Electrolessly Deposited Carbon-Supported CuNiSn Electrocatalysts for the Electrochemical Reduction of CO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Electroless Deposition Time on the Structure of CuNiSn/CS Electrocatalysts
2.2. Effect of the Electroless Deposition Time on the CO2-ERR Performance of the CuNiSn/CS Electrocatalysts
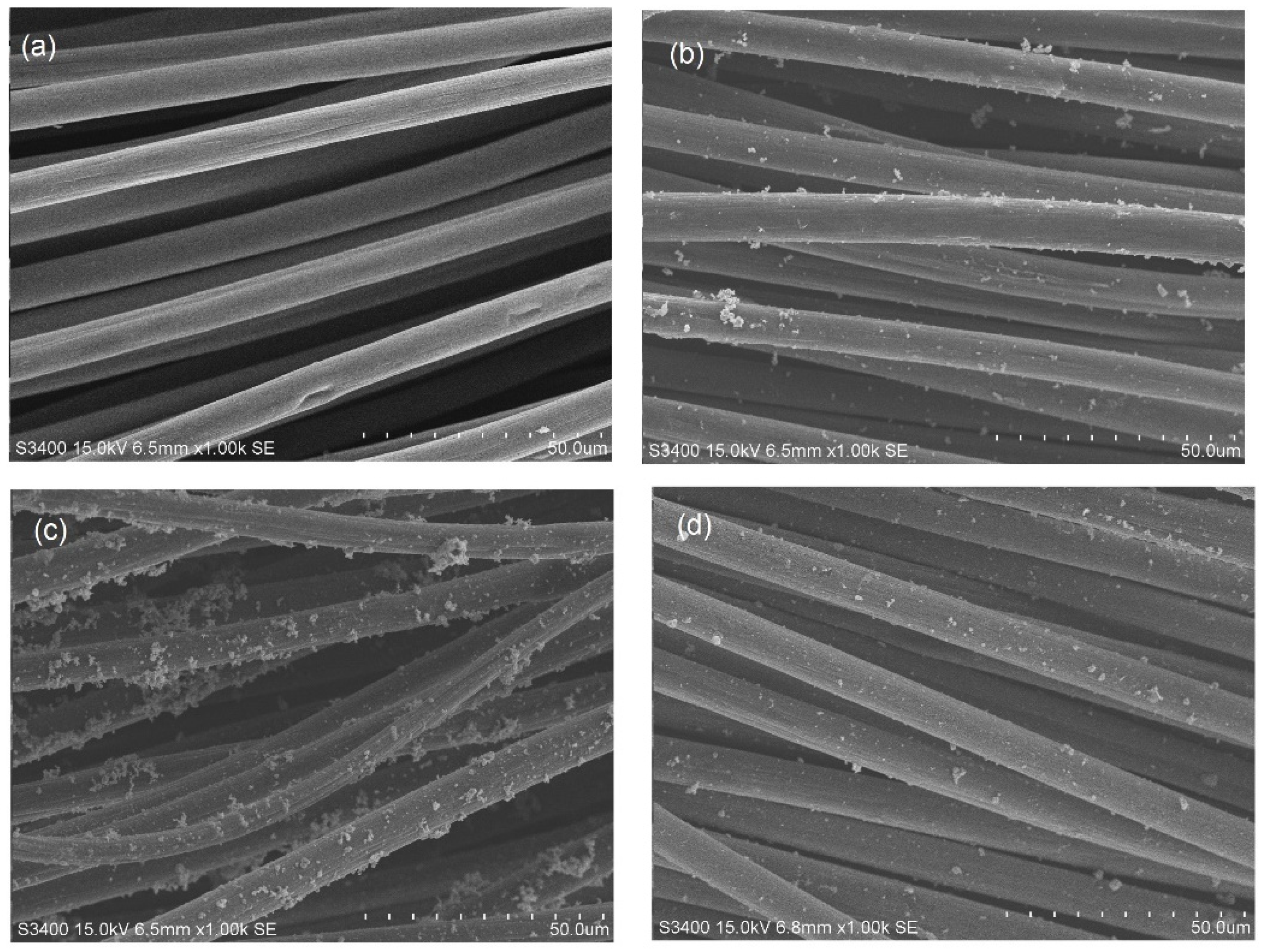
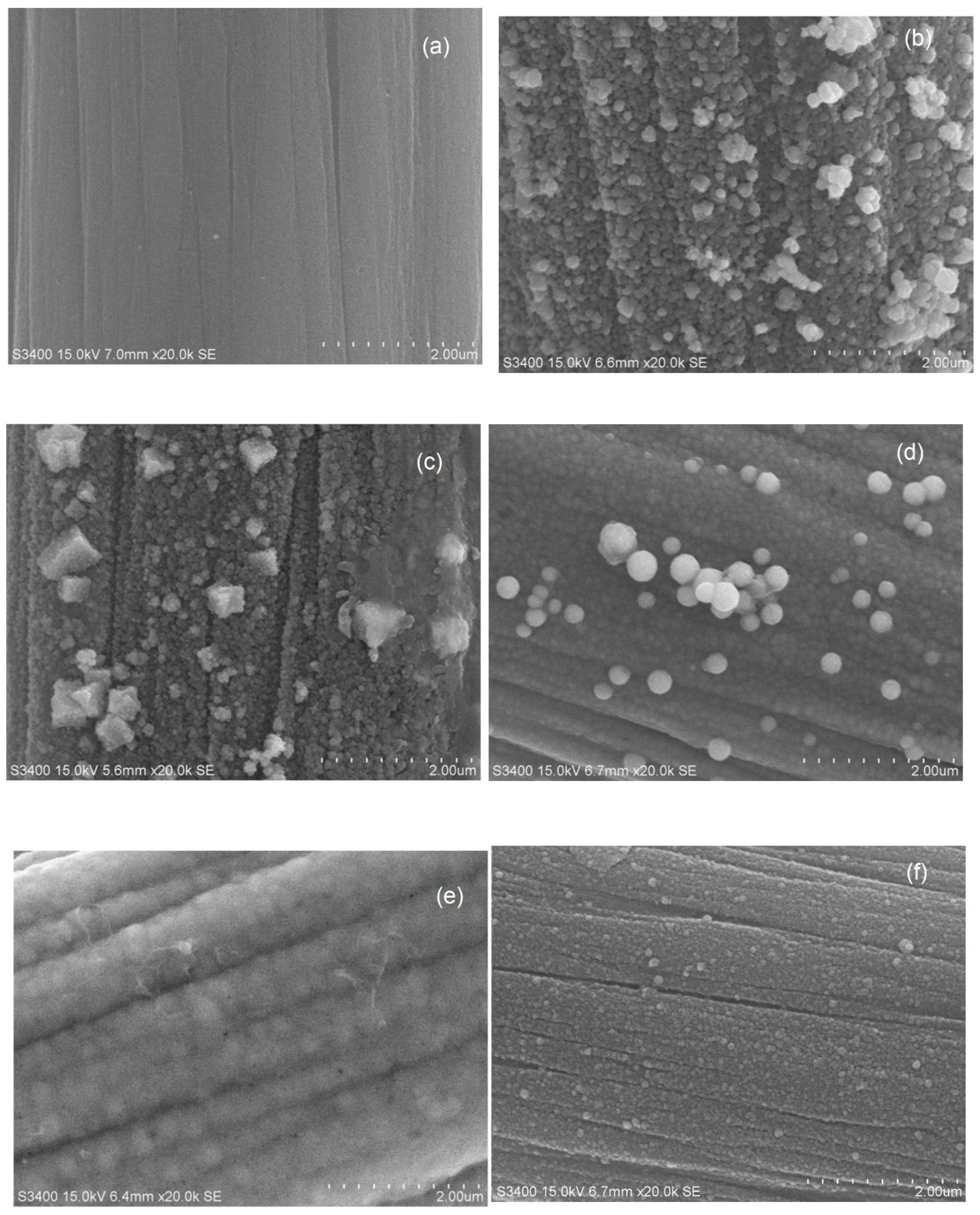
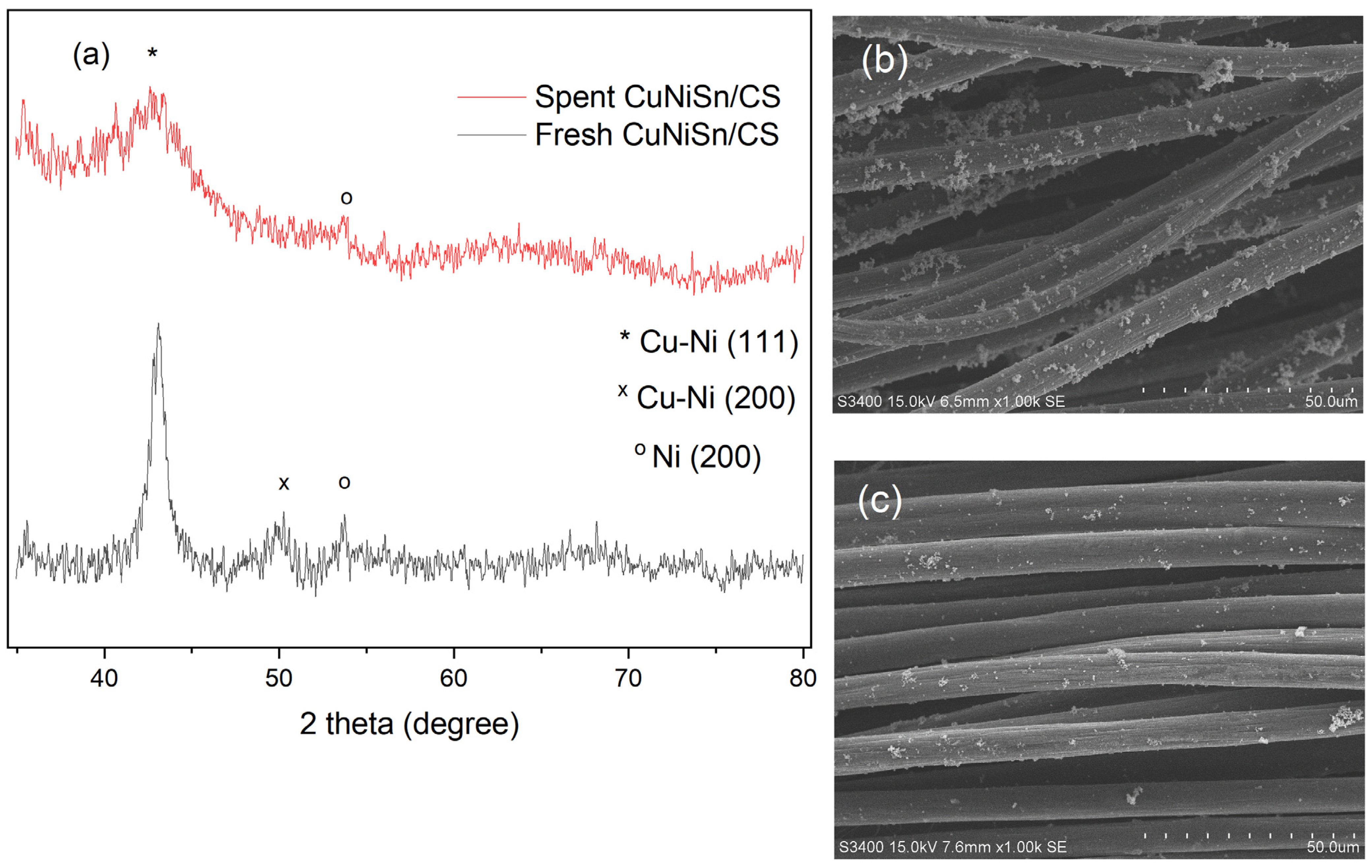
2.3. SEM–EDX Analysis of Tri-, Bi-, and Monometallic Alloy Electrocatalysts
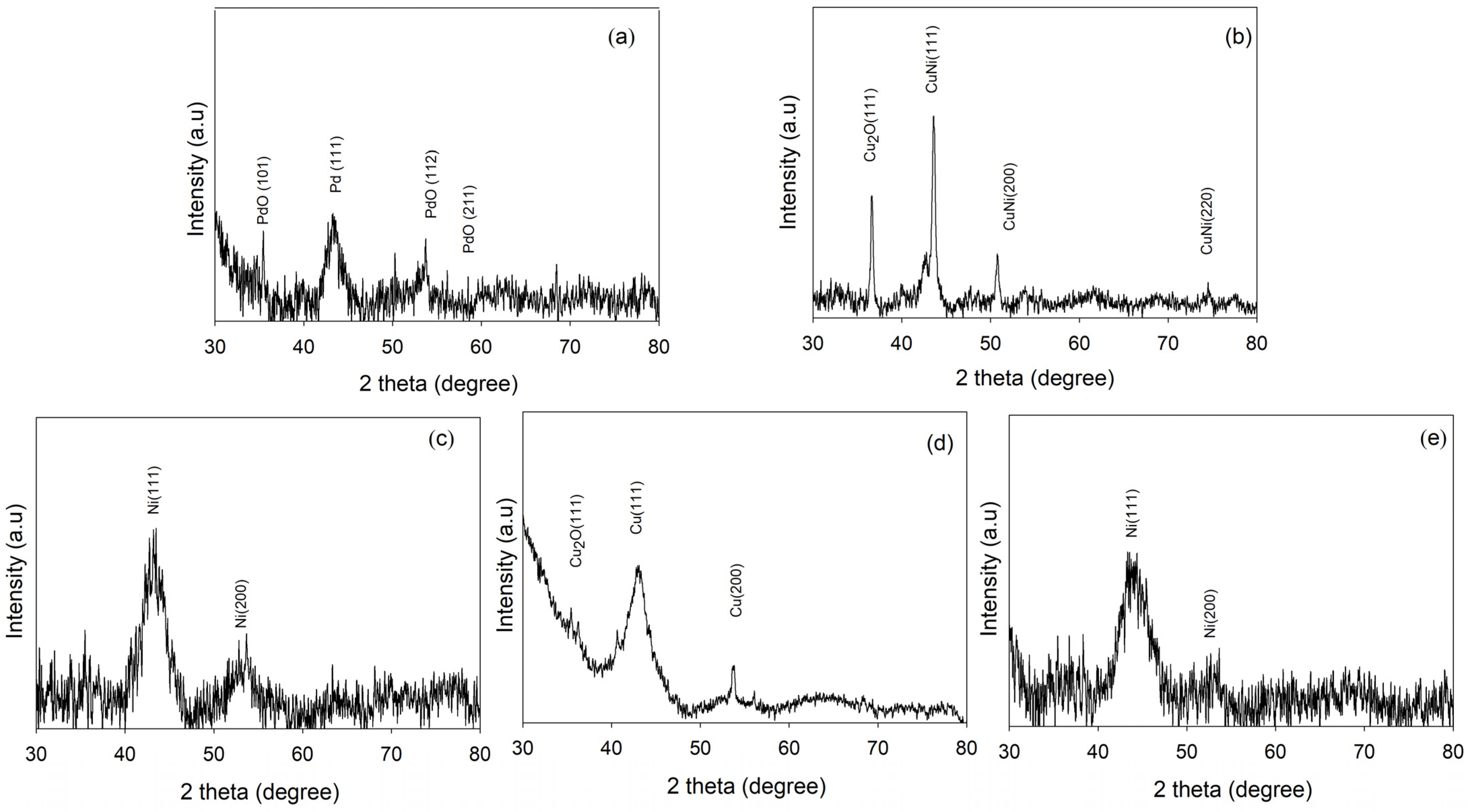
2.4. XRD Analysis of Tri-, Bi-, and Monometallic Alloy Electrocatalysts
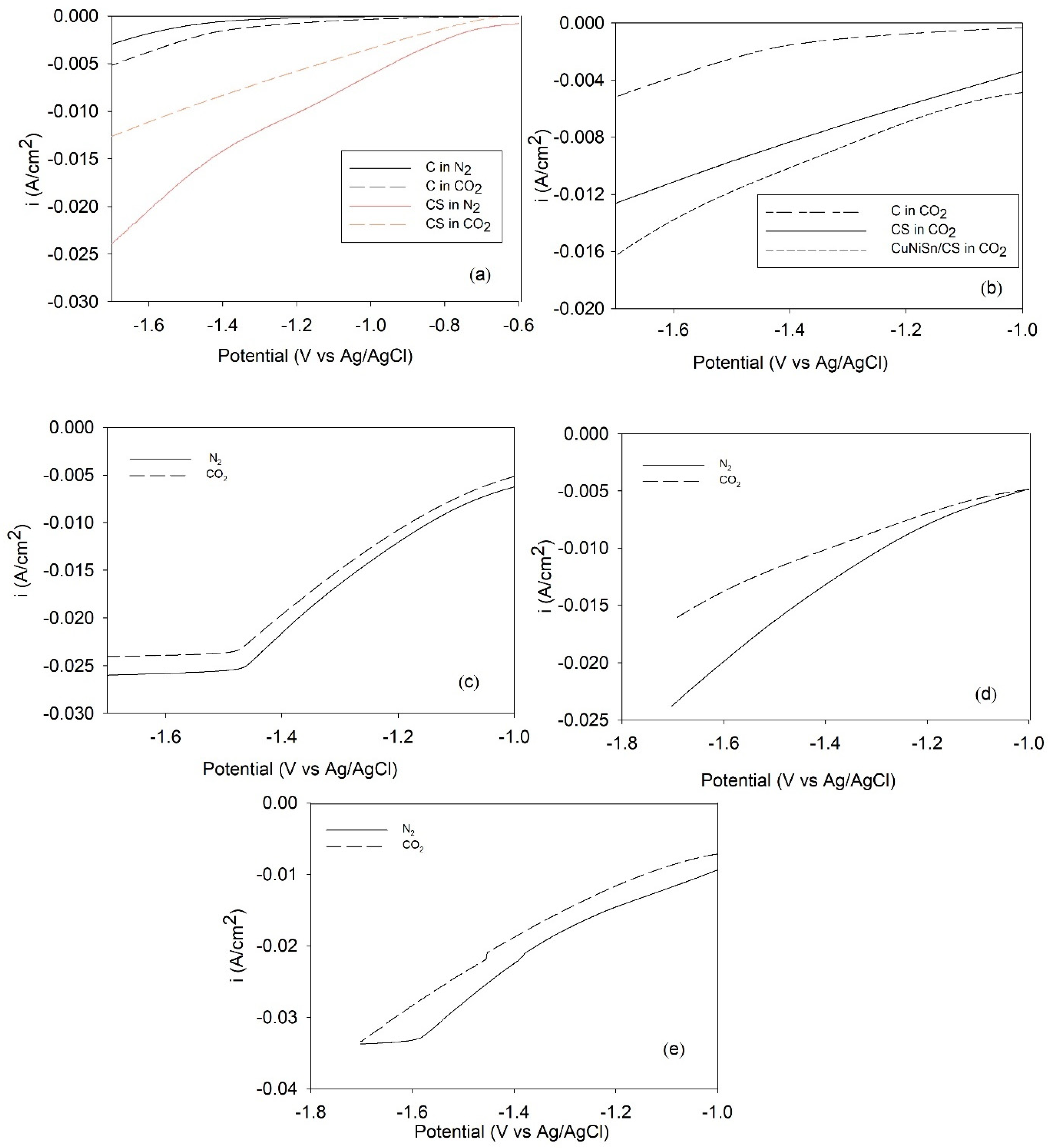
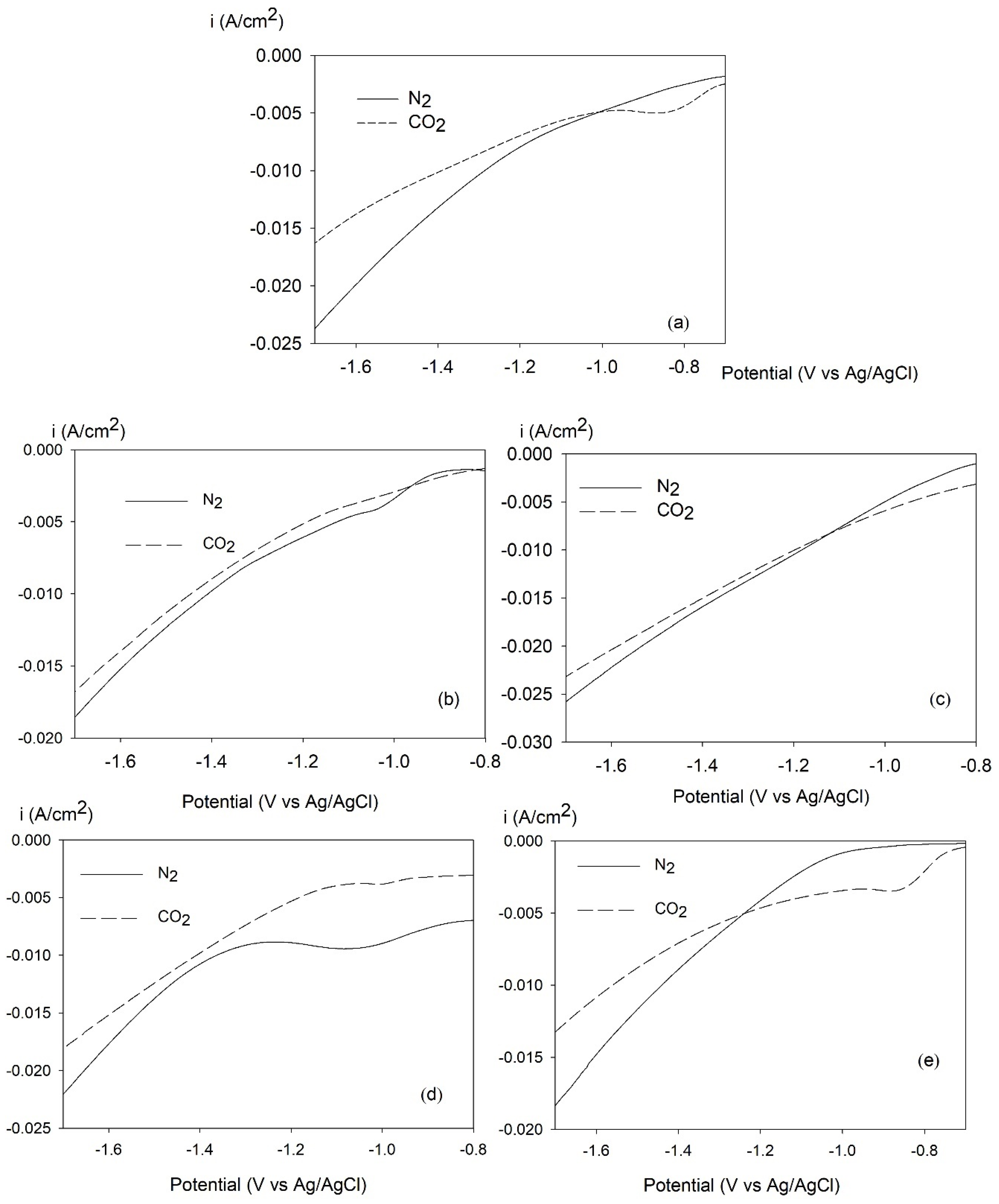
2.5. LSV for CO2-ERR over Tri-, Bi-, and Monometallic Alloy Electrocatalysts
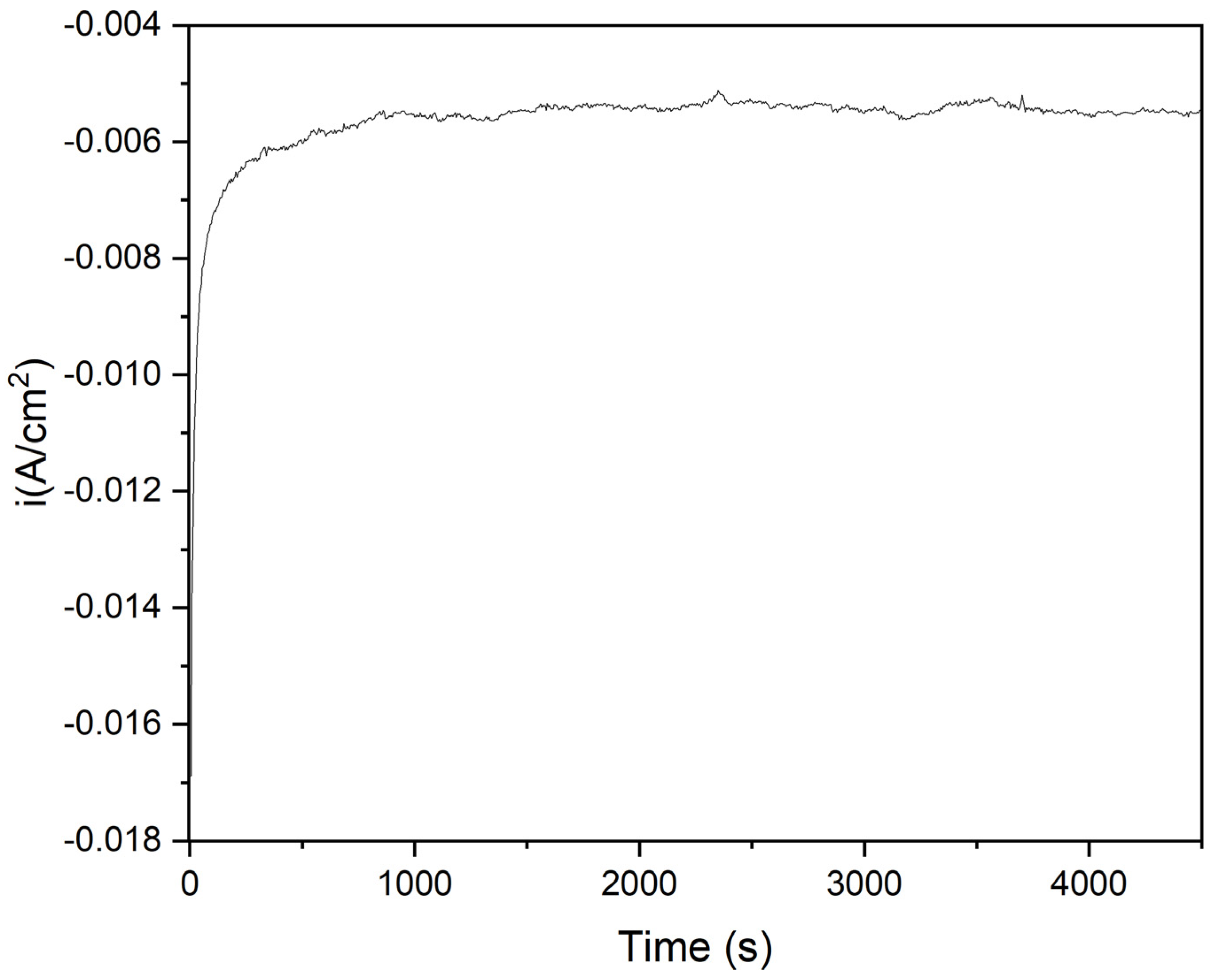
2.6. CO2-ERR Tests
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Palladium (Pd) Polymer Ink
3.3. Preparation of the Electrocatalysts
3.4. CO2-ERR Tests
3.5. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mikkelsen, M.; Jørgensena, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusikb, R.; Maroto-Valerac, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daza, Y.A.; Kent, R.A.; Yung, M.M.; Kuhn, J.N. Carbon Dioxide Conversion by Reverse Water–Gas Shift Chemical Looping on Perovskite-Type Oxides. Ind. Eng. Chem. Res. 2014, 53, 5828–5837. [Google Scholar] [CrossRef]
- Bushuyev, O.; Luna, P.D.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.; Sargent, E. What Should We Make with CO2 and How Can We Make It. Joule 2018, 2, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Poudel, M.B.; Kim, A.A.; Lohani, P.C.; Yoo, D.J.; Kim, H.J. Assembling zinc cobalt hydroxide/ternary sulfides heterostructure and iron oxide nanorods on three-dimensional hollow porous carbon nanofiber as high energy density hybrid supercapacitor. J. Energy Storage 2023, 60, 106713. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, A.R.; Ramakrishan, S.; Logeshwaran, N.; Ramasamy, S.K.; Kim, H.J.; Yoo, D.J. Integrating the essence of metal organic framework-derived ZnCoTe–N–C/MoS2 cathode and ZnCo-NPS-N-CNT as anode for high-energy density hybrid supercapacitors. Compos. B 2022, 247, 110339. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr(VI) and Pb(II) ions by MOF derived Co-Al layered double hydroxide @hematite nanorods on 3D porous carbon nanofiber network. J. Chem. Eng 2021, 417, 129312. [Google Scholar] [CrossRef]
- Jeon, Y.; Choi, I.; Kim, J.J. Facile electrochemical fabrication of Ag/Cu bi-metallic catalysts and the dependence of their selectivity for electrochemical CO2 reduction on the surface composition. Thin Solid Films 2022, 726, 138674. [Google Scholar] [CrossRef]
- Ye, K.; Cao, A.; Shao, J.; Wang, G.; Si, R.; Ta, N.; Xiao, J.; Wang, G. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity. Sci. Bull. 2020, 65, 711–719. [Google Scholar] [CrossRef]
- Suzuki, T.M.; Ishizaki, T.; Kosaka, S.; Takahashi, N.; Isomura, N.; Seki, J.; Matsuoka, Y.; Oh-ishi, K.; Oshima, A.; Kitazumi, K.; et al. Electrochemical CO2 reduction over nanoparticles derived from an oxidized Cu–Ni intermetallic alloy. Chem. Commun. 2020, 96, 15008–15011. [Google Scholar] [CrossRef]
- Feaster, J.T.; Shi, C.; Cave, E.R.; Hatsukade, T.; Abram, D.N.; Kuhl, K.P.; Hahn, C.; Nørskov, J.K.; Jaramillo, T.F. Understanding Selectivity for the Electrochemical Reduction of Carbon Dioxide to Formic Acid and Carbon Monoxide on Metal Electrodes. ACS Catal. 2017, 7, 4822–4827. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Pastor, E.; Lázaro, M.J. Noble metal-free catalysts supported on carbon for CO2 electrochemical reduction. J. CO2 Util. 2017, 18, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Du, J.; Sun, H.; Ye, F.; Wang, X.; Xu, P.; Hu, C.; Zhang, L.; Liu, D. Boron, nitrogen co-doped carbon with abundant mesopores for efficient CO2 electroreduction. Appl. Catal. B Environ 2021, 298, 120543. [Google Scholar] [CrossRef]
- Song, Y.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.; Wu, Z.; Meyer, H.M.; Chi, M.; Ma, C.; Sumpter, B.G.; et al. High-Selectivity Electrochemical Conversion of CO2 to Ethanol using a Copper Nanoparticle/N-Doped Graphene Electrode. ChemistrySelect 2016, 1, 6055–6061. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S. Electroless Copper Deposition: A Critical Review. Thin Solid Films 2019, 669, 641–658. [Google Scholar] [CrossRef]
- Walls, J.M.; Sagu, J.S.; Wijayantha, K.G.U. Microwave synthesised Pd-TiO2 for photocatalytic ammonia production. RSC Adv. 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, R.Y.; Kareem, A.A. Characteristics of electroless copper plating on modified carbon fiber. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2290. [Google Scholar] [CrossRef]
- Dai, G.; Wu, S.; Huang, X. Preparation process for high-entropy alloy coatings based on electroless plating and thermal diffusion. J. Alloys Compd. 2022, 902, 163736. [Google Scholar] [CrossRef]
- Badawy, S.M.; Elkhashab, R.A.; Nayl, A.A. Synthesis, Characterization and Catalytic Activity of Cu/Cu2O Nanoparticles Prepared in Aqueous Medium. Bull. Chem. React. Eng. 2015, 10, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Liao, F.; Han, X.; Zhang, Y.; Xu, C.; Chen, H. Carbon fabrics coated with nickel film through alkaline electroless plating technique. Mater. Lett. 2017, 205, 165–168. [Google Scholar] [CrossRef]
- Phuc, T.-V.; Chung, J.-S.; Hur, S.-H. Highly CO Selective Trimetallic Metal-Organic Framework Electrocatalyst for the Electrochemical Reduction of CO2. Catalysts 2021, 11, 537. [Google Scholar] [CrossRef]
- Chen, C.; Kotyk, J.F.K.; Sheehan, S.W. Progress toward Commercial Application of Electrochemical Carbon Dioxide Reduction. Chem 2018, 4, 2571–2586. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, H.L.A.; Symes, M.D. Recent progress in CO2 reduction using bimetallic electrodes containing copper. Electrochem. Commun. 2022, 135, 107212. [Google Scholar] [CrossRef]
- Kim, M.-G.; Choi, Y.; Park, E.; Cheon, C.-H.; Kim, N.; Min, B.K.; Kim, W. Crystalline/Amorphous Ni2P/Ho2O3 Core/Shell Nanoparticles for Electrochemical Reduction of CO2 to Acetone. ACS Appl. Energy Mater. 2020, 3, 11516–11522. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Kim, D.; Kley, C.S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl. Acad. Sci. USA 2017, 114, 10560–10565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.; Lee, S.Y.; Lee, C.W.; Cho, M.K.; Won, D.H.; Kim, C.; Oh, H.-S.; Min, B.K.; Hwang, Y.J. Electrochemical Fragmentation of Cu2O Nanoparticles Enhancing Selective C–C Coupling from CO2 Reduction Reaction. Am. Chem. Soc. 2019, 141, 4624–4633. [Google Scholar] [CrossRef] [PubMed]

| Electrocatalysts | Concentrations of Metals in the Catalyst (mg/g) | ||
|---|---|---|---|
| Cu | Ni | Sn | |
| CuNiSn/CS_15 | 12.4 | 2.2 | 1.1 |
| CuNiSn/CS_30 | 17.8 | 4.0 | 2.1 |
| CuNiSn/CS_45 | 51.1 | 1.0 | 0.4 |
| CuNi/CS | 11.0 | 2.9 | NA |
| NiSn/CS | NA | 7.3 | 1.6 |
| Cu/CS | 20.9 | NA | NA |
| Ni/CS | NA | 5.5 | NA |
| Electrocatalyst | Rate of Gas Products (µmol/min) | Faradaic Efficiency (%FE) | Rate of Liquid Products (µmol/min) | Faradaic Efficiency (%FE) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | H2 | CO | Formate | Ethylene Glycol | Acetone | Ethanol | Acetate | 1-Buthanol | Formate | Ethylene Glycol | Acetone | Ethanol | Acetate | 1-Buthanol | |
| CuNiSn/CS | 3.50 | - | 26.4% | - | 0.036 | 0.0483 | 0.0776 | 0.0441 | 0.129 | 0.028 | 0.70 | 4.64 | 11.91 | 5.80 | 8.34 | 6.42 |
| CuNi/CS | 10.90 | - | 100% | - | 0.0400 | 0.0590 | 0.0080 | 0.0250 | 0.127 | 0.068 | 0.74 | 5.78 | 1.31 | 2.94 | 9.94 | 16.0 |
| NiSn/CS | 9.90 | - | 100% | - | 0.0009 | 0.0130 | 0.0100 | 0.0200 | 0.083 | 0.004 | 0.02 | 1.22 | 1.49 | 2.38 | 6.5 | 0.98 |
| Cu/CS | 9.50 | - | 100% | - | 0.1655 | 0.0034 | 0.0037 | 0.0079 | 0.022 | 0.004 | 2.42 | 0.25 | 0.44 | 0.69 | 1.31 | 0.82 |
| Ni/CS | 13.40 | - | 100% | - | 0.0090 | 0.0130 | 0.0100 | 0.0050 | 0.055 | 0.004 | 0.16 | 1.07 | 1.33 | 0.48 | 3.74 | 0.86 |
| Electrocatalysts | Electrolyte | Potentials (vs. Ag/AgCl) | Faradic Efficiency (FE, %) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Formate | Ethylene Glycol | Acetone | Ethanol | ||||
| CuNiSn/CS | 0.1 M KHCO3 | −1.6 V | 0.70 | 4.64 | 11.91 | 5.80 | This work |
| CuNi/CS | 0.74 | 5.78 | 1.31 | 2.94 | |||
| NiSn/CS | 0.02 | 1.22 | 1.49 | 2.38 | |||
| Ni2P/Ho2O3 | 0.1 M KHCO3 | −1.51 V | NA | NA | 20 | NA | [24] |
| Cu foil | 0.1 M KHCO3 | −1.55 V | NA | NA | NA | 2.5 | [25] |
| Cu NP | 0.1 M KHCO3 | −1.16 V | NA | NA | NA | 1.9 | [26] |
| Cu2O NP/C | 0.1 M KHCO3 | −1.52 V | 32 | NA | 3.2 | 0 | [27] |
| Chemicals | CuNiSn | CuNi | NiSn | Cu | Ni |
|---|---|---|---|---|---|
| 1. Tin (II) sulfate (0.05 g) | ✓ | ✓ | |||
| 2. Potassium sodium tartrate tetrahydrate (2.50 g) | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3. Potassium D-gluconate(2.50 g) | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4. Boric acid (1.25 g) | ✓ | ✓ | ✓ | ✓ | ✓ |
| 5. Nickel (II) sulfate hexahydrate(1.75 g) | ✓ | ✓ | ✓ | ✓ | |
| 6. Sodium citrate dihydrate (2.50 g) | ✓ | ✓ | ✓ | ✓ | ✓ |
| 7. Copper (II) sulfate Pentahydrate (0.55 g) | ✓ | ✓ | ✓ | ||
| 8. Sodium hypophosphite monohydrate (2.50 g) | ✓ | ✓ | ✓ | ✓ | ✓ |
| 9. EDTA (1.25 g) | ✓ | ✓ | ✓ | ✓ | ✓ |
| 10. Formaldehyde(10 mL) | ✓ | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaitree, W.; Hongmeuan, A.; Pinthong, P.; Panpranot, J. Electrolessly Deposited Carbon-Supported CuNiSn Electrocatalysts for the Electrochemical Reduction of CO2. Catalysts 2023, 13, 1020. https://doi.org/10.3390/catal13061020
Chaitree W, Hongmeuan A, Pinthong P, Panpranot J. Electrolessly Deposited Carbon-Supported CuNiSn Electrocatalysts for the Electrochemical Reduction of CO2. Catalysts. 2023; 13(6):1020. https://doi.org/10.3390/catal13061020
Chicago/Turabian StyleChaitree, Wasu, Atikom Hongmeuan, Piriya Pinthong, and Joongjai Panpranot. 2023. "Electrolessly Deposited Carbon-Supported CuNiSn Electrocatalysts for the Electrochemical Reduction of CO2" Catalysts 13, no. 6: 1020. https://doi.org/10.3390/catal13061020






