Metallic–Organic Cages (MOCs) with Heterometallic Character: Flexibility-Enhancing MOFs
Abstract
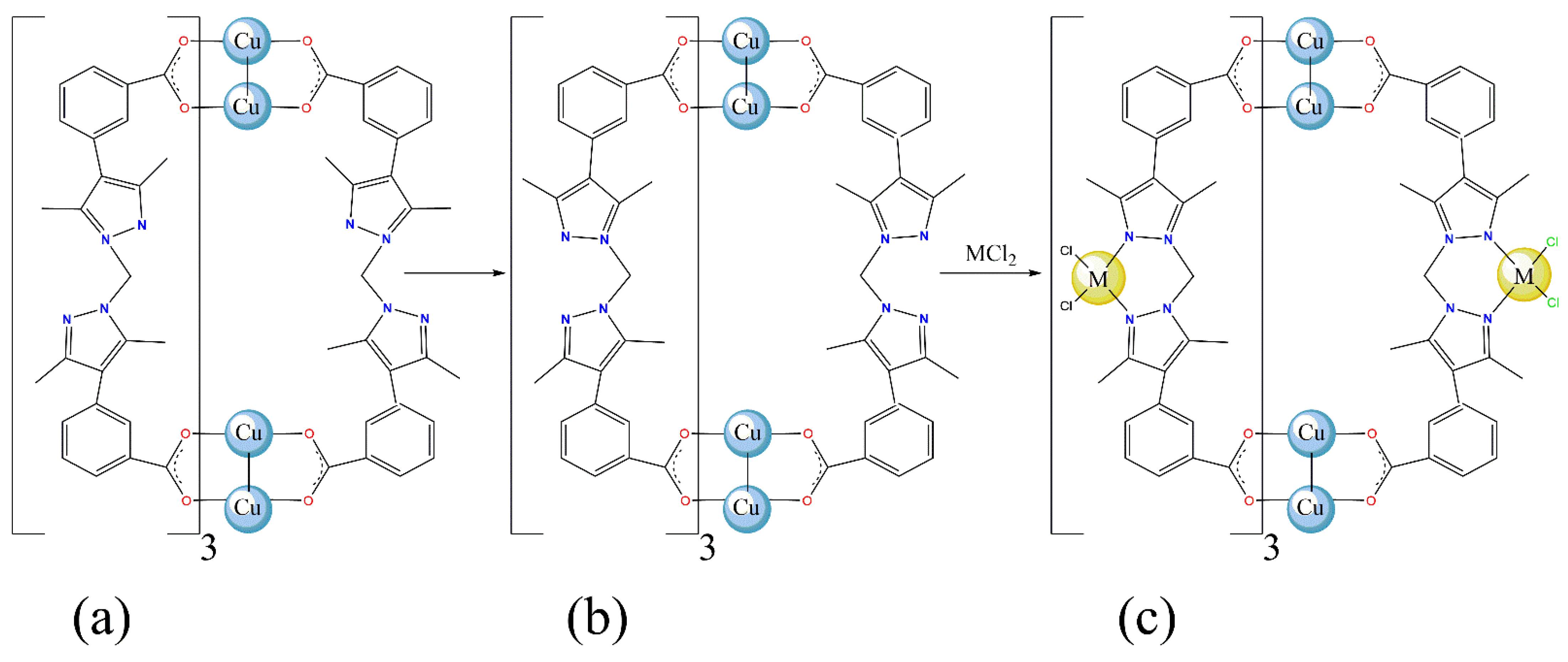
:1. Introduction
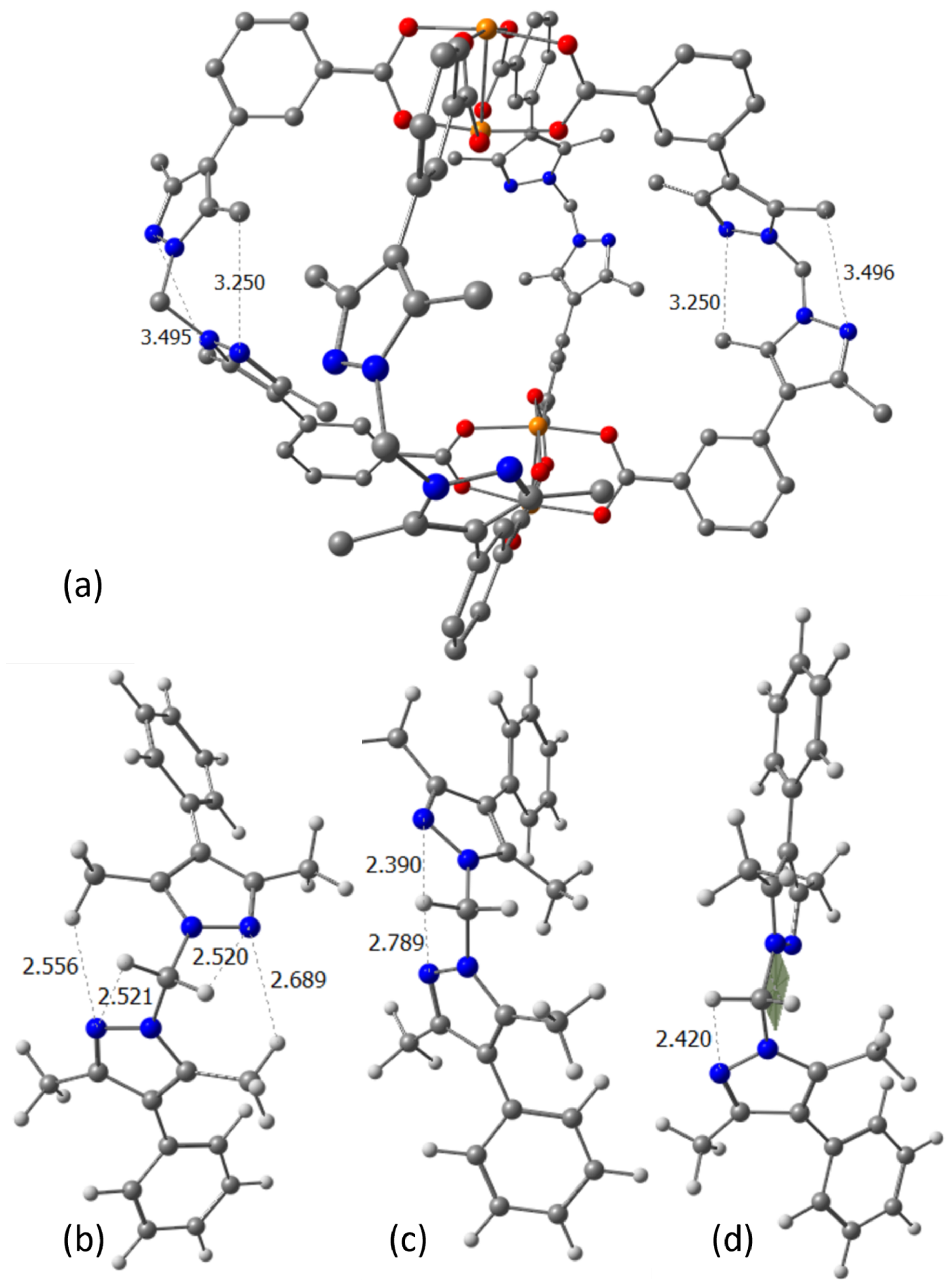
2. Results
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Chemical Society National Historic Chemical Landmarks. The Houdry Process for Catalytic Cracking. Available online: http://www.acs.org/content/acs/en/education/whatischemistry/landmaks/houdry.html (accessed on 24 December 2022).
- Spitz, P.H. Petrochemicals: The Rise of an Industry; John Wiley and Sons, Inc.: New York, NY, USA, 1988; pp. 184–191. [Google Scholar]
- Weirsselmel, K.; Arpe, H.J. Industrial Organic Chemistry, 3rd ed.; VCH Publishers, Inc.: New York, NY, USA, 1997; p. 218. [Google Scholar]
- Worthy, W. Canadian chemical firms: Another good year. Chem. Eng. News 1979, 57, 17. [Google Scholar] [CrossRef]
- History. Available online: http://www.dupont.com/corporate-functions/our-company/dupont-history.html (accessed on 24 December 2022).
- Schröder, G. Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2012; Volume 28, pp. 481–485. [Google Scholar]
- Hilal, M.E.; Aboulouard, A.; Akbar, A.R.; Younus, H.A.; Horzum, N.; Verpoort, F. Progress of MOF-derived functional materials toward industrialization in solar cells and metal-air batteries. Catalysts 2020, 10, 897. [Google Scholar] [CrossRef]
- Hosono, N.; Kitagawa, S. Modular Design of Porous Soft Materials via Self-Organization of Metal—Organic Cages. Acc. Chem. Res. 2018, 51, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Pareras, G.; Detiana, D.; Poater, A. MOF encapsulation of Ru olefin metathesis catalysts to block catalyst decomposition. Catalysts 2020, 10, 687. [Google Scholar] [CrossRef]
- Poater, J.; Gimferrer, M.; Poater, A. Covalent and Ionic Capacity of MOFs To Sorb Small Gas Molecules. Inorg. Chem. 2018, 57, 6981–6990. [Google Scholar] [CrossRef]
- Colomban, C.; Szalóki, G.; Allain, M.; Gómez, L.; Goeb, S. Reversible C60 Ejection from a Metallocage through the Redox-Dependent Binding of a Competitive Guest. Chem. Eur. J. 2018, 23, 3016–3022. [Google Scholar] [CrossRef]
- Sánchez-González, E.; Tsang, M.Y.; Troyano, J.; Craig, G.A.; Furukawa, S. Assembling Metal—Organic Cages as Porous Mate-rials. Chem. Soc. Rev. 2022, 51, 4876–4889. [Google Scholar] [CrossRef]
- Gosselin, E.J.; Rowland, C.A.; Bloch, E.D. Permanently Microporous Metal—Organic Polyhedra. Chem. Rev. 2020, 120, 8987–9014. [Google Scholar] [CrossRef]
- Rizzuto, F.J.; von Krbek, L.K.S.; Nitschke, J.R. Strategies for Binding Multiple Guests in Metal—Organic Cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent Developments in the Preparation and Chemistry of Metallacycles and Metallacages via Coor-dination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Dalgarno, S.J.; Power, N.P.; Atwood, J.L. Metallo-Supramolecular Capsules. Coord. Chem. Rev. 2008, 252, 825–841. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, J.; Ju, Y.; Serda, R.E.; Croissant, J.G.; Shang, J.; Coker, E.; Agola, J.O.; Zhong, Q.-Z.; Ping, Y.; et al. Modular Metal—Organic Polyhedra Superassembly: From Molecular-Level Design to Targeted Drug Delivery. Adv. Mater. 2019, 31, 1806774. [Google Scholar] [CrossRef] [PubMed]
- Zava, O.; Mattsson, J.; Therrien, B.; Dyson, P.J. Evidence for Drug Release from a Metalla-Cage Delivery Vector Following Cellular Internalisation. Chem. Eur. J. 2010, 16, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.M.; Gavey, E.L.; Cameron, S.A.; Crowley, J.D. Stimuli-Responsive Pd2L4 Metallosupramolecular Cages: Towards Targeted Cisplatin Drug Delivery. Chem. Sci. 2012, 3, 778–784. [Google Scholar] [CrossRef]
- Deegan, M.M.; Dworzak, M.R.; Gosselin, A.J.; Korman, K.J.; Bloch, E.D. Gas Storage in Porous Molecular Materials. Chem. Eur. J. 2021, 27, 4531–4547. [Google Scholar] [CrossRef]
- Duriska, M.B.; Neville, S.M.; Lu, J.; Iremonger, S.S.; Boas, J.F.; Kepert, C.J.; Batten, S.R. Systematic Metal Variation and Solvent and Hydrogen-Gas Storage in Supramolecular Nanoballs. Angew. Chem. Int. Ed. 2009, 48, 8919–8922. [Google Scholar] [CrossRef]
- Lorzing, G.R.; Trump, B.A.; Brown, C.M.; Bloch, E.D. Selective Gas Adsorption in Highly Porous Chromium(II)-Based Metal—Organic Polyhedra. Chem. Mater. 2017, 29, 8583–8587. [Google Scholar] [CrossRef]
- Fan, W.; Peh, S.B.; Zhang, Z.; Yuan, H.; Yang, Z.; Wang, Y.; Chai, K.; Sun, D.; Zhao, D. Tetrazole-Functionalized Zirconium Metal-Organic Cages for Efficient C2H2/C2H4 and C2H2/CO2 Separations. Angew. Chem. Int. Ed. 2021, 60, 17338–17343. [Google Scholar] [CrossRef]
- Zhu, J.-L.; Zhang, D.; Ronson, T.K.; Wang, W.; Xu, L.; Yang, H.-B.; Nitschke, J.R. A Cavity-Tailored Metal-Organic Cage Entraps Gases Selectively in Solution and the Amorphous Solid State. Angew. Chem. Int. Ed. 2021, 60, 11789–11792. [Google Scholar] [CrossRef]
- Yan, X.; Cook, T.R.; Wang, P.; Huang, F.; Stang, P.J. Highly Emissive Platinum(II) Metallacages. Nat. Chem. 2015, 7, 342–348. [Google Scholar] [CrossRef]
- Brzechwa-Chodzyńska, A.; Drożdż, W.; Harrowfield, J.; Stefankiewicz, A.R. Fluorescent Sensors: A Bright Future for Cages. Coord. Chem. Rev. 2021, 434, 213820. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Lawrence, A.L.; Lusby, P.J. High Activity and Efficient Turnover by a Simple, Self-Assembled “Artificial Diels—Alderase”. J. Am. Chem. Soc. 2018, 140, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular Catalysis in Metal—Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hang, X.; Ding, J.; Li, B.; Zhu, R.; Pang, H.; Xu, Q. Catalysis within Coordination Cages. Coord. Chem. Rev. 2021, 430, 213656. [Google Scholar] [CrossRef]
- Gosselin, A.J.; Antonio, A.M.; Korman, K.J.; Deegan, M.M.; Yap, G.P.A.; Bloch, E.D. Elaboration of Porous Salts. J. Am. Chem. Soc. 2021, 143, 14956–14961. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A.M.; Korman, K.J.; Yap, G.P.A.; Bloch, E.D. Porous Metal−Organic Alloys Based on Soluble Coordination Cages. Chem. Sci. 2020, 11, 12540–12546. [Google Scholar] [CrossRef]
- Li, T.-T.; Liu, S.-N.; Wu, L.-H.; Cai, S.-L.; Zheng, S.-R. Strategies for the Construction of Functional Materials Utilizing Presynthesized Metal-Organic Cages (MOCs). ChemPlusChem 2022, 87, e202200172. [Google Scholar] [CrossRef]
- Zhu, G.; O’Nolan, D.; Lively, R.P. Molecularly Mixed Composite Membranes: Challenges and Opportunities. Chem. Eur. J. 2020, 26, 3464–3473. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, G.; Yuan, Y.D.; Peh, S.B.; Ying, Y.; Fan, W.; Yu, X.; Yang, H.; Wu, Z.; Zhao, D. Homoporous Hybrid Membranes Containing Metal-Organic Cages for Gas Separation. J. Memb. Sci. 2021, 636, 119564. [Google Scholar] [CrossRef]
- Legrand, A.; Liu, L.-H.; Royla, P.; Aoyama, T.; Craig, G.A.; Carné-Sánchez, A.; Urayama, K.; Weigand, J.J.; Lin, C.-H.; Fu-rukawa, S. Spatiotemporal Control of Supramolecular Polymerization and Gelation of Metal—Organic Polyhedra. J. Am. Chem. Soc. 2021, 143, 3562–3570. [Google Scholar] [CrossRef]
- Oldenhuis, N.J.; Qin, K.P.; Wang, S.; Ye, H.-Z.; Alt, E.A.; Willard, A.P.; Van Voorhis, T.; Craig, S.L.; Johnson, J.A. Pho-toswitchable Sol—Gel Transitions and Catalysis Mediated by Polymer Networks with Coumarin-Decorated Cu24L24 Met-al—Organic Cages as Junctions. Angew. Chem. Int. Ed. 2020, 59, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Grancha, T.; Carné-Sánchez, A.; Zarekarizi, F.; Hernández-López, L.; Albalad, J.; Khobotov, A.; Guillerm, V.; Morsali, A.; Juanhuix, J.; Gándara, F.; et al. Synthesis of Polycarboxylate Rhodium(II) Metal—Organic Polyhedra (MOPs) and Their Use as Building Blocks for Highly Connected Metal−Organic Frameworks (MOFs). Angew. Chem. Int. Ed. 2021, 60, 5729–5733. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Rao, C.; Tan, X.; Ling, Y.; Singh, A.; Kumar, A.; Li, B.; Liu, J. Cobalt-seamed C-methylpyrogallol[4]arene nanocapsules-derived magnetic carbon cubes as advanced adsorbent toward drug contaminant removal. Chem. Eng. J. 2022, 433, 133857. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, H.; Nam, D.; Lah, M.S.; Choe, W. The Rise of Metal—Organic Polyhedra. Chem. Soc. Rev. 2021, 50, 528–555. [Google Scholar] [CrossRef]
- Hardy, M.; Tessarolo, J.; Holstein, J.J.; Struch, N.; Wagner, N.; Weisbarth, R.; Engeser, M.; Beck, J.; Horiuchi, S.; Clever, G.H.; et al. A Family of Heterobimetallic Cubes Shows Spin-Crossover Behaviour Near Room Temperature. Angew. Chem. Int. Ed. 2021, 60, 22562–22569. [Google Scholar] [CrossRef]
- Hu, X.; Han, M.; Shao, L.; Zhang, C.; Zhang, L.; Kelley, S.P.; Zhang, C.; Lin, J.; Dalgarno, S.J.; Atwood, D.A.; et al. Self-Assembly of a Semiconductive and Photoactive Heterobimetallic Metal—Organic Capsule. Angew. Chem. Int. Ed. 2021, 60, 10516–10520. [Google Scholar] [CrossRef]
- Bloch, W.M.; Clever, G.H. Integrative Self-Sorting of Coordination Cages Based on “naked” Metal Ions. Chem. Commun. 2017, 53, 8506–8516. [Google Scholar] [CrossRef]
- Bloch, W.M.; Holstein, J.J.; Hiller, W.; Clever, G.H. A “Doubly-Bridged Figure-Eight:” Morphological Control of Heteroleptic Cis- and Trans-Pd2L2L′2 Cages. Angew. Chem. Int. Ed. 2017, 56, 8285–8289. [Google Scholar] [CrossRef]
- Walker, S.E.; Boer, S.A.; Malcomson, T.; Paterson, M.J.; Tuck, K.L.; Turner, D.R. Steric Control of Sorting Regimes in Self-Assembled Cages. Chem. Commun. 2021, 57, 12456–12459. [Google Scholar] [CrossRef]
- Preston, D.; Barnsley, J.E.; Gordon, K.C.; Crowley, J.D. Controlled Formation of Heteroleptic [Pd2(La)2(Lb)2]4+ Cages. J. Am. Chem. Soc. 2016, 138, 10578–10585. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Zhou, H.C. Bridging-Ligand-Substitution Strategy for the Preparation of Metal-Organic Polyhedra. Nat. Chem. 2010, 2, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Markwell-Heys, A.W.; Schneider, M.L.; Marie, J.; Madridejos, L.; Metha, G.F.; Bloch, W.M. Self-Sorting of Porous Cu4L2L’2 Metal-Organic Cages Composed of Isomerisable Ligands. Chem. Commun. 2021, 57, 2915–2918. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.M.; Crowley, J.D. Metallo-Supramolecular Self-Assembly with Reduced-Symmetry Ligands. ChemPlusChem 2020, 85, 815–827. [Google Scholar] [CrossRef]
- Hardy, M.; Lützen, A. Better Together: Functional Heterobimetallic Macrocyclic and Cage-like Assemblies. Chem. Eur. J. 2020, 26, 13332–13346. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.-J.; Liu, D.; Jin, G.-X. Multi-Component Coordination-Driven Self-Assembly toward Heterometallic Macrocycles and Cages. Coord. Chem. Rev. 2015, 293–294, 139–157. [Google Scholar]
- Liu, S.; Qiu, Y.; Liu, Y.; Zhang, W.; Dai, Z.; Srivastava, D.; Kumar, A.; Pan, Y.; Liu, J. Recent advances in bimetallic metal—Organic frameworks (BMOFs): Synthesis, applications and challenges. New J. Chem. 2022, 46, 13818–13837. [Google Scholar] [CrossRef]
- Barreda, O.; Bannwart, G.; Yap, G.P.A.; Bloch, E.D. Ligand-Based Phase Control in Porous Molecular Assemblies. ACS Appl. Mater. Interfaces 2018, 10, 11420–11424. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Jiménez, A.; Nitschke, J.R. Integrative Self-Sorting Synthesis of a Fe8Pt6L24 Cubic Cage. Angew. Chem. Int. Ed. 2012, 51, 6681–6685. [Google Scholar] [CrossRef]
- Teo, J.M.; Coghlan, C.J.; Evans, J.D.; Tsivion, E.; Head-Gordon, M.; Sumby, C.J.; Doonan, C.J. Hetero-Bimetallic Metal-Organic Polyhedra. Chem. Commun. 2016, 52, 276–279. [Google Scholar] [CrossRef]
- Maity, M.; Howlader, P.; Mukherjee, P.S. Coordination-Driven Self-Assembly of Cyclopentadienyl-Capped Heterometallic Zr—Pd Cages. Cryst. Growth Des. 2018, 18, 6956–6964. [Google Scholar] [CrossRef]
- Li, F.; Lindoy, L.F. Metalloligand Strategies for Assembling Heteronuclear Nanocages—Recent Developments. Aust. J. Chem. 2019, 72, 731–741. [Google Scholar] [CrossRef]
- Planes, O.M.; Jansze, S.M.; Scopelliti, R.; Fadaei-Tirani, F.; Severin, K. Two-Step Synthesis of Linear and Bent Dicarboxylic Acid Metalloligands with Lengths of up to 3 Nm. Inorg. Chem. 2020, 59, 14544–14548. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Struch, N.; Topić, F.; Schnakenburg, G.; Rissanen, K.; Lützen, A. Stepwise Construction of Heterobimetallic Cages by an Extended Molecular Library Approach. Inorg. Chem. 2018, 57, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.; Clegg, J.K.; Gloe, K.; Gloe, K.; Weigand, J.J.; Reynolds, J.K.; Li, C.-G.; Aldrich-Wright, J.R.; Kepert, C.J.; Lindoy, L.F. Self-Assembly of an Imidazolate-Bridged FeIII/CuII Heterometallic Cage. Inorg. Chem. 2014, 53, 688–690. [Google Scholar] [CrossRef]
- Lisboa, L.S.; Findlay, J.A.; Wright, L.J.; Hartinger, C.G.; Crowley, J.D. A Reduced-Symmetry Heterobimetallic [PdPtL4]4+ Cage: Assembly, Guest Binding, and Stimulus-Induced Switching. Angew. Chem. Int. Ed. 2020, 59, 11101–11107. [Google Scholar] [CrossRef]
- Carpenter, J.P.; Ronson, T.K.; Rizzuto, F.J.; Héliot, T.; Grice, P.; Nitschke, J.R. Incorporation of a Phosphino(Pyridine) Subcomponent Enables the Formation of Cages with Homobimetallic and Heterobimetallic Vertices. J. Am. Chem. Soc. 2022, 144, 8467–8473. [Google Scholar] [CrossRef]
- Liu, G.; Zeller, M.; Su, K.; Pang, J.; Ju, Z.; Yuan, D.; Hong, M. Controlled Orthogonal Self-Assembly of Heterometal-Decorated Coordination Cages. Chem. Eur. J. 2016, 22, 17345–17350. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Cheng, P.; Zaworotko, M.J.; Chen, Y.; Zhang, Z. Post-Synthetic Modifications of Metal—Organic Cages. Nat. Rev. Chem. 2022, 6, 339–356. [Google Scholar] [CrossRef]
- Markwell-Heys, A.W.; Roemelt, M.; Slattery, A.D.; Linder-Patton, O.M.; Bloch, W.M. Linking Metal—Organic Cages Pairwise as a Design Approach for Assembling Multivariate Crystalline Materials. Chem. Sci. 2021, 13, 68–73. [Google Scholar] [CrossRef]
- Albalad, J.; Hernández-López, L.; Carné-Sánchez, A.; Maspoch, D. Surface Chemistry of Metal—Organic Polyhedra. Chem. Commun. 2022, 58, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.L.; Linder-Patton, O.M.; Bloch, W.M. A Covalent Deprotection Strategy for Assembling Supramolecular Coor-dination Polymers from Metal-Organic Cages. Chem. Commun. 2020, 56, 12969–12972. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.L.; Markwell-Heys, A.W.; Linder-Patton, O.M.; Bloch, W.M. Assembly and Covalent Cross-Linking of an Amine-Functionalised Metal-Organic Cage. Front. Chem. 2021, 9, 696081. [Google Scholar] [CrossRef]
- Carné-Sánchez, A.; Craig, G.A.; Larpent, P.; Hirose, T.; Higuchi, M.; Kitagawa, S.; Matsuda, K.; Urayama, K.; Furukawa, S. Self-Assembly of Metal—Organic Polyhedra into Supramolecular Polymers with Intrinsic Microporosity. Nat. Commun. 2018, 9, 2506. [Google Scholar] [CrossRef]
- Yong, M.T.; Linder-Patton, O.M.; Bloch, W.M. Assembly of a Heterometallic Cu(II)-Pd(II) Cage by Post-assembly Metal Insertion. Inorg. Chem. 2022, 61, 12863–12869. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, C.; Pettinari, R. Metal Derivatives of Poly(Pyrazolyl)-Alkanes: II. Bis(Pyrazolyl)Alkanes and Related Systems. Coord. Chem. Rev. 2005, 249, 663–691. [Google Scholar] [CrossRef]
- Bloch, W.M.; Doonan, C.J.; Sumby, C.J. Using Hinged Ligands to Target Structurally Flexible Copper (Ii) MOFs. CrystEngComm 2013, 15, 9663–9671. [Google Scholar] [CrossRef]
- Bloch, W.M.; Doonan, C.J.; Sumby, C.J. Tuning Packing, Structural Flexibility, and Porosity in 2D Metal—Organic Frameworks by Metal Node Choice. Aust. J. Chem. 2019, 72, 797–804. [Google Scholar] [CrossRef]
- Bloch, W.M.; Burgun, A.; Coghlan, C.J.; Lee, R.; Coote, M.L.; Doonan, C.J.; Sumby, C.J. Capturing Snapshots of Post-Synthetic Metallation Chemistry in Metal—Organic Frameworks. Nat. Chem. 2014, 6, 906–912. [Google Scholar] [CrossRef]
- Bloch, W.M.; Babarao, R.; Hill, M.R.; Doonan, C.J.; Sumby, C.J. Post-Synthetic Structural Processing in a Metal—Organic Framework Material as a Mechanism for Exceptional CO2/N2 Selectivity. J. Am. Chem. Soc. 2013, 135, 10441–10448. [Google Scholar] [CrossRef]
- Evans, J.D.; Sumby, C.J.; Doonan, C.J. Post-Synthetic Metalation of Metal-Organic Frameworks. Chem. Soc. Rev. 2014, 43, 5933–5951. [Google Scholar] [CrossRef]
- Martín Díaz, A.E.; Lewis, J.E.M. Structural Flexibility in Metal-Organic Cages. Front. Chem. 2021, 9, 706462. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Panahi, F.; Bahri-Laleh, N.; Sabzi, M.; Pareras, G.; Falcone, B.N.; Poater, A. pH-Responsive Gelation in Metallo-Supramolecular Polymers Based on the Protic Pyridinedicarboxamide Ligand. Chem. Mater. 2022, 13, 6155–6169. [Google Scholar] [CrossRef]
- De, S.; Mahata, K.; Schmittel, M. Metal-Coordination-Driven Dynamic Heteroleptic Architectures. Chem. Soc. Rev. 2010, 39, 1555–1575. [Google Scholar] [CrossRef]
- Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 1983, 97, 270–274. [Google Scholar] [CrossRef]
- Poater, A.; Gallegos Saliner, A.; Solà, M.; Cavallo, L.; Worth, A.P. Computational Methods to Predict the Reactivity of Nanoparticles Through Structure-Property Relationships. Expert Opin. Drug Deliv. 2010, 7, 295–305. [Google Scholar] [CrossRef]
- Poater, A.; Moradell, S.; Pinilla, E.; Poater, J.; Solà, M.; Martínez, M.A.; Llobet, A. A trinuclear Pt(II) compound with short Pt–Pt–Pt contacts. An analysis of the influence of π–π stacking interactions on the strength and length of the Pt–Pt bond. Dalton Trans. 2006, 9, 1188–1196. [Google Scholar] [CrossRef]
- Asadi, Z.; Sadjadi, S.; Nekoomanesh-Haghighi, M.; Posada-Pérez, S.; Solà, M.; Bahri-Laleh, N.; Poater, A. Lubricant hydrogenation over a functionalized clay-based Pd catalyst: A combined computational and experimental study. Appl. Organomet. Chem. 2022, 36, e6850. [Google Scholar] [CrossRef]
- Bosson, J.; Poater, A.; Cavallo, L.; Nolan, S.P. Mechanism of Racemization of Chiral Alcohols Mediated by 16-Electron Ruthenium Complexes. J. Am. Chem. Soc. 2010, 132, 13146–13149. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W.T. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W.T. NCIPLOT: A program for plotting noncovalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Lattanzi, A.; De Fusco, C.; Russo, A.; Poater, A.; Cavallo, L. Hexafluorobenzene: A powerful solvent for a noncovalent stereoselective organocatalytic Michael addition reaction. Chem. Commun. 2012, 48, 1650–1652. [Google Scholar] [CrossRef]
- Lal, G.; Gelfand, B.S.; Lin, J.-B.; Banerjee, A.; Trudel, S.; Shimizu, G.K.H. Three Sequential Hydrolysis Products of the Ubiq-uitous Cu24 Isophthalate Metal—Organic Polyhedra. Inorg. Chem. 2019, 58, 9874–9881. [Google Scholar] [CrossRef]
- Mallick, A.; Garai, B.; Díaz, D.D.; Banerjee, R. Hydrolytic Conversion of a Metal—Organic Polyhedron into a Metal—Organic Framework. Angew. Chem. Int. Ed. 2013, 52, 13755–13759. [Google Scholar] [CrossRef]
- Poater, A.; Vummaleti, S.V.C.; Pump, E.; Cavallo, L. Comparing Ru and Fe-catalyzed olefin metathesis. Dalton Trans. 2014, 43, 11216–11220. [Google Scholar] [CrossRef]
- Luque-Urrutia, J.A.; Solà, M.; Milstein, D.; Poater, A. Mechanism of the Manganese-Pincer Catalyzed Acceptorless Dehydrogenative Coupling of Nitriles and Alcohols. J. Am. Chem. Soc. 2019, 141, 2398–2403. [Google Scholar] [CrossRef]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Group IV diamine bis(phenolate) catalysts for 1-decene oligomerization. Mol. Catal. 2020, 493, 111047. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Wang, C.; Shang, J.; Tian, L.; Zhao, H.-W.; Wang, P.; Feng, K.; He, G.-K.; Liu, J.Z.; Zhu, W.; Li, G.-T. Direct identification of HMX via guest-induced fluorescence turn-on of molecular cage. Chin. Chem. Lett. 2022, 32, 4006–4010. [Google Scholar] [CrossRef]
- Lisboa, L.S.; Preston, D.; McAdam, C.J.; Wright, L.J.; Hartinger, C.G.; Crowley, J.D. Heterotrimetallic Double Cavity Cages: Syntheses and Selective Guest Binding. Angew. Chem. Int. Ed. 2022, 61, e202201700. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posada-Pérez, S.; Poater, J.; Bahri-Laleh, N.; Poater, A. Metallic–Organic Cages (MOCs) with Heterometallic Character: Flexibility-Enhancing MOFs. Catalysts 2023, 13, 317. https://doi.org/10.3390/catal13020317
Posada-Pérez S, Poater J, Bahri-Laleh N, Poater A. Metallic–Organic Cages (MOCs) with Heterometallic Character: Flexibility-Enhancing MOFs. Catalysts. 2023; 13(2):317. https://doi.org/10.3390/catal13020317
Chicago/Turabian StylePosada-Pérez, Sergio, Jordi Poater, Naeimeh Bahri-Laleh, and Albert Poater. 2023. "Metallic–Organic Cages (MOCs) with Heterometallic Character: Flexibility-Enhancing MOFs" Catalysts 13, no. 2: 317. https://doi.org/10.3390/catal13020317
APA StylePosada-Pérez, S., Poater, J., Bahri-Laleh, N., & Poater, A. (2023). Metallic–Organic Cages (MOCs) with Heterometallic Character: Flexibility-Enhancing MOFs. Catalysts, 13(2), 317. https://doi.org/10.3390/catal13020317









