Synthesis of Rape Pollen-Fe2O3 Biohybrid Catalyst and Its Application on Photocatalytic Degradation and Antibacterial Properties
Abstract
:1. Introduction
2. Results
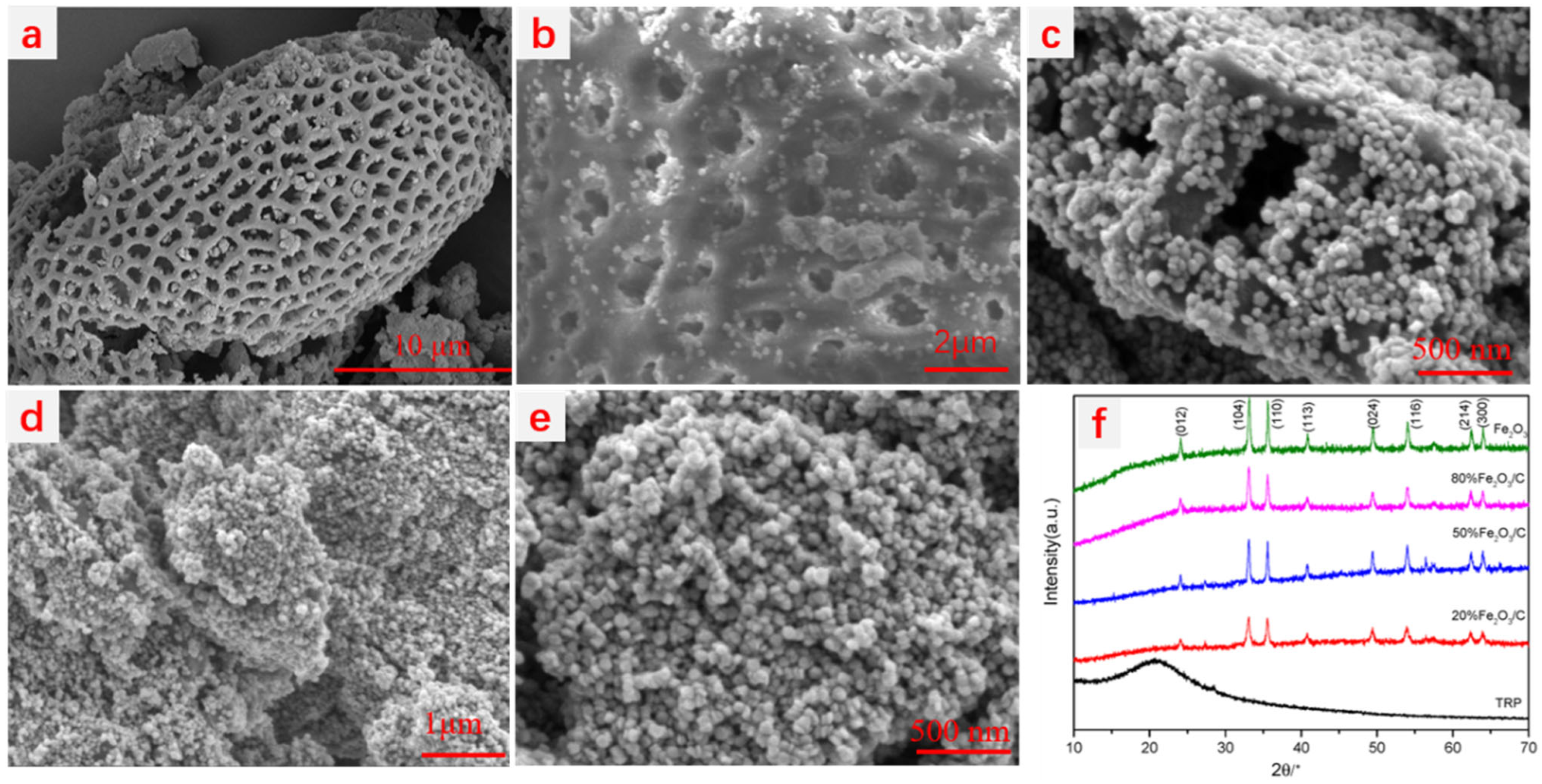
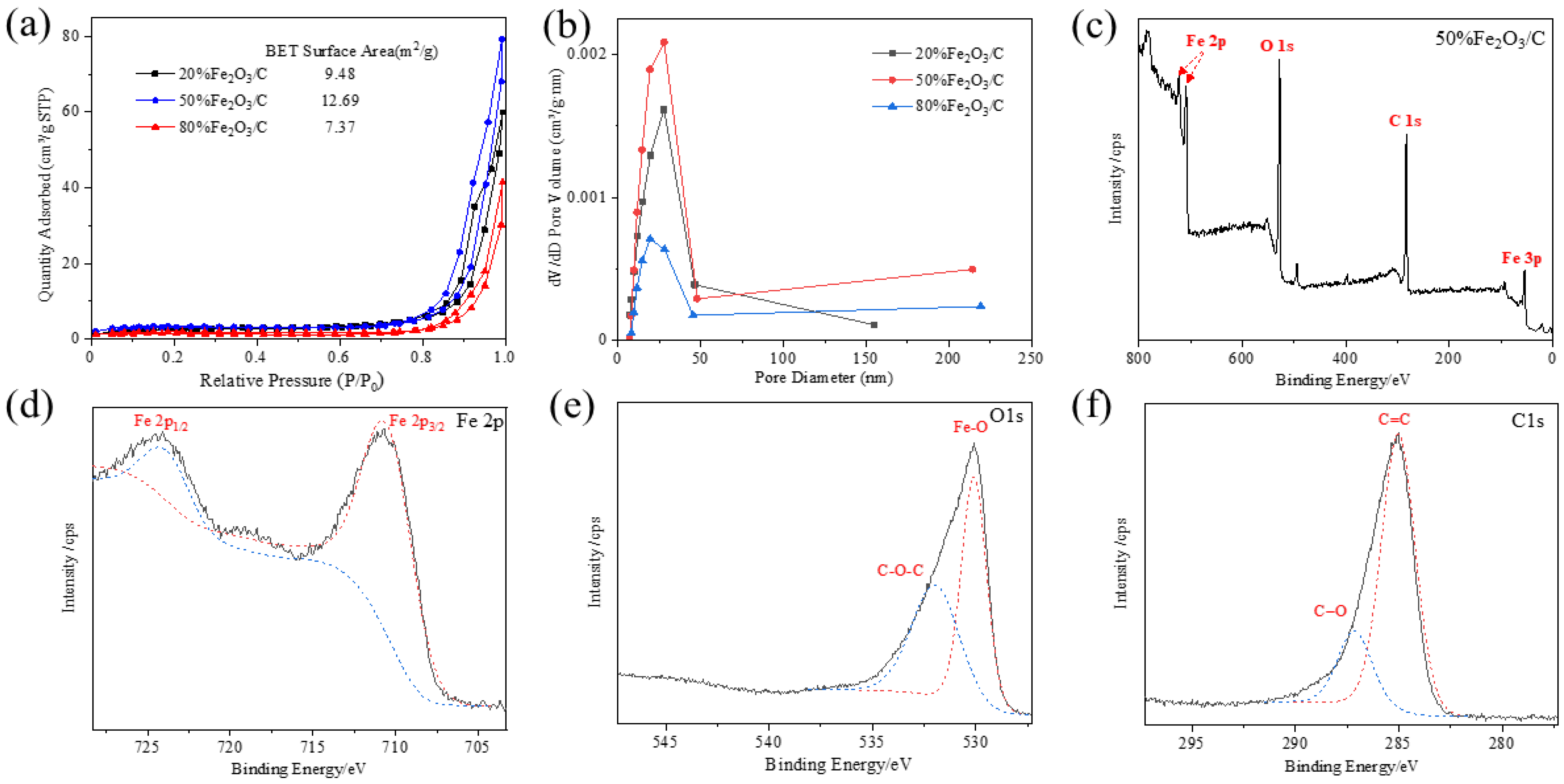
2.1. Characterization of the Fe2O3/C Heterojunction
2.2. Optical Property of Samples
2.3. Photocatalytic Activity of Degrade Methylene Blue
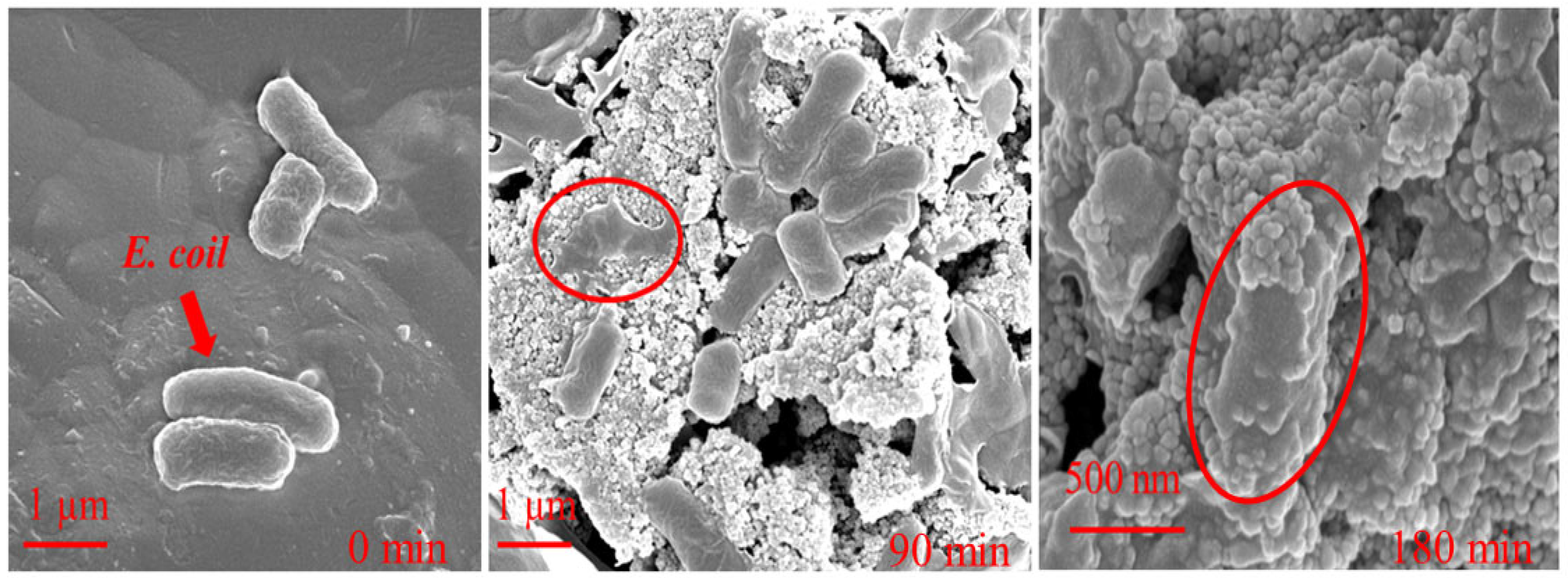
2.4. Antibacterial Activity of Samples
2.5. Mechanism
3. Materials and Methods
3.1. Preparation of Carbonized Rape Pollen Loaded with Granular Fe2O3
3.2. Characterization of Photocatalysts
3.3. Photocatalytic Degradation of MB Conditions
3.4. Photocatalytic Disinfection and Microscopic Images of Bacteria
3.5. Microscopic Observation of E. coli
3.6. Effective ROS Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Kausor, M.; Chakrabortty, D. Graphene oxide based semiconductor photocatalysts for degradation of organic dye in waste water: A review on fabrication, performance enhancement and challenges. Inorg. Chem. Commun. 2021, 129, 108630. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Cui, L.; Tian, Y.; Xia, L.; Wu, Y.; Yu, J.; Bagley, D.M.; Sun, J.; Fan, M. A novel and high-performance double Z-scheme photocatalyst ZnO-SnO2-Zn2SnO4 for effective removal of the biological toxicity of antibiotics. J. Hazard. Mater. 2020, 399, 123017. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, J.; García-Encina, P.A.; Jiménez, J.J.; López-Serna, R.; Irusta-Mata, R. Photolytic and photocatalytic removal of a mixture of four veterinary antibiotics. J. Water Process Eng. 2022, 48, 102841. [Google Scholar] [CrossRef]
- Lima, M.L.; Romanelli, A.; Massone, H.E. Assessing groundwater pollution hazard changes under different socio-economic and environmental scenarios in an agricultural watershed. Sci. Total Environ. 2015, 530, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Lutze, H.V.; Tuerk, J.; Schmidt, T.C. Influence of water matrix on the degradation of organic micropollutants by ozone based processes: A review on oxidant scavenging mechanism. J. Hazard. Mater. 2022, 429, 128189. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; He, Y.; Zhi, D.; Luo, L.; Sun, Y.; Khan, E.; Wang, L.; Peng, Y.; Zhou, Y.; Tsang, D.C. Current progress in treatment techniques of triclosan from wastewater: A review. Sci. Total Environ. 2019, 696, 133990. [Google Scholar] [CrossRef]
- Zewde, A.A.; Li, Z.; Zhang, L.; Odey, E.A.; Xiaoqin, Z. Utilisation of appropriately treated wastewater for some further beneficial purposes: A review of the disinfection method of treated wastewater using UV radiation technology. Rev. Environ. Health 2022, 352, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Song, R.; Chi, H.; Ma, Q.; Li, D.; Wang, X.; Gao, W.; Wang, H.; Wang, X.; Li, Z.; Li, C. Highly efficient degradation of persistent pollutants with 3D nanocone TiO2-based photoelectrocatalysis. J. Am. Chem. Soc. 2021, 143, 13664–13674. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Tian, L.; Guan, X.; Cheng, C.; Shi, J.; Guo, L. Photocatalytic overall water splitting without noble-metal: Decorating CoP on Al-doped SrTiO3. J. Colloid Interf. Sci. 2022, 606, 491–499. [Google Scholar] [CrossRef]
- Ali, A.M.; Sayed, M.A.; Algarni, H.; Ganesh, V.; Aslam, M.; Ismail, A.A.; El-Bery, H.M. Synthesis, characterization and photoelectric properties of Fe2O3 incorporated TiO2 photocatalyst nanocomposites. Catalysts 2021, 11, 1062. [Google Scholar] [CrossRef]
- Asif, A.H.; Wang, S.; Sun, H. Hematite-based nanomaterials for photocatalytic degradation of pharmaceuticals and personal care products (PPCPs): A short review. Curr. Opin. Green Sustain. Chem. 2021, 281, 100447. [Google Scholar] [CrossRef]
- Katoch, V.; Sharma, N.; Sharma, M.; Baghoria, M.; Panda, J.J.; Singh, M.; Prakash, B. Microflow synthesis and enhanced photocatalytic dye degradation performance of antibacterial Bi2O3 nanoparticles. Environ. Sci. Pollut. Res. 2021, 2815, 19155–19165. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, K.S.; Labhane, P.K.; Dhake, R.B.; Sonawane, G.H. Solvothermal synthesis of activated carbon loaded CdS nanoflowers: Boosted photodegradation of dye by adsorption and photocatalysis synergy. Chem. Phys. Lett. 2020, 744, 137202. [Google Scholar] [CrossRef]
- Sharma, B.; Boruah, P.K.; Yadav, A.; Das, M.R. TiO2–Fe2O3 nanocomposite heterojunction for superior charge separation and the photocatalytic inactivation of pathogenic bacteria in water under direct sunlight irradiation. J. Environ. Chem. Eng. 2018, 6, 134–145. [Google Scholar] [CrossRef]
- Sze-Mun, L.; Man-Kit, C.; Jin-Chung, S.; Honghu, Z.; Liangliang, H.; Lin, H.; Haixiang, L.; Haider, J.Z.; Hwa, C.K. Construction of delaminated Ti3C2 MXene/NiFe2O4/V2O5 ternary composites for expeditious pollutant degradation and bactericidal property. J. Environ. Chem. Eng. 2022, 10, 108284. [Google Scholar]
- Liu, H.; Zhang, Z.; Wang, X.; Nie, G.; Zhang, J.; Zhang, S.; Cao, N.; Yan, S.; Long, Y. Highly flexible Fe2O3/TiO2 composite nanofibers for photocatalysis and ultraviolet detection. J. Phys. Chem. Solids 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Hu, X.; Han, S.; Zhu, Y. Facet-controlled synthesis of polyhedral hematite/carbon composites with enhanced photoactivity. Appl. Surf. Sci. 2018, 443, 227–235. [Google Scholar] [CrossRef]
- Khasawneh, O.; Palaniandy, P. Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: A review. Environ. Technol. Innov. 2021, 21, 101–230. [Google Scholar]
- Zhu, D.; Zhang, J.; Song, J.; Wang, H.; Yu, Z.; Shen, Y.; Xie, A. Efficient one-pot synthesis of hierarchical flower-like α-Fe2O3 hollow spheres with excellent adsorption performance for water treatment. Appl. Surf. Sci. 2013, 284, 855–861. [Google Scholar] [CrossRef]
- Ji, M.; Cai, J.; Ma, Y.; Qi, L. Controlled growth of ferrihydrite branched nanosheet arrays and their transformation to hematite nanosheet arrays for photoelectrochemical water splitting. ACS Appl. Mater. Inter. 2016, 86, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Das, S.; Jana, S. Doping of Ni in α-Fe2O3 nanoclews to boost oxygen evolution electrocatalysis. ACS Sustain. Chem. Eng. 2019, 7, 12117–12124. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Zhang, L.; Zhang, P.; Dong, E.; Ma, J.; Wang, G. Design and fabrication of an α-Fe2O3/TiO2/Si 3D hierarchical photoanode for improved photoelectrochemical water splitting. J. Alloys Compd. 2019, 773, 597–604. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, C.; Niu, G.; Zhang, W.; Wang, F. Construction of 1D/2D α-Fe2O3/SnO2 hybrid nanoarrays for sub-ppm acetone detection. Research 2020, 2020, 2196063. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.M.; Shaban, M. Highly sensitive Au-Fe2O3-Au and Fe2O3-Au-Fe2O3 biosensors utilizing strong surface plasmon resonance. Appl. Phys. B 2020, 126, 57. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Venkataramana, R.; Watts, A.; Anpo, M.; Neppolian, B. Facile synthesis of Fe2O3/Cu2O nanocomposite and its visible light photocatalytic activity for the degradation of cationic dyes. Res. Chem. Intermed. 2017, 43, 5091–5102. [Google Scholar] [CrossRef]
- Narendhran, S.; Shakila, P.B.; Manikandan, M.; Vinoth, V.; Rajiv, P. Spectroscopic investigation on photocatalytic degradation of methyl orange using Fe2O3/WO3/FeWO4 nanomaterials. Spectrochim. Acta A 2020, 232, 118164. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Fu, H.; Jia, H.; Jin, H.; Cheng, X.; Wang, W.; Liu, M.; Zhou, Q. Ultrathin Ti3C2 nanosheets served as a highly efficient hole transport layer on a Fe2O3 photoanode for photoelectrochemical water oxidation. New J. Chem. 2021, 45, 20537–20541. [Google Scholar] [CrossRef]
- Fard, A.K.; Mckay, G.; Preud’Homme, H.; Kochkodan, V.; Atieh, M.A. Fabrication and evaluation of activated carbon/Fe2O3 nano-composite on the removal of strontium ions from water. Desalin. Water Treat. 2017, 73, 399–408. [Google Scholar] [CrossRef]
- Muhajir, M.; Puspitasari, P.; Razak, J.A. Synthesis and applications of Hematite α-Fe2O3: A Review. J. Mech. Eng. Sci. 2020, 3, 51–58. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, B.; Wu, F.; Zhang, Q.; Chu, X.; Ge, M.; Zhou, N.; Shen, J. Wound healing acceleration by antibacterial biodegradable black phosphorus nanosheets loaded with cationic carbon dots. J. Mater. Sci. 2021, 56, 6411–6426. [Google Scholar] [CrossRef]
- Sahani, S.; Tripathi, K.M.; Lee, T.I.; Dubal, D.P.; Wong, C.P.; Sharma, Y.C.; Kim, T.Y. Recent advances in photocatalytic carbon-based materials for enhanced water splitting under visible-light irradiation. Energ. Convers. Manag. 2022, 252, 115133. [Google Scholar] [CrossRef]
- Xu, J.; Yao, Q.; Li, P.; Zhang, X.; Wang, S.; Pan, C. Construction of amorphous carbon-coated alpha-Fe2O3 core–shell nanostructure for efficient photocatalytic performance. J. Mater. Sci. Mater. Electron. 2022, 33, 22549–22559. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, S.; Yang, Y.; Liu, S.; Cao, Q.; Wang, Y.; Wagberg, T.; Hu, G. Artificial chloroplast-like phosphotungstic acid-iron oxide microbox heterojunctions penetrated by carbon nanotubes for solar photocatalytic degradation of tetracycline antibiotics in wastewater. Adv. Compos. Hybrid Mater. 2022, 5, 3158–3175. [Google Scholar] [CrossRef]
- Sun, S.; Sun, M.; Kong, Y.; Fang, Y.; Yao, Y. MoS2 and graphene as dual, cocatalysts for enhanced visible light photocatalytic activity of Fe2O3. J. Sol-Gel Sci. Technol. 2016, 80, 719–727. [Google Scholar] [CrossRef]
- Shin, J.; Hutomo, C.A.; Kim, J.; Jang, J.; Park, C.B. Natural pollen exine-templated synthesis of photocatalytic metal oxides with high surface area and oxygen vacancies. Appl. Surf. Sci. 2022, 599, 154064. [Google Scholar] [CrossRef]
- Xia, D.; Chen, Q.; Jiao, Y.; Lian, Q.; Sun, M.; He, C.; Shang, J.; Wang, T. A modified flower pollen-based photothermocatalytic process for enhanced solar water disinfection: Photoelectric effect and bactericidal mechanisms. Water Res. 2022, 217, 118423. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Feng, M.; Chen, X.; Zhao, D.; Sun, J. One-pot construction of chitin-derived carbon/g-C3N4 heterojunction for the improvement of visible-light photocatalysis. Appl. Surf. Sci. 2020, 527, 146737. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Zhang, M.; Antonietti, M.; Fu, X.; Wang, X. Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun. 2012, 3, 1139. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, H.; Wang, T.; Wang, B.; Wei, W.; Li, H.; Yuan, S.; An, T.; Zhao, H.; Yu, J.; et al. Nature-based catalyst for visible-light-driven photocatalytic CO2 reduction. Energy Environ. Sci. 2018, 11, 2382–2389. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, B.; Jiang, C.; Bei, C.; Yu, J. Constructing 2D/2D Fe2O3/g-C3N4 direct Z-scheme photocatalysts with enhanced H2 generation performance. Sol. RRL 2018, 2, 1800006. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Vasanthakumar, V.; Shanavas, S.; Karthikeyan, S.; Anbarasan, P. Crumpled sheet like graphene based WO3-Fe2O3 nanocomposites for enhanced charge transfer and solar photocatalysts for environmental remediation. Appl. Surf. Sci. 2019, 470, 114–128. [Google Scholar] [CrossRef]
- Pradhan, G.; Padhi, D.; Parida, K. Fabrication of α-Fe2O3 Nanorod/RGOcomposite: A novel hybrid photocatalyst for phenol degradation. ACS Appl. Mater. Interf. 2013, 5, 9101–9110. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Ajayan, P.M. High efficiency photocatalytic water splitting using 2D α-Fe2O3/g-C3N4 Z-scheme catalysts. Adv. Energy Mater. 2017, 7, 170025. [Google Scholar] [CrossRef]
- Feng, H.; Wang, W.; Zhang, M.; Zhu, S.; Wang, Q.; Liu, J.; Chen, S. 2D titanium carbide-based nanocomposites for photocatalytic bacteriostatic applications. Appl. Catal. B Environ. 2020, 266, 118609. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Z.; Su, J.; Guo, L. Metal-Free Flexible Protonated g-C3N4/Carbon Dots Photoanode for Photoelectrochemical Water Splitting. ChemElectroChem 2018, 5, 2734–2737. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Efficient reduction of Cr (VI) by a BMO/Bi2S3 heterojunction via synergistic adsorption and photocatalysis under visible light. J. Hazard. Mater. 2020, 400, 123243. [Google Scholar] [CrossRef]
- Nikitenko, S.I.; Chave, T.; Cau, C.; Brau, H.P.; Flaud, V. Photothermal hydrogen production using noble-metal-free Ti@TiO2 core–shell nanoparticles under visible–NIR light irradiation. ACS Catal. 2015, 5, 4790–4795. [Google Scholar] [CrossRef]
- Jiang, R.; Wu, D.; Lu, G.; Yan, Z.; Liu, J. Modified 2D-2D ZnIn2S4/BiOCl van der Waals heterojunctions with CQDs: Accelerated charge transfer and enhanced photocatalytic activity under vis-and NIR-light. Chemosphere 2019, 227, 82–92. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Liu, X.; Cui, Z.; Chen, D.F.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. The rapid photoresponsive bacteria-killing of Cu-doped MoS2. Biomater. Sci. 2020, 8, 4216–4224. [Google Scholar] [CrossRef]
- Rajendran, A.; Alsawalha, M.; Alomayri, T. Biogenic synthesis of husked rice-shaped iron oxide nanoparticles using coconut pulp (Cocos nucifera L.) extract for photocatalytic degradation of rhodamine b dye and their in vitro antibacterial and anticancer activity. J. Saudi Chem. Soc. 2021, 25, 101307. [Google Scholar] [CrossRef]
- Haseena, S.; Shanavas, S.; Duraimurugan, J.; Ahamad, T.; Jayamani, N. Study on photocatalytic and antibacterial properties of phase pure fe2o3 nanostructures synthesized using caralluma fimbriata and achyranthes aspera leaves. Optik 2020, 203, 164047. [Google Scholar] [CrossRef]
- Tharani, K.; Christy, A.J.; Sagadevan, S.; Nehru, L.C. Photocatalytic and antibacterial performance of iron oxide nanoparticles formed by the combustion method. Chem. Phys. Lett. 2021, 771, 138524. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, T.; Sun, M.; Hanif, A.; Gu, Q.; Tian, B.; Jiang, Z.; Wang, B.; Sun, H.; Shang, J.; et al. Photocatalytic bacterial inactivation by a rape pollen-MoS2 biohybrid catalyst: Synergetic effects and inactivation mechanisms. Environ. Sci. Technol. 2019, 54, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, Z.; Jimmy, C.Y. Treated rape pollen: A metal-free visible-light-driven photocatalyst from nature for efficient water disinfection. J. Mater. Chem. A 2019, 7, 9335–9344. [Google Scholar] [CrossRef]
- Liu, F.; Nie, C.; Dong, Q.; Ma, Z.; Liu, W.; Tong, M. AgI modified covalent organic frameworks for effective bacterial disinfection and organic pollutant degradation under visible light irradiation. J. Hazard. Mater. 2020, 398, 122865. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Easton, C.D.; Li, Q.; Xia, Y.; Yin, Y.; Hu, X.; Hu, J.; Xia, D.; McCarthy, D.T.; et al. An in situ assembled WO3–TiO2 vertical heterojunction for enhanced Z-scheme photocatalytic activity. Nanoscale 2020, 12, 8775–8784. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, F.; Li, M.; Liu, W.; Tong, M. Facile synthesis of magnetic Fe3O4@BiOI@AgI for water decontamination with visible light irradiation: Different mechanisms for different organic pollutants degradation and bacterial disinfection. Water Res. 2018, 137, 120–129. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Ma, Y.; Li, X.; Li, S.; Chen, S.; Cao, Y.; Lu, Y.; Zhang, R.; Zhou, W.; Wang, H.; et al. Synthesis of Rape Pollen-Fe2O3 Biohybrid Catalyst and Its Application on Photocatalytic Degradation and Antibacterial Properties. Catalysts 2023, 13, 358. https://doi.org/10.3390/catal13020358
Gu J, Ma Y, Li X, Li S, Chen S, Cao Y, Lu Y, Zhang R, Zhou W, Wang H, et al. Synthesis of Rape Pollen-Fe2O3 Biohybrid Catalyst and Its Application on Photocatalytic Degradation and Antibacterial Properties. Catalysts. 2023; 13(2):358. https://doi.org/10.3390/catal13020358
Chicago/Turabian StyleGu, Jialin, Yanping Ma, Xinshang Li, Shuwen Li, Siyi Chen, Yuxuan Cao, Yifan Lu, Rui Zhang, Wenquan Zhou, He Wang, and et al. 2023. "Synthesis of Rape Pollen-Fe2O3 Biohybrid Catalyst and Its Application on Photocatalytic Degradation and Antibacterial Properties" Catalysts 13, no. 2: 358. https://doi.org/10.3390/catal13020358
APA StyleGu, J., Ma, Y., Li, X., Li, S., Chen, S., Cao, Y., Lu, Y., Zhang, R., Zhou, W., Wang, H., & Jiang, J. (2023). Synthesis of Rape Pollen-Fe2O3 Biohybrid Catalyst and Its Application on Photocatalytic Degradation and Antibacterial Properties. Catalysts, 13(2), 358. https://doi.org/10.3390/catal13020358





