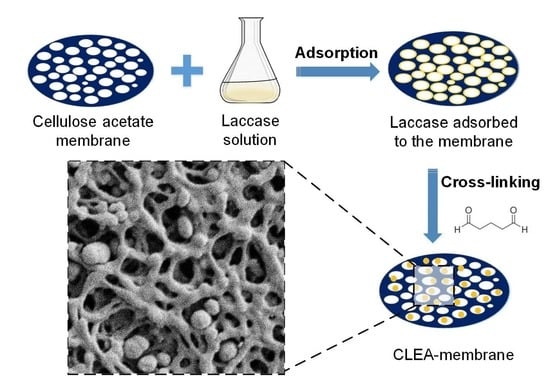
Design and Optimization of Laccase Immobilization in Cellulose Acetate Microfiltration Membrane for Micropollutant Remediation
Abstract
:1. Introduction
2. Results
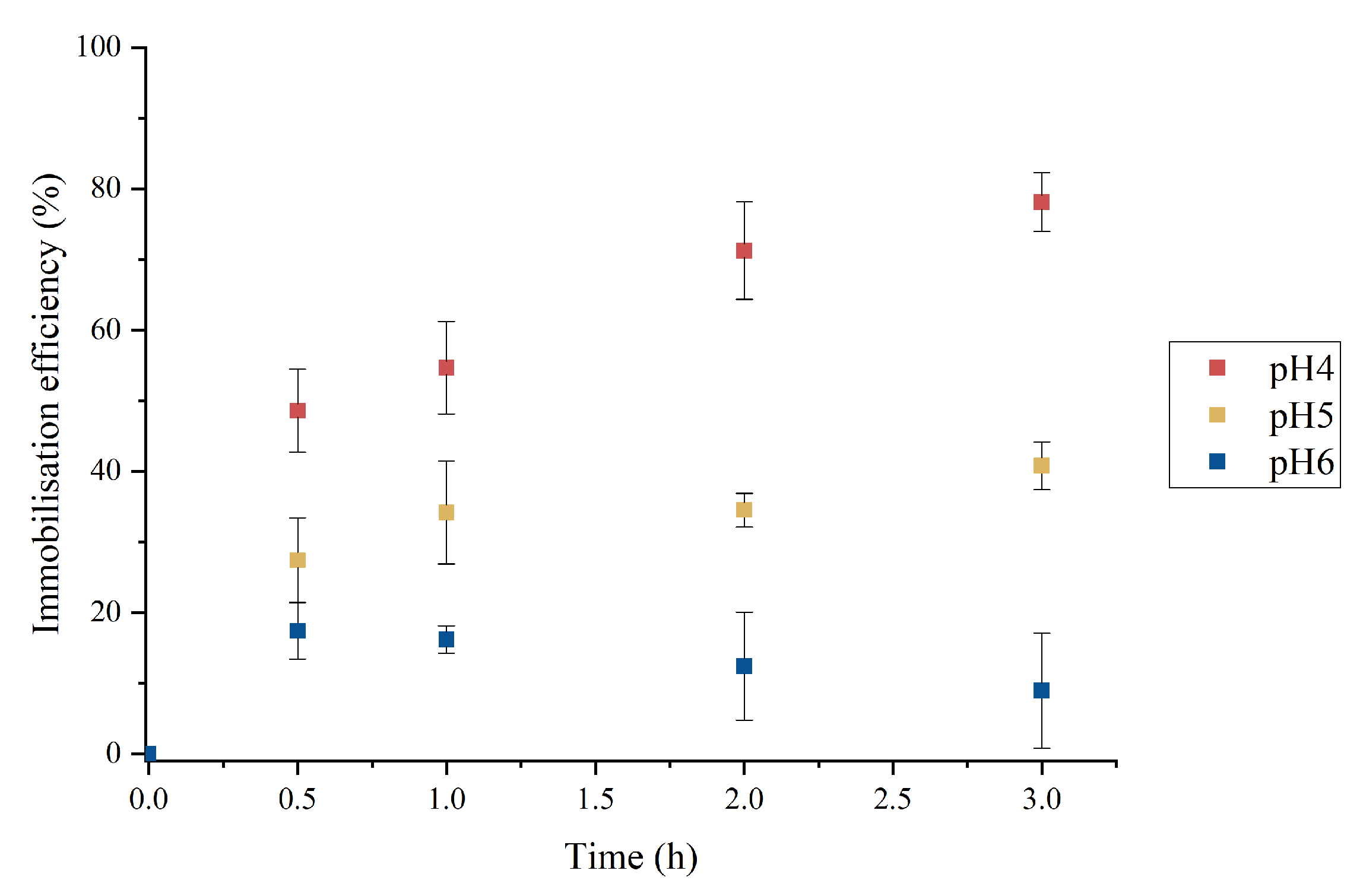
2.1. Effect of the pH
2.2. Thermal Denaturation of the Laccase
2.3. Optimization of Laccase Adsorption on the Membrane
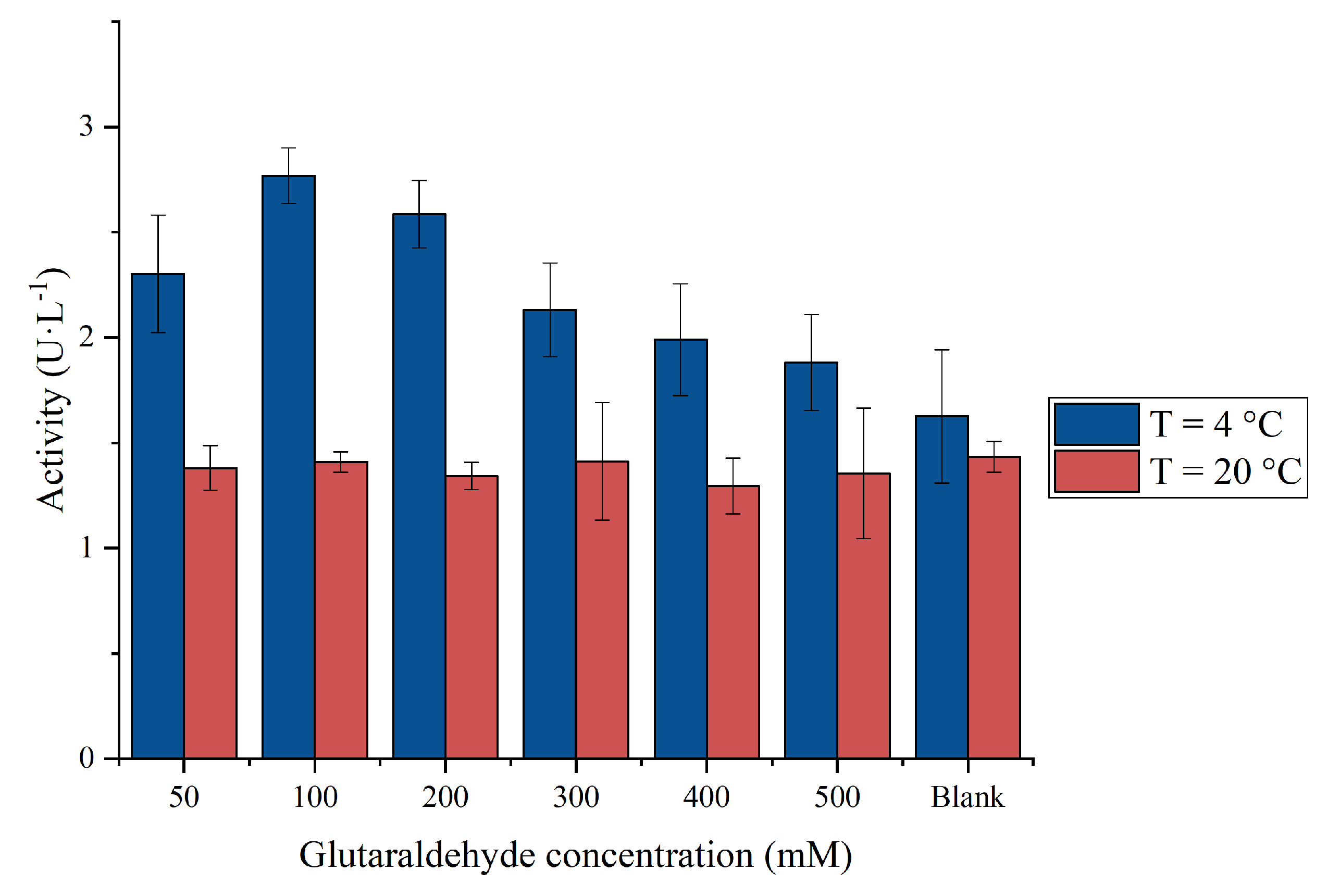
2.4. Effect of Cross-Linker Concentration
2.5. Storage Stability
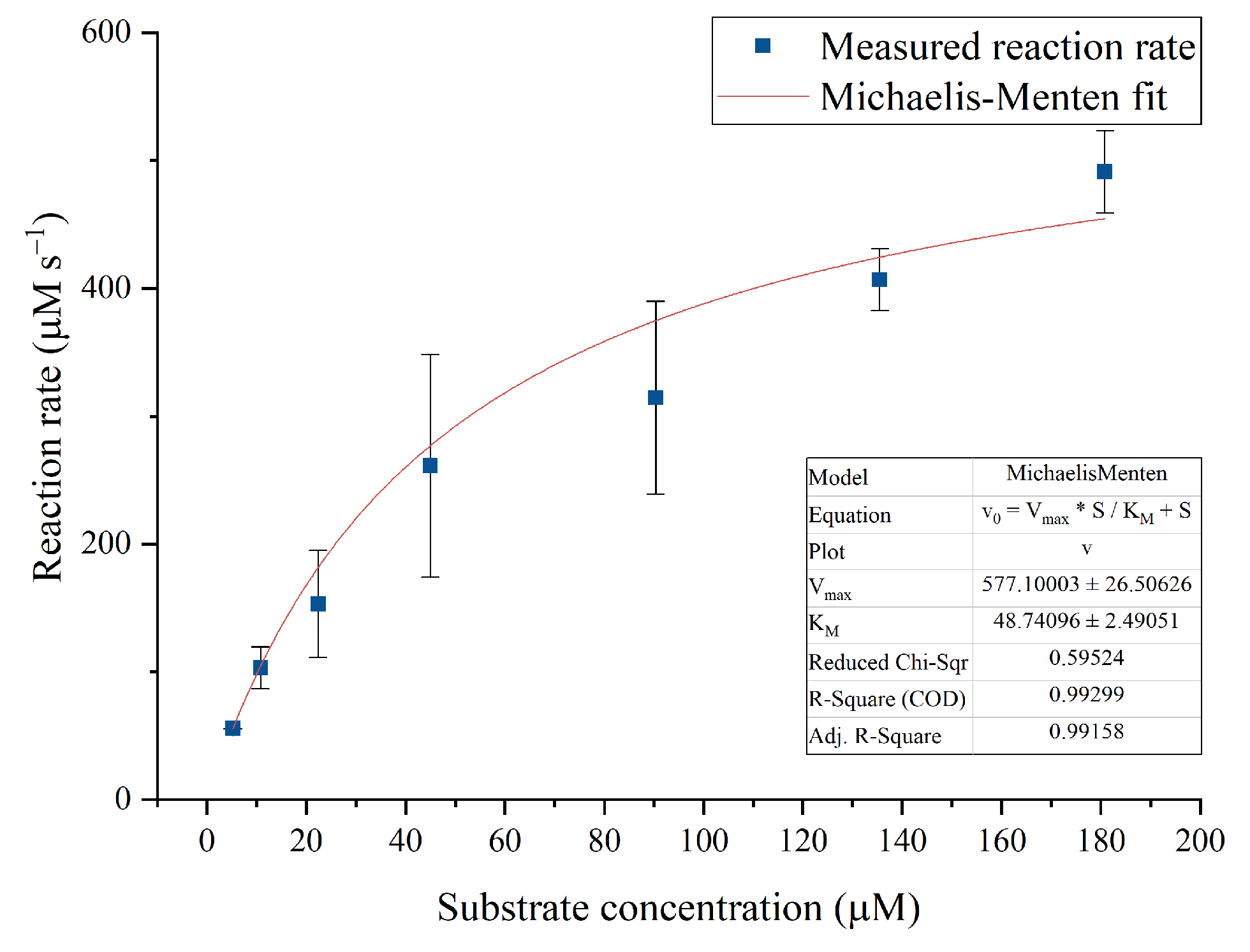
2.6. Membrane Activity and Enzyme Kinetics
2.7. Surface Analysis by Electron Microscopy
2.8. Effect of Transmembrane Pressure on Flux and Activity
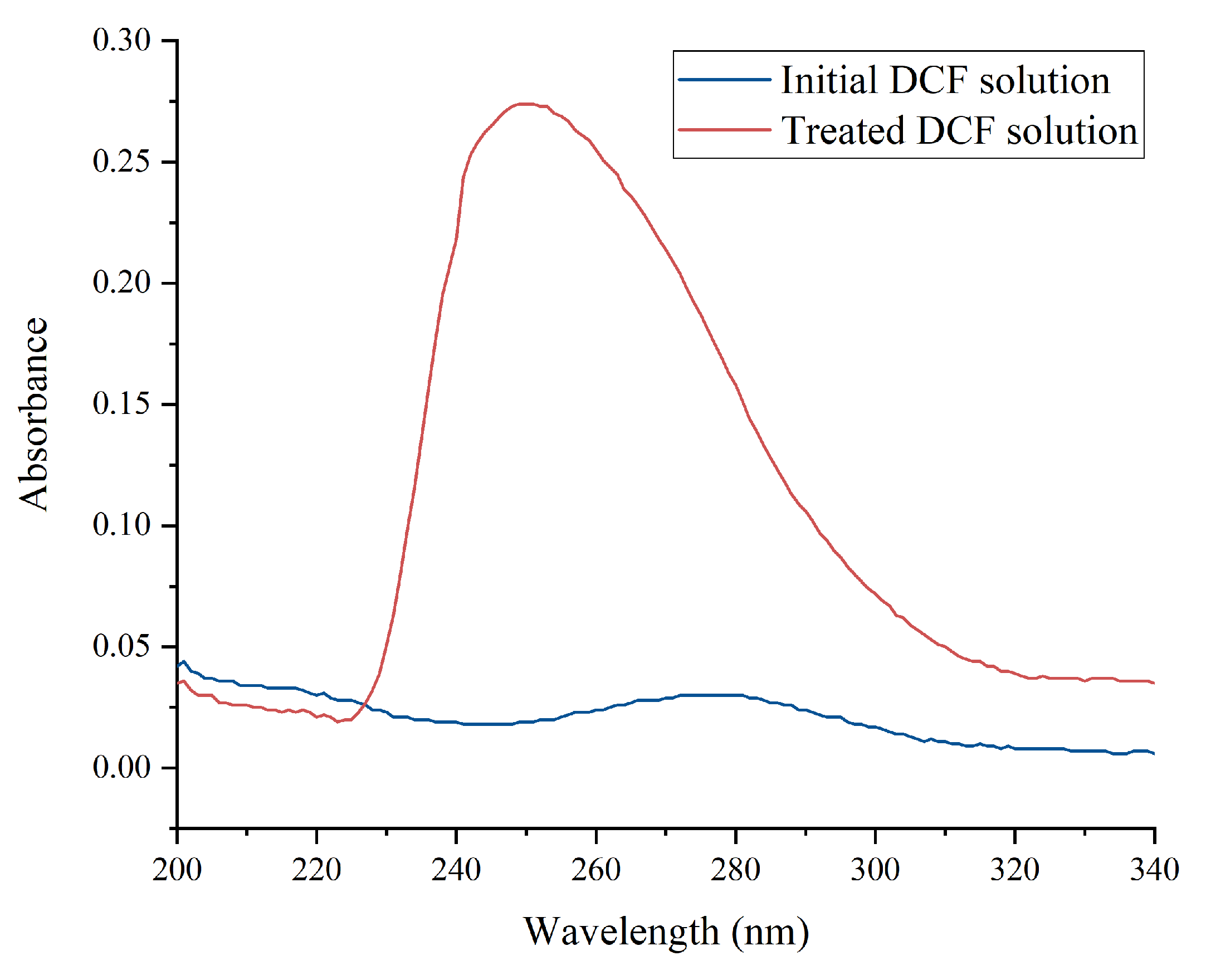
2.9. Degradation of Diclofenac
3. Materials and Methods
3.1. Materials
3.2. Activity Measurement
3.3. Immobilisation of Laccase
3.4. Optimisation
3.5. Surface Analysis
3.6. Effect of Substrate Concentration
3.7. Storage Stability
3.8. Transformation of Diclofenac Using Enzymatic Membrane
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | laccase enzyme activity measured with ABTS |
| Activity measured during the experiment (t). | |
| Activity measured at the beginning of the experiment (t = 0). | |
| ABTS | 2,2-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) |
| concentration in the permeate (M) | |
| concentration in the feed (M) | |
| EMR | enzymatic membrane reactor |
| k | first-order decay constant |
| Michaelis–Menten kinetic constant (M) | |
| OFAT | one factor at time method |
| Q | mass flow rate (g·s) |
| RSM | response surface methodology |
| S | membrane surface (cm) |
| SD | standard deviation |
| t | time (s) |
| membrane volume (cm) | |
| maximal reaction rate (M·) | |
| Extinction coefficient (Mcm) | |
| membrane porosity |
Appendix A
| A | B | C |
|---|---|---|
| −1 | −1 | −1 |
| 1 | −1 | −1 |
| −1 | 1 | −1 |
| 1 | 1 | −1 |
| −1 | −1 | 1 |
| 1 | −1 | 1 |
| −1 | 1 | 1 |
| 1 | 1 | 1 |
| −1 | 0 | 0 |
| 1 | 0 | 0 |
| 0 | −1 | 0 |
| 0 | 1 | 0 |
| 0 | 0 | −1 |
| 0 | 0 | 1 |
| 0 | 0 | 0 |
| 0 | 0 | 0 |
| 0 | 0 | 0 |
| 0 | 0 | 0 |
| 0 | 0 | 0 |
| 0 | 0 | 0 |
| Estimated Coefficients | Estimate | SE | t Stat | p Value |
|---|---|---|---|---|
| (Intercept) | 371.23 | 10.524 | 35.276 | |
| A | 92.8 | 9.0903 | 10.209 | |
| B | 46.1 | 9.0903 | 5.0714 | 0.00096339 |
| C | 45.5 | 9.0903 | 5.0053 | 0.0010458 |
| A:B | 25.625 | 10.163 | 2.5213 | 0.035734 |
| A:C | 23.875 | 10.163 | 2.3492 | 0.046743 |
| B:C | −16.875 | 10.163 | −1.6604 | 0.13541 |
| A | −51.01 | 17.39 | −2.9333 | 0.018903 |
| B | −9.5103 | 17.39 | −0.54688 | 0.59938 |
| C | −53.51 | 17.39 | −3.077 | 0.015185 |
| A:B:C | −7.375 | 10.163 | −0.72565 | 0.48873 |
| Sum SQ | DF | Mean SQ | F | p Value | |
|---|---|---|---|---|---|
| 1. Total | 18 | NaN | NaN | ||
| 2. Model | 10 | 22.6663 | 0.0001 | ||
| 3. Linear | 3 | 51.6633 | 0.0000 | ||
| 4. Nonlinear | 7 | 10.2390 | 0.0019 | ||
| 5. Residual | 8 | 826.3318 | NaN | NaN | |
| 6. Lack of fit | 4 | 10.6549 | 0.0208 | ||
| 7. Pure error | 567.2000 | 4 | 141.8000 | NaN | NaN |
References
- Barbosa, M.O.; Moreira, N.F.; Ribeiro, A.R.; Pereira, M.F.; Silva, A.M. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.F.; Lange, L.C.; Amaral, M.C. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process. Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Becker, D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.; et al. Removal of endocrine disrupting chemicals in wastewater by enzymatic treatment with fungal laccases. Org. Process. Res. Dev. 2017, 21, 480–491. [Google Scholar] [CrossRef]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D.; Al Aukidy, M.; Zambello, E. Removal of selected pharmaceuticals from domestic wastewater in an activated sludge system followed by a horizontal subsurface flow bed—Analysis of their respective contributions. Sci. Total Environ. 2013, 454–455, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Liu, J.; Cai, Y.; Liao, X.; Huang, Q.; Hao, Z.; Hu, M.; Zhang, D.; Li, Z. Efficiency of laccase production in a 65-L air-lift reactor for potential green industrial and environmental application. J. Clean. Prod. 2013, 39, 154–160. [Google Scholar] [CrossRef]
- Ardila-Leal, L.D.; Alvarado-Ramírez, M.F.; Gutiérrez-Rojas, I.S.; Poutou-Piñales, R.A.; Quevedo-Hidalgo, B.; Pérez-Flórez, A.; Pedroza-Rodríguez, A.M. Low-cost media statistical design for laccase rPOXA 1B production in P. pastoris. Heliyon 2020, 6, e03852. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Laccase immobilization for water purification: A comprehensive review. Chem. Eng. J. 2021, 403, 126272. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 2018, 234, 190–213. [Google Scholar] [CrossRef]
- Minteer, S.D. (Ed.) Enzyme Stabilization and Immobilization: Methods and Protocols; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1504. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R. Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci. Total Environ. 2017, 584−585, 393–401. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Meiczinger, M.; Salman, J.M.; Al-Juboori, R.A.; Hashim, K.S.; Somogyi, V.; Jakab, M. Ketoprofen and aspirin removal by laccase immobilized on date stones. Chemosphere 2023, 311, 137133. [Google Scholar] [CrossRef]
- Varga, B.; Meiczinger, M.; Somogyi, V. Immobilization of Laccase in Alginate Beads. Hung. J. Ind. Chem. 2019, 47, 17–23. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Chen, G.; Wang, Z. An Improved Method to Encapsulate Laccase from Trametes versicolor with Enhanced Stability and Catalytic Activity. Catalysts 2018, 8, 286. [Google Scholar] [CrossRef] [Green Version]
- Şahutoğlu, A.S.; Akgül, C. One-phase synthesis of single enzyme nanoparticles (SENs) of Trametes versicolor laccase by in situ acrylamide polymerisation. Biocatal. Biotransformation 2020, 38, 64–74. [Google Scholar] [CrossRef]
- Piao, M.; Zou, D.; Yang, Y.; Ren, X.; Qin, C.; Piao, Y. Multi-Functional Laccase Immobilized Hydrogel Microparticles for Efficient Removal of Bisphenol A. Materials 2019, 12, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matijošytė, I.; Arends, I.W.; de Vries, S.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B Enzym. 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Voběrková, S.; Solčány, V.; Vršanská, M.; Adam, V. Immobilization of ligninolytic enzymes from white-rot fungi in cross-linked aggregates. Chemosphere 2018, 202, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, X.; Yang, X.; Ye, X.; Lin, J. Cross-linked enzyme aggregates of Cerrena laccase: Preparation, enhanced NaCl tolerance and decolorization of Remazol Brilliant Blue Reactive. J. Taiwan Inst. Chem. Eng. 2016, 65, 1–7. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement—A review. Biocatalysis 2016, 1, 166–177. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Seow, N.; Yang, K.L. Hollow cross-linked enzyme aggregates (h-CLEA) of laccase with high uniformity and activity. Colloids Surf.B Biointerfaces 2017, 151, 88–94. [Google Scholar] [CrossRef]
- Qing, W.; Li, X.; Shao, S.; Shi, X.; Wang, J.; Feng, Y.; Zhang, W.; Zhang, W. Polymeric catalytically active membranes for reaction-separation coupling: A review. J. Membr. Sci. 2019, 583, 118–138. [Google Scholar] [CrossRef]
- Kujawa, J.; Głodek, M.; Li, G.; Al-Gharabli, S.; Knozowska, K.; Kujawski, W. Highly effective enzymes immobilization on ceramics: Requirements for supports and enzymes. Sci. Total Environ. 2021, 801, 149647, MAG ID: 3195348418. [Google Scholar] [CrossRef] [PubMed]
- Rios, G.; Belleville, M.; Paolucci, D.; Sanchez, J. Progress in enzymatic membrane reactors—A review. J. Membr. Sci. 2004, 242, 189–196. [Google Scholar] [CrossRef]
- Catherine, H.; Penninckx, M.; Frédéric, D. Product formation from phenolic compounds removal by laccases: A review. Environ. Technol. Innov. 2016, 5, 250–266. [Google Scholar] [CrossRef]
- Asif, M.B.; Nguyen, L.N.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Integration of an enzymatic bioreactor with membrane distillation for enhanced biodegradation of trace organic contaminants. Int. Biodeterior. Biodegrad. 2017, 124, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Pype, R.; Septavaux, J.; Oulad Haj Amar, B.; Debaste, F. Polymerization and formation of insoluble byproducts of diclofenac using Trametes versicolor laccases—Experimental study and modelling. J. Water Process. Eng. 2019, 32, 100948. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Neo, K.R.S.; Yang, K.L. Continuous hydrolysis of carboxymethyl cellulose with cellulase aggregates trapped inside membranes. Enzym. Microb. Technol. 2015, 78, 34–39. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.; Roddick, F.; McAdam, E.J.; Magram, S.F.; Nghiem, L.D. Continuous biotransformation of bisphenol A and diclofenac by laccase in an enzymatic membrane reactor. Int. Biodeterior. Biodegrad. 2014, 95, 25–32, Challenges in Environmental Science and Engineering, CESE-2013. [Google Scholar] [CrossRef] [Green Version]
- de Cazes, M.; Belleville, M.P.; Mougel, M.; Kellner, H.; Sanchez-Marcano, J. Characterization of laccase-grafted ceramic membranes for pharmaceuticals degradation. J. Membr. Sci. 2015, 476, 384–393, MAG ID: 2017584998. [Google Scholar] [CrossRef]
- Edwards, W.; Leukes, W.; Rose, P.; Burton, S. Immobilization of polyphenol oxidase on chitosan-coated polysulphone capillary membranes for improved phenolic effluent bioremediation. Enzym. Microb. Technol. 1999, 25, 769–773. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; He, H.; Zhu, Y.; Dionysiou, D.D.; Liu, Z.; Zhao, C. Do membrane filtration systems in drinking water treatment plants release nano/microplastics? Sci. Total Environ. 2021, 755, 142658. [Google Scholar] [CrossRef] [PubMed]
- Masjoudi, M.; Golgoli, M.; Ghobadi Nejad, Z.; Sadeghzadeh, S.; Borghei, S.M. Pharmaceuticals removal by immobilized laccase on polyvinylidene fluoride nanocomposite with multi-walled carbon nanotubes. Chemosphere 2021, 263, 128043. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Enzymatic degradation of bisphenol-A with immobilized laccase on TiO2 sol–gel coated PVDF membrane. J. Membr. Sci. 2014, 469, 19–30. [Google Scholar] [CrossRef]
- Uragami, T.; Chakraborty, S.; Piemonte, V.; Di Paola, L. Biocatalytic membrane reactors: Principles, preparation and biotechnological, pharmaceutical and medical applications. In Handbook of Membrane Reactors; Woodhead Publishing: Cambridge, UK, 2013; pp. 846–887. [Google Scholar] [CrossRef]
- Abejón, R.; Belleville, M.; Sanchez-Marcano, J. Design, economic evaluation and optimization of enzymatic membrane reactors for antibiotics degradation in wastewaters. Sep. Purif. Technol. 2015, 156, 183–199. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef] [Green Version]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Choi, J.; Fukushi, K.; Yamamoto, K. A submerged nanofiltration membrane bioreactor for domestic wastewater treatment: The performance of cellulose acetate nanofiltration membranes for long-term operation. Sep. Purif. Technol. 2007, 52, 470–477. [Google Scholar] [CrossRef]
- Saoudi, O.; Ghaouar, N. Biocatalytic characterization of free and immobilized laccase from Trametes versicolor in its activation zone. Int. J. Biol. Macromol. 2019, 128, 681–691. [Google Scholar] [CrossRef]
- Rogalski, J.; Dawidowicz, A.L.; Leonowicz, A. Purification and immobilization of the inducible form of extracellular laccase of the fungus trametes versicolor. Acta Biotechnol. 1990, 10, 261–269. [Google Scholar] [CrossRef]
- Chea, V.; Paolucci-Jeanjean, D.; Belleville, M.; Sanchez, J. Optimization and characterization of an enzymatic membrane for the degradation of phenolic compounds. Catal. Today 2012, 193, 49–56. [Google Scholar] [CrossRef]
- Kurniawati, S.; Nicell, J.A. Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour. Technol. 2008, 99, 7825–7834. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hlady, V.; Buijs, J.; Jennissen, H.P. Methods for studying protein adsorption. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 309, pp. 402–429. [Google Scholar] [CrossRef] [Green Version]
- Koromilas, N.D.; Anastasopoulos, C.; Oikonomou, E.K.; Kallitsis, J.K. Preparation of Porous Polymeric Membranes Based on a Pyridine Containing Aromatic Polyether Sulfone. Polymers 2019, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Loos, R.; Mariani, G.; Paracchini, B.; Ricci, M.; Suurkuusk, G.; Gawlik, B.; Tavazzi, S. Water Framework Directive, Watch List Method: Analysis of Diclofenac in Water: Validation Report, According to ISO 17025 Requirements; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar] [CrossRef]
- Saarinen, T.; Orelma, H.; Grönqvist, S.; Andberg, M.; Holappa, S.; Laine, J. Adsorption of different laccases on cellulose and lignin surfaces. BioResources 2009, 4, 94–110. [Google Scholar]
- Bertrand, B.; Martínez-Morales, F.; Tinoco-Valencia, R.; Rojas, S.; Acosta-Urdapilleta, L.; Trejo-Hernández, M.R. Biochemical and molecular characterization of laccase isoforms produced by the white-rot fungus Trametes versicolor under submerged culture conditions. J. Mol. Catal. B Enzym. 2015, 122, 339–347. [Google Scholar] [CrossRef]
- Norde, W. Driving forces for protein adsorption at solid surfaces. Macromol. Symp. 1996, 103, 5–18. [Google Scholar] [CrossRef]
- Li, N.; Zheng, J.; Hadi, P.; Yang, M.; Huang, X.; Ma, H.; Walker, H.; Hsiao, B. Synthesis and Characterization of a High Flux Nanocellulose–Cellulose Acetate Nanocomposite Membrane. Membranes 2019, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Pentari, C.; Topakas, E. Crosslinked Enzyme Aggregates (CLEAs) of Laccases from Pleurotus citrinopileatus Induced in Olive Oil Mill Wastewater (OOMW). Molecules 2020, 25, 2221. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Boeuf, G.; Zhu, K.; Jia, Z.; Kanaev, A.; Azouani, R.; Wu, Z.; Traore, M.; Elm’selmi, A. Laccase Cross-Linked Ultraporous Aluminas for Sustainable Biodegradation of Remazol Brilliant Blue R. Catalysts 2022, 12, 744. [Google Scholar] [CrossRef]
- Lante, A.; Crapisi, A.; Krastanov, A.; Spettoli, P. Biodegradation of phenols by laccase immobilised in a membrane reactor. Process. Biochem. 2000, 36, 51–58. [Google Scholar] [CrossRef]
- Nagy, E.; Lepossa, A.; Prettl, Z. Mass Transfer through a Biocatalytic Membrane Reactor. Ind. Eng. Chem. Res. 2012, 51, 1635–1646. [Google Scholar] [CrossRef]
- Lorenzo, M.; Moldes, D.; Rodriguez Couto, S.; Sanromán, M. Inhibition of laccase activity from Trametes versicolor by heavy metals and organic compounds. Chemosphere 2005, 60, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.M.; Padrão, J.; Faria, J.; Silva, J.P.; Silva, C.J.; Dourado, F.; Zille, A. Laccase immobilization on bacterial nanocellulose membranes: Antimicrobial, kinetic and stability properties. Carbohydr. Polym. 2016, 145, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurniawati, S.; Nicell, J.A. Kinetic model of laccase-catalyzed oxidation of aqueous phenol. Biotechnol. Bioeng. 2005, 91, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Gea, M.; Bicchi, C.; Cordero, C.; Schilirò, T.; Varese, G.C. Ecofriendly laccases treatment to challenge micropollutants issue in municipal wastewaters. Environ. Pollut. 2020, 257, 113579–113589. [Google Scholar] [CrossRef]
- Hilal, N.; Nigmatullin, R.; Alpatova, A. Immobilization of cross-linked lipase aggregates within microporous polymeric membranes. J. Membr. Sci. 2004, 238, 131–141. [Google Scholar] [CrossRef]
- Jochems, P.; Satyawali, Y.; Diels, L.; Dejonghe, W. Enzyme immobilization on/in polymeric membranes: Status, challenges and perspectives in biocatalytic membrane reactors (BMRs). Green Chem. 2011, 13, 1609. [Google Scholar] [CrossRef]
- Ezike, T.C.; Udeh, J.O.; Joshua, P.E.; Ezugwu, A.L.; Isiwu, C.V.; Eze, S.O.; Chilaka, F.C. Substrate specificity of a new laccase from Trametes polyzona WRF03. Heliyon 2021, 7, e06080. [Google Scholar] [CrossRef]
- Primožič, M.; Kravanja, G.; Željko, K.; Crnjac, A.; Leitgeb, M. Immobilized laccase in the form of (magnetic) cross-linked enzyme aggregates for sustainable diclofenac (bio)degradation. J. Clean. Prod. 2020, 275, 124121. [Google Scholar] [CrossRef]
- Nair, R.R.; Demarche, P.; Agathos, S.N. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. New Biotechnol. 2013, 30, 814–823, Biotechnology for the Bio and Green Economy. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar] [CrossRef] [PubMed]
- Meiczinger, M.; Varga, B.; Wolmarans, L.; Hajba, L.; Somogyi, V. Stability improvement of laccase for micropollutant removal of pharmaceutical origins from municipal wastewater. Clean Technol. Environ. Policy 2022, 24, 3213–3223. [Google Scholar] [CrossRef]
- Margot, J.; Maillard, J.; Rossi, L.; Barry, D.; Holliger, C. Influence of treatment conditions on the oxidation of micropollutants by Trametes versicolor laccase. New Biotechnol. 2013, 30, 803–813, Biotechnology for the Bio and Green Economy. [Google Scholar] [CrossRef] [PubMed]


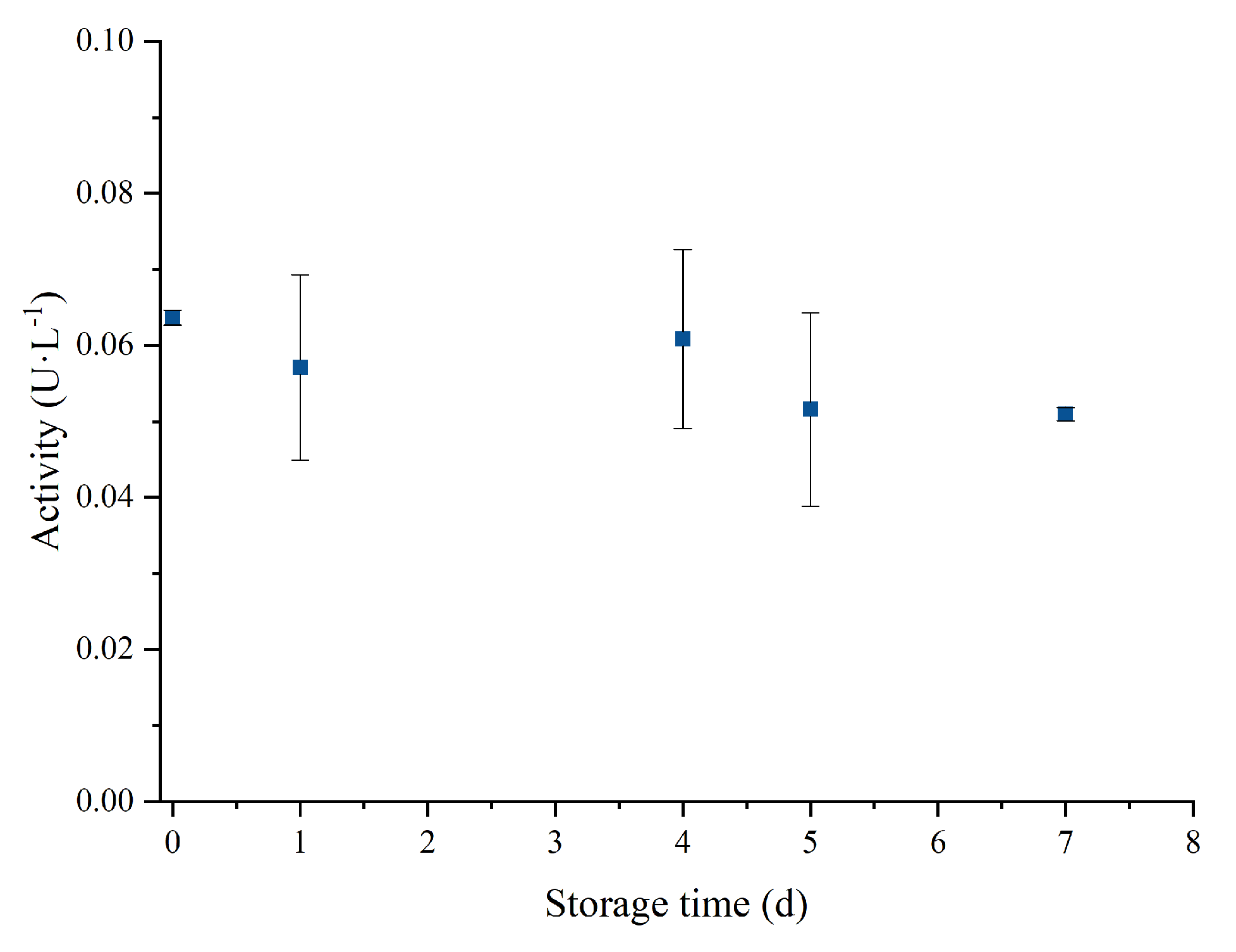


| No.1 | Laccase Concentration (mg·mL) | Adsorption Time (h) | Temperature (C) | Immobilisation Efficiency | Immobilized Activity (U·L) |
|---|---|---|---|---|---|
| 1 | 1 | 3 | 15 | 0.38 | 108 |
| 2 | 3 | 3 | 15 | 0.20 | 172 |
| 3 | 1 | 9 | 15 | 0.57 | 162 |
| 4 | 3 | 9 | 15 | 0.42 | 358 |
| 5 | 1 | 3 | 35 | 0.65 | 185 |
| 6 | 3 | 3 | 35 | 0.44 | 374 |
| 7 | 1 | 9 | 35 | 0.71 | 201 |
| 8 | 3 | 9 | 35 | 0.54 | 463 |
| 9 | 1 | 6 | 25 | 0.81 | 229 |
| 10 | 3 | 6 | 25 | 0.52 | 446 |
| 11 | 2 | 3 | 25 | 0.57 | 321 |
| 12 | 2 | 9 | 25 | 0.77 | 437 |
| 13 | 2 | 6 | 15 | 0.56 | 319 |
| 14 | 2 | 6 | 35 | 0.62 | 351 |
| 15 | 2 | 6 | 25 | 0.66 | 375 |
| 16 | 2 | 6 | 25 | 0.64 | 364 |
| 17 | 2 | 6 | 25 | 0.61 | 346 |
| 18 | 2 | 6 | 25 | 0.62 | 350 |
| 19 | 2 | 6 | 25 | 0.62 | 352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, B.; Meiczinger, M.; Jakab, M.; Somogyi, V. Design and Optimization of Laccase Immobilization in Cellulose Acetate Microfiltration Membrane for Micropollutant Remediation. Catalysts 2023, 13, 222. https://doi.org/10.3390/catal13020222
Varga B, Meiczinger M, Jakab M, Somogyi V. Design and Optimization of Laccase Immobilization in Cellulose Acetate Microfiltration Membrane for Micropollutant Remediation. Catalysts. 2023; 13(2):222. https://doi.org/10.3390/catal13020222
Chicago/Turabian StyleVarga, Béla, Mónika Meiczinger, Miklós Jakab, and Viola Somogyi. 2023. "Design and Optimization of Laccase Immobilization in Cellulose Acetate Microfiltration Membrane for Micropollutant Remediation" Catalysts 13, no. 2: 222. https://doi.org/10.3390/catal13020222
APA StyleVarga, B., Meiczinger, M., Jakab, M., & Somogyi, V. (2023). Design and Optimization of Laccase Immobilization in Cellulose Acetate Microfiltration Membrane for Micropollutant Remediation. Catalysts, 13(2), 222. https://doi.org/10.3390/catal13020222