Simultaneous Photocatalytic Sugar Conversion and Hydrogen Production Using Pd Nanoparticles Decorated on Iron-Doped Hydroxyapatite
Abstract
1. Introduction
2. Results and Discussion
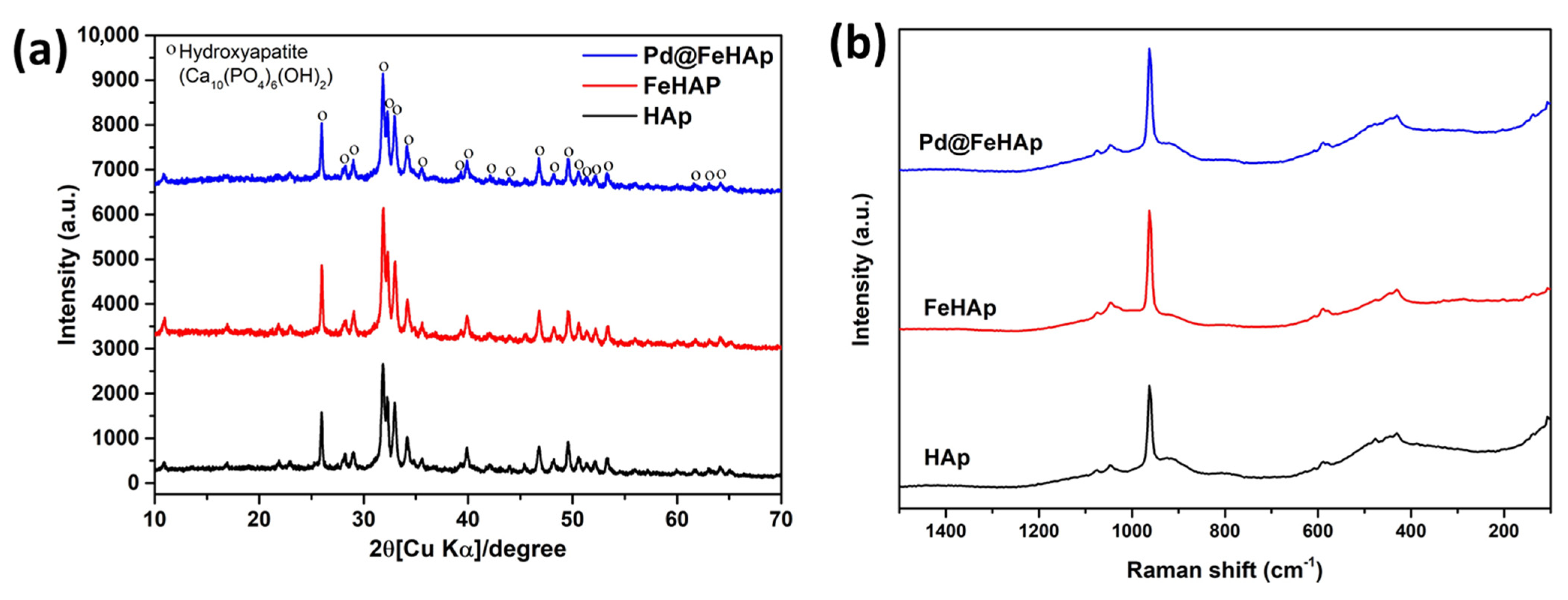
2.1. Phase Structure and Chemical Composition
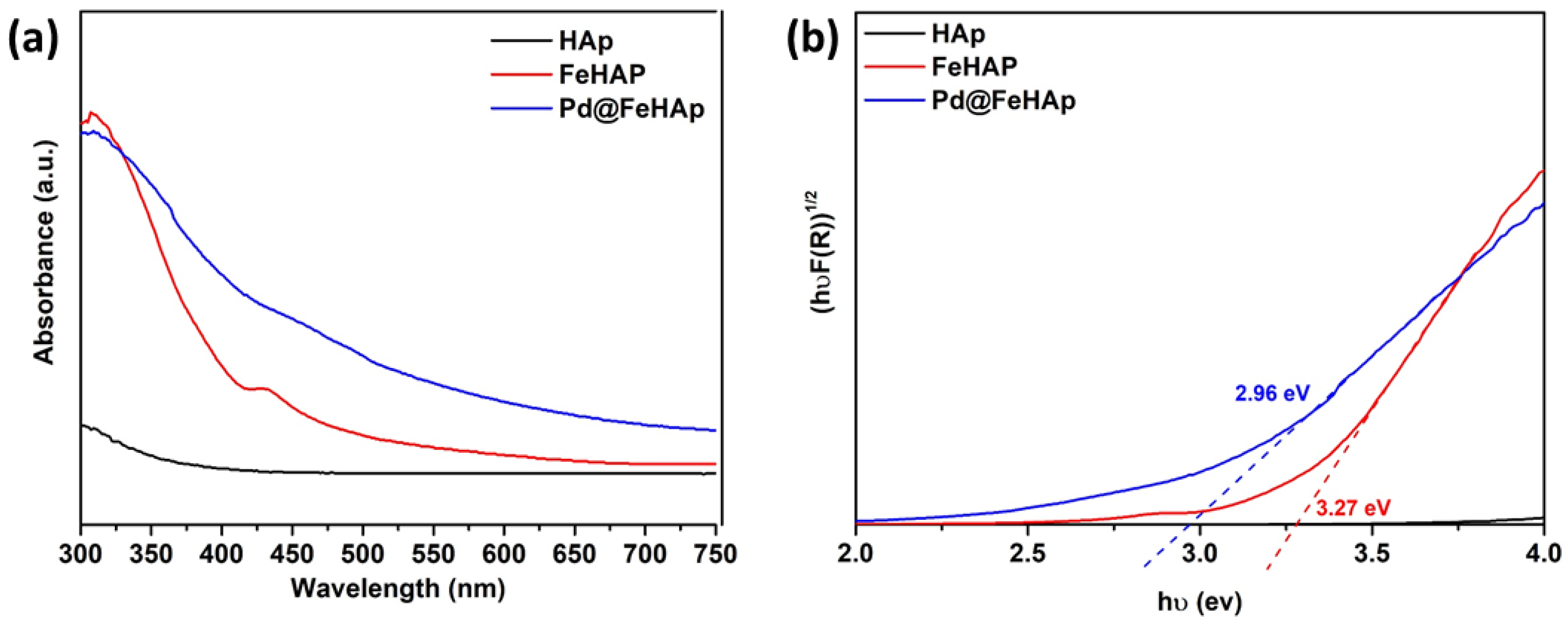
2.2. Optical and Surface Electronic Properties
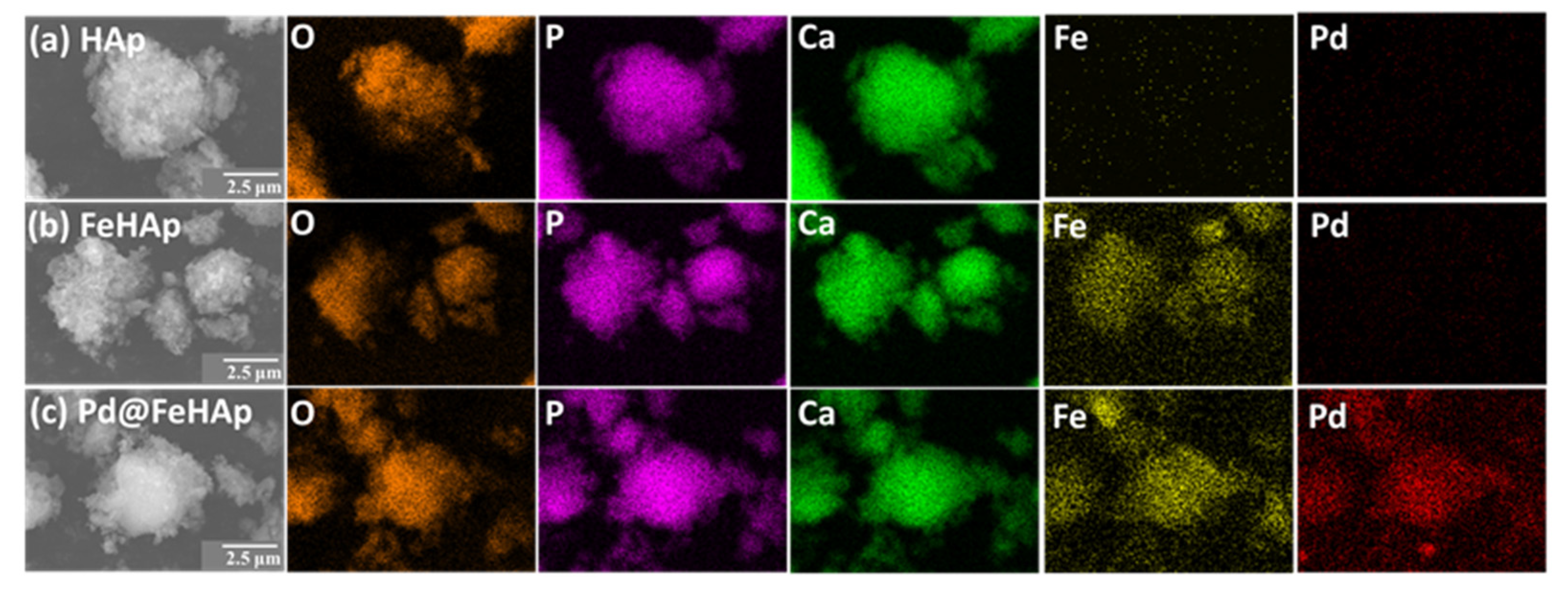
2.3. Morphology Results
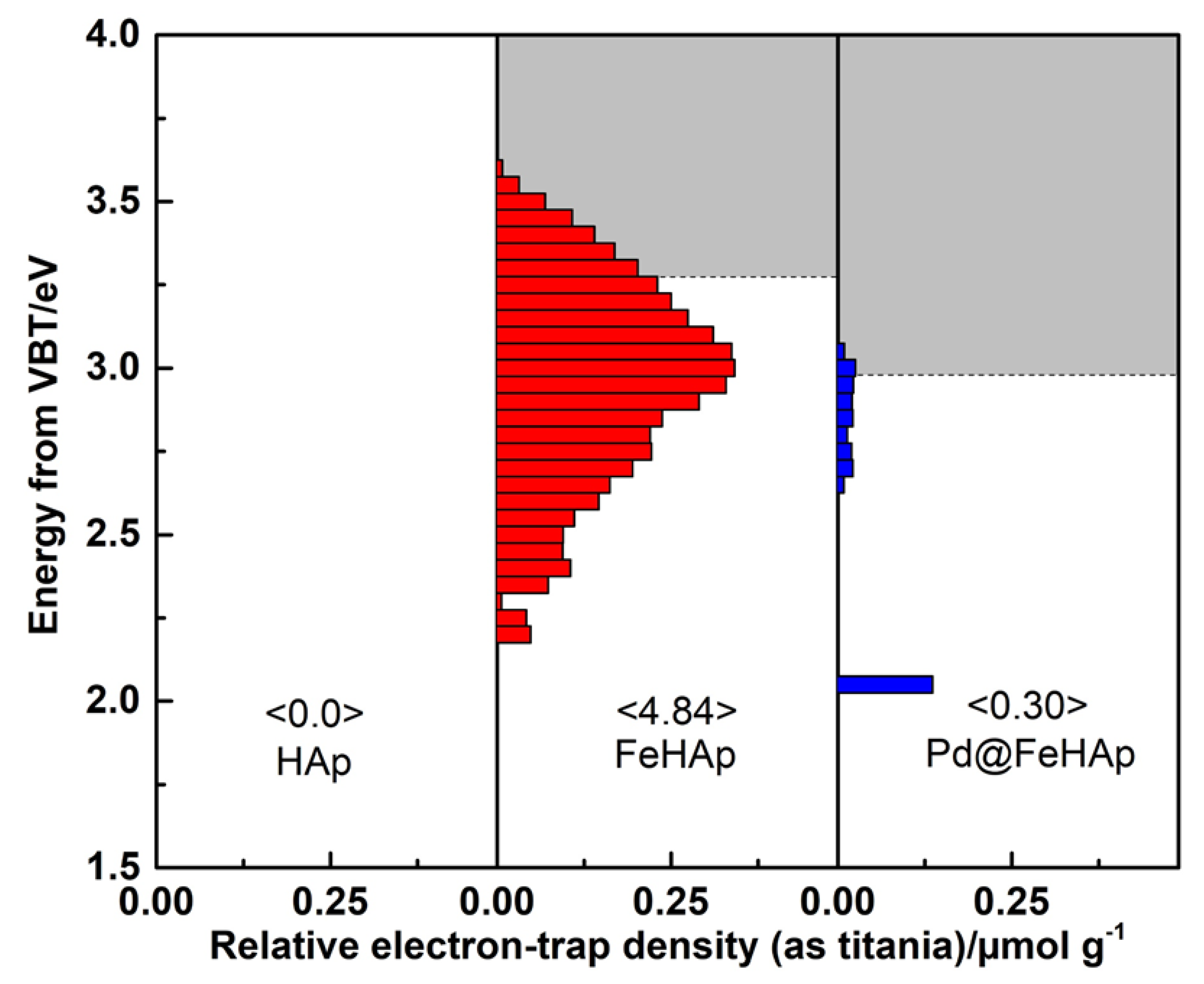
2.4. Chemical State and Band Positions
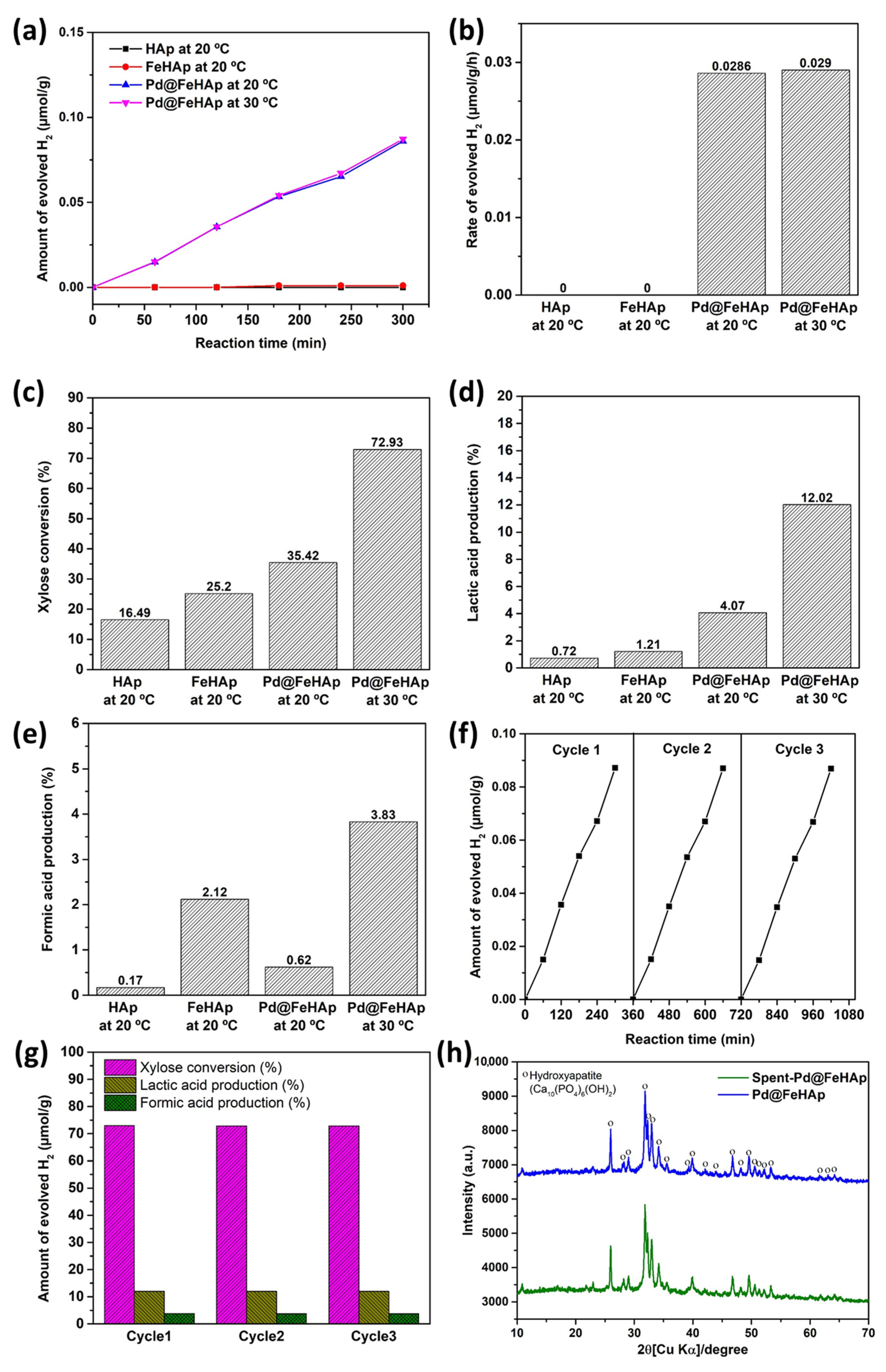
2.5. Simultaneous Photocatalytic Xylose Conversion and Hydrogen Evolution
2.6. Examination of the Transfer and Separation of Charges
2.7. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of HAp and FeHAp
3.3. Preparation of Pd@HAp and Pd@FeHAp
3.4. Characterization
3.5. Simultaneous Photocatalytic Sugar Conversion and Hydrogen Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Heo, Y.-J.; Lee, J.-W.; Lee, J.-H.; BajGai, J.; Lee, K.-J.; Park, S.-J. Photocatalytic Hydrogen Evolution via Water Splitting: A Short Review. Catalysts 2018, 8, 655. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Ramis, G.; Bahadori, E.; Rossetti, I. Design of efficient photocatalytic processes for the production of hydrogen from biomass derived substrates. Int. J. Hydrogen Energy 2021, 46, 12105–12116. [Google Scholar] [CrossRef]
- Cai, M.; Cao, S.; Zhuo, Z.; Wang, X.; Shi, K.; Cheng, Q.; Xue, Z.; Du, X.; Shen, C.; Liu, X.; et al. Fabrication of Ni2P Cocatalyzed CdS Nanorods with a Well-Defined Heterointerface for Enhanced Photocatalytic H2 Evolution. Catalysts 2022, 12, 417. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Jeon, T.H.; Koo, M.S.; Kim, H.; Choi, W. Dual-Functional Photocatalytic and Photoelectrocatalytic Systems for Energy- and Resource-Recovering Water Treatment. ACS Catal. 2018, 8, 11542–11563. [Google Scholar] [CrossRef]
- Granone, L.I.; Sieland, F.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Photocatalytic conversion of biomass into valuable products: A meaningful approach? Green Chem. 2018, 20, 1169–1192. [Google Scholar] [CrossRef]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta-Zamora, P. Use of scavenger agents in heterogeneous photocatalysis: Truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, Y.; Shi, B.; Ran, J.; Davey, K.; Qiao, S.Z. Photocatalysts for Hydrogen Evolution Coupled with Production of Value-Added Chemicals. Small Methods 2020, 4, 1499–1506. [Google Scholar] [CrossRef]
- Antonietti, M.; Savateev, A. Splitting Water by Electrochemistry and Artificial Photosynthesis: Excellent Science but a Nightmare of Translation? Chem. Rec. 2018, 18, 969–972. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.-F.; Yong, X.; Kumar, P.; Palma, B.; Hu, Z.-Y.; Van Tendeloo, G.; Siahrostami, S.; Larter, S.; Zheng, D.; et al. Coproduction of hydrogen and lactic acid from glucose photocatalysis on band-engineered Zn1-xCdxS homojunction. iScience 2021, 24, 102109. [Google Scholar] [CrossRef] [PubMed]
- Rohini, B.; Hebbar, H.U. Photocatalytic Conversion of Xylose to Xylitol over Copper Doped Zinc Oxide Catalyst. Catal. Lett. 2021, 151, 2583–2594. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Zou, R.; Ma, J.; Yang, Y.; Li, T.; Li, M.; Hao, Q.; Xie, H.; Peng, X. Regulating TiO2/MXenes catalysts to promote photocatalytic performance of highly selective oxidation of d-xylose. Green Chem. 2021, 23, 1382–1388. [Google Scholar] [CrossRef]
- Liu, K.; Ma, J.; Yang, X.; Jin, D.; Li, Y.; Jiao, G.; Yao, S.; Sun, S.; Sun, R. Boosting electron kinetics of anatase TiO2 with carbon nanosheet for efficient photo-reforming of xylose into biomass-derived organic acids. J. Alloys Compd. 2022, 906, 164276. [Google Scholar] [CrossRef]
- Sanwald, K.E.; Berto, T.F.; Eisenreich, W.; Jentys, A.; Gutiérrez, O.Y.; Lercher, J.A. Overcoming the Rate-Limiting Reaction during Photoreforming of Sugar Aldoses for H2-Generation. ACS Catal. 2017, 7, 3236–3244. [Google Scholar] [CrossRef]
- Imizcoz, M.; Puga, A.V. Assessment of Photocatalytic Hydrogen Production from Biomass or Wastewaters Depending on the Metal Co-Catalyst and Its Deposition Method on TiO2. Catalysts 2019, 9, 584. [Google Scholar] [CrossRef]
- Puga, A.V.; Barka, N.; Imizcoz, M. Simultaneous H2 Production and Bleaching via Solar Photoreforming of Model Dye-polluted Wastewaters on Metal/Titania. ChemCatChem 2020, 13, 1513–1529. [Google Scholar] [CrossRef]
- Uekert, T.; Bajada, M.A.; Schubert, T.; Pichler, C.M.; Reisner, E. Scalable Photocatalyst Panels for Photoreforming of Plastic, Biomass and Mixed Waste in Flow. ChemSusChem 2021, 14, 4190–4197. [Google Scholar] [CrossRef]
- Kasap, H.; Achilleos, D.S.; Huang, A.; Reisner, E. Photoreforming of Lignocellulose into H(2) Using Nanoengineered Carbon Nitride under Benign Conditions. J. Am. Chem. Soc. 2018, 140, 11604–11607. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Yao, S.; Guo, Y.; Sun, R. Photocatalytic Biorefinery to Lactic Acid: A Carbon Nitride Framework with O Atoms Replacing the Graphitic N Linkers Shows Fast Migration/Separation of Charge. ChemCatChem 2022, 14, e202200097. [Google Scholar] [CrossRef]
- Qin, H.-J.; Zhang, Y.-H.; Wang, Z.; Yang, G.-H. Photocatalytic Conversion of Fructose to Lactic Acid by BiOBr/Zn@SnO2 Material. Catalysts 2022, 12, 719. [Google Scholar] [CrossRef]
- Ma, J.; Jin, D.; Li, Y.; Xiao, D.; Jiao, G.; Liu, Q.; Guo, Y.; Xiao, L.; Chen, X.; Li, X.; et al. Photocatalytic conversion of biomass-based monosaccharides to lactic acid by ultrathin porous oxygen doped carbon nitride. Appl. Catal. B Environ. 2021, 283, 119520. [Google Scholar] [CrossRef]
- Suhag, M.H.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Khatun, A.; Kaneco, S. Photocatalytic Hydrogen Production from Formic Acid Solution with Titanium Dioxide with the Aid of Simultaneous Rh Deposition. ChemEngineering 2022, 6, 43. [Google Scholar] [CrossRef]
- Jin, B.; Yao, G.; Wang, X.; Ding, K.; Jin, F. Photocatalytic Oxidation of Glucose into Formate on Nano TiO2 Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 6377–6381. [Google Scholar] [CrossRef]
- Roongraung, K.; Chuangchote, S.; Laosiripojana, N. Enhancement of Photocatalytic Oxidation of Glucose to Value-Added Chemicals on TiO2 Photocatalysts by A Zeolite (Type Y) Support and Metal Loading. Catalysts 2020, 10, 423. [Google Scholar] [CrossRef]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic Acid as an Antimicrobial for Poultry Production: A Review. Front. Vet. Sci. 2020, 7, 563. [Google Scholar] [CrossRef]
- García, V.; Catalá-Gregori, P.; Hernández, F.; Megías, M.D.; Madrid, J. Effect of Formic Acid and Plant Extracts on Growth, Nutrient Digestibility, Intestine Mucosa Morphology, and Meat Yield of Broilers. J. Appl. Poult. Res. 2007, 16, 555–562. [Google Scholar] [CrossRef]
- Piccirillo, C.; Castro, P.M.L. Calcium hydroxyapatite-based photocatalysts for environment remediation: Characteristics, performances and future perspectives. J. Environ. Manag. 2017, 193, 79–91. [Google Scholar] [CrossRef]
- Zhang, L.; Chuaicham, C.; Balakumar, V.; Sekar, K.; Ohtani, B.; Sasaki, K. Determination of the roles of FeIII in the interface between titanium dioxide and montmorillonite in FeIII-doped montmorillonite/titanium dioxide composites as photocatalysts. Appl. Clay Sci. 2022, 227, 106577. [Google Scholar] [CrossRef]
- Saxena, V.; Sharma, S.; Pandey, L.M. Fe(III) doped ZnO nano-assembly as a potential heterogeneous nano-catalyst for the production of biodiesel. Mater. Lett. 2019, 237, 232–235. [Google Scholar] [CrossRef]
- Khan, H.; Swati, I.K. Fe3+-doped Anatase TiO2 with d–d Transition, Oxygen Vacancies and Ti3+ Centers: Synthesis, Characterization, UV–vis Photocatalytic and Mechanistic Studies. Ind. Eng. Chem. Res. 2016, 55, 6619–6633. [Google Scholar] [CrossRef]
- Dos Santos Silva, D.; Villegas, A.E.C.; Bonfim, R.d.P.F.; Salim, V.M.M.; De Resende, N.S. Iron-substituted hydroxyapatite as a potential photocatalyst for selective reduction of CO2 with H2. J. CO2 Util. 2022, 63, 102102. [Google Scholar] [CrossRef]
- Boukha, Z.; Choya, A.; Cortés-Reyes, M.; de Rivas, B.; Alemany, L.J.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Influence of the calcination temperature on the activity of hydroxyapatite-supported palladium catalyst in the methane oxidation reaction. Appl. Catal. B Environ. 2020, 277, 119280. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Yek, S.M.-G.; Nasrollahzadeh, M.; Kim, A.; Varma, R.S. Palladium Nanocatalysts on Hydroxyapatite: Green Oxidation of Alcohols and Reduction of Nitroarenes in Water. Appl. Sci. 2019, 9, 4183. [Google Scholar] [CrossRef]
- Ulas, B.; Yilmaz, Y.; Koc, S.; Kivrak, H. Hydroxyapatite supported PdxIn100-x as a novel electrocatalyst for high-efficiency glucose electrooxidation. Int. J. Hydrogen Energy 2023, 48, 6798–6810. [Google Scholar] [CrossRef]
- Patria, R.D.; Islam, M.K.; Luo, L.; Leu, S.-Y.; Varjani, S.; Xu, Y.; Wong, J.W.-C.; Zhao, J. Hydroxyapatite-based catalysts derived from food waste digestate for efficient glucose isomerization to fructose. Green Synth. Catal. 2021, 2, 356–361. [Google Scholar] [CrossRef]
- Jung, K.; Kim, Y.; Chung, W.-J.; Kwon, K.-Y. Hydroxyapatite Supported Ruthenium Catalysts for Hydrogen Generation from Borane Dimethyl Amine. Bull. Korean Chem. Soc. 2015, 36, 2797–2798. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Su, S.; Yin, D. Efficient Oxidation of Glucose into Sodium Gluconate Catalyzed by Hydroxyapatite Supported Au Catalyst. Catal. Lett. 2016, 147, 383–390. [Google Scholar] [CrossRef]
- Nur, A.; Nazriati, N.; Fajaroh, F.; Arthaningrum, A.; Nurcahyani, I.; Cipto, D.L.R.P.; Kurniawan, F. Electrosynthesis of Cu/hydroxyapatite as the catalyst for hydrogen production via NaBH4 hydrolysis. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1070, 012023. [Google Scholar] [CrossRef]
- Hou, Q.; Laiq Ur Rehman, M.; Bai, X.; Qian, H.; Lai, R.; Xia, T.; Yu, G.; Tang, Y.; Xie, H.; Ju, M. Enhancing the reusability of hydroxyapatite by barium modification for efficient isomerization of glucose to fructose in ethanol. Fuel 2023, 338, 127308. [Google Scholar] [CrossRef]
- Malpica-Maldonado, J.J.; Melo-Banda, J.A.; Martínez-Salazar, A.L.; Garcia-Hernández, M. Synthesis and characterization of Ni-Mo2C particles supported over hydroxyapatite for potential application as a catalyst for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 12446–12454. [Google Scholar] [CrossRef]
- Mitsudome, T.; Urayama, T.; Kiyohiro, T.; Maeno, Z.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. On-demand Hydrogen Production from Organosilanes at Ambient Temperature Using Heterogeneous Gold Catalysts. Sci. Rep. 2016, 6, 37682. [Google Scholar] [CrossRef] [PubMed]
- Yaemsunthorn, K.; Randorn, C. Hydrogen production using economical and environmental friendly nanoparticulate hydroxyapatite and its ion doping. Int. J. Hydrogen Energy 2017, 42, 5056–5062. [Google Scholar] [CrossRef]
- Yan, T.; Li, N.; Jiang, Z.; Guan, W.; Qiao, Z.; Huang, B. Self-sacrificing template synthesis of CdS quantum dots/Cd-Hap composite photocatalysts for excellent H2 production under visible light. Int. J. Hydrogen Energy 2018, 43, 20616–20626. [Google Scholar] [CrossRef]
- Liang, Z.; Ouyang, B.; Wang, T.; Liu, X.; Huo, H.; Liu, D.; Feng, H.; Ma, J.; Deng, K.; Li, A.; et al. Pt modified TiO2/NiO p-n junction with enhanced surface reaction and charge separation for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 10868–10876. [Google Scholar] [CrossRef]
- Prekajski, M.; Jokic, B.; Kalijadis, A.; Maletaskic, J.; Stankovic, N.; Lukovic, J.; Matovic, B. Synthesis of silver doped hydroxyapatite nanospheres using Ouzo effect. Process. Appl. Ceram. 2016, 10, 169–174. [Google Scholar] [CrossRef]
- Mehta, S.K.; Kumar, S.; Chaudhary, S.; Bhasin, K.K. Nucleation and growth of surfactant-passivated CdS and HgS nanoparticles: Time-dependent absorption and luminescence profiles. Nanoscale 2010, 2, 145–152. [Google Scholar] [CrossRef]
- Chang, M.C.; Tanaka, J. XPS study for the microstructure development of hydroxyapatite–collagen nanocomposites cross-linked using glutaraldehyde. Biomaterials 2002, 23, 3879–3885. [Google Scholar] [CrossRef]
- Gomes, G.C.; Borghi, F.F.; Ospina, R.O.; López, E.O.; Borges, F.O.; Mello, A. Nd:YAG (532 nm) pulsed laser deposition produces crystalline hydroxyapatite thin coatings at room temperature. Surf. Coat. Technol. 2017, 329, 174–183. [Google Scholar] [CrossRef]
- Govindasamy, P.; Kandasamy, B.; Thangavelu, P.; Barathi, S.; Thandavarayan, M.; Shkir, M.; Lee, J. Biowaste derived hydroxyapatite embedded on two-dimensional g-C3N4 nanosheets for degradation of hazardous dye and pharmacological drug via Z-scheme charge transfer. Sci. Rep. 2022, 12, 11572. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Hussain, S.; Jia, L.; Lin, D.; Yin, X.; Lin, Y.; Cheng, Z.; Wang, L. FeS2/carbon hybrids on carbon cloth: A highly efficient and stable counter electrode for dye-sensitized solar cells. Sustain. Energy Fuels 2019, 3, 1749–1756. [Google Scholar] [CrossRef]
- Li, D.; Zhao, X.; Zhou, Q.; Ding, B.; Zheng, A.; Peng, Q.; Hou, Z. Vicinal hydroxyl group-inspired selective oxidation of glycerol to glyceric acid on hydroxyapatite supported Pd catalyst. Green Energy Environ. 2022, 7, 691–703. [Google Scholar] [CrossRef]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Mittraphab, Y.; Shimizu, K.; Ohtani, B.; Sasaki, K. Fabrication of graphitic carbon nitride/ZnTi-mixed metal oxide heterostructure: Robust photocatalytic decomposition of ciprofloxacin. J. Alloys Compd. 2022, 906, 164294. [Google Scholar] [CrossRef]
- Chong, R.; Li, J.; Ma, Y.; Zhang, B.; Han, H.; Li, C. Selective conversion of aqueous glucose to value-added sugar aldose on TiO2-based photocatalysts. J. Catal. 2014, 314, 101–108. [Google Scholar] [CrossRef]
- Unwiset, P.; Chen, G.; Ohtani, B.; Chanapattharapol, K.C. Correlation of the Photocatalytic Activities of Cu, Ce and/or Pt-Modified Titania Particles with their Bulk and Surface Structures Studied by Reversed Double-Beam Photoacoustic Spectroscopy. Catalysts 2019, 9, 1010. [Google Scholar] [CrossRef]
- Ratova, M.; Tosheva, L.; Kelly, P.J.; Ohtani, B. Characterisation and properties of visible light-active bismuth oxide-titania composite photocatalysts. Sustain. Mater. Technol. 2019, 22, e00112. [Google Scholar] [CrossRef]
- Chuaicham, C.; Inoue, T.; Balakumar, V.; Tian, Q.; Ohtani, B.; Sasaki, K. Visible light-driven ZnCr double layer oxide photocatalyst composites with fly ashes for the degradation of ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 106970. [Google Scholar] [CrossRef]
- Li, L.; Shen, F.; Smith, R.L.; Qi, X. Quantitative chemocatalytic production of lactic acid from glucose under anaerobic conditions at room temperature. Green Chem. 2017, 19, 76–81. [Google Scholar] [CrossRef]




| Materials | Applications | Processes | Ref. |
|---|---|---|---|
| HAp | Sugar conversion | Isomerization of glucose to fructose | [36] |
| Ru/HAp | H2 production | Hydrolysis of borane dimethyl amine | [37] |
| Au/HAp | Sugar conversion | Glucose oxidation to sodium gluconate | [38] |
| Cu/HAp | H2 production | Hydrolysis of NaBH4 | [39] |
| Ba/HAp | Sugar conversion | Isomerization of glucose to fructose | [40] |
| Ni-Mo2C/HAp | H2 production | Biomass gasification | [41] |
| Au/HAp | H2 production | Hydrolytic oxidation of organosilanes | [42] |
| PaIn/HAp | Sugar conversion | Glucose electrooxidation for direct glucose fuel cells | [35] |
| Ti-HAp | H2 production | Photocatalytic water splitting | [43] |
| CdS quantum dots/Cd-HAp | H2 production | Photocatalytic H2 evolution | [44] |
| O | P | Ca | Fe | Pd | |
|---|---|---|---|---|---|
| HAp | 47.49 | 17.60 | 34.91 | - | - |
| FeHAp | 48.25 | 16.35 | 31.95 | 3.45 | - |
| Pd@FeHAp | 48.27 | 16.39 | 31.89 | 3.21 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuaicham, C.; Noguchi, Y.; Shenoy, S.; Shu, K.; Trakulmututa, J.; Srikhaow, A.; Sekar, K.; Sasaki, K. Simultaneous Photocatalytic Sugar Conversion and Hydrogen Production Using Pd Nanoparticles Decorated on Iron-Doped Hydroxyapatite. Catalysts 2023, 13, 675. https://doi.org/10.3390/catal13040675
Chuaicham C, Noguchi Y, Shenoy S, Shu K, Trakulmututa J, Srikhaow A, Sekar K, Sasaki K. Simultaneous Photocatalytic Sugar Conversion and Hydrogen Production Using Pd Nanoparticles Decorated on Iron-Doped Hydroxyapatite. Catalysts. 2023; 13(4):675. https://doi.org/10.3390/catal13040675
Chicago/Turabian StyleChuaicham, Chitiphon, Yuto Noguchi, Sulakshana Shenoy, Kaiqian Shu, Jirawat Trakulmututa, Assadawoot Srikhaow, Karthikeyan Sekar, and Keiko Sasaki. 2023. "Simultaneous Photocatalytic Sugar Conversion and Hydrogen Production Using Pd Nanoparticles Decorated on Iron-Doped Hydroxyapatite" Catalysts 13, no. 4: 675. https://doi.org/10.3390/catal13040675
APA StyleChuaicham, C., Noguchi, Y., Shenoy, S., Shu, K., Trakulmututa, J., Srikhaow, A., Sekar, K., & Sasaki, K. (2023). Simultaneous Photocatalytic Sugar Conversion and Hydrogen Production Using Pd Nanoparticles Decorated on Iron-Doped Hydroxyapatite. Catalysts, 13(4), 675. https://doi.org/10.3390/catal13040675











