S-Scheme Heterojunction Photocatalyst for Photocatalytic H2O2 Production: A Review
Abstract
:1. Introduction
2. Mechanism of Photocatalytic H2O2 Production Reaction
3. S-Scheme Heterojunctions
3.1. Mechanism of S-Scheme Heterojunctions
3.2. Characterization of S-Scheme Heterojunctions
3.3. Synthesis Method
4. H2O2 Production by S-Scheme Heterojunction Photocatalysts
4.1. Photocatalytic H2O2 Production
4.2. Water Splitting
4.3. Coupling of H2O2 Production and Organic Synthesis
4.4. Pollutant Degradation with In Situ H2O2 Production
5. Conclusions and Outlook
- Modification of the pore size, porosity and particle size of S-scheme heterojunction photocatalysts to increase their surface area, which is conducive to improving the adsorption of reactants (H2O, O2) by the photocatalysts;
- Construction of multiphase catalytic systems. At present, there are few studies on enhancing H2O2 yield by constructing multiphase S-scheme heterojunction photocatalytic systems. The disadvantage of slow gas transport kinetics of bi-phase catalysts can be avoided by constructing multiphase catalytic systems, which can promote the adsorption of O2 by solid photocatalysts and further improve the efficiency of photocatalytic reactions;
- Combining photocatalysis with electrocatalysis. S-scheme heterojunctions are used to promote the separation of photogenerated charges by using intrinsic electric fields (IEF) at the interface, and other electric fields can be superimposed to further improve their separation efficiency. The introduction of an external electric field by applying a voltage can induce surface charge redistribution of the photocatalyst and can also facilitate the adsorption and activation of O2 and H2O;
- To construct the relationship between the Fermi energy level difference and redox potential. Modulation of redox potential by controlling the Fermi energy level positions of semiconductors and constructing S-scheme heterojunctions to avoid four-electron competition reactions and improve the selectivity of H2O2 products;
- Optimize the model for theoretical calculations to pre-select semiconductors with suitable Fermi energy levels and energy band structures by theoretical calculations. Meanwhile, theoretical calculations combined with in situ characterization results can also enhance the investigation of the mechanism of photocatalytic H2O2 production and contribute to the deeper comprehension of interfacial charge transfer in S-scheme heterojunctions, which is important for the design of efficient S-scheme heterojunction photocatalysts;
- Considering future commercialization, in addition to the dual-channel pathway of photocatalytic H2O2 production, the cost of S-scheme photocatalysts should be controlled and recyclable and reusable photocatalysts should be designed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campos-Martin, J.M.; Blanco-Brieva, G.; Fierro Jose, L.G. Wasserstoffperoxid-synthese: Perspektiven jenseits des Anthrachinon-Verfahrens. Angew. Chem. 2006, 118, 7116–7139. [Google Scholar] [CrossRef]
- Zhang, P.; Tong, Y.; Liu, Y.; Vequizo, J.J.M.; Sun, H.; Yang, C.; Yamakata, A.; Fan, F.; Lin, W.; Wang, X.; et al. Heteroatom dopants promote two-electron O2 reduction for photocatalytic production of H2O2 on polymeric carbon nitride. Angew. Chem. Int. Ed. Engl. 2020, 59, 16209–16217. [Google Scholar] [CrossRef] [PubMed]
- Keigo Kamata, K.Y. Yasutaka Sumida, Kazuya Yamaguchi, Shiro Hikichi, Noritaka Mizuno. Efficient epoxidation of olefins with >99% selectivity and use of hydrogen peroxide. Science 2003, 300, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pinto, A.; Sampaio, M.J.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Recent strategies for hydrogen peroxide production by metal-free carbon nitride photocatalysts. Catalysts 2019, 9, 990. [Google Scholar] [CrossRef]
- Zheng, L.; Su, H.; Zhang, J.; Walekar, L.S.; Vafaei Molamahmood, H.; Zhou, B.; Long, M.; Hu, Y.H. Highly selective photocatalytic production of H2O2 on sulfur and nitrogen co-doped graphene quantum dots tuned TiO2. Appl. Catal. B-Environ. 2018, 239, 475–484. [Google Scholar] [CrossRef]
- Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366, 226–231. [Google Scholar] [CrossRef]
- Lei, J.; Chen, B.; Lv, W.; Zhou, L.; Wang, L.; Liu, Y.; Zhang, J. Robust photocatalytic H2O2 production over inverse opal g-C3N4 with carbon vacancy under visible light. ACS Sustain. Chem. Eng. 2019, 7, 16467–16473. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Cao, J.; Wang, H.; Shao, M.; Huang, H.; Liu, Y.; Kang, Z. Efficient production of H2O2 via two-channel pathway over ZIF-8/C3N4 composite photocatalyst without any sacrificial agent. Appl. Catal. B-Environ. 2020, 278, 119289. [Google Scholar] [CrossRef]
- Feng, C.; Tang, L.; Deng, Y.; Wang, J.; Liu, Y.; Ouyang, X.; Yang, H.; Yu, J.; Wang, J. A novel sulfur-assisted annealing method of g-C3N4 nanosheet compensates for the loss of light absorption with further promoted charge transfer for photocatalytic production of H2 and H2O2. Appl. Catal. B-Environ. 2021, 281, 119539. [Google Scholar] [CrossRef]
- Liu, S.; Qi, W.; Adimi, S.; Guo, H.; Weng, B.; Attfield, J.P.; Yang, M. Titanium nitride-supported platinum with metal–support interaction for boosting photocatalytic H2 evolution of indium sulfide. ACS Appl. Mater. Inter. 2021, 13, 7238–7247. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, F.; Zheng, L.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B. Boron containing metal-organic framework for highly selective photocatalytic production of H2O2 by promoting two-electron O2 reduction. Mater. Horiz. 2021, 8, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Jung, J.; Suenobu, T.; Fukuzumi, S. Production of hydrogen peroxide as a sustainable solar fuel from water and dioxygen. Energy Environ. Sci. 2013, 6, 3756. [Google Scholar] [CrossRef]
- Kaynan, N.; Berke, B.A.; Hazut, O.; Yerushalmi, R. Sustainable photocatalytic production of hydrogen peroxide from water and molecular oxygen. J. Mater. Chem. A 2014, 2, 13822–13826. [Google Scholar] [CrossRef]
- Zhuang, H.; Yang, L.; Xu, J.; Li, F.; Zhang, Z.; Lin, H.; Long, J.; Wang, X. Robust photocatalytic H2O2 production by octahedral Cd3(C3N3S3)2 coordination polymer under visible light. Sci. Rep. 2015, 5, 16947. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, G.; Hailili, R.; Yang, L.; Li, Y.; Wang, F.; Zeng, Y.; Wang, C. Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B-Environ. 2016, 190, 26–35. [Google Scholar] [CrossRef]
- Yang, L.; Dong, G.; Jacobs, D.L.; Wang, Y.; Zang, L.; Wang, C. Two-channel photocatalytic production of H2O2 over g-C3N4 nanosheets modified with perylene imides. J. Catal. 2017, 352, 274–281. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.T.; Zeng, G.M.; Huang, D.L.; Xiao, R.; Zhang, C.; Zhou, C.Y.; Xiong, W.P.; Wang, W.J.; Cheng, M.; et al. Ti3C2 Mxene/porous g-C3N4 interfacial Schottky junction for boosting spatial charge separation in photocatalytic H2O2 production. Appl. Catal. B-Environ. 2019, 258, 117956. [Google Scholar] [CrossRef]
- Che, H.; Gao, X.; Chen, J.; Hou, J.; Ao, Y.; Wang, P. Iodide-induced fragmentation of polymerized hydrophilic carbon nitride for high-performance Quasi-Homogeneous photocatalytic H2O2 production. Angew. Chem. Int. Ed. Engl. 2021, 60, 25546–25550. [Google Scholar] [CrossRef]
- Liu, B.; Du, J.; Ke, G.; Jia, B.; Huang, Y.; He, H.; Zhou, Y.; Zou, Z. Boosting O2 reduction and H2O dehydrogenation kinetics: Surface N-hydroxymethylation of g-C3N4 photocatalysts for the efficient production of H2O2. Adv. Funct. Mater. 2021, 32, 2111125. [Google Scholar] [CrossRef]
- Wang, P.; Fan, S.; Li, X.; Duan, J.; Zhang, D. Modulating the molecular structure of graphitic carbon nitride for identifying the impact of the piezoelectric effect on photocatalytic H2O2 production. ACS Catal. 2023, 13, 9515–9523. [Google Scholar] [CrossRef]
- Xue, L.; Sun, H.; Wu, Q.; Yao, W. P-doped melon-carbon nitride for efficient photocatalytic H2O2 production. J. Colloid Interface Sci. 2022, 615, 87–94. [Google Scholar] [CrossRef]
- Che, H.; Wang, J.; Gao, X.; Chen, J.; Wang, P.; Liu, B.; Ao, Y. Regulating directional transfer of electrons on polymeric g-C3N5 for highly efficient photocatalytic H2O2 production. J. Colloid Interface Sci. 2022, 627, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shi, C.; Li, Q.; Wang, X.; Zeng, G.; Ye, S.; Jiang, B.; Liu, J. Nitrogen vacancy-rich porous carbon nitride nanosheets for efficient photocatalytic H2O2 production. Mater. Today Energy 2022, 24, 100926. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, H.; Zhao, L.; Jian, L.; Liang, Q.; Xiao, X. Insight into facet-dependent photocatalytic H2O2 production on BiOCl nanosheets. New J. Chem. 2021, 45, 3335–3342. [Google Scholar] [CrossRef]
- Zhu, H.; Xue, Q.; Zhu, G.; Liu, Y.; Dou, X.; Yuan, X. Decorating Pt@cyclodextrin nanoclusters on C3N4/MXene for boosting the photocatalytic H2O2 production. J. Mater. Chem. A 2021, 9, 6872–6880. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, X. Protonated g-C3N4 coated Co9S8 heterojunction for photocatalytic H2O2 production. J. Colloid Interface Sci. 2022, 627, 541–553. [Google Scholar] [CrossRef]
- Zhao, X.; You, Y.; Huang, S.; Wu, Y.; Ma, Y.; Zhang, G.; Zhang, Z. Z-scheme photocatalytic production of hydrogen peroxide over Bi4O5Br2/g-C3N4 heterostructure under visible light. Appl. Catal. B-Environ. 2020, 278, 119251. [Google Scholar] [CrossRef]
- Wu, S.; Yu, H.; Chen, S.; Quan, X. Enhanced photocatalytic H2O2 production over carbon nitride by doping and defect engineering. ACS Catal. 2020, 10, 14380–14389. [Google Scholar] [CrossRef]
- Xu, Y.; Liao, J.; Zhang, L.; Sun, Z.; Ge, C. Dual sulfur defect engineering of Z-scheme heterojunction on Ag-CdS1-x@ZnIn2S4-x hollow core-shell for ultra-efficient selective photocatalytic H2O2 production. J. Colloid Interface Sci. 2023, 647, 446–455. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, H. Facet-dependent photocatalytic H2O2 production of single phase Ag3PO4 and Z-scheme Ag/ZnFe2O4-Ag-Ag3PO4 composites. Chem. Eng. J. 2022, 429, 132373. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B-Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhu, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B-Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In situ irradiated XPS investigation on S-scheme TiO2@ZnIn2S4 photocatalyst for efficient photocatalytic CO2 reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2 reduction selectivity. Appl. Catal. B-Environ. 2021, 289, 120039. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Miao, S.; Chen, F.; Guo, C.; Ye, P.; Ning, J.; Zhong, Y.; Hu, Y. Nitric acid-assisted growth of InVO4 nanobelts on protonated ultrathin C3N4 nanosheets as an S-scheme photocatalyst with tunable oxygen vacancies for boosting CO2 conversion. Chem. Eng. J. 2022, 434, 133867. [Google Scholar] [CrossRef]
- Han, X.; Lu, B.; Huang, X.; Liu, C.; Chen, S.; Chen, J.; Zeng, Z.; Deng, S.; Wang, J. Novel p- and n-type S-scheme heterojunction photocatalyst for boosted CO2 photoreduction activity. Appl. Catal. B-Environ. 2022, 316, 121587. [Google Scholar] [CrossRef]
- Jin, Z.; Jiang, X.; Guo, X. Hollow tubular Co9S8 grown on In2O3 to form S-scheme heterojunction for efficient and stable hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 1669–1682. [Google Scholar] [CrossRef]
- Hao, X.; Xiang, D.; Jin, Z. Zn-vacancy engineered S-scheme ZnCdS/ZnS photocatalyst for highly efficient photocatalytic H2 evolution. ChemCatChem 2021, 13, 4738–4750. [Google Scholar] [CrossRef]
- Shen, R.; Lu, X.; Zheng, Q.; Chen, Q.; Ng, Y.H.; Zhang, P.; Li, X. Tracking S-scheme charge transfer pathways in Mo2C/CdS H2-evolution photocatalysts. Solar RRL 2021, 5, 2100177. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Zhang, P.; Li, X.; Wang, S. ZnWO4-ZnIn2S4 S-scheme heterojunction for enhanced photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. [Google Scholar] [CrossRef]
- Feng, K.; Tian, J.; Hu, X.; Fan, J.; Liu, E. Active-center-enriched Ni0.85Se/g-C3N4 S-scheme heterojunction for efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 2022, 47, 4601–4613. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, B.; Yu, J.; Wang, L.; Ho, W. TiO2/In2S3 S-scheme photocatalyst with enhanced H2O2-production activity. Nano Res. 2021, 16, 4506–4514. [Google Scholar] [CrossRef]
- Wu, S.; Yu, X.; Zhang, J.; Zhang, Y.; Zhu, Y.; Zhu, M. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J. 2021, 411, 128555. [Google Scholar] [CrossRef]
- He, R.; Ou, S.; Liu, Y.; Liu, Y.; Xu, D. In situ fabrication of Bi2Se3/g-C3N4 S-scheme photocatalyst with improved photocatalytic activity. Chin. J. Catal. 2022, 43, 370–378. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Wang, J.; Wu, X.; Zhang, G. Sb2WO6/BiOBr 2D nanocomposite S-scheme photocatalyst for NO removal. J. Mater. Sci. Technol. 2020, 56, 236–243. [Google Scholar] [CrossRef]
- Le, S.; Ma, Y.; He, D.; Wang, X.; Guo, Y. CdS/NH4V4O10 S-scheme photocatalyst for sustainable photo-decomposition of amoxicillin. Chem. Eng. J. 2021, 426, 130354. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, H.; Park, S.O.; Hwang, J.M.; Hong, Y.; Sharma, P.; Jeon, W.C.; Cho, Y.; Yang, C.; Kwak, S.K.; et al. High performance H2O2 production achieved by sulfur-doped carbon on CdS photocatalyst via inhibiting reverse H2O2 decomposition. Appl. Catal. B-Environ. 2021, 284, 119690. [Google Scholar] [CrossRef]
- Pan, C.; Bian, G.; Zhang, Y.; Lou, Y.; Zhang, Y.; Dong, Y.; Xu, J.; Zhu, Y. Efficient and stable H2O2 production from H2O and O2 on BiPO4 photocatalyst. Appl. Catal. B-Environ. 2022, 316, 121675. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhao, J.; Chen, M.; Huang, X.; Xu, Y. Efficient production of H2O2 on Au/WO3 under visible light and the influencing factors. Appl. Catal. B-Environ. 2021, 284, 119691. [Google Scholar] [CrossRef]
- Xie, H.; Zheng, Y.; Guo, X.; Liu, Y.; Zhang, Z.; Zhao, J.; Zhang, W.; Wang, Y.; Huang, Y. Rapid microwave synthesis of mesoporous oxygen-doped g-C3N4 with carbon vacancies for efficient photocatalytic H2O2 production. ACS Sustain. Chem. Eng. 2021, 9, 6788–6798. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Y.; Fan, C.; Tang, L.; Yang, S.; Liu, M.; Wang, M.; Feng, C.; Ouyang, X.; Wang, L.; et al. Direct attack and indirect transfer mechanisms dominated by reactive oxygen species for photocatalytic H2O2 production on g-C3N4 possessing nitrogen vacancies. ACS Catal. 2021, 11, 11440–11450. [Google Scholar] [CrossRef]
- He, B.; Wang, Z.; Xiao, P.; Chen, T.; Yu, J.; Zhang, L. Cooperative coupling of H2O2 production and organic synthesis over a floatable polystyrene-sphere-supported TiO2 /Bi2O3 S-scheme photocatalyst. Adv. Mater. 2022, 34, e2203225. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Zhang, L.; Feng, L.; Zhang, C.; Jiang, J.; Wang, H. Construction of porous tubular In2S3@In2O3 with plasma treatment-derived oxygen vacancies for efficient photocatalytic H2O2 production in pure water via two-electron reduction. ACS Appl. Mater. Interfaces 2021, 13, 25868–25878. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Wang, Z.; Ma, Y.; Zhou, Y.; Shi, X.; Wu, Q.; Wang, X.; Shao, M.; Huang, H.; et al. Carbon nitride assisted 2D conductive metal-organic frameworks composite photocatalyst for efficient visible light-driven H2O2 production. Appl. Catal. B-Environ. 2021, 289, 120035. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, R.-T.; Hong, L.-F.; Ji, X.-Y.; Lin, Z.-D.; Li, Z.-S.; Pan, W.-G. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Li, X.; Garlisi, C.; Guan, Q.; Anwer, S.; Al-Ali, K.; Palmisano, G.; Zheng, L. A review of material aspects in developing direct Z-scheme photocatalysts. Mater. Today 2021, 47, 75–107. [Google Scholar] [CrossRef]
- Bao, Y.; Song, S.; Yao, G.; Jiang, S. S-Scheme Photocatalytic Systems. Solar RRL 2021, 5, 2100118. [Google Scholar] [CrossRef]
- Hasija, V.; Kumar, A.; Sudhaik, A.; Raizada, P.; Singh, P.; Van Le, Q.; Le, T.T.; Nguyen, V.-H. Step-scheme heterojunction photocatalysts for solar energy, water splitting, CO2 conversion, and bacterial inactivation: A review. Environ. Chem. Lett. 2021, 19, 2941–2966. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, e2107668. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tan, H.; Fan, J.; Cheng, B.; Yu, J.; Ho, W. Tuning the strength of built-in electric field in 2D/2D g-C3N4/SnS2 and g-C3N4/ZrS2 S-scheme heterojunctions by nonmetal doping. J. Mater. 2021, 7, 988–997. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Cheng, B.; Zhang, J.; Zhang, L.; Yu, J. In-situ preparation of TiO2/N-doped graphene hollow sphere photocatalyst with enhanced photocatalytic CO2 reduction performance. Chin. J. Catal. 2021, 42, 1648–1658. [Google Scholar] [CrossRef]
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An inorganic/organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Adv. Mater. 2021, 33, 2100317. [Google Scholar] [CrossRef]
- Wang, X.; Sayed, M.; Ruzimuradov, O.; Zhang, J.; Fan, Y.; Li, X.; Bai, X.; Low, J. A review of step-scheme photocatalysts. Appl. Mater. Today 2022, 29, 101609. [Google Scholar] [CrossRef]
- Deng, X.; Wang, D.; Li, H.; Jiang, W.; Zhou, T.; Wen, Y.; Yu, B.; Che, G.; Wang, L. Boosting interfacial charge separation and photocatalytic activity of 2D/2D g-C3N4/ZnIn2S4 S-scheme heterojunction under visible light irradiation. J. Alloys Compd. 2022, 894, 162209. [Google Scholar] [CrossRef]
- Dou, L.; Jin, X.; Chen, J.; Zhong, J.; Li, J.; Zeng, Y.; Duan, R. One-pot solvothermal fabrication of S-scheme OVs-Bi2O3/Bi2SiO5 microsphere heterojunctions with enhanced photocatalytic performance toward decontamination of organic pollutants. Appl. Surf. Sci. 2020, 527, 146775. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.F.; Dai, K. Organic amine surface modified one-dimensional CdSe0.8S0.2-diethylenetriamine/two-dimensional SnNb2O6 S-scheme heterojunction with promoted visible-light-driven photocatalytic CO2 reduction. Chin. J. Catal. 2022, 43, 255–264. [Google Scholar] [CrossRef]
- Liao, X.; Li, T.T.; Ren, H.T.; Zhang, X.; Shen, B.; Lin, J.H.; Lou, C.W. Construction of BiOI/TiO2 flexible and hierarchical S-scheme heterojunction nanofibers membranes for visible-light-driven photocatalytic pollutants degradation. Sci. Total Environ. 2022, 806, 150698. [Google Scholar] [CrossRef]
- Xia, P.; Cao, S.; Zhu, B.; Liu, M.; Shi, M.; Yu, J.; Zhang, Y. Designing a 0D/2D S-scheme heterojunction over polymeric carbon nitride for visible-light photocatalytic inactivation of bacteria. Angew. Chem. Int. Ed. Engl. 2020, 59, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, X.; Jin, Z.; Tsubaki, N. Spatially separated catalytic sites supplied with the CdS–MoS2–In2O3 ternary dumbbell S-scheme heterojunction for enhanced photocatalytic hydrogen production. J. Mater. Chem. A 2022, 10, 10715–10728. [Google Scholar] [CrossRef]
- Wang, K.; Liu, S.; Li, Y.; Wang, G.; Yang, M.; Jin, Z. Phosphorus ZIF-67@NiAl LDH S-scheme heterojunction for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2022, 601, 154174. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Shen, H.; Li, J.; Yang, C.; Xie, B.; Xia, S. Photocatalytic degradation of rhodamine B by Bi2O3@LDHs S–scheme heterojunction: Performance, kinetics and mechanism. Appl. Surf. Sci. 2021, 567, 150760. [Google Scholar] [CrossRef]
- Kamali, M.; Sheibani, S.; Ataie, A. Magnetic MgFe2O4-CaFe2O4 S-scheme photocatalyst prepared from recycling of electric arc furnace dust. J. Environ. Manag. 2021, 290, 112609. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, C.; Li, J.; Dong, B.; Zhao, L.; Wang, S. 1D/2D TiO2/ZnIn2S4 S-scheme heterojunction photocatalyst for efficient hydrogen evolution. Chin. J. Catal. 2022, 43, 339–349. [Google Scholar] [CrossRef]
- Yu, W.; Hu, C.; Bai, L.; Tian, N.; Zhang, Y.; Huang, H. Photocatalytic hydrogen peroxide evolution: What is the most effective strategy? Nano Energy 2022, 104, 107906. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Zhang, Y.; Yu, H.; Qu, Y.; Yu, J. Inorganic metal-oxide photocatalyst for H2O2 production. Small 2022, 18, e2104561. [Google Scholar] [CrossRef]
- Hou, H.; Zeng, X.; Zhang, X. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. Engl. 2020, 59, 17356–17376. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, B.; Zhang, Y.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Wang, L. S-scheme ZnO/WO3 heterojunction photocatalyst for efficient H2O2 production. J. Mater. Sci. Technol. 2022, 124, 193–201. [Google Scholar] [CrossRef]
- Lai, C.; Xu, M.; Xu, F.; Li, B.; Ma, D.; Li, Y.; Li, L.; Zhang, M.; Huang, D.; Tang, L.; et al. An S-scheme CdS/K2Ta2O6 heterojunction photocatalyst for production of H2O2 from water and air. Chem. Eng. J. 2023, 452, 139070. [Google Scholar] [CrossRef]
- Liu, B.; Bie, C.; Zhang, Y.; Wang, L.; Li, Y.; Yu, J. Hierarchically porous ZnO/g-C3N4 S-scheme heterojunction photocatalyst for efficient H2O2 production. Langmuir 2021, 37, 14114–14124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, J.; Macyk, W.; Wageh, S.; Al-Ghamdi, A.A.; Wang, L. C3N4/PDA S-scheme heterojunction with enhanced photocatalytic H2O2 production performance and its mechanism. Adv. Sustain. Syst. 2022, 7, 2200113. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Zhu, B.; Fedin, M.V.; Cheng, B.; Yu, J.; Zhang, L. ZnO/COF S-scheme heterojunction for improved photocatalytic H2O2 production performance. Chem. Eng. J. 2022, 444, 136584. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Yu, H.; Patir, I.H.; Li, Y.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. Dynamics of photogenerated charge carriers in inorganic/organic S-scheme heterojunctions. J. Phys. Chem. Lett. 2022, 13, 4695–4700. [Google Scholar] [CrossRef]
- Bariki, R.; Das, K.; Pradhan, S.K.; Prusti, B.; Mishra, B.G. MOF-derived hollow tubular In2O3/MIIIn2S4 (MII: Ca, Mn, and Zn) heterostructures: Synergetic charge-transfer mechanism and excellent photocatalytic performance to boost activation of small atmospheric molecules. ACS Appl. Energy Mater. 2022, 5, 11002–11017. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X.; Jiang, x.; Feng, Y.; Ding, X.-L.; Jiang, N.; Wang, J. Internal electric field and interfacial S-C bonds jointly accelerate S-scheme charge transfer achieving efficient sunlight-driven photocatalysis. J. Mater. Chem. A 2022, 10, 25279–25294. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Ranjith, K.S.; Park, B.; Hwang, S.-K.; Hosseini, R.; Behjatmanesh-Ardakani, R.; Pourmortazavi, S.M.; Lee, H.U.; Son, B.; Mirsadeghi, S.; et al. Full-spectrum-responsive Bi2S3@CdS S-scheme heterostructure with intimated ultrathin RGO toward photocatalytic Cr(VI) reduction and H2O2 production: Experimental and DFT studies. Chem. Eng. J. 2021, 419, 129530. [Google Scholar] [CrossRef]
- Han, G.; Xu, F.; Cheng, B.; Li, Y.; Yu, J.; Zhang, L. Enhanced photocatalytic H2O2 production over inverse opal ZnO@Polydopamine S-scheme heterojunctions. Acta Phys. Chim. Sin. 2022, 38, 2112037. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies. Nano Energy 2021, 81, 105671. [Google Scholar] [CrossRef]
- Xia, C.; Yuan, L.; Song, H.; Zhang, C.; Li, Z.; Zou, Y.; Li, J.; Bao, T.; Yu, C.; Liu, C. Spatial specific janus S-scheme photocatalyst with enhanced H2O2 production Performance. Small 2023, 19, 2300292. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Long, Q.; Cheng, B.; He, R.; Wang, L. 3D ordered macroporous sulfur-doped g-C3N4/TiO2 S-scheme photocatalysts for efficient H2O2 production in pure water. J. Mater. Sci. Technol. 2023, 162, 1–10. [Google Scholar] [CrossRef]
- Cao, S.; Chan, T.-S.; Lu, Y.-R.; Shi, X.; Fu, B.; Wu, Z.; Li, H.; Liu, K.; Alzuabi, S.; Cheng, P.; et al. Photocatalytic pure water splitting with high efficiency and value by Pt/porous brookite TiO2 nanoflutes. Nano Energy 2020, 67, 104287. [Google Scholar] [CrossRef]
- Xue, F.; Si, Y.; Wang, M.; Liu, M.; Guo, L. Toward efficient photocatalytic pure water splitting for simultaneous H2 and H2O2 production. Nano Energy 2019, 62, 823–831. [Google Scholar] [CrossRef]
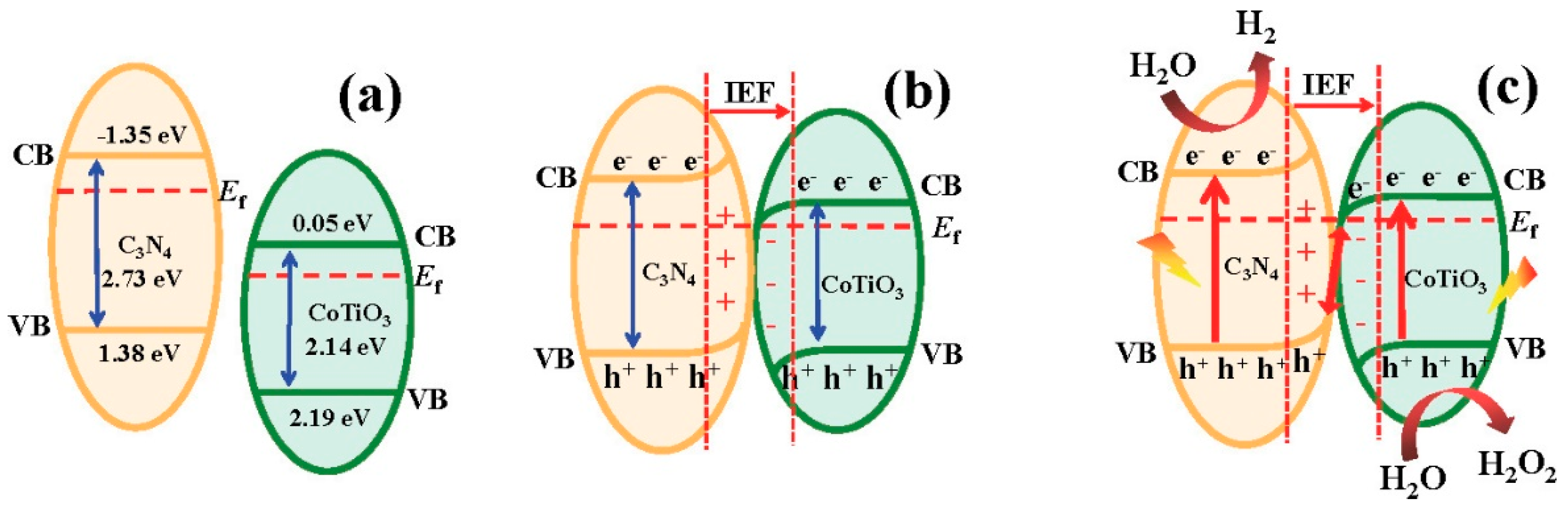
- Meng, A.; Zhou, S.; Wen, D.; Han, P.; Su, Y. g-C3N4/CoTiO3 S-scheme heterojunction for enhanced visible light hydrogen production through photocatalytic pure water splitting. Chin. J. Catal. 2022, 43, 2548–2557. [Google Scholar] [CrossRef]
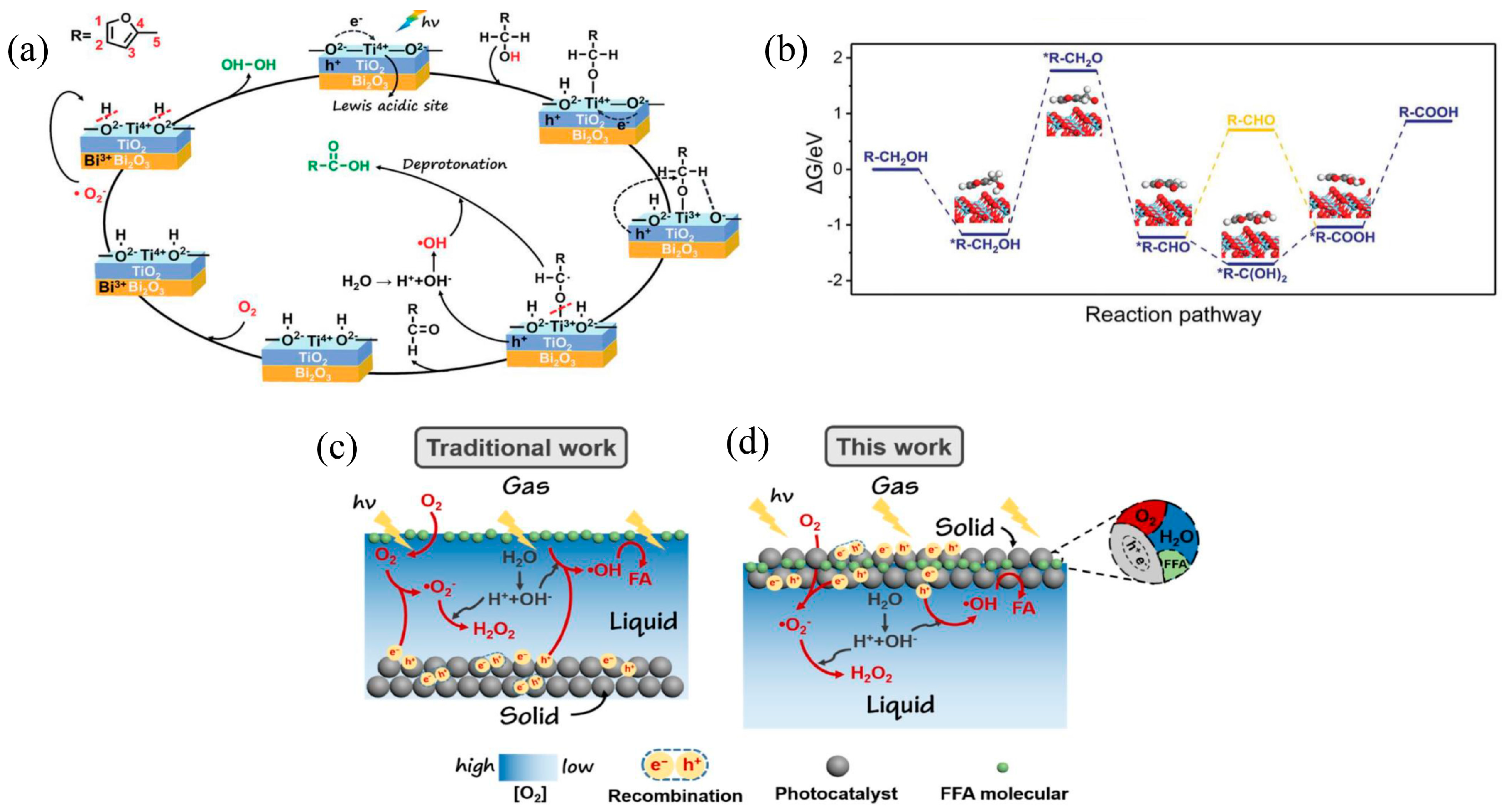
- He, R.; Xu, D.; Li, X. Floatable S-scheme photocatalyst for H2O2 production and organic synthesis. J. Mater. Sci. Technol. 2023, 138, 256–258. [Google Scholar] [CrossRef]
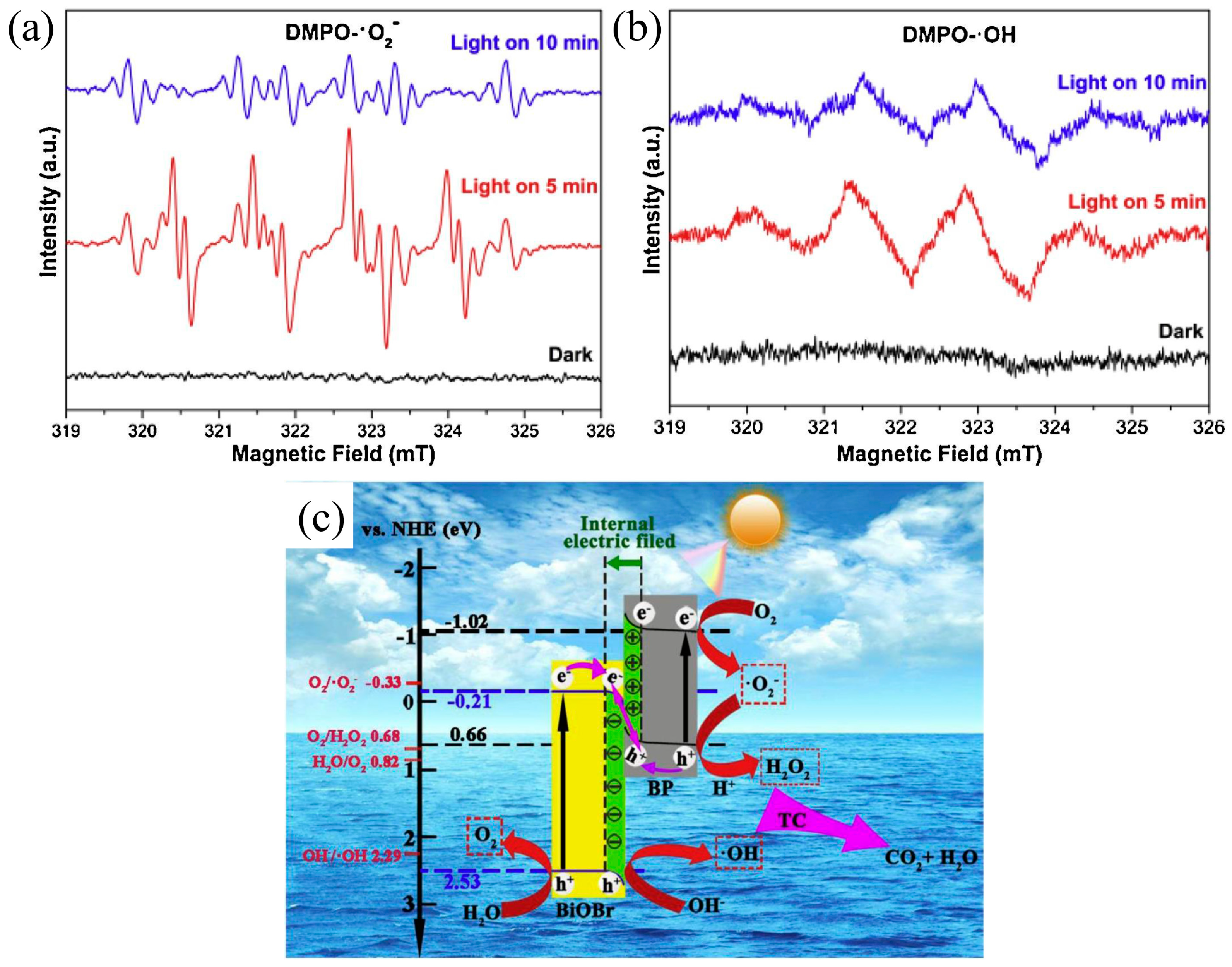
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Deng, F.; Liu, J.; Gao, X.; Huang, J.; Xu, J.; Feng, Z.; Chen, Z.; Han, L. Novel organic/inorganic PDI-Urea/BiOBr S-scheme heterojunction for improved photocatalytic antibiotic degradation and H2O2 production. Chin. Chem. Lett. 2022, 33, 5200–5207. [Google Scholar] [CrossRef]
- Khamesan, A.; Esfahani, M.M.; Ghasemi, J.B.; Farzin, F.; Parsaei-Khomami, A.; Mousavi, M. Graphitic-C3N4/ZnCr-layered double hydroxide 2D/2D nanosheet heterojunction: Mesoporous photocatalyst for advanced oxidation of azo dyes with in situ produced H2O2. Adv. Power Technol. 2022, 33, 103777. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Deng, J.; Li, L.; Zhou, Z.; Zheng, J.; Su, L.; Yang, L. π-π Stacked step-scheme PDI/g-C3N4/TiO2@Ti3C2 photocatalyst with enhanced visible photocatalytic degradation towards atrazine via peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 131809. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Chi, Y.; Yin, P.; Wei, L.; Liu, W.; Wang, X.; Zhang, H.; Song, H. Construction of S-scheme p-n heterojunction between protonated g-C3N4 and α-MnS nanosphere for photocatalytic H2O2 production and in situ degradation of oxytetracycline. J. Environ. Chem. Eng. 2023, 11, 109968. [Google Scholar] [CrossRef]
- Sun, X.; He, K.; Chen, Z.; Yuan, H.; Guo, F.; Shi, W. Construction of visible-light-response photocatalysis-self-Fenton system for the efficient degradation of amoxicillin based on industrial waste red mud/CdS S-scheme heterojunction. Sep. Purif. Technol. 2023, 324, 124600. [Google Scholar] [CrossRef]
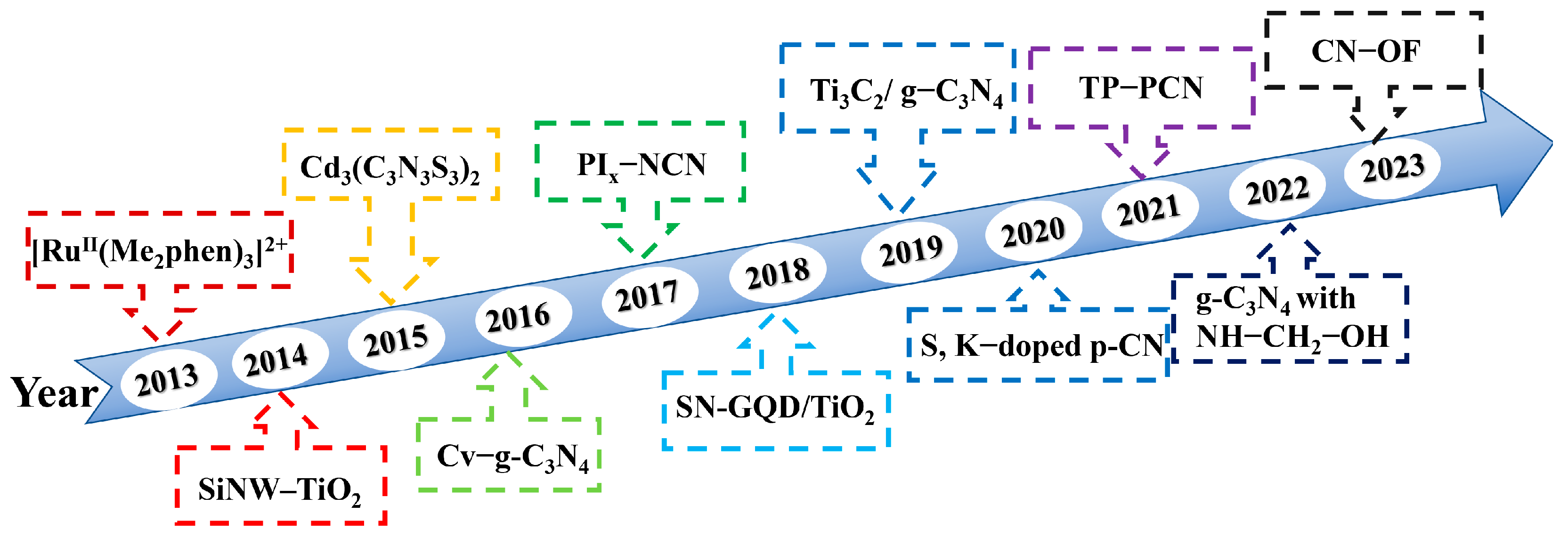

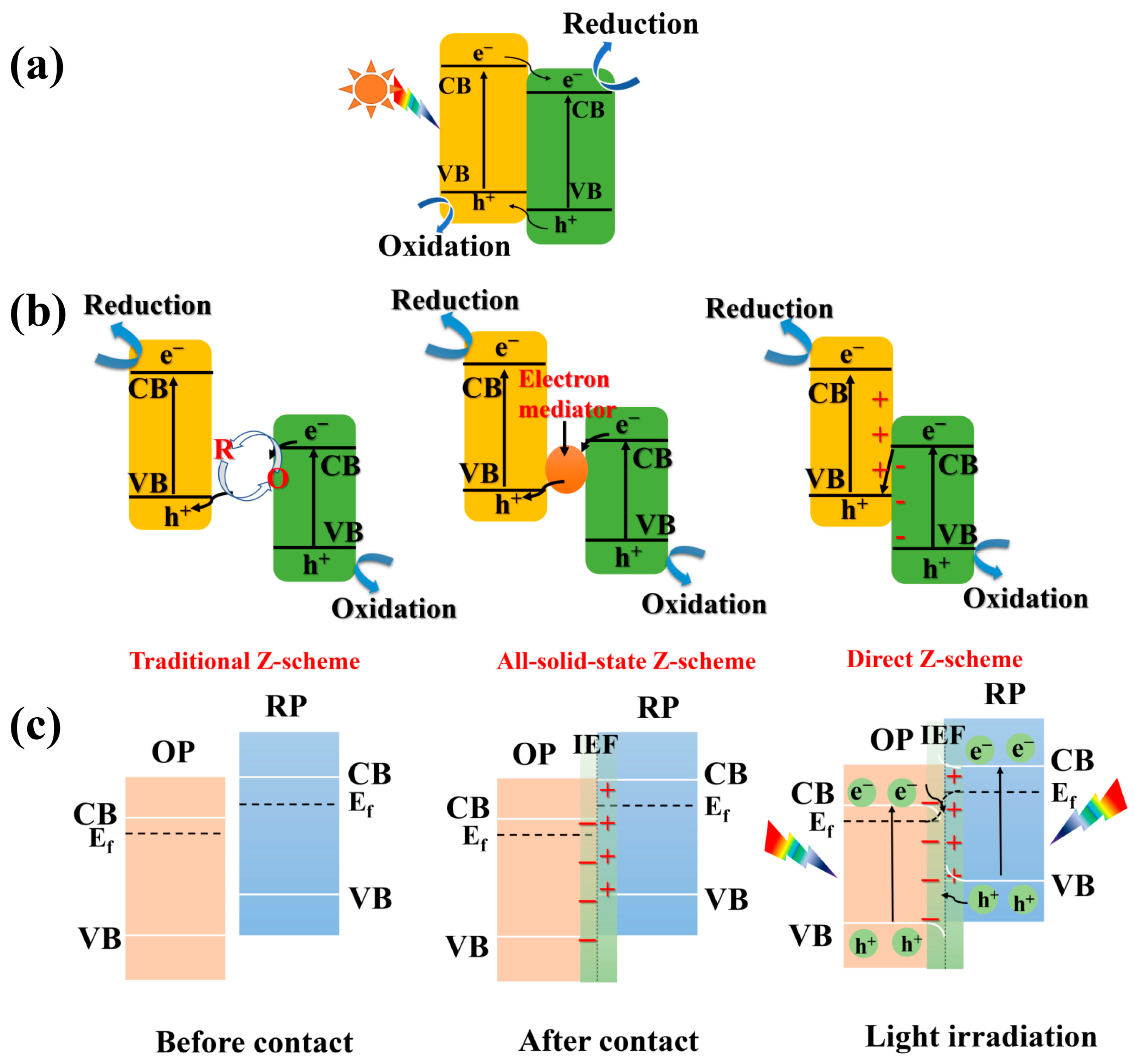

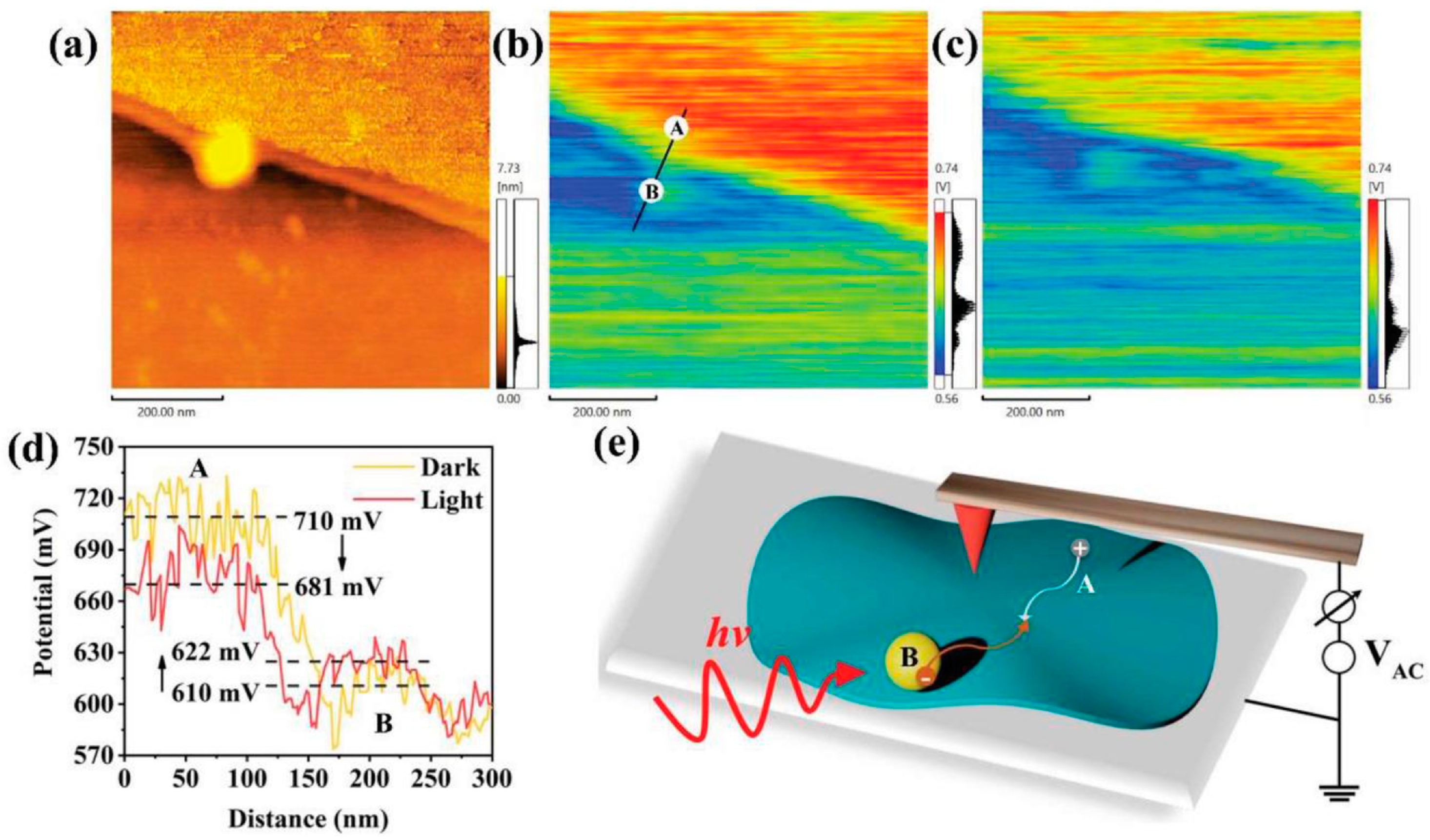

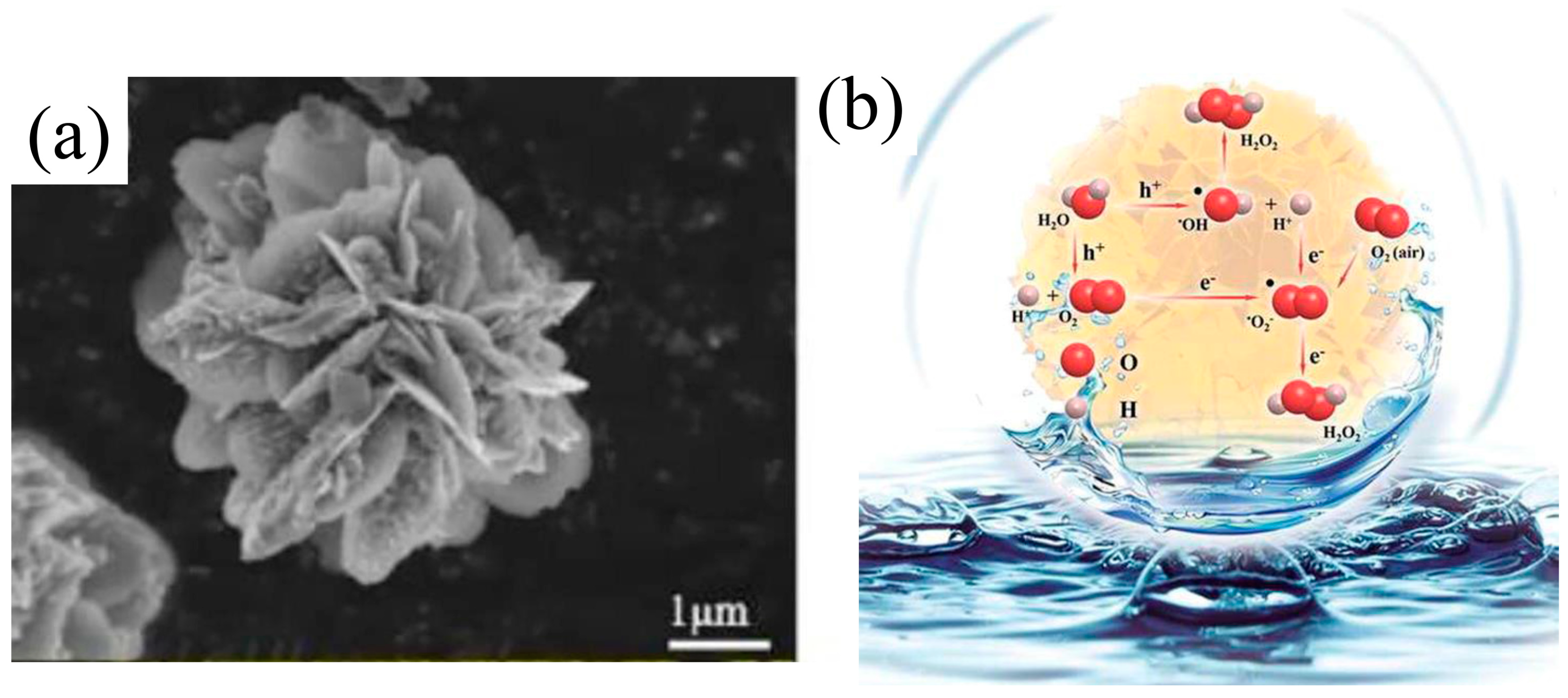
| Photocatalyst | Morphology | Light Source | Reaction Solution | Pathway | Concentration of Photocatalyst/g·L−1 | H2O2 Yield | Ref. |
|---|---|---|---|---|---|---|---|
| ZnO/WO3 | Hierarchical microsphere structure | 300 W Xe lamp | 50 mL of 10 vol% ethanol | Direct 2e− ORR and indirect 2e− ORR pathways | 1.0 | 6788 μmol·L−1·h−1 | [80] |
| CdS/K2Ta2O6 | Flower−like structure | 300 W Xe lamp (λ > 420 nm) | Ultra−pure water | 2e− ORR and WOR pathways | 0.6 | 160.89 μmol·L−1·h−1; 346.31 μmol·L−1·h−1 with saturated O2 | [81] |
| ZnO/g-C3N4 | ZnO NPs dispersed on the CN nanosheet | 300 W Xe lamp (λ > 350 nm) | 50 mL of 10 vol% ethanol | ORR pathway | 0.4 | 1544 μmol·L−1·h−1 | [82] |
| TiO2/In2S3 | Core–shell structure | 300 W Xe arc lamp | 40 mL of 10 vol% ethanol | Indirect 2e− ORR pathway | 0.5 | 376 μmol·L−1·h−1 | [45] |
| C3N4/PDA | Nanosheet | 300 W Xe arc lamp (λ > 350 nm) | 50 mL of 20 vol% ethanol | Indirect 2e− ORR pathway | 0.4 | 3801.25 µmol·g−1·h−1 | [83] |
| ZnO/COF (TpPa−Cl) | ZnO nanoparticles distributed on the surface of TpPa−Cl | 300 W Xe lamp | 10 vol% ethanol | Indirect 2e− ORR pathway | 0.5 | 2443 µmol·g−1·h−1 | [84] |
| TiO2/PDA | Inverse opals | 300 W Xe arc lamp | 40 mL of 10 vol% ethanol | ORR pathway | 0.5 | ~2.2 mmol·g−1·h−1 | [85] |
| In2O3/ZnIn2S4 | Ordered hollow structure | 250 W Xe lamp (λ > 420 nm) | 50 mL of 5 vol% ethanol | ORR pathway | 0.4 | 5716 µmol·g−1·h−1 | [86] |
| Sv−ZIS/CN | Three−dimensional flower-like structure and agaric shaped with a microporous structure | 300 W Xe lamp (λ > 420 nm) | 50 mL of 10 vol% isopropanol | Direct 2e− ORR and indirect 2e− ORR pathways | 0.4 | 1310.18 μmol·L−1·h−1 | [87] |
| Bi2S3@CdS@RGO | Flaky RGO is wrapped onto the CdS nanoparticles and Bi2S3 rod−aggregate morphology | 300 W Xe lamp (λ > 420 nm) | 50 mL of 10 vol% isopropanol | Indirect 2e− ORR pathway | 1.0 | 212.82 μmol·L−1 within 180 min | [88] |
| ZnO@PDA | Inverse Opal | 300 W Xe arc lamp | 50 mL of 4 vol% glycol | Direct 2e− ORR and indirect 2e− ORR pathways | 0.4 | 1011.4 μmol·L−1·h−1 | [89] |
| S-pCN/WO2.72 | Uniform porous sheet−like two−dimensional structure | 300 W Xe lamp (λ > 420 nm) | 100 mL water | Direct 2e− ORR and indirect 2e− ORR pathways | 1.0 | 90 μmol·L−1 within 180 min | [90] |
| TiO2@RF | Core–shell structure | 300 W Xe lamp | 15 mL water | 2e− ORR pathway | ~0.67 | 66.6 mmol·g−1·h−1 | [91] |
| sulfur-doped g-C3N4/TiO2 | Well-ordered macroporous framework | 300 W Xe lamp | 50 mL water | 2e− ORR and WOR pathways | 0.2 | 2128 µmol·g−1·h−1 | [92] |
| Photocatalyst | Morphology | Contaminant or Organics | Light Source | Reaction System | Concentration of Photocatalyst/g·L−1 | H2O2 Yield | Degradation Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| PDI−Urea/BiOBr | BiOBr nanospheres dispersed on the PDI−Urea lamellar layer | Ofloxacin (OFLO), tetracycline (TC) | 300 W Xe lamp (λ > 420 nm) | 50 mL of TC (50 mg/L) and OFLO (10 mg/L) | 1.0 | 71 μmol·L−1·h−1 after 3 h irradiation | 93%(~65%) photocatalytic degradation rate for OFLO (TC) after 150 (90) min | [98] |
| BP/BiOBr | Two−dimensional structure | Tetracycline (TC) | 300 W Xe arc lamp (λ > 420 nm) | 100 mL of TC (50 mg/L) | 1.0 | 1.62 μmol·L−1·min−1 | ~85% photocatalytic degradation rate for TC after 90 min | [97] |
| Graphitic−C3N4/ZnCr | Layered structures | Rhodamine B(RhB) | Xe lamp | 100 mL of RhB (5 ppm) | 1.0 | − | 99.8% photocatalytic degradation rate for RhB after 210 min | [99] |
| PDI/g-C3N4/TiO2@Ti3C2 | Multi-layered 2D frame | Atrazine (ATZ) | 300 W Xe lamp (λ > 420 nm) | 50 mL of ATZ (10 ppm) | 0.8 | ~160 μmol·L−1·h−1 | 75% removal rate of ATZ within one hour | [100] |
| g-C3N4/α-MnS | Inhomogeneous morphology with a rough surface | Oxytetracycline (OTC) | 300 W Xe lamp (λ > 420 nm) | 50 mL of OTC hydrochloride (20 mg·L−1) | 1.0 | 111.6 μmol·L−1·h−1 | 82.2% degradation of OTC in water within 80 min | [101] |
| Red mud/CdS | RM particles loaded on the surface of CdS nanospheres | Amoxicillin (AMX) | LED lamp (410 < λ < 760 nm) | 50 mL of AMX (20 mg·L−1) | 0.5 | 1.05 mg·L−1·h−1 | 73.0% degradation of AMX within 120 min | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, W.; Wang, L. S-Scheme Heterojunction Photocatalyst for Photocatalytic H2O2 Production: A Review. Catalysts 2023, 13, 1325. https://doi.org/10.3390/catal13101325
Fang W, Wang L. S-Scheme Heterojunction Photocatalyst for Photocatalytic H2O2 Production: A Review. Catalysts. 2023; 13(10):1325. https://doi.org/10.3390/catal13101325
Chicago/Turabian StyleFang, Weili, and Liang Wang. 2023. "S-Scheme Heterojunction Photocatalyst for Photocatalytic H2O2 Production: A Review" Catalysts 13, no. 10: 1325. https://doi.org/10.3390/catal13101325






