Abstract
In this study, a series of zeolite-X-supported different crystal phases of MnO2 (α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2) catalysts were prepared via a solid-state diffusion method and high-heat treatment method to explore their low-temperature NH3-SCR performance. All of the catalysts featured typical octahedral zeolite X structures and manganese dioxides species of various crystal types dispersed across the support surface. Throughout the entire temperature range of the reaction, γ-MnO2/X catalyst had the highest NO conversion. Additionally, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts had nearly 100% of N2 selectivity, whereas the α-MnO2/X catalyst had the lowest N2 selectivity (about 90%) below 125 °C. Moreover, the γ-MnO2/X catalyst demonstrated superior acidity capacity and reduction ability compared with the other three catalysts. All the catalysts contained the essential intermediates NH2NO and NH4NO3 species, which are essential to the SCR reaction. More acid sites and nitrate species existed on the γ-MnO2/X catalyst than on the other catalysts, thereby boosting the SCR reaction.
1. Introduction
Nitrogen oxides (NOx), which are emitted by the natural gas and the combustion of fossil fuels, are one of the primary air pollutants responsible for grave environmental issues, such as acid rain, ozone depletion, and photochemical smog [1,2,3]. As the most effective method for reducing NOx, selective catalytic reduction via NH3 technology (NH3-SCR) has been applied extensively [4]. Due to the exceptional catalytic activity, the commercial V2O5-WO3/TiO2 (VWT) catalyst is widely utilized [5]. Regrettably, the VWT catalyst has some flaws, such as a high reacted temperature of 300–400 °C and the environmental hazard of vanadium, which prevents it from being used optimally with low-temperature flue gas [6]. Consequently, the search for more active, efficient, and eco-friendly low-temperature SCR catalysts is required.
Generally, rare earth and transition metal oxides are used as the active component for catalysts; among them, Mn-based catalysts have exhibited ideal low-temperature SCR activity due to various chemical valence states of Mn element (Mn2+, Mn3+, Mn4+) [7,8]. Our previous work investigated the SCR performance and N2O pathways in the SCR process over manganese oxides with different valence states (MnO2, Mn2O3, Mn3O4), and discovered that MnO2 demonstrated the highest SCR activity at low temperature [9]. Furthermore, the MnO2 four common crystal phases and structures are α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2 [10]. In particular, pure Mn-based catalysts generated an amount of byproduct of N2O within the SCR reaction and reduced the N2 selectivity of the catalysts, which affected the catalytic activity. Then, the N2O formation for different crystal phases of manganese oxide (α-MnO2, β-MnO2, γ-MnO2 and σ-MnO2) catalysts during a low-temperature NH3-SCR reaction was investigated in our study using DRIFT measurement, which generated a higher content of N2O even at a low temperature [11]. Guo et al. [12] investigated the atomic-level mechanism of N2O formation on the α-MnO2 catalyst via DFT calculations and thermodynamics/kinetic analysis and pointed out that the (310) plane of α-MnO2 owned the highest N2 selectivity by avoiding NH3 dissociation. Some studies have attempted to enhance the catalyst activity and N2 selectivity at the same times. Gan et al. [13] prepared the α-MnO2 @CeO2 catalyst with the core shell-like structure and found that the CeO2 hall could improve the N2 selectivity of the catalysts. However, there is few research compared with the N2 selectivity enhancement of α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2 catalysts, and the previous prepared methods for the catalysts are complex. Thereby, it is vital to find a new way to improve the catalytic activity of the different crystal phases of MnO2.
It is common knowledge that zeolite-supported catalysts have been used extensively in NH3-SCR condition owing to their porous structure and high specific surface area [14,15,16,17,18]. Our previous study showed that zeolite X supported and doped with Mn or Ce oxides catalysts had excellent low-temperature SCR activity [19]. Resultantly, in this study, zeolite-X-supported different crystal phases of MnO2 (α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2) catalysts were synthesized and their SCR performance was measured, and then the SCR reaction process of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts was investigated via in situ DRIFTS study and their possible mechanism was pointed out.
2. Results
2.1. Microtopography of Catalysts
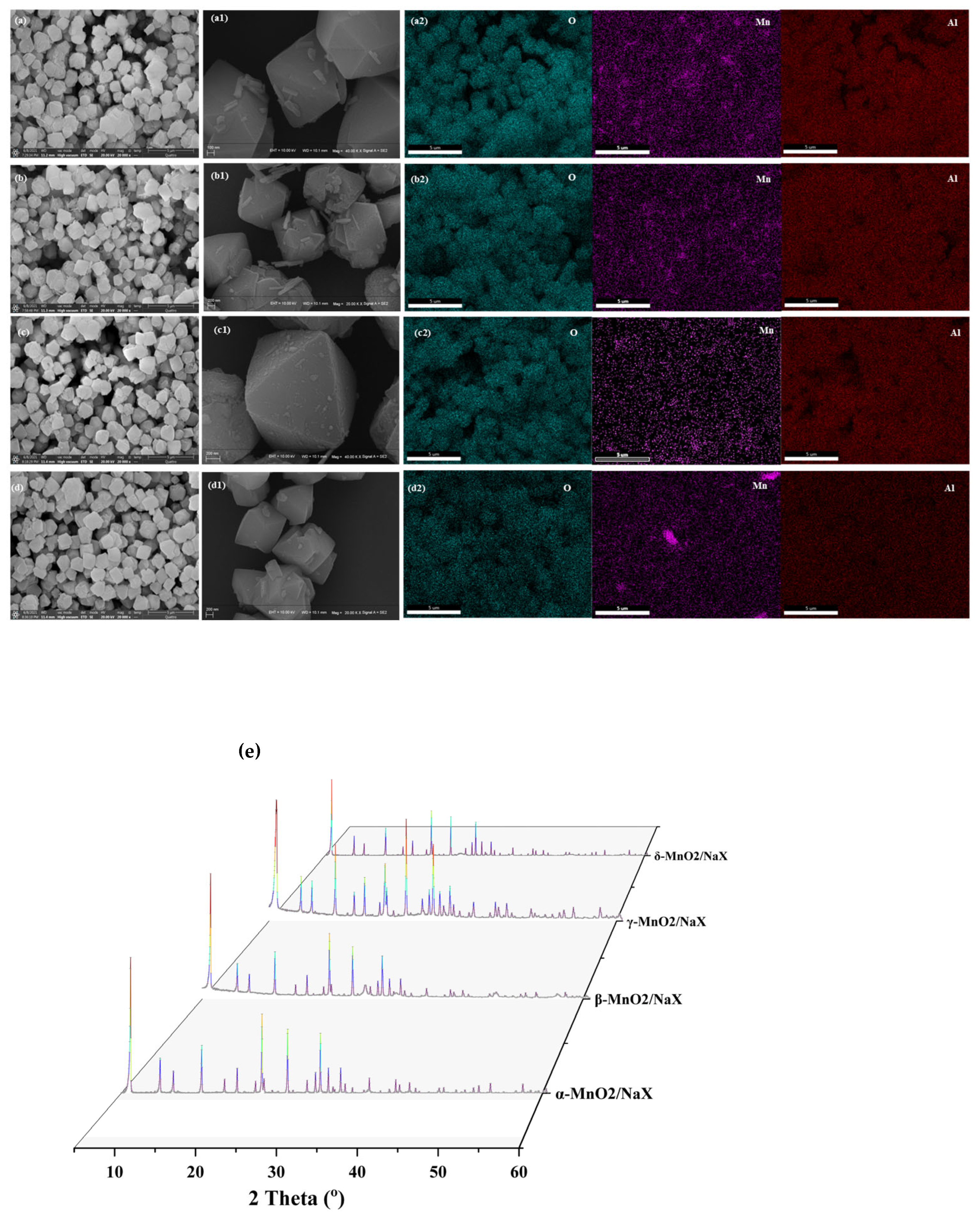
The microtopography of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts was measured by SEM, and the surface element distribution was observed by EDS mapping (Figure 1). It was found that all of the catalysts possessed the regular octahedral zeolite X structures [8], and manganese dioxide species with different crystal types distributed on the support surface [11], indicating that the catalysts were synthesized successfully. According to the EDS mapping results, some Mn elements aggregated together in α-MnO2/X and σ-MnO2/X, whereas Mn element on β-MnO2/X and γ-MnO2/X distributed uniformly, which may impact the different SCR activity of the catalysts. From the XRD pattern in Figure 1d, all the catalysts showed the characterized peaks of zeolite X, and no peaks belonged to manganese species.
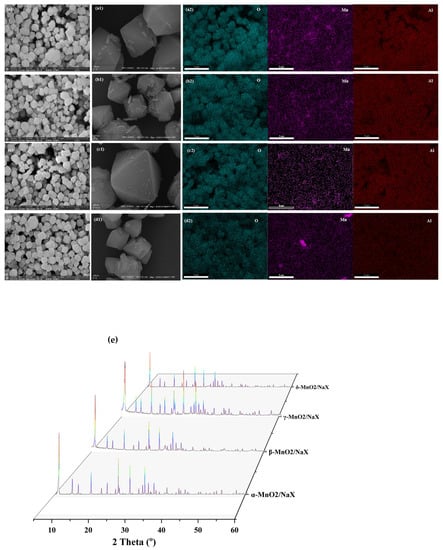
Figure 1.
SEM images of (a), (a1) α-MnO2/X, (b), (b1) β-MnO2/X, (c), (c1) γ-MnO2/X, and (d), (d1) σ-MnO2/X catalysts, and element EDS mapping of (a2) α-MnO2/X, (b2) β-MnO2/X, (c2) γ-MnO2/X, and (d2) σ-MnO2/X catalysts; (e) XRD pattern of the catalysts.
2.2. Catalytic Performance
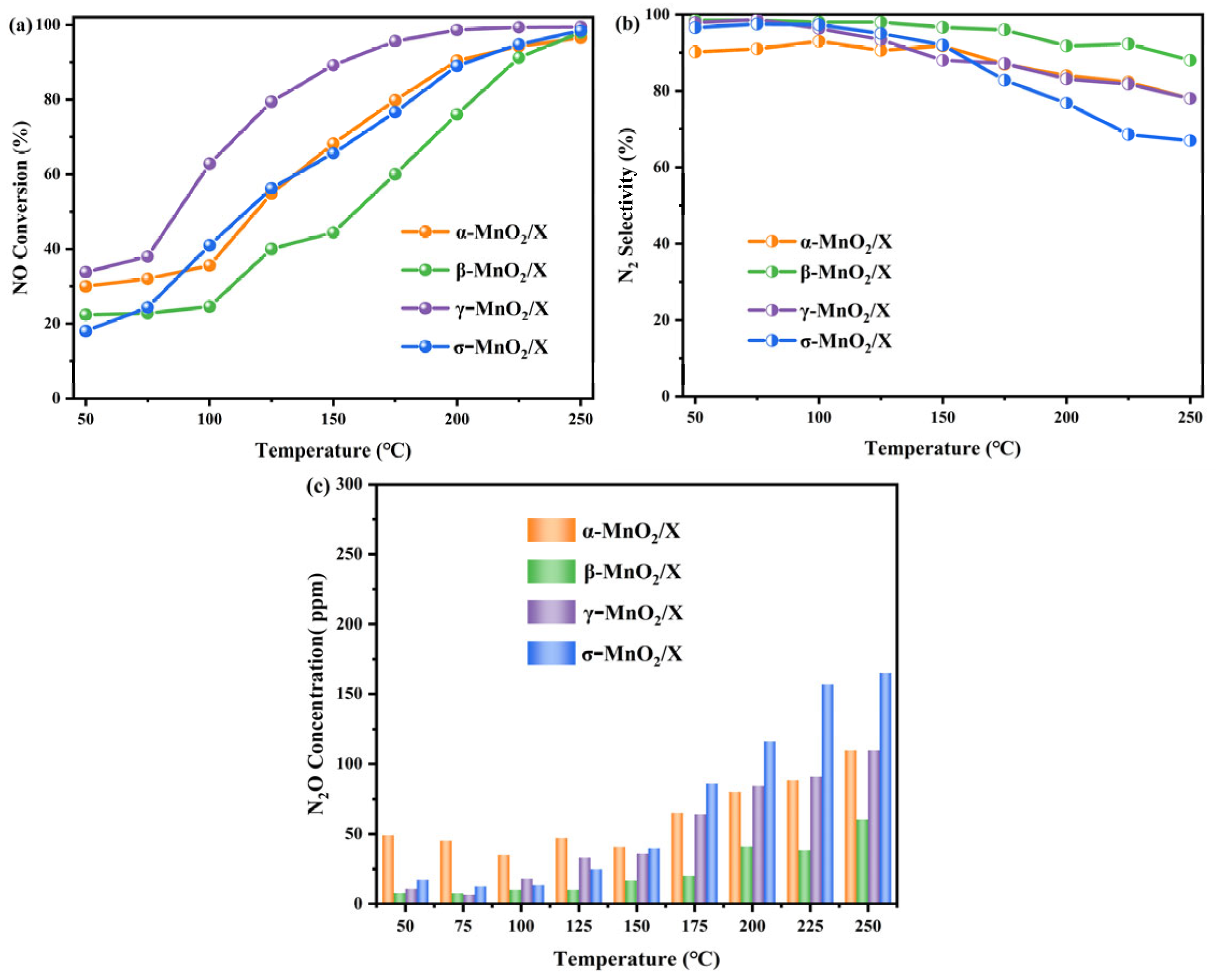
NH3-SCR activity of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts were measured in the temperature range of 50–250 °C, and the results are depicted in Figure 2. NO conversion of all the catalysts increased with increasing temperature, as shown in Figure 2a. The γ-MnO2/X catalyst had the highest NO conversion across the entire reaction temperature range, and the NO conversion was nearly 35% at 50 °C and then increased to 100% at 200 °C. α-MnO2/X and σ-MnO2/X catalysts exhibited nearly the same NO conversion from 100–250 °C, which was both 100% at 250 °C. While β-MnO2/X had the lowest NO conversion rate from 75 °C to 225 °C, it also reached 100% at 250 °C. According to Figure 2b, N2 selectivity of all the catalysts declined with increasing temperature. The β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts displayed nearly 100% N2 selectivity below 125 °C then decreased gradually under the higher temperature range, and the σ-MnO2/X catalyst showed the poorest N2 selectivity at higher temperature (over 175 °C). Meanwhile, the α-MnO2/X catalyst had the lowest N2 selectivity (about 90%) at 50–125 °C. From Figure 2c, it could be found that N2O concentration of all the catalysts increased with increasing temperature. In addition, the α-MnO2/X catalyst had the highest N2O concentration during 50–150 °C, and the σ-MnO2/X catalyst had the highest N2O concentration above 175 °C, while β-MnO2/X had the lowest N2O concentration over the whole reaction temperature range. Combined with pure manganese dioxides with different crystal types [11], the support of zeolite X could enhance the N2 selectivity of the catalysts and reduce the generation of N2O byproduct.
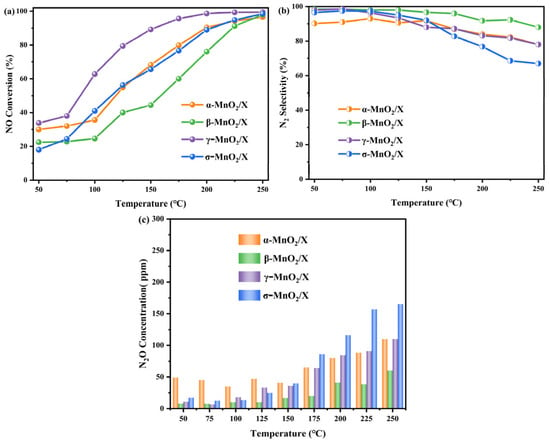
Figure 2.
NH3-SCR activity of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts at different temperature: (a) NO conversion of all the catalysts at different temperature; (b) N2 selectivity of all the catalysts at different temperature; (c) N2O concentration of all the catalysts at different temperature. Reaction conditions: 500 ppm of NO, 500 ppm of NH3, 11% O2, N2 as balance, and GHSV of 36,000 h−1.
2.3. Surface Acidity and Reducibility
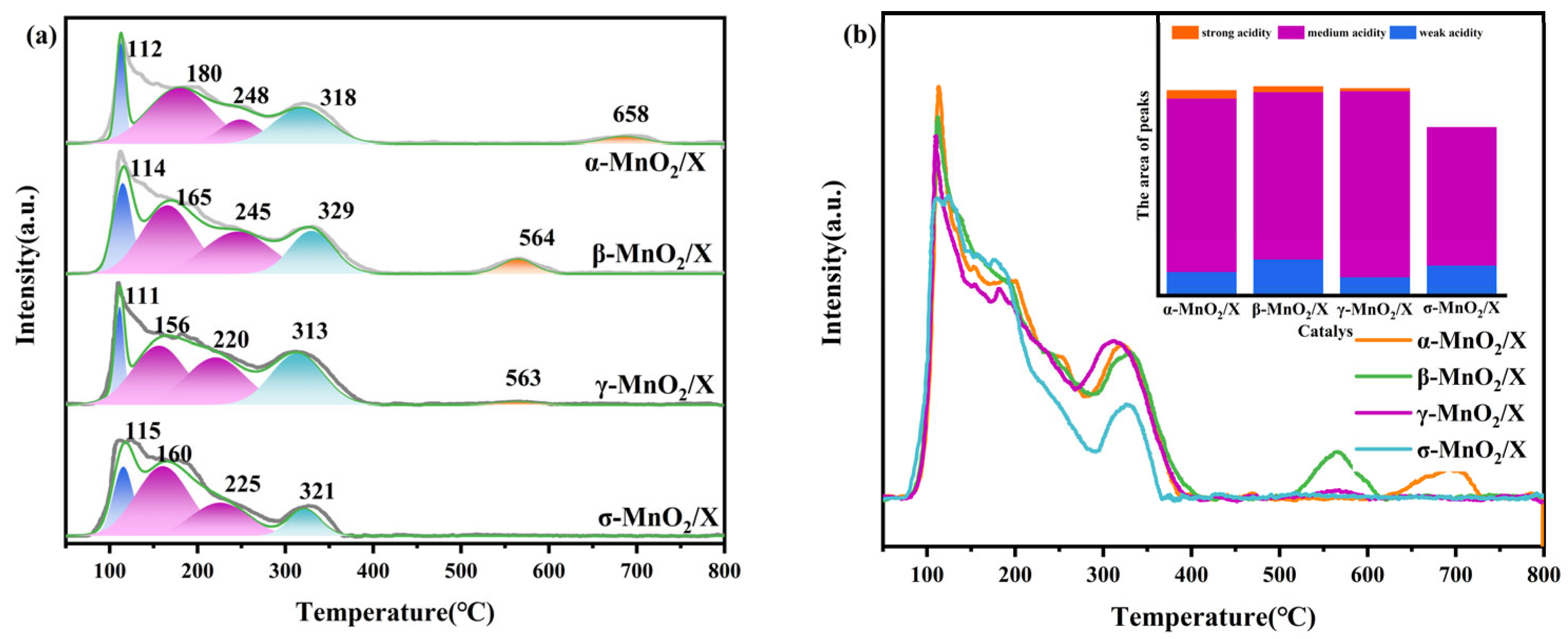
Not only did the structure of the catalysts affect its ability to act as a catalyst, but its chemical properties were also important for the SCR reaction. The surface acidity of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts was studied using NH3-TPD, and the results are displayed in Figure 3. There were several peaks at approximately 100–600 °C; among them, the peak at approximately 100 °C belonged to the weak acid sites for NH3 physical adsorption, the peaks at 150–400 °C related to the medium acid sites for NH3 chemical adsorption, and the peaks over 400 °C were assigned to NH3 adsorption on the strong acid sites [8,20,21]. The α-MnO2/X catalyst possessed the highest peak of weak acid sites, and the γ-MnO2/X catalyst had the highest peak of medium acid sites. It was observed that α-MnO2/X, β-MnO2/X, and γ-MnO2/X catalysts had stronger surface acidity than that of the σ-MnO2/X catalyst, which was correlated with the SCR activity results, indicating that the surface acidity played a significant role in SCR reaction. Furthermore, the peaks of acid sites for the γ-MnO2/X catalyst shifted to a lower temperature. It could be found that the γ-MnO2/X catalyst showed better acidity capacity than that of other catalysts, which might be one of reasons for its enhanced catalytic activity at lower temperatures.
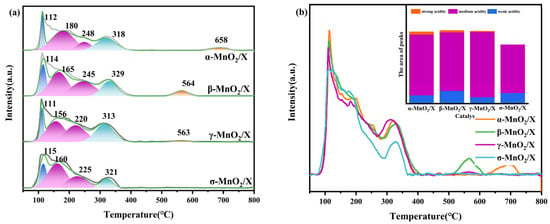
Figure 3.
(a) NH3-TPD profiles and (b) the related peaks analysis of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts, and all the curves for NH3-TPD over catalysts are shown in the same baseline, which could be a better way to compare the peaks’ intensity and position.
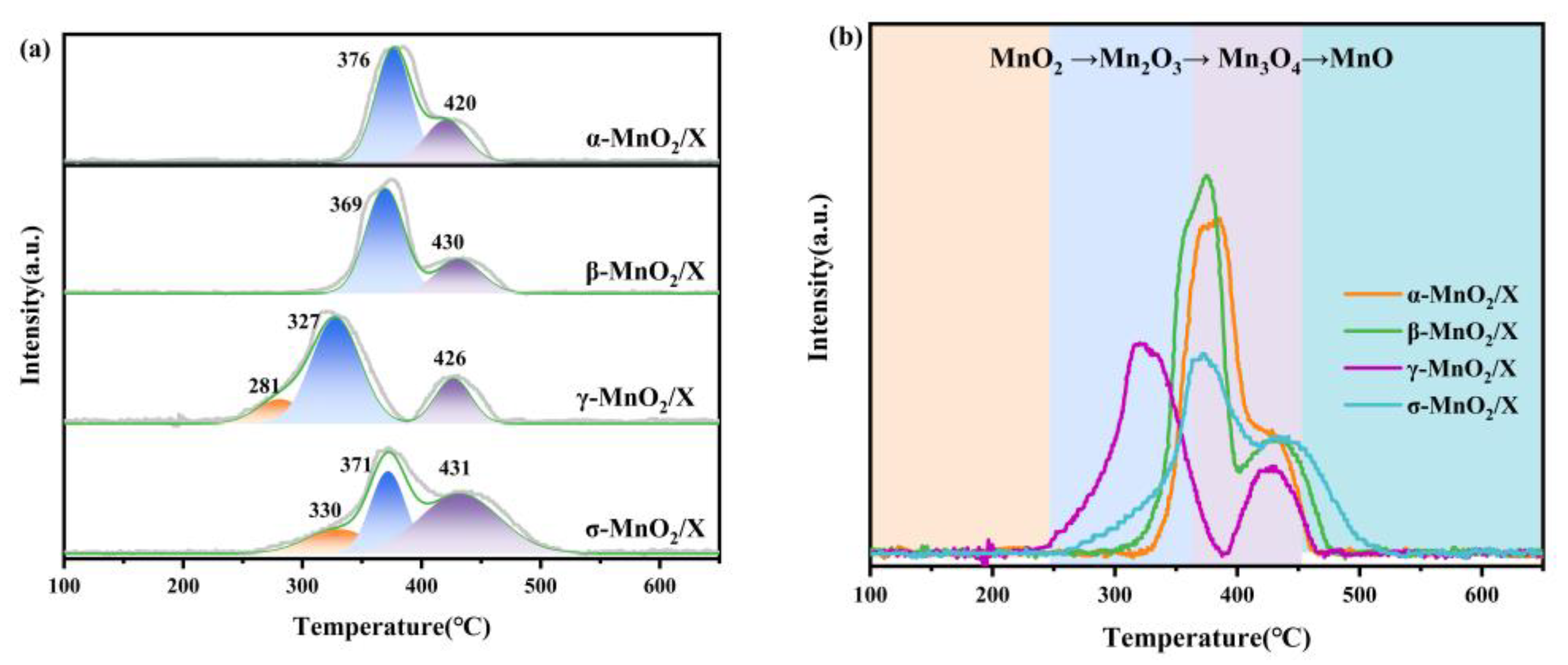
The redox capacity of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts was detected by H2-TPR, and the results are depicted in Figure 4. There were several peaks existing at 200–500 °C for all the catalysts. For the α-MnO2/X catalyst, there were two peaks at 376 °C and 420 °C, corresponding to the reduction of Mn2O3 to Mn3O4 and Mn3O4 to MnO, respectively [9,22]. As for the β-MnO2/X catalyst, two peaks appeared at 369 °C and 430 °C, also related to Mn2O3 to Mn3O4 and Mn3O4 to MnO, respectively [9,22]. The two abroad peaks on the γ-MnO2/X catalyst could be fitted into three peaks at 281 °C, 327 °C, and 426 °C, assigned to MnO2 to Mn2O3, Mn2O3 to Mn3O4, and Mn3O4 to MnO, respectively [9,19,22]. It could be found that the reduction peaks of the γ-MnO2/X catalyst had lower temperature than that of the other three catalysts, illustrating that the γ-MnO2/X catalyst was more reducible. There were also three fitting peaks at 330 °C, 371 °C, and 431 °C on the σ-MnO2/X catalyst, attributed to MnO2 to Mn2O3, Mn2O3 to Mn3O4, and Mn3O4 to MnO, respectively.
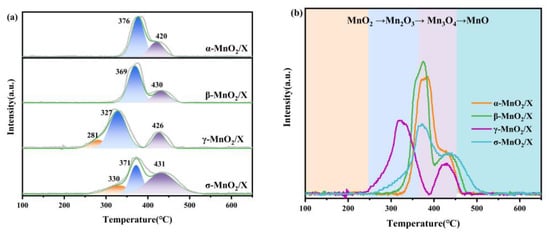
Figure 4.
(a) H2-TPR profiles and (b) related peaks analysis of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts.
2.4. In Situ DRIFTS Studies on the Catalysts
In order to further investigate the reaction of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts, the in situ DRIFTS studies were measured. Combined with the SCR activity of the catalysts, the reaction temperature was 200 °C, and the in situ DRIFTS spectra of only NH3 adsorption, reactions between NO+O2 and pre-adsorbed NH3 species, NO+O2 adsorption, reactions between NH3 and pre-adsorbed NO+O2 species, and reactions between NH3+NO+O2 were tested.
2.4.1. Reactions between NO+O2 and Pre-Adsorbed NH3 Species on the Catalysts
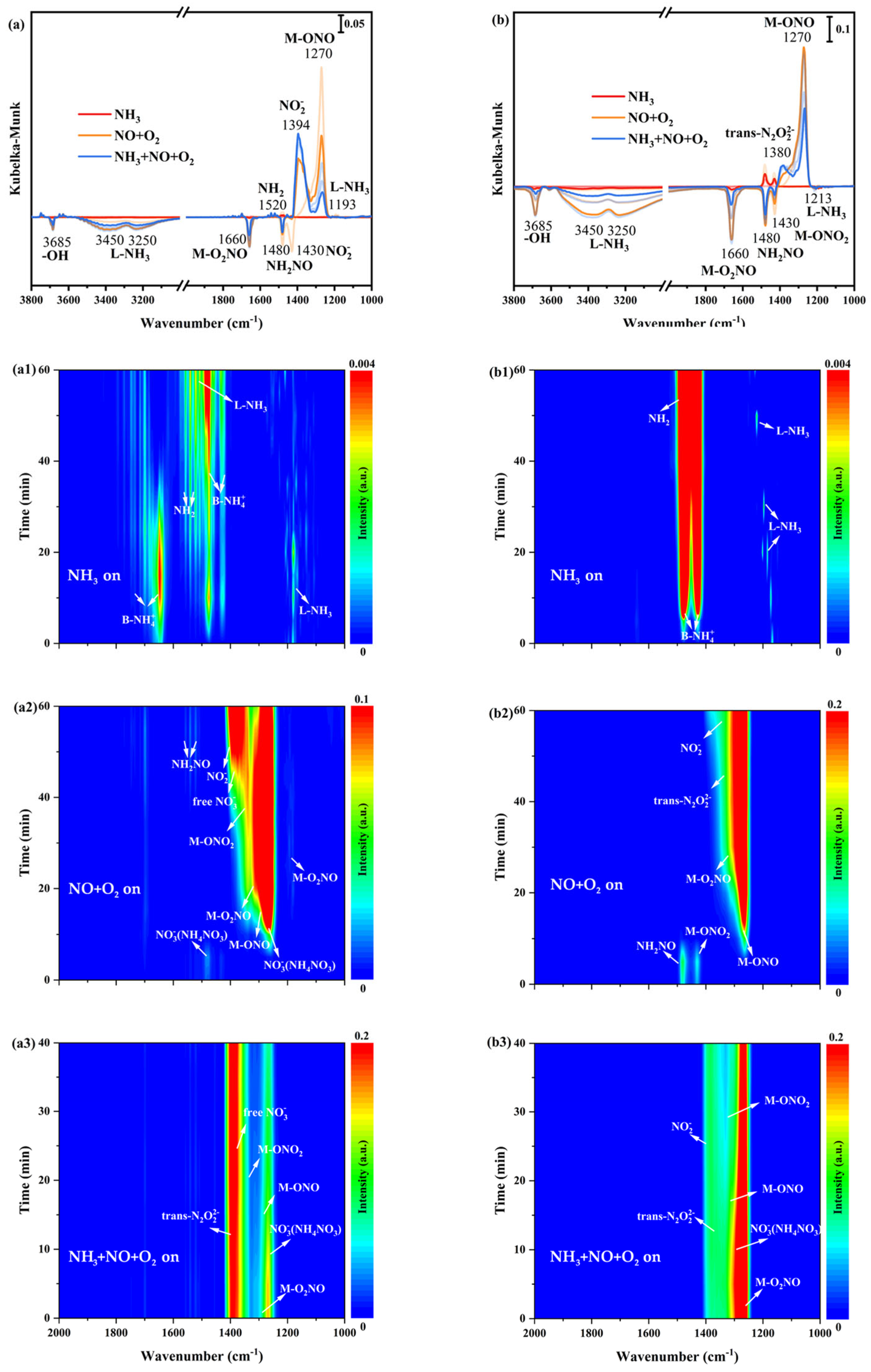
In order to research the nature of adsorbed NH3 species and some potential intermediates, in situ DRIFTS spectra of only NH3 adsorption at 200 °C on α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts were collected. From Figure 5(a1), there were several bands at 1000–2000 cm−1 on the α-MnO2/X catalyst, which were assigned to Brønsted acid sites (1640 cm−1 and 1480 cm−1), Lewis acid sites (1610 cm−1 and 1193 cm−1), and -NH2 species (1520 cm−1) [23,24,25]. It could be found that the bands’ intensity of 1640 cm−1 and 1193 cm−1 increased at first, but declined from 40 min, while the bands’ intensity of Brønsted acid sites (1480 cm−1), Lewis acid sites (1610 cm−1), and -NH2 species (1520 cm−1) increased with the increasing time of NH3 introducing. For the β-MnO2/X catalyst (in Figure 5(b1)), the bands of -NH2 species, Brønsted acid sites, and Lewis acid sites could be observed at 1520 cm−1, 1480 cm−1, and 1213 cm−1, respectively [26,27,28], and the bands intensity of -NH2 species and Brønsted acid sites became strengthened with increasing time. As for the γ-MnO2/X catalyst (in Figure 5(c1)), it also could be found that the bands of Brønsted acid sites (1640 cm−1 and 1480 cm−1), Lewis acid sites (1610 cm−1 and 1193 cm−1), and -NH2 species (1520 cm−1) existed. The bands of Brønsted acid sites (1640 cm−1 and 1480 cm−1), Lewis acid sites (1610 cm−1 and 1193 cm−1), and -NH2 species (1520 cm−1) were also detected on the σ-MnO2/X catalyst (in Figure 5(d1)) [23,24,25,26,27,28]. It could be found that the bands’ intensity of -NH2 species on β-MnO2/X was stronger than that of other catalysts, which might speed up the combination -NH2 species with NO species and reduce the byproduct of N2O generation. Moreover, the types of acid bands on the γ-MnO2/X catalyst were more than those of other catalysts, which facilitated the SCR reaction of the catalyst.
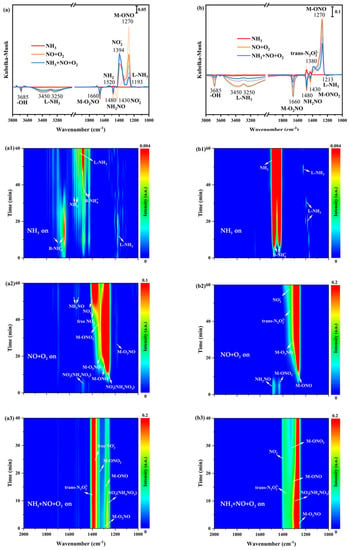
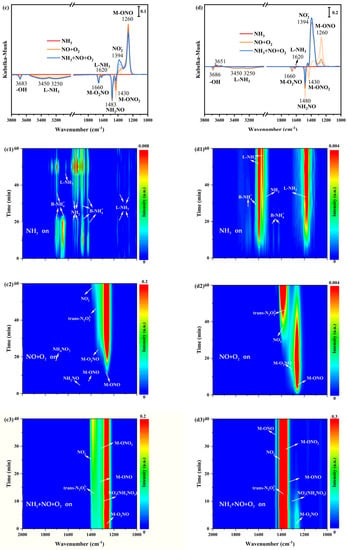
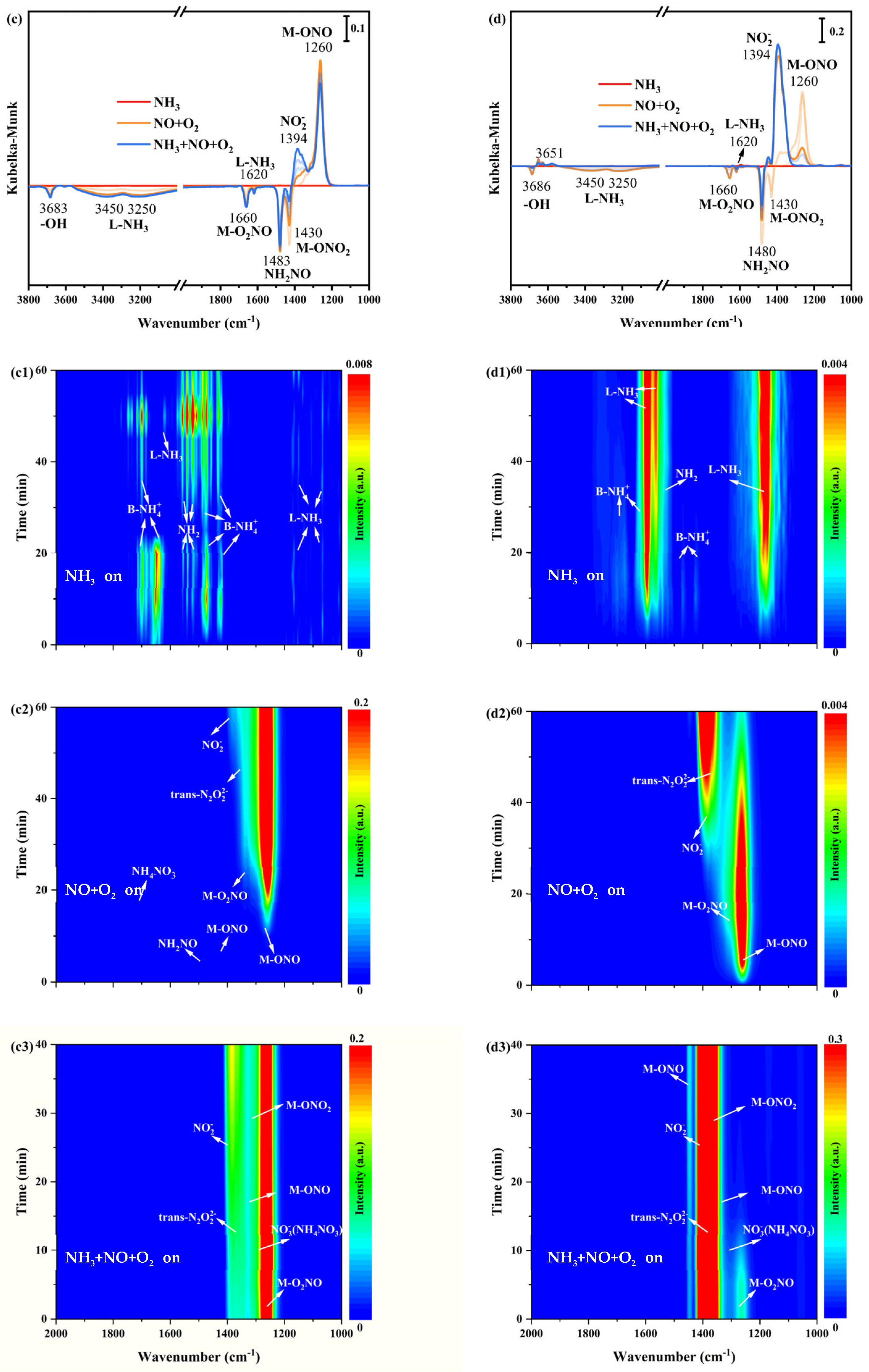
Figure 5.
In situ DRIFTS spectra of NH3 adsorption at 200 °C over (a1) α-MnO2/X, (b1) β-MnO2/X, (c1) γ-MnO2/X, and (d1) σ-MnO2/X catalysts; in situ DRIFTS spectra of NO+O2 reacted with pre-adsorbed NH3 species at 200 °C over (a2) α-MnO2/X, (b2) β-MnO2/X, (c2) γ-MnO2/X, and (d2) σ-MnO2/X catalysts; in situ DRIFTS spectra of NH3 +NO+O2 species at 200 °C over (a3) α-MnO2/X, (b3) β-MnO2/X, (c3) γ-MnO2/X, and (d3) σ-MnO2/X catalysts; the summary of in situ DRIFTS spectra of NH3 adsorption, NO+O2 reacted with pre-adsorbed NH3 species, and NH3 +NO+O2 species at 200 °C over (a) α-MnO2/X, (b) β-MnO2/X, (c) γ-MnO2/X, and (d) σ-MnO2/X catalysts; conditions: 500 ppm NH3 (when used), 500 ppm NO, 11% O2 (when used), and N2 as balance.
After NO+O2 introducing, in situ DRIFTS spectra of reactions between NO+O2 and pre-adsorbed NH3 species on catalysts were obtained. In Figure 5(a2), the bands’ intensity of the acid sites was weak and hardly found when NO+O2 was introduced on α-MnO2/X catalysts, and then some bands at 1178 cm−1, 1394 cm−1, 1400 cm−1, 1430 cm−1, 1460 cm−1, and 1660 cm−1 belonging to bridging nitrate species (M-O2NO), nitro species (M-NO2), free nitrate species, monodentate nitrate species (M-ONO2), monodentate nitrite species (M-ONO), and bidentate nitrate species (M-O2NO) were observed, respectively [29,30,31]. Meanwhile, the bands of NH2NO and NH4NO3 species were found at 1480 cm−1 and 1380 cm−1, respectively, which played an essential role in SCR reaction [8,14]. The bands’ intensity of nitrate species increased with the increasing time of NO+O2 purging. For the β-MnO2/X catalyst (Figure 5(b2)), the bands of monodentate nitrite species (M-ONO), monodentate nitrate species (M-ONO2), nitro species (M-NO2), trans-N2O22− species, bidentate nitrate species (M-O2NO), and NH2NO species were observed; moreover, the bands’ intensity of nitrate species increased. To the γ-MnO2/X catalyst (in Figure 5(c2)), the bands of the monodentate nitrite species (M-ONO), monodentate nitrate species (M-ONO2), nitro species (M-NO2), trans-N2O22− species, bidentate nitrate species (M-O2NO), and NH2NO species also could be found, and the bands’ intensity of nitrate were the most strengthened among the four catalysts [9,29,30,31]. There were bands of monodentate nitrite species (M-ONO), bidentate nitrate species (M-O2NO), nitro species (M-NO2), trans-N2O22− species on the σ-MnO2/X catalyst (Figure 5(d2)) when NO+O2 was introduced, but fewer types of nitrate species generated.
To better determine the intermediates of the SCR reaction over the four catalysts, NH3, NO, and O2 were introduced into the reaction container under 200 °C to simulate the SCR reaction process, and in situ DRIFTS spectra of NH3, NO, and O2 reaction were measured at the same time (Figure 5(a3,b3,c3,d3)). At 1200–1400 cm−1, all catalysts exhibited bands belonging to bridging nitrate species (M-O2NO), nitro species (M-NO2), free nitrate species, monodentate nitrate species (M-ONO2), monodentate nitrite species (M-ONO), and bidentate nitrate species (M-O2NO). All the intensities of nitrate species’ bands strengthened when NH3, NO, and O2 were introduced, Additionally, it could be discovered that some negative bands appeared when NH3, NO, and O2 reacted, such as bridging nitrate species and NH2NO species, indicating that the SCR reaction occurred on the catalyst surface. At the same time, the N2 and H2O were produced, which followed both the Eley–Rideal (E-R) and Langmuir–Hinshelwood (L-H) mechanism [32,33].
2.4.2. Reactions between NH3 and Pre-Adsorbed NO+O2 Species on Catalysts
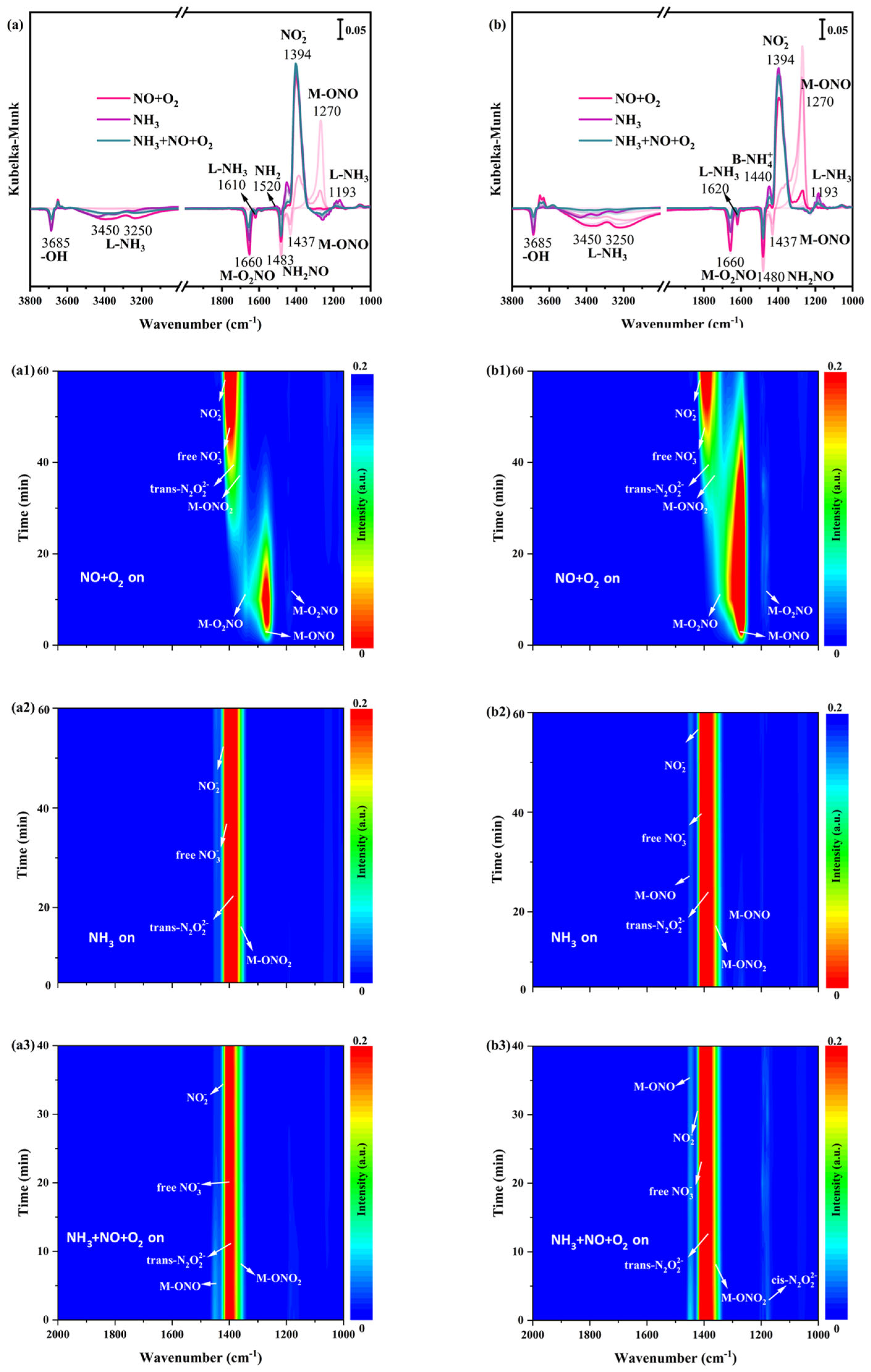
In situ DRIFTS spectra of NO+O2 adsorption at 200 °C on α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts were detected. Some bands were observed at 1178 cm−1, 1394 cm−1, 1400 cm−1, 1430 cm−1, 1460 cm−1, and 1660 cm−1 over four catalysts (Figure 6(a1,b1,c1,d1)), which were correlated to bridging nitrate species (M-O2NO), nitro species (M-NO2), free nitrate species, monodentate nitrate species (M-ONO2), monodentate nitrite species (M-ONO), trans-N2O22− species, and bidentate nitrate species (M-O2NO), respectively [9,29,30,31]. The intensity of bands belonged to monodentate nitrite species and bridging nitrate species at 1200–1300 cm−1 over α-MnO2/X, β-MnO2/X, and σ-MnO2/X catalysts strengthened in the first 20 min, and then decreased gradually. Meanwhile, bands of nitro species, trans-N2O22− species, free nitrate specie, and bidentate nitrate species at 1350–1450 cm−1 appeared at approximately 15 min and the intensity of these bands increased with NO+O2 adsorption. However, for the γ-MnO2/X catalyst (Figure 6(c1)), the intensity of the bands for nitrate species increased with the introduction of NO+O2. Consequently, the nitrate species on the γ-MnO2/X catalyst was more stable than those of the other three catalysts.
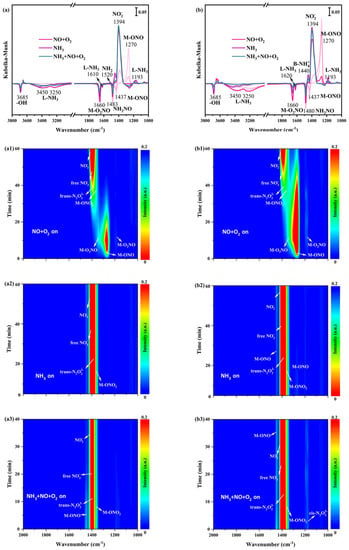
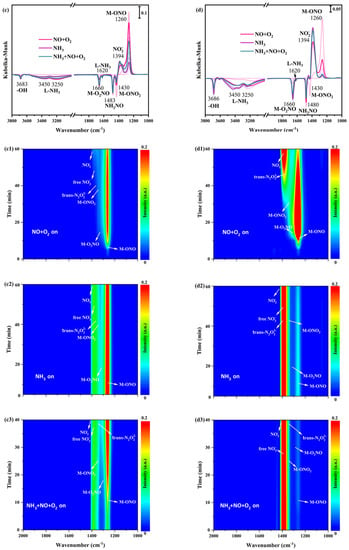
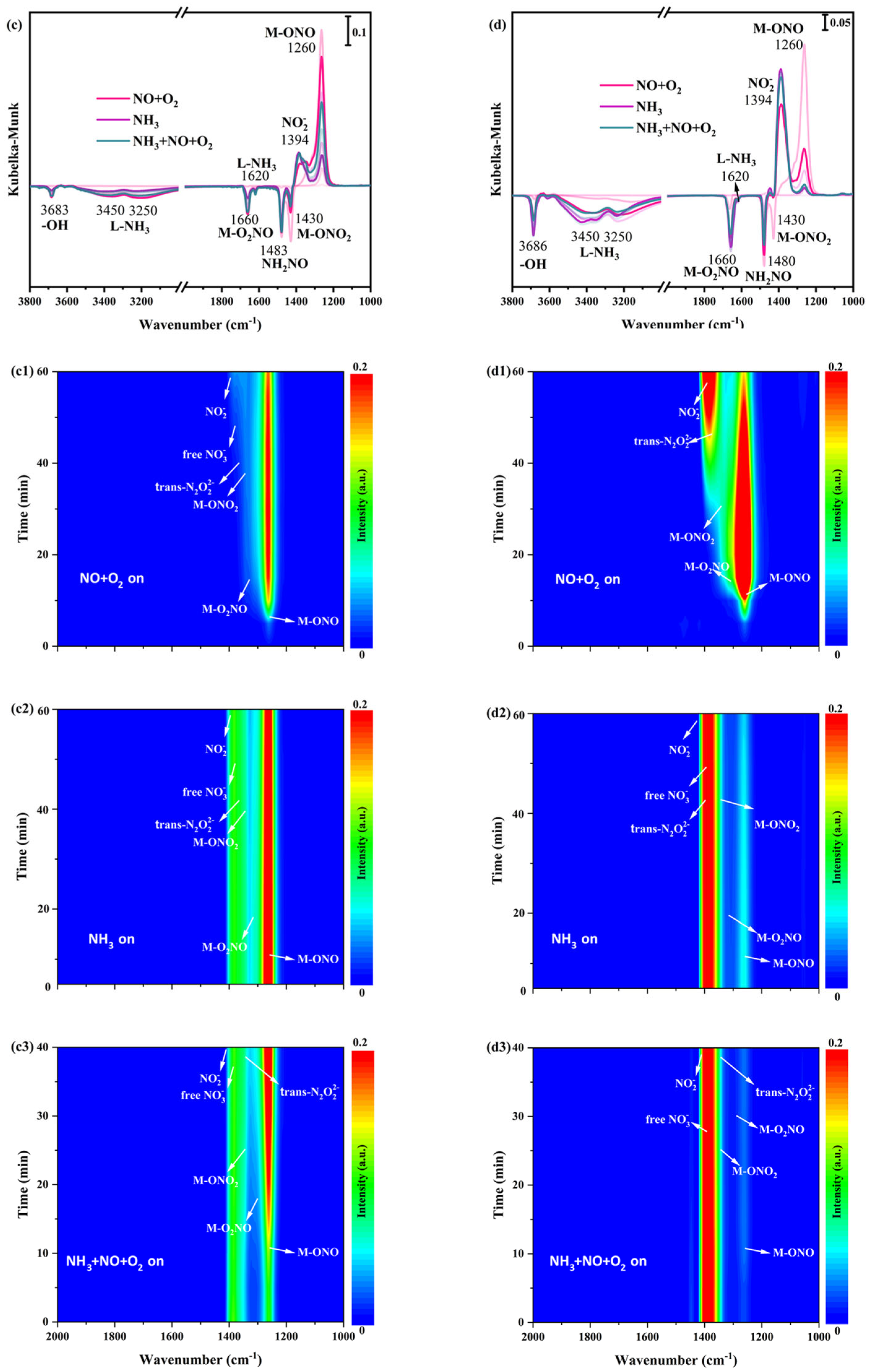
Figure 6.
In situ DRIFTS spectra of NO+O2 adsorption at 200 °C over (a1) α-MnO2/X, (b1) β-MnO2/X, (c1) γ-MnO2/X, and (d1) σ-MnO2/X catalysts; in situ DRIFTS spectra of NH3 reacted with pre-adsorbed NO+O2 species at 200 °C over (a2) α-MnO2/X, (b2) β-MnO2/X, (c2) γ-MnO2/X, and (d2) σ-MnO2/X catalysts; in situ DRIFTS spectra of NO+O2 +NH3 species at 200 °C over (a3) α-MnO2/X, (b3) β-MnO2/X, (c3) γ-MnO2/X, and (d3) σ-MnO2/X catalysts; the summary of in situ DRIFTS spectra of NO+O2 adsorption, NH3 reacted with pre-adsorbed NO+O2 species, and NH3 +NO+O2 species at 200 °C over (a) α-MnO2/X, (b) β-MnO2/X, (c) γ-MnO2/X, and (d) σ-MnO2/X catalysts; conditions: 500 ppm NH3 (when used), 500 ppm NO, 11% O2 (when used), and N2 as balance.
After NO+O2 adsorption for 60 min and N2 purging for 10 min, NH3 was introduced and reacted with the pre-adsorbed NO+O2 species at 200 °C on α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts, and the in situ DRIFTS spectra were collected. There was a broad band at 1350–1450 cm−1 on α-MnO2/X, β-MnO2/X, and σ-MnO2/X catalysts (Figure 6(a2,b2,d2)), which was assigned to nitro species, trans-N2O22− species, free nitrate specie, and bidentate nitrate species [30,31,34]. Meanwhile, the broad band on the γ-MnO2/X catalyst was at 1200–1450 cm−1, attributed to bridging nitrate species (M-O2NO), nitro species (M-NO2), free nitrate species, monodentate nitrate species (M-ONO2), monodentate nitrite species (M-ONO), trans-N2O22− species, and bidentate nitrate species (M-O2NO), which indicated that the types of nitrate species on the γ-MnO2/X catalyst were more than that of the other three catalysts. Moreover, two negative bands could be observed at 1660 and 1483 cm−1 on the four catalysts, owing to the consumption of bridging nitrate species and NH2NO species. When NH3, NO, and O2 were introduced over α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts (Figure 6(a1,b1,c1,d1)), the intensity and position of bands on α-MnO2/X, β-MnO2/X, and σ-MnO2/X catalysts were nearly unchanged. While the intensity of bands varied on the γ-MnO2/X catalyst, the intensity of some bands at approximately 1300 cm−1 become weak in the first 20 min and then increased gradually over 20 min. Two negative bands of the bridging nitrate species and NH2NO species were detected, suggesting the effective reaction pathway of the SCR process [35,36].
3. Discussion
All the catalysts had regular octahedral zeolite X structures; α-MnO2/X and σ-MnO2/X catalysts exhibited Mn elements aggregation, whereas β-MnO2/X and γ-MnO2/X catalysts had uniform Mn element distribution. This suggested that the aggregation of α-MnO2 or σ-MnO2 species on the zeolite X support could reduce the active sites for the SCR reaction, resulting in the low SCR activity. For β-MnO2/X and γ-MnO2/X catalysts, the distribution of β-MnO2 and γ-MnO2 species on the support could provide an abundance of active sites for reacted gas adsorption and the SCR reaction. From the NH3-TPD results, the α-MnO2/X catalyst possessed the highest peak of weak acid sites, and the γ-MnO2/X catalyst had the highest peak of medium acid sites, while the σ-MnO2/X catalyst had the lower surface acidity, which was associated with the lowest SCR activity of the σ-MnO2/X catalyst among the four catalysts. All the four catalysts had four peaks from approximately 250 °C to 500 °C, which belonged to MnO2 to Mn2O3, Mn2O3 to Mn3O4, and Mn3O4 to MnO, respectively. It was noted that the reduction peaks of the γ-MnO2/X catalyst had lower temperature than that of the other three catalysts, illustrating that the γ-MnO2/X catalyst had a stronger reduction capacity.
Through in situ DRIFTS spectra of NH3 adsorption, the bands of Brønsted acid sites, Lewis acid sites, and -NH2 species were observed on α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts, respectively, which followed reaction (1)–(3). When NO+O2 was introduced, following reaction (4)–(6), especially, the bands intensity of -NH2 species on β-MnO2/X was stronger than that of other catalysts, which might have speeded up the combination -NH2 species with NO species and reduced the byproduct of N2O generation, shown as reaction (7). Moreover, the varieties of acid bands on the γ-MnO2/X catalyst were more than those of other catalysts which facilitated the SCR reaction of the catalyst after NO+O2 addition, and the bands’ intensity of nitrate species on the γ-MnO2/X catalyst were the most strengthened among the four catalysts. However, the σ-MnO2/X catalyst produced fewer types of nitrate species when NO+O2 was added. When NH3, NO, and O2 were introduced, all the intensities of nitrate species bands strengthened for all four catalysts. In addition, some negative bands (bridging nitrate species and NH2NO species) appeared when NH3, NO, and O2 reacted, following reaction (8)–(11), which indicated that the SCR reaction occurred on the catalyst surface; the N2 and H2O were produced at the same time, following both the Eley-Rideal (E-R) and Langmuir–Hinshelwood (L-H) mechanism. As for NO+O2 adsorption, bridging nitrate species (M-O2NO), nitro species (M-NO2), free nitrate species, monodentate nitrate species (M-ONO2), monodentate nitrite species (M-ONO), trans-N2O22− species, and bidentate nitrate species (M-O2NO) were detected on the four catalysts (seen as reaction (4)–(6)); particularly, the nitrate species on the γ-MnO2/X catalyst was more stable than those of the other three catalysts. When NH3 was introduced and reacted with pre-adsorbed NO+O2 species, two negative bands could be observed at 1660 and 1483 cm−1 on the four catalysts, due to the consumption of bridging nitrate species and NH2NO species, and the types of nitrate species on the γ-MnO2/X catalyst were more than that of the other three catalysts. Two negative bands of the bridging nitrate species and NH2NO species were also detected when NH3, NO, and O2 were introduced over the γ-MnO2/X catalyst, suggesting that the effective reaction pathway of the SCR process happened, as shown in reaction (7)–(11).
4. Materials and Methods
4.1. Catalyst Preparation
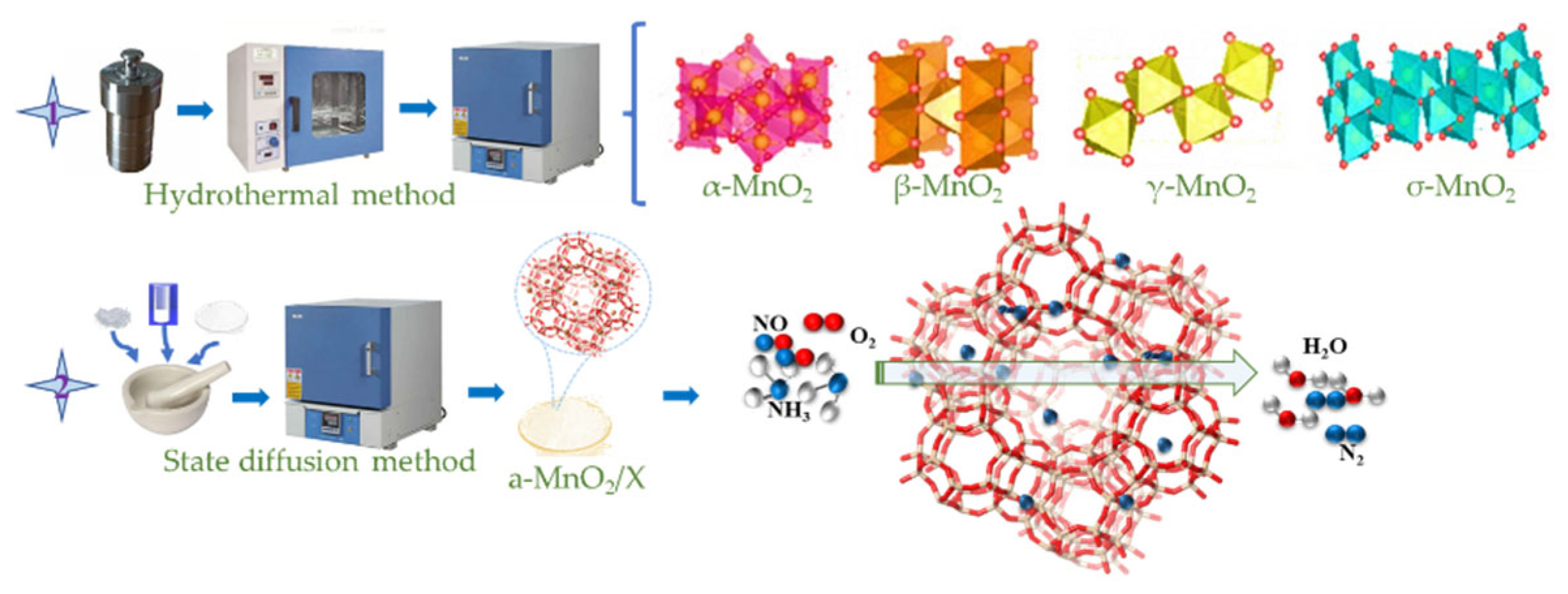
The different crystal phases of MnO2 (α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2) were prepared by a redox hydrothermal method as in our previous studies [11,22]. A certain amount of KMnO4 and MnSO4·H2O, MnSO4·H2O and (NH4)2S2O8, MnSO4·H2O and (NH4)2S2O8, and MnSO4·H2O and KMnO4 were mixed in 72 mL H2O, respectively, and then transferred to Teflon-lined stainless-steel autoclaves under 160 °C for 12 h, 160 °C for 12 h, 90 °C for 24 h, and 180 °C for 24 h, respectively, and finally washed by deionized water to obtain α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2, respectively.
Different crystal phases of MnO2/X catalysts were synthesized via a solid-state diffusion method and high-heat treatment. A certain amount of α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2, and 1 g of zeolite X and 10 mL of anhydrous ethanol were mixed in an agate quartz mortar. After ultrasonic grinding until the anhydrous ethanol volatilized, the mixture dried at 80 °C for 12 h and then calcined at 300 °C for 2 h in a muffle furnace, finally obtained α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts, respectively, and the synthesis procedures and experimental steps of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts are shown in Figure 7.
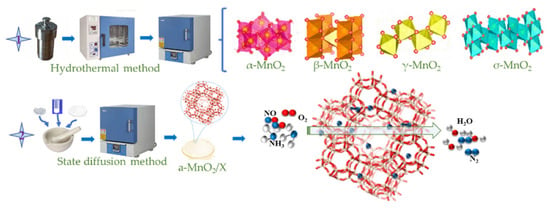
Figure 7.
The synthesis procedures and experimental steps of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts.
4.2. Catalytic Activity Tests
NH3-SCR catalytic performance of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts were measured by a catalytic testing system under the following conditions: 0.5 mL of sample (40–60 mesh), 500 ppm of NH3, 500 ppm of NO, 11% O2, GHSV of 36,000 h−1, and N2 as balance. The concentration of outlet gases was tested by the flue gas analyzer (Thermo Scientific, Antaris IGS). NO conversion and N2 selectivity of all catalysts were calculated as Equations (12) and (13).
4.3. Catalysts Characterization
Scanning electron microscope (SEM) tests were used to investigate the surface microstructure of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts via the Thermo Scientific Quattro SEM. The distribution of surface elements was measured by energy dispersive X-ray spectrometry (EDS) mapping.
NH3-TPD measurements were used to investigate the surface acidity of all catalysts on a ChemBET Pulsar TPR/TPD. For each test, 0.15 g of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts were carried into the U-shaped quartz tube, pretreated under helium atmosphere for 5 min, then heated at 300 °C for 30 min, followed by cooling to 60 °C, and purged by helium atmosphere for 60 min. After that, the sample was exposed under NH3/Ar atmosphere for 15 min, and then heated to 650 °C at a constant heating rate of 5 °C/min. At the same time, TPR analysis was performed and the TCD signal was recorded by the detector. H2-TPR was conducted, being similar to that of NH3-TPD. Firstly, the sample was exposed under H2/Ar atmosphere for 15 min, and then heated to 800 °C at a constant heating rate of 5 °C/min.
In situ DRIFTS tests were measured on a Thermo Fisher Nicolet iS50 spectrometer. A certain weight of α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts (80–100 mesh) was carried into solid reaction in situ cell, respectively, and all catalysts were pretreated at 300 °C for 30 min under N2 atmosphere (100 mL/min of flow rate). After cooling to 200 °C, the background spectrum was collected. Then, α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts were exposed under the gas atmosphere in turn: 500 ppm of NH3 for 60 min, N2 atmosphere for 10 min, 500 ppm of NO2, 11 vol% O2, and N2 for 60 min, and 500 ppm of NH3, 500 ppm of NO2, 11 vol% O2, and N2 for 40 min, and finally flushed under N2 gas for 60 min, respectively. At the same time, the spectrum of all the catalysts were recorded by the instrument. All the catalysts were also exposed under the gas atmosphere in turn: 500 ppm of NO2 and 11 vol% O2 for 60 min, N2 atmosphere for 10 min, 500 ppm of NH3 for 60 min, 500 ppm of NO2 and 11 vol% O2 for 40 min, finally flushed under N2 gas for 60 min, respectively, and the related spectra were collected.
5. Conclusions
In this study, a series of zeolite-X-supported different crystal phases of MnO2 (α-MnO2, β-MnO2, γ-MnO2, and σ-MnO2) catalysts were synthesized using a solid-state diffusion method and their low-temperature NH3-SCR performance was evaluated. The results showed that the α-MnO2/X, β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts had the regular octahedral zeolite X structures and different crystal types of MnO2 on the supported surface. Furthermore, the γ-MnO2/X catalyst had the highest NO conversion over the whole reaction temperature range. β-MnO2/X, γ-MnO2/X, and σ-MnO2/X catalysts had nearly 100% of N2 selectivity, while the α-MnO2/X catalyst had the lowest N2 selectivity (approximately 90%) below 125 °C. Furthermore, the γ-MnO2/X catalyst had better acidity capacity and was more reducible than the other three catalysts via NH3-TPD and H2-TPR tests. From the in situ DRIFTS study, all the catalysts both followed E-R and L-H mechanism, and the acid sites and nitrate species on the γ-MnO2/X catalyst were more than that of the other three catalysts, which enhanced the SCR reaction.
Author Contributions
L.C.: conceptualization, methodology, writing—original draft, investigation, formal analysis; S.R.: conceptualization, supervision, methodology, resources, funding acquisition, writing—review and editing, resources; T.C.: investigation, formal analysis; X.L.: validation, writing—review and editing; M.W.: investigation, formal analysis; Z.C.: investigation, formal analysis, validation; Q.L.: conceptualization, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52174298.
Data Availability Statement
We are glad to share our research data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, S.; Xia, Z.; Zou, Y.; Zhang, M.; Qu, Y. Spatial intimacy of binary active-sites for selective sequential hydrogenation-condensation of nitriles into secondary imines. Nat. Commun. 2021, 12, 3382. [Google Scholar] [CrossRef] [PubMed]
- Wuebbles, D.J. Atmosphere. Nitrous oxide: No laughing matter. Science 2009, 326, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Anenberg, S.C.; Miller, J.; Minjares, R.; Du, L.; Henze, D.K.; Lacey, F.; Malley, C.S.; Emberson, L.; Franco, V.; Klimont, Z.; et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 2017, 545, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Liu, X.; Chen, J.; Dong, Y.; Tang, X.; Chen, Y. Single-atom catalysts reveal the dinuclear characteristic of active sites in NO selective reduction with NH3. Nat. Commun. 2020, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Inomata, Y.; Kubota, H.; Hata, S.; Kiyonaga, E.; Morita, K.; Yoshida, K.; Sakaguchi, N.; Toyao, T.; Shimizu, K.I.; Ishikawa, S.; et al. Bulk tungsten-substituted vanadium oxide for low-temperature NOx removal in the presence of water. Nat. Commun. 2021, 12, 557. [Google Scholar] [CrossRef]
- Kang, K.; Yao, X.; Huang, Y.; Cao, J.; Rong, J.; Zhao, W.; Luo, W.; Chen, Y. Insights into the co-doping effect of Fe3+ and Zr4+ on the anti-K performance of CeTiOx catalyst for NH3-SCR reaction. J. Hazard. Mater. 2021, 416, 125821. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, D.; Yu, G.; Xu, R.; Dai, C.; Liu, N.; Wang, N.; Chen, B. N2O Catalytic Decomposition and NH3-SCR Coupling Reactions over Fe-SSZ-13 Catalyst: Mechanisms and Interactions Unraveling via Experiments and DFT Calculations. ACS Catal. 2022, 13, 934–947. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Jiang, Y.; Liu, L.; Wang, M.; Yang, J.; Chen, Z.; Liu, W.; Liu, Q. Effect of Mn and Ce oxides on low-temperature NH3-SCR performance over blast furnace slag-derived zeolite X supported catalysts. Fuel 2022, 320, 123969. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhou, Y.; Su, Z.; Yao, L.; Cao, J.; Jiang, L.; Hu, G.; Kong, M.; Yang, J.; et al. In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3–SCR of NO. Chem. Eng. J. 2020, 397, 125446. [Google Scholar] [CrossRef]
- Yang, W.; Su, Z.a.; Xu, Z.; Yang, W.; Peng, Y.; Li, J. Comparative study of α-, β-, γ- and δ-MnO2 on toluene oxidation: Oxygen vacancies and reaction intermediates. Appl. Catal. B 2020, 260, 118150. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Su, B.; Zhou, Y.; Hu, G.; Jiang, L.; Cao, J.; Liu, W.; Yao, L.; Kong, M.; et al. Insight into N2O formation over different crystal phases of MnO2 during low-temperature NH3–SCR of NO. Catal. Lett. 2021, 151, 2964–2971. [Google Scholar] [CrossRef]
- Guo, J.; Gan, F.; Zhao, Y.; He, J.; Wang, B.; Gao, T.; Jiang, X.; Ma, S. Revealing the crystal facet effect on N(2)O formation during the NH(3)-SCR over alpha-MnO(2) catalysts. RSC Adv. 2023, 13, 4032–4039. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Li, K.; Yang, W.; Chen, J.; Peng, Y.; Li, J. Core-shell-like structured α-MnO2@CeO2 catalyst for selective catalytic reduction of NO: Promoted activity and SO2 tolerance. Chem. Eng. J. 2020, 391, 123473. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Xing, X.; Yang, J.; Yang, J.; Wang, M.; Chen, Z.; Liu, Q. Low-Cost CuX Catalyst from Blast Furnace Slag Waste for Low-Temperature NH3-SCR: Nature of Cu Active Sites and Influence of SO2/H2O. ACS Sustain. Chem. Eng. 2022, 10, 7739–7751. [Google Scholar] [CrossRef]
- Chen, Z.; Bian, C.; Guo, Y.; Pang, L.; Li, T. Efficient strategy to regenerate phosphorus-poisoned Cu-SSZ-13 catalysts for the NH3-SCR of NOx: The deactivation and promotion mechanism of phosphorus. ACS Catal. 2021, 11, 12963–12976. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Janssens, T.V.W.; Vennestrøm, P.N.R.; Jansson, J.; Skoglundh, M.; Grönbeck, H. First-principles microkinetic model for low-temperature NH3-assisted selective catalytic reduction of NO over Cu-CHA. ACS Catal. 2021, 11, 14395–14407. [Google Scholar] [CrossRef]
- Peng, H.; Dong, T.; Yang, S.; Chen, H.; Yang, Z.; Liu, W.; He, C.; Wu, P.; Tian, J.; Peng, Y.; et al. Intra-crystalline mesoporous zeolite encapsulation-derived thermally robust metal nanocatalyst in deep oxidation of light alkanes. Nat. Commun. 2022, 13, 295. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Zhao, Z.; Liao, J.; Chen, C.; Li, Q. Recent progress of metal-exchanged zeolites for selective catalytic reduction of NOx with NH3 in diesel exhaust. Fuel 2021, 305, 121482. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Liu, L.; Su, B.; Yang, J.; Chen, Z.; Wang, M.; Liu, Q. Catalytic performance over Mn-Ce catalysts for NH3-SCR of NO at low temperature: Different zeolite supports. J. Environ. Chem. Eng. 2022, 10, 107167. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Liu, Y.; Guo, G.; Dai, H.; Cui, S.; Deng, J. Support promotion effect on the SO2 and K(+) co-poisoning resistance of MnO2/TiO2 for NH3-SCR of NO. J. Hazard Mater. 2021, 416, 126117. [Google Scholar] [CrossRef]
- Xue, H.; Guo, X.; Meng, T.; Mao, D.; Ma, Z. Poisoning effect of K with respect to Cu/ZSM-5 used for NO reduction. Colloid Interface Sci. Commun. 2021, 44, 100465. [Google Scholar] [CrossRef]
- Chen, L.; Ren, S.; Xing, X.D.; Yang, J.; Li, J.L.; Yang, J.; Liu, Q.C. Effect of MnO2 crystal types on CeO2@MnO2 oxides catalysts for low-temperature NH3-SCR. J. Environ. Chem. Eng. 2022, 10, 108239. [Google Scholar] [CrossRef]
- Hu, W.; Iacobone, U.; Gramigni, F.; Zhang, Y.; Wang, X.; Liu, S.; Zheng, C.; Nova, I.; Gao, X.; Tronconi, E. Unraveling the Hydrolysis of Z2Cu2+ to ZCu2+(OH)− and Its Consequences for the Low-Temperature Selective Catalytic Reduction of NO on Cu-CHA Catalysts. ACS Catal. 2021, 11, 11616–11625. [Google Scholar] [CrossRef]
- Hu, W.; Gramigni, F.; Nasello, N.D.; Usberti, N.; Iacobone, U.; Liu, S.; Nova, I.; Gao, X.; Tronconi, E. Dynamic Binuclear CuII Sites in the Reduction Half-Cycle of Low-Temperature NH3–SCR over Cu-CHA Catalysts. ACS Catal. 2022, 12, 5263–5274. [Google Scholar] [CrossRef]
- Liu, C.; Malta, G.; Kubota, H.; Toyao, T.; Maeno, Z.; Shimizu, K.-i. Mechanism of NH3–Selective Catalytic Reduction (SCR) of NO/NO2 (Fast SCR) over Cu-CHA Zeolites Studied by In Situ/Operando Infrared Spectroscopy and Density Functional Theory. J. Phys. Chem. C 2021, 125, 21975–21987. [Google Scholar] [CrossRef]
- Song, I.; Lee, H.; Jeon, S.W.; Kim, D.H. Understanding the dynamic behavior of acid sites on TiO2-supported vanadia catalysts via operando DRIFTS under SCR-relevant conditions. J. Catal. 2020, 382, 269–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Li, K.; Liu, S.; Chen, J.; Li, J.; Gao, F.; Peden, C.H.F. Using Transient FTIR Spectroscopy to Probe Active Sites and Reaction Intermediates for Selective Catalytic Reduction of NO on Cu/SSZ-13 Catalysts. ACS Catal. 2019, 9, 6137–6145. [Google Scholar] [CrossRef]
- Marberger, A.; Ferri, D.; Elsener, M.; Krocher, O. The significance of Lewis acid sites for the selective catalytic reduction of nitricoxide on vanadium-based catalysts. Angew. Chem. Int. Ed. Engl. 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Negri, C.; Hammershoi, P.S.; Janssens, T.V.W.; Beato, P.; Berlier, G.; Bordiga, S. Investigating the low temperature formation of Cu(II) -(N,O) species on Cu-CHA zeolites for the selective catalytic reduction of NOx. Chemistry 2018, 24, 12044–12053. [Google Scholar] [CrossRef]
- Xie, S.; Li, L.; Jin, L.; Wu, Y.; Liu, H.; Qin, Q.; Wei, X.; Liu, J.; Dong, L.; Li, B. Low temperature high activity of M (M = Ce, Fe, Co, Ni) doped M-Mn/TiO2 catalysts for NH3-SCR and in situ DRIFTS for investigating the reaction mechanism. Appl. Surf. Sci. 2020, 515, 146014. [Google Scholar] [CrossRef]
- Yu, C.; Huang, B.; Dong, L.; Chen, F.; Liu, X. In situ FT-IR study of highly dispersed MnOx /SAPO-34 catalyst for low-temperature selective catalytic reduction of NOx by NH3. Catal. Today 2017, 281, 610–620. [Google Scholar] [CrossRef]
- Chen, Z.C.; Guo, R.T.; Ren, S.; Chen, L.; Li, X.D.; Wang, M.M. Comparative analysis of the dual origins of the N2O byproduct on MnOx, FeOx, and MnFeOx sphere catalysts for a low-temperature SCR of NO with NH3. J. Mater. Chem. A 2022, 10, 21474–21491. [Google Scholar] [CrossRef]
- Xie, R.; Ma, L.; Li, Z.; Qu, Z.; Yan, N.; Li, J. Review of Sulfur Promotion Effects on Metal Oxide Catalysts for NOx Emission Control. ACS Catal. 2021, 11, 13119–13139. [Google Scholar] [CrossRef]
- Wang, H.; Jia, J.; Liu, S.; Chen, H.; Wei, Y.; Wang, Z.; Zheng, L.; Wang, Z.; Zhang, R. Highly efficient NO abatement over Cu-ZSM-5 with special nanosheet features. Env. Sci Technol 2021, 55, 5422–5434. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Z.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. Understanding the role of redox properties and NO adsorption over MnFeOx for NH3-SCR. Catal. Sci. Technol. 2022, 12, 2030–2041. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, S.; Liao, Y.; Xiao, X.; Qi, F.; Peng, Y.; Fu, Y.; Shan, W.; Li, J. Mechanism of N2O formation during the low-temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel. Environ. Sci. Technol. 2014, 48, 10354–10362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).