A Core-Shell Structured Na/Fe@Co Bimetallic Catalyst for Light-Hydrocarbon Synthesis from CO2 Hydrogenation
Abstract
:1. Introduction
2. Results and Discussion
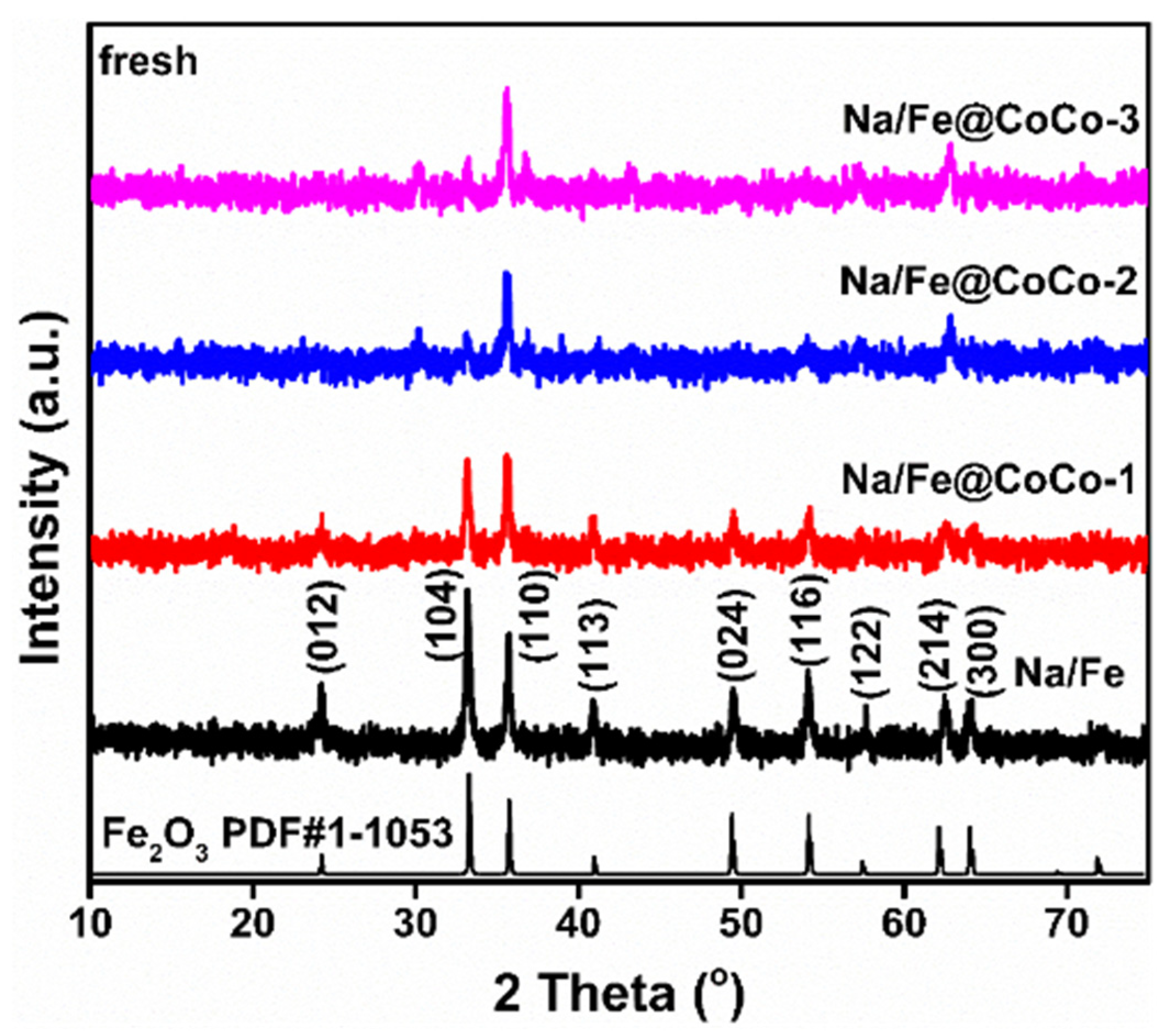
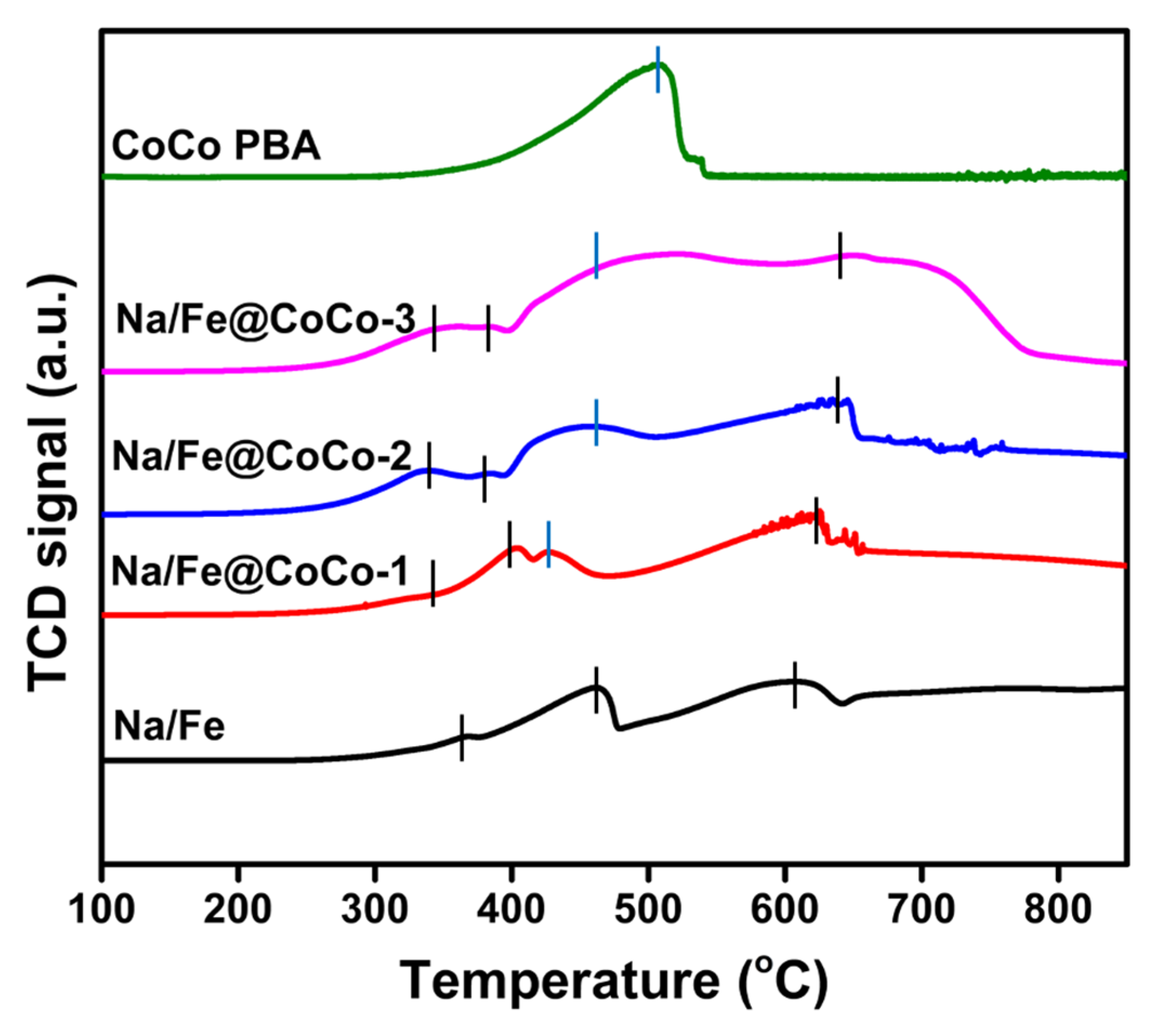
2.1. Structural Characterization
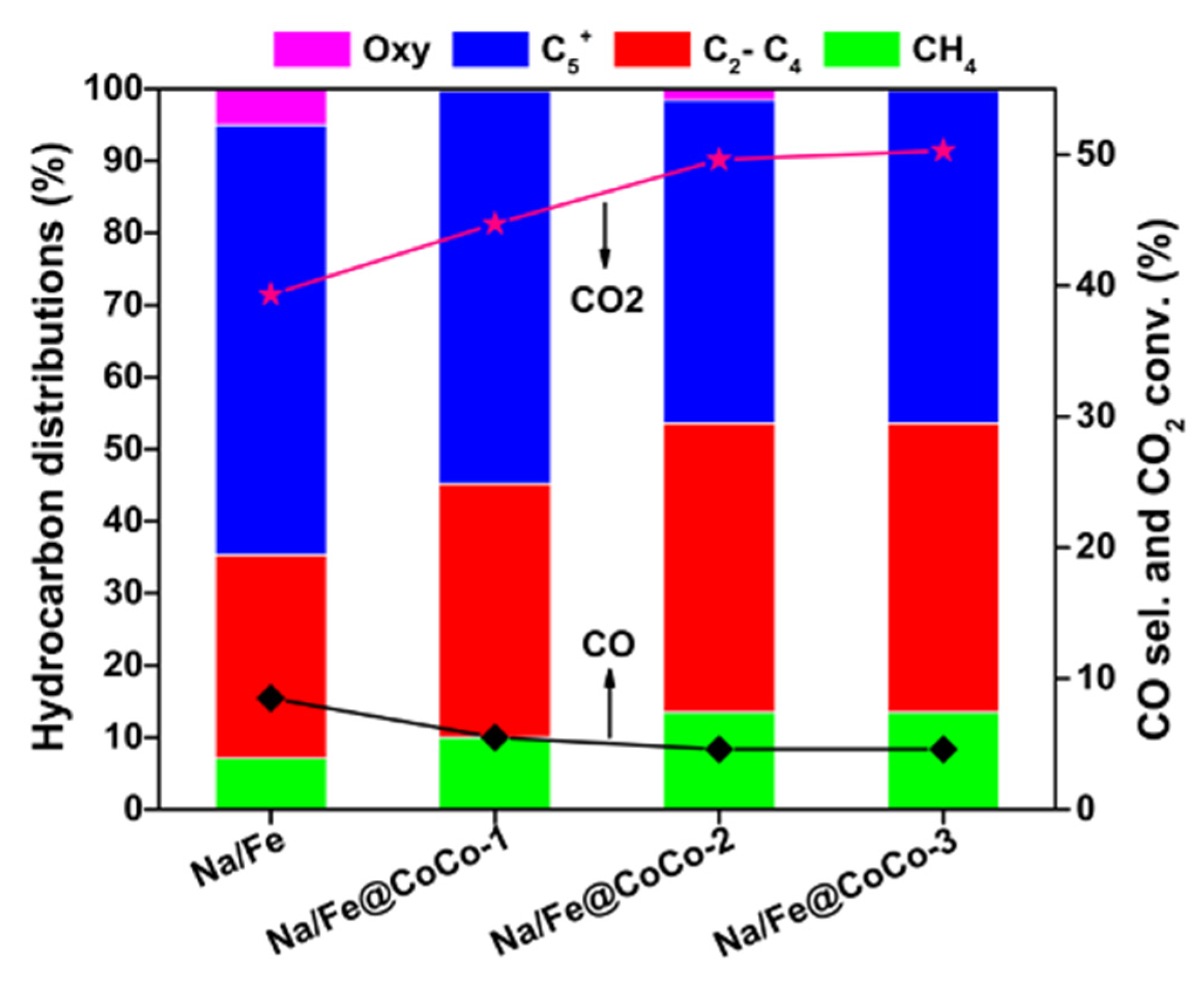
2.2. CO2 Hydrogenation Performance
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
3.3. Catalytic Performance Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Guo, L.; Gao, W.; Chen, F.; Wu, X.; Wang, K.; He, Y.; Zhang, P.; Yang, G.; Tsubaki, N. Multi-Promoters Regulated Iron Catalyst with Well-Matching Reverse Water-Gas Shift and Chain Propagation for Boosting CO2 Hydrogenation. J. CO2 Util. 2021, 52, 101700. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Wu, M.; Tsubaki, N. Thermocatalytic hydrogenation of CO2 into aromatics by tailor-made catalysts: Recent advancements and perspectives. EcoMat 2021, 3, 12080. [Google Scholar] [CrossRef]
- Song, C. CO2 conversion and utilization: An overview. In CO2 Conversion and Utilization; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2002; Volume 809, pp. 2–30. [Google Scholar]
- Li, F.; Thevenon, A.; Rosas-Hernández, A.; Wang, Z.; Li, Y.; Gabardo, C.M.; Ozden, A.; Dinh, C.T.; Li, J.; Wang, Y.; et al. Molecular tuning of CO2-to-ethylene conversion. Nature 2020, 577, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Yao, R.; Han, Y.; Ge, Q.; Sun, J. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons. Chem. Soc. Rev. 2021, 50, 10764–10805. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Liu, X.; Hu, C.; Xiao, D.; Ma, D. Catalytic Transformation of PET and CO2 into High-Value Chemicals. Angew. Chem. 2022, 134, e202117205. [Google Scholar] [CrossRef]
- Guo, L.; Guo, X.; He, Y.; Tsubaki, N. CO2 heterogeneous hydrogenation to carbon-based fuels: Recent key developments and perspectives. J. Mater. Chem. A 2023, 11, 11637–11669. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhang, B.; Peng, X.; Gao, X.; Yang, G.; Hu, H.; Wu, M.; Tsubaki, N. Direct Conversion of CO2 to Ethanol Boosted by Intimacy-Sensitive Multifunctional Catalysts. ACS Catal. 2021, 11, 11742–11753. [Google Scholar] [CrossRef]
- Li, Z.; Qu, Y.; Wang, J.; Liu, H.; Li, M.; Miao, S.; Li, C. Highly Selective Conversion of Carbon Dioxide to Aromatics over Tandem Catalysts. Joule 2019, 3, 570–583. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, W.; Kazumi, S.; Li, H.; Yang, G.; Tsubaki, N. Direct and Oriented Conversion of CO2 into Value-Added Aromatics. Chem. A Eur. J. 2019, 25, 5149–5153. [Google Scholar] [CrossRef]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, R.; Ma, S.; Xu, K.; Chen, Y.; Jiang, K.; Fang, Y.; Zhu, C.; Liu, X.; Tang, Y.; et al. The role of Cu1–O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 2022, 5, 818–831. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, H.; Wang, L.; Wang, L.; Wang, C.; Wang, H.; Fang, W.; He, M.; Wu, Q.; Xiao, F.-S. Enhanced CO2 utilization in dry reforming of methane achieved through nickel-mediated hydrogen spillover in zeolite crystals. Nat. Catal. 2022, 5, 1030–1037. [Google Scholar] [CrossRef]
- Chen, F.; Liang, J.; Wang, F.; Gao, W.; Kugue, Y.; He, Y.; Guo, X.; Yang, G.; Liu, G.; Wu, J.; et al. Alcohol Solvent Assisted Synthesis of Metallic and Metal Oxide Catalysts: As-Prepared Cu/ZnO/Al2O3 Catalysts for Low-Temperature Methanol Synthesis with an Ultrahigh Yield. ACS Catal. 2023, 13, 6169–6184. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Xiao, F.-S. Silica-modulated Cu-ZnO-Al2O3 catalyst for efficient hydrogenation of CO2 to methanol. Catal. Today 2023, 418, 114051. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, G.; Jiang, X.; Wang, J.; Song, C.; Guo, X. Insight into the role of Fe5C2 in CO2 catalytic hydrogenation to hydrocarbons. Catal. Today 2021, 371, 162–170. [Google Scholar] [CrossRef]
- Liang, J.; Guo, L.; Gao, W.; Wang, C.; Guo, X.; He, Y.; Yang, G.; Tsubaki, N. Direct Conversion of CO2 to Aromatics over K–Zn–Fe/ZSM-5 Catalysts via a Fischer–Tropsch Synthesis Pathway. Ind. Eng. Chem. Res. 2022, 61, 10336–10346. [Google Scholar] [CrossRef]
- Han, Y.; Fang, C.; Ji, X.; Wei, J.; Ge, Q.; Sun, J. Interfacing with Carbonaceous Potassium Promoters Boosts Catalytic CO2 Hydrogenation of Iron. ACS Catal. 2020, 10, 12098–12108. [Google Scholar] [CrossRef]
- Wang, C.; Fang, W.; Liu, Z.; Wang, L.; Liao, Z.; Yang, Y.; Li, H.; Liu, L.; Zhou, H.; Qin, X.; et al. Fischer–Tropsch synthesis to olefins boosted by MFI zeolite nanosheets. Nat. Nanotechnol. 2022, 17, 714–720. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, K.; Wang, Y.; Liu, Z.; Gao, X.; Zhang, J.; Ma, Q.; Fan, S.; Zhao, T.-S.; Yao, M. Recent advances in thermocatalytic hydrogenation of carbon dioxide to light olefins and liquid fuels via modified Fischer-Tropsch pathway. J. CO2 Util. 2023, 67, 102321. [Google Scholar] [CrossRef]
- Guo, L.; Gao, X.; Gao, W.; Wu, H.; Wang, X.; Sun, S.; Wei, Y.; Kugue, Y.; Guo, X.; Sun, J.; et al. High-yield production of liquid fuels in CO2 hydrogenation on a zeolite-free Fe-based catalyst. Chem. Sci. 2023, 14, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Krause, S.; Krans, N.A.; Hernández Mejía, C.; Kaskel, S.; De Jong, K.P. Influence of precursor porosity on sodium and sulfur promoted iron/carbon Fischer–Tropsch catalysts derived from metal–organic frameworks. Chem. Commun. 2017, 53, 10204–10207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhai, P.; Deng, Y.; Xie, J.; Liu, X.; Wang, S.; Ma, D. Highly Selective Olefin Production from CO2 Hydrogenation on Iron Catalysts: A Subtle Synergy between Manganese and Sodium Additives. Angew. Chem. 2020, 132, 21920–21928. [Google Scholar] [CrossRef]
- Yang, Q.; Kondratenko, V.A.; Petrov, S.A.; Doronkin, D.E.; Saraçi, E.; Lund, H.; Arinchtein, A.; Kraehnert, R.; Skrypnik, A.S.; Matvienko, A.A.; et al. Identifying Performance Descriptors in CO2 Hydrogenation over Iron-Based Catalysts Promoted with Alkali Metals. Angew. Chem. Int. Ed. 2022, 61, e202116517. [Google Scholar] [CrossRef]
- Mango, F.D. The light hydrocarbons in petroleum: A critical review. Org. Geochem. 1997, 26, 417–440. [Google Scholar] [CrossRef]
- Mango, F.D. The origin of light hydrocarbons. Geochim. Cosmochim. Acta 2000, 64, 1265–1277. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, P.; Cui, Y.; Suzuki, Y.; Li, H.; Guo, L.; Yang, G.; Tsubaki, N. Direct CO2 hydrogenation to light olefins by suppressing CO by-product formation. Fuel Process. Technol. 2019, 196, 106174. [Google Scholar] [CrossRef]
- Li, H.; Zhang, P.; Guo, L.; He, Y.; Zeng, Y.; Thongkam, M.; Natakaranakul, J.; Kojima, T.; Reubroycharoen, P.; Vitidsant, T.; et al. A Well-Defined Core–Shell-Structured Capsule Catalyst for Direct Conversion of CO2 into Liquefied Petroleum Gas. ChemSusChem 2020, 13, 2060–2065. [Google Scholar] [CrossRef]
- Yang, G.; Xing, C.; Hirohama, W.; Jin, Y.; Zeng, C.; Suehiro, Y.; Wang, T.; Yoneyama, Y.; Tsubaki, N. Tandem catalytic synthesis of light isoparaffin from syngas via Fischer–Tropsch synthesis by newly developed core–shell-like zeolite capsule catalysts. Catal. Today 2013, 215, 29–35. [Google Scholar] [CrossRef]
- Bao, J.; He, J.; Zhang, Y.; Yoneyama, Y.; Tsubaki, N. A Core/Shell Catalyst Produces a Spatially Confined Effect and Shape Selectivity in a Consecutive Reaction. Angew. Chem. 2008, 120, 359–362. [Google Scholar] [CrossRef]
- Song, F.; Yong, X.; Wu, X.; Zhang, W.; Ma, Q.; Zhao, T.; Tan, M.; Guo, Z.; Zhao, H.; Yang, G.; et al. FeMn@HZSM-5 capsule catalyst for light olefins direct synthesis via Fischer-Tropsch synthesis: Studies on depressing the CO2 formation. Appl. Catal. B Environ. 2022, 300, 120713. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, W.; Wang, F.; Wang, C.; Liang, J.; Guo, X.; He, Y.; Yang, G.; Tsubaki, N. Highly selective synthesis of light aromatics from CO2 by chromium-doped ZrO2 aerogels in tandem with HZSM-5@SiO2 catalyst. Appl. Catal. B Environ. 2023, 328, 122535. [Google Scholar] [CrossRef]
- Amoo, C.C.; Xing, C.; Tsubaki, N.; Sun, J. Tandem Reactions over Zeolite-Based Catalysts in Syngas Conversion. ACS Cent. Sci. 2022, 8, 1047–1062. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Wu, Q.; Wang, C.; Guo, X.; He, Y.; Zhang, P.; Yang, G.; Liu, G.; Wu, J.; et al. Capsule-like zeolite catalyst fabricated by solvent-free strategy for para-Xylene formation from CO2 hydrogenation. Appl. Catal. B Environ. 2022, 303, 120906. [Google Scholar] [CrossRef]
- Lemine, O.M. Microstructural characterisation of α-Fe2O3 nanoparticles using, XRD line profiles analysis, FE-SEM and FT-IR. Superlattices Microstruct. 2009, 45, 576–582. [Google Scholar] [CrossRef]
- Kashyap, S.J.; Sankannavar, R.; Madhu, G.M. Iron oxide (Fe2O3) synthesized via solution-combustion technique with varying fuel-to-oxidizer ratio: FT-IR, XRD, optical and dielectric characterization. Mater. Chem. Phys. 2022, 286, 126118. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Wang, J.; Chen, S.; Wang, H.; Zhang, J. Interfacial Scaffolding Preparation of Hierarchical PBA-Based Derivative Electrocatalysts for Efficient Water Splitting. Adv. Energy Mater. 2019, 9, 1802939. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liu, X.; Bai, J.; Wang, H.; Wang, G. Prussian blue analogs (PBA) derived porous bimetal (Mn, Fe) selenide with carbon nanotubes as anode materials for sodium and potassium ion batteries. Chem. Eng. J. 2020, 382, 123050. [Google Scholar] [CrossRef]
- Wu, X.; Ru, Y.; Bai, Y.; Zhang, G.; Shi, Y.; Pang, H. PBA composites and their derivatives in energy and environmental applications. Coord. Chem. Rev. 2022, 451, 214260. [Google Scholar] [CrossRef]
- Zhai, P.; Xu, C.; Gao, R.; Liu, X.; Li, M.; Li, W.; Fu, X.; Jia, C.; Xie, J.; Zhao, M.; et al. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5C2 Catalyst. Angew. Chem. 2016, 128, 10056–10061. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer–Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef]
- Song, C.; Liu, X.; Xu, M.; Masi, D.; Wang, Y.; Deng, Y.; Zhang, M.; Qin, X.; Feng, K.; Yan, J.; et al. Photothermal Conversion of CO2 with Tunable Selectivity Using Fe-Based Catalysts: From Oxide to Carbide. ACS Catal. 2020, 10, 10364–10374. [Google Scholar] [CrossRef]
- Wang, L.-X.; Wang, L.; Xiao, F.-S. Tuning product selectivity in CO2 hydrogenation over metal-based catalysts. Chem. Sci. 2021, 12, 14660–14673. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.-L.; Gao, X.-H.; Zhao, T.-S. Research progress on iron-based catalysts for CO2 hydrogenation to long-chain linear α-olefins. J. Fuel Chem. Technol. 2023, 51, 67–85. [Google Scholar] [CrossRef]


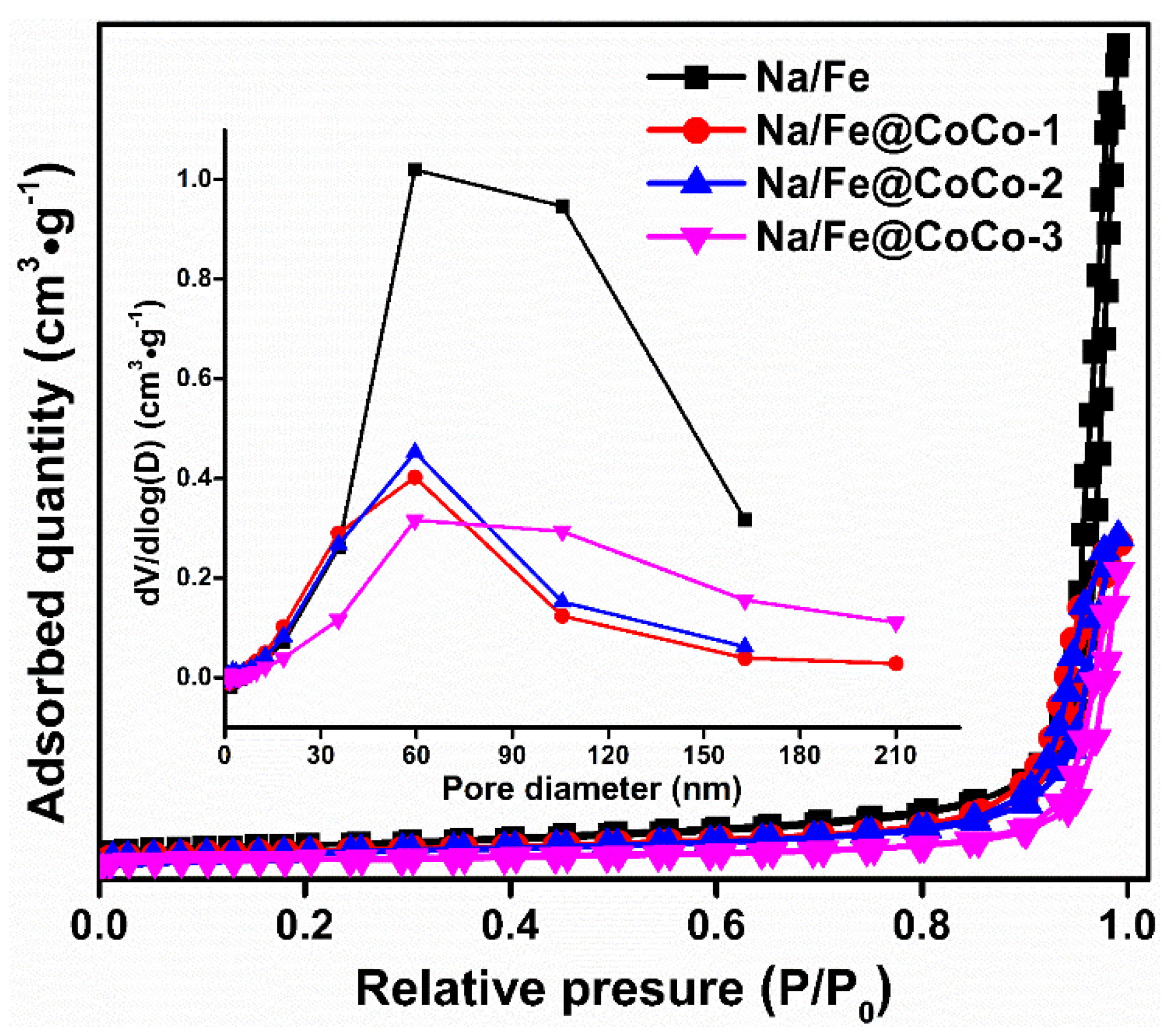

| Catalysts | SBET/(m2/g) a | Total Pore Volume/(cm3/g) b | Average Pore Diameter/(nm) b |
|---|---|---|---|
| Na/Fe | 40 | 0.59 | 67 |
| Na/Fe@CoCo-1 | 30 | 0.24 | 32 |
| Na/Fe@CoCo-2 | 28 | 0.24 | 33 |
| Na/Fe@CoCo-3 | 16 | 0.22 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; He, Y.; Fujihara, K.; Wang, C.; Sun, X.; Gao, W.; Guo, X.; Yasuda, S.; Yang, G.; Tsubaki, N. A Core-Shell Structured Na/Fe@Co Bimetallic Catalyst for Light-Hydrocarbon Synthesis from CO2 Hydrogenation. Catalysts 2023, 13, 1090. https://doi.org/10.3390/catal13071090
Li Y, He Y, Fujihara K, Wang C, Sun X, Gao W, Guo X, Yasuda S, Yang G, Tsubaki N. A Core-Shell Structured Na/Fe@Co Bimetallic Catalyst for Light-Hydrocarbon Synthesis from CO2 Hydrogenation. Catalysts. 2023; 13(7):1090. https://doi.org/10.3390/catal13071090
Chicago/Turabian StyleLi, Yanbing, Yingluo He, Kensei Fujihara, Chengwei Wang, Xu Sun, Weizhe Gao, Xiaoyu Guo, Shuhei Yasuda, Guohui Yang, and Noritatsu Tsubaki. 2023. "A Core-Shell Structured Na/Fe@Co Bimetallic Catalyst for Light-Hydrocarbon Synthesis from CO2 Hydrogenation" Catalysts 13, no. 7: 1090. https://doi.org/10.3390/catal13071090






