Fe-N-C Catalyst Derived from MOFs with Enhanced Catalytic Performance for Selective Oxidation of Emerging Contaminants
Abstract
:1. Introduction
2. Results and Discussion
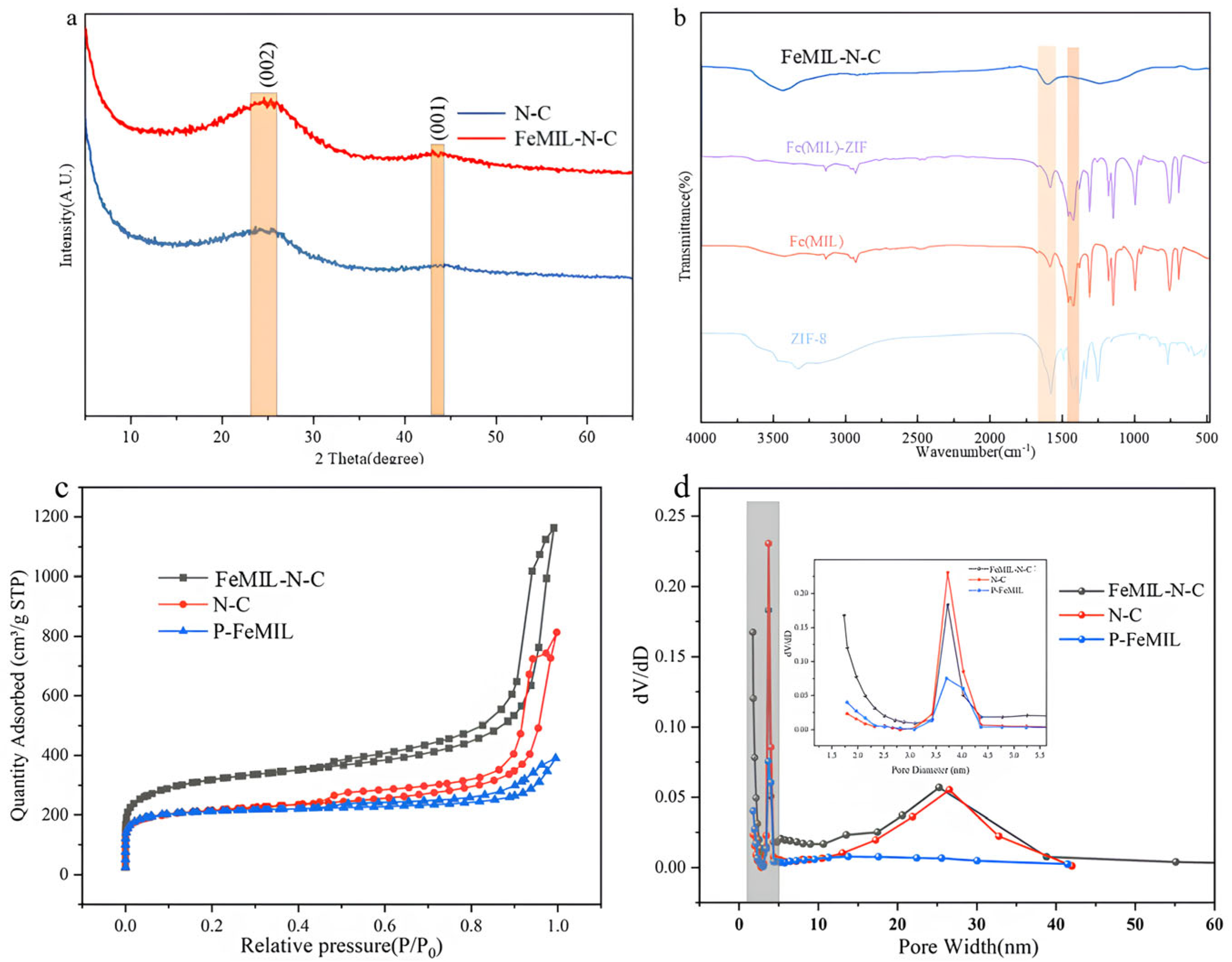
2.1. Characterization of Catalysts
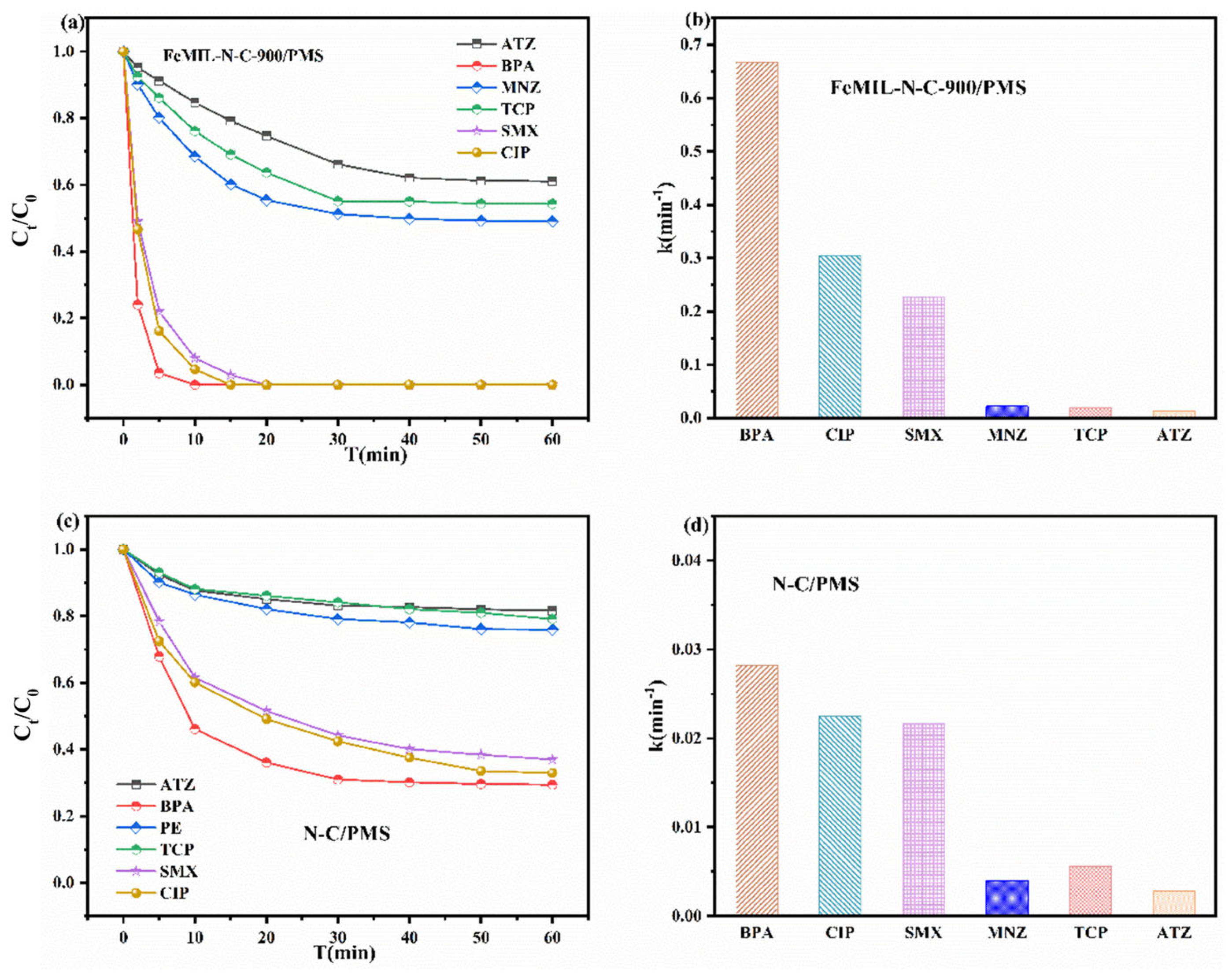
2.2. Catalytic Oxidation of Different Pollutants in the FeMIL-N-C/PMS System
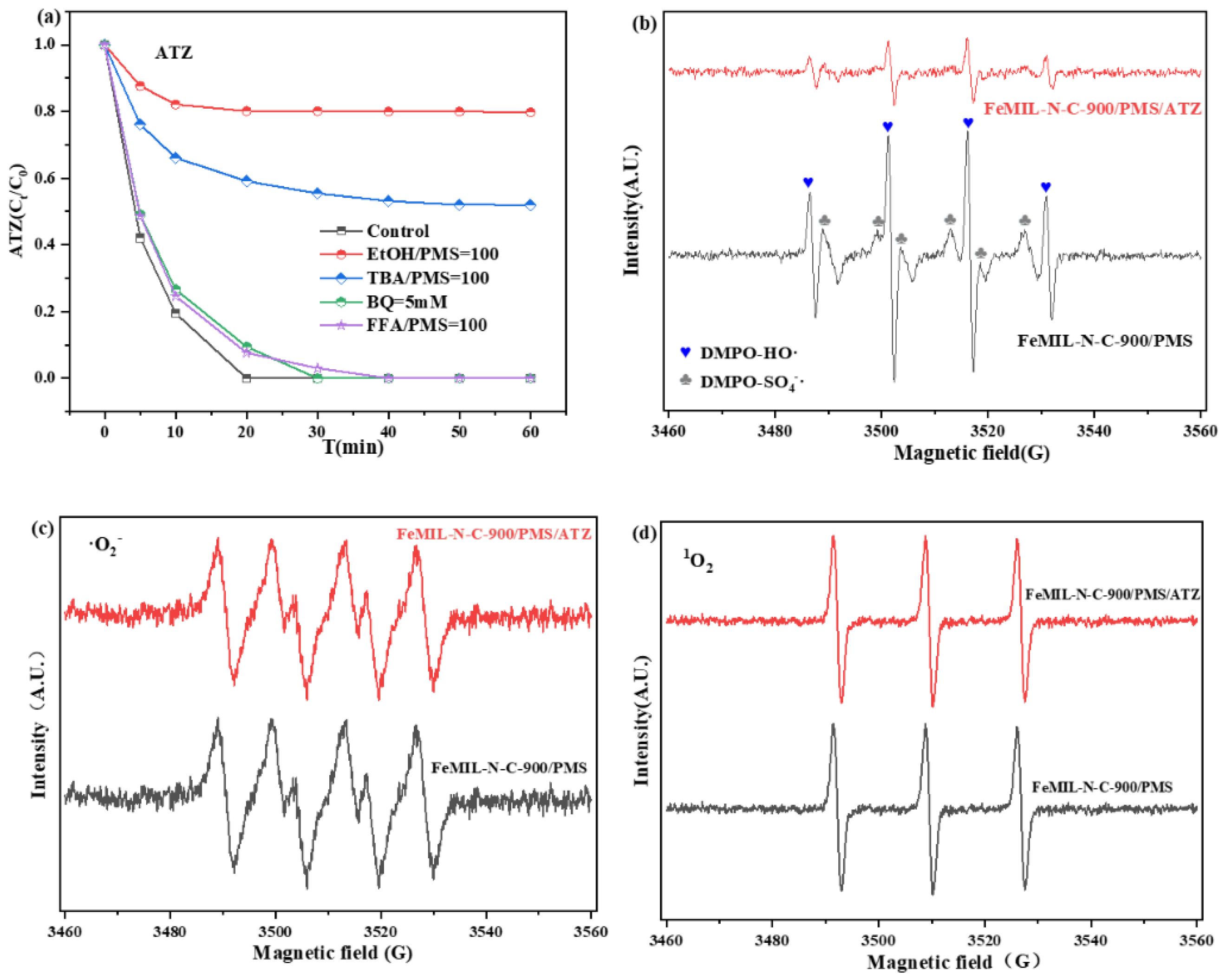
2.3. ROS Determination
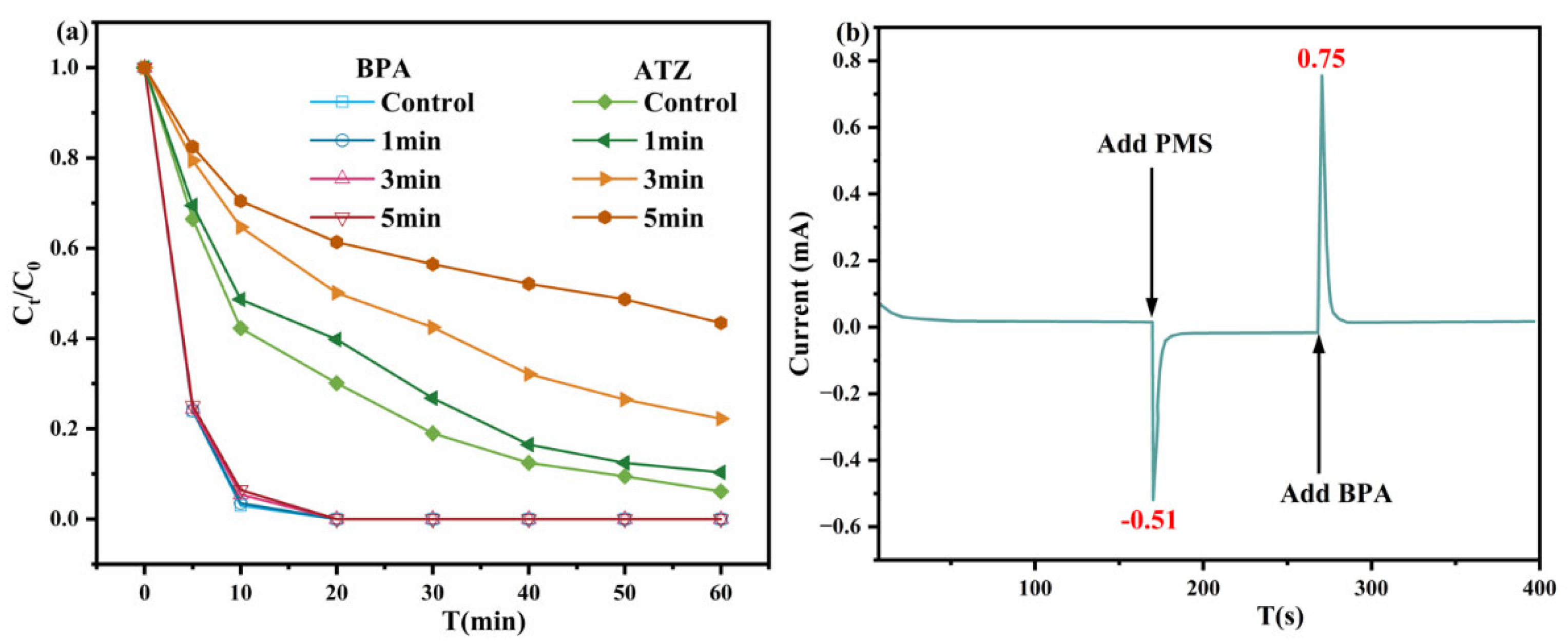
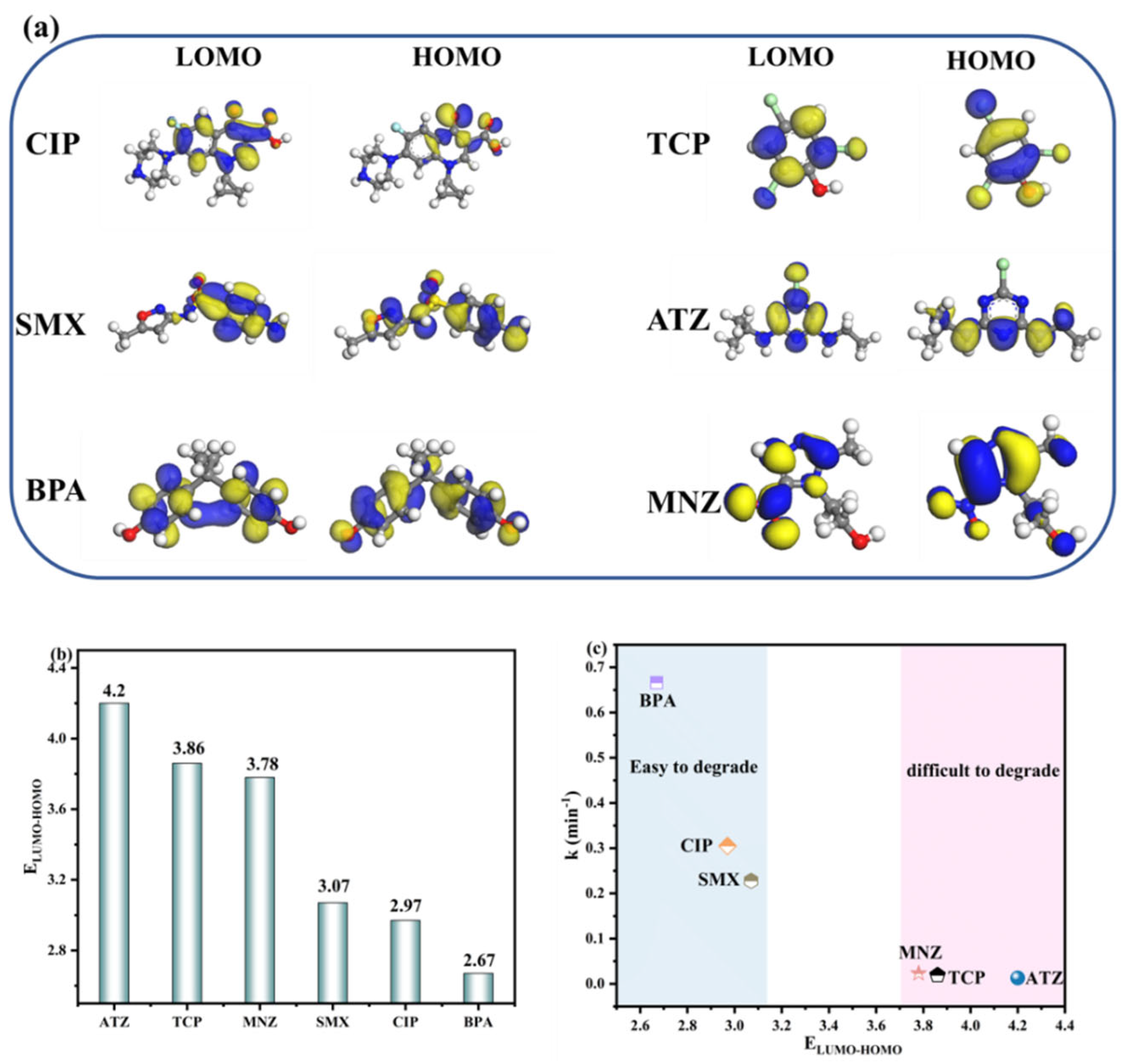
2.4. Study of Degradation Mechanisms
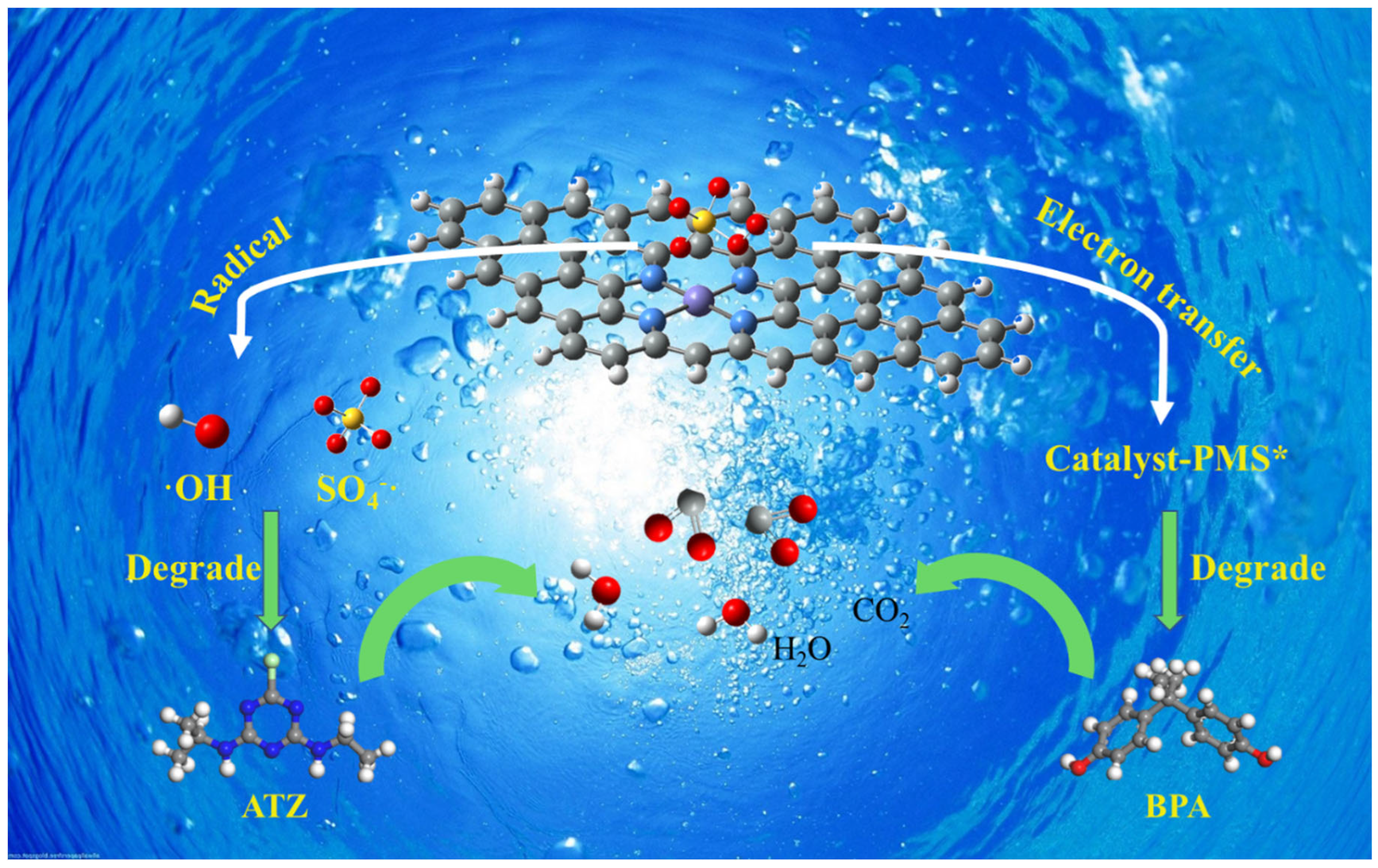
2.5. Selective Oxidation Mechanism
3. Experimental Section
3.1. Chemicals
3.2. Synthesis of Catalyst
3.3. Characterization
3.4. Experimental Procedure
3.5. Analytical Methods
3.6. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Chu, L.; Wojnarovits, L.; Takacs, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Du, H.; Yang, Z.; Tian, Z.; Huang, M.; Yang, W.; Zhang, L.; Li, A. Enhanced removal of trace antibiotics from turbid water in the coexistence of natural organic matters using phenylalanine-modified-chitosan flocculants: Effect of flocculants’ molecular architectures. Chem. Eng. J. 2018, 333, 310–319. [Google Scholar] [CrossRef]
- Rozas, O.; Vidal, C.; Baeza, C.; Jardim, W.F.; Rossner, A.; Mansilla, H.D. Organic micropollutants (OMPs) in natural waters: Oxidation by UV/H2O2 treatment and toxicity assessment. Water Res. 2016, 98, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, T.; Zuo, S.; Wan, J.; Yan, Z.; Zhu, B.; Zhang, X. Exploring degradation properties and mechanisms of emerging contaminants via enhanced directional electron transfer by polarized electric fields regulation in Fe-N4-Cx. J. Hazard. Mater. 2023, 446, 130698. [Google Scholar] [CrossRef]
- Du, Z.; Qin, J.; Zhang, K.; Jia, L.; Tian, K.; Zhang, J.; Xie, H. N-doped carbon nanosheets supported-single Fe atom for p-nitrophenol degradation via peroxymonosulfate activation. Appl. Surf. Sci. 2022, 591, 153124. [Google Scholar] [CrossRef]
- Xu, X.; Zhan, F.; Pan, J.; Zhou, L.; Su, L.; Cen, W.; Li, W.; Tian, C. Engineering single-atom Fe-Pyridine N4 sites to boost peroxymonosulfate activation for antibiotic degradation in a wide pH range. Chemosphere 2022, 294, 133735. [Google Scholar] [CrossRef]
- Lu, D.L.; Chen, Z.; Yang, Q.D.; Han, S. Efficient novel FeOCl/C with high singlet oxygen generation for TCH degradation. Chem. Phys. Lett. 2022, 800, 139664. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, Y.; Wan, J.; Ma, Y.; Yan, Z.; Huang, S.; Zeng, C. Fe-N-C catalyst with Fe-N-X sites anchored nano carboncubes derived from Fe-Zn-MOFs activate peroxymonosulfate for high-effective degradation of ciprofloxacin: Thermal activation and catalytic mechanism. J. Hazard. Mater. 2022, 424, 127380. [Google Scholar] [CrossRef] [PubMed]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Li, H.X.; Wan, J.Q.; Ma, Y.W.; Wang, Y. Reaction pathway and oxidation mechanisms of dibutyl phthalate by persulfate activated with zero-valent iron. Sci. Total Environ. 2016, 562, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Ai-Abed, S.R.; Dionysiou, D.D. Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B-Environ. 2009, 85, 171–179. [Google Scholar] [CrossRef]
- Waclawek, S.; Lutze, H.V.; Grubel, K.; Padil, V.V.T.; Cernik, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Rastogi, A.; Al-Abed, S.R.; Dionysiou, D.D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols. Water Res. 2009, 43, 684–694. [Google Scholar] [CrossRef]
- Zhang, R.C.; Yang, Y.K.; Huang, C.H.; Zhao, L.; Sun, P.Z. Kinetics and modeling of sulfonamide antibiotic degradation in wastewater and human urine by UV/H2O2 and UV/PDS. Water Res. 2016, 103, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Z.; Li, L.; Lin, K.F.; Zhang, W.; Lu, S.G.; Luo, Q.S. Removal of 1,1,1-trichloroethane from aqueous solution by a sono-activated persulfate process. Ultrason. Sonochemistry 2013, 20, 855–863. [Google Scholar] [CrossRef]
- Ji, Y.F.; Fan, Y.; Liu, K.; Kong, D.Y.; Lu, J.H. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ji, Y.F.; Kong, D.Y.; Lu, J.H.; Zhou, Q.S. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.G.; Sun, H.Q.; Shao, Z.P.; Wang, S.B. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B-Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Zhou, L.; Sun, H.Q.; Wang, G.X.; Wang, S.B. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl. Catal. B-Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Shang, Y.N.; Xu, X.; Gao, B.Y.; Wang, S.B.; Duan, X.G. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281–5322. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Wei, X.; Gao, E.; Zhang, C.; Hu, X.-M.; Chen, Y.; Liu, Z.; Finck, N.; Luetzenkirchen, J.; Dionysiou, D.D. Single Fe atoms confined in two-dimensional MoS2 for sulfite activation: A biomimetic approach towards efficient radical generation. Appl. Catal. B-Environ. 2020, 268, 118459. [Google Scholar] [CrossRef]
- Qian, K.; Chen, H.; Li, W.; Ao, Z.; Wu, Y.-N.; Guan, X. Single-Atom Fe Catalyst Outperforms Its Homogeneous Counterpart for Activating Peroxymonosulfate to Achieve Effective Degradation of Organic Contaminants. Environ. Sci. Technol. 2021, 55, 7034–7043. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, T.; Qiu, S.; Lin, W.; Yan, J.; Fan, S.; Zhou, Q. Uniform N-coordinated single-atomic iron sites dispersed in porous carbon framework to activate PMS for efficient BPA degradation via high-valent iron-oxo species. Chem. Eng. J. 2020, 389, 124382. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Xiao, M.; Xing, Z.; Jin, Z.; Liu, C.; Ge, J.; Zhu, J.; Wang, Y.; Zhao, X.; Chen, Z. Preferentially Engineering FeN4 Edge Sites onto Graphitic Nanosheets for Highly Active and Durable Oxygen Electrocatalysis in Rechargeable Zn–Air Batteries. Adv. Mater. 2020, 32, 2004900. [Google Scholar] [CrossRef] [PubMed]
- Nabae, Y.; Nagata, S.; Kusaba, K.; Aoki, T.; Hayakawa, T.; Tanida, H.; Imai, H.; Hori, K.; Yamamoto, Y.; Arai, S.; et al. Magnetic purification of non-precious metal fuel cell catalysts for obtaining atomically dispersed Fe centers. Catal. Sci. Technol. 2020, 10, 493–501. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.K.; Yu, H.J.; Wang, L.; Wang, M.Y.; Liu, X.W.; Shen, D.; Shen, S.D.; Ren, S.N.; Lin, T.F.; Lei, S.Y. Ferrocene doped ZIF-8 derived Fe-N-C single atom catalyst to active peroxymonosulfate for removal of bisphenol A. Sep. Purif. Technol. 2023, 305, 122402. [Google Scholar] [CrossRef]
- Miao, J.; Zhu, Y.; Lang, J.Y.; Zhang, J.Z.; Cheng, S.X.; Zhou, B.X.; Zhang, L.Z.; Alvarez, P.J.J.; Long, M.C. Spin-State-Dependent Peroxymonosulfate Activation of Single-Atom M-N Moieties via a Radical-Free Pathway. ACS Catal. 2021, 11, 9569–9577. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Gao, Y.W.; Zhu, Y.; Li, T.; Chen, Z.H.; Jiang, Q.K.; Zhao, Z.Y.; Liang, X.Y.; Hu, C. Unraveling the High-Activity Origin of Single-Atom Iron Catalysts for Organic Pollutant Oxidation via Peroxymonosulfate Activation. Environ. Sci. Technol. 2021, 55, 8318–8328. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xiong, L.L.; Nie, G.; Zhang, H.; Duan, X.G.; Wang, S.B. Insights into the Electron-Transfer Regime of Peroxydisulfate Activation on Carbon Nanotubes: The Role of Oxygen Functional Groups. Environ. Sci. Technol. 2020, 54, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.G.; Wang, S.B. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef]
- Jiao, D.X.; Liu, Y.J.; Cai, Q.H.; Zhao, J.X. Coordination tunes the activity and selectivity of the nitrogen reduction reaction on single-atom iron catalysts: A computational study. J. Mater. Chem. A 2021, 9, 1240–1251. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.-L.; Xu, Q. Nanomaterials derived from metal-organic frameworks. Nat. Rev. Mater. 2018, 3, 17075. [Google Scholar] [CrossRef]
- Wu, W.; Lei, W.; Wang, L.; Wang, S.; Zhang, H. Preparation of Single Atom Catalysts. Prog. Chem. 2020, 32, 23–32. [Google Scholar] [CrossRef]
- Li, X.; Wan, J.; Wang, Y.; Chi, H.; Yan, Z.; Ding, S. Selective removal and persulfate catalytic decomposition of diethyl phthalate from contaminated water on modified MIL100 through surface molecular imprinting. Chemosphere 2020, 240, 124875. [Google Scholar] [CrossRef]
- Wang, L.; Wan, X.; Liu, S.Y.; Xu, L.; Shui, J.L. Fe-N-C catalysts for PEMFC: Progress towards the commercial application under DOE reference. J. Energy Chem. 2019, 39, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Xie, Y.; Li, C.; Zheng, J.; Si, M.; Xiao, R.; Liao, Q.; Yang, W. Non-radical dominated catalytic degradation of chlorophenol by a structure-tailored catalyst of high nitrogen doping carbon matrix with nano-CuO. J. Environ. Chem. Eng. 2022, 10, 107559. [Google Scholar] [CrossRef]
- He, J.; Wan, Y.; Zhou, W. ZIF-8 derived Fe-N coordination moieties anchored carbon nanocubes for efficient peroxymonosulfate activation via non-radical pathways: Role of FeNx sites. J. Hazard. Mater. 2021, 405, 124199. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Wang, B.; Kumar, A.; Qin, Y.; Jin, J.; Wang, X.; Yang, C.; Dong, B.; Jia, Y.; Liu, J.; et al. Recent Advances for MOF-Derived Carbon-Supported Single-Atom Catalysts. Small Methods 2019, 3, 1800471. [Google Scholar] [CrossRef]
- An, S.F.; Zhang, G.H.; Wang, T.W.; Zhan, W.N.; Li, K.Y.; Song, C.S.; Miller, J.T.; Miao, S.; Wang, J.H.; Guo, X.W. High-Density Ultra-small Clusters and Single-Atom Fe Sites Embedded in Graphitic Carbon Nitride (g-C3N4) for Highly Efficient Catalytic Advanced Oxidation Processes. ACS Nano 2018, 12, 9441–9450. [Google Scholar] [CrossRef]
- Pu, M.J.; Niu, J.F.; Brusseau, M.L.; Sun, Y.L.; Zhou, C.Z.; Deng, S.; Wan, J.Q. Ferrous metal-organic frameworks with strong electron-donating properties for persulfate activation to effectively degrade aqueous sulfamethoxazole. Chem. Eng. J. 2020, 394, 125044. [Google Scholar] [CrossRef]
- Wang, F.L.; Wang, Y.F.; Li, Y.Y.; Cui, X.H.; Zhang, Q.X.; Xie, Z.J.; Liu, H.J.; Feng, Y.P.; Lv, W.Y.; Liu, G.G. The facile synthesis of a single atom-dispersed silver-modified ultrathin g-C3N4 hybrid for the enhanced visible-light photocatalytic degradation of sulfamethazine with peroxymonosulfate. Dalton Trans. 2018, 47, 6924–6933. [Google Scholar] [CrossRef]
- Ao, X.W.; Liu, W.J. Degradation of sulfamethoxazole by medium pressure UV and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]
- Du, N.; Liu, Y.; Li, Q.; Miao, W.; Wang, D.; Mao, S. Peroxydisulfate activation by atomically-dispersed Fe-Nx on N-doped carbon: Mechanism of singlet oxygen evolution for nonradical degradation of aqueous contaminants. Chem. Eng. J. 2021, 413, 127545. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Huang, M.; Wang, Y.; Chen, Y. New insights into the role of zero-valent iron surface oxidation layers in persulfate oxidation of dibutyl phthalate solutions. Chem. Eng. J. 2014, 250, 137–147. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, F.-L.; Mi, Y.; Yan, C.; Zhao, S. Single-metal-atom catalysts: An emerging platform for electrocatalytic oxygen reduction. Chem. Eng. J. 2021, 406, 127135. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, D.; Li, J.; Dong, L.; Ong, W.-J.; He, Y. A highly efficient Fenton-like catalyst based on isolated diatomic Fe-Co anchored on N-doped porous carbon. Chem. Eng. J. 2021, 404, 126376. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; Wang, Y.; Wan, J.; Yan, Z. Fe-N-C Catalyst Derived from MOFs with Enhanced Catalytic Performance for Selective Oxidation of Emerging Contaminants. Catalysts 2023, 13, 1021. https://doi.org/10.3390/catal13061021
Zeng C, Wang Y, Wan J, Yan Z. Fe-N-C Catalyst Derived from MOFs with Enhanced Catalytic Performance for Selective Oxidation of Emerging Contaminants. Catalysts. 2023; 13(6):1021. https://doi.org/10.3390/catal13061021
Chicago/Turabian StyleZeng, Cheng, Yan Wang, Jinquan Wan, and Zhicheng Yan. 2023. "Fe-N-C Catalyst Derived from MOFs with Enhanced Catalytic Performance for Selective Oxidation of Emerging Contaminants" Catalysts 13, no. 6: 1021. https://doi.org/10.3390/catal13061021
APA StyleZeng, C., Wang, Y., Wan, J., & Yan, Z. (2023). Fe-N-C Catalyst Derived from MOFs with Enhanced Catalytic Performance for Selective Oxidation of Emerging Contaminants. Catalysts, 13(6), 1021. https://doi.org/10.3390/catal13061021



