. 2016, 188, 351–359. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.L.; Hu, C.; Xing, X.C.; Yan, B.; Gao, Y.W.; Zhou, L. Enhanced azo dye decolorization through charge transmission by sigma-Sb3+-azo complexes on amorphous Sb2S3 under visible light irradiation. Appl. Catal. B-Environ. 2019, 240, 132–140. [Google Scholar] [CrossRef]
- Sun, J.X.; Yuan, Y.P.; Qiu, L.G.; Jiang, X.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, J.Q.; Yu, X.; Fu, X.H.; Zhu, Y.; Zhang, Y.M. Enhanced removal of organic pollutant by separable and recyclable rGH-PANI/BiOI photocatalyst via the synergism of adsorption and photocatalytic degradation under visible light. J. Mater. Sci. Technol. 2021, 77, 19–27. [Google Scholar] [CrossRef]
- Dou, W.Y.; Hu, X.Y.; Kong, L.H.; Peng, X.J. Photo-induced dissolution of Bi2O3 during photocatalysis reactions: Mechanisms and inhibition method. J. Hazard. Mater. 2021, 412, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Wang, Z.; Wang, X.R.; Yang, G.H.; Liu, Y. Adsorption-photocatalysis performance of polyaniline/dicarboxyl acid cellulose@graphene oxide for dye removal. Int. J. Biol. Macromol. 2021, 182, 492–501. [Google Scholar] [CrossRef]
- Mallappa, M.; Nagaraju, K.; Shivaraj, Y. Enhanced Photocatalytic degradation of methylene blue dye using CuS-CdS nanocomposite under visible light irradiation. Appl. Surf. Sci. 2019, 475, 828–838. [Google Scholar] [CrossRef]
- Dogar, S.; Nayab, S.; Farooq, M.Q.; Said, A.; Kamran, R.; Duran, H.; Yameen, B. Utilization of Biomass Fly Ash for Improving Quality of Organic Dye-Contaminated Water. ACS Omega 2020, 5, 15850–15864. [Google Scholar] [CrossRef]
- Liang, C.; Cui, M.Y.; Zhao, W.; Dong, L.Y.; Ma, S.S.; Liu, X.T.; Wang, D.K.; Jiang, Z.J.; Wang, F. Hybridizing electron-mediated H5PMo10V2O40 with CdS/g-C3N4 for efficient photocatalytic performance of Z-scheme heterojunction in wastewater treatment. Chemosphere 2022, 305, 12. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, Z.W.; Cai, Y.H.; Song, M.X.; Qi, K.M.; Xie, X.Y. S-scheme BiVO4/CQDs/beta-FeOOH photocatalyst for efficient degradation of ofloxacin: Reactive oxygen species transformation mechanism insight. Chemosphere 2022, 295, 13. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, T.Y.; Zhang, L.; Che, S.Y.; Liu, H.; Liu, S.X.; Wang, C.Y.; Su, D.W.; Teng, Z.Y. Highly efficient Ag2O/Na-g-C3N4 heterojunction for photocatalytic desulfurization of thiophene in fuel under ambient air conditions. Appl. Catal. B-Environ. 2022, 316, 13. [Google Scholar] [CrossRef]
- Ma, L.J.; Wu, H.Q.; Chen, B.Y.; Wang, G.; Lei, B.X.; Zhang, D.; Kuang, D.B. 0D/2D CsPbBr3 Nanocrystal/BiOCl Nanoplate Heterostructure with Enhanced Photocatalytic Performance. Adv. Mater. Interfaces 2022, 9, 9. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Hussain, N.; Silla, Y.; Das, M.R. Specific ion effect on the surface properties of Ag/reduced graphene oxide nanocomposite and its influence on photocatalytic efficiency towards azo dye degradation. Appl. Surf. Sci. 2017, 423, 752–761. [Google Scholar] [CrossRef]
- Zhou, H.; Qiu, Y.; Yang, C.; Zang, J.; Song, Z.; Yang, T.; Li, J.; Fan, Y.; Dang, F.; Wang, W. Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System. Molecules 2022, 27, 8642. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, J.; Guo, H.; Li, H.; Liu, E.; Zhao, Y.; Ji, Y.; Fan, J. Preparation of a Recyclable and High-Performance Photocatalyst Agins2/Cn/Pan for Rhb and Phenol Degradation. J. Environ. Chem. Eng. 2023, 11, 3. [Google Scholar] [CrossRef]
- Liang, Z.; Cheng, H.; Zhang, X.; Mao, Q. Two Polyoxometalates Based on {P2mo5} Catalysts: Synthesis, Characterization, and Photocatalytic Degradation of Rhb. J. Mol. Liq. 2023, 377, 121483. [Google Scholar] [CrossRef]
- Liu, S.; Ge, Y.; Wang, C.; Li, K.; Mei, Y. Tio2/Bp/G-C3n4 Heterojunction Photocatalyst for the Enhanced Photocatalytic Degradation of Rhb. Environ. Sci. Pollut. Res. 2023, 30, 84452–84461. [Google Scholar] [CrossRef] [PubMed]
- Smok, W.; Zaborowska, M.; Tański, T.; Radoń, A. Novel In2o3/Sno2 Heterojunction 1d Nanostructure Photocatalyst for Mb Degradation. Opt. Mater. 2023, 139, 113757. [Google Scholar] [CrossRef]
- Athavale, S.; Barai, D.P.; Bhanvase, B.A.; Pandharipande, S.L. Investigation on Performance of Eu:Go:Ksrpo4 Nanocomposite for Mb Dye Photocatalytic Degradation: Experimental and Ann Modeling. Optik 2023, 275, 170561. [Google Scholar] [CrossRef]
- Rastgar, S.; Rezaei, H.; Younesi, H.; Abyar, H.; Kordrostami, A. Photocatalytic Degradation of Methylene Blue (Mb) Dye under Uv Light Irradiation by Magnetic Diesel Tank Sludge (Mdts). Biomass Convers. Biorefin. 2023; in press. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Wang, D.; Li, L.; Song, W.; Pang, X.; Liang, C. A Novel Organic/Inorganic Dual Z-Scheme Photocatalyst with Visible-Light Response for Organic Pollutants Degradation. Catalysts 2023, 13, 1391. https://doi.org/10.3390/catal13111391
Yu T, Wang D, Li L, Song W, Pang X, Liang C. A Novel Organic/Inorganic Dual Z-Scheme Photocatalyst with Visible-Light Response for Organic Pollutants Degradation. Catalysts. 2023; 13(11):1391. https://doi.org/10.3390/catal13111391
Chicago/Turabian StyleYu, Taoming, Doudou Wang, Lili Li, Wenjing Song, Xuan Pang, and Ce Liang. 2023. "A Novel Organic/Inorganic Dual Z-Scheme Photocatalyst with Visible-Light Response for Organic Pollutants Degradation" Catalysts 13, no. 11: 1391. https://doi.org/10.3390/catal13111391