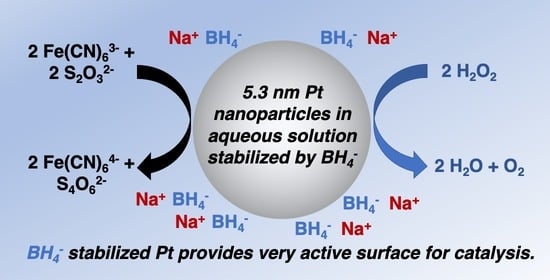
Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications
Abstract
:1. Introduction
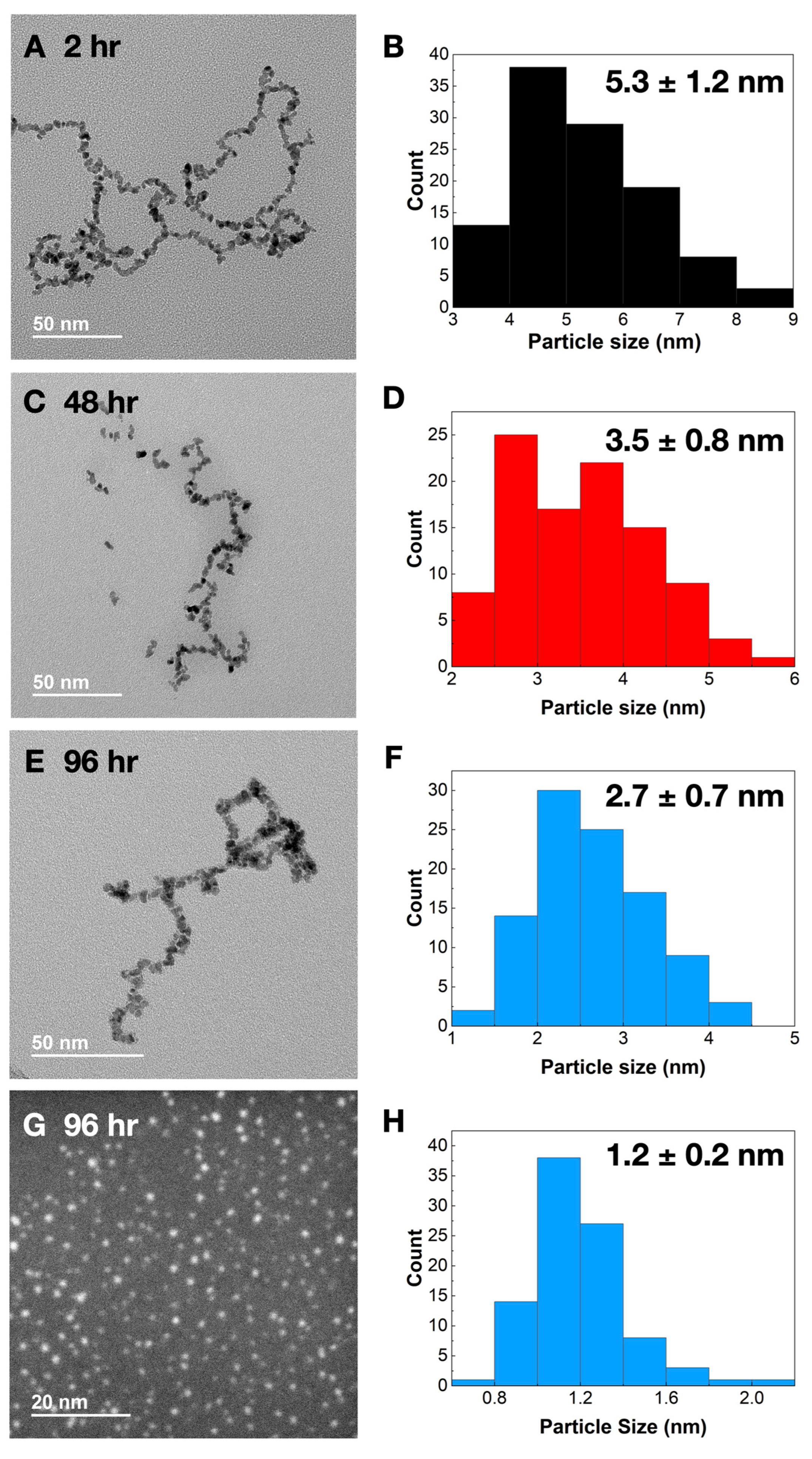
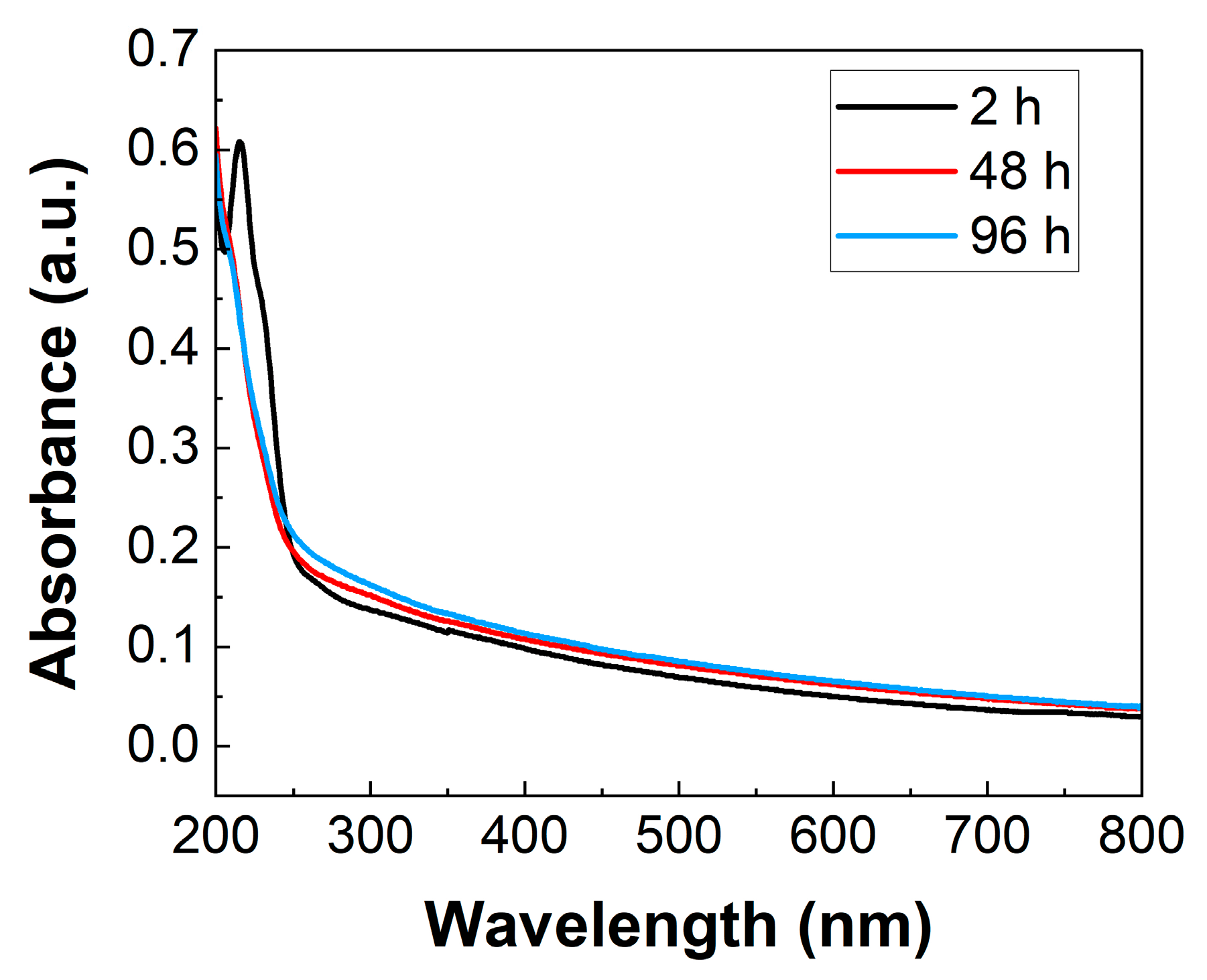
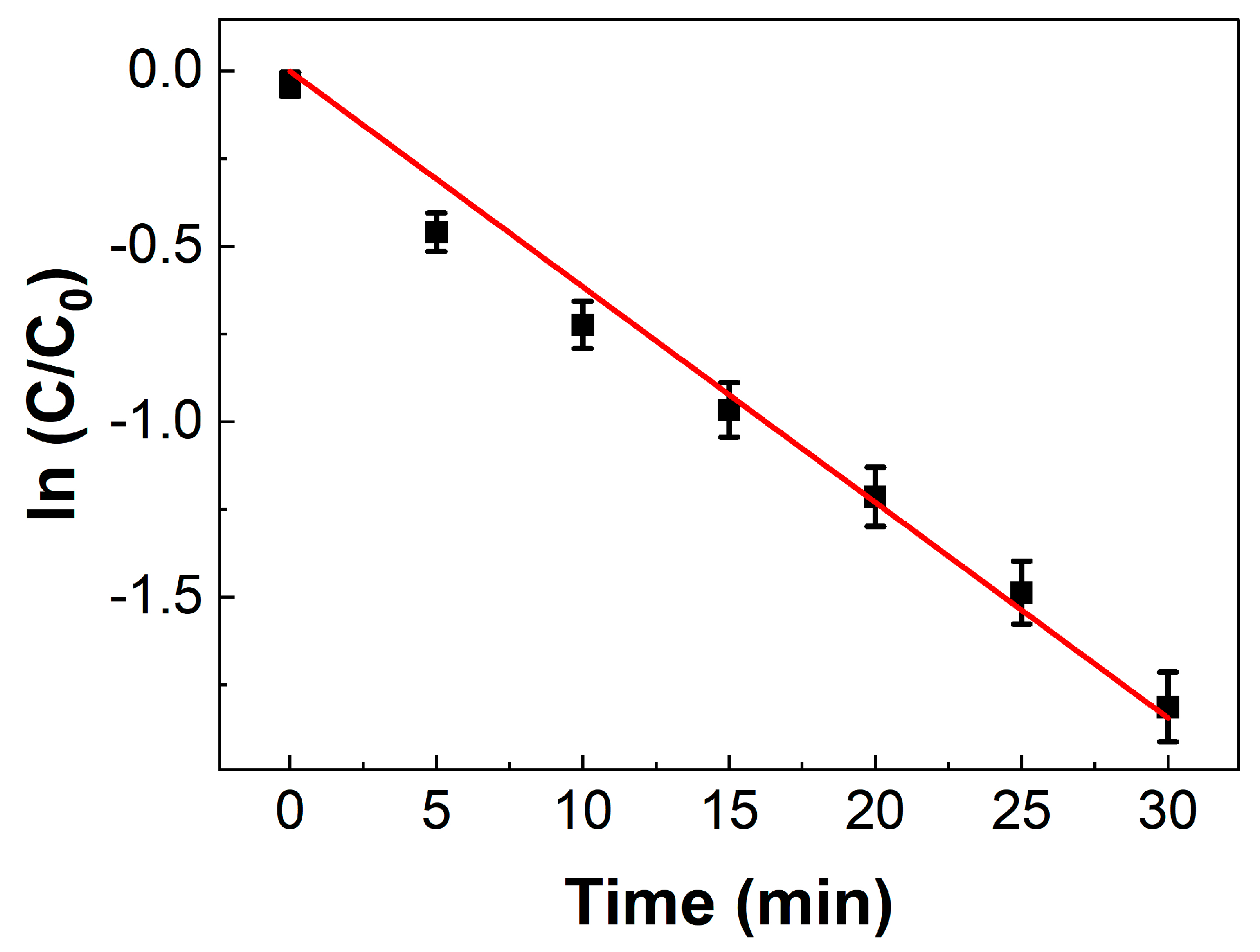
2. Results
2.1. Synthesis and Characterization
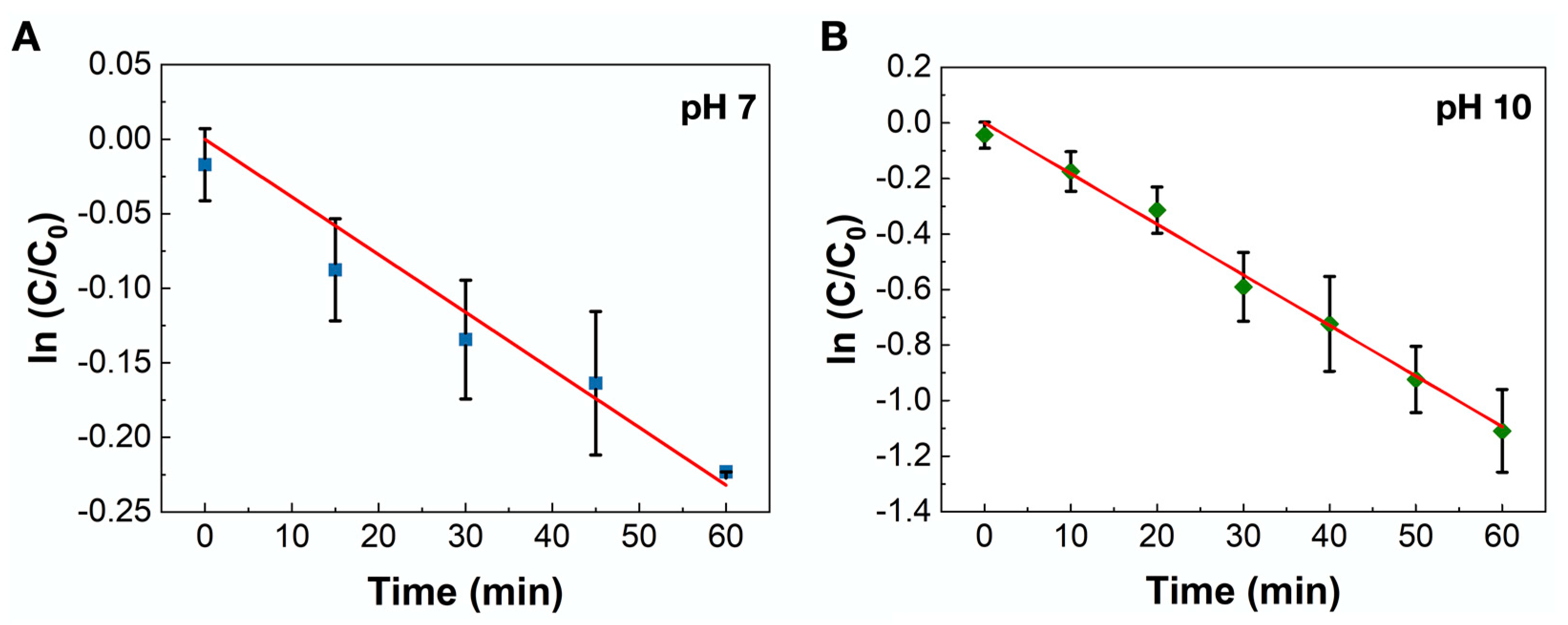
2.2. Catalytic Reaction Testing
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Platinum Nanoparticle Synthesis
3.3. UV-Vis Spectroscopic Measurements
3.4. Transmission Electron Microscopy Measurements
3.5. Pt-Catalyzed Hydrogen Peroxide Decomposition
3.6. Pt-Catalyzed Electron-Transfer Reaction between Hexacyanoferrate(III) and Thiosulfate Ions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Wang, Z.; Ye, B.; Huang, X.; Deng, H. Ligand effect over gold nanocatalysts towards enhanced gas-phase oxidation of alcohols. J. Catal. 2021, 400, 274–282. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Lei, Z.; Guan, Z.-J.; Wang, Q.-M. Atomically Precise Preorganization of Open Metal Sites on Gold Nanoclusters with High Catalytic Performance. Angew. Chem. Int. Ed. 2021, 60, 5225–5229. [Google Scholar] [CrossRef] [PubMed]
- Brindle, J.; Sufyan, S.A.; Nigra, M.M. Support, composition, and ligand effects in partial oxidation of benzyl alcohol using gold–copper clusters. Catal. Sci. Technol. 2022, 12, 3846–3855. [Google Scholar] [CrossRef]
- Tsunoyama, H.; Sakurai, H.; Negishi, Y.; Tsukuda, T. Size-Specific Catalytic Activity of Polymer-Stabilized Gold Nanoclusters for Aerobic Alcohol Oxidation in Water. J. Am. Chem. Soc. 2005, 127, 9374–9375. [Google Scholar] [CrossRef]
- Sufyan, S.A.; van Devener, B.; Perez, P.; Nigra, M.M. Electronic Tuning of Gold Nanoparticle Active Sites for Reduction Catalysis. ACS Appl. Mater. Interfaces 2023, 15, 1210–1218. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, G.; Jiang, D.-e.; Mullins, D.R.; Zhang, Q.-F.; Allard, L.F.; Wang, L.-S.; Overbury, S.H. Diphosphine-Protected Au22 Nanoclusters on Oxide Supports Are Active for Gas-Phase Catalysis without Ligand Removal. Nano Lett. 2016, 16, 6560–6567. [Google Scholar] [CrossRef]
- Kapil, N.; Weissenberger, T.; Cardinale, F.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.-O. Precisely Engineered Supported Gold Clusters as a Stable Catalyst for Propylene Epoxidation. Angew. Chem. Int. Ed. 2021, 60, 18185–18193. [Google Scholar] [CrossRef]
- Kapil, N.; Cardinale, F.; Weissenberger, T.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.-O. Gold nanoparticles with tailored size through ligand modification for catalytic applications. Chem. Commun. 2021, 57, 10775–10778. [Google Scholar] [CrossRef]
- Liu, Y.; Tsunoyama, H.; Akita, T.; Xie, S.; Tsukuda, T. Aerobic Oxidation of Cyclohexane Catalyzed by Size-Controlled Au Clusters on Hydroxyapatite: Size Effect in the Sub-2 nm Regime. ACS Catal. 2011, 1, 2–6. [Google Scholar] [CrossRef]
- Cargnello, M.; Chen, C.; Diroll, B.T.; Doan-Nguyen, V.V.T.; Gorte, R.J.; Murray, C.B. Efficient Removal of Organic Ligands from Supported Nanocrystals by Fast Thermal Annealing Enables Catalytic Studies on Well-Defined Active Phases. J. Am. Chem. Soc. 2015, 137, 6906–6911. [Google Scholar] [CrossRef]
- Lu, L.; Zou, S.; Fang, B. The Critical Impacts of Ligands on Heterogeneous Nanocatalysis: A Review. ACS Catal. 2021, 11, 6020–6058. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, L.; Kirkensgaard, J.J.K.; Jensen, K.M.Ø.; Oezaslan, M.; et al. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Kacenauskaite, L.; Quinson, J.; Schultz, H.; Kirkensgaard, J.J.K.; Kunz, S.; Vosch, T.; Arenz, M. UV-Induced Synthesis and Stabilization of Surfactant-Free Colloidal Pt Nanoparticles with Controlled Particle Size in Ethylene Glycol. ChemNanoMat 2017, 3, 89–93. [Google Scholar] [CrossRef]
- Quinson, J.; Dworzak, A.; Simonsen, S.B.; Theil Kuhn, L.; Jensen, K.M.Ø.; Zana, A.; Oezaslan, M.; Kirkensgaard, J.J.K.; Arenz, M. Surfactant-free synthesis of size controlled platinum nanoparticles: Insights from in situ studies. Appl. Surf. Sci. 2021, 549, 149263. [Google Scholar] [CrossRef]
- Quinson, J.; Neumann, S.; Wannmacher, T.; Kacenauskaite, L.; Inaba, M.; Bucher, J.; Bizzotto, F.; Simonsen, S.B.; Theil Kuhn, L.; Bujak, D.; et al. Colloids for Catalysts: A Concept for the Preparation of Superior Catalysts of Industrial Relevance. Angew. Chem. Int. Ed. 2018, 57, 12338–12341. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Kacenauskaite, L.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, L.; Oezaslan, M.; Kunz, S.; Arenz, M. Controlled Synthesis of Surfactant-Free Water-Dispersible Colloidal Platinum Nanoparticles by the Co4Cat Process. ChemSusChem 2019, 12, 1229–1239. [Google Scholar] [CrossRef] [Green Version]
- Quinson, J.; Mathiesen, J.K.; Schröder, J.; Dworzak, A.; Bizzotto, F.; Zana, A.; Simonsen, S.B.; Theil Kuhn, L.; Oezaslan, M.; Jensen, K.M.Ø.; et al. Teaching old precursors new tricks: Fast room temperature synthesis of surfactant-free colloidal platinum nanoparticles. J. Colloid Interface Sci. 2020, 577, 319–328. [Google Scholar] [CrossRef]
- Quinson, J.; Neumann, S.; Kacenauskaite, L.; Bucher, J.; Kirkensgaard, J.J.K.; Simonsen, S.B.; Theil Kuhn, L.; Zana, A.; Vosch, T.; Oezaslan, M.; et al. Solvent-Dependent Growth and Stabilization Mechanisms of Surfactant-Free Colloidal Pt Nanoparticles. Chem. A Eur. J. 2020, 26, 9012–9023. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, H.; Li, Y.; Zhou, Y.; Fang, B. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2023, 451, 138668. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of Surfactant-Free Colloidal Nanoparticles in Heterogeneous Catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Quinson, J. Colloidal surfactant-free syntheses of precious metal nanoparticles for electrocatalysis. Curr. Opin. Electrochem. 2022, 34, 100977. [Google Scholar] [CrossRef]
- Arce-Sarria, A.; Aldana-Villegas, K.M.; Betancourt-Buitrago, L.A.; Colina-Márquez, J.Á.; Machuca-Martínez, F.; Mueses, M.A. Degradation of Hexacyanoferrate (III) from Gold Mining Wastewaters via UV-A/LED Photocatalysis Using Modified TiO2 P25. Water 2020, 12, 2531. [Google Scholar] [CrossRef]
- Brindle, J.S.; Nelson, P.S.; Charde, R.P.; Sufyan, S.A.; Nigra, M.M. Catalytic cooperativity between glucose oxidase and gold nanoparticles in the sequential oxidation of glucose to saccharic acid. Green Chem. 2022, 24, 5162–5170. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Q.; Li, M.; Chao, D.; Huang, L.; Wu, W.; Fang, Y.; Dong, S. Glucose-oxidase like catalytic mechanism of noble metal nanozymes. Nat. Commun. 2021, 12, 3375. [Google Scholar] [CrossRef] [PubMed]
- Hermans, S.; Deffernez, A.; Devillers, M. Au–Pd/C catalysts for glyoxal and glucose selective oxidations. Appl. Catal. A Gen. 2011, 395, 19–27. [Google Scholar] [CrossRef]
- Lewis, R.J.; Bara-Estaun, A.; Agarwal, N.; Freakley, S.J.; Morgan, D.J.; Hutchings, G.J. The Direct Synthesis of H2O2 and Selective Oxidation of Methane to Methanol Using HZSM-5 Supported AuPd Catalysts. Catal. Lett. 2019, 149, 3066–3075. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.; Carter, J.H.; Dummer, N.F.; Chow, Y.K.; Morgan, D.J.; Yacob, S.; Serna, P.; Willock, D.J.; Meyer, R.J.; Taylor, S.H.; et al. Selective Oxidation of Methane to Methanol Using Supported AuPd Catalysts Prepared by Stabilizer-Free Sol-Immobilization. ACS Catal. 2018, 8, 2567–2576. [Google Scholar] [CrossRef]
- McVicker, R.; Agarwal, N.; Freakley, S.J.; He, Q.; Althahban, S.; Taylor, S.H.; Kiely, C.J.; Hutchings, G.J. Low temperature selective oxidation of methane using gold-palladium colloids. Catal. Today 2020, 342, 32–38. [Google Scholar] [CrossRef]
- Agarwal, N.; Freakley, S.J.; McVicker, R.U.; Althahban, S.M.; Dimitratos, N.; He, Q.; Morgan, D.J.; Jenkins, R.L.; Willock, D.J.; Taylor, S.H.; et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 2017, 358, 223–227. [Google Scholar] [CrossRef]
- Huang, J.; Takei, T.; Ohashi, H.; Haruta, M. Propene epoxidation with oxygen over gold clusters: Role of basic salts and hydroxides of alkalis. Appl. Catal. A Gen. 2012, 435-436, 115–122. [Google Scholar] [CrossRef]
- Huang, J.; Akita, T.; Faye, J.; Fujitani, T.; Takei, T.; Haruta, M. Propene epoxidation with dioxygen catalyzed by gold clusters. Angew. Chem. Int. Ed. Engl. 2009, 48, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Neurock, M.; Manzer, L.E. Theoretical insights on the mechanism of alkene epoxidation by H2O2 with titanium silicalite. Chem. Commun. 1996, 1133–1134. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, W.-J.; Wu, H.; Liebens, A.; Wu, P. Selective synthesis of ethylene oxide through liquid-phase epoxidation of ethylene with titanosilicate/H2O2 catalytic systems. Appl. Catal. A Gen. 2016, 515, 51–59. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, X.; Feng, Z.; Lu, Z.; Zhang, Z.; Huang, W.; Li, Y.; Vuckovic, D.; Li, Y.; Dai, S.; et al. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 2021, 4, 233–241. [Google Scholar] [CrossRef]
- Agrawal, S.; Mysko, R.A.; Nigra, M.M.; Mohanty, S.K.; Hoepfner, M.P. Plasmonic Photocatalytic Enhancement of L-Cysteine Self-Assembled Gold Nanoparticle Clusters for Fenton Reaction Catalysis. Langmuir 2021, 37, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Y.; Yao, Q.; Wang, Y.; Fang, X.; Shen, C.; Li, F.; Huang, M.; Wang, Z.; Sand, W.; et al. Supported Atomically-Precise Gold Nanoclusters for Enhanced Flow-through Electro-Fenton. Environ. Sci. Technol. 2020, 54, 5913–5921. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, S.; You, M.; Hong, S.; Wu, T.-S.; Soo, Y.-L.; Zhao, Z.; Jiang, G.; Jieshan, Q.; et al. Photocatalytic Fixation of Nitrogen to Ammonia by Single Ru Atom Decorated TiO2 Nanosheets. ACS Sustain. Chem. Eng. 2019, 7, 6813–6820. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Vetter, T.A.; Colombo, D.P. Kinetics of Platinum-Catalyzed Decomposition of Hydrogen Peroxide. J. Chem. Educ. 2003, 80, 788. [Google Scholar] [CrossRef]
- van Wyk, P.-H.; Gerber, W.J.; Koch, K.R. A robust method for speciation, separation and photometric characterization of all [PtCl6−nBrn]2− (n=0–6) and [PtCl4−nBrn]2− (n=0–4) complex anions by means of ion-pairing RP-HPLC coupled to ICP-MS/OES, validated by high resolution 195Pt NMR spectroscopy. Anal. Chim. Acta 2011, 704, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Menumerov, E.; Hughes, R.A.; Golze, S.D.; Neal, R.D.; Demille, T.B.; Campanaro, J.C.; Kotesky, K.C.; Rouvimov, S.; Neretina, S. Identifying the True Catalyst in the Reduction of 4-Nitrophenol: A Case Study Showing the Effect of Leaching and Oxidative Etching Using Ag Catalysts. ACS Catal. 2018, 8, 8879–8888. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Shape-Dependent Catalytic Activity of Platinum Nanoparticles in Colloidal Solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- McKee, D.W. Catalytic decomposition of hydrogen peroxide by metals and alloys of the platinum group. J. Catal. 1969, 14, 355–364. [Google Scholar] [CrossRef]
- Richards, T.; Lewis, R.J.; Morgan, D.J.; Hutchings, G.J. The Direct Synthesis of Hydrogen Peroxide Over Supported Pd-Based Catalysts: An Investigation into the Role of the Support and Secondary Metal Modifiers. Catal. Lett. 2022, 153, 32–40. [Google Scholar] [CrossRef]
- Brehm, J.; Lewis, R.J.; Morgan, D.J.; Davies, T.E.; Hutchings, G.J. The Direct Synthesis of Hydrogen Peroxide over AuPd Nanoparticles: An Investigation into Metal Loading. Catal. Lett. 2022, 152, 254–262. [Google Scholar] [CrossRef]
- Freakley, S.J.; Agarwal, N.; McVicker, R.U.; Althahban, S.; Lewis, R.J.; Morgan, D.J.; Dimitratos, N.; Kiely, C.J.; Hutchings, G.J. Gold–palladium colloids as catalysts for hydrogen peroxide synthesis, degradation and methane oxidation: Effect of the PVP stabiliser. Catal. Sci. Technol. 2020, 10, 5935–5944. [Google Scholar] [CrossRef]
- Bianchi, G.; Mazza, F.; Mussini, T. Catalytic decomposition of acid hydrogen peroxide solutions on platinum, iridium, palladium and gold surfaces. Electrochim. Acta 1962, 7, 457–473. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Michel, F.M.; Douglas, T.A.; Kang, Y.; Stach, E.A. Mechanism and Kinetics of Methane Oxidation to Methanol Catalyzed by AuPd Nanocatalysts at Low Temperature. ACS Catal. 2021, 11, 2837–2845. [Google Scholar] [CrossRef]
- MacInnes, D.A. The Mechanism of the Catalysis of the Decomposition of Hydrogen Peroxide by Colloidal Platinum. J. Am. Chem. Soc. 1914, 36, 878–881. [Google Scholar] [CrossRef]
- Eley, D.D.; Macmahon, D.M. The decomposition of hydrogen peroxide catalysed by palladium-gold alloy wires. J. Colloid Interface Sci. 1972, 38, 502–510. [Google Scholar] [CrossRef]
- Naya, S.-i.; Teranishi, M.; Kimura, K.; Tada, H. A strong support-effect on the catalytic activity of gold nanoparticles for hydrogen peroxide decomposition. Chem. Commun. 2011, 47, 3230–3232. [Google Scholar] [CrossRef] [PubMed]
- Serra-Maia, R.; Winkler, C.; Murayama, M.; Tranhuu, K.; Michel, F.M. Abundance and Speciation of Surface Oxygen on Nanosized Platinum Catalysts and Effect on Catalytic Activity. ACS Appl. Energy Mater. 2018, 1, 3255–3266. [Google Scholar] [CrossRef]
- Weiss, J. The catalytic decomposition of hydrogen peroxide on different metals. Trans. Faraday Soc. 1935, 31, 1547–1557. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Aoki, T.; Kaibara, D.; Seino, S.; Mori, O.; Sasaki, R.; Endo, K.; Yamamura, K. Strong Biomimetic Immobilization of Pt-Particle Catalyst on ABS Substrate Using Polydopamine and Its Application for Contact-Lens Cleaning with H2O2. Nanomaterials 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Radiolytic Synthesis of Pt-Particle/ABS Catalysts for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2017, 7, 235. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, Y.; Aoki, T.; Seino, S.; Mori, O.; Ito, I.; Endo, K.; Yamamura, K. Improved Catalytic Durability of Pt-Particle/ABS for H2O2 Decomposition in Contact Lens Cleaning. Nanomaterials 2019, 9, 342. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.S.W.; Rouquerol, J.; Bergeret, G.; Gallezot, P.; Vaarkamp, M.; Koningsberger, D.C.; Datye, A.K.; Niemantsverdriet, J.W.; Butz, T.; Engelhardt, G.; et al. Handbook of Heterogeneous Catalysis; Wiley: Hoboken, NJ, USA, 1997; pp. 427–582. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Shape-Dependent Catalytic Activity of Platinum Nanoparticles in Colloidal Solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Winkler, C.; Murayama, M.; Tranhuu, K.; Michel, F.M. Abundance and Speciation of Surface Oxygen on Nanosized Platinum Catalysts and Effect on Catalytic Activity. ACS Appl. Energy Mater. 2018, 1, 3255–3266. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charde, R.P.; Devener, B.v.; Nigra, M.M. Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications. Catalysts 2023, 13, 246. https://doi.org/10.3390/catal13020246
Charde RP, Devener Bv, Nigra MM. Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications. Catalysts. 2023; 13(2):246. https://doi.org/10.3390/catal13020246
Chicago/Turabian StyleCharde, Rashmi P., Brian van Devener, and Michael M. Nigra. 2023. "Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications" Catalysts 13, no. 2: 246. https://doi.org/10.3390/catal13020246