Selective Oxidation of Tetrahydrofuran to Gamma-Butyrolactone over Spinel ZnFe2O4 Nanoparticle Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
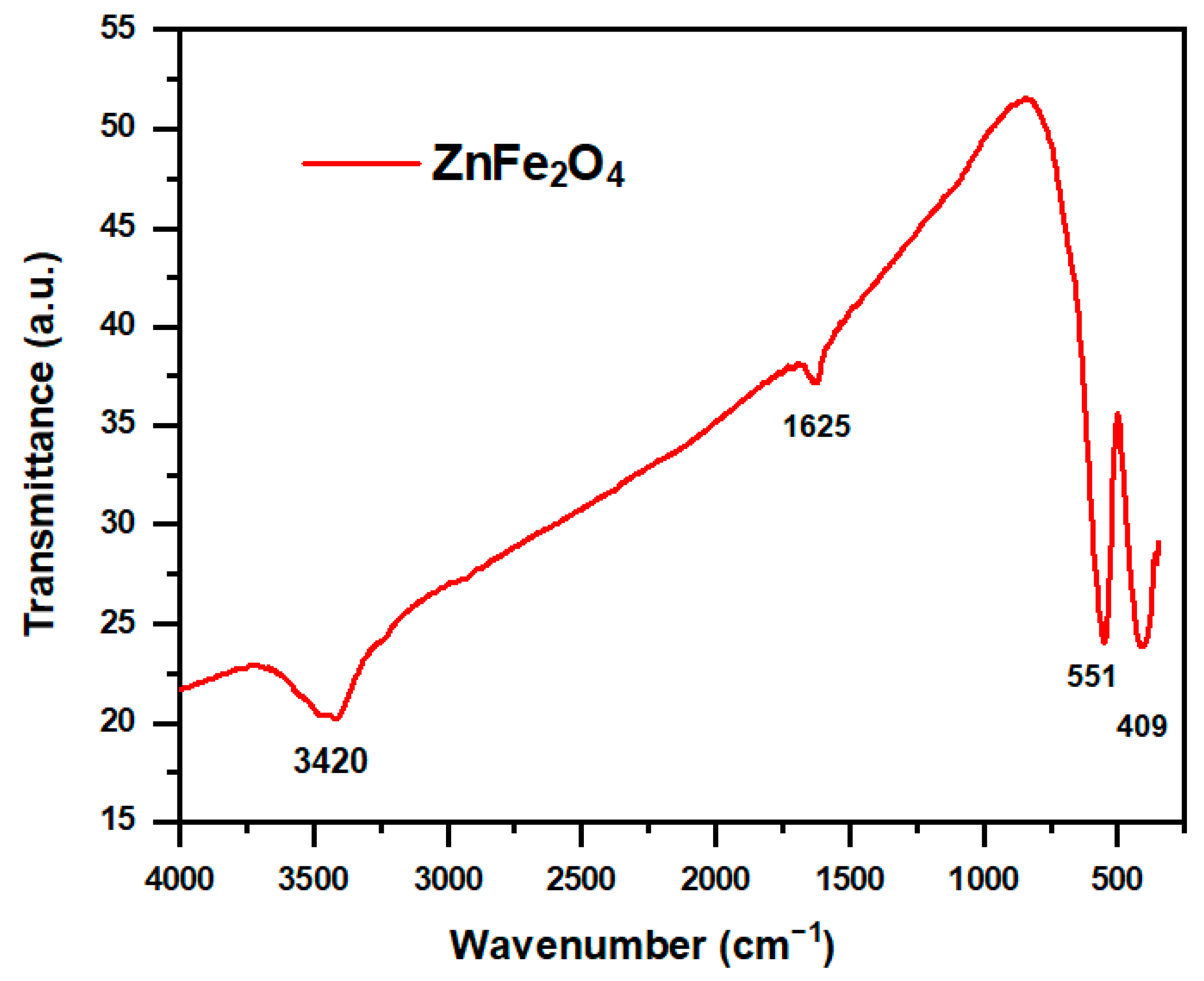
2.1.1. FTIR Analysis
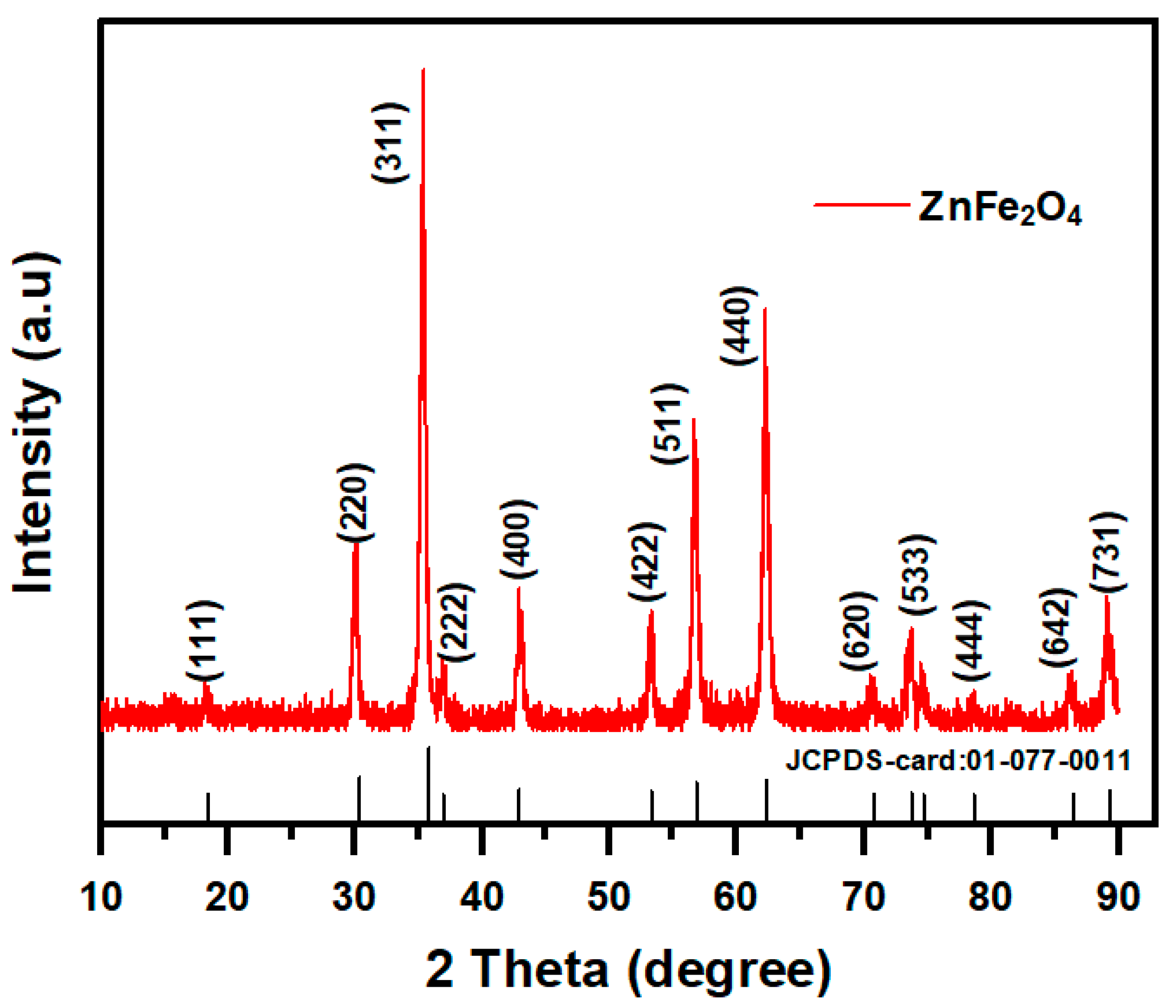
2.1.2. XRD Analysis
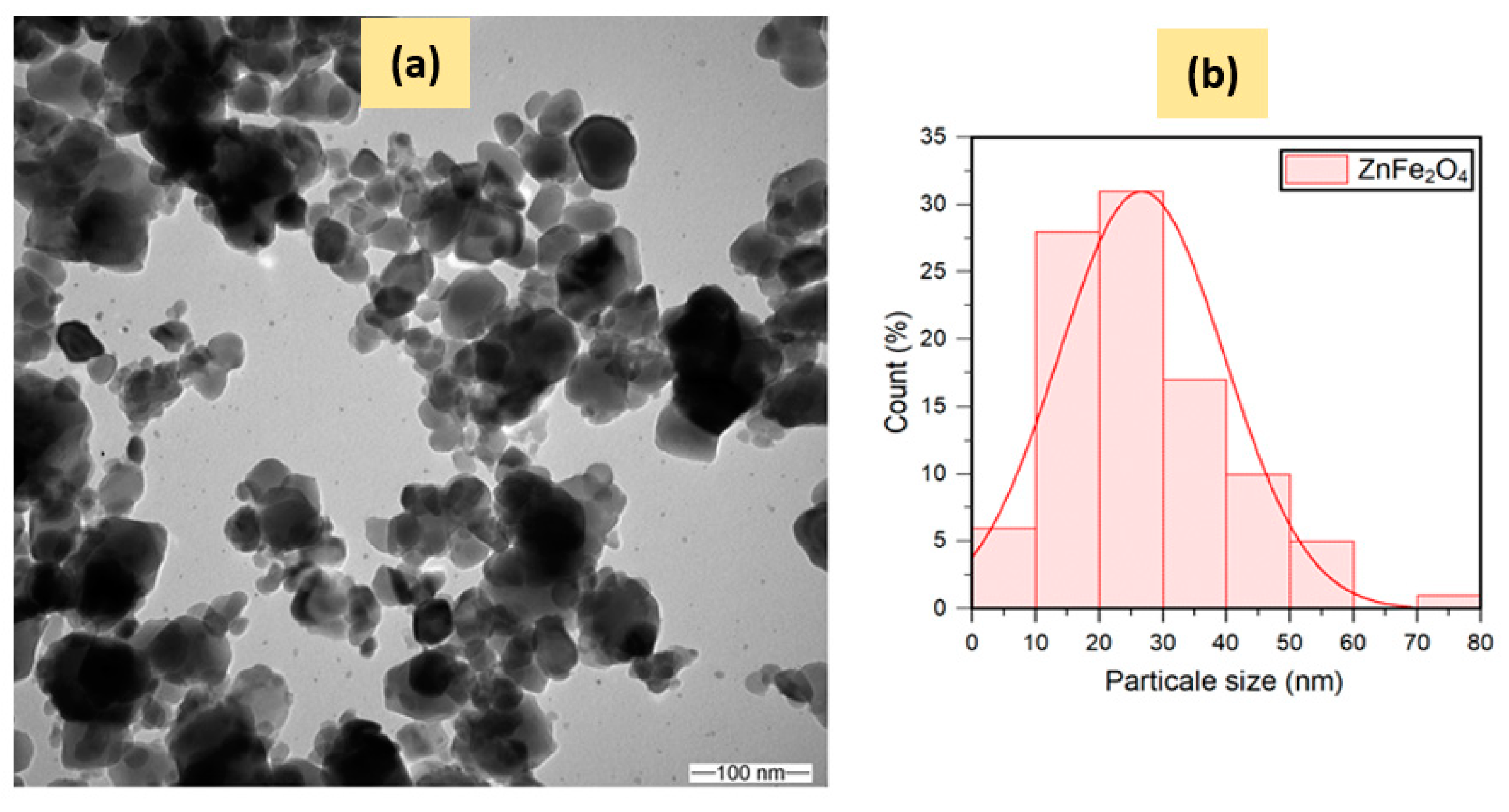
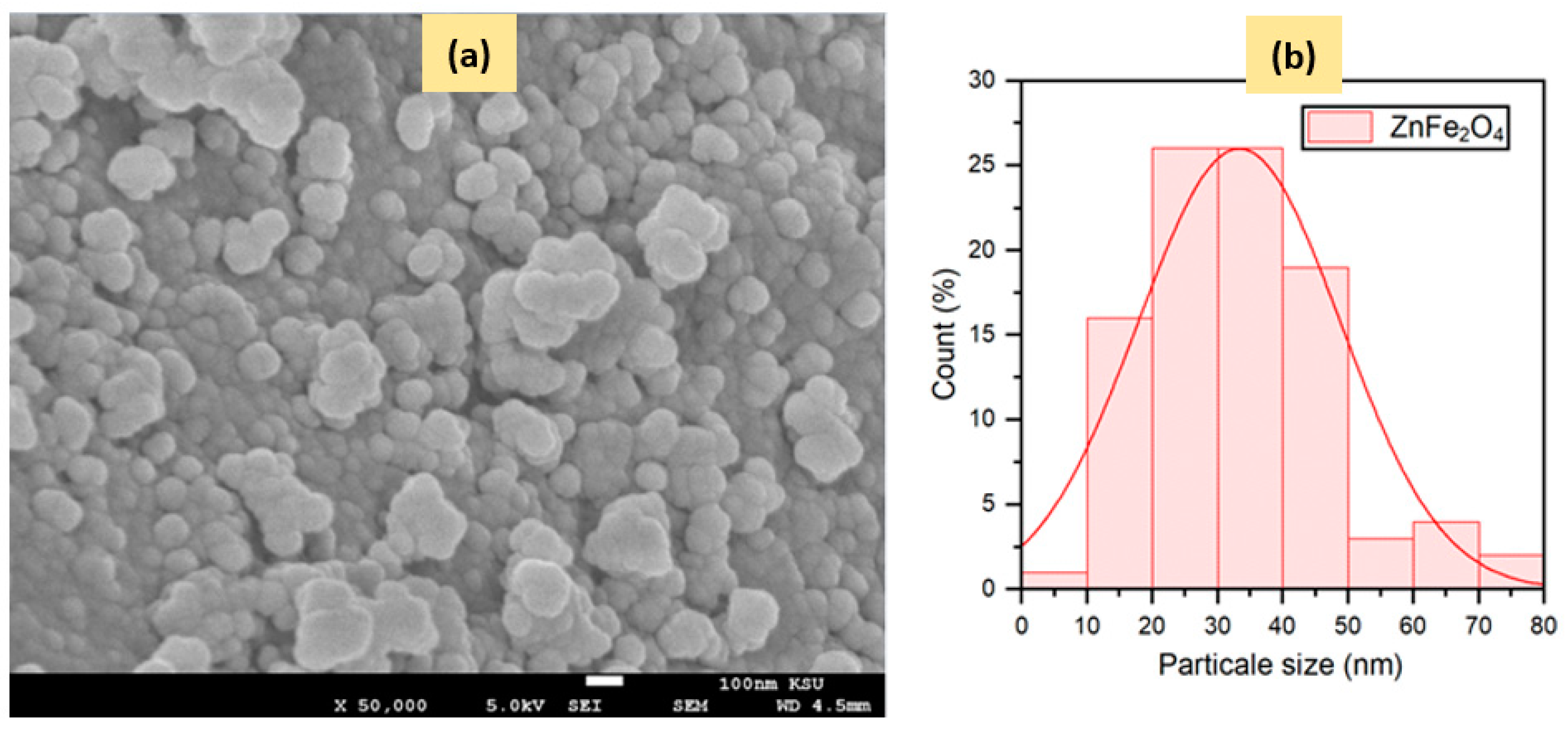
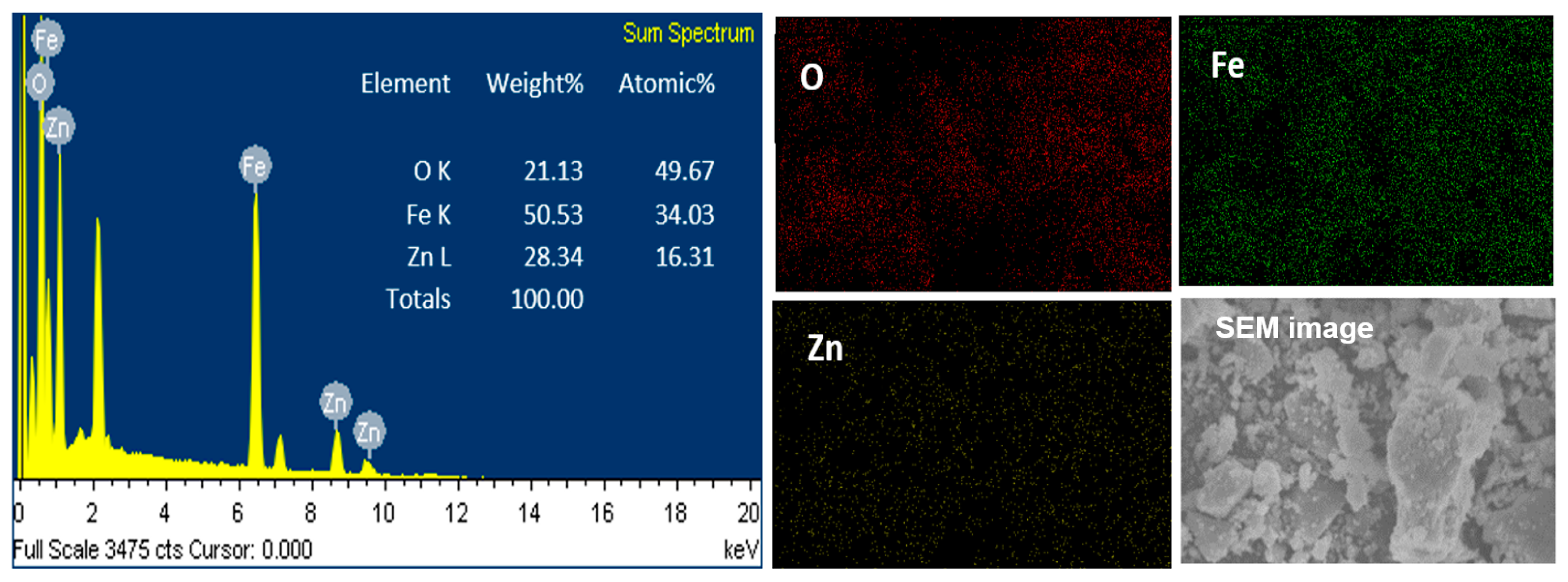
2.1.3. Electronic Microscopy Analysis
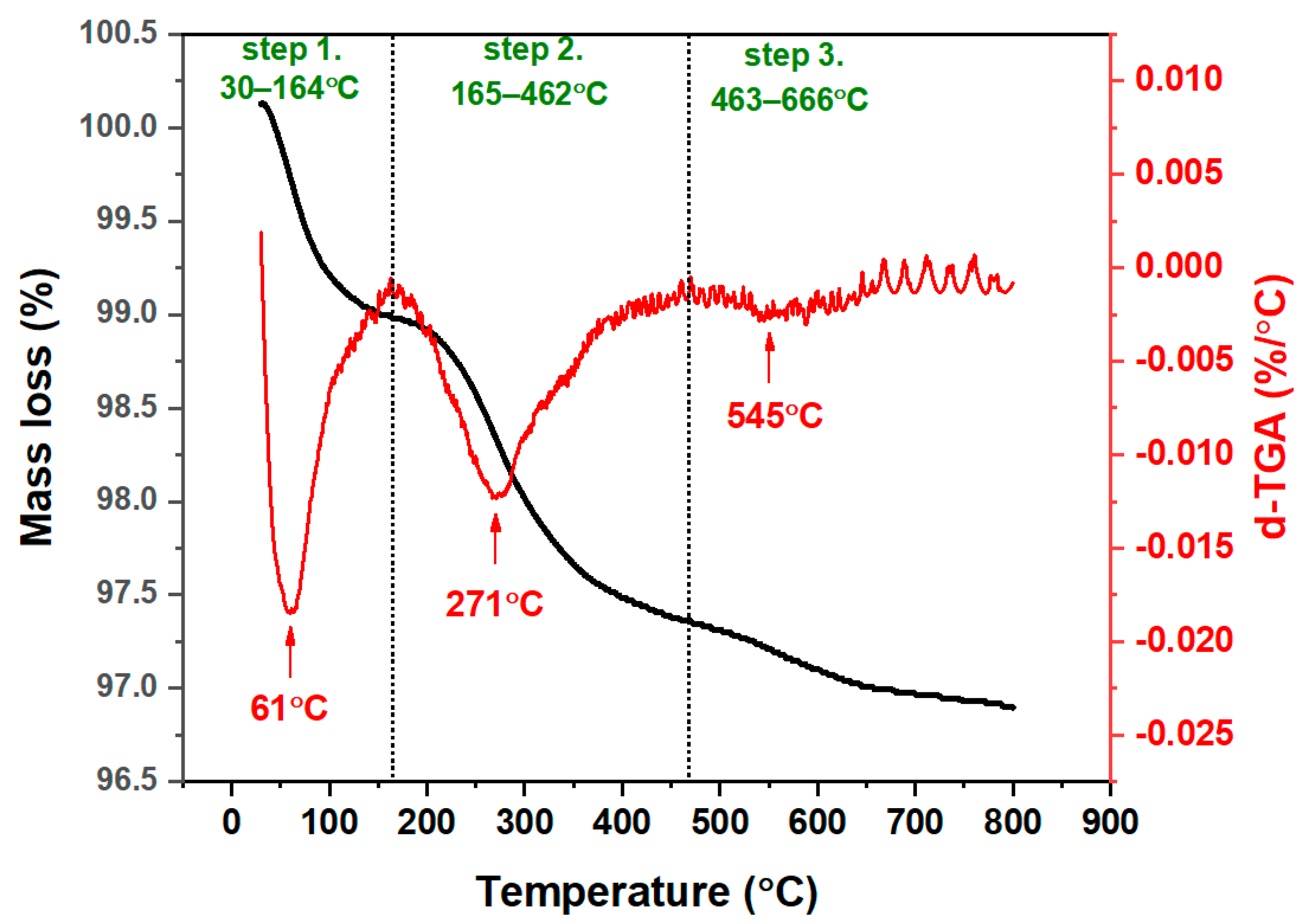
2.1.4. Thermogravimetric Analysis
2.1.5. X-ray Photoelectron Spectroscopy
2.1.6. BET Surface Area
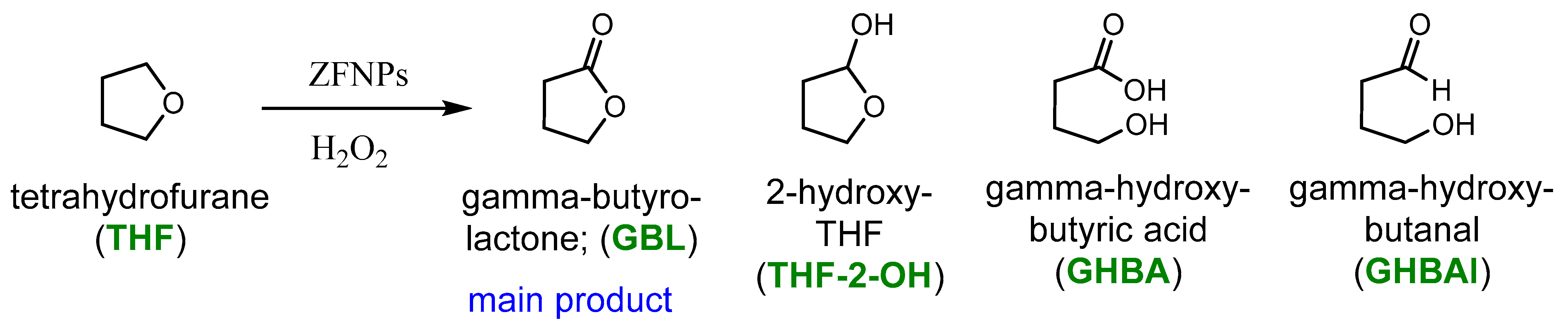
2.2. Catalytic Activity
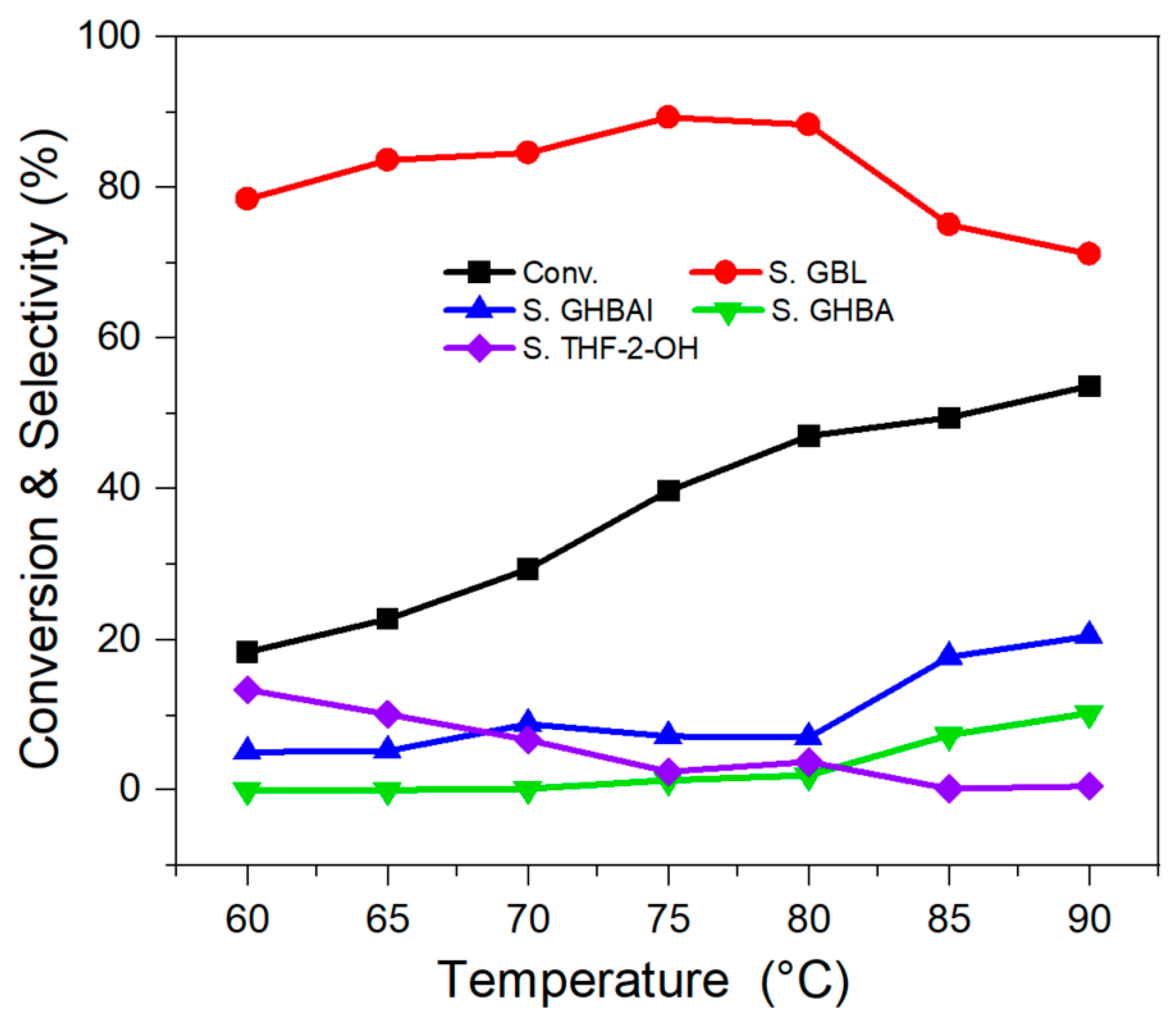
2.2.1. Effect of Reaction Temperature
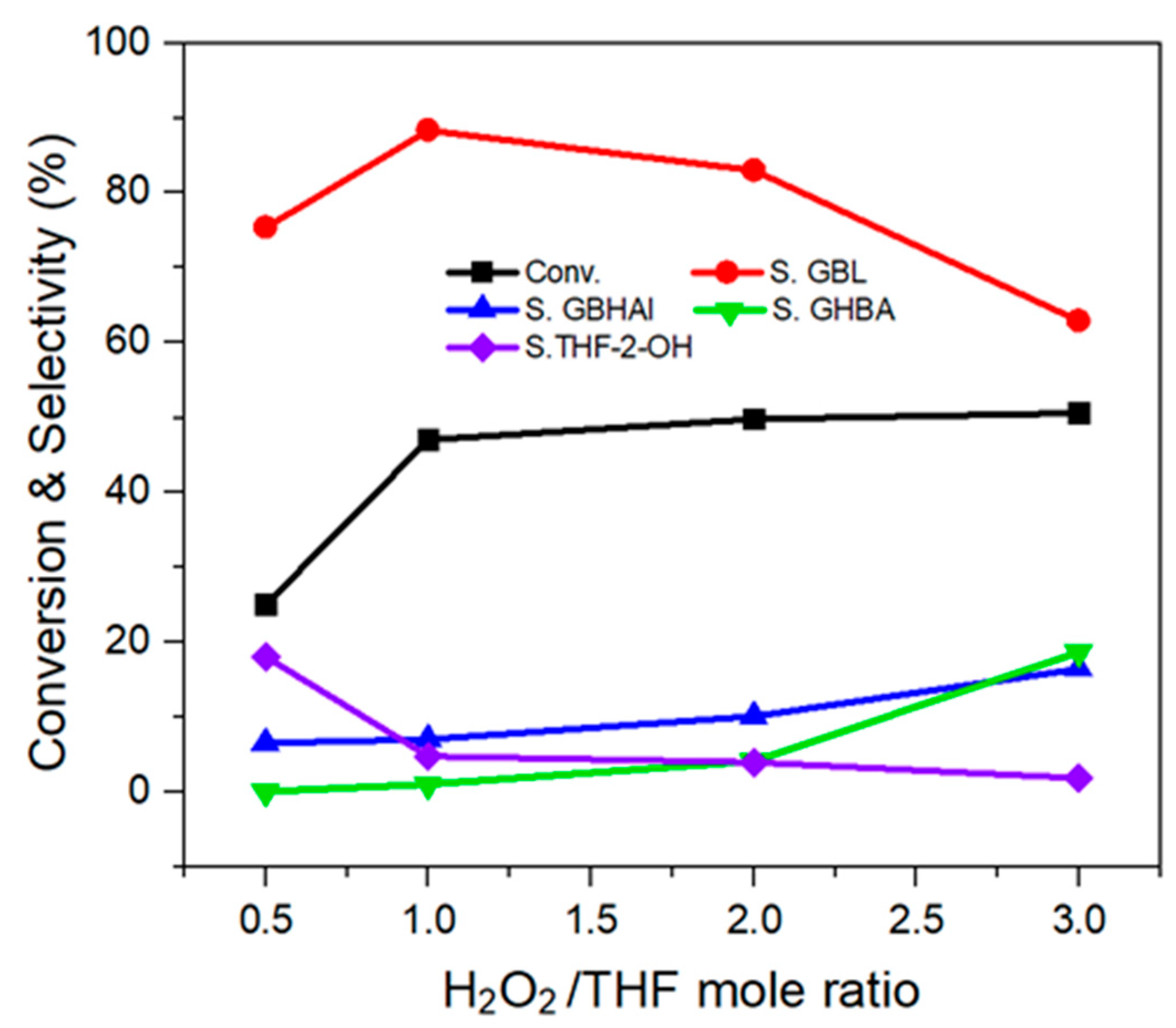
2.2.2. Effect of H2O2/THF Mole Ratio
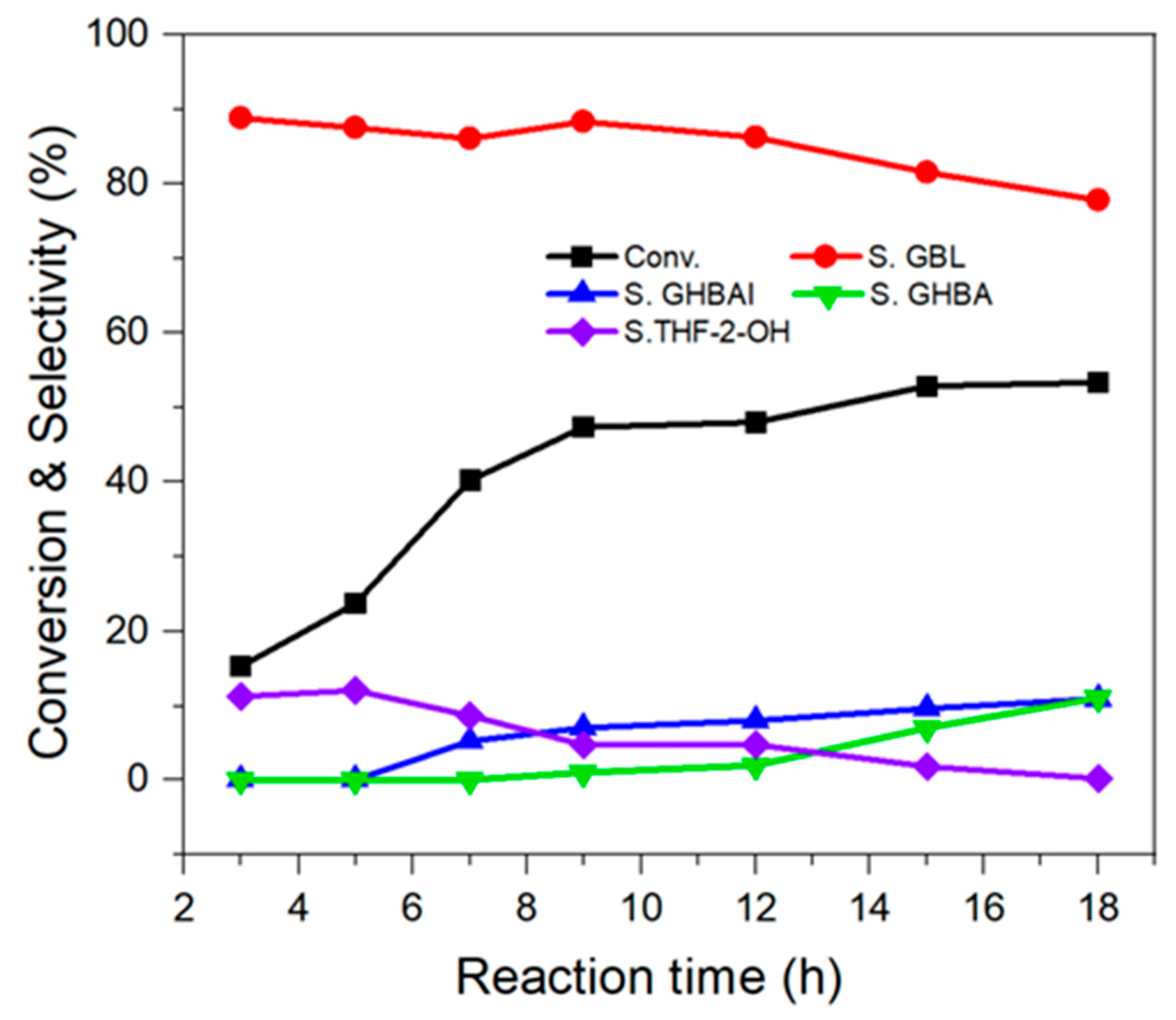
2.2.3. Effect of Reaction Time
2.2.4. Effect of Catalyst Amount
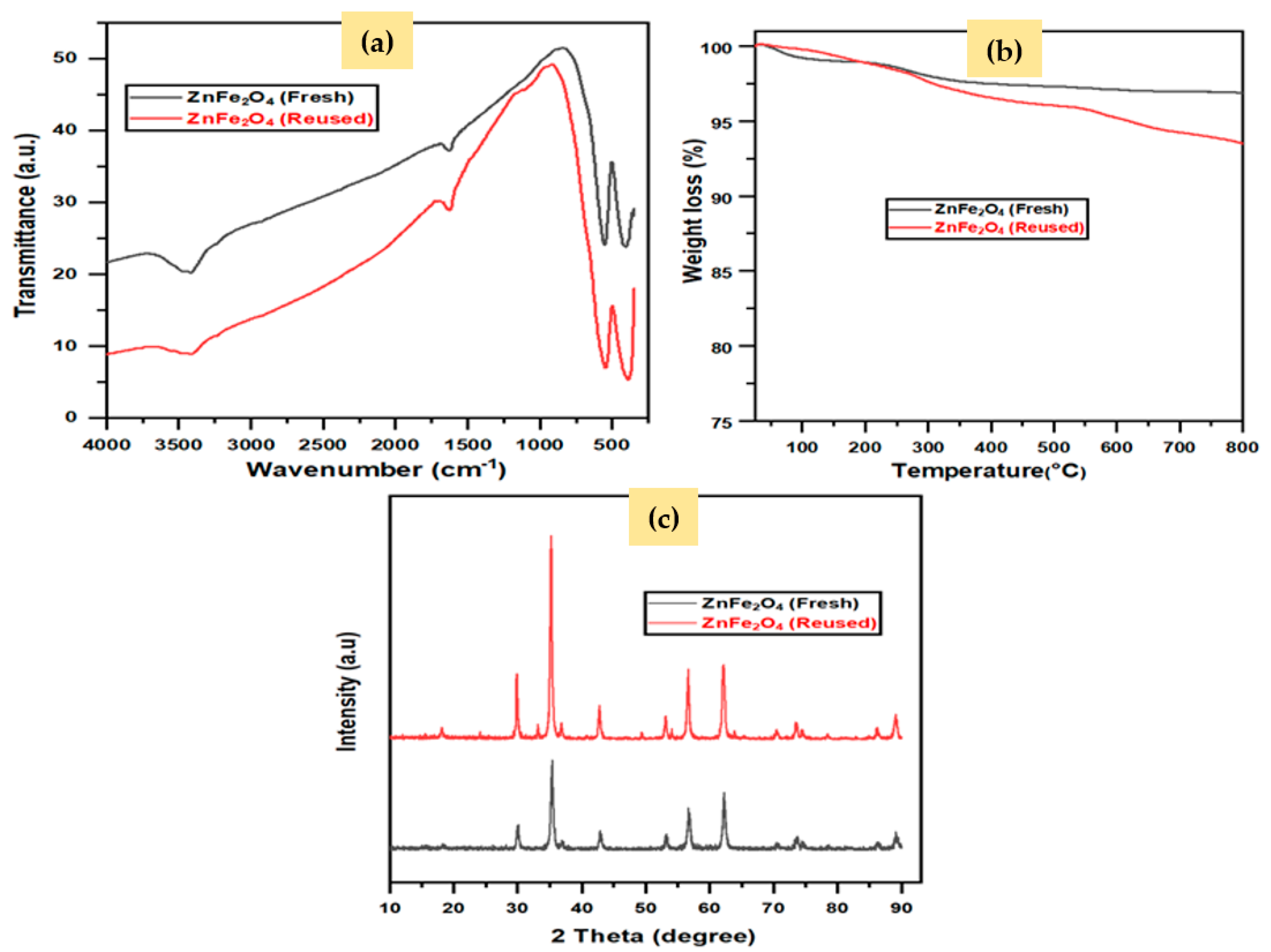
2.2.5. Reusability
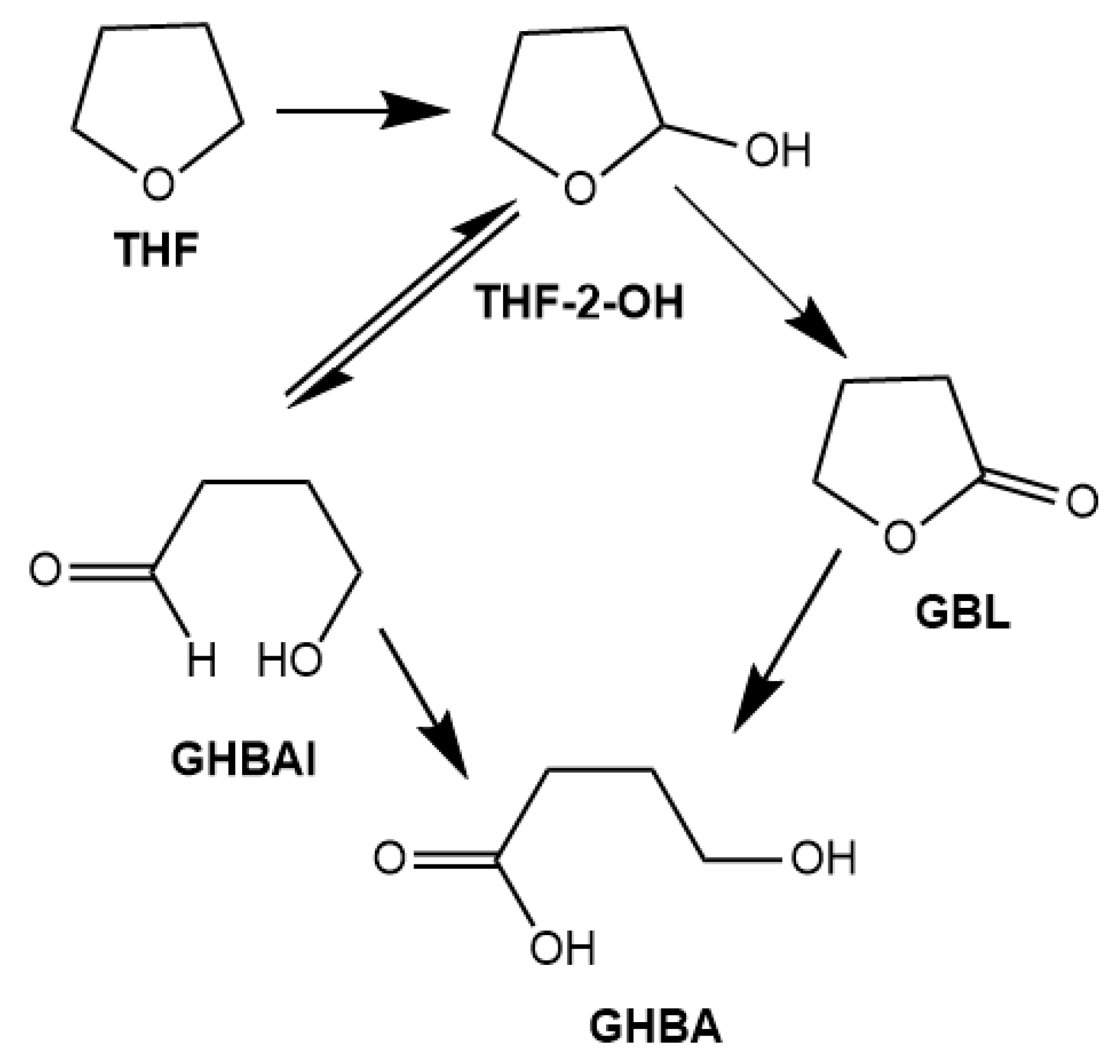
2.2.6. Proposed Catalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Experimental Procedure
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bińczak, J.; Dziuba, K.; Chrobok, A. Recent Developments in Lactone Monomers and Polymer Synthesis and Application. Materials 2021, 14, 2881. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Varma, I.K. Recent developments in ring opening polymerization of lactones for biomedical applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Şardan, M.; Sezer, S.; Günel, A.; Akkaya, M.; Tanyeli, C. Synthesis and biological evaluation of optically active conjugated γ-and δ-lactone derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5814–5818. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Sánchez, P.; Pàmies, O. Metal-π-allyl mediated asymmetric cycloaddition reactions. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2021; Volume 69, pp. 103–180. [Google Scholar]
- Sartori, S.K.; Diaz, M.A.N.; Diaz-Muñoz, G. Lactones: Classification, synthesis, biological activities, and industrial applications. Tetrahedron 2021, 84, 132001. [Google Scholar] [CrossRef]
- Venkatesan, S.; Su, S.-C.; Hung, W.-N.; Liu, I.-P.; Teng, H.; Lee, Y.-L. Printable electrolytes based on polyacrylonitrile and gamma-butyrolactone for dye-sensitized solar cell application. J. Power Sources 2015, 298, 385–390. [Google Scholar] [CrossRef]
- Phattanarudee, S.; Maher, T.J.; Towiwat, P. Catalepsy and Comparing Gamma-Hydroxybutyrate, 1, 4-Butanediol, and Gamma-Butyrolactone. In Neuropathology of Drug Addictions and Substance Misuse; Elsevier: Amsterdam, The Netherlands, 2016; pp. 484–490. [Google Scholar]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an enabling science for sustainable polymers. Chem. Rev. 2018, 118, 839–885. [Google Scholar] [CrossRef] [PubMed]
- Ritzoulis, G.; Missopolinou, D.; Doulami, S.; Panayiotou, C. Relative permittivities, densities, refractive indices and ultrasound velocities of the binary systems of γ-butyrolactone with methanol, ethanol, 1-butanol, and 1-octanol. J. Chem. Eng. Data 2000, 45, 636–641. [Google Scholar] [CrossRef]
- Świergiel, J.; Jadżyn, J. Temperature behavior of electric relaxational effects due to ionic conductivity in liquid lactones. Int. J. Thermophys. 2012, 33, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Mbaiwa, F.; Nyepetsi, M. Molecular dynamics and density functional theory studies of γ-butyrolactone (GBL)+ ethanol and γ-valerolactone (GVL)+ ethanol liquid mixtures. J. Mol. Liq. 2020, 319, 114128. [Google Scholar] [CrossRef]
- Gagliardi, M.; Bifone, A. Ring-opening copolymerization thermodynamics and kinetics of γ-valerolactone/ϵ-caprolactone. PLoS ONE 2018, 13, e0199231. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Zhao, J.; Zhang, G.; Peruch, F.; Carlotti, S. Ring-opening (co) polymerization of γ-butyrolactone: A review. Polym. J. 2020, 52, 3–11. [Google Scholar] [CrossRef]
- Duereh, A.; Sato, Y.; Smith, R.L., Jr.; Inomata, H. Methodology for replacing dipolar aprotic solvents used in API processing with safe hydrogen-bond donor and acceptor solvent-pair mixtures. Org. Process Res. Dev. 2017, 21, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Pillai, U.R.; Sahle-Demessie, E.; Young, D. Maleic anhydride hydrogenation over Pd/Al2O3 catalyst under supercritical CO2 medium. Appl. Catal. B Environ. 2003, 43, 131–138. [Google Scholar] [CrossRef]
- Javaid, A.; Bildea, C.S. Integrated process for γ-butyrolactone production. UPB Sci. Bull. 2014, 76, 33–42. [Google Scholar]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Eseyin, A.E.; Steele, P.H. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Hay, M.T.; Geib, S.J.; Pettner, D.A. Aerobic oxidation of tetrahydrofuran by a series of iron (III) containing POSS compounds. Polyhedron 2009, 28, 2183–2186. [Google Scholar] [CrossRef]
- Straub, T.; Koskinen, A.M. Catalytic oxidation of THF with homo and hetero metallic Mo/Ru complexes and molecular oxygen. Inorg. Chem. Commun. 2002, 5, 1052–1055. [Google Scholar] [CrossRef] [Green Version]
- Salavati-Niasari, M.; Bazarganipour, M. Bis(macrocyclic) copper(II) complexes containing aromatic nitrogen–nitrogen linkers produced by in situ one pot template condensation reaction (IOPTCR): Synthesis, characterization and catalytic oxidation of tetrahydrofuran. Inorg. Chem. Commun. 2006, 9, 332–336. [Google Scholar] [CrossRef]
- Salavati-Niasari, M. Oxidation of tetrahydrofuran with hydrogen peroxide in the presence of host (zeolite Y)/guest (1,9-dialkyl-1,3,7,9,11,15-hexaazacyclohexadecane copper(II) complexes, [Cu(R2[16]aneN6)]2+) nanocomposite materials. Inorg. Chem. Commun. 2006, 9, 628–633. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Z.-g.; Li, H.; Yang, N.-y.; Leung, K.M.; Wang, Y.-z.; Yu, R.-z.; Zhang, L.; Wang, W.-h.; Jiao, C.-y. Progress of environmental management and risk assessment of industrial chemicals in China. Environ. Pollut. 2012, 165, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, R.; Worden, S.; Cao, H.; Li, C. Public participation in environmental governance initiatives of chemical industrial parks. J. Clean. Prod. 2021, 305, 127092. [Google Scholar] [CrossRef]
- Chen, H.; Wen, Y.; Waters, M.D.; Shonnard, D.R. Design guidance for chemical processes using environmental and economic assessments. Ind. Eng. Chem. Res. 2002, 41, 4503–4513. [Google Scholar] [CrossRef]
- Védrine, J.C. Metal oxides in heterogeneous oxidation catalysis: State of the art and challenges for a more sustainable world. ChemSusChem 2019, 12, 577–588. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous catalysis on metal oxides. Catalysts 2017, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Vozniuk, O.; Tabanelli, T.; Tanchoux, N.; Millet, J.-M.M.; Albonetti, S.; Di Renzo, F.; Cavani, F. Mixed-oxide catalysts with spinel structure for the valorization of biomass: The chemical-loop reforming of bioethanol. Catalysts 2018, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A. Application of spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Al-Najar, B.; Bououdina, M.; Ahmed, M.A. Catalytic and photocatalytic properties of oxide spinels. In Handbook of Ecomaterials; Springer: New York, NY, USA, 2019; Volume 3, pp. 1701–1750. [Google Scholar]
- Hao, A.; Ning, X. Recent advances in spinel ferrite-based thin films: Synthesis, performances, applications, and beyond. Front. Mater. 2021, 8, 501. [Google Scholar] [CrossRef]
- Linh, N.L.M.; Giang, H.T.B.; Thang, L.Q.; Tien, T.D.; Thu, N.T.A.; Duong, T.; Van Duc, H.; Hoa, L.T.; Nguyen, V.T.; Nhiem, D.N. The Benzylation of p-Xylene Using ZnFe2O4 Nanoparticles as Heterogeneous Catalyst. J. Nanomater. 2022, 2022, 6490334. [Google Scholar] [CrossRef]
- Hazarika, R.; Garg, A.; Chetia, S.; Phukan, P.; Kulshrestha, A.; Kumar, A.; Bordoloi, A.; Kalita, A.J.; Guha, A.K.; Sarma, D. Magnetically separable ZnFe2O4 nanoparticles: A low cost and sustainable catalyst for propargyl amine and NH-triazole synthesis. Appl. Catal. A Gen. 2021, 625, 118338. [Google Scholar] [CrossRef]
- De, K.; Mukhopadhyay, C. ZnFe2O4 Nanoparticles: An Efficient and Recyclable Catalyst for the Synthesis of Isatinylidenethiazol-4-one Derivatives. ChemistrySelect 2018, 3, 6873–6879. [Google Scholar] [CrossRef]
- Ramazani, A.; Sadri, F.; Massoudi, A.; Khoobi, M.; Joo, S.W.; Dolatyari, L.; Dayyani, N. Magnetic ZnFe2O4 nanoparticles as an efficient catalyst for the oxidation of alcohols to carbonyl compounds in the presence of oxone as an oxidant. Iran. J. Catal. 2015, 5, 285–291. [Google Scholar]
- Lee, D.G.; Engh, M.V.D. The oxidation of tetrahydrofuran by ruthenium tetroxide. Can. J. Chem. 1972, 50, 3129–3134. [Google Scholar] [CrossRef]
- Metsger, L.; Bittner, S. Autocatalytic oxidation of ethers with sodium bromate. Tetrahedron 2000, 56, 1905–1910. [Google Scholar] [CrossRef]
- Podgoršek, A.; Zupan, M.; Iskra, J. Oxidative halogenation with “green” oxidants: Oxygen and hydrogen peroxide. Angew. Chem. Int. Ed. 2009, 48, 8424–8450. [Google Scholar] [CrossRef] [PubMed]
- AlGarni, T.S.; Abduh, N.A.; Al Kahtani, A.; Aouissi, A. Preparation of α-MoO3 from H3PMo12O40 precursor: Synthesis of 1,2-cyclohexanediol from cyclohexene over α-MoO3-TiO2 catalyst. Mater. Res. Express 2022, 9, 085003. [Google Scholar] [CrossRef]
- Hone, C.A.; Kappe, C.O. The use of molecular oxygen for liquid phase aerobic oxidations in continuous flow. In Accounts on Sustainable Flow Chemistry; Springer: Cham, Switzerland, 2020; pp. 67–110. [Google Scholar]
- Gonzalez-de-Castro, A.; Xiao, J. Green and efficient: Iron-catalyzed selective oxidation of olefins to carbonyls with O2. J. Am. Chem. Soc. 2015, 137, 8206–8218. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Shen, X.; Wang, Y.; Du, J.; Chen, B.; Han, B.; Liu, H. Selective aerobic oxidation of cyclic ethers to lactones over Au/CeO2 without any additives. Chem. Commun. 2020, 56, 2638–2641. [Google Scholar] [CrossRef]
- Dou, J.; Tao, F.F. Selective epoxidation of cyclohexene with molecular oxygen on catalyst of nanoporous Au integrated with MoO3 nanoparticles. Appl. Catal. A Gen. 2017, 529, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Heravi, M.M.; Ghalavand, N.; Hashemi, E. Hydrogen peroxide as a green oxidant for the selective catalytic oxidation of benzylic and heterocyclic alcohols in different media: An overview. Chemistry 2020, 2, 101–178. [Google Scholar] [CrossRef] [Green Version]
- Noyori, R.; Aoki, M.; Sato, K. Green oxidation with aqueous hydrogen peroxide. Chem. Commun. 2003, 34, 1977–1986. [Google Scholar] [CrossRef]
- Sasidharan, M.; Bhaumik, A. Catalytic oxidation of cyclic ethers to lactones over various titanosilicates. J. Mol. Catal. A Chem. 2011, 338, 105–110. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Sooknoi, T. Oxidation of tetrahydrofuran to butyrolactone catalyzed by iron-containing clay. Green Chem. 2015, 17, 435–441. [Google Scholar] [CrossRef]
- Saafan, S.A.; El-Nimr, M.K.; Hussein, M.M.; Omar, M.K. FTIR, DC, and AC electrical measurements of Mg Zn Nano-ferrites and their composites with Polybenzoxazine. Appl. Phys. A 2021, 127, 800. [Google Scholar] [CrossRef]
- Andhare, D.; Jadhav, S.; Khedkar, M.; Somvanshi, S.B.; More, S.; Jadhav, K. Structural and chemical properties of ZnFe2O4 nanoparticles synthesised by chemical co-precipitation technique. J. Phys. Conf. Ser. 2020, 1644, 012014. [Google Scholar] [CrossRef]
- Ghanbari, D.; BandehAli, S.; Moghadassi, A. Embedded three spinel ferrite nanoparticles in PES-based nano filtration membranes with enhanced separation properties. Main Group Met. Chem. 2022, 45, 1–10. [Google Scholar] [CrossRef]
- Rana, A.; Kumar, V. Investigation on anneal-tuned properties of ZnFe2O4 nanoparticles for use in humidity sensors. Appl. Phys. A 2021, 127, 609. [Google Scholar]
- Silambarasu, A.; Manikandan, A.; Balakrishnan, K. Room-temperature superparamagnetism and enhanced photocatalytic activity of magnetically reusable spinel ZnFe2O4 nanocatalysts. J. Supercond. Nov. Magn. 2017, 30, 2631–2640. [Google Scholar] [CrossRef]
- Lemine, O.; Bououdina, M.; Sajieddine, M.; Al-Saie, A.; Shafi, M.; Khatab, A.; Al-Hilali, M.; Henini, M. Synthesis, structural, magnetic and optical properties of nanocrystalline ZnFe2O4. Phys. B Condens. Matter 2011, 406, 1989–1994. [Google Scholar] [CrossRef]
- Algarni, T.S.; Al-Mohaimeed, A.M.; Al-Odayni, A.-B.; Abduh, N.A. Activated Carbon/ZnFe2O4 Nanocomposite Adsorbent for Efficient Removal of Crystal Violet Cationic Dye from Aqueous Solutions. Nanomaterials 2022, 12, 3224. [Google Scholar] [CrossRef]
- Abbasian, A.R.; Shafiee Afarani, M. One-step solution combustion synthesis and characterization of ZnFe2O4 and ZnFe1.6O4 nanoparticles. Appl. Phys. A 2019, 125, 721. [Google Scholar] [CrossRef]
- Roshani, R.; Tadjarodi, A. Synthesis of ZnFe2O4 nanoparticles with high specific surface area for high-performance supercapacitor. J. Mater. Sci. Mater. Electron. 2020, 31, 23025–23036. [Google Scholar] [CrossRef]
- Khanmohammadi-Sarabi, F.; Ghorbani-Choghamarani, A.; Aghavandi, H.; Zolfigol, M.A. ZnFe2O4@ SiO2-ascorbic acid: Green, magnetic, and versatile catalyst for the synthesis of chromeno [2, 3-d] pyrimidine-8-amine and quinazoline derivatives. Appl. Organomet. Chem. 2022, 36, e6768. [Google Scholar] [CrossRef]
- Briceño, S.; Brämer-Escamilla, W.; Silva, P.; Delgado, G.E.; Plaza, E.; Palacios, J.; Cañizales, E. Effects of synthesis variables on the magnetic properties of CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 2012, 324, 2926–2931. [Google Scholar] [CrossRef]
- Borade, R.M.; Somvanshi, S.B.; Kale, S.B.; Pawar, R.P.; Jadhav, K. Spinel zinc ferrite nanoparticles: An active nanocatalyst for microwave irradiated solvent free synthesis of chalcones. Mater. Res. Express 2020, 7, 016116. [Google Scholar] [CrossRef]
- Doiphode, V.; Vairale, P.; Sharma, V.; Waghmare, A.; Punde, A.; Shinde, P.; Shah, S.; Pandharkar, S.; Hase, Y.; Aher, R. Solution-processed electrochemical synthesis of ZnFe2O4 photoanode for photoelectrochemical water splitting. J. Solid State Electrochem. 2021, 25, 1835–1846. [Google Scholar] [CrossRef]
- Song, H.; Zhu, L.; Li, Y.; Lou, Z.; Xiao, M.; Ye, Z. Preparation of ZnFe2O4 nanostructures and highly efficient visible-light-driven hydrogen generation with the assistance of nanoheterostructures. J. Mater. Chem. A 2015, 3, 8353–8360. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Wang, G.; Wang, H. Zinc Ferrite Nanorod-Assembled Mesoporous Microspheres as Advanced Anode Materials for Sodium-Ion Batteries. Energy Technol. 2019, 7, 1900479. [Google Scholar] [CrossRef]
- Morsi, R.E.; Mohamed, R.S. Nanostructured mesoporous silica: Influence of the preparation conditions on the physical-surface properties for efficient organic dye uptake. R. Soc. Open Sci. 2018, 5, 172021. [Google Scholar] [CrossRef] [Green Version]
- Weng, Z.; Wang, J.; Zhang, S.; Jian, X. Selective oxidation of benzyl alcohol by heteropolytungstate as reaction-controlled phase-transfer catalyst with hydrogen peroxide. Bull. Chem. Soc. Jpn. 2008, 81, 525–529. [Google Scholar] [CrossRef]
- Cánepa, A.L.; Elías, V.R.; Vaschetti, V.M.; Sabre, E.V.; Eimer, G.A.; Casuscelli, S.G. Selective oxidation of benzyl alcohol through eco-friendly processes using mesoporous V-MCM-41, Fe-MCM-41 and Co-MCM-41 materials. Appl. Catal. A Gen. 2017, 545, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Sagayaraj, R.; Aravazhi, S.; Praveen, P.; Chandrasekaran, G. Structural, morphological and magnetic characters of PVP coated ZnFe2O4 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 2151–2158. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Kumar, R.; Grinblat, F.; Aphesteguy, J.C.; Saccone, F.D.; Errandonea, D. In-situ high-pressure X-ray diffraction study of zinc ferrite nanoparticles. Solid State Sci. 2016, 56, 68–72. [Google Scholar] [CrossRef] [Green Version]




| Catalyst | Total Surface Area (m2/g) | External Surface Area (m2/g) | Average Pore Volume (cm3/g) | Average Pore Width (nm) |
|---|---|---|---|---|
| ZFNPs | 22.05 | 14.65 | 0.076 | 13.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduh, N.A.Y.; Al-Kahtani, A.; Algarni, T.S.; Al-Odayni, A.-B. Selective Oxidation of Tetrahydrofuran to Gamma-Butyrolactone over Spinel ZnFe2O4 Nanoparticle Catalyst. Catalysts 2023, 13, 692. https://doi.org/10.3390/catal13040692
Abduh NAY, Al-Kahtani A, Algarni TS, Al-Odayni A-B. Selective Oxidation of Tetrahydrofuran to Gamma-Butyrolactone over Spinel ZnFe2O4 Nanoparticle Catalyst. Catalysts. 2023; 13(4):692. https://doi.org/10.3390/catal13040692
Chicago/Turabian StyleAbduh, Naaser A. Y., Abdullah Al-Kahtani, Tahani Saad Algarni, and Abdel-Basit Al-Odayni. 2023. "Selective Oxidation of Tetrahydrofuran to Gamma-Butyrolactone over Spinel ZnFe2O4 Nanoparticle Catalyst" Catalysts 13, no. 4: 692. https://doi.org/10.3390/catal13040692
APA StyleAbduh, N. A. Y., Al-Kahtani, A., Algarni, T. S., & Al-Odayni, A.-B. (2023). Selective Oxidation of Tetrahydrofuran to Gamma-Butyrolactone over Spinel ZnFe2O4 Nanoparticle Catalyst. Catalysts, 13(4), 692. https://doi.org/10.3390/catal13040692








