Applications of Brewer’s Spent Grain Hemicelluloses in Biorefineries: Extraction and Value-Added Product Obtention
Abstract
:1. Introduction
2. Productive Chain and Physicochemical Composition of Brewer’s Spent Grain
3. The Use of Brewer’s Spent Grain in Biorefineries
3.1. Pretreatments for Extraction of Hemicelluloses from Brewer’s Spent Grain
| Method of Extraction | Yield/Recovery | The Product Obtained from BSG Hemicelluloses | Reference |
|---|---|---|---|
| Diluted acid (o.c. 1: 130 °C, 1% H2SO4, 26 mi) | 34 g/L, average | Xylose/arabinose monomers (subjected to fermentation to obtain bioethanol) | [18] |
| Two-step acidic hydrolysis (o.c.: 90 °C, 1.85% H2SO4, 19.5 min) | 1° hydrolysis: 29.9% total xylose yield and 69.7% total arabinose yield. 2° hydrolysis: 89.7% xylose yield, complete arabinose solubilization. | Xylose/arabinose monomers and arabinoxylan-oligosaccharides | [34] |
| Ultrasound-assisted extraction w/1.5 M NaOH | 58.47 ± 3.17% max. | Arabinoxylan solution w/increased emulsifying properties | [51] |
| Direct fermentation (o.c.: T. reesei, 20 g/L of BSG, pH 7.0, 30 °C, 72 h) | 326.2 mg XOS/g max. | Arabino-xylooligosaccharides (AXOS) w/degree of polymerization from 2 to 5. | [52] |
| Steam Explosion (o.c.: 180 °C, 10 min, 25% initial dry matter) | >73.1% | Xylooligosaccharides (XOS) | [53] |
| Nixtamalization + thermal treatment w/CaO, 100 °C | 6.43% | Arabinoxylan chains | [54] |
| Hydrothermal pretreatment (160 °C for 20 min) | 20 g/L max. | Xylitol and 2G ethanol | [55] |
| Thermal extraction (100 °C for 30 min) followed by NaOH 0.5 mol/L solution (25 °C 100 rpm, 8 h) | - | Thermoplastic films | [56] |
| Alkaline extraction (4 M KOH + 5 mM Na2S2O5) followed by ethanol precipitation and enzymatic hydrolysis | 63.6% max. | Xylose monomers | [57] |
| SSF w/Fusarium oxysporum (o.c.: BSG extruded, 48 h fermentation) | 63.28% | Soluble Arabinoxylans | [58] |
| Microwave-assisted alkali pretreatment (o.c.: 172 °C, 0.38 M NaOH) followed by ethanol precipitation/enzymatic hydrolysis | 133 kg AX/t BSG | Arabino-xylooligosacharides (AXOS) and biobutanol | [59] |
| Organosolv pretreatment (o.c.: 180 °C, 10 min, 50% ethanol) | 50% average | Arabino-xylooligosaccharides (AXOS) | [60] |
| Subcritical water hydrolysis (174 °C, 60 min and 5% (w/v) of dry BSG) | >80% | Arabinoxylan-rich hydrolysate | [61] |
| Peroxide alkaline pretreatment (o.c.: 5% H2O2, pH 11.5, 50 °C, 150 rpm) followed by enzymatic hydrolysis | 30% max. | Arabino-xylooligosaccharides (AXOS) and monosaccharides | [44] |
3.2. Obtaining Value-Added Products from Brewer’s Spent Grain Hemicelluloses
3.2.1. Xylooligosaccharides
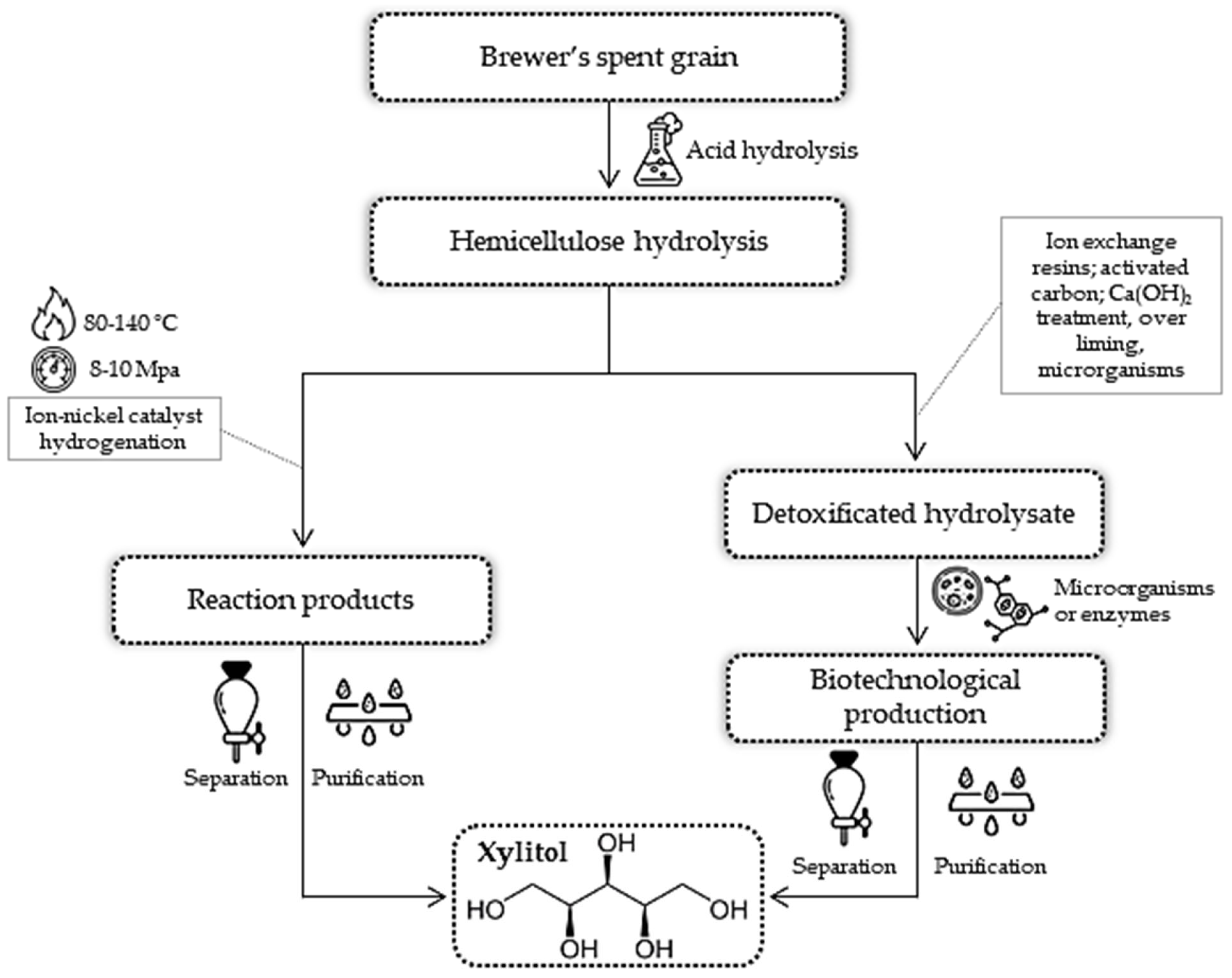
3.2.2. Xylitol
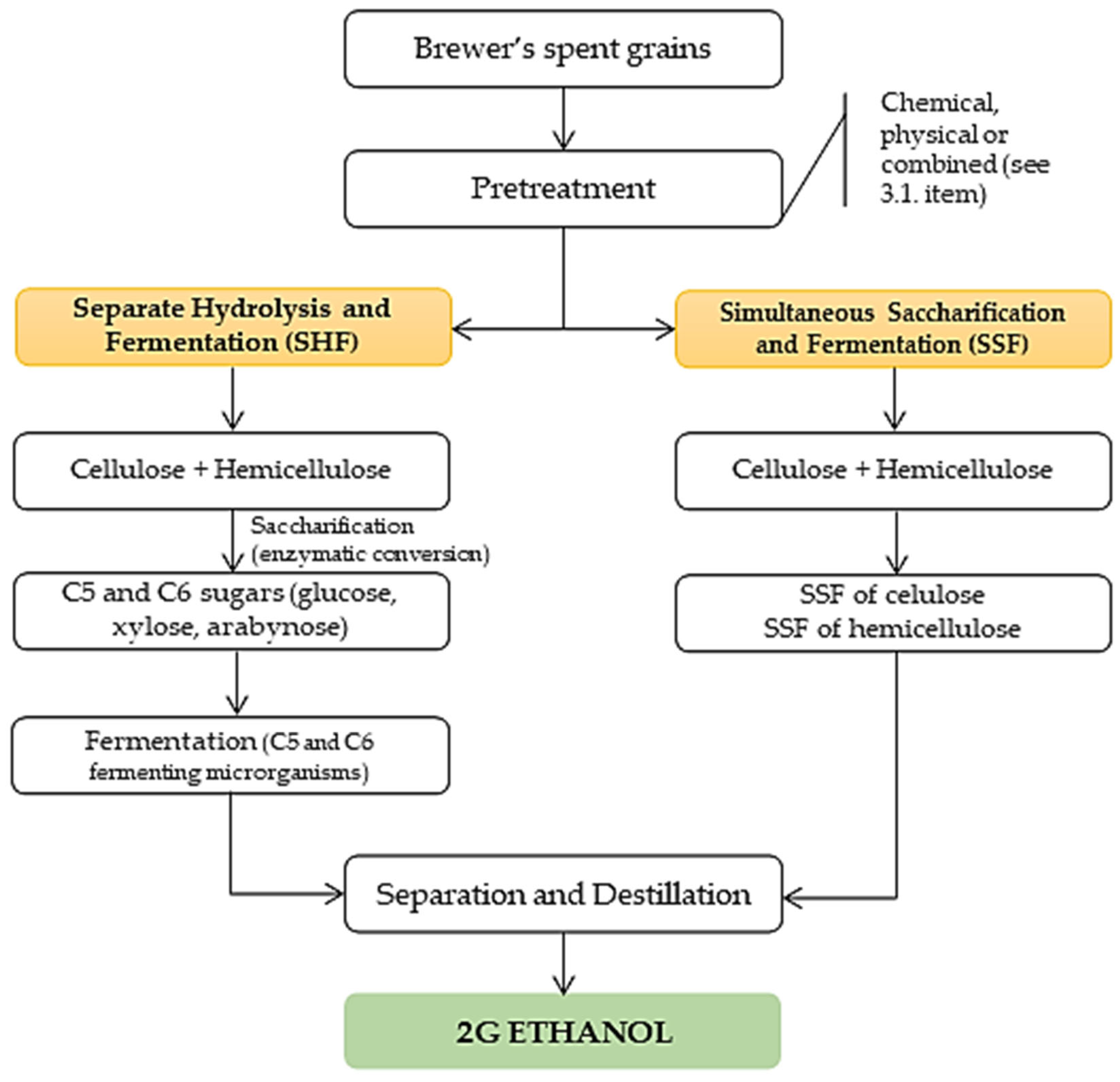
3.2.3. 2G Ethanol
3.2.4. Enzymes
3.2.5. Biofilms and Bioplastics
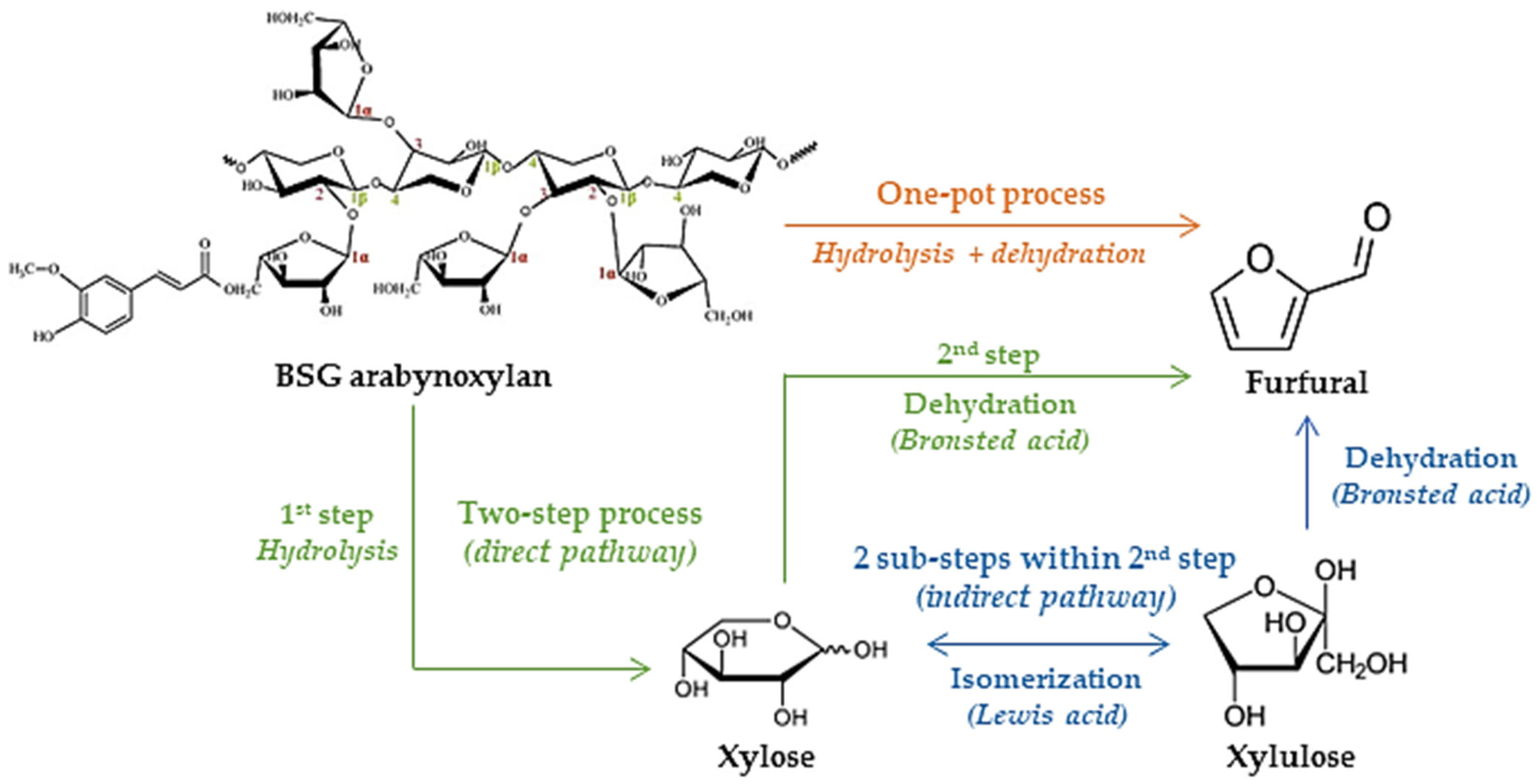
3.2.6. Furan Derivatives (Furfural and Furfuryl Alcohol)
3.3. The Use of Catalysts in Hemicellulosic Biorefinery: What’s Next?
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated Lignocellulosic Value Chains in a Growing Bioeconomy: Status Quo and Perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane Bagasse: A Biomass Sufficiently Applied for Improving Global Energy, Environment and Economic Sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications of Lignocellulose-Based Polyurethanes: A Review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, C.; Meng, X.; Wang, L.; Zhou, X. Editorial: Isolation, Modification, and Characterization of the Constituents (Cellulose, Hemicellulose, Lignin; et al.) in Biomass and Their Bio-Based Applications. Front. Bioeng. Biotechnol. 2022, 10, 616. [Google Scholar] [CrossRef]
- Candido, J.P.; Freitas, C.; Schmatz, A.A.; Felipuci, J.P.; de Oliveira Leite, D.A.N.; de Franceschi de Angelis, D.; Brienzo, M. Hemicellulose Sugar Fermentation: Hydrolysate Challenges, Microorganisms, and Value-Added Products. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 337–360. [Google Scholar]
- Melati, R.B.; de Freitas, C.; Brienzo, M. Analytical Techniques Applied to Hemicellulose Structure and Functional Characterization. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 139–170. [Google Scholar]
- Gómez Millán, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent Advances in the Catalytic Production of Platform Chemicals from Holocellulosic Biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic Conversion of Lignocellulosic Biomass into Chemicals and Fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- de Jesus Costa, T.; Inô, M.M.O.; Kunz, V.R.; Barros, F.C. De Reaproveitamento De Resíduos Sólidos Da Indústria Cervejeira: Bagaço De Malte Extrusado Para A Produção De Produtos Alimentícios. In Avanços e Desafios da Nutrição 4; Atena Editora: Ponta Grossa, Brazil, 2019; pp. 269–278. [Google Scholar]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [Green Version]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The Potential of Brewer’s Spent Grain in the Circular Bioeconomy: State of the Art and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 959. [Google Scholar] [CrossRef]
- Lisci, S.; Tronci, S.; Grosso, M.; Karring, H.; Hajrizaj, R.; Errico, M. Brewer’s Spent Grain: Its Value as Renewable Biomass and Its Possible Applications. Chem. Eng. Trans. 2022, 92, 259–264. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Chiș, M.S.; Pop, O.L.; Fogarasi, M.; Păucean, A.; Igual, M.; Michiu, D. Reintegration of Brewers Spent Grains in the Food Chain: Nutritional, Functional and Sensorial Aspects. Plants 2021, 10, 2504. [Google Scholar] [CrossRef]
- Macias-Garbett, R.; Serna-Hernández, S.O.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Phenolic Compounds From Brewer’s Spent Grains: Toward Green Recovery Methods and Applications in the Cosmetic Industry. Front. Sustain. Food Syst. 2021, 5, 196. [Google Scholar] [CrossRef]
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The Role of Microorganisms on Biotransformation of Brewers’ Spent Grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef] [PubMed]
- Massardi, M.M.; Massini, R.M.M.; de Jesus Silva, D. Caracterização química do bagaço de malte e avaliação do seu potencial para obtenção de produtos de valor agregado. J. Eng. Exact Sci. 2020, 6, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Smart, K.A.; Cook, D.J. A Comparison of Dilute Acid- and Alkali-Catalyzed Hydrothermal Pretreatments for Bioethanol Production from Brewers’ Spent Grains. J. Am. Soc. Brew. Chem. 2018, 72, 143–153. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Romero, I.; López-Linares, J.C.; Castro, E. Brewer’s Spent Grain as a Source of Renewable Fuel through Optimized Dilute Acid Pretreatment. Renew. Energy 2020, 148, 81–90. [Google Scholar] [CrossRef]
- Silbir, S.; Goksungur, Y. Natural Red Pigment Production by Monascus Purpureus in Submerged Fermentation Systems Using a Food Industry Waste: Brewer’s Spent Grain. Foods 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Yadav, J.; Gaur, R.; Singh, R.; Verma, T.; Yadav, J.S.; Pandey, P.K.; Rath, S.K. Multistep Structural and Chemical Evaluation of Sugarcane Baggase, Pretreated with Alkali for Enhancing the Enzymatic Saccharification by Cellulase and Xylanase of the Pseudomonas Sp. CVB-10 (MK443365) and Bacillus Paramycoides T4 (MN370035) Mix-Culture System. Front. Energy Res. 2022, 9, 891. [Google Scholar]
- Saeed, H.A.M.; Liu, Y.; Chen, H. Exploring Sudanese Agricultural Residues as Alternative Fibres for Pulp and Paper Manufacturing. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012030. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Ramakrishnan, K.; Dinesh Kirupha, S.; Sivanesan, S. Thermodynamic and Kinetic Studies of Cadmium Adsorption from Aqueous Solution onto Rice Husk. Braz. J. Chem. Eng. 2010, 27, 347–355. [Google Scholar] [CrossRef]
- Pabbathi, N.P.P.; Velidandi, A.; Pogula, S.; Gandam, P.K.; Baadhe, R.R.; Sharma, M.; Sirohi, R.; Thakur, V.K.; Gupta, V.K. Brewer’s Spent Grains-Based Biorefineries: A Critical Review. Fuel 2022, 317, 123435. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Ampese, L.C.; Mussatto, S.I.; Forster-Carneiro, T. A Bibliometric Analysis on Potential Uses of Brewer’s Spent Grains in a Biorefinery for the Circular Economy Transition of the Beer Industry. Biofuels Bioprod. Biorefining 2021, 15, 1965–1988. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Recent Advances in Biotechnological Valorization of Brewers’ Spent Grain. Food Sci. Biotechnol. 2021, 30, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.I. Pretreatment Strategies for Enhanced Biogas Production from Lignocellulosic Biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Buller, L.S.; Sganzerla, W.G.; Lima, M.N.; Muenchow, K.E.; Timko, M.T.; Forster-Carneiro, T. Ultrasonic Pretreatment of Brewers’ Spent Grains for Anaerobic Digestion: Biogas Production for a Sustainable Industrial Development. J. Clean Prod. 2022, 355, 131802. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, H.; Wang, Y.; He, B.; Lu, J.; Zhu, W.; Peng, L.; Wang, Y. Challenges and Perspectives of Green-like Lignocellulose Pretreatments Selectable for Low-Cost Biofuels and High-Value Bioproduction. Bioresour. Technol. 2023, 369, 128315. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and Modification of Hemicellulose from Lignocellulosic Biomass: A Review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Ujor, V.C.; Okonkwo, C.C. Microbial Detoxification of Lignocellulosic Biomass Hydrolysates: Biochemical and Molecular Aspects, Challenges, Exploits and Future Perspectives. Front. Bioeng. Biotechnol. 2022, 10, 2125. [Google Scholar] [CrossRef]
- Arranz, J.I.; Miranda, M.T.; Sepúlveda, F.J.; Montero, I.; Rojas, C.V. Analysis of Drying of Brewers’ Spent Grain. Proceedings 2018, 2, 1467. [Google Scholar] [CrossRef] [Green Version]
- Mitri, S.; Salameh, S.J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A Comparative Analysis of Pretreatment Strategies on the Properties and Hydrolysis of Brewers’ Spent Grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedő, S.; Rozbach, M.; Nagy, L.; Fehér, A.; Fehér, C. Optimised Fractionation of Brewer’s Spent Grain for a Biorefinery Producing Sugars, Oligosaccharides, and Bioethanol. Processes 2021, 9, 366. [Google Scholar] [CrossRef]
- Guo, W.; Bruining, H.C.; Heeres, H.J.; Yue, J. Efficient Synthesis of Furfural from Xylose over HCl Catalyst in Slug Flow Microreactors Promoted by NaCl Addition. AIChE J. 2022, 68, e17606. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery-Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Nzediegwu, E.; Pérez-Venegas, M.; Auclair, K.; Dumont, M.J. Semisynthetic Production of Hydroxymethylfurfural and Furfural: The Benefits of an Integrated Approach. J. Environ. Chem. Eng. 2022, 10, 108515. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, X.; Lin, Q.; Xin, F.; Sun, R.; Wang, X.; Ren, J. A New Approach to Recycle Oxalic Acid during Lignocellulose Pretreatment for Xylose Production. Biotechnol. Biofuels 2018, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid Hydrolysis of Lignocellulosic Biomass: Sugars and Furfurals Formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Zhang, M.; Zhu, L.; Zhao, X.; Chen, J.; Chen, W.; Chang, C. Hydrolysis of Lignocellulose to Succinic Acid: A Review of Treatment Methods and Succinic Acid Applications. Biotechnol. Biofuels Bioprod. 2023, 16, 1. [Google Scholar] [CrossRef]
- Shao, H.; Zhao, Y.; Sun, H.; Yang, B.; Fan, B.; Zhang, H.; Weng, Y. Barrier Film of Etherified Hemicellulose from Single-Step Synthesis. Polymers 2020, 12, 2199. [Google Scholar] [CrossRef]
- Kavalopoulos, M.; Stoumpou, V.; Christofi, A.; Mai, S.; Barampouti, E.M.; Moustakas, K.; Malamis, D.; Loizidou, M. Sustainable Valorisation Pathways Mitigating Environmental Pollution from Brewers’ Spent Grains. Environ. Pollut. 2021, 270, 116069. [Google Scholar] [CrossRef]
- Martins, J.R.; Abe, M.M.; Brienzo, M. Chemical Modification Strategies for Developing Functionalized Hemicellulose. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 171–205. [Google Scholar]
- Fernández-Delgado, M.; Plaza, P.E.; Coca, M.; García-Cubero, M.T.; González-Benito, G.; Lucas, S. Comparison of Mild Alkaline and Oxidative Pretreatment Methods for Biobutanol Production from Brewer’s Spent Grains. Ind. Crops Prod. 2019, 130, 409–419. [Google Scholar] [CrossRef]
- De Freitas, C.; Terrone, C.C.; Forsan, C.F.; Milagres, A.M.F.; Brienzo, M. Oligosaccharides from Lignocellulosic Biomass and Their Biological and Physicochemical Properties. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 275–309. [Google Scholar]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and Protein Recovery from Brewer’s Spent Grain Using Hydrothermal Pretreatment and Their Conversion to Edible Filamentous Fungi—A Brewery Biorefinery Concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef]
- Scapini, T.; dos Santos, M.S.N.; Bonatto, C.; Wancura, J.H.C.; Mulinari, J.; Camargo, A.F.; Klanovicz, N.; Zabot, G.L.; Tres, M.V.; Fongaro, G.; et al. Hydrothermal Pretreatment of Lignocellulosic Biomass for Hemicellulose Recovery. Bioresour. Technol. 2021, 342, 126033. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 148. [Google Scholar] [CrossRef]
- Cardoso, F.; Castrillo, V.S.; Bueno, D.; Schmatz, A.A.; de Freitas, C.; Brienzo, M. Processo Organosolv Modificado Para Obtenção de Hemicelulose e Lignina. 2022. Available online: https://repositorio.unesp.br/handle/11449/237046 (accessed on 27 February 2023).
- Felipuci, J.P.; Angelis, D.A.D.; Brienzo, M. Método de Extração de Hemicelulose Por Associação de Processo Biológico e Químico. 2022. Available online: https://repositorio.unesp.br/handle/11449/237049 (accessed on 27 February 2023).
- Liu, L.; Chen, M.; Coldea, T.E.; Yang, H.; Zhao, H. Emulsifying Properties of Arabinoxylans Derived from Brewers’ Spent Grain by Ultrasound-Assisted Extraction: Structural and Functional Properties Correlation. Cellulose 2023, 30, 359–372. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Rodrigues, L.R. One-Step Process for Producing Prebiotic Arabino-Xylooligosaccharides from Brewer’s Spent Grain Employing Trichoderma Species. Food Chem. 2019, 270, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Swart, L.J.; Bedzo, O.K.K.; van Rensburg, E.; Görgens, J.F. Pilot-Scale Xylooligosaccharide Production through Steam Explosion of Screw Press–Dried Brewers’ Spent Grains. Biomass Convers. Biorefin. 2022, 12, 1295–1309. [Google Scholar] [CrossRef]
- Martínez-Encinas, E.G.; Carvajal-Millán, E.; Calderón de la Barca, A.M.; Rascón-Chu, A.; Martínez-Porchas, M.; Márquez-Escalante, J.A.; Islas-Rubio, A.R. Extraction and Characterization of Arabinoxylans Obtained from Nixtamalized Brewers’ Spent Grains. Food Sci. Technol. Int. 2021, 29, 40–49. [Google Scholar] [CrossRef]
- Da Silva, E.G.; Borges, A.S.; Maione, N.R.; Castiglioni, G.L.; Suarez, C.A.G.; Montano, I.D.C. Fermentation of Hemicellulose Liquor from Brewer’s Spent Grain Using Scheffersomyces Stipitis and Pachysolen Tannophilus for Production of 2G Ethanol and Xylitol. Biofuels Bioprod. Biorefining 2020, 14, 127–137. [Google Scholar] [CrossRef]
- Jaguey-Hernández, Y.; Tapia-Ignacio, C.; Aguilar-Arteaga, K.; González-Olivares, L.G.; Castañeda-Ovando, E.P.; Cruz-Cansino, N.; Ojeda-Ramirez, D.; Castañeda-Ovando, A. Thermoplastic Biofilms Obtained from an Arabinoxylan-Rich Fraction from Brewers’ Spent Grain: Physicochemical Characterization and Thermal Analysis. Biomass Convers. Biorefin. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Rojas-Pérez, L.C.; Narváez-Rincón, P.C.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A. Production of Xylose through Enzymatic Hydrolysis of Glucuronoarabinoxylan from Brewers’ Spent Grain. Bioresour. Bioprocess. 2022, 9, 105. [Google Scholar] [CrossRef]
- Cervantes-Ramirez, J.G.; Vasquez-Lara, F.; Sanchez-Estrada, A.; Troncoso-Rojas, R.; Heredia-Olea, E.; Islas-Rubio, A.R. Arabinoxylans Release from Brewers’ Spent Grain Using Extrusion and Solid-State Fermentation with Fusarium Oxysporum and the Antioxidant Capacity of the Extracts. Foods 2022, 11, 1415. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; Lucas, S.; García-Cubero, M.T.; Jiménez, J.J.; Coca, M. A Biorefinery Based on Brewer`s Spent Grains: Arabinoxylans Recovery by Microwave Assisted Pretreatment Integrated with Butanol Production. Ind. Crops Prod. 2020, 158, 113044. [Google Scholar] [CrossRef]
- Parchami, M.; Agnihotri, S.; Taherzadeh, M.J. Aqueous Ethanol Organosolv Process for the Valorization of Brewer’s Spent Grain (BSG). Bioresour. Technol. 2022, 362, 127764. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; He, J.; Pan, H.; Alonso-Riaño, P.; Amândio, M.S.T.; Xavier, A.M.R.B.; Beltrán, S.; Sanz, M.T.; Bañuelos, M. Subcritical Water as Pretreatment Technique for Bioethanol Production from Brewers’ Spent Grain within a Biorefinery Concept. Polymers 2022, 14, 5218. [Google Scholar] [CrossRef]
- Vilcocq, L.; Castilho, P.C.; Carvalheiro, F.; Duarte, L.C. Hydrolysis of Oligosaccharides Over Solid Acid Catalysts: A Review. ChemSusChem 2014, 7, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Li, W.; Ogunbiyi, A.T.; An, S. Efficient Catalytic Conversion of Corn Stover to Furfural and 5-Hydromethylfurfural Using Glucosamine Hydrochloride Derived Carbon Solid Acid in Ƴ-Valerolactone. Ind. Crops Prod. 2021, 161, 113173. [Google Scholar] [CrossRef]
- Salmi, T.; Murzin, D.; Wärnå, J.; Mäki-Arvela, P.; Kusema, B.; Holmbom, B.; Willför, S. Hemicellulose Hydrolysis in the Presence of Heterogeneous Catalysts. Top Catal. 2014, 57, 1470–1475. [Google Scholar] [CrossRef]
- Yuan, Z.; Dai, W.; Zhang, S.; Wang, F.; Jian, J.; Zeng, J.; Zhou, H. Heterogeneous Strategies for Selective Conversion of Lignocellulosic Polysaccharides. Cellulose 2022, 29, 3059–3077. [Google Scholar] [CrossRef]
- Qin, L.; Ma, J.; Tian, H.; Ma, Y.; Wu, Q.; Cheng, S.; Fan, G. Production of Xylooligosaccharides from Jiuzao by Autohydrolysis Coupled with Enzymatic Hydrolysis Using a Thermostable Xylanase. Foods 2022, 11, 2663. [Google Scholar] [CrossRef]
- Sajib, M.; Falck, P.; Sardari, R.R.R.; Mathew, S.; Grey, C.; Karlsson, E.N.; Adlercreutz, P. Valorization of Brewer’s Spent Grain to Prebiotic Oligosaccharide: Production, Xylanase Catalyzed Hydrolysis, in-Vitro Evaluation with Probiotic Strains and in a Batch Human Fecal Fermentation Model. J. Biotechnol. 2018, 268, 61–70. [Google Scholar] [CrossRef]
- Swart, L.J.; Bedzo, O.K.K.; van Rensburg, E.; Görgens, J.F. Intensification of Xylo-Oligosaccharides Production by Hydrothermal Treatment of Brewer’s Spent Grains: The Use of Extremely Low Acid Catalyst for Reduction of Degradation Products Associated with High Solid Loading. Appl. Biochem. Biotechnol. 2021, 193, 1979–2003. [Google Scholar] [CrossRef] [PubMed]
- Poletto, P.; Pereira, G.N.; Monteiro, C.R.M.; Pereira, M.A.F.; Bordignon, S.E.; de Oliveira, D. Xylooligosaccharides: Transforming the Lignocellulosic Biomasses into Valuable 5-Carbon Sugar Prebiotics. Process Biochem. 2020, 91, 352–363. [Google Scholar] [CrossRef]
- Delgado Arcaño, Y.; Valmaña García, O.D.; Mandelli, D.; Carvalho, W.A.; Magalhães Pontes, L.A. Xylitol: A Review on the Progress and Challenges of Its Production by Chemical Route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Xu, Y.; Chi, P.; Bilal, M.; Cheng, H. Biosynthetic Strategies to Produce Xylitol: An Economical Venture. Appl. Microbiol. Biotechnol. 2019, 103, 5143–5160. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Mishra, D.K.; Hwang, J.S. Catalytic Hydrogenation of Xylose to Xylitol Using Ruthenium Catalyst on NiO Modified TiO2 Support. Appl. Catal. A Gen. 2012, 425–426, 110–116. [Google Scholar] [CrossRef]
- Ullah, N.; Jérôme, F.; De Oliveira Vigier, K. Hydrogenation of Xylose to Xylitol in the Presence of Bimetallic Nanoparticles Ni3Fe Catalyst in the Presence of Choline Chloride. Catalysts 2022, 12, 841. [Google Scholar] [CrossRef]
- Audemar, M.; Ramdani, W.; Junhui, T.; Raluca Ifrim, A.; Ungureanu, A.; Jérôme, F.; Royer, S.; de Oliveira Vigier, K. Selective Hydrogenation of Xylose to Xylitol over Co/SiO2 Catalysts. ChemCatChem 2020, 12, 1973–1978. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, L.; Hu, H.; Zuo, S.; Yang, L. Efficient Hydrogenation of Xylose and Hemicellulosic Hydrolysate to Xylitol over Ni-Re Bimetallic Nanoparticle Catalyst. Nanomaterials 2019, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and Applications-A Review. Front. Sustain. 2022, 3, 2. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rempel, A.; Cerqueira, M.A.; Camargo, A.F.; Gullón, P.; Scapini, T.; Rodríguez-Jasso, R.M.; Colla, L.; Gullón, B.; Treichel, H. Sustainable Biorefinery Processing for Hemicellulose Fractionation and Bio-Based Products in a Circular Bioeconomy. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 39–69. [Google Scholar]
- Mussatto, S.I.; Roberto, I.C. Acid Hydrolysis and Fermentation of Brewer’s Spent Grain to Produce Xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A.; Dávila, J.A.; Rosenberg, M.; Cardona, C.A. A Biorefinery Approach for the Production of Xylitol, Ethanol and Polyhydroxybutyrate from Brewer’s Spent Grain. AIMS Agric. Food 2016, 1, 52–66. [Google Scholar] [CrossRef]
- Swart, L.J.; Petersen, A.M.; Bedzo, O.K.K.; Görgens, J.F. Techno-Economic Analysis of the Valorization of Brewers Spent Grains: Production of Xylitol and Xylo-Oligosaccharides. J. Chem. Technol. Biotechnol. 2021, 96, 1632–1644. [Google Scholar] [CrossRef]
- Araújo, D.; Costa, T.; Freitas, F. Biovalorization of Lignocellulosic Materials for Xylitol Production by the Yeast Komagataella Pastoris. Appl. Sci. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Pascual, A.R.; Víctor, E.E.; Martín, C.; Broda, M.; Yelle, D.J.; Serwá Nska, K. Bioethanol Production from Lignocellulosic Biomass—Challenges and Solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Correa, C.; Alves, Y.A.; Souza, C.G.; Boloy, R.A.M. Brazil and the World Market in the Development of Technologies for the Production of Second-Generation Ethanol. Alex. Eng. J. 2023, 67, 153–170. [Google Scholar] [CrossRef]
- Ha, S.J.; Galazka, J.M.; Kim, S.R.; Choi, J.H.; Yang, X.; Seo, J.H.; Glass, N.L.; Cate, J.H.D.; Jin, Y.S. Engineered Saccharomyces Cerevisiae Capable of Simultaneous Cellobiose and Xylose Fermentation. Proc. Natl. Acad. Sci. USA 2011, 108, 504–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadioto, V.; Deoti, J.R.; Müller, C.; de Souza, B.R.; Fogolari, O.; Purificação, M.; Giehl, A.; Deoti, L.; Lucaroni, A.C.; Matsushika, A.; et al. Prospecting and Engineering Yeasts for Ethanol Production under Inhibitory Conditions: An Experimental Design Analysis. Bioprocess Biosyst. Eng. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Narisetty, V.; Cox, R.; Bommareddy, R.; Agrawal, D.; Ahmad, E.; Pant, K.K.; Chandel, A.K.; Bhatia, S.K.; Kumar, D.; Binod, P.; et al. Valorisation of Xylose to Renewable Fuels and Chemicals, an Essential Step in Augmenting the Commercial Viability of Lignocellulosic Biorefineries. Sustain. Energy Fuels 2021, 6, 29–65. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Cara, C.; Romero, I.; Ruiz, E.; Romero-García, J.M.; Mussatto, S.I.; Castro, E. Ethanol Production from Brewers’ Spent Grain Pretreated by Dilute Phosphoric Acid. Energy Fuels 2018, 32, 5226–5233. [Google Scholar] [CrossRef]
- Marcus, A.; Fox, G.; Grassi, S.; Paciulli, M. Fungal Biovalorization of a Brewing Industry Byproduct, Brewer’s Spent Grain: A Review. Foods 2021, 10, 2159. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, G.; lo Curto, R.B.; Waldron, K.W.; Faulds, C.B. Production of Feruloyl Esterases and Xylanases by Talaromyces Stipitatus and Humicola Grisea Var. Thermoidea on Industrial Food Processing byproducts. Bioresour. Technol. 2008, 99, 5130–5133. [Google Scholar] [CrossRef] [PubMed]
- Terrasan, C.R.F.; Temer, B.; Duarte, M.C.T.; Carmona, E.C. Production of Xylanolytic Enzymes by Penicillium Janczewskii. Bioresour. Technol. 2010, 101, 4139–4143. [Google Scholar] [CrossRef] [PubMed]
- Terrasan, C.R.F.; Carmona, E.C. Solid-State Fermentation of Brewer’s Spent Grain for Xylanolytic Enzymes Production by Penicillium Janczewskii and Analyses of the Fermented Substrate. Biosci. J. 2015, 31, 1826–1836. [Google Scholar] [CrossRef] [Green Version]
- Faria, N.T.; Marques, S.; Ferreira, F.C.; Fonseca, C. Production of Xylanolytic Enzymes by Moesziomyces Spp. Using Xylose, Xylan and Brewery’s Spent Grain as Substrates. New Biotechnol. 2019, 49, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dilokpimol, A.; Kabel, M.A.; de Vries, R.P. Fungal Xylanolytic Enzymes: Diversity and Applications. Bioresour. Technol. 2022, 344, 126290. [Google Scholar] [CrossRef]
- Macedo, J.V.C.; Martins, J.R.; Abe, M.M.; Branciforti, M.C.; Brienzo, M. Hemicellulose Application for the Production of Bioplastics and Biomaterials. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 231–273. [Google Scholar]
- Qazanfarzadeh, Z.; Ganesan, A.R.; Mariniello, L.; Conterno, L.; Kumaravel, V. Valorization of Brewer’s Spent Grain for Sustainable Food Packaging. J. Clean Prod. 2023, 385, 135726. [Google Scholar] [CrossRef]
- De Lucena, C.A.A.; da Costa, S.C.; Eleamen, G.R.D.A.; Mendonça, E.A.D.M.; Oliveira, E.E. Desenvolvimento de Biofilmes à Base de Xilana e Xilana/Gelatina Para Produção de Embalagens Biodegradáveis. Polímeros 2017, 27, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and Antimicrobial Films Based on Brewers Spent Grain Arabinoxylans, Nanocellulose and Feruloylated Compounds for Active Packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Pérez-Flores, J.G.; Contreras-López, E.; Castañeda-Ovando, A.; Pérez-Moreno, F.; Aguilar-Arteaga, K.; Álvarez-Romero, G.A.; Téllez-Jurado, A. Physicochemical Characterization of an Arabinoxylan-Rich Fraction from Brewers’ Spent Grain and Its Application as a Release Matrix for Caffeine. Food Res. Int. 2019, 116, 1020–1030. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and Disadvantages of Bioplastics Production from Starch and Lignocellulosic Components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Urruzola, I.; Labidi, J. Xylan—Cellulose Films: Improvement of Hydrophobicity, Thermal and Mechanical Properties. Carbohydr. Polym. 2014, 112, 56–62. [Google Scholar] [CrossRef]
- Kayserilioǧlu, B.Ş.; Bakir, U.; Yilmaz, L.; Akkaş, N. Use of Xylan, an Agricultural byproduct, in Wheat Gluten Based Biodegradable Films: Mechanical, Solubility and Water Vapor Transfer Rate Properties. Bioresour. Technol. 2003, 87, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Shen, F.; Wang, F.; Tian, D.; Hu, J. Synthesis, Characterization and Enzymatic Surface Roughing of Cellulose/Xylan Composite Films. Carbohydr. Polym. 2019, 213, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.H.; Yang, H.J.; Song, K.B. Preparation and Characterization of Brewer’s Spent Grain Protein-Chitosan Composite Films. J. Food Sci. Technol. 2015, 52, 7549–7555. [Google Scholar] [CrossRef]
- Xu, J.; Xia, R.; Zheng, L.; Yuan, T.; Sun, R. Plasticized Hemicelluloses/Chitosan-Based Edible Films Reinforced by Cellulose Nanofiber with Enhanced Mechanical Properties. Carbohydr. Polym. 2019, 224, 115164. [Google Scholar] [CrossRef] [PubMed]
- Global Information Inc. Furfuryl Alcohol Market Size, Share & Trends Analysis Report by Application (Resins, Solvents, Corrosion Inhibitors), by End Use (Foundry, Agriculture, Food & Beverages), by Region, and Segment Forecasts, 2021–2028. Available online: https://www.giiresearch.com/report/grvi1044783-furfuryl-alcohol-market-size-share-trends-analysis.html (accessed on 23 February 2023).
- Liu, H.; Lin, Q.; Li, R.; Chang, M.; Ren, J. Synthesis of Furan Compounds from Hemicelluloses. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 399–445. [Google Scholar]
- Mazar, A.; Ajao, O.; Benali, M.; Jemaa, N.; Wafa Al-Dajani, W.; Paleologou, M. Integrated Multiproduct Biorefinery for Furfural Production with Acetic Acid and Lignin Recovery: Design, Scale-Up Evaluation, and Technoeconomic Analysis. ACS Sustain. Chem. Eng. 2020, 8, 17345–17358. [Google Scholar] [CrossRef]
- Bariani, M.; Boix, E.; Cassella, F.; Cabrera, M.N. Furfural Production from Rice Husks within a Biorefinery Framework. Biomass Convers. Biorefin. 2021, 11, 781–794. [Google Scholar] [CrossRef]
- Cousin, E.; Namhaed, K.; Pérès, Y.; Cognet, P.; Delmas, M.; Hermansyah, H.; Gozan, M.; Alaba, P.A.; Aroua, M.K. Towards Efficient and Greener Processes for Furfural Production from Biomass: A Review of the Recent Trends. Sci. Total Environ. 2022, 847, 157599. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Wang, X.; Lu, X.; Qi, X.; Yu, H. Recent Progress in Furfural Production from Hemicellulose and Its Derivatives: Conversion Mechanism, Catalytic System, Solvent Selection. Mol. Catal. 2021, 515, 111899. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Si, C.; Lu, L.; Luo, C.; Gu, X.; Liu, W.; Lu, X. Green and Efficient Production of Furfural from Corn Cob over H-ZSM-5 Using γ-Valerolactone as Solvent. Ind. Crops Prod. 2018, 120, 343–350. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; An, S.; Huang, F.; Li, X.; Liu, J.; Pei, G.; Liu, Q. Efficient Transformation of Corn Stover to Furfural Using P-Hydroxybenzenesulfonic Acid-Formaldehyde Resin Solid Acid. Bioresour. Technol. 2018, 264, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pan, D.; Wu, Y.; Song, X.; Gao, L.; Li, W.; Das, L.; Xiao, G. Efficient Production of Furfural from Xylose and Wheat Straw by Bifunctional Chromium Phosphate Catalyst in Biphasic Systems. Fuel Process. Technol. 2018, 175, 90–96. [Google Scholar] [CrossRef]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of Sugars and Biomass to Furans Using Heterogeneous Catalysts in Biphasic Solvent Systems. ChemCatChem 2018, 10, 4805–4816. [Google Scholar] [CrossRef] [Green Version]
- Upare, P.P.; Hong, D.Y.; Kwak, J.; Lee, M.; Chitale, S.K.; Chang, J.S.; Hwang, D.W.; Hwang, Y.K. Direct Chemical Conversion of Xylan into Furfural over Sulfonated Graphene Oxide. Catal. Today 2019, 324, 66–72. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Lin, Q.; Li, R.; Li, W.; Peng, F.; Ren, J. Efficient Catalytic Conversion of Dilute-Oxalic Acid Pretreated Bagasse Hydrolysate to Furfural Using Recyclable Ironic Phosphates Catalysts. Bioresour. Technol. 2019, 290, 121764. [Google Scholar] [CrossRef]
- Sweygers, N.; Harrer, J.; Dewil, R.; Appels, L. A Microwave-Assisted Process for the in-Situ Production of 5-Hydroxymethylfurfural and Furfural from Lignocellulosic Polysaccharides in a Biphasic Reaction System. J. Clean Prod. 2018, 187, 1014–1024. [Google Scholar] [CrossRef]
- Wang, Z.K.; Shen, X.J.; Chen, J.J.; Jiang, Y.Q.; Hu, Z.Y.; Wang, X.; Liu, L. Lignocellulose Fractionation into Furfural and Glucose by AlCl3-Catalyzed DES/MIBK Biphasic Pretreatment. Int. J. Biol. Macromol. 2018, 117, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Luo, Y.; Jiang, Z.; Fang, Q.; Hu, C. The Promotion Effect of NaCl on the Conversion of Xylose to Furfural†. Chin. J. Chem. 2020, 38, 178–184. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Mao, J.; Ramaswamy, S.; Zhang, X.; Xu, F. Insights on the Efficiency of Bifunctional Solid Organocatalysts in Converting Xylose and Biomass into Furfural in a GVL-Water Solvent. Ind. Crops Prod. 2019, 138, 111454. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.; Lusi, A.; Jacoby, W.A.; Wan, C. One-Pot Selective Conversion of Lignocellulosic Biomass into Furfural and Co-Products Using Aqueous Choline Chloride/Methyl Isobutyl Ketone Biphasic Solvent System. Bioresour. Technol. 2019, 289, 121708. [Google Scholar] [CrossRef]
- Rozenfelde, L.; Puke, M.; Vedernikovs, N.; Scherbaka, R.; Rapoport, A. Catalytic Treatment of Rapeseed Straw for Enhanced Production of Furfural and Glucose for Bioethanol Production. Process Biochem. 2021, 102, 102–107. [Google Scholar] [CrossRef]
- Tongtummachat, T.; Jaree, A.; Akkarawatkhoosith, N. Continuous Hydrothermal Furfural Production from Xylose in a Microreactor with Dual-Acid Catalysts. RSC Adv. 2022, 12, 23366–23378. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cai, T.; Yin, X.; Liang, J.; Jia, S.; Zhang, X.; Xu, J.; Hu, J.; Jiang, J.; Wang, K. A Sustainable and Profitable Biorefinery Strategy for Efficiently Converting Lignocellulose to Furfural, Glucose and Phenolic Compounds. Green Chem. 2022, 24, 8494–8502. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, S.; Wu, Y. Recent Advances in Aqueous-Phase Catalytic Conversions of Biomass Platform Chemicals Over Heterogeneous Catalysts. Front. Chem. 2019, 7, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilpmann, G.; Steudler, S.; Ayubi, M.M.; Pospiech, A.; Walther, T.; Bley, T.; Lange, R. Combining Chemical and Biological Catalysis for the Conversion of Hemicelluloses: Hydrolytic Hydrogenation of Xylan to Xylitol. Catal. Lett. 2019, 149, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, E.M.; Fraga, M.A. Production of Platform Chemicals and High Value Products from Hemicellulose. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Singapore, 2022; pp. 361–397. [Google Scholar]
- Sheldon, R.A. Biocatalysis and Biomass Conversion: Enabling a Circular Economy. Philos. Trans. R. Soc. A 2020, 378, 20190274. [Google Scholar] [CrossRef] [PubMed]
- Mariño, M.A.; Fulaz, S.; Tasic, L. Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications. Magnetochemistry 2021, 7, 133. [Google Scholar] [CrossRef]
- Hu, J.; Davies, J.; Mok, Y.K.; Arato, C.; Saddler, J.N. The Potential of Using Immobilized Xylanases to Enhance the Hydrolysis of Soluble, Biomass Derived Xylooligomers. Materials 2018, 11, 2005. [Google Scholar] [CrossRef] [Green Version]
- Bayer, E.A.; Laura, A.; Benatti, T.; De, M.; Teixeira, L.; Polizeli, M. Lignocellulolytic Biocatalysts: The Main Players Involved in Multiple Biotechnological Processes for Biomass Valorization. Microorganisms 2023, 11, 162. [Google Scholar] [CrossRef]



| Database | Brewers Spent Grain + Biorefinery | Brewers Spent Grain + Hemicellulose + Hydrolysis | Brewers Spent Grain + Catalyst | Brewers Spent Grain + Hemicellulose + Catalyst | Hemicellulose + Biorefinery + Catalyst | Catalysis + Mechanism + Hemicellulose |
|---|---|---|---|---|---|---|
| Scopus | 61 | 35 | 21 | 3 | 136 | 145 |
| Web of Science | 103 | 47 | 12 | 1 | 196 | 134 |
| Science Direct | 328 | 561 | 381 | 253 | 3441 | 4720 |
| Wiley | 148 | 367 | 1062 | 226 | 1476 | 3183 |
| Springer | 127 | 188 | 157 | 73 | 1235 | 1632 |
| Component | BSG [16] | BSG [17] | BSG [18] | BSG [19] | SCB [20] | PM [21] | RH [22] |
|---|---|---|---|---|---|---|---|
| Cellulose | 15.99 ± 0.88 | 19.2 ± 1.40 | 15.2 ± 0.50 | 19.24 | 39.52 ± 0.66 | 40.99 ± 2.50 | 31.12 |
| Hemicellulose | 29.92 ± 1.60 | 18.4 ± 3.70 | 25.1 ± 0.70 | 53.09 | 25.63 ± 0.44 | 20.90 ± 2.00 | 22.48 |
| Xylan | 25.22 ± 1.46 | 11.3 ± 1.20 | 16.9 ± 0.70 | - | - | - | - |
| Arabinan | 4.71 ± 0.14 | - | 6.6 ± 0.30 | - | - | - | - |
| Total Lignin | 20.80 ± 0.42 | - | 12.5 ± 0.80 | 8.53 | 30.36 ± 0.13 | 18.3 ± 1.80 | 22.34 |
| Soluble Lignin | 3.02 ± 0.01 | 9.9 ± 1.40 | 5.5 ± 0.30 | - | 3.60 ± 0.90 | 18.20 ± 1.80 | - |
| Insoluble Lignin | 17.78 ± 0.41 | - | 7.0 ± 0.50 | - | 26.40 ± 0.20 | 0.06 ± 0.01 | - |
| Starch | - | 4.8 ± 0.46 | 5.3 ± 0.20 | - | - | - | - |
| Lipids | - | 5.2 ± 2.10 | - | - | - | - | - |
| Proteins | 21.16 ± 0.61 | 26.6 ± 0.38 | 21.2 ± 0.20 | 17.25 | - | - | - |
| Ashes | 3.76 ± 0.05 | 2.7 ± 0.07 | 2.3 ± 0.10 | 3.68 | 1.45 ± 0.21 | 5.96 ± 0.50 | 13.87 |
| Extractives | 8.33 ± 0.76 | - | 18.5 ± 1.00 | - | 3.04 ± 0.17 | 19.30 ± 1.40 | 2.33 |
| Substrate | Catalyst | Type of Catalyst | Yield (%) | Reference |
|---|---|---|---|---|
| Cornbob | H-ZSM-5 | Heterogeneous | 71.7 | [111] |
| Corn stover | MSPFR 1 | Heterogeneous | 50.3 | [112] |
| Wheat straw | CrPO4 | Heterogeneous | 67.0 | [113] |
| Bagasse | H-USY | Heterogeneous | 55.0 | [114] |
| Xylan | GO-SO3H 2 | Heterogeneous | 87.0 | [115] |
| Xylose | FePO4 | Heterogeneous | 88.7 | [116] |
| Bamboo | HCl | Homogeneous | 46.0 | [117] |
| Eucalyptus | AlCl3 | Homogeneous | 70.3 | [118] |
| Xylose | NaCl | Homogeneous | 76.7 | [119] |
| Tung shell | H3NSO3 | Homogeneous | 92.2 | [120] |
| Switchgrass | H2SO4 | Homogeneous | 84.0 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, A.R.; Dresch, A.P.; Alves Junior, S.L.; Bender, J.P.; Treichel, H. Applications of Brewer’s Spent Grain Hemicelluloses in Biorefineries: Extraction and Value-Added Product Obtention. Catalysts 2023, 13, 755. https://doi.org/10.3390/catal13040755
Schmidt AR, Dresch AP, Alves Junior SL, Bender JP, Treichel H. Applications of Brewer’s Spent Grain Hemicelluloses in Biorefineries: Extraction and Value-Added Product Obtention. Catalysts. 2023; 13(4):755. https://doi.org/10.3390/catal13040755
Chicago/Turabian StyleSchmidt, Aline Ruth, Aline Perin Dresch, Sergio Luiz Alves Junior, João Paulo Bender, and Helen Treichel. 2023. "Applications of Brewer’s Spent Grain Hemicelluloses in Biorefineries: Extraction and Value-Added Product Obtention" Catalysts 13, no. 4: 755. https://doi.org/10.3390/catal13040755









