Abstract
Cu-based catalysts, modified by gallium addition via the stepwise co-precipitation method, were studied for the liquid phase hydrogenation of hydroxypivalaldehyde (HPA) to neopentyl glycol (NPG). Through physico-chemical techniques, the effects of gallium introduction on the Cu trimetallic catalyst performance and the reaction mechanism of HPA hydrogenation were discussed. The characterization results showed that gallium introduction can influence the dispersion, reduction, and distribution of active Cu species, as well as their reactivity. Herein, the catalyst with 2 wt% gallium addition exhibited excellent catalytic performance with HPA conversion rate and NPG selectivity of 93.5% and 95.5%, at a reaction pressure of 3 MPa, temperature of 110 °C, hydrogen-aldehyde ratio (molar ratio) 10:1, and liquid space-time at a speed of 8.4 h−1. The good performance could be attributed to gallium doping tending to dynamically tune the interaction between the components, increasing Cu dispersion and the distributions of Cu+ and Cu0 species on the catalyst surfaces.
1. Introduction
Neopentyl glycol (NPG) is an important organic chemical raw material that can be used as an intermediate for producing polyurethane, ibuprofen, unsaturated polyester resin, etc. Because of the better chemical and thermal stability, NPG-derived products are widely used in our daily life [1]. With the continuous development of NPG downstream products and application fields, the demand for NPG will further increase. However, there are few related studies on NPG synthesis in the current literature. Commercially, NPG can be produced from the reduction of hydroxypivalaldehyde (HPA), which was obtained from the aldol condensation reaction of formaldehyde and isobutyraldehyde. Currently, the condensation process is very mature, and the subsequent reduction process is the key to restricting the industrial production of NPG. The sodium formate method and catalytic hydrogenation method [2,3] are two commonly used HPA reduction methods; the latter has become the main NPG production process at home and abroad due to its high product yield, low material consumption, and outstanding environmental protection advantages. As one of the core technologies of the hydrogenation process, the performance of the catalyst has become the key to restricting the technological progress of this process. Nowadays, most of the reported hydrogenation catalyst systems are based on heterogeneous transition or noble metals catalysts [3,4,5,6]. Overall, these systems, the copper-based catalyst has received substantial attention as a promising HPA hydrogenation catalyst due to its high selectivity in C−O hydrogenation. Given its hopeful catalytic activity, low cost, and easily available features compared with noble catalysts, there are certain advantages in choosing copper catalysts for C=O hydrogenation in terms of environmental and economic prospects [6,7,8,9]. Many factors can affect the catalytic performance of Cu-based catalyst, such as the synthetic method, crystallite size of metals, reduction conditions, the operating conditions, and so on. Therefore, research efforts continue to focus on the development of efficient and novel commercial catalyst.
It is widely accepted that the dispersion and distribution of the reduced copper compared with the interactions between each component are two prominent factors influencing the catalytic activity and stability. Much work based the catalyst modification has been conducted to tune its chemical and physical properties [8,9,10,11,12]. Yuan et al. [9] reported that suitable H3BO3 modified Cu-SiO2 catalysts exhibited good dimethyl oxalate hydrogenation activity and the boric acid introduction amount greatly affected the dispersion, stability and reduction of Cu component. They presumed that an appropriate distribution of Cu0/Cu+ should be a crucial prerequisite for gaining excellent hydrogenation performance. The outstanding catalytic performance is ascribed to the strong interaction between boric oxide and active copper component, which is beneficial for maintaining a high proportion of Cu+/Cu0 and retarding the transmigration of copper nanoparticles on the surface of Cu-SiO2 catalysts. Chen et al. [10] supposed that the monovalent copper on the catalyst surface is favorable for the enhancement of catalytic performance. They proposed that the monovalent cationic defects generated by the introduction of trivalent metal promoters into ZnO are beneficial to stabilize the Cu+ species during the reduction and reaction process. Dong et al. [11] investigated the effect of lanthanum group promoters on Copper-based catalysts for methanol synthesis. They found that the lanthanum-modified catalyst showed smaller copper particle size and stronger anti-sintering ability. Ma et al. [12] reported a series of highly comparable Cu/SiO2 catalysts prepared by the ammonia evaporation method, and their catalytic performance of the hydrogenation of esters to alcohols was researched. They attribute the differences in catalyst activity to the different roles played by copper species in the acyl hydrogenation reaction, where Cu0 mainly assisted the activation of H2, while Cu+ species were accountable for the adsorption of methoxy and acyl groups. Sun [13] investigated the effect of silicon content on the reaction performance of slurry copper-based catalysts for the synthesis of dimethyl ether. They demonstrated that the specific activity of Si promoted catalysts was mainly ascribed to the modification of electronic interaction between active component and other components, which could inhibit excessive reduction of copper and result in more Cu+ species on the catalyst surface. Previous research by the Uchijima group [14] investigated the role of metal oxides over Cu-based catalysts and found that the specific activity for methanol synthesis was accredited to the distribution of reduced Cu species on the catalyst surface. The results indicated that both surface oxygen and foreign elements can stabilize the surface Cu+. These reports strongly evidenced the doping effect on the catalytic performance of Cu catalyst. Through these reports, we also can see that Cu-based catalysts have undergone considerable research in the hydrogenation of carbon oxides, aromatic aldehydes, dimethyl oxalate, etc. However, to our acknowledgement, only less detailed and in-depth investigation of the effects of Cu-based catalysts has been reported in the hydrogenation of fatty aldehyde to produce the corresponding alcohols.
Adding specific metal (oxides) to catalysts is one of the most efficient and practical methods to tune the catalytic performance. In the current work, gallium modified Cu-based catalyst prepared by fractional precipitation technique have been applied in the hydrogenation of HPA to NPG. The physicochemical properties of as-prepared catalysts were investigated to get an insight into the structure–activity relationship. The results manifested that Ga introduction can affect the distribution ratio of copper species, leading to a well-performing catalyst for the synthesis of NPG from HPA. This can provide a certain basis for the development and fulfilment of novel industrial catalysts for aldehyde hydrogenation.
2. Experimental
2.1. Materials and Methods
Copper nitrate (Cu(NO3)2·3H2O, 99%), aluminum nitrate (Al(NO3)3·9H2O, 99%), and sodium carbonate (Na2CO3, 99.8%) were purchased from Sinopharm Chemical Reagent limited corporation. Zinc nitrate (Zn(NO3)2·6H2O, 99%) was from Da Mao Chemical Reagent Factory. Gallium nitrate (Ga(NO3)3·xH2O, 99.8%) was supplied from Aladdin reagent.
2.2. Catalyst Preparation
Under controlled temperature and pH, a series of Cu-Zn-Al catalysts with and without Ga modification containing a fixed concentration (The molar ratio of Cu:Zn:Al = 2.8:1:0.9) were prepared by the urea-promoted step co-precipitation method. Typically, Urea/Na2CO3 precipitant and an aqueous copper-zinc mixed solution were added parallelly to hot water under vigorous stirring at 343 K. Followed by precipitation of aluminum nitrate solution and gallium nitrate at the same conditions. Then, the mixture was further aged for 2 h under constant pH (~7). The obtained precipitate was washed with deionized water for 5–6 times, recovered by filtration, dried at 110 °C for 12 h, and calcined at 723 K for 5 h. The obtained solids were mentioned as CZAGa-x, where x represents the weight percentage of Ga2O3.
2.3. Catalyst Characterization
N2 physisorption isotherms were measured on an automated TriStar II 3020 device at 77 K. X-ray diffraction (XRD) patterns were carried on a Rigaku D/max 2500 V/PC X-ray diffractometer using Cu Kα radiation. Scanning electron microscopy (SEM) was conducted on a Philips XL30 ESEM. The specific surface area of copper (SCu0) on the catalysts was measured according to the method described in the literature [9]. Firstly, the samples were reduced in a mixed gas of 10 vol% H2/N2 at 573 K, then cooled down to 353 K under He atmosphere. Subsequently, the measurement was started by switching from He to N2O and maintained for 1.5 h, ensuring that surface Cu atoms were completely oxidized according to the reaction 2 Cu(s) + N2O=Cu2O(s) + N2. After He purging for 0.5 h, the temperature was raised to 300 °C and pulsed titration was performed with 10% H2/He until the pulse peak area of TCD signal did not change significantly, ensuring that Cu2O on the catalyst surface was reduced to Cu again. The total specific surface area of copper on catalyst surface was calculated according to hydrogen consumption.
Hydrogen temperature programmed reduction (H2-TPR) experiments were tested on a Micromeritics AutChem II 2920 apparatus equipped with TCD. Firstly, the sample was swept at 523 K for 1 h under argon atmosphere. After cooling to ambient temperature, the argon gas was switched to a mixed gas of 10 vol% H2–Ar and the temperature was raised to 873 K at a heating rate of 10 K·min−1.
X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (XAES) were conducted on a Quantum 2000 Scanning ESCA Microprob instrument (Physical Electronics) equipped with an Al Ka X-ray radiation source (hν = 1486.6 eV). Generally, the samples were pretreated at a mixed gas of 10 vol% H2-N2 at 573 K for 6 h, and carefully transferred to the holder after cooling to room temperature. The C1s peak at 284.6 eV was selected as an internal reference to calibrate the binding energies of all catalysts.
2.4. Measurement of Catalytic Activity
The activity test of the catalyst is conducted on a fixed-bed reactor filled with 1 mL catalyst. Firstly, the catalysts were reduced at 573 K for 12 h in a 10% volH2/N2 gas stream under atmospheric pressure. After cooling to 383 K, HPA (10 wt% methanol solution) and H2 were fed into the reactor with hydrogen-aldehyde ratio (molar ratio) 10:1, a liquid hourly space velocity (LHSV) 8.4 h−1 and reaction pressure 3.5 MPa. The effluent products were analyzed on a gas chromatograph with OV-1701 capillary. The activity data of catalyst within 12 h are summarized in Table 1. Heat treatment at higher temperatures was performed to investigate the thermal resistance of the catalysts. After reaction for 12 h, catalysts CZAGa-2 and CZAGa-0 were treated at 673 k for 4 h and then returned to the reaction condition to investigate the catalyst stability.

Table 1.
Activities and physicochemical properties of different catalysts.
3. Results
3.1. Catalytic Activity and Stability
The catalytic performances and surface texture of each sample with different amount of Ga introduction are displayed in Table 1. As can be seen that the catalysts modified with Ga show a relatively higher catalytic activity compared with the unmodified one, which displayed the lowest HPA conversion of 81.2%. The HPA conversion increases as the content of Ga increases from 1 to 2 wt%, and then decreases with the continue increase of Ga to 3%. Under the specific evaluation conditions, the sample CZAGa-2 with 2.0 wt% Ga modification exhibited the highest HPA conversion of 93.5%, being higher than that of the traditional catalyst CZAGa-0 by about 15.1%. By comparison, CZAGa-1 and CZAGa-3 with lower and higher Ga doping amount exhibit relatively poorer catalytic performance, and the HPA conversions of these two catalysts are 89.5 and 91.9%, respectively. The selectivity of NPG is also listed in Table 1, and it presents a same trend as the activity data. Moreover, the focus of our next work is to explore the influencing factors and further improve the selectivity of the catalyst. As is known to all, the addition of promoters may affect the physicochemical properties of the catalyst, such as the reduction degree, metallic surface area and dispersion, which were related to the catalytic performance. The experimental results show that an appropriate amount of gallium introduction can increase the Cu surface area, which is conducive to the improvement of catalytic performance. However, with a higher amount of gallium introduction, the catalytic performance decreased. As described in Table 1, similar values of the BET surface area and pore volume were obtained for all samples. Moreover, Ga addition has no obvious effect on the physical properties. The sample CZAGa-2 was observed to possess the highest catalytic activity perhaps due to the change of catalyst chemical properties caused by heteroatom doping.
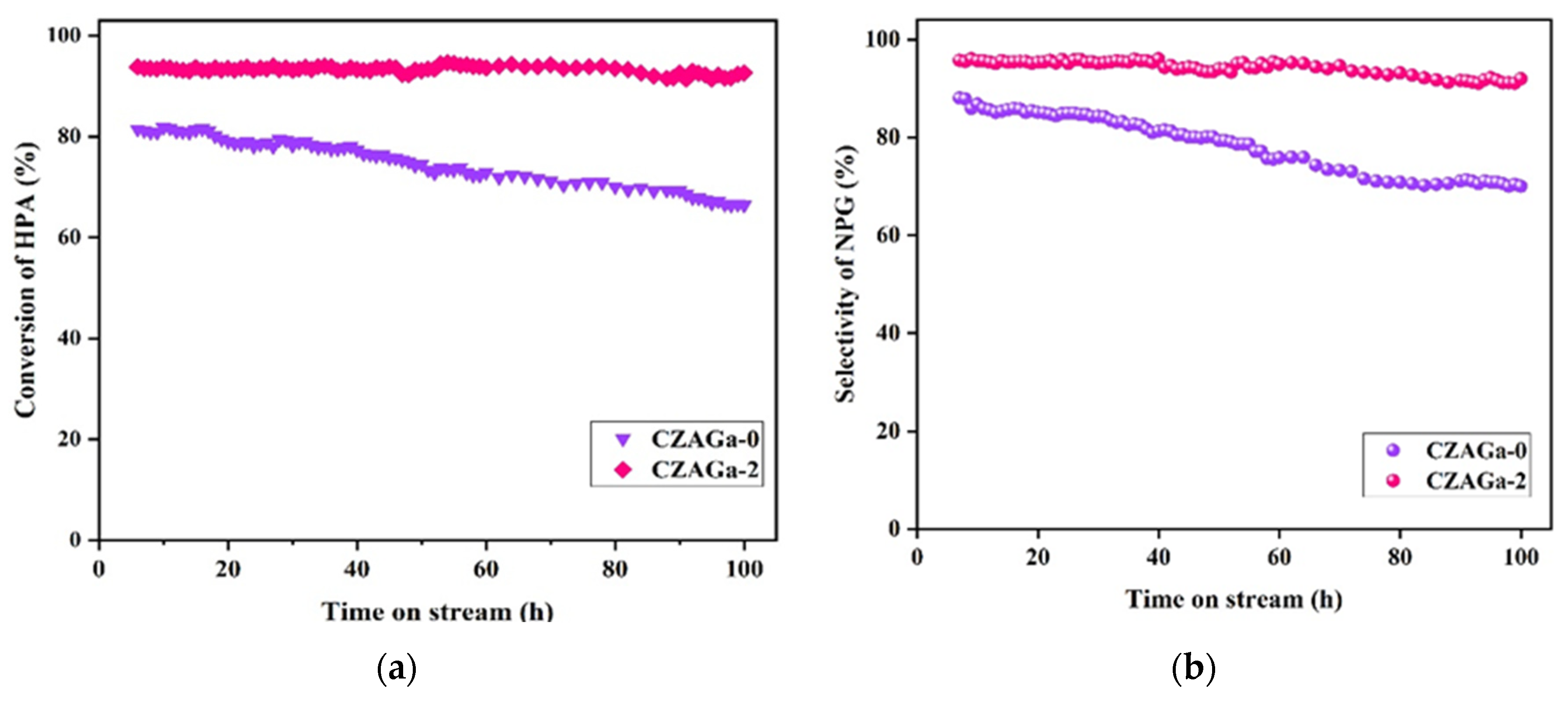
The operational stability of the catalyst is an important indicator for its industrial application. It’s reported that the lifetime of the catalyst is closely related to the stability of the Cu active phase during the hydrogenation process [15]. Catalytic behaviors of the CZAGa-0 and CZAGa-2 catalyst as a function of reaction time are displayed in Figure 1. The catalytic activity of CZAGa-2 maintained relatively stable performance during 100 h test. Compared with CZAGa-2, the deactivation rate of CZAGa-0 catalyst was relatively fast under the same reaction conditions. This indicates that the introduction of a suitable amount of gallium introduction is conducive to maintain the stability of the Cu active phases. Jun’s previous work also showed that the introduction of Ga could stabilize the catalytic performance [16].
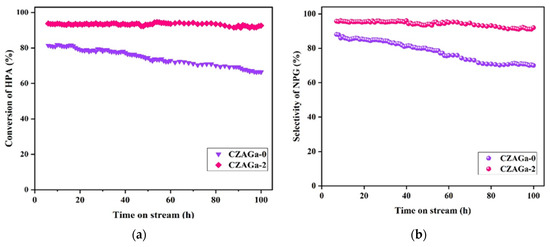
Figure 1.
Catalytic performance of CZAGa-0 and CZAGa-2 as a function of reaction time. (a) HPA conversion with time on stream; (b) NPG selectivity with time on stream The reaction conditions are the same as in Table 1.
Concerning the copper surface area of each sample, as showing in Table 1, the Ga modified catalysts exhibit a larger metallic copper surface area than the unmodified catalyst, and the sample CZAGa-2 shows the largest metallic copper surface area. The dispersion and reduction degree are two key factors that affect the copper surface area, which also strongly affect the catalytic activity. These results demonstrate that the introduction of additives can better adjust the balance between the active component dispersion and reduction degree. From this observation, we can induce that appropriate amount of Ga addition drastically modifies the catalysts surface properties. We infer that the amount of gallium introduction may impact the interaction between the active species of copper and other components of Cu-based catalysts, leading to changes in the reducibility and dispersion of the active components.
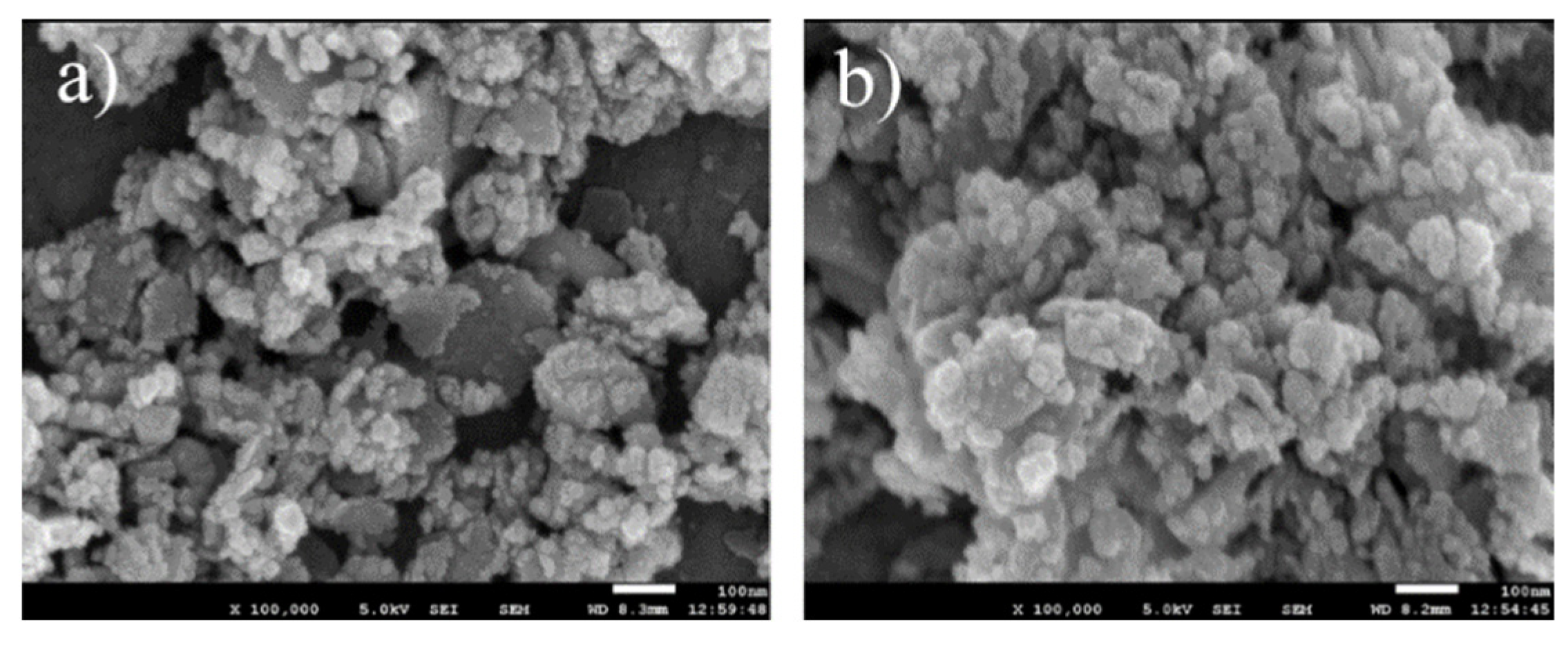
The morphology of the calcined samples CZAGa-0 and CZAGa-2 were probed by SEM Characterization. As shown in Figure 2, lots of isolated or agglomerated particles located on decomposed lamellar platelets were prevalent on both catalysts. The particles are basically spherical with the particle size less than 20 nm. Compared with CZAGa-0, fewer large plate-shaped crystals and smaller particles can be observed for CZAGa-2. This demonstrates that the copper particles on the Ga-modified catalyst maintain a higher dispersibility. The results of Grunwaldt’s [17] studies on the morphology of copper zinc system showed that copper particles shape changed with the different chemical environment. On the surface of partially reduced ZnO, the Cu particles were disc-shaped, while on the surface of fully oxidized zinc oxide, due to the weak interaction of Cu-ZnO, the particles became more spherical. The SEM micrographs illustrate that the introduction of an appropriate amount of Ga may tune the interaction between Cu and other components, resulting in a more uniform catalyst structure.
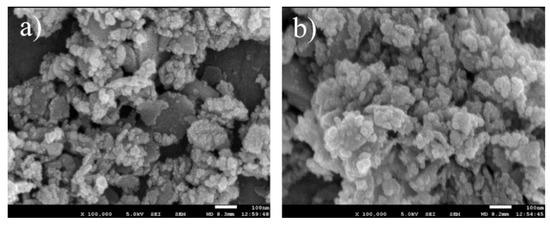
Figure 2.
SEM images of catalysts: (a) CZAGa-0; (b) CZAGa-2.
3.2. XRD Characterization
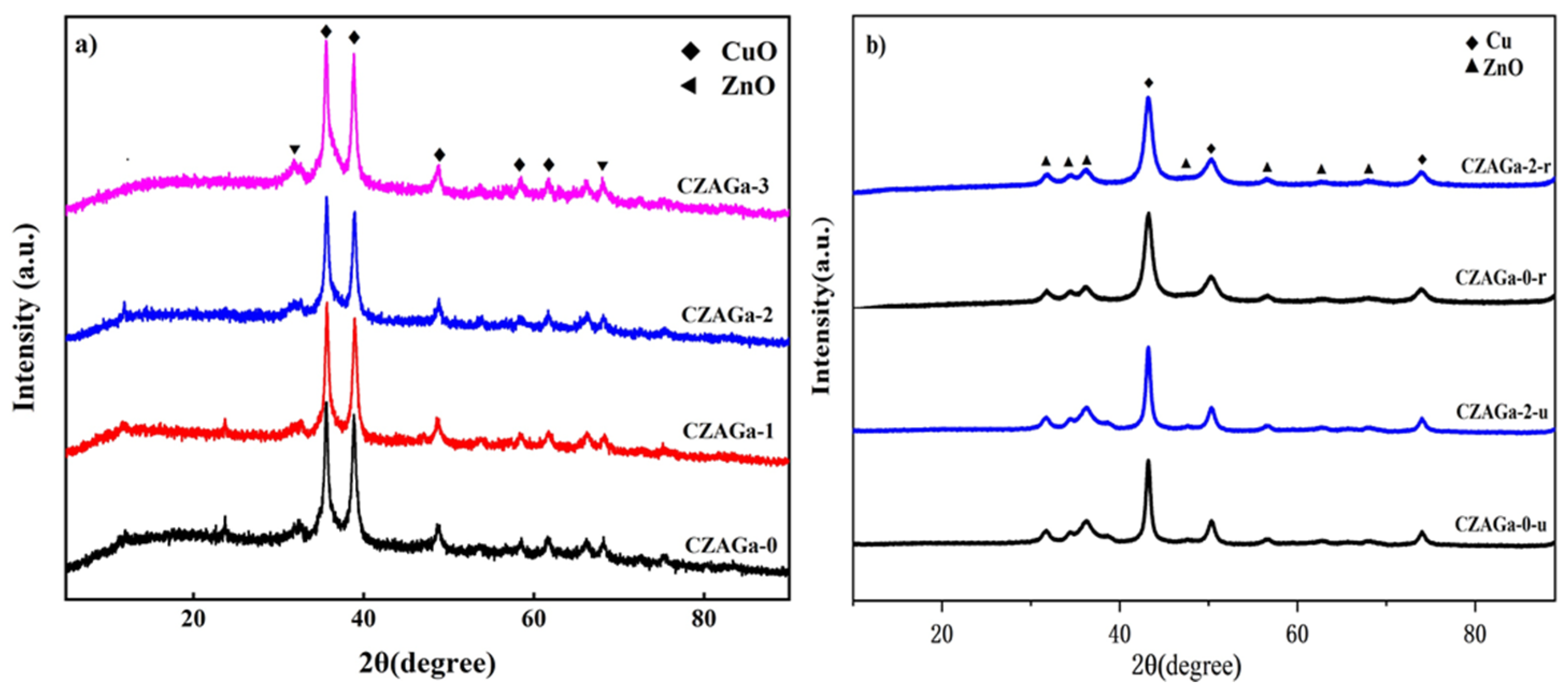
The XRD patterns of the Ga-modified and unmodified catalysts are displayed in Figure 3a. For all samples, the characteristic peaks belong to CuO and ZnO can be observed, but there are no peaks corresponding to Al2O3 and Ga2O3, maybe due to their good dispersion or the amorphous state. Scherrer’s equation can be used to calculate the CuO particle size of each catalyst, and the results are shown in Table 1. As shown in Table 1, with the increase of Ga additives, the crystallite sizes of catalysts decreased at first and then increased. The particle size of gallium-modified samples increased with increasing gallium content, and the CuO particle size of in CZAGa-3 with 3 wt% gallium addition is the largest. It is reported that the catalyst particle size may have some relationship with the interaction between the metal components [18,19]. So, we infer that this phenomenon may be due to the Ga doping effects has adjusted the interaction between copper oxide and other components in the catalyst. As the activity data in Table 1 show, the catalyst with the best catalytic performance has neither the largest nor the smallest copper oxide crystalline size. The CuO crystalline size will affect the position and coordination of surface-active atoms, which in turn affects the catalytic activity. This implies that a suitable catalyst particle size is needed for a highly active NPG synthesis catalyst.
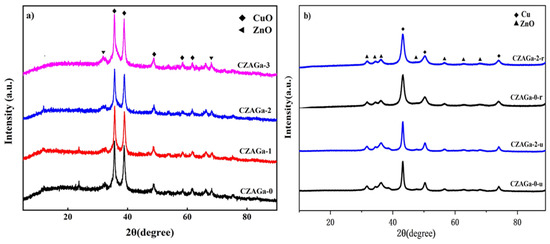
Figure 3.
XRD patterns of the samples: (a) calcined catalysts, (b) reduced (r) and used (u) catalysts.
The XRD patterns of the functioning catalysts CZAGa-0 and CZAGa-2 after reduced and used are shown in Figure 3b. For the two samples, a diffraction peak at 43.2°, which is characteristic of Cu (1 1 1), became observable due to the Cu2+ species were reduced to Cu0 after reduction. Cu+ is not observed because of overlap or high dispersion. After thermal treatment and reaction, the characteristic peaks of both samples become narrow, but the catalyst CZAGa-0 is more obvious. According to the XRD results, we can deduce that the Ga addition could suppress the aggregation of metal oxides and thus lead to the improvement of the stability of the catalyst.
3.3. H2-TPR Analysis
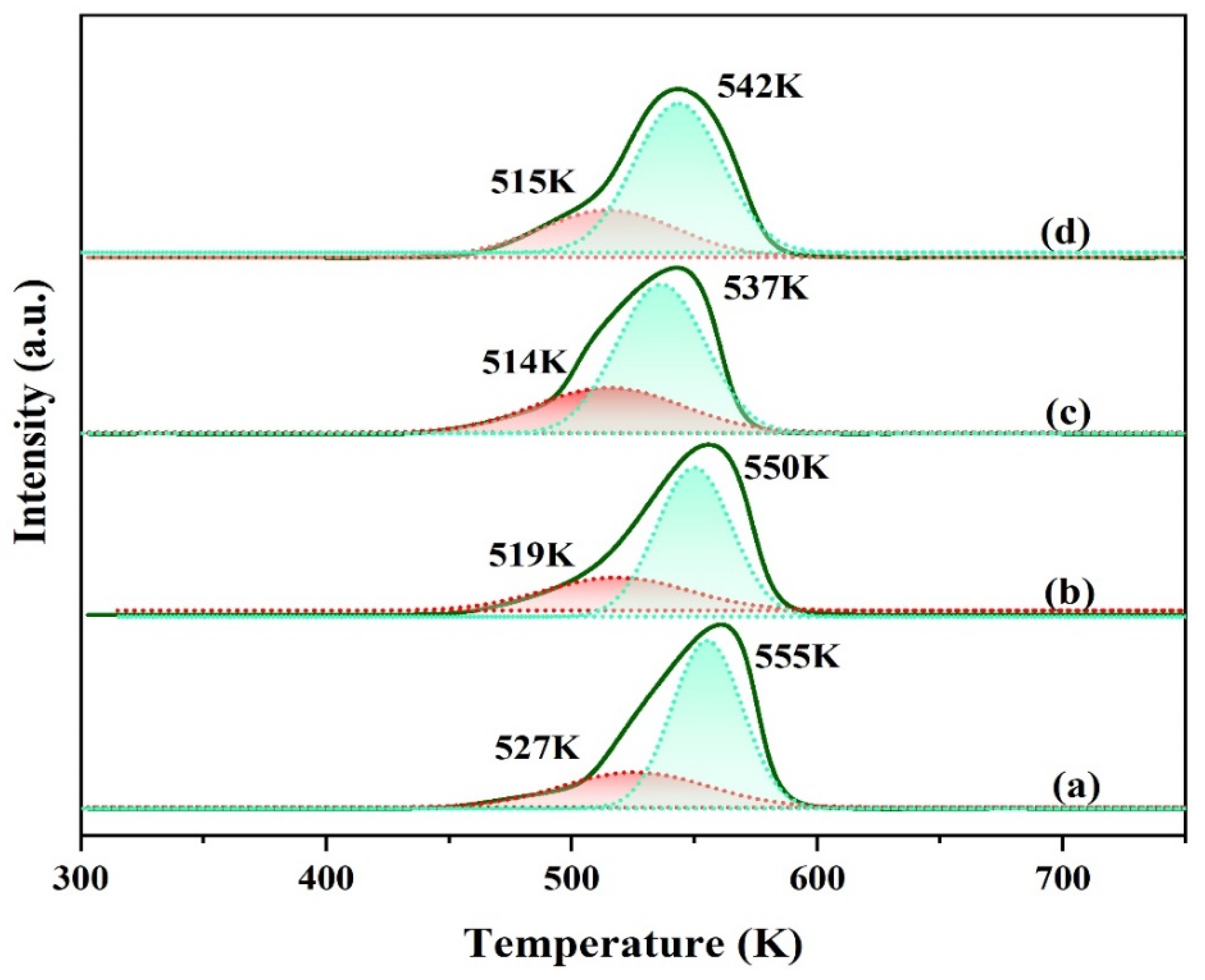
The reduction patterns of copper species for various Cu-based catalysts were measured by H2-TPR technique. The reduction profiles with different amounts of Ga doping are shown in Figure 4. It can be seen that all the catalysts show a broad reduction peak with shoulders peaked at 450–610 K. The reduction temperature of the Ga-modified catalyst is about 5–13 K lower than that of the unmodified one. To further dissect the TPR results, the wide H2 consumption peak can be deconvoluted into two reduction peaks with different temperatures. Generally, the low-temperature reduction peak is ascribed to the reduction of surface highly dispersed copper oxide, while the high-temperature reduction peak is ascribed to the reduction of bulk-like CuO phases [20]. The proportion of low temperature peak area of each catalyst was 25.1%, 28.9%, 33.3%, and 30.1%, respectively. It can be seen that the introduction of promoter gallium increases the proportion of low-temperature reduction peaks in the catalyst. This implies that the content of highly dispersed copper on the modified catalyst surface has increased. Improved Cu dispersion possessed by Ga modified catalysts, as depicted in Table 1, should also be accounted for such findings. The catalyst CZAGa-2 with the highest low temperature peak area ratio shows the optimal catalytic performance.
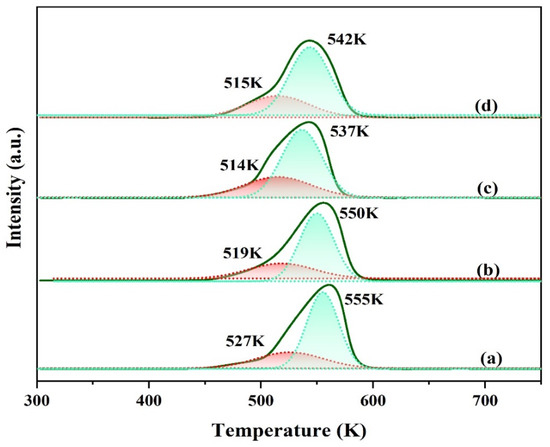
Figure 4.
H2-TPR profiles of the calcined catalysts. (a) CZA, (b) CZAGa-1, (c) CZAGa-2, (d) CZAGa-3.
The order of the highest reduction peak temperature of each catalyst is as follows: TCZA > TCZAGa-1 > T CZAGa-3 > TCZAGa-2. Compared with the CZA catalyst, the modified samples showed relatively lower reduction temperature which gradually decreased at first and then increased with increasing the amount of Ga. The result indicates that the reducibility of active components showed an increasing trend from CZA to CZAGa-2. As is known all, the reducibility of CuO is related to the interaction between the components. The tendency of Ga introduction to decrease the reduction temperature was probably owing to the change of the interaction of multi metallic species. This implies that Ga modified Cu-based catalyst has a weaker interaction between CuO and other components. Studies have shown that the reduction temperature of the catalyst has a certain relationship with the particle size. Generally, the smaller the CuO particles are, the lower the reduction temperature should be [21,22]. The combined effect of the particle size and the interaction between the components causes the CZAGa-3 reduction temperature to be slightly higher than CZAGa-2. Combining the activity and copper dispersion data in Table 1, we infer that the well-adjusted balance between reducibility and dispersion is conducive to the improvement of catalytic performance.
3.4. XPS Test Analysis
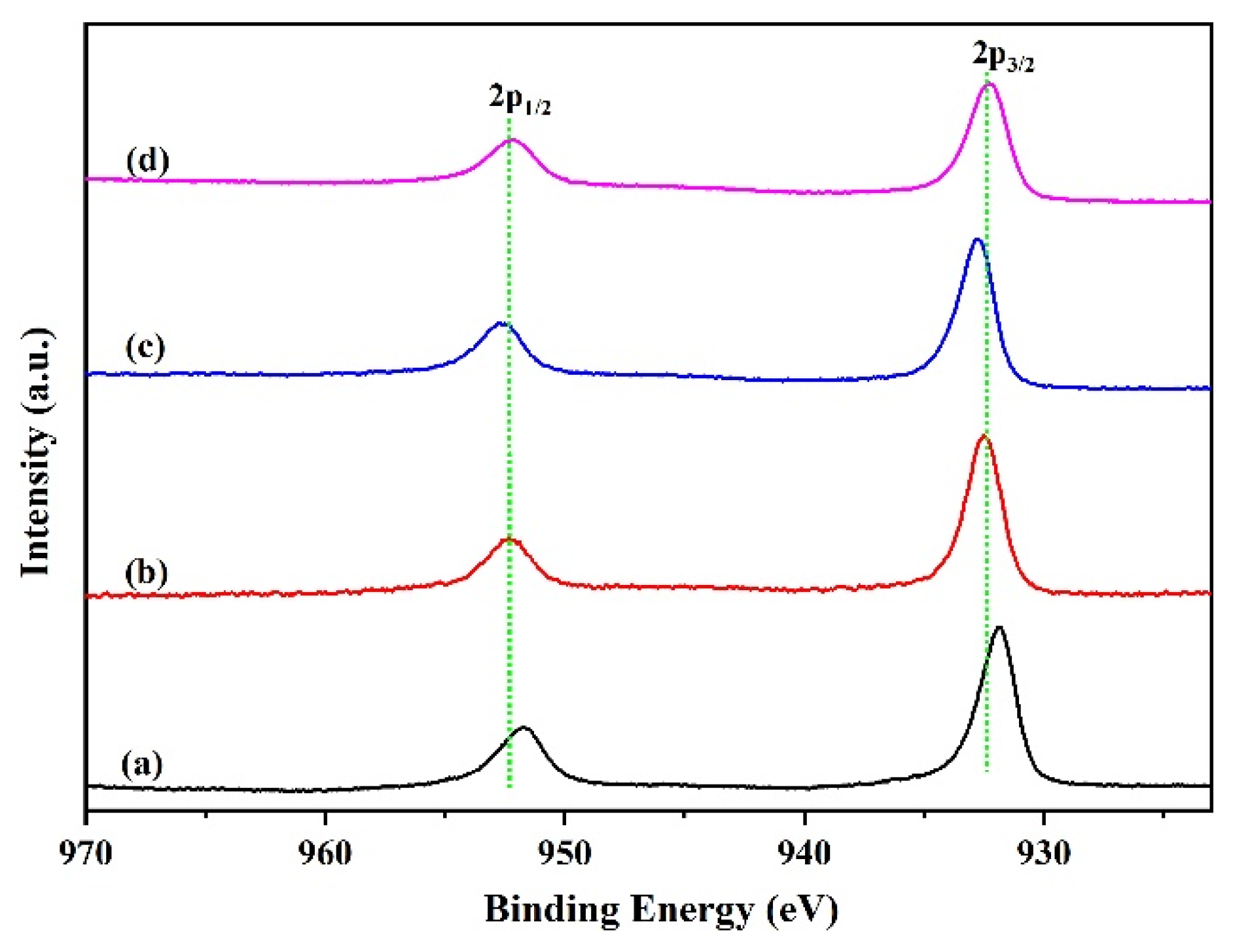
As mentioned above, the increased activity of Ga modified catalyst can be attributed to its superior dispersion and reducibility, which is indicated by the XRD and TPR data. Lots of research indicates that the distribution of copper species on the catalyst surface also have a significant impact on the catalyst activity. Regardless of numerous reports on Cu-based catalysts, exploring the factual role of the promoters in this catalyst and the actual chemical state of the catalytically active species during hydrogenation is still a challenging job [23,24,25,26,27]. So, the in situ XPS analysis (Figure 5) of the reduced samples were undertaken to ascertain the chemical states of active copper species. As is shown in Figure 5, the spectral features of the catalysts are very similar. Only two principal peaks of Cu2p3/2 and Cu2p1/2 centered at ~932 eV and ~952 eV were observed over the reduced samples, which was the characteristic peak of reduced Cu+ and/or metallic Cu. Generally, the binding energy of Cu2p3/2 at 934 eV and 940–950 eV is attributed to characteristic peak of Cu2+ species. The absence of Cu2+ characteristic peak indicates that the Cu species on the reduced catalyst surface mainly exist in the form of a low valence state. For Ga-modified catalysts, the binding energies migrated slightly to high energy, suggesting a certain electronic effect that occurred between Cu and Ga species.
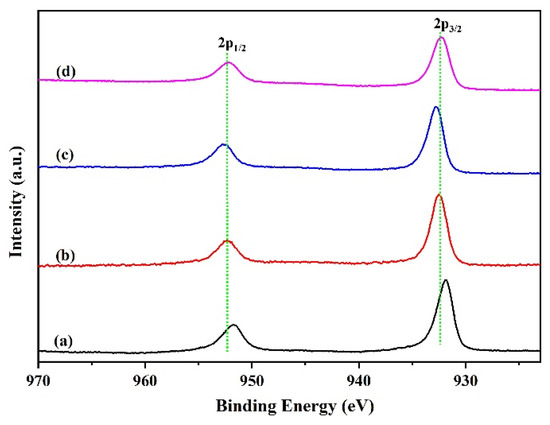
Figure 5.
Cu2p XPS spectra of the reduced catalysts. (a) CZA, (b) CZAGa-1, (c) CZAGa-2, (d) CZAGa-3.
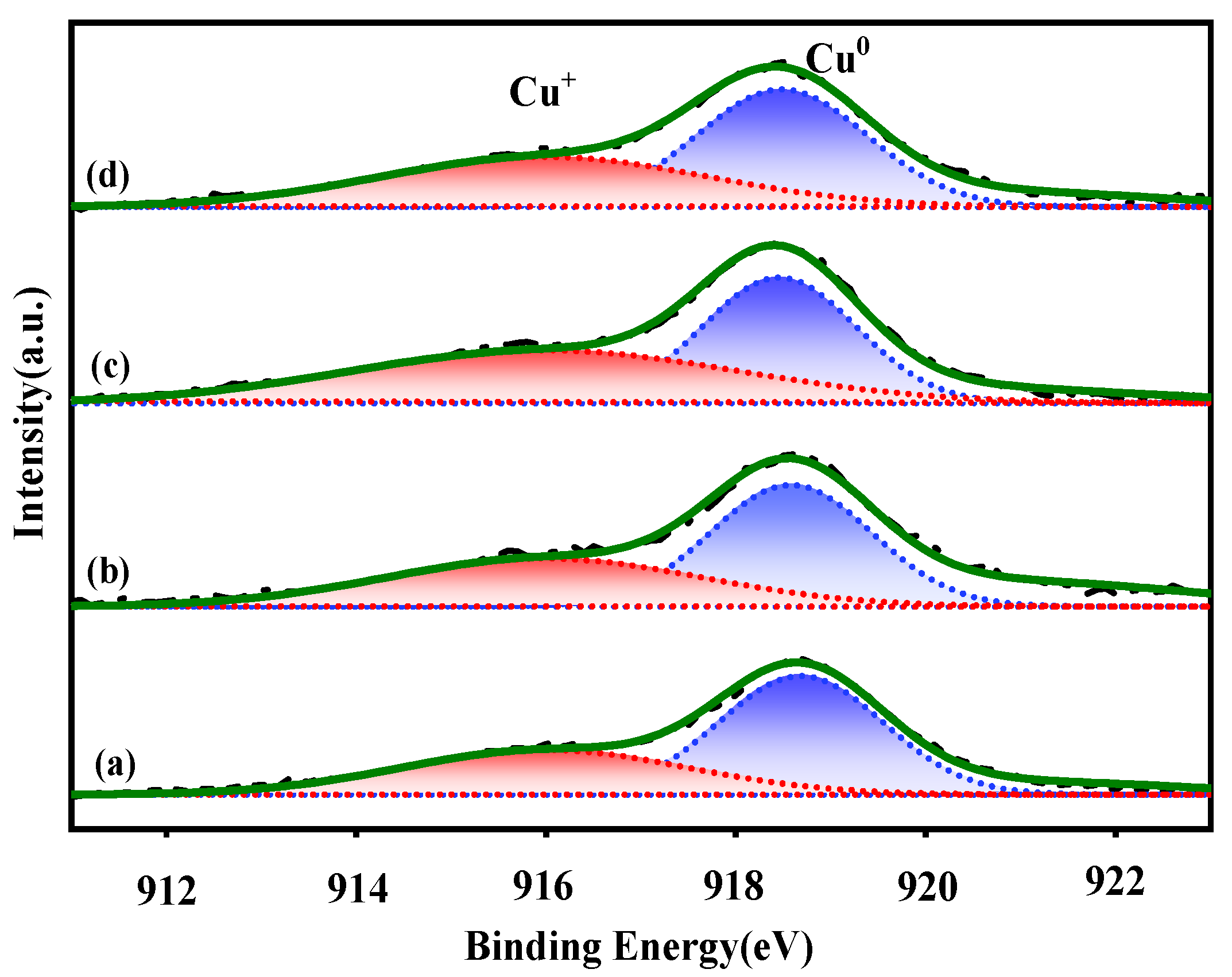
Because of the minor binding energy values of Cu0 and Cu+ species, it is difficult to identify them based on the Cu2p level. The auger electron spectra of CuLMM were used to further distinguish the surface Cu0 and Cu+. As presented in Figure 6, all the catalysts exhibited broad and asymmetric auger electron spectra, demonstrating the presence of both Cu0 and Cu+ species on the catalyst surface. By deconvolving these wide spectra, the relative surface content of copper species with different valence can be obtained, with peaks at ~916.0 and ~918.6 eV corresponding to Cu+ and Cu0, respectively [27,28].
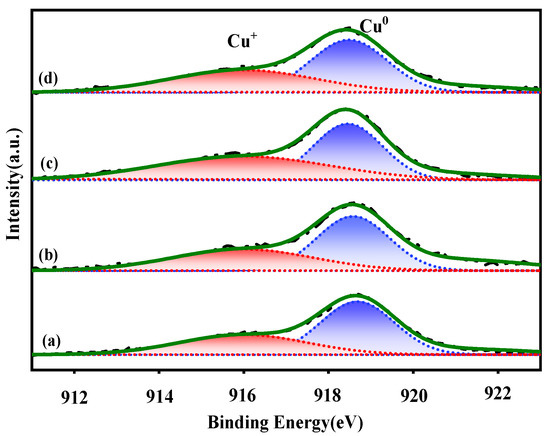
Figure 6.
Cu LMM XAES spectra of the reduced catalysts. (a) CZA, (b) CZAGa-1, (c) CZAGa-2, (d) CZAGa-3.
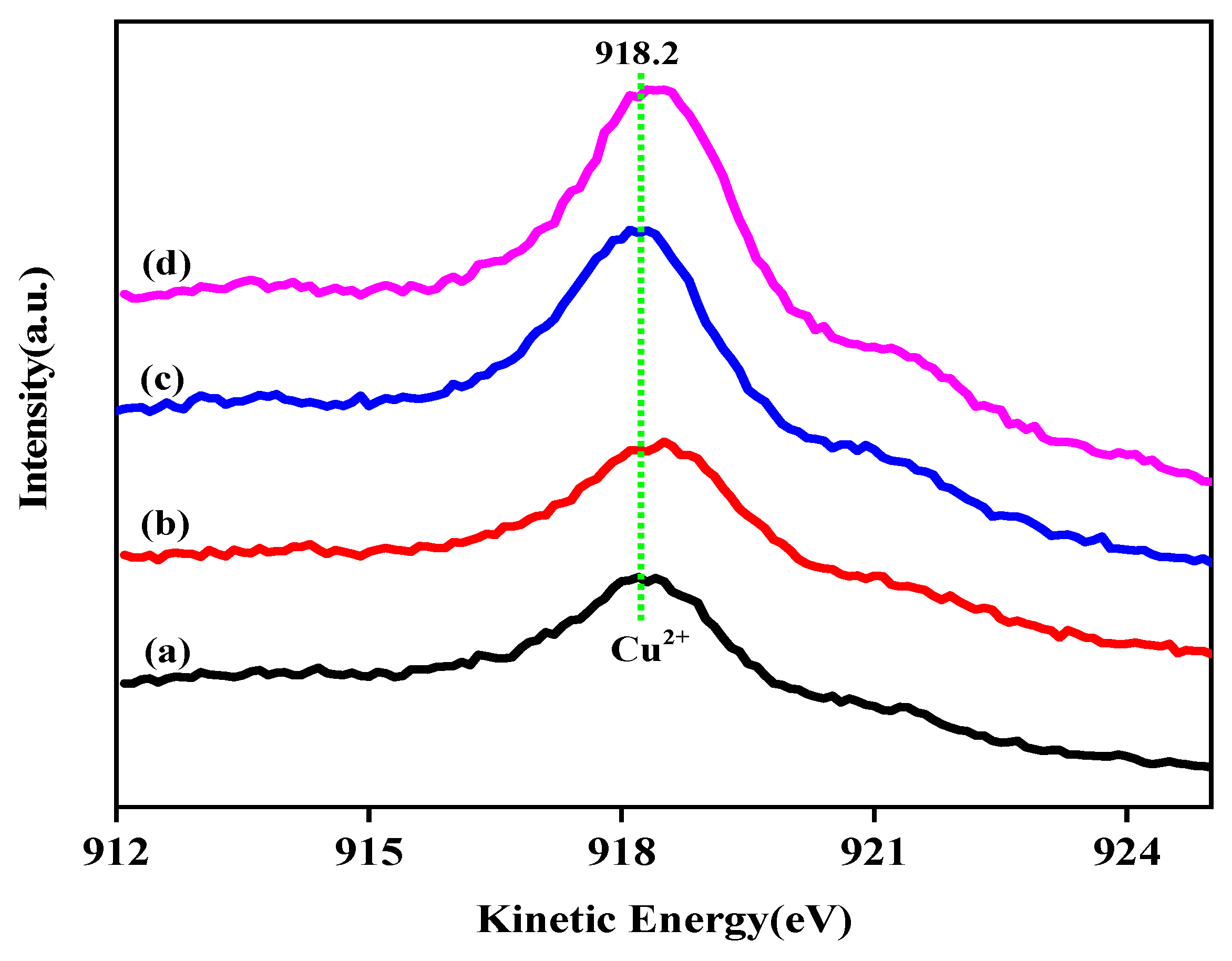
For comparison, the auger electron spectra of the calcined samples are displayed in Figure 7. Only an Auger kinetic energy peak at 918.2 eV attributed to the Cu2+ species can be observed. It is known that valence distribution balance of Cu species has a great impact on the original activity of Cu-based catalyst during the reaction. Thus, the ratio of surface Cu+ and Cu0, calculated from their corresponding auger peak areas, is shown in Table 2. As can be seen from Table 2, Ga loading has a certain impact on the surface distribution of mono-/zero-valent copper. With the increase of gallium addition, the monovalent Cu content on the catalyst surface increased at first and then decreased. The surface Cu+ content reaches the highest value when the addition of gallium is 2 wt%. It can be seen that the Cu+ content of the sample CZAGa-2 is 9% higher than that of CZAGa-0. This indicates more monovalent Cu appears on the surface of CZAGa-2 catalyst. Studies have shown that Cu species can act as Lewis acidic or electrophilic sites to polarize the C=O bond by bonding with the lone electron pair of oxygen in the aldehyde group, thereby enhancing the catalytic performance [29]. As mentioned above, suitable heteroatoms doping can regulate the surface Cu+ content. Studies have shown that the introduction of a small amount of high-valent (tri- or tetre-) metal ions can promote the formation of cationic defects in the Cu-ZnO catalyst, thereby enriching and stabilizing the univalent copper on the catalyst surface [30]. So, we inferred that gallium introduction was beneficial to the formation of monovalent cationic defects on ZnO surface, which might enhance and stabilize Cu+ species during reduction and reaction process. In addition, gallium addition has some effect on the Cu2p binding energy and the reduction temperature of calcined catalyst, which is shown in XPS (Figure 5) and H2-TPR (Figure 4). These results indicated the introduction of gallium might alter the electron interactions among the catalyst components, and thus influence the electron density of copper. Ai’s research about the promotional roles of cerium on Cu/SiO2 catalysts found that the surface Cu+ content was related to the amount of cerium doping, which implied the existence of electron interaction between Cu species and Ce promoter [31]. Therefore, the higher proportion of Cu+/Cu0 ratio on the gallium modified catalyst surface may be caused by the combined effect of monovalent cationic defects and electronic effects.
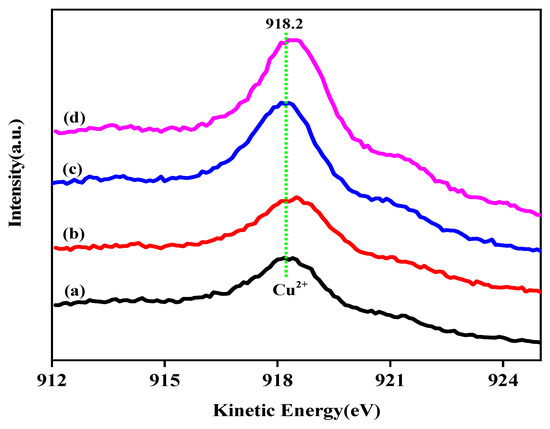
Figure 7.
Cu LMM XAES spectra of the calcined catalysts. (a) CZA, (b) CZAGa-1, (c) CZAGa-2, (d) CZAGa-3.

Table 2.
Deconvolution of Cu LMM XAES of the reduced catalysts.
4. Discussion
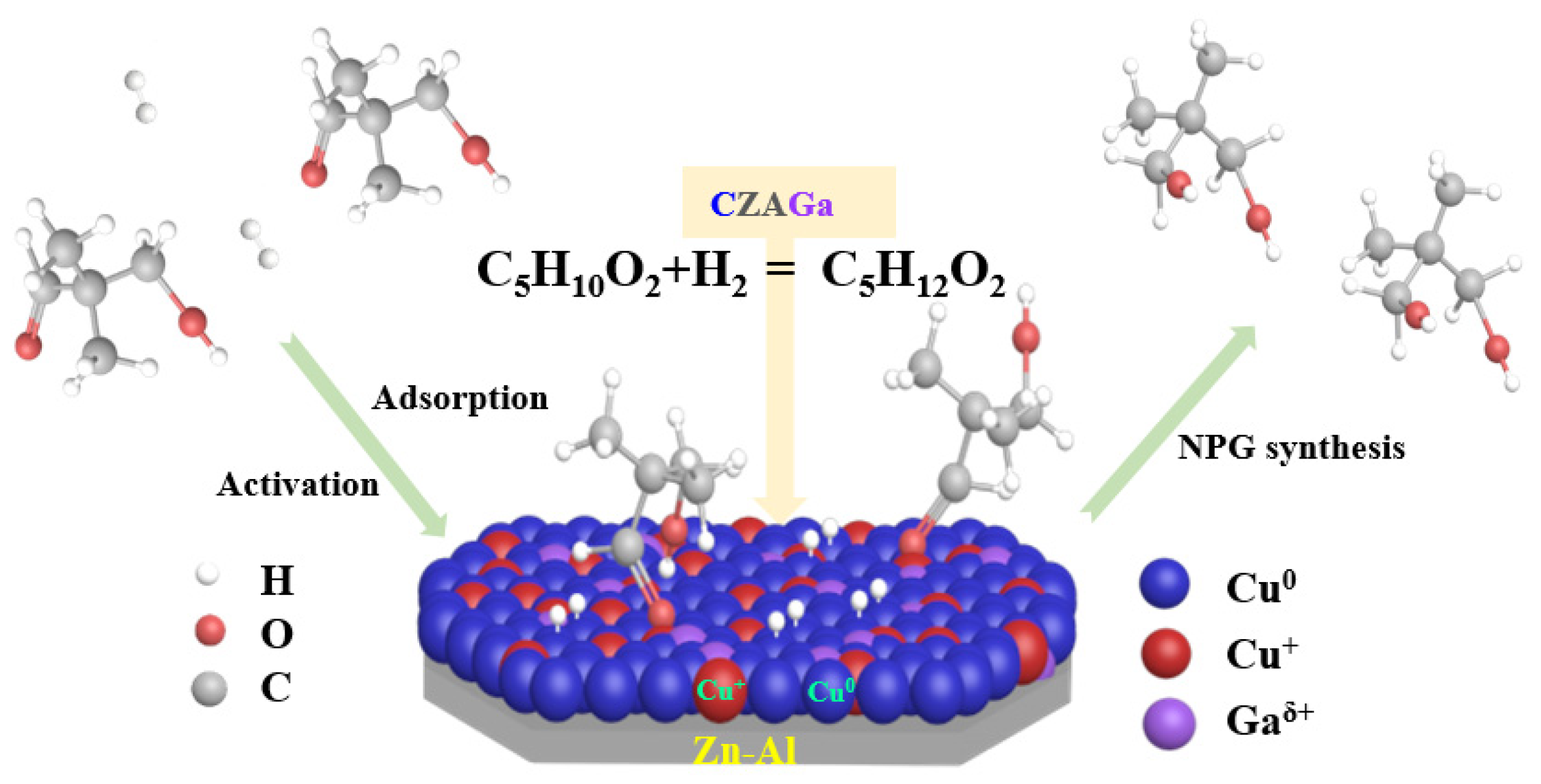
Although Cu-based catalysts for hydrogenation were extensively studied, reports related to the impact of gallium introduction on Cu-based catalyst for hydrogenation of HPA to NPG remain limited. The research on the catalytic hydrogenation of benzaldehyde shows that the hydrogenation reaction follows two reaction paths according to the different reaction atmosphere; one is the Cannizzaro mechanism under an inert atmosphere and the other is 1,2 nucleophilic addition/direct hydrogenation under a hydrogen environment [32]. A large number of studies on copper-based catalysts have opined that the active site of the hydrogenation reaction is the reduced copper [29,30,31,32]. Moreover, the catalytic activity is closely associated to the physical and chemical structure of the catalyst, the reducibility, the dispersion, and distribution of copper species, etc. However, with regard to the properties of active species, there is no consensus on the exact role of Cu0 and Cu+ species in hydrogenation reactions. Based on the catalyst characterization results, we propose that both reduced Cu+/Cu0 species are indispensable for an active catalyst and their relative content is pivotal for the catalytic performance of NPG synthesis. Considering literature [33] and our ongoing experiment, a possible mechanism for hydrogenation of HPA to NPG is proposed (Scheme 1). As illustrated in Scheme 1, H2 is adsorbed and dissociated on the surface of reduced Cu catalyst, then followed by reacting with the activated reactant molecule HPA. During the hydrogenation process, HPA underwent the breakage of C=O bond and the addition of two activated hydrogen atoms. The reaction pathway mainly includes the following two routes. Firstly, the hydrogen atom attacks the oxygen atom in C=O group to form a hydroxyalkyl intermediate, and then the carbon atom is hydrogenated to form NPG.
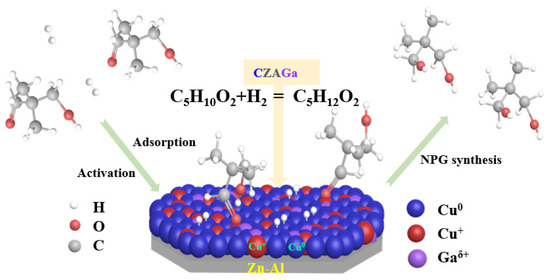
Scheme 1.
Mechanism of the catalytic hydrogenation of HPA to NPG on Cu-based catalysts.
5. Conclusions
In summary, NPG manufactured from HPA hydrogenation over gallium modified Cu-based catalysts synthesized by the stepwise co-precipitation method was investigated. It was found that the proper Ga promoted catalyst exhibited good active site dispersion, and higher reactant activation capacity. The introduction of Ga promoters can regulate the interaction of the catalyst components, which is conducive to improving the dispersion and concentration of active Cu0 and Cu+ sites on the catalyst surface. Both Cu+ and Cu0 sites are demanded for an optimal Cu-based catalyst, and a suitable specific Cu+/Cu0 ratio is integral for excellent catalytic performance in the hydrogenation of HPA to NPG.
Author Contributions
Methodology, J.Z.; conceptualization, J.Z.; investigation, J.Z., Z.W. and Z.W.; Writing—Original draft preparation, J.Z. and H.L.; data curation, H.L. and X.Z.; formal analysis, X.Z. and Z.W.; visualization, D.H.; funding acquisition, L.B. and F.W.; resources, L.B. and F.W.; supervision, G.W.; writing-reviewing, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2021MB134, ZR2022MB019) and National Natural Science Foundation of China (22008131).
Data Availability Statement
All relevant data are within the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aguieiras, E.C.G.; Cavalcanti, E.D.C.; Silva, d.P.R.; Soares, V.F.; Fernandez-Lafuente, R.; Assunçao, C.L.B.; Silva, d.J.A.C.; Freire, D.M.G. Enzymatic synthesis of neopentyl glycol-bases biolubricants using biodiesel from soybean and castor bean as raw materials. Renew. Energ. 2020, 148, 689–696. [Google Scholar] [CrossRef]
- Nousiainen, H. US06545189B1; Process for the preparation of neopentyl glycol; Neste Chemicals Oy: Espoo, Finland, 2003. [Google Scholar]
- Monasterska, E.; Chrobok, A.; Pankalla, E.; Siewniak, A. Development of Methods for the Synthesis of Neopentyl Glycol by Hydrogenation of Hydroxypivaldehyde. Molecules 2021, 26, 5822. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Insyani, R.; Cahyadi, H.S.; Park, J.Y.; Kim, S.M.; Chao, S.M.; Bae, J.W.; Kim, J. Ga-doped Cu/H-nanozeolite-Y catalyst for selective hydrogenation and hydrodeoxygenation of lignin-derived chemicals. Green Chem. 2018, 20, 3253–3270. [Google Scholar] [CrossRef]
- Kalong, M.; Srifa, A.; Hongmanorom, P.; Cholsuk, C.; Klysubun, W.; Ratchahat, S.; Koo-Amornpattana, W.; Khemthong, P.; Assabumrungrat, S.; Kawi, S. Catalytic transfer hydrogenation of furfural to furfuryl alcohol and 2-methylfuran over CuFe catalysts: Ex situ observation of simultaneous structural phase transformation. Fuel Process. Technol. 2022, 231, 107256. [Google Scholar] [CrossRef]
- Lan, X.C.; Xue, K.C.; Wang, T.F. Combined synergetic and steric effects for highly selective hydrogenation of unsaturated aldehyde. J. Catal. 2019, 372, 49–60. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced activity, selectivity and stability of a CuO-ZnO-ZrO2 catalyst by adding graphene oxide for CO2 hydrogenation to methanol. Chem. Eng. J. 2018, 334, 1781–1791. [Google Scholar] [CrossRef]
- Yang, W.T.; Li, A.T.; Yang, Y.W.; Hai, Y.H.; Zhen, Z.H.; Li, Z.S.; Lv, J.; Wang, Y.; Ma, X.B. Low-Temperature Hydrogenation of Methyl Acetate to Ethanol over a Manganese-Modified Cu/SiO2 Catalyst. Ind. Eng. Chem. Res. 2022, 61, 11718–11726. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.Q.; He, P.; Yuan, Y.Z. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Chen, H.B.; Liao, D.W.; Yu, L.J.; Lin, Y.J.; Yi, J.; Zhang, H.B.; Tsai, T.R. Influence of trivalent metal ions on the surface structure of a copper-based catalyst for methanol synthesis. Appl. Surf. Sci. 1999, 147, 85–93. [Google Scholar] [CrossRef]
- Song, H.T.; Fazelia, A.; Kim, H.D.; Eslami, A.A.; Noh, Y.S.; Saeidabad, N.G.; Moon, D.J. Effect of lanthanum group promoters on Cu/(mixture of ZnO and Zn-Al-spinnel-oxides) catalyst for methanol synthesis by hydrogenation of CO and CO2 mixtures. Fuel 2021, 283, 118987. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Zhao, Y.; Lv, J.; Wang, S.; Ma, X. Insight into the Balancing Effect of Active Cu Species for Hydrogenation of Carbon—Oxygen Bonds. ACS Catal. 2015, 5, 6200–6208. [Google Scholar] [CrossRef]
- Sun, K.; Bian, Z.K.; Cheng, S.Y.; Wang, G.R.; Zhang, L.; Huang, W. Effect of Si content on the performance of direct synthesis of dimethyl ether over slurry CuZnAl catalyst prepared by complete liquid phase technology. J. Fuel Chem. Technol. 2021, 49, 791–798. [Google Scholar] [CrossRef]
- Fujitani, T.; Saito, M.; Kanai, Y.; Kakumoto, T.; Watanabe, T.; Nakamura, J.; Uchijima, T. The role of metal oxides in promoting a copper catalyst for methanol synthesis. Catal. Lett. 1994, 25, 271–276. [Google Scholar] [CrossRef]
- Si, C.C.; Ban, H.Y.; Chen, K.; Wang, X.; Cao, R.; Yi, Q.; Li, C. Insight into the positive effect of Cu0/Cu+ ratio on the stability of Cu-ZnO-CeO2 catalyst for syngas hydrogenation. Appl. Catal. A Gen. 2020, 594, 117466. [Google Scholar] [CrossRef]
- Kang, S.H.; Bae, J.W.; Prasad, P.S.S.; Oh, J.H.; Jun, K.W.; Song, S.L.; Min, K.S. Influence of Ga addition on the methanol synthesis activity of Cu/ZnO catalyst in the presence and absence of alumina. J. Ind. Eng. Chem. 2009, 15, 665–669. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; Molenbroek, A.M.; Topsoe, A.-Y.; Topsoe, H.; Clausen, B.C. In Situ Investigations of Structural Changes in Cu/ZnO Catalysts. J. Catal. 2000, 194, 452–460. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.W.; Gan, Y.H.; Ding, W.; Fang, W.P.; Yang, Y.Q. Study on the modification of Cu-based catalysts with cupric silicate for methanol synthesis from synthesis gas. Fuel Process. Technol. 2013, 110, 190–196. [Google Scholar] [CrossRef]
- Toyir, J.; De La Piscina, P.R.; Fierro, J.L.G.; Homs, N. Catalytic performance for CO2 conversion to methanol of gallium-promoted copper-based catalysts: Influence of metallic precursors. Appl. Catal. B Environ. 2001, 34, 255–266. [Google Scholar] [CrossRef]
- Guo, X.M.; Mao, D.S.; Lu, G.Z.; Wang, S.; Wu, G.S. Glycine-nitrate combustion synthesis of CuO-ZnO-ZrO2 catalysts for methanol synthesis from CO2 hydrogenation. J. Cataly. 2010, 271, 178–185. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Seela, K.K.; Sagar, G.V.; Sreedhar, B. Characterization and Reactivity of Niobia Supported Copper Oxide Catalysts. J. Phys. Chem. B 2004, 108, 658–663. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Matralis, H. Influence of the preparation method on the performance of CuO-CeO2 catalysts for the selective oxidation of CO. Appl. Catal. B Environ. 2005, 56, 87–93. [Google Scholar] [CrossRef]
- Lunkenbein, T.; Schumann, J.; Behrens, M.; Schlögl, R.; Willinger, M.G. Formation of a ZnO Overlayer in Industrial Cu/ZnO/Al2O3 Catalysts Induced by Strong Metal-Support Interactions Angew. Chem. Int. Ed. 2015, 54, 4544–4548. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.W.; Huang, L.L.; Cui, C.H.; Chen, Z.C.; Liu, X.F.; Duan, X.; Zheng, L.S. Ambient-pressure synthesis of ethylene glycol catalyzed by C60-buffered Cu/SiO2. Science 2022, 376, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Narkhede, N.; Zheng, H.Y.; Zhang, H.C.L.; Zhang, G.Q.; Li, Z. Group 13 metal doped Cu/ZnO catalysts from phase pure precursors via an isomorphous substitution route: Mechanistic insights into promotional effects for syngas hydrogenation to methanol. Catal. Sci. Technol. 2020, 10, 7386. [Google Scholar] [CrossRef]
- Liu, Z.; An, X.Q.; Song, M.; Wang, Z.J.; Wei, Y.F.; Mintova, S.; Giordano, G.; Yan, Z.F. Dry gel assisting crystallization of bifunctional CuO-ZnO-Al2O3/SiO2-Al2O3 catalysts for CO2 hydrogenation. Biomass. Bioenerg. 2022, 163, 106525. [Google Scholar] [CrossRef]
- Gu, Y.T.; Han, C.; Huang, J.H.; Vinokurov, V.A.; Huang, W. CuZnAlOOH catalysts with Cu0/Cu+ constructed by two-step hydrolysis for ethanol production from syngas. Fuel 2022, 322, 124111. [Google Scholar] [CrossRef]
- Ye, R.; Lin, L.; Yang, J.; Sun, M.; Li, F.; Li, B.; Yao, Y. A new low-cost and effective method for enhancing the catalytic performance of Cu–SiO2 catalysts for the synthesis of ethylene glycol via the vapor-phase hydrogenation of dimethyl oxalate by coating the catalysts with dextrin. J. Catal. 2017, 350, 122–132. [Google Scholar] [CrossRef]
- Rao, R.S.; Baker, R.; Terry, K.; Vannice, M.A. Furfural hydrogenation over carbon-supported copper. Catal. Lett. 1999, 60, 51–57. [Google Scholar] [CrossRef]
- Liu, X.M.; Lu, G.Q.; Yan, Z.F.; Beltramini, J. Assessment of economic considerations for air pollution control technologies. Ind. Eng. Chem. Res. 2003, 42, 6518–6530. [Google Scholar] [CrossRef]
- Ai, P.P.; Tan, M.H.; Reubroycharoen, P.; Wang, Y.; Feng, X.B.; Liu, G.G.; Yang, G.H.; Tsubaki, N. Catalytic Hydrogenation of Benzaldehyde for Selective Synthesis of Benzyl Alcohol: A Review. Catal. Sci. Technol. 2018, 8, 6441–6451. [Google Scholar] [CrossRef]
- Bhanushali, J.T.; Kainthla, I.; Keri, R.S.; Nagaraja, B.M. Probing the promotional roles of cerium in the structure and performance of Cu/SiO2 catalysts for ethanol production. ChemistrySelect 2016, 1, 3839–3853. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wu, D.F. Aqueous phase catalytic hydrogenation of furfural to furfuryl alcohol over in-situ synthesized Cu-Zn/SiO2 catalysts, Mater. Chem. Phys. 2021, 260, 124152. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).