CO2-Assisted Dehydrogenation of Propane to Propene over Zn-BEA Zeolites: Impact of Acid–Base Characteristics on Catalytic Performance
Abstract
:1. Introduction
2. Results and Discussion
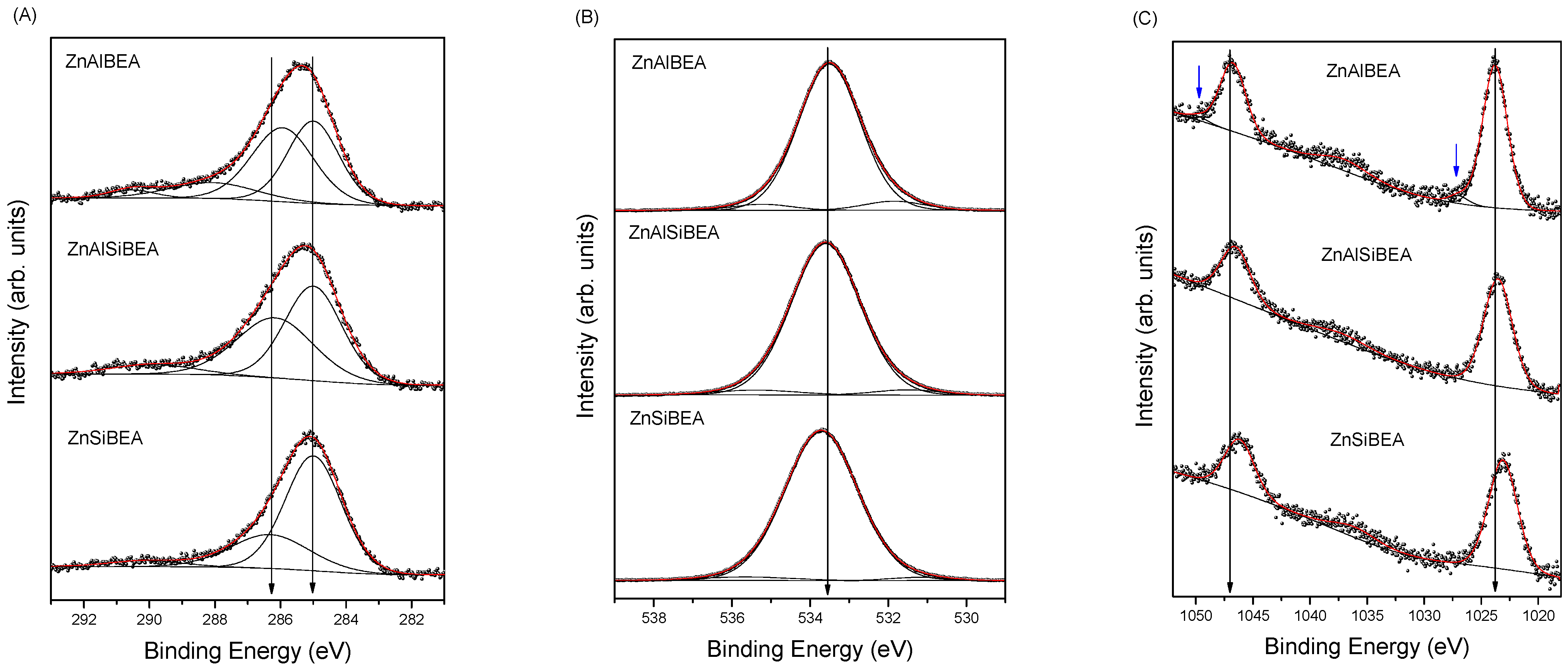
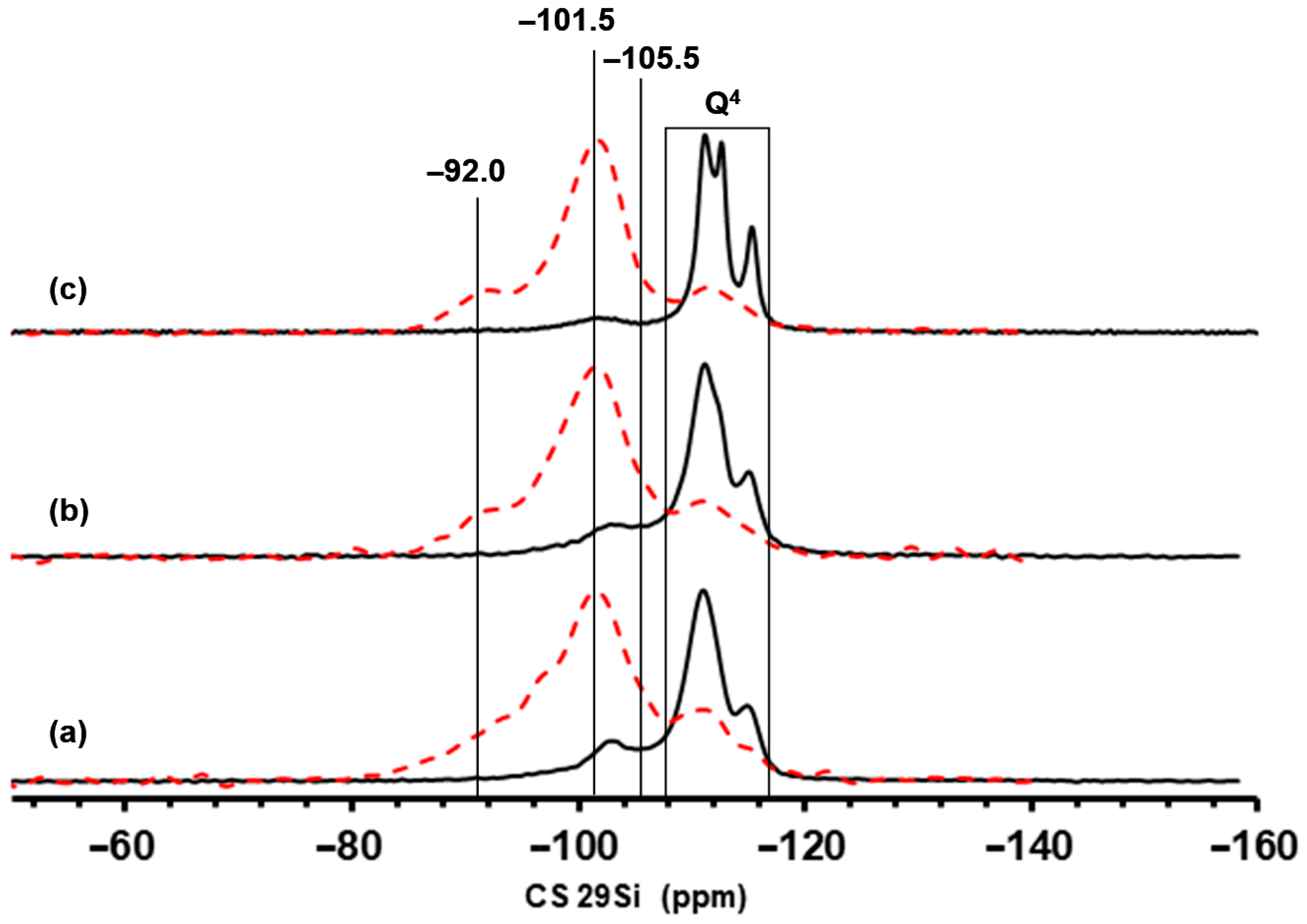
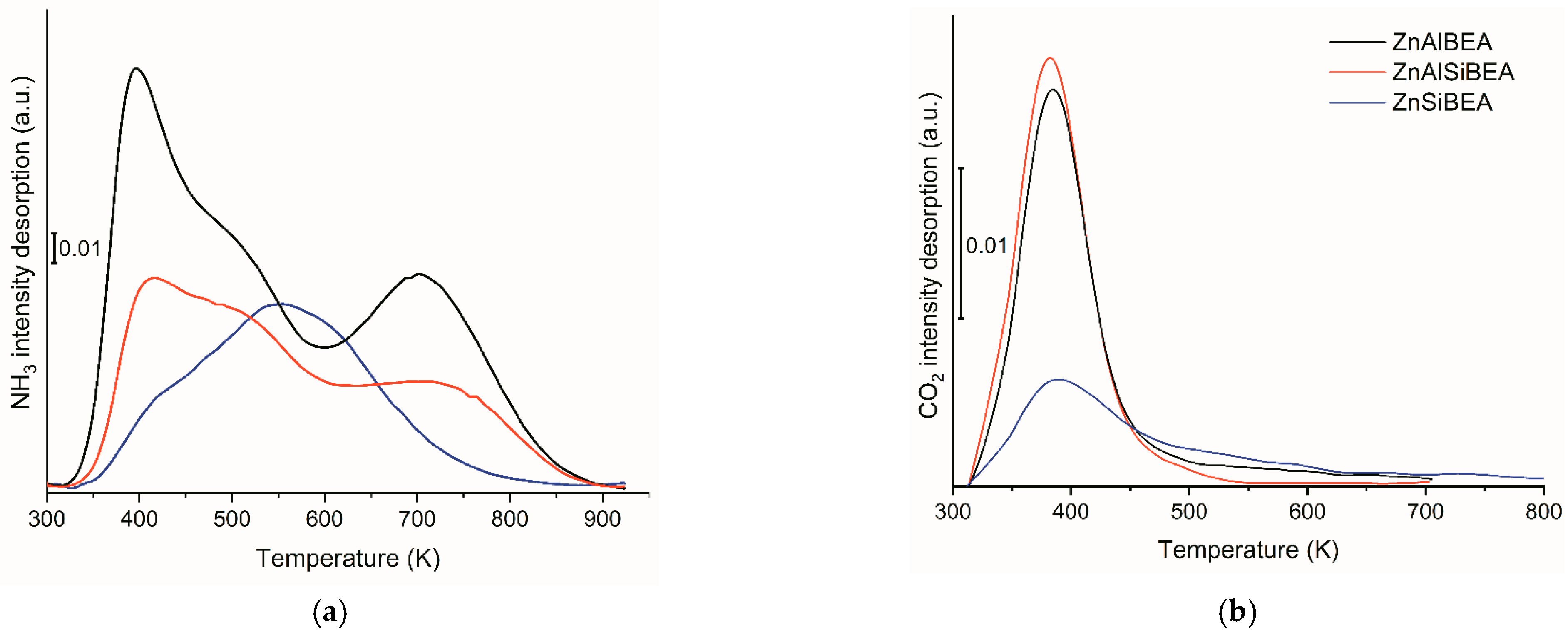
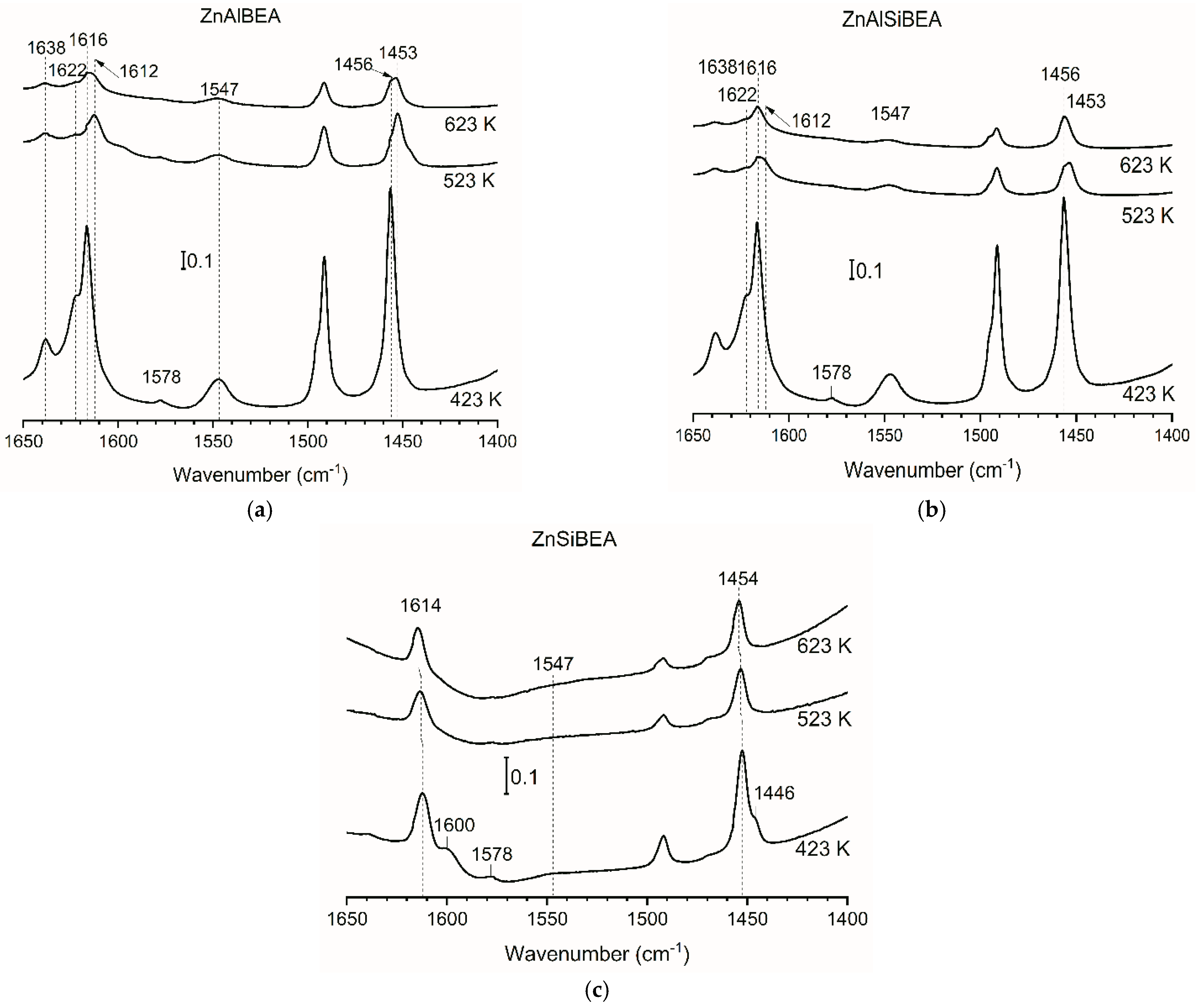
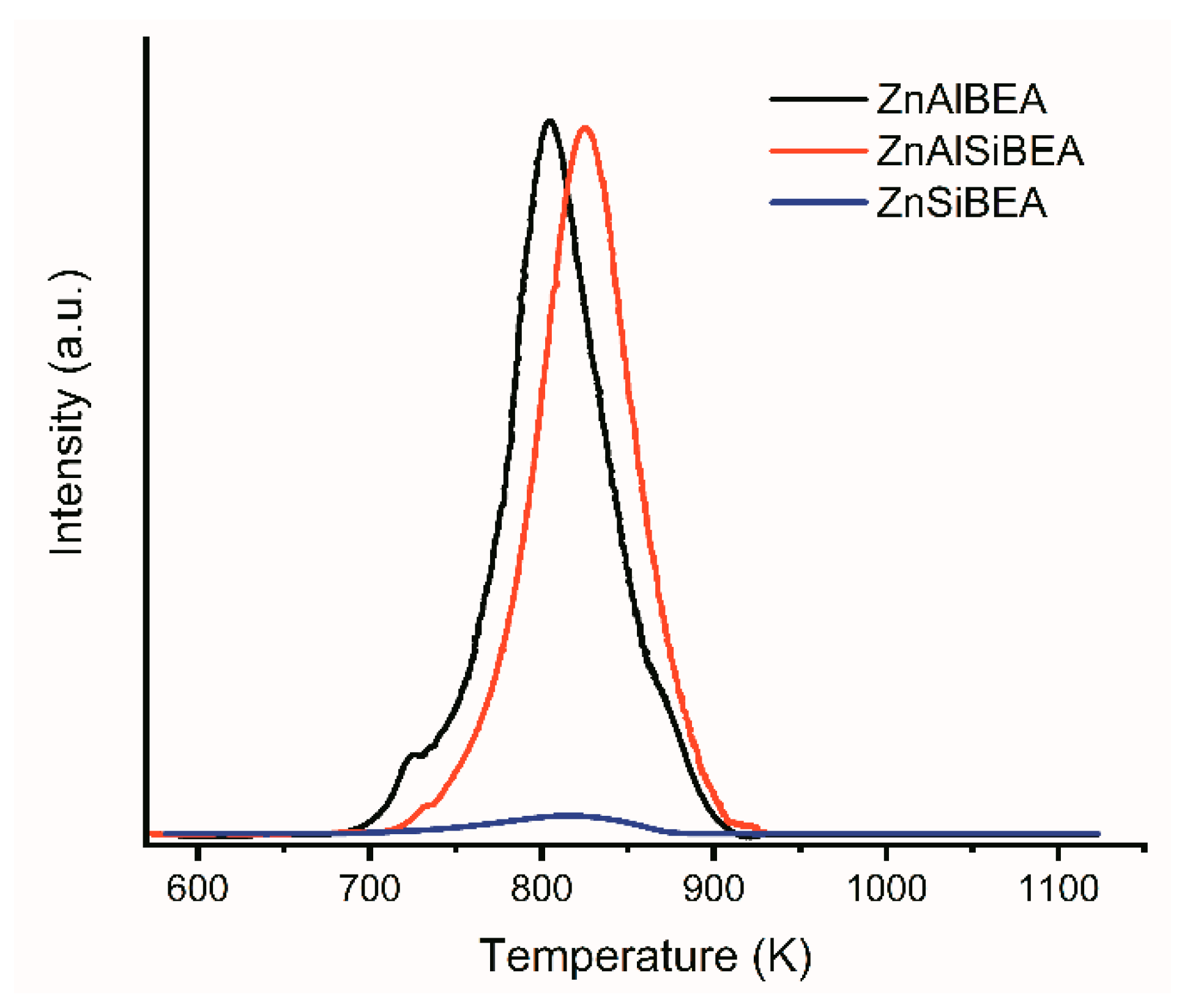
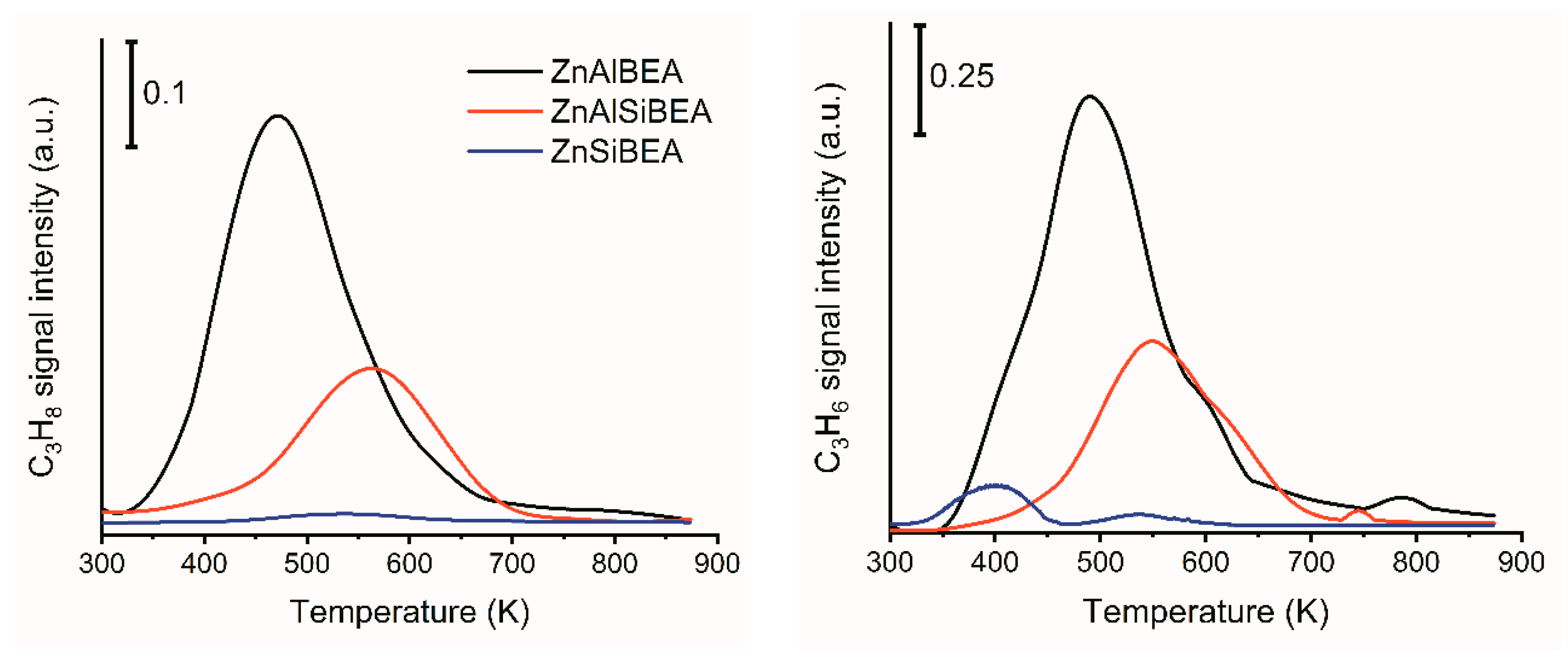
2.1. Structure, Texture, and Acid–Base Characteristics of Zn-BEA Zeolites with Different Si/Al Ratios
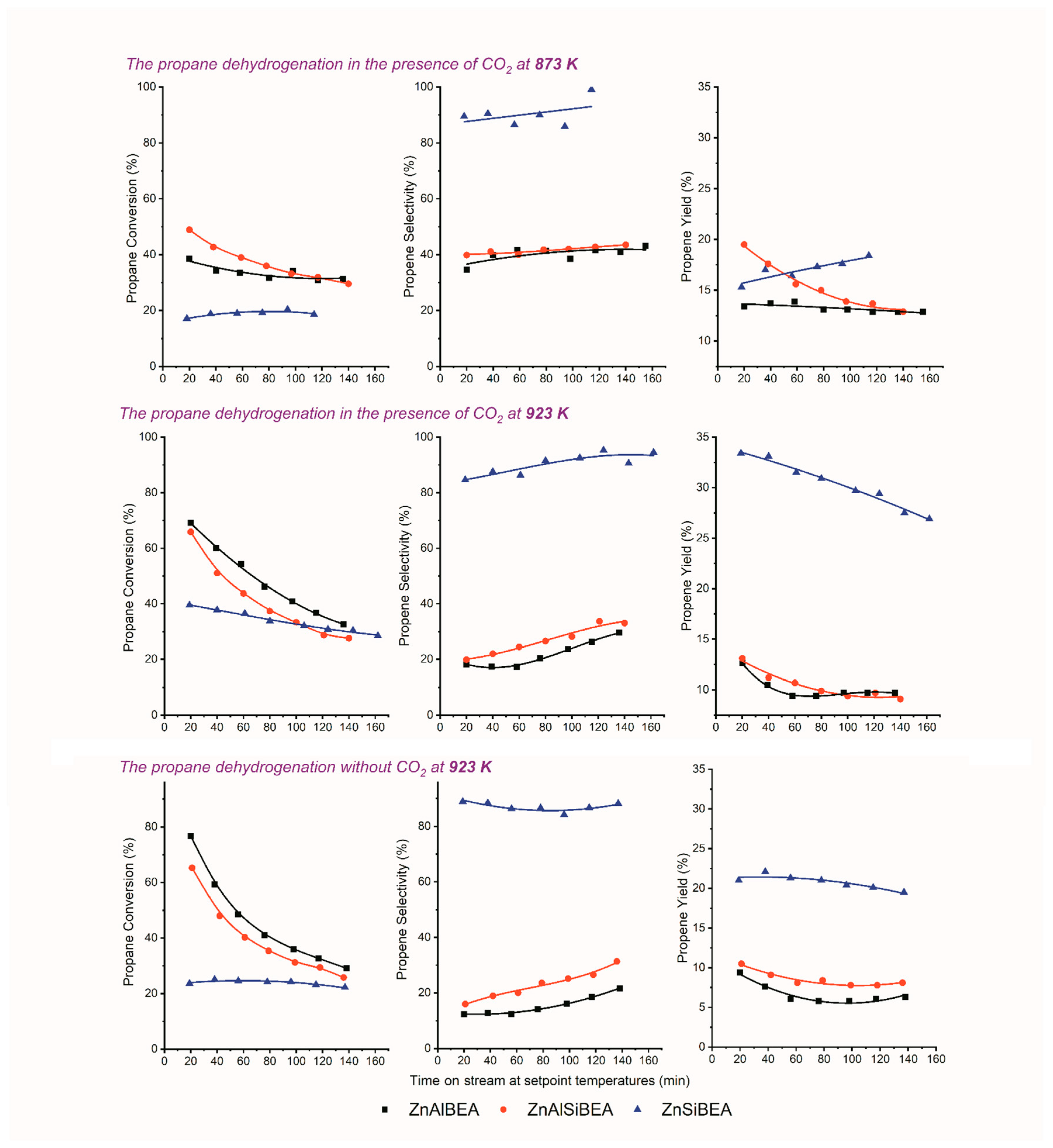
2.2. Catalytic Properties of Zn-BEA Zeolites in Propane Dehydrogenation
3. Materials and Methods
3.1. Zeolite Sample Preparation and Characterization
3.2. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.P.; Yang, D.; Wang, Z.; Yuan, Z.Y. State-of-the-art catalysts for direct dehydrogenation of propane to propylene. Chinese J. Catal. 2019, 40, 1233–1254. [Google Scholar] [CrossRef]
- Gomez, E.; Yan, B.; Kattel, S.; Chen, J.G. Carbon dioxide reduction in tandem with light-alkane dehydrogenation. Nat. Rev. Chem. 2019, 3, 638–649. [Google Scholar] [CrossRef]
- Atanga, M.A.; Rezaei, F.; Jawad, A.; Fitch, M.; Rownaghi, A.A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B Environ. 2018, 220, 429–445. [Google Scholar] [CrossRef]
- Gambo, Y.; Adamu, S.; Tanimu, G.; Abdullahi, I.M.; Lucky, R.A.; Ba-Shammakh, M.S.; Hossain, M.M. CO2-mediated oxidative dehydrogenation of light alkanes to olefins: Advances and perspectives in catalyst design and process improvement. Appl. Catal. A Gen. 2021, 623, 118273. [Google Scholar] [CrossRef]
- Schreiber, M.W.; Plaisance, C.P.; Baumgärtl, M.; Reuter, K.; Jentys, A.; Bermejo-Deval, R.; Lercher, J.A. Lewis-Brønsted acid pairs in Ga/H-ZSM-5 to catalyze dehydrogenation of light alkanes. J. Am. Chem. Soc. 2018, 140, 4849–4859. [Google Scholar] [CrossRef]
- Michorczyk, P.; Zeńczak-Tomera, K.; Michorczyk, B.; Wȩgrzyniak, A.; Basta, M.; Millot, Y.; Valentin, L.; Dzwigaj, S. Effect of dealumination on the catalytic performance of Cr-containing Beta zeolite in carbon dioxide assisted propane dehydrogenation. J. CO2 Util. 2020, 36, 54–63. [Google Scholar] [CrossRef]
- Ni, L.; Khare, R.; Bermejo-Deval, R.; Zhao, R.; Tao, L.; Liu, Y.; Lercher, J.A. Highly active and selective sites for propane dehydrogenation in zeolite Ga-BEA. J. Am. Chem. Soc. 2022, 144, 12347–12356. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; He, Z.-H.; Li, L.-Y.; Yang, S.-Y.; He, M.-X.; Sun, Y.-C.; Wang, K.; Chen, J.-G.; Liu, Z.-T. Research progress of CO2 oxidative dehydrogenation of propane to propylene over Cr-free metal catalysts. Rare Met. 2022, 41, 2129–2152. [Google Scholar] [CrossRef]
- Castro-Fernández, P.; Mance, D.; Liu, C.; Abdala, P.M.; Willinger, E.; Rossinelli, A.A.; Serykh, A.I.; Pidko, E.A.; Copéret, C.; Fedorov, A.; et al. Bulk and surface transformations of Ga2O3 nanoparticle catalysts for propane dehydrogenation induced by a H2 treatment. J. Catal. 2022, 408, 155–164. [Google Scholar] [CrossRef]
- Ye, T.; Carter, J.H.; Chen, B.; Li, X.; Ye, Y.; Taylor, S.H.; Hutchings, G.J. Iron-chromium mixed metal oxides catalyse the oxidative dehydrogenation of propane using carbon dioxide. Catal. Commun. 2022, 162, 106383. [Google Scholar] [CrossRef]
- Li, L.-Y.; Wang, Z.-Y.; Yang, S.-Y.; Chen, J.-G.; He, Z.-H.; Wang, K.; Luo, Q.-X.; Liu, Z.-W.; Liu, Z.-T. Understanding the role of Fe doping in tuning the size and dispersion of GaN nanocrystallites for CO2-assisted oxidative dehydrogenation of propane. ACS Catal. 2022, 12, 8527–8543. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Z.P.; Ren, J.T.; Zhang, S.; Wang, Z.; Yuan, Z.Y. ZnO supported on high-silica HZSM-5 as efficient catalysts for direct dehydrogenation of propane to propylene. Mol. Catal. 2019, 476, 110508. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, F.; Hua, W.; Yue, Y.; Gao, Z. ZnO supported on high silica HZSM-5 as new catalysts for dehydrogenation of propane to propene in the presence of CO2. Catal. Today 2009, 148, 316–322. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Z.; Ren, J.; Zhang, S.; Wang, Z.; Yuan, Z.Y. ZnO nanoclusters supported on dealuminated zeolite β as a novel catalyst for direct dehydrogenation of propane to propylene. ChemCatChem 2019, 11, 868–877. [Google Scholar] [CrossRef]
- Orlyk, S.M.; Kantserova, M.R.; Chedryk, V.I.; Kyriienko, P.I.; Balakin, D.Y.; Millot, Y.; Dzwigaj, S. Ga(Nb,Ta)SiBEA zeolites prepared by two-step postsynthesis method: Acid–base characteristics and catalytic performance in the dehydrogenation of propane to propylene with CO2. J. Porous Mater. 2021, 28, 1511–1522. [Google Scholar] [CrossRef]
- Liu, J.; He, N.; Zhang, Z.; Yang, J.; Jiang, X.; Zhang, Z.; Su, J.; Shu, M.; Si, R.; Xiong, G.; et al. Highly-dispersed zinc species on zeolites for the continuous and selective dehydrogenation of ethane with CO2 as a soft oxidant. ACS Catal. 2021, 11, 2819–2830. [Google Scholar] [CrossRef]
- Zhao, D.; Tian, X.; Doronkin, D.E.; Han, S.; Kondratenko, V.A.; Grunwaldt, J.D.; Perechodjuk, A.; Vuong, T.H.; Rabeah, J.; Eckelt, R.; et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 2021, 599, 234–238. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.; Han, S.; Zhang, Y.; Jiang, G.; Wang, Y.; Guo, K.; Zhao, Z.; Xu, C.; Li, R.; et al. ZnO nanoparticles encapsulated in nitrogen-doped carbon material and silicalite-1 composites for efficient propane dehydrogenation. iScience 2019, 13, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhou, Y.; Huang, L.; Zhou, S.; Sheng, X.; Wang, Q.; Zhang, C. Structure and catalytic properties of the Zn-modified ZSM-5 supported platinum catalyst for propane dehydrogenation. Chem. Eng. J. 2015, 270, 352–361. [Google Scholar] [CrossRef]
- Xie, L.; Chai, Y.; Sun, L.; Dai, W.; Wu, G.; Guan, N.; Li, L. Optimizing zeolite stabilized Pt-Zn catalysts for propane dehydrogenation. J. Energy Chem. 2021, 57, 92–98. [Google Scholar] [CrossRef]
- Qi, L.; Babucci, M.; Zhang, Y.; Lund, A.; Liu, L.; Li, J.; Chen, Y.; Hoffman, A.S.; Bare, S.R.; Han, Y.; et al. Propane dehydrogenation catalyzed by isolated Pt atoms in ≡SiOZn–OH nests in dealuminated zeolite Beta. J. Am. Chem. Soc. 2021, 143, 21364–21378. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Han, D.; Guan, L.; Zhu, L.; Mei, Y.; He, D.; Zu, Y. Bimetallic Ni-Zn site anchored in siliceous zeolite framework for synergistically boosting propane dehydrogenation. Fuel 2022, 307, 121790. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Fan, Q.; Zeng, L.; Mayoral, A.; Miao, S.; Yang, R.; Jiang, Z.; Zhou, W.; Zhang, J.; et al. Subnanometer bimetallic platinum–zinc clusters in zeolites for propane dehydrogenation. Angew. Chemie Int. Ed. 2020, 59, 19450–19459. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Arzumanov, S.S.; Toktarev, A.V.; Danilova, I.G.; Prosvirin, I.P.; Kriventsov, V.V.; Zaikovskii, V.I.; Freude, D.; Stepanov, A.G. Different efficiency of Zn2+ and ZnO species for methane activation on Zn-modified zeolite. ACS Catal. 2017, 7, 1818–1830. [Google Scholar] [CrossRef] [Green Version]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interf. Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Bandala, E.R.; Sadek, R.; Gurgul, J.; Łątka, K.; Zimowska, M.; Valentin, L.; Rodriguez-Narvaez, O.M.; Dzwigaj, S. Assessment of the capability of Fe and Al modified BEA zeolites to promote advanced oxidation processes in aqueous phase. Chem. Eng. J. 2021, 409, 127379. [Google Scholar] [CrossRef]
- Chalupka, K.A.; Sadek, R.; Szkudlarek, L.; Mierczynski, P.; Maniukiewicz, W.; Rynkowski, J.; Gurgul, J.; Casale, S.; Brouri, D.; Dzwigaj, S. The catalytic activity of microporous and mesoporous NiCoBeta zeolite catalysts in Fischer–Tropsch synthesis. Res. Chem. Intermed. 2021, 47, 397–418. [Google Scholar] [CrossRef]
- Pamin, K.; Gurgul, J.; Mordarski, G.; Millot, Y.; Nogier, J.-P.; Valentin, L.; Dzwigaj, S. Efficient transformation of cyclohexanone to ε-caprolactone in the oxygen-aldehyde system over single-site titanium BEA zeolite. Microporous Mesoporous Mater. 2021, 322, 111159. [Google Scholar] [CrossRef]
- Kocemba, I.; Rynkowski, J.; Gurgul, J.; Socha, R.P.; Łątka, K.; Krafft, J.-M.; Dzwigaj, S. Nature of the active sites in CO oxidation on FeSiBEA zeolites. Appl. Catal. A Gen. 2016, 519, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Iyoki, K.; Yamada, H.; Yanaba, Y.; Ohara, K.; Katada, N.; Wakihara, T. Synthesis and characterization of MFI-type zincosilicate zeolites with high zinc content using mechanochemically treated Si–Zn oxide composite. Microporous Mesoporous Mater. 2019, 288, 109594. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, J.; Chen, Z.; Yang, L.; Liu, Y.; Han, Y. Effects of amorphous-zinc-silicate-catalyzed ozonation on the degradation of p-chloronitrobenzene in drinking water. Appl. Catal. A Gen. 2011, 403, 112–118. [Google Scholar] [CrossRef]
- Hastir, A.; Kohli, N.; Singh, R.C. Comparative study on gas sensing properties of rare earth (Tb, Dy and Er) doped ZnO sensor. Phys. Chem. Solids 2017, 105, 23–34. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS characterization of ALD Al2O3 coated porous silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Sygellou, L.; Gianneta, V.; Xanthopoulos, N.; Skarlatos, D.; Georga, S.; Krontiras, C.; Ladas, S.; Kennou, S. ZrO2 and Al2O3 thin films on Ge(100) grown by ALD: An XPS investigation. Surf. Sci. Spectra. 2011, 18, 58–67. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Hunsicker, R.A.; Klier, K.; Gaffney, T.S.; Kirner, J.G. Framework zinc-substituted zeolites: synthesis, and core-level and valence-band XPS. Chem. Mater. 2002, 14, 4807–4811. [Google Scholar] [CrossRef]
- Wöll, C. The chemistry and physics of zinc oxide surfaces. Progr. Surf. Sci. 2007, 82, 55–120. [Google Scholar] [CrossRef]
- Tamiyakul, S.; Ubolcharoen, W.; Tungasmita, D.N.; Jongpatiwut, S. Conversion of glycerol to aromatic hydrocarbons over Zn-promoted HZSM-5 catalysts. Catal. Today 2015, 256, 325–335. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.J. Wang. Influence of preparation method on the performance of Zn-containing HZSM-5 catalysts in methanol-to-aromatics. Microporous Mesoporous Mater. 2014, 197, 252–261. [Google Scholar] [CrossRef]
- Santos, R.C.; Almeida, D.F.; de Aguiar Pontes, D.; Lau, L.Y.; Magalhães Pontes, L.A. Thiophene cracking on zinc modified beta zeolite. Mol. Catal. 2019, 470, 112–119. [Google Scholar] [CrossRef]
- Zhang, N.; Li, R.; Zhang, G.; Dong, L.; Zhang, D.; Wang, G.; Li, T. Zn-modified Hβ zeolites used in the adsorptive removal of organic chloride from model naphtha. ACS Omega 2020, 5, 11987–11997. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Qin, L.; Lu, J.; Feng, H. ZnO modified ZSM-5 and Y zeolites fabricated by atomic layer deposition for propane conversion. Phys. Chem. Chem. Phys. 2016, 18, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zan, W.; Bai, X.; Wang, G.; Wu, W. Synthesis of microscale and nanoscale ZSM-5 zeolites: Effect of particle size and acidity of Zn modified ZSM-5 zeolites on aromatization performance. Catal. Sci. Technol. 2017, 7, 1943–1952. [Google Scholar] [CrossRef]
- Almutairi, S.M.T.; Mezari, B.; Magusin, P.C.M.M.; Pidko, E.A.; Hensen, E.J.M. Structure and reactivity of Zn-Modified ZSM-5 zeolites: The importance of clustered cationic Zn complexes. ACS Catal. 2012, 2, 71–83. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Strobl, H.; Kokotailo, G.T.; Pasztor, C.T.; Barlow, G.E.; Bradley, S. Correlations between lattice structures of zeolites and their 29Si MAS n.m.r. spectra: Zeolites KZ-2, ZSM-12, and Beta. Zeolites 1988, 8, 132–136. [Google Scholar] [CrossRef]
- Pérez-Pariente, J.; Sanz, J.; Fornés, V.; Corma, A. 29Si and 27Al MAS NMR study of zeolite β with different Si/Al ratios. J. Catal. 1990, 124, 217–223. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Millot, Y.; Méthivier, C.; Che, M. Incorporation of Nb(V) into BEA zeolite investigated by XRD, NMR, IR, DR UV–vis, and XPS. Microporous Mesoporous Mater. 2010, 130, 162–166. [Google Scholar] [CrossRef]
- Popovych, N.O.; Kyriienko, P.I.; Soloviev, S.O.; Orlyk, S.M.; Dzwigaj, S. Influence of partial dealumination of BEA zeolites on physicochemical and catalytic properties of AgAlSiBEA in H2-promoted SCR of NO with ethanol. Microporous Mesoporous Mater. 2016, 226, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Dzwigaj, S.; Popovych, N.; Kyriienko, P.; Krafft, J.M.; Soloviev, S. The similarities and differences in structural characteristics and physico-chemical properties of AgAlBEA and AgSiBEA zeolites. Microporous Mesoporous Mater. 2013, 182, 16–24. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Popovych, N.; Kyriienko, P.; Soloviev, S.; Orlyk, S.; Dzwigaj, S. Catalytic properties of AgAlBEA and AgSiBEA zeolites in H2-promoted selective reduction of NO with ethanol. Microporous Mesoporous Mater. 2015, 203, 163–169. [Google Scholar] [CrossRef]
- Penzien, J.A.L.J.; Abraham, A.; van Bokhoven, J.A.; Jentys, A.; Müller, T.E.; Sievers, C. Generation and characterization of well-defined Zn2+ Lewis acid sites in ion exchanged zeolite BEA. J. Phys. Chem. B 2004, 108, 4116–4126. [Google Scholar] [CrossRef]
- Larina, O.V.; Shcherban, N.D.; Kyriienko, P.I.; Remezovskyi, I.M.; Yaremov, P.S.; Khalakhan, I.; Mali, G.; Soloviev, S.O.; Orlyk, S.M.; Dzwigaj, S. Design of effective catalysts based on ZnLaZrSi oxide systems for obtaining 1,3-butadiene from aqueous ethanol. ACS Sustain. Chem. Eng. 2020, 8, 16600–16611. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Bondil, M.; Head-Gordon, M.; Bell, A.T. Mechanism and kinetics of propane dehydrogenation and cracking over Ga/H-MFI prepared via vapor-phase exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 2019, 141, 1614–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gounder, R.; Iglesia, E. Catalytic hydrogenation of alkenes on acidic zeolites: Mechanistic connections to monomolecular alkane dehydrogenation reactions. J. Catal. 2011, 277, 36–45. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, J.; Hua, W.; Yue, Y.; Gao, Z. Ga2O3/HZSM-48 for dehydrogenation of propane: Effect of acidity and pore geometry of support. J. Ind. Eng. Chem. 2012, 18, 731–736. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Gambo, Y.; Adamu, S.; Abdulrasheed, A.A.; Lucky, R.A.; Ba-Shammakh, M.S.; Hossain, M.M. Catalyst design and tuning for oxidative dehydrogenation of propane–A review. Appl. Catal. A Gen. 2021, 8, 117914. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.H.; Lu, J.; Fan, K. Periodic density functional theory study of propane dehydrogenation over perfect Ga2O3(100) surface. J. Phys. Chem. C 2008, 112, 20382–20392. [Google Scholar] [CrossRef]
- Copéret, C. C-H bond activation and organometallic intermediates on isolated metal centers on oxide surfaces. Chem. Rev. 2010, 110, 656–680. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.B.; Park, S.-E. Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ. Sci. 2012, 5, 9419–9437. [Google Scholar] [CrossRef]
- Mukherjee, D.; Park, S.-E.; Reddy, B.M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An eco benign process for industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Nowicka, E.; Reece, C.; Althahban, S.; Mohammed, K.; Kondrat, S.; John, D. Elucidating the role of CO2 in the soft oxidative dehydrogenation of propane over ceria-based catalysts. ACS Catal. 2018, 8, 3454–3468. [Google Scholar] [CrossRef] [Green Version]
- Michorczyk, P.; Zeńczak, K.; Niekurzak, R.; Ogonowski, J. Dehydrogenation of propane with CO2–a new green process for propene and synthesis gas production. Polish J. Chem. Technol. 2012, 14, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Nozik, D.; Tinga, F.M.P.; Bell, A.T. Propane dehydrogenation and cracking over Zn/H-MFI prepared by solid-state ion exchange of ZnCl2. ACS Catal. 2021, 11, 14489–14506. [Google Scholar] [CrossRef]
- Otroshchenko, T.P.; Kondratenko, V.A.; Rodemerck, U.; Linke, D.; Kondratenko, E.V. Non-oxidative dehydrogenation of propane, n-butane, and isobutane over bulk ZrO2-based catalysts: Effect of dopant on the active site and pathways of product formation. Catal. Sci. Technol. 2017, 7, 4499–4510. [Google Scholar] [CrossRef]
- Dzwigaj, S.; Peltre, M.J.; Massiani, P.; Davidson, A.; Che, M.; Sen, T.; Sivasanker, S. Incorporation of vanadium species in a dealuminated β zeolite. Chem. Commun. 1998, 1, 87–88. [Google Scholar] [CrossRef]
- Kyriienko, P.I.; Larina, O.V.; Balakin, D.Y.; Stetsuk, A.O.; Nychiporuk, Y.M.; Soloviev, S.O.; Orlyk, S.M. 1,3-Butadiene production from aqueous ethanol over ZnO/MgO-SiO2 catalysts: Insight into H2O effect on catalytic performance. Appl. Catal. A Gen. 2021, 616, 118081. [Google Scholar] [CrossRef]


| Sample | Micropores | Mesopores | SBET, m2/g | Adsorption Volume at p/p0 = 1, cm3/g | |||
|---|---|---|---|---|---|---|---|
| Volume Vmi, cm3/g | Diameter dmi *, nm | Volume Vme, cm3/g | Diameter dme, nm | (Sme + Souter), m2/g | |||
| ZnAlBEA | 0.19 | 1.00 | 0.37 | 32 ± 5 | 70 | 535 | 0.58 |
| ZnAlSiBEA | 0.18 | 1.01 | 0.32 | 50 ± 15 | 60 | 505 | 0.52 |
| ZnSiBEA | 0.21 | 1.05 | 0.33 | ~60 * | 80 | 605 | 0.56 |
| Sample | Zn | Si | Al | O | C |
|---|---|---|---|---|---|
| ZnAlBEA | 0.23 | 33.24 | 2.07 | 58.53 | 5.92 |
| ZnAlSiBEA | 0.25 | 34.53 | 0.59 | 59.68 | 4.95 |
| ZnSiBEA | 0.24 | 36.86 | 0.11 | 57.95 | 4.84 |
| Core Excitation | ZnAlBEA | ZnAlSiBEA | ZnSiBEA | ||||
|---|---|---|---|---|---|---|---|
| BE (eV) | Area (%) | BE (eV) | Area (%) | BE (eV) | Area (%) | ||
| Zn 2p3/2 | 1023.8 1027.0 | 95.9 4.1 | 1023.5 | 100 | 1023.1 | 100 | A B |
| Si 2p3/2 | 104.0 | 100 | 104.2 2 | 100 | 104.3 | 100 | |
| Al 2p3/2 | 75.6 | 100 | 75.55 | 100 | 75.0 | 100 | |
| Sample | Concentration of Acidic Sites, rel. un. 1 | Concentration of Basic Sites, rel. un. 1 | |||||
|---|---|---|---|---|---|---|---|
| Weak (293–423 K) 2 | Medium Strength (423–673 K) 2 | Strong (>673 K) 2 | Total | Weak (293–423 K) 2 | Medium Strength (423–673 K) 2 | Total | |
| ZnAlBEA | 0.26 | 0.36 | 0.38 | 1.00 | 0.78 | 0.10 | 0.88 |
| ZnAlSiBEA | 0.14 | 0.38 | 0.08 | 0.60 | 0.89 | 0.11 | 1.00 |
| ZnSiBEA | 0.04 | 0.31 | 0.08 | 0.43 | 0.22 | 0.26 | 0.48 |
| Catalyst | TOS, min | 823 K | 873 K | 923 K | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XC3H8 | SC3H6 | YC3H6 | XC3H8 | SC3H6 | YC3H6 | XC3H8 | SC3H6 | YC3H6 | ||
| ZnAlBEA | 30 | 38 | 25 | 9.5 | 36 | 37 | 13.3 | 64 | 17 | 10.9 |
| 60 | 34 | 29 | 9.9 | 33 | 41 | 13.5 | 52 | 18 | 9.4 | |
| 120 | – | – | – | 31 | 42 | 13.0 | 35 | 27 | 9.5 | |
| ZnAlSiBEA | 30 | 29 | 36 | 10.4 | 45 | 40 | 18.0 | 58 | 21 | 12.2 |
| 60 | 23 | 42 | 9.7 | 39 | 41 | 16.0 | 44 | 24 | 10.6 | |
| 120 | – | – | – | 31 | 43 | 13.3 | 29 | 33 | 9.6 | |
| ZnSiBEA | 30 | 8 | 57 | 4.6 | 18 | 90 | 16.2 | 38 | 86 | 32.7 |
| 60 | 9 | 57 | 5.1 | 20 | 90 | 18.0 | 36 | 88 | 31.7 | |
| 120 | – | – | – | 19 | 94 | 17.9 | 32 | 94 | 30.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlyk, S.; Kyriienko, P.; Kapran, A.; Chedryk, V.; Balakin, D.; Gurgul, J.; Zimowska, M.; Millot, Y.; Dzwigaj, S. CO2-Assisted Dehydrogenation of Propane to Propene over Zn-BEA Zeolites: Impact of Acid–Base Characteristics on Catalytic Performance. Catalysts 2023, 13, 681. https://doi.org/10.3390/catal13040681
Orlyk S, Kyriienko P, Kapran A, Chedryk V, Balakin D, Gurgul J, Zimowska M, Millot Y, Dzwigaj S. CO2-Assisted Dehydrogenation of Propane to Propene over Zn-BEA Zeolites: Impact of Acid–Base Characteristics on Catalytic Performance. Catalysts. 2023; 13(4):681. https://doi.org/10.3390/catal13040681
Chicago/Turabian StyleOrlyk, Svitlana, Pavlo Kyriienko, Andriy Kapran, Valeriy Chedryk, Dmytro Balakin, Jacek Gurgul, Malgorzata Zimowska, Yannick Millot, and Stanislaw Dzwigaj. 2023. "CO2-Assisted Dehydrogenation of Propane to Propene over Zn-BEA Zeolites: Impact of Acid–Base Characteristics on Catalytic Performance" Catalysts 13, no. 4: 681. https://doi.org/10.3390/catal13040681
APA StyleOrlyk, S., Kyriienko, P., Kapran, A., Chedryk, V., Balakin, D., Gurgul, J., Zimowska, M., Millot, Y., & Dzwigaj, S. (2023). CO2-Assisted Dehydrogenation of Propane to Propene over Zn-BEA Zeolites: Impact of Acid–Base Characteristics on Catalytic Performance. Catalysts, 13(4), 681. https://doi.org/10.3390/catal13040681










