Mass Transfer in the Processes of Native Lignin Oxidation into Vanillin via Oxygen
Abstract
:1. Introduction
2. Results and Discussion
- Influence of the reaction mixture volume on the dynamics of oxygen consumption and the formation of the principal products during the oxidation of mucilage-free flax shives. Absence of the mucilage in the reaction mass avoids unnecessary complications of the mass transfer.
- Influence of the reaction mixture volume and the stirring speed on the rate of homogenization of the aqueous phase, and on sorption of alkali via the powdered shives’ suspension.
- Reasons for differing rates of oxygen consumption in the initial (one minute) and main (2–40 min) sections of the kinetic curves.
- Influence of the stirring speed on the nature of the rate-limiting stage of the process of the oxidation of flax shives into vanillin and cellulose.
- Comparison of the oxidation processes in reactors with low stirring power density (stirrer engine power: 8 W) and with intense stirring (engine power: 200 W).
2.1. Influence of the Reaction Mixture Volume on the Process of the Catalytic Oxidation of Flax Shives
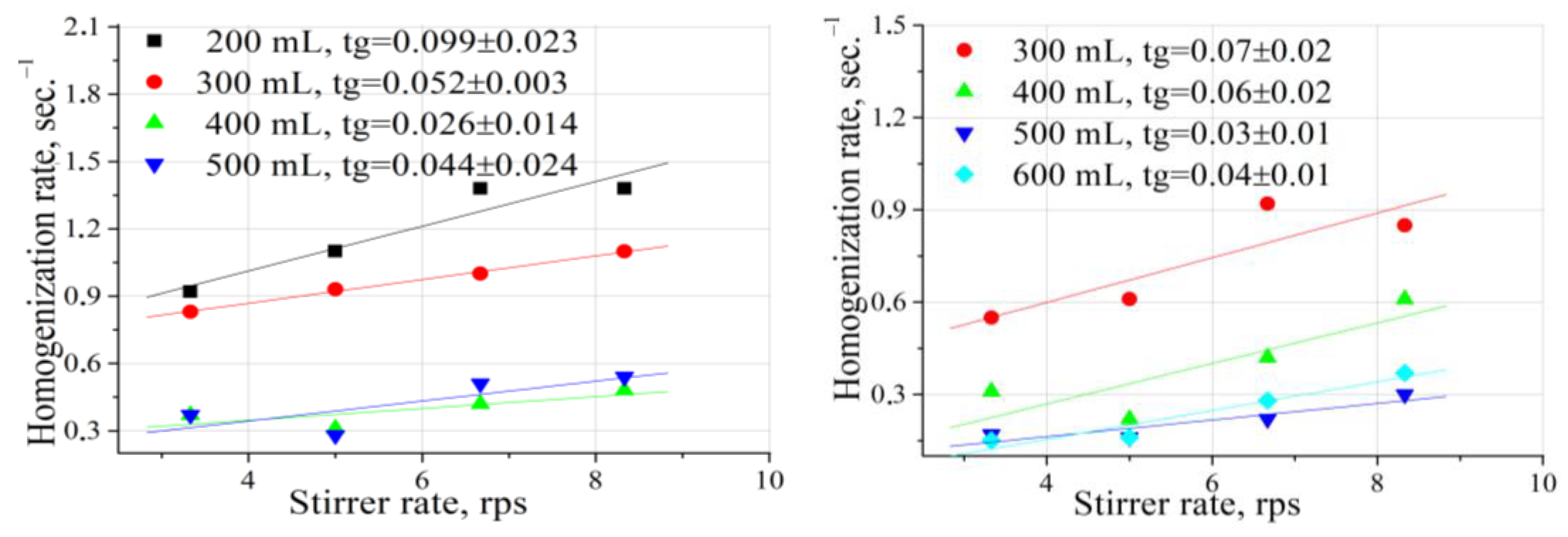
2.2. Influence of the Stirring Speed and the Reaction Mixture Volume on the Liquid Phase Homogenization Rate
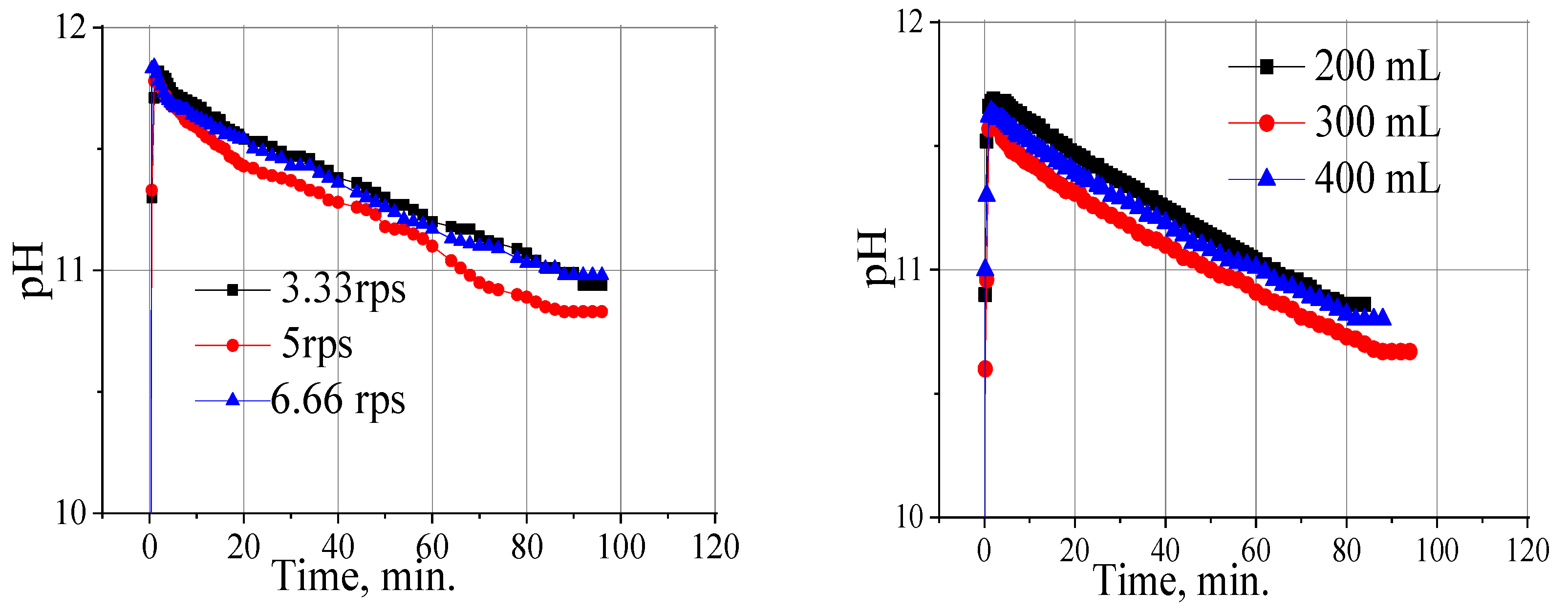
2.3. Influence of the Stirring Speed and the Reaction Mixture Volume on the Rate of Alkali Sorption by Powdered Flax Shives
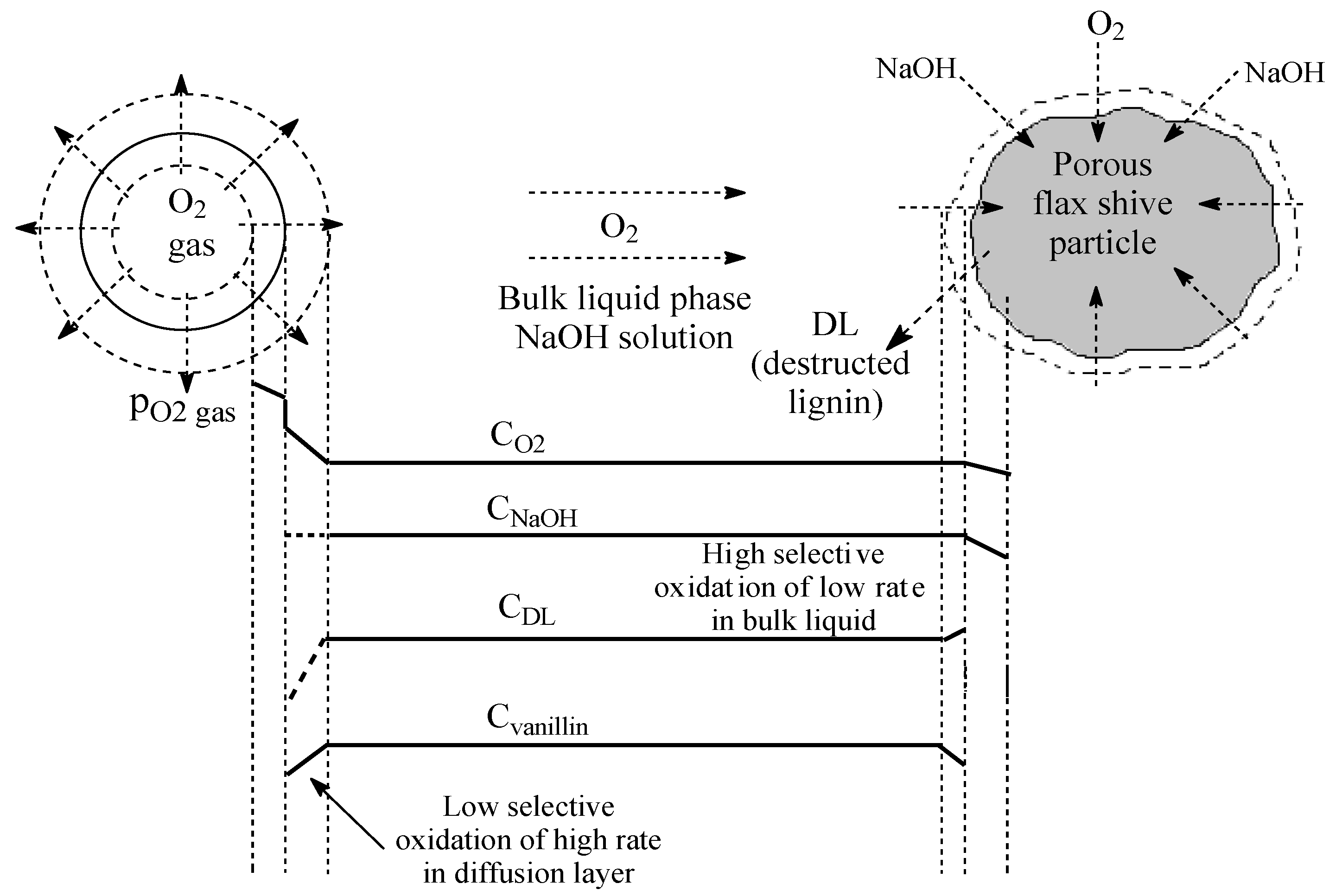
2.4. Mass Transfer near the Catalytic Surface
2.5. Diffusion Limitation in Oxygen Transfer through the Gas–Liquid Phase Boundary
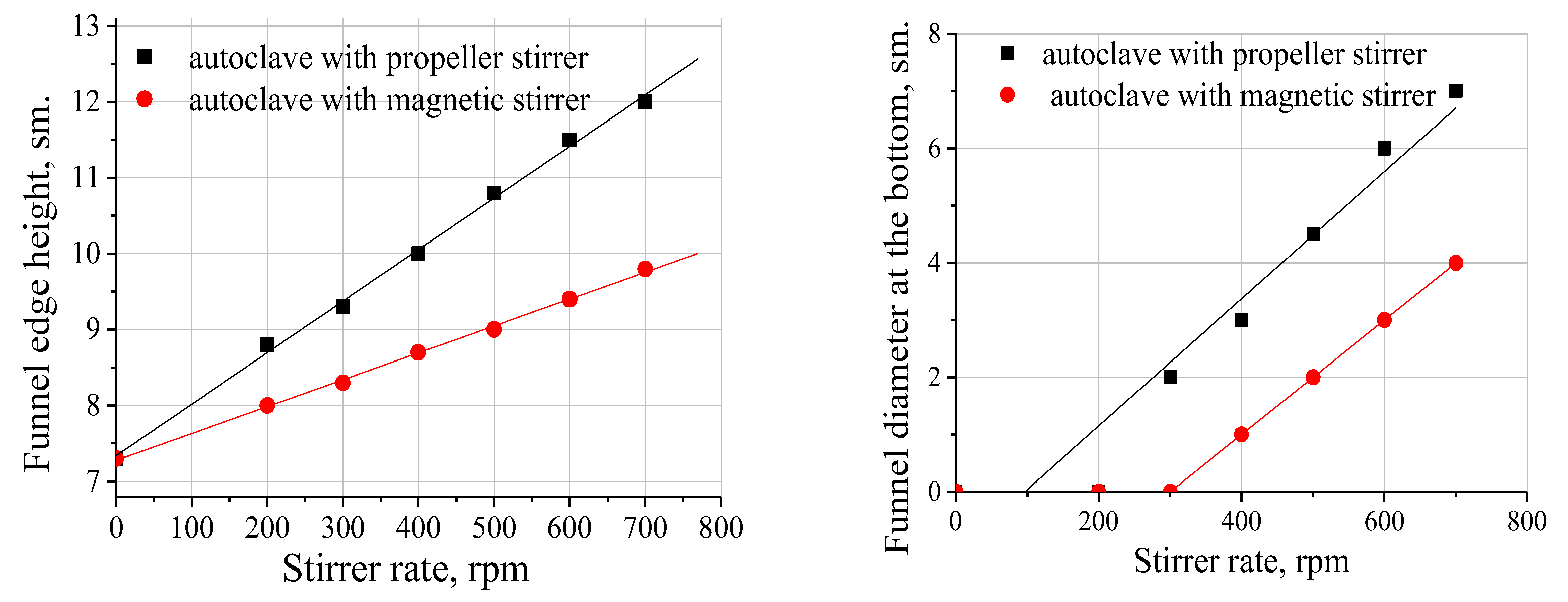
2.6. Oxidation with Intensified Mass Transfer
3. Materials and Methods
4. Conclusions
- (1)
- The homogenization stage (equalization of reactant concentrations across the liquid bulk) proceeded very fast in terms of characteristic time (1–4 s), and the oxidation reactions took place, for the most part, in this isotropic (homogeneous) aqueous medium.
- (2)
- The internal diffusion of alkali (and by extension, of oxygen) into the porous solid flax shive particles and the diffusion of fragments of the lignin macromolecules outward of the particles proceeded far slower than the liquid homogenization stage, with rates comparable to those of the overall process. However, under low stirring power density, the internal diffusion process did not limit the process rate. In the solid phase at 160 °C, delignification reactions and lignin solubilization occurred under the effects of alkali and oxygen. We believe that the slowest process at this phase boundary is the diffusion of the soluble lignin fragments inside the shive particles towards their outer surface, the external diffusion layer. The transfer from this diffusion layer into the liquid bulk was much faster and does not limit the overall process rate.
- (3)
- The overall rate of the studied process with a low stirring power density (engine power: 8 W) was controlled via the diffusion transfer of oxygen through the diffusion layer at the gas–liquid phase boundary.
- (4)
- At higher mass transfer intensities (engine power: 200 W), the stage of oxygen diffusion through the gas–liquid boundary accelerated and ceased to limit the overall oxidation process rate. However, this process did not enter the chemically controlled kinetic mode; the limiting via the internal diffusion of the reactants and intermediate products inside the particles of the flax shives (or other lignocellulosic materials) set in, whose characteristic time was dozens of minutes, was not affected by the stirring speed. For this reason, a hypothesis can be formulated in that the processes of lignin oxidation by oxygen at 160 °C requiring dozens of minutes exhibit diffusion-controlled kinetics.
- (5)
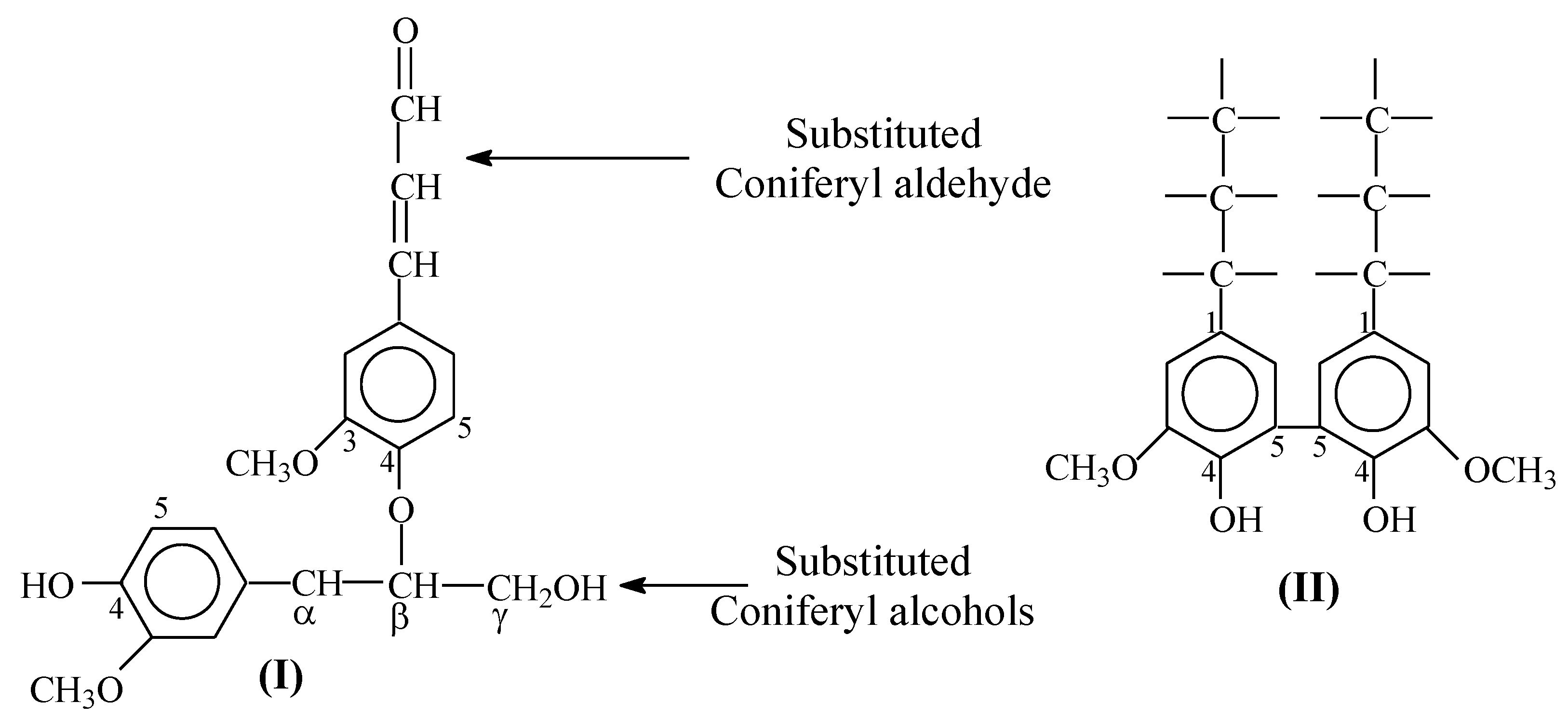
- Finally, we looked into the question formulated in the Introduction Section regarding the reasons for high selectivity towards the aromatic aldehydes in the diffusion-controlled oxidation of native lignins via oxygen. The theoretical limit of vanillin yield was attained in the three-stage oxidation process of pine-native lignin via molecular oxygen [27]. Attaining this limit indicated that under these conditions, the rates of the lignin depolymerization process, the transfer of the fragments into the bulk solution, and their subsequent oxidation all considerably exceed the rate of the lignin condensation process. This is one reason for the high selectivity.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varfolomeev, S.D.; Moiseev, I.I.; Myasoedov, B.F. Energy carriers from renewable sources: Chemical aspects. Her. Russ. Acad. Sci. 2009, 79, 334–344. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Tarabanko, N. Catalytic oxidation of lignins into the aromatic aldehydes: General process trends and development prospects. Int. J. Mol. Sci. 2017, 18, 2421. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.; Mazza, G. Characteristics of lignin from flax shives as affected by extraction conditions. Int. J. Mol. Sci. 2010, 11, 4035–4050. [Google Scholar] [CrossRef] [PubMed]
- Karpunin, I.I. Nitrobenzene oxidation of flax shives lignin. Rus. J. Appl. Chem. 1978, 51, 2387–2389. [Google Scholar]
- Kazachenko, A.S.; Miroshnikova, A.V.; Tarabanko, V.E.; Skripnikov, A.M.; Malyar, Y.N.; Borovkova, V.S.; Sychev, V.V.; Taran, O.P. Thermal conversion of flax shives in sub- and supercritical ethanol in the presence of Ru/C catalyst. Catalysts 2021, 11, 970. [Google Scholar] [CrossRef]
- Lugovoy, Y.V.; Chalov, K.V.; Tarabanko, V.E.; Stepacheva, A.A.; Kosivtsov, Y.Y. Fast pyrolysis of flax shive in a screw-type reactor. Chem. Eng. Technol. 2021, 44, 2056–2063. [Google Scholar] [CrossRef]
- Leopold, B.; Malmstrom, I.L. Studies on lignin. IV. Investigation on nitrobenzene oxidation products of lignin from different woods by paper partition chromatography. Acta Chem. Scand. 1952, 6, 38–54. [Google Scholar] [CrossRef]
- Kurshner, K. The difficulties in the production of vanillin from sulphite liquors. Russ. J. Appl. Chem. 1955, 28, 957–968. [Google Scholar]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the oxidative valorization of lignin to high-value chemicals: A critical review of opportunities and challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawase, K.; Hirano, Y.; Ikeuchi, M.; Miyafuji, H. Alkaline aerobic oxidation of native softwood lignin in the presence of Na+-cyclic polyether complexes. J. Wood. Chem. Technol. 2022, 42, 1–14. [Google Scholar] [CrossRef]
- Xu, X.; Li, P.; Zhong, Y.; Yu, J.; Miao, C.; Tong, G. Review on the oxidative catalysis methods of converting lignin into vanillin. Int. J. Biol. Macromol. 2023, 243, 125203. [Google Scholar] [CrossRef]
- Sales, F.G.; Maranhão, L.C.A.; Lima Filho, N.M.; Abreu, C.A.M. Kinetic evaluation and modeling of lignin catalytic wet oxidation to selective production of aromatic aldehydes. Ind. Eng. Chem. Res. 2006, 45, 6627–6631. [Google Scholar] [CrossRef]
- Santos, S.G.; Marques, A.P.; Lima, D.L.D.; Evtuguin, D.V.; Esteves, V.I. Kinetics of eucalypt lignosulfonate oxidation to aromatic aldehydes by oxygen in alkaline medium. Ind. Eng. Chem. Res. 2011, 50, 291–298. [Google Scholar] [CrossRef]
- Fargues, C.; Mathias, A.; Rodrigues, A. Kinetics of vanillin production from kraft lignin oxidation. Ind. Eng. Chem. Res. 1996, 35, 28–36. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, Y.; Wei, L.; Liu, J.; Song, X.B.; Chen, L.; Wang, C.; Sels, B.F.; Ma, L. Complementing vanillin and cellulose production by oxidation of lignocellulose with stirring control. ACS Sust. Chem. Eng. 2020, 8, 2361–2374. [Google Scholar] [CrossRef]
- Pacek, A.W.; Ding, P.; Garrett, M.; Sheldrake, G.; Nienow, A.W. Catalytic conversion of sodium lignosulfonate to vanillin: Engineering aspects. Part 1. Effects of processing conditions on vanillin yield and selectivity. Ind. Eng. Chem. Res. 2013, 52, 8361–8372. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Pervishina, E.P.; Hendogina, Y.V. Kinetics of aspen wood oxidation by oxygen in alkaline media. React. Kinet. Catal. Lett. 2001, 72, 153–162. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Vigul, D.O.; Kaygorodov, K.L.; Kosivtsov, Y.; Tarabanko, N.; Chelbina, Y.V. Influence of mass transfer and acid prehydrolysis on the process of flax shives catalytic oxidation into vanillin and pulp. Biomass Conv. Bioref. 2022, 1–11. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Vigul, D.O.; Kaygorodov, K.L.; Chelbina, Y.V.; Mazurova, E.V. Catalytic oxidation of flax shives into Vanillin and Pulp. Catalysts 2022, 12, 1003–1014. [Google Scholar] [CrossRef]
- Hughmark, G. Power requirements and interfacial area in gas-liquid turbine agitated aystems. Ind. Eng. Chem. Proc. Des. Dev. 1980, 19, 638–641. [Google Scholar] [CrossRef]
- Joshi, J.B.; Pandit, A.B.; Sharma, M.M. Mechanically agitated gas-liquid reactors. Chem. Eng. Sci. 1982, 37, 813–844. [Google Scholar] [CrossRef]
- Perry, J.H. Chemical Engineers’ Handbook, 4th ed.; McGRAW-Hill Book Company: New York, NY, USA, 1963; p. 93. [Google Scholar]
- Buwa, V.V.; Roy, S.; Ranade, V.V. Three-phase slurry reactors. In Multiphase Catalytic Reactors (Theory, Design, Manufacturing, and Applications); Önsan, Z.I., Avci, A.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 132–155. [Google Scholar] [CrossRef]
- Dardelet, S.; Froment, P.; Lacoste, N.; Robert, A. Vanilline et aldehyde syringique. Stabilite a l’oxydation en milieu alcalin par l’oxygene. Revue ATIP 1985, 39, 369–376. [Google Scholar]
- Casimiro, F.M.; Costa, C.A.E.; Botelho, C.M.; Barreiro, M.F.; Rodrigues, A.E. Kinetics of oxidative degradation of lignin-based phenolic compounds in batch reactor. Ind. Eng. Chem. Res. 2019, 58, 16442–16449. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Kaygorodov, K.L.; Vigul, D.O.; Tarabanko, N.; Chelbina, Y.V.; Smirnova, M.A. Influence of acid prehydrolysis on the process of wood oxidation into vanillin and pulp. J. Wood Chem. Technol. 2020, 40, 421–433. [Google Scholar] [CrossRef]
- Evstigneyev, E.I.; Zakusilo, D.N.; Ryabukhin, D.S.; Vasilyev, A.V. Recent advances in lignins: Fundamentals and applications. Russ. Chem. Rev. 2023, 92, RCR5082. [Google Scholar] [CrossRef]
- Mañas, A.H.; Vilcocq, L.; Fongarland, P.; Djakovitch, L. Lignin catalytic oxidation by CuO/TiO2: Role of catalyst in phenolics formation. Waste Biom. Valorization 2023, 14, 3789–3809. [Google Scholar] [CrossRef]
- Ramachandran, P.A.; Chaudhari, R.V. Three Phase Catalytic Reactors; Gordon and Breach Science Publishers: New York, NY, USA, 1983. [Google Scholar]
- Braginsky, L.N.; Begachev, V.I.; Barabash, E.M. Mixing in Liquid Media; -Khimiya: Leningrad, Russia, 1984; p. 91. [Google Scholar]
- Xing, W.; Yin, M.; Lv, Q.; Hu, Y.; Liu, C.; Zhang, J. Oxygen solubility, diffusion coefficient, and solution viscosity. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar] [CrossRef]
- Krylov, O.V. Heterogeneous Catalysis; Akademkniga: Moscow, Russia, 2004; pp. 98–101. [Google Scholar]

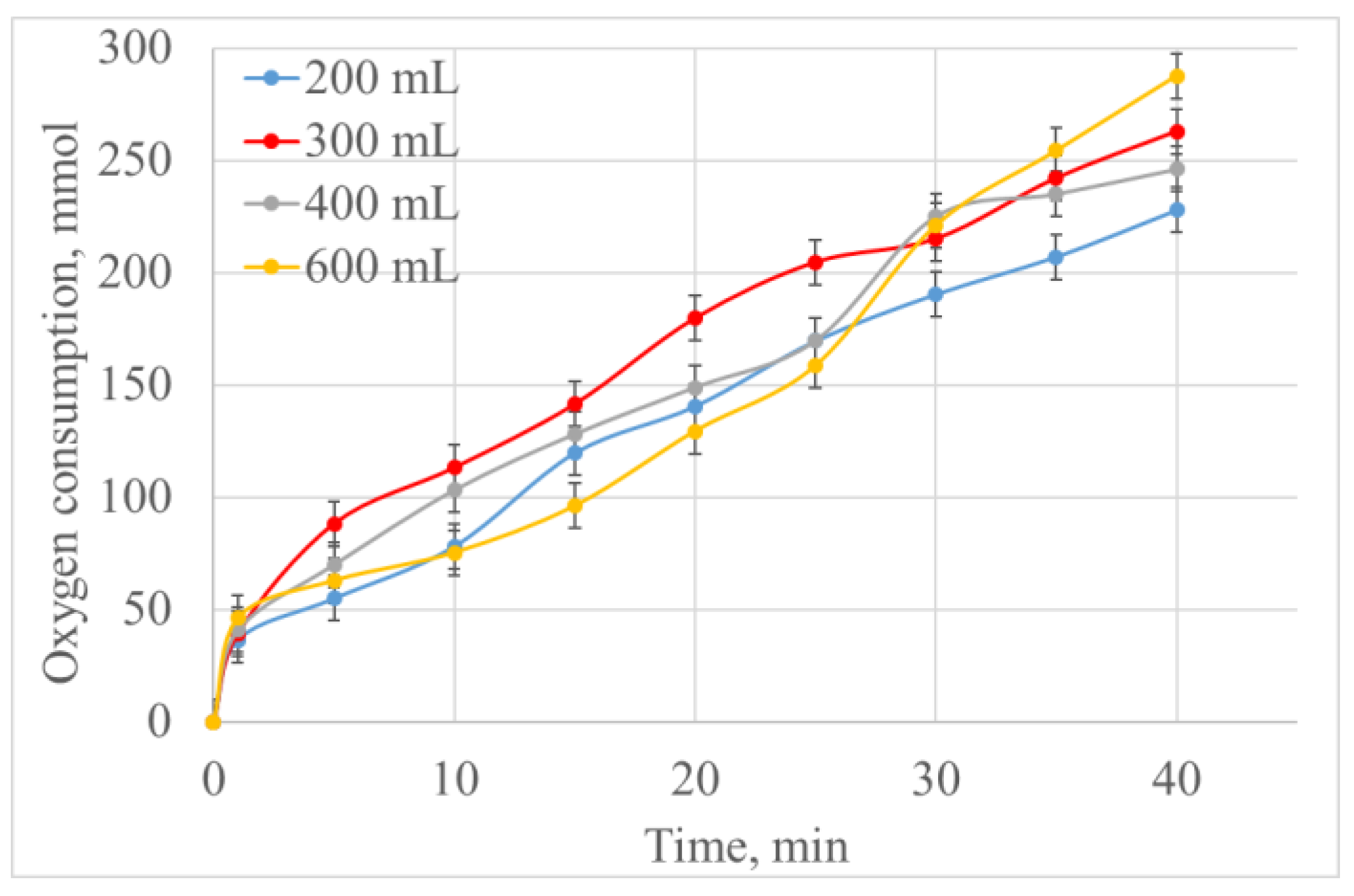

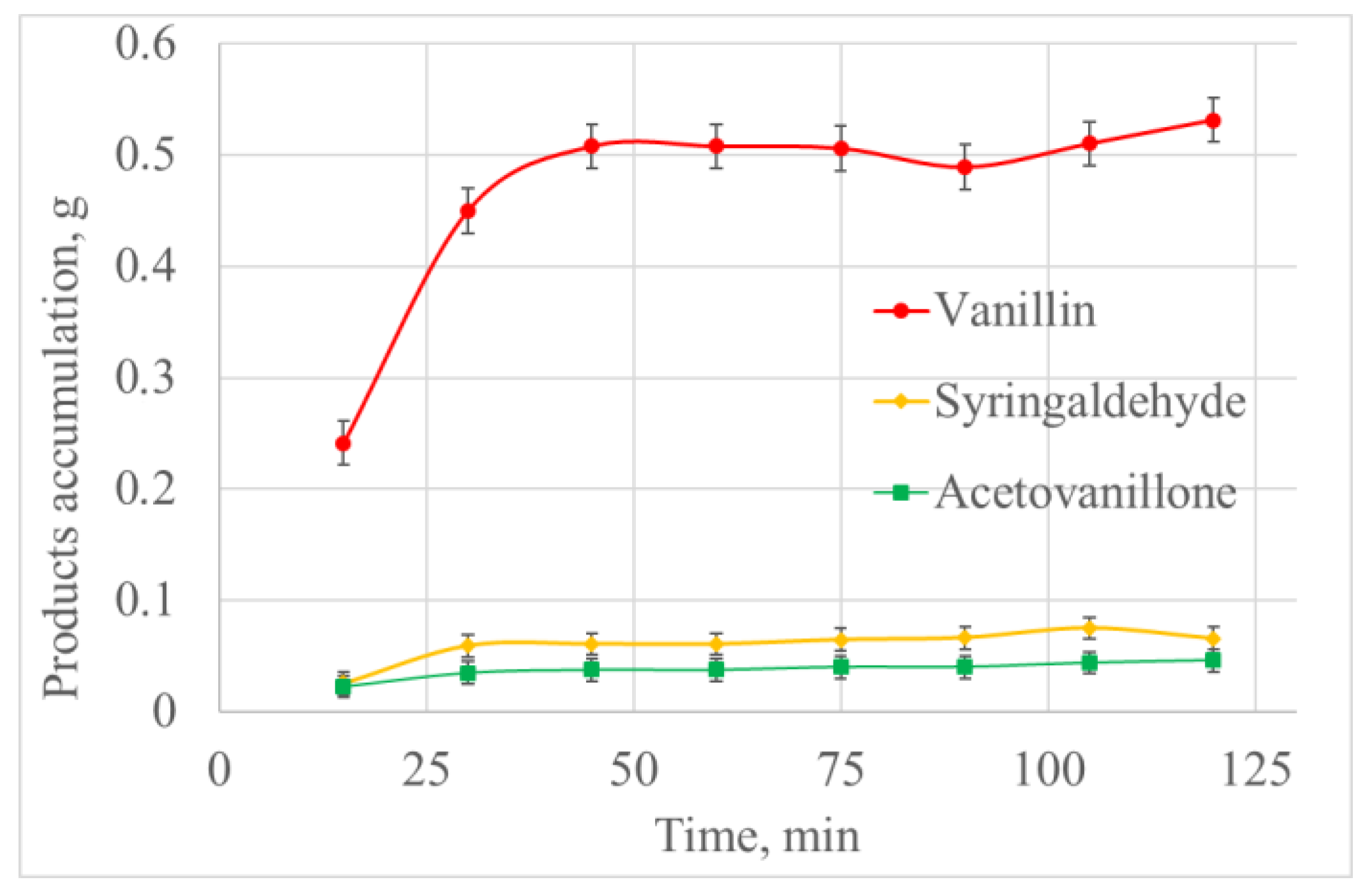
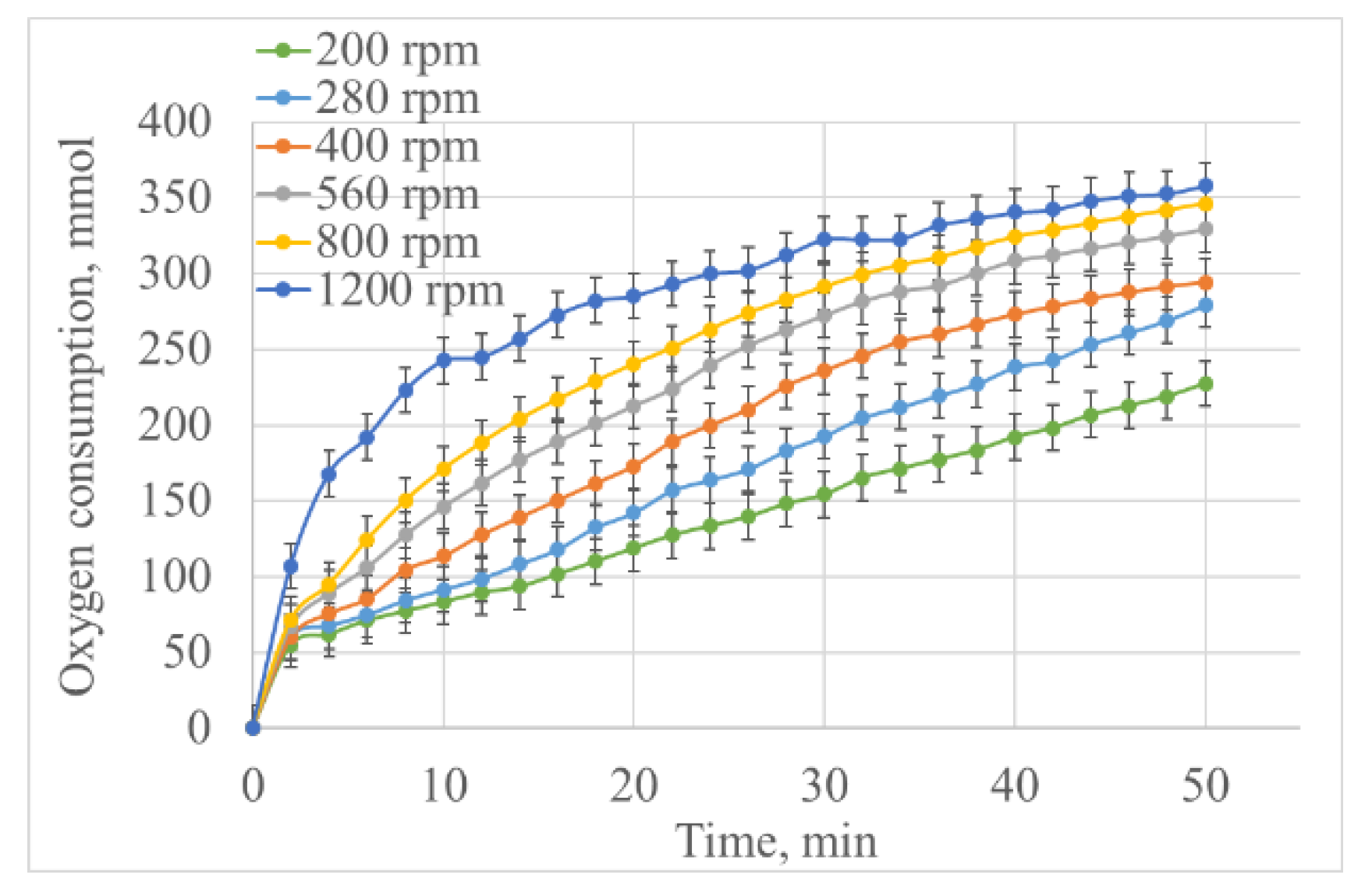
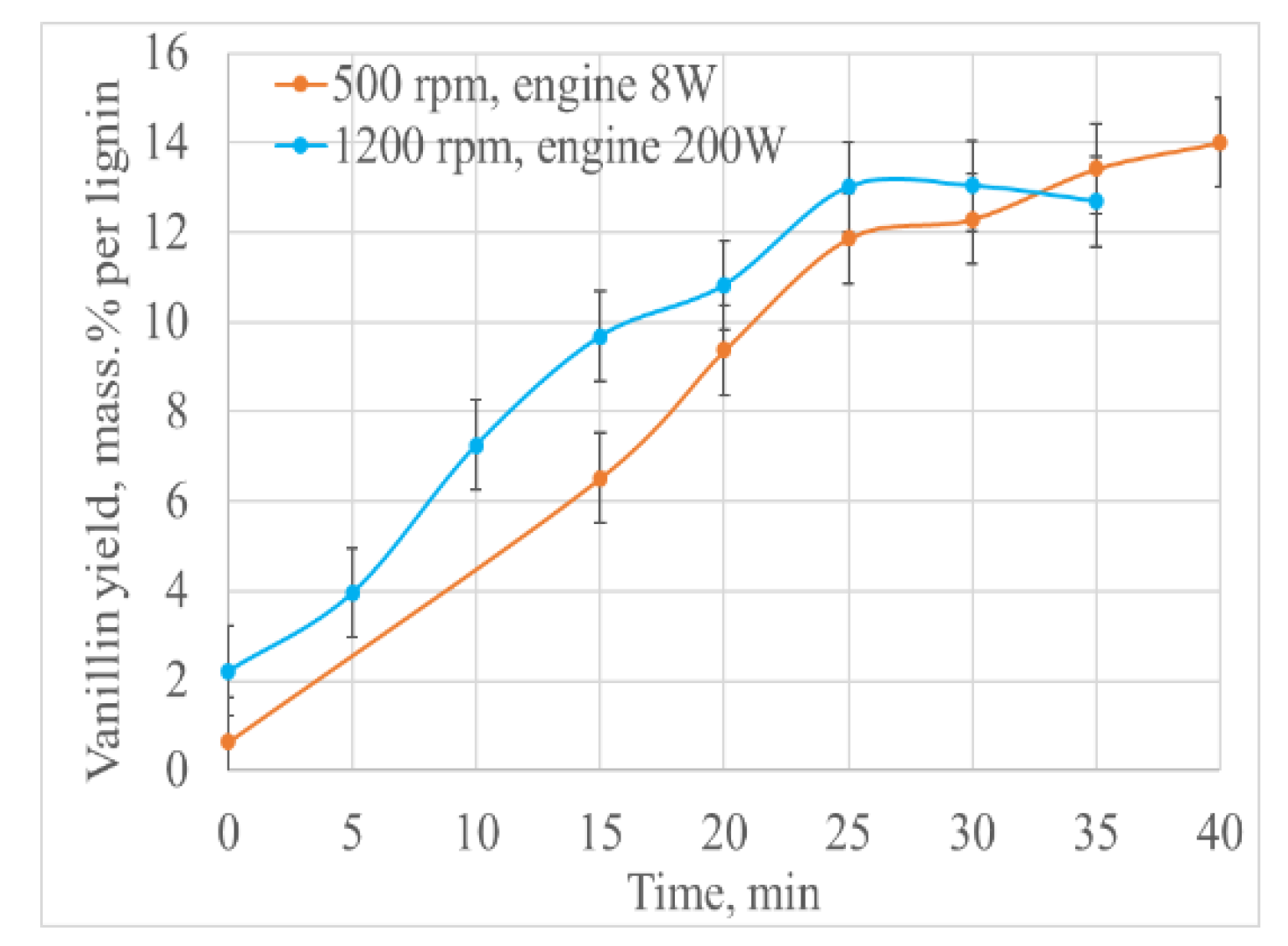

| Volume (mL) | Oxygen Consumption at 40 min (mmol) | Oxygen Consumption Rate (mmol/min) | Vanillin Yield (g) | Lignoacid Yield (g) | Vanillin Yield Based on the Initial Lignin (wt.%) | Lignoacid Yield Based on the Initial Lignin (wt.%) |
|---|---|---|---|---|---|---|
| 200 | 228 | 4.79 | 0.302 | 1.36 | 12.3 | 49 |
| 300 | 263 | 4.99 | 0.528 | 1.89 | 14.4 | 45 |
| 400 | 246 | 5.06 | 0.492 | 2.2 | 10.0 | 39 |
| 600 | 288 | 6.03 | 0.508 | 2.36 | 6.9 | 28 |
| Average results (300–600 mL) | 266 ± 14 | 5.36 ± 0.45 | 0.51 ± 0.02 | 2.13 ± 0.23 | - | - |
| Wstirrer (s−1) | Reaction Mixture Volume (mL) | k (min−1) | τ1/2 (min) |
|---|---|---|---|
| 3.33 | 300 | 0.021 | 32.9 |
| 5 | 300 | 0.026 | 26.5 |
| 6.66 | 300 | 0.023 | 30.0 |
| 8.33 | 300 | 0.025 | 27.6 |
| 8.33 | 200 | 0.024 | 28.75 |
| 8.33 | 300 | 0.025 | 27.6 |
| 8.33 | 400 | 0.024 | 28.75 |
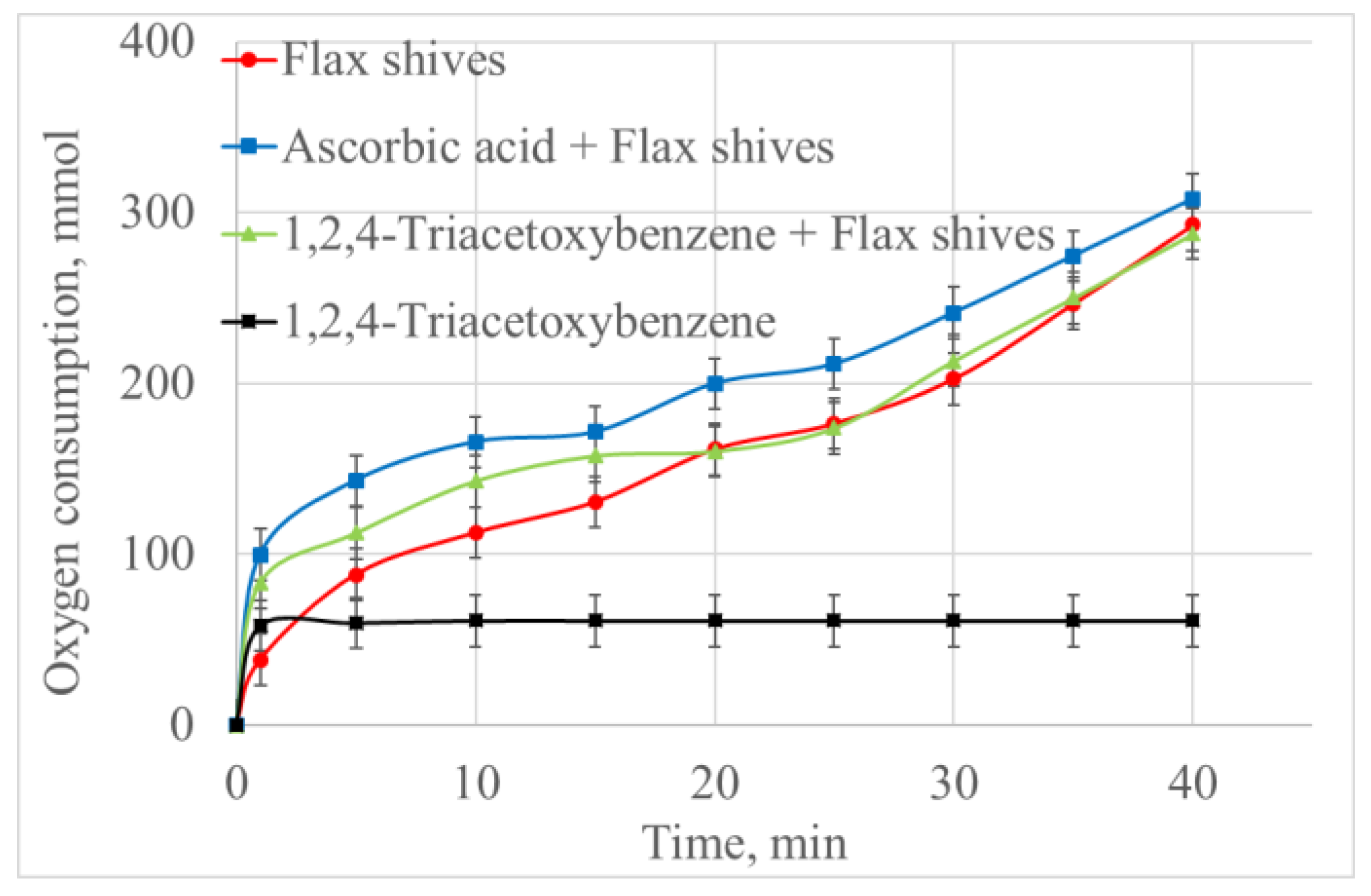
| t, min | Flax Shive | Ascorbic Acid + Flax shive | Pyrogallol A + Flax Shive | Pyrogallol A |
|---|---|---|---|---|
| Oxygen Consumption, mmol (Rate of Consumption, mmol/L∙s) | ||||
| 0 | 0 | 0 | 0 | 0 |
| 1 | 38.3 (2.1) | 99.9 (5.5) | 83.2 (4.6) | 58.2 (3.2) |
| 5 | 88.2 | 143.2 | 112.4 | 59.8 (0) |
| 10 | 112.7 (0.46) | 165.7 (0.41) | 142.77 (0.6) | 61.1 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarabanko, V.E.; Kaygorodov, K.L.; Kazachenko, A.S.; Smirnova, M.A.; Chelbina, Y.V.; Kosivtsov, Y.; Golubkov, V.A. Mass Transfer in the Processes of Native Lignin Oxidation into Vanillin via Oxygen. Catalysts 2023, 13, 1490. https://doi.org/10.3390/catal13121490
Tarabanko VE, Kaygorodov KL, Kazachenko AS, Smirnova MA, Chelbina YV, Kosivtsov Y, Golubkov VA. Mass Transfer in the Processes of Native Lignin Oxidation into Vanillin via Oxygen. Catalysts. 2023; 13(12):1490. https://doi.org/10.3390/catal13121490
Chicago/Turabian StyleTarabanko, Valery E., Konstantin L. Kaygorodov, Aleksandr S. Kazachenko, Marina A. Smirnova, Yulia V. Chelbina, Yury Kosivtsov, and Viktor A. Golubkov. 2023. "Mass Transfer in the Processes of Native Lignin Oxidation into Vanillin via Oxygen" Catalysts 13, no. 12: 1490. https://doi.org/10.3390/catal13121490
APA StyleTarabanko, V. E., Kaygorodov, K. L., Kazachenko, A. S., Smirnova, M. A., Chelbina, Y. V., Kosivtsov, Y., & Golubkov, V. A. (2023). Mass Transfer in the Processes of Native Lignin Oxidation into Vanillin via Oxygen. Catalysts, 13(12), 1490. https://doi.org/10.3390/catal13121490









