The Promotional Effect of Na on Ru for pH-Universal Hydrogen Evolution Reactions
Abstract
1. Introduction
2. Results and Discussion
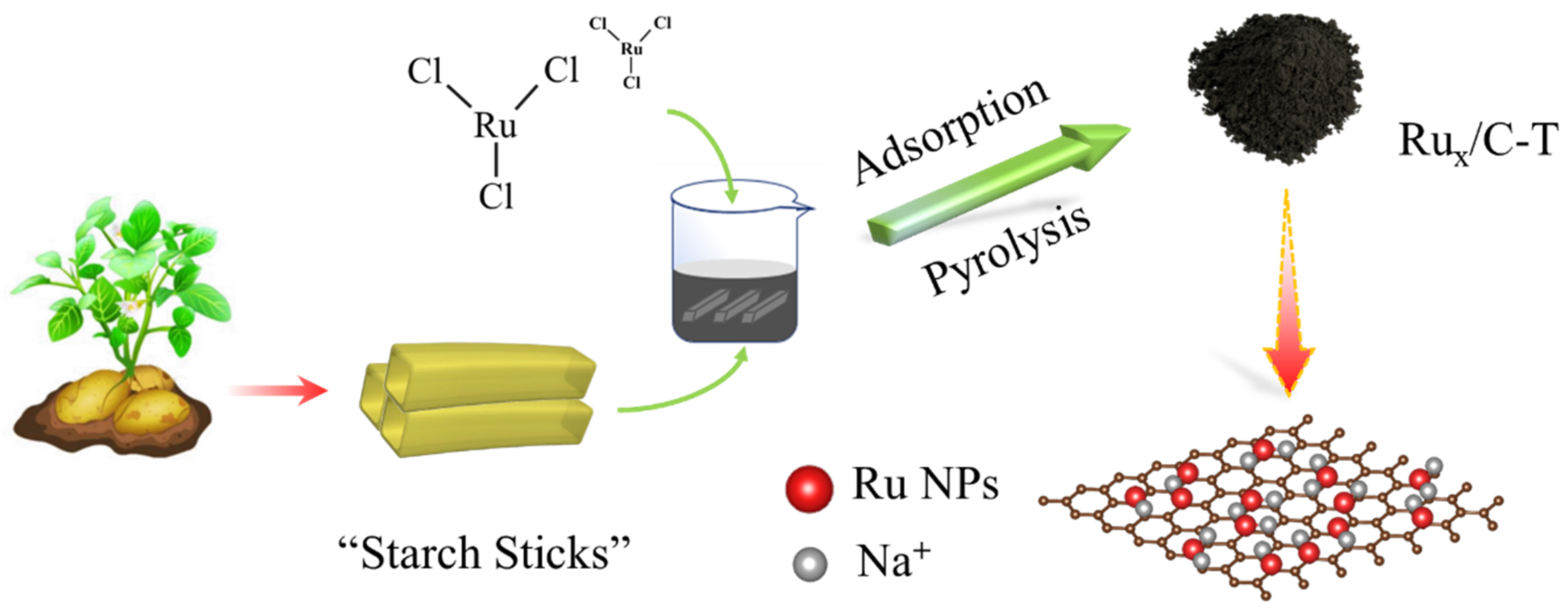
2.1. Catalyst Synthesis and Characterization
2.2. Electrochemical Characterization
3. Experimental
3.1. Chemicals
3.2. Synthesis of Electrocatalysts
3.2.1. Synthesis of Ru0.3/C−800
3.2.2. Synthesis of C−800, Ru0.2/C−800, and Ru0.4/C−800
3.2.3. Synthesis of Ru0.3/C−700, Ru0.3/C−900, and Ru0.3/C−1000
3.2.4. Synthesis of Ru0.3/C−800−WF (Wash First)
3.3. Material Characterization
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, B.; Wang, Z.; Cheng, L.; Chen, Z.; Dong, Y.; Wang, X.; Wang, T.; Mao, X.; Gao, Y.; Xu, Z.; et al. Regulating charge distribution of Ru atoms in ruthenium phosphide/carbon nitride/carbon for promoting hydrogen evolution reaction. J. Alloy. Compd. 2023, 939, 168717. [Google Scholar] [CrossRef]
- Abdollahi, A.; Ghaffarinejad, A.; Arabi, M. Electrodeposition of Ni-Fe on graphite rod as an efficient and binder-free electrocatalyst for oxygen and hydrogen evolution reactions. J. Alloy. Compd. 2023, 937, 168400. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Zhang, D.; Qin, Y.; Wang, M.; Han, Y.; Zhan, T.; Yang, B.; Li, S.; Lai, J.; et al. Solvent-free microwave synthesis of ultra-small Ru-Mo2C@CNT with strong metal-support interaction for industrial hydrogen evolution. Nat. Commun. 2021, 12, 4018. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Luo, M.; Liu, Z.; Peng, M.; Chen, D.; Lu, Y.-R.; Chan, T.-S.; de Groot, F.M.F.; Tan, Y. Rational strain engineering of single-atom ruthenium on nanoporous MoS2 for highly efficient hydrogen evolution. Nat. Commun. 2021, 12, 1687. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, W.; Wu, H.; Lu, S. Carbon Dots Enhance Ruthenium Nanoparticles for Efficient Hydrogen Production in Alkaline. Acta Phys. Chim. Sin. 2021, 37, 2009082. [Google Scholar] [CrossRef]
- Zhou, S.; Jang, H.; Qin, Q.; Li, Z.; Kim, M.G.; Li, C.; Liu, X.; Cho, J. Three-dimensional hierarchical Co(OH)F nanosheet arrays decorated by single-atom Ru for boosting oxygen evolution reaction. Sci. China Mater. 2021, 64, 1408–1417. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, X.; Wang, S.; Liang, L.; Cao, M.; Wang, L.; Li, G.; Xu, Y.; Huang, X. Superlattice in a Ru Superstructure for Enhancing Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202116867. [Google Scholar] [CrossRef]
- Feng, T.; Yu, G.; Tao, S.; Zhu, S.; Ku, R.; Zhang, R.; Zeng, Q.; Yang, M.; Chen, Y.; Chen, W.; et al. A highly efficient overall water splitting ruthenium-cobalt alloy electrocatalyst across a wide pH range via electronic coupling with carbon dots. J. Mater. Chem. A 2020, 8, 9638–9645. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Appropriate Use of Electrochemical Impedance Spectroscopy in Water Splitting Electrocatalysis. ChemElectroChem 2020, 7, 2297–2308. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Z.; Jiang, J.; Wang, J.; Song, X.; He, Q.; Ding, W.; Wei, Z. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 2020, 3, 454–462. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Dang, N.K.; Sultan, S.; Thangavel, P.; Jeong, H.Y.; Kim, K.S. Multi-heteroatom-doped carbon from waste-yeast biomass for sustained water splitting. Nat. Sustain. 2020, 3, 556–563. [Google Scholar] [CrossRef]
- Cai, C.; Liu, K.; Zhu, Y.; Li, P.; Wang, Q.; Liu, B.; Chen, S.; Li, H.; Zhu, L.; Li, H.; et al. Optimizing Hydrogen Binding on Ru Sites with RuCo Alloy Nanosheets for Efficient Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202113664. [Google Scholar] [CrossRef]
- Ren, R.; Wang, X.; Chen, H.; Miller, H.A.; Salam, I.; Varcoe, J.R.; Wu, L.; Chen, Y.; Liao, H.; Liu, E.; et al. Reshaping the Cathodic Catalyst Layer for Anion Exchange Membrane Fuel Cells: From Heterogeneous Catalysis to Homogeneous Catalysis. Angew. Chem. Int. Ed. 2021, 60, 4049–4054. [Google Scholar] [CrossRef]
- Ha, Y.; Fei, B.; Yan, X.; Xu, H.; Chen, Z.; Shi, L.; Fu, M.; Xu, W.; Wu, R. Atomically Dispersed Co-Pyridinic N-C for Superior Oxygen Reduction Reaction. Adv. Energy Mater. 2020, 10, 2002592. [Google Scholar] [CrossRef]
- Rapson, T.D.; Ju, H.; Marshall, P.; Devilla, R.; Jackson, C.J.; Giddey, S.; Sutherland, T.D. Engineering a solid-state metalloprotein hydrogen evolution catalyst. Sci. Rep. 2020, 10, 3774. [Google Scholar] [CrossRef]
- Tong, Y.; Sun, Q.; Chen, P.; Chen, L.; Fei, Z.; Dyson, P.J. Nitrogen-Incorporated Cobalt Sulfide/Graphene Hybrid Catalysts for Overall Water Splitting. ChemSusChem 2020, 13, 5112–5118. [Google Scholar] [CrossRef]
- Ding, R.; Lin, L.; Pei, C.; Yu, X.; Sun, Q.; Park, H.S. Hierarchical Architectures Based on Ru Nanoparticles/Oxygen-Rich-Carbon Nanotubes for Efficient Hydrogen Evolution. Chem.—A Eur. J. 2021, 27, 11150–11157. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, N.; Zhang, C.; Sun, N.; Pan, Y.; Chen, C.; Li, J.; Tan, M.; Cui, R.; Shi, Z.; et al. Doping Ruthenium into Metal Matrix for Promoted pH-Universal Hydrogen Evolution. Adv. Sci. 2022, 9, 2200010. [Google Scholar] [CrossRef]
- Su, P.; Pei, W.; Wang, X.; Ma, Y.; Jiang, Q.; Liang, J.; Zhou, S.; Zhao, J.; Liu, J.; Lu, G.Q. Exceptional Electrochemical HER Performance with Enhanced Electron Transfer between Ru Nanoparticles and Single Atoms Dispersed on a Carbon Substrate. Angew. Chem. Int. Ed. 2021, 60, 16044–16050. [Google Scholar] [CrossRef]
- Wang, C.; Qi, L. Heterostructured Inter-Doped Ruthenium–Cobalt Oxide Hollow Nanosheet Arrays for Highly Efficient Overall Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 17219–17224. [Google Scholar] [CrossRef]
- Rao, Y.; Xu, L.; Zhou, M.; Yin, B.; Osuka, A.; Song, J. Expanded Azaporphyrins Consisting of Multiple BODIPY Units: Global Aromaticity and High Affinities Towards Alkali Metal Ions. Angew. Chem. Int. Ed. 2022, 61, e202206899. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A. Review of ammonia catalysis. Catal. Rev. 1971, 4, 1–26. [Google Scholar] [CrossRef]
- Williams, F.J.; Palermo, A.; Tracey, S.; Tikhov, M.S.; Lambert, R.M. Electrochemical Promotion by Potassium of the Selective Hydrogenation of Acetylene on Platinum: Reaction Studies and XP Spectroscopy. J. Phys. Chem. B 2002, 106, 5668–5672. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Dongil, A.B.; Conesa, J.M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Promotion of Ru or Ni on Alumina Catalysts with a Basic Metal for CO2 Hydrogenation: Effect of the Type of Metal (Na, K, Ba). Nanomaterials 2022, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Liu, L.; Zhuang, J.; Wang, H.; Li, Y.; Shen, W.; Xu, Y.; Bao, X. Creating Mesopores in ZSM-5 Zeolite by Alkali Treatment: A New Way to Enhance the Catalytic Performance of Methane Dehydroaromatization on Mo/HZSM-5 Catalysts. Catal. Lett. 2003, 91, 155–167. [Google Scholar] [CrossRef]
- Zhao, B.; Zhai, P.; Wang, P.; Li, J.; Li, T.; Peng, M.; Zhao, M.; Hu, G.; Yang, Y.; Li, Y.-W.; et al. Direct Transformation of Syngas to Aromatics over Na-Zn-Fe5C2 and Hierarchical HZSM-5 Tandem Catalysts. Chem 2017, 3, 323–333. [Google Scholar] [CrossRef]
- Chen, I.; Chen, F.L. Effect of alkali and alkaline-earth metals on the resistivity to coke formation and sintering of nickel-alumina catalysts. Ind. Eng. Chem. Res. 1990, 29, 534–539. [Google Scholar] [CrossRef]
- Gentner, T.X.; Mulvey, R.E. Alkali-Metal Mediation: Diversity of Applications in Main-Group Organometallic Chemistry. Angew. Chem. Int. Ed. 2021, 60, 9247–9262. [Google Scholar] [CrossRef]
- Qin, R.; Zhou, L.; Liu, P.; Gong, Y.; Liu, K.; Xu, C.; Zhao, Y.; Gu, L.; Fu, G.; Zheng, N. Alkali ions secure hydrides for catalytic hydrogenation. Nat. Catal. 2020, 3, 703–709. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, M.; Yin, J.; Abou-Hamad, E.; Schwingenschlögl, U.; Costa, P.M.F.J.; Alshareef, H.N. A Cyclized Polyacrylonitrile Anode for Alkali Metal Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 1355–1363. [Google Scholar] [CrossRef]
- Zimmermann, P.; Ar, D.; Rößler, M.; Holze, P.; Cula, B.; Herwig, C.; Limberg, C. Selective Transformation of Nickel-Bound Formate to CO or C−C Coupling Products Triggered by Deprotonation and Steered by Alkali-Metal Ions. Angew. Chem. Int. Ed. 2021, 60, 2312–2321. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, X.; Qian, Y.; Gao, H.; Weber, D.H.; Zhang, L.; Goodenough, J.B.; Yu, G. In Situ Formation of Liquid Metals via Galvanic Replacement Reaction to Build Dendrite-Free Alkali-Metal-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 12170–12177. [Google Scholar] [CrossRef]
- Zhang, L.; Jang, H.; Liu, H.; Kim, M.G.; Yang, D.; Liu, S.; Liu, X.; Cho, J. Sodium-Decorated Amorphous/Crystalline RuO2 with Rich Oxygen Vacancies: A Robust pH-Universal Oxygen Evolution Electrocatalyst. Angew. Chem. Int. Ed. 2021, 60, 18821–18829. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Liu, X.; Shao, Z.; Xia, L.; Zhong, L.; Wang, H.; Sun, Y. Tuning the interaction between Na and Co2C to promote selective CO2 hydrogenation to ethanol. Appl. Catal. B Environ. 2021, 293, 120207. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Gong, Y.; Wang, Y. Directly immobilizing a Ru–tannic acid linkage coordination complex on carbon cloth: An efficient and ultrastable catalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 11038–11043. [Google Scholar] [CrossRef]
- Sun, S.-W.; Wang, G.-F.; Zhou, Y.; Wang, F.-B.; Xia, X.-H. High-Performance Ru@C4N Electrocatalyst for Hydrogen Evolution Reaction in Both Acidic and Alkaline Solutions. ACS Appl. Mater. Interfaces 2019, 11, 19176–19182. [Google Scholar] [CrossRef]
- Xu, C.; Ming, M.; Wang, Q.; Yang, C.; Fan, G.; Wang, Y.; Gao, D.; Bi, J.; Zhang, Y. Facile synthesis of effective Ru nanoparticles on carbon by adsorption-low temperature pyrolysis strategy for hydrogen evolution. J. Mater. Chem. A 2018, 6, 14380–14386. [Google Scholar] [CrossRef]
- Kweon, D.H.; Okyay, M.S.; Kim, S.-J.; Jeon, J.-P.; Noh, H.-J.; Park, N.; Mahmood, J.; Baek, J.-B. Ruthenium anchored on carbon nanotube electrocatalyst for hydrogen production with enhanced Faradaic efficiency. Nat. Commun. 2020, 11, 1278. [Google Scholar] [CrossRef]
- Li, J.-S.; Huang, M.-J.; Kong, L.-X.; Chen, X.-N.; Zhou, Y.-W.; Li, J.-L.; Wang, M.-Y. Ruthenium Nanoparticles Anchored on Graphene Hollow Nanospheres Superior to Platinum for the Hydrogen Evolution Reaction in Alkaline Media. Inorg. Chem. 2020, 59, 930–936. [Google Scholar] [CrossRef]
- Chithaiah, P.; Binwal, D.C.; Raos, C.N.R. Simple Synthesis of 2D Molybdenum Carbide Nanosheets and Their Application in the Hydrogen Evolution Reaction. Eur. J. Inorg. Chem. 2022, 2022, e202101086. [Google Scholar] [CrossRef]
- Song, H.; Wu, M.; Tang, Z.; Tse, J.S.; Yang, B.; Lu, S. Single Atom Ruthenium-Doped CoP/CDs Nanosheets via Splicing of Carbon-Dots for Robust Hydrogen Production. Angew. Chem. Int. Ed. 2021, 60, 7234–7244. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Xu, K.; Zhang, C.; Dai, J.; Oliaee, S.N.; Li, L.; Zeng, X.; Wu, C.; Peng, Z. Free-Standing Two-Dimensional Ru Nanosheets with High Activity toward Water Splitting. ACS Catal. 2016, 6, 1487–1492. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Jaroniec, M.; Qiao, S.-Z. High Electrocatalytic Hydrogen Evolution Activity of an Anomalous Ruthenium Catalyst. J. Am. Chem. Soc. 2016, 138, 16174–16181. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, Y.; Zhao, Z.; Li, R.; He, J.; Yin, J.; Yan, B.; Zhang, X. A Review of the Application of Heterostructure Catalysts in Hydrogen Evolution Reaction. ChemistrySelect 2022, 7, e202104041. [Google Scholar] [CrossRef]
- Lu, E.; Zhang, Z.; Tao, J.; Hou, Y.; Zhang, J.; Yu, Z. Enhanced Metal-Semiconductor Interaction for Photocatalytic Hydrogen Evolution Reaction. Chem.—A Eur. J. 2022, 28, e202201590. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Shen, T.; Wu, C.; Zu, L.; Zhang, L. Porous NiFe alloys synthesized via freeze casting as bifunctional electrocatalysts for oxygen and hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 37736–37745. [Google Scholar] [CrossRef]
- Wang, T.; Tao, L.; Zhu, X.; Chen, C.; Chen, W.; Du, S.; Zhou, Y.; Zhou, B.; Wang, D.; Xie, C.; et al. Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat. Catal. 2022, 5, 66–73. [Google Scholar] [CrossRef]
- Liu, L.; Qin, C.; Yu, M.; Wang, Q.; Wang, J.; Hou, B.; Jia, L.; Li, D. Morphology Evolution of Hcp Cobalt Nanoparticles Induced by Ru Promoter. ChemCatChem 2020, 12, 2083–2090. [Google Scholar] [CrossRef]
- Niyitanga, T.; Jeong, H.K. Modification of thermally reduced graphite oxide and molybdenum disulfide by solution plasma for hydrogen evolution reaction. Mater. Chem. Phys. 2021, 263, 124345. [Google Scholar] [CrossRef]
- Ortiz-Restrepo, J.E.; Loaiza, O.A.; Urresta, J.D.; Velasquez, J.D.; Pastor, E.; Chaur, M.N.; Lizcano-Valbuena, W.H. A comparative study of different carbon materials as metal-free catalysts for oxygen reduction and hydrogen evolution reactions in alkaline media. Diam. Relat. Mater. 2021, 117, 108464. [Google Scholar] [CrossRef]
- Pham, N.N.; Kang, S.G.; Kim, H.-J.; Pak, C.; Han, B.; Lee, S.G. Catalytic activity of Ni3Mo surfaces for hydrogen evolution reaction: A density functional theory approach. Appl. Surf. Sci. 2021, 537, 147894. [Google Scholar] [CrossRef]
- Zhu, T.; Huang, J.; Huang, B.; Zhang, N.; Liu, S.; Yao, Q.; Haw, S.; Chang, Y.; Pao, C.; Chen, J.; et al. High-Index Faceted RuCo Nanoscrews for Water Electrosplitting. Adv. Energy Mater. 2020, 10, 2002860. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, B.; Zhao, C.; Zhou, Y.; Guo, J.; Wei, Z.; Wang, J. The Promotional Effect of Na on Ru for pH-Universal Hydrogen Evolution Reactions. Catalysts 2023, 13, 552. https://doi.org/10.3390/catal13030552
Guo B, Zhao C, Zhou Y, Guo J, Wei Z, Wang J. The Promotional Effect of Na on Ru for pH-Universal Hydrogen Evolution Reactions. Catalysts. 2023; 13(3):552. https://doi.org/10.3390/catal13030552
Chicago/Turabian StyleGuo, Bingxin, Chengfei Zhao, Yingshuang Zhou, Junjie Guo, Zhongzhe Wei, and Jing Wang. 2023. "The Promotional Effect of Na on Ru for pH-Universal Hydrogen Evolution Reactions" Catalysts 13, no. 3: 552. https://doi.org/10.3390/catal13030552
APA StyleGuo, B., Zhao, C., Zhou, Y., Guo, J., Wei, Z., & Wang, J. (2023). The Promotional Effect of Na on Ru for pH-Universal Hydrogen Evolution Reactions. Catalysts, 13(3), 552. https://doi.org/10.3390/catal13030552





