Walnut Shell Biomass Triggered Formation of Fe3C-Biochar Composite for Removal of Diclofenac by Activating Percarbonate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. XRD of the Synthesized Catalyst
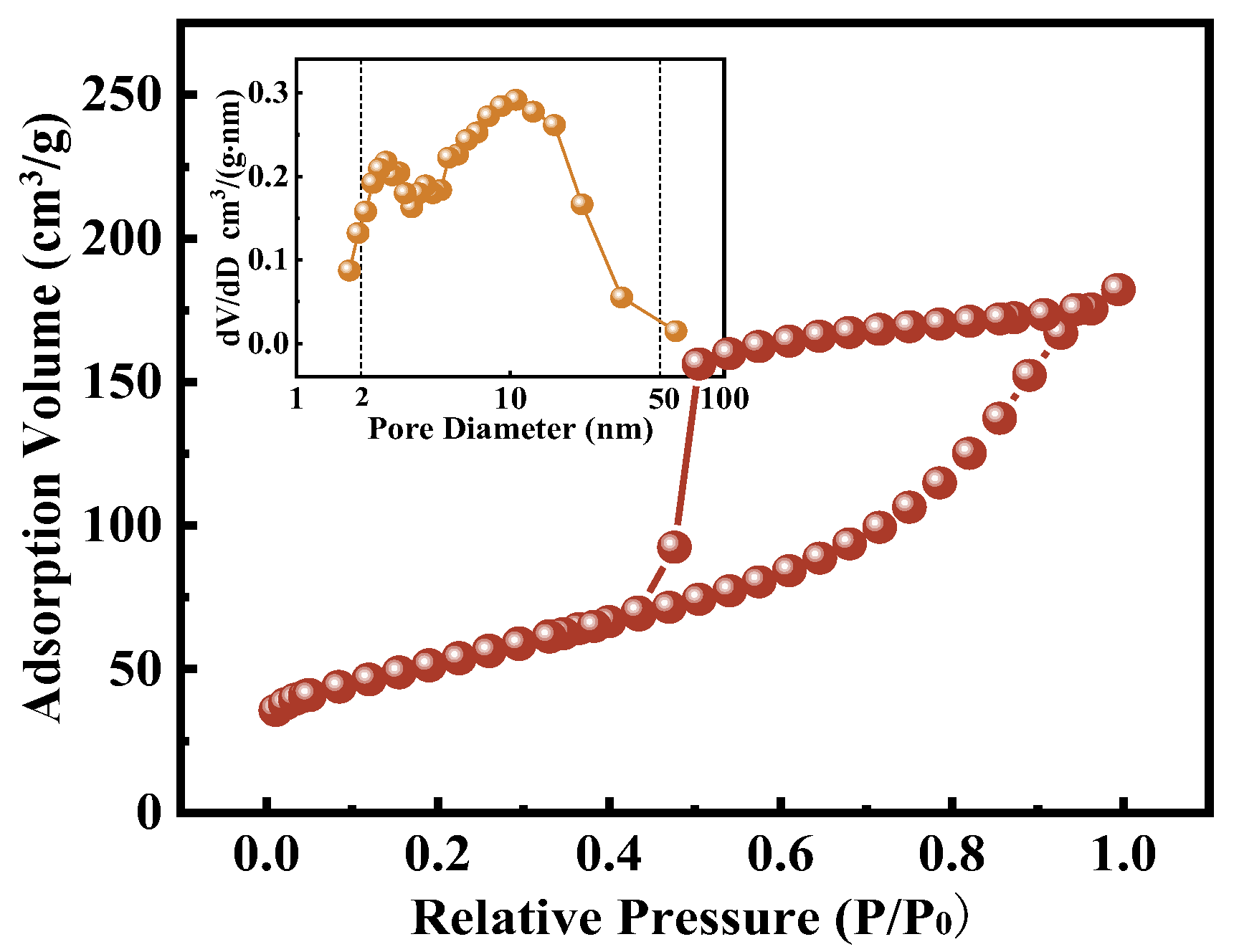
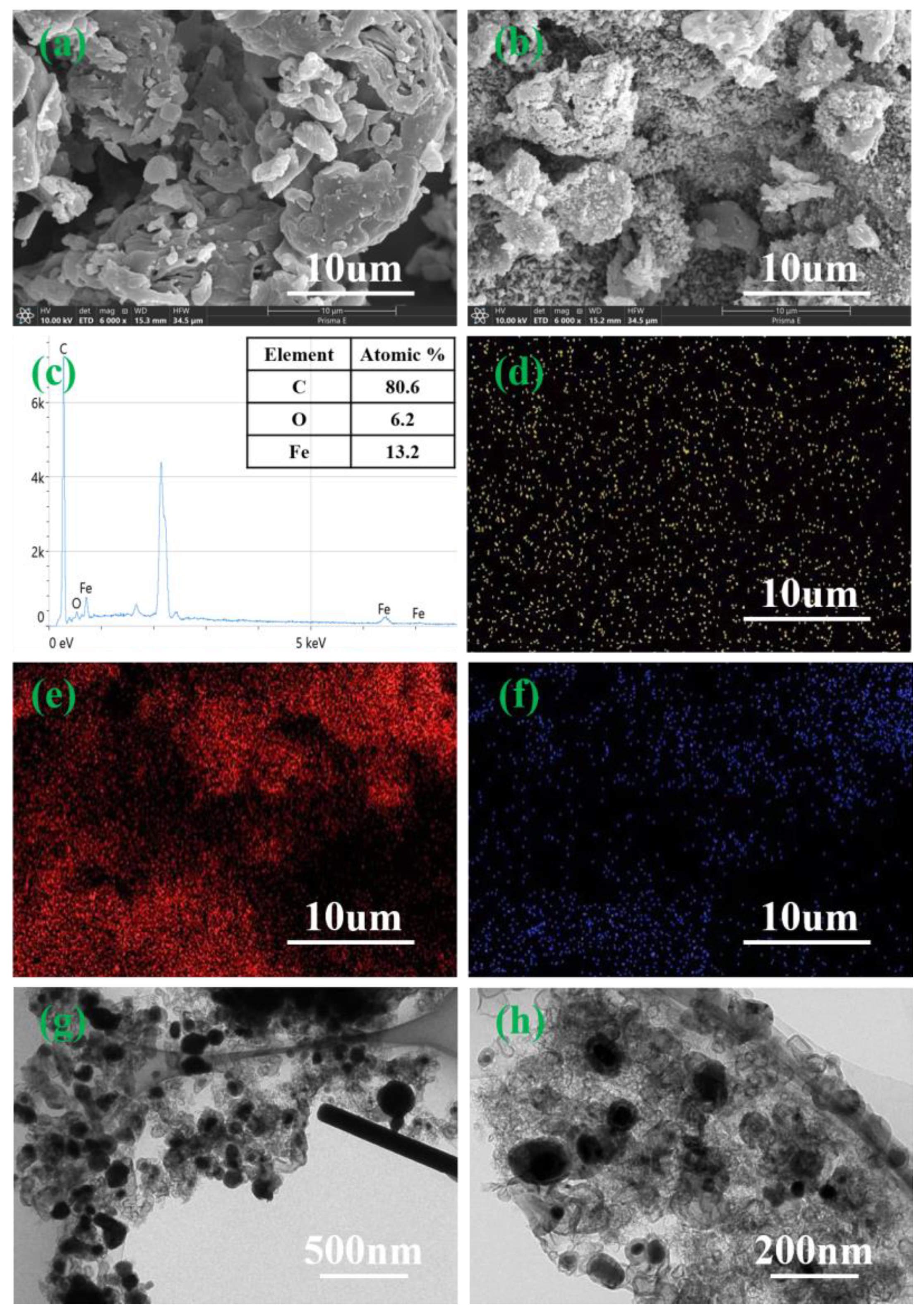
2.1.2. Morphology and Porosity Characterization
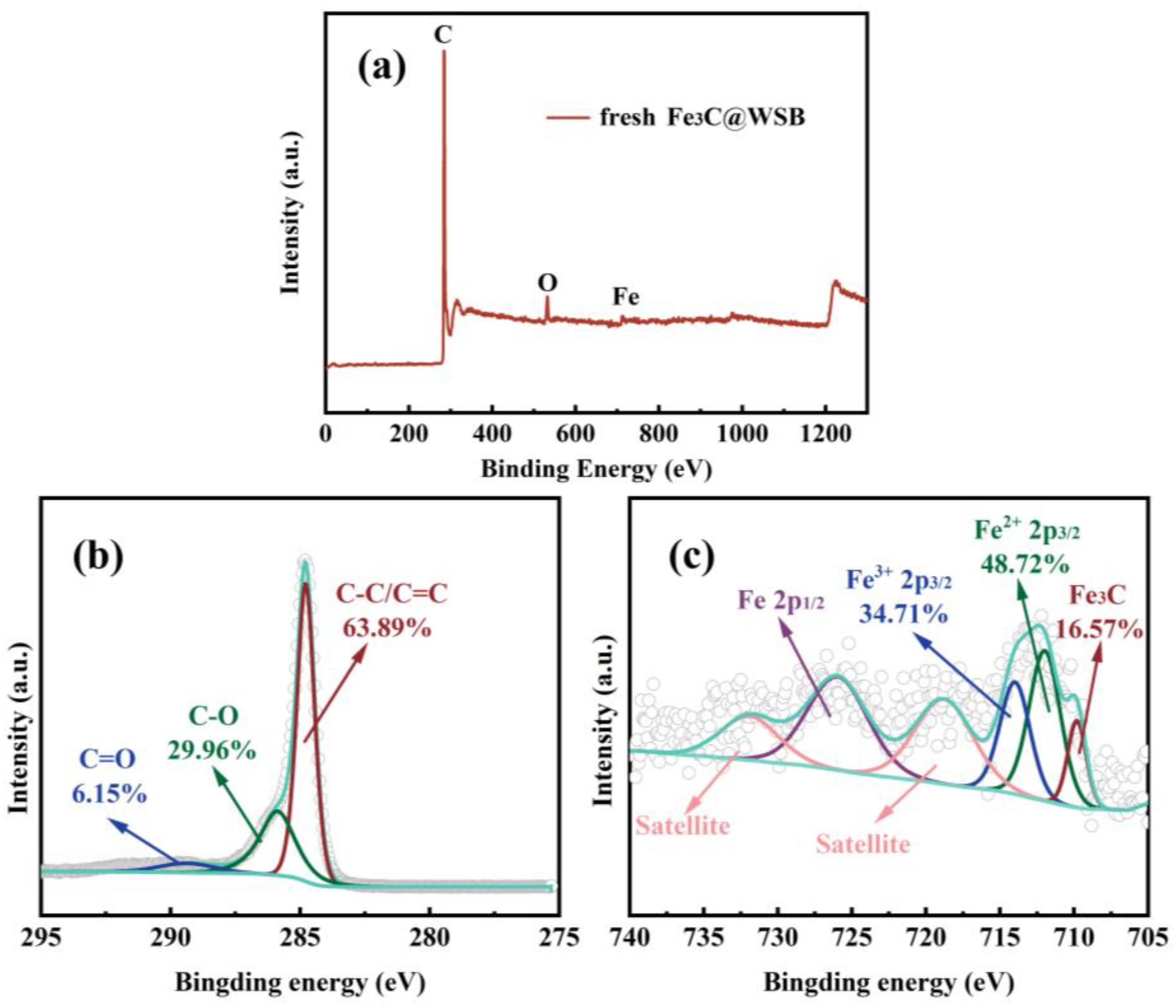
2.1.3. The Chemical State Analysis
2.2. DCF Removal Performance of Fe3C@WSB
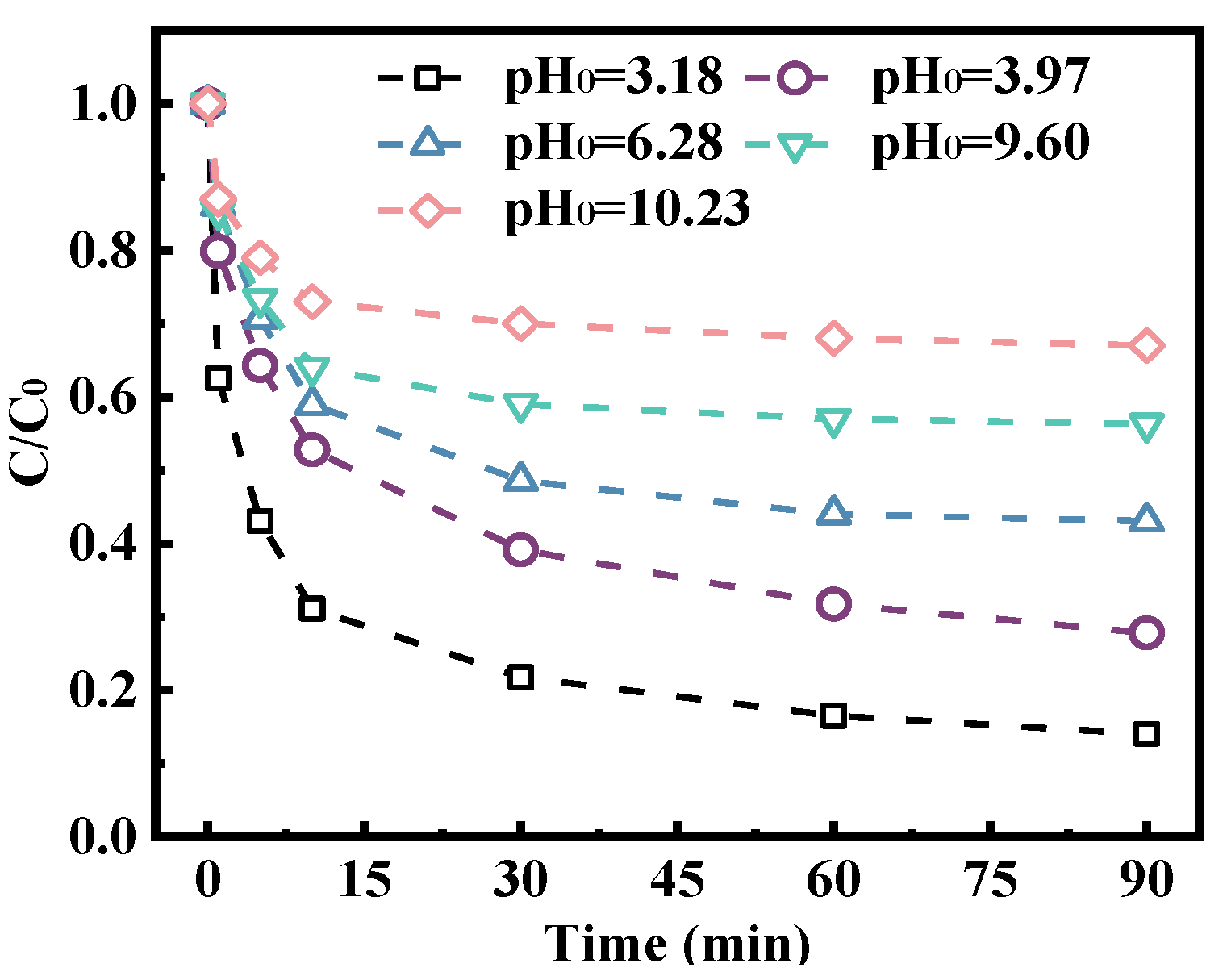
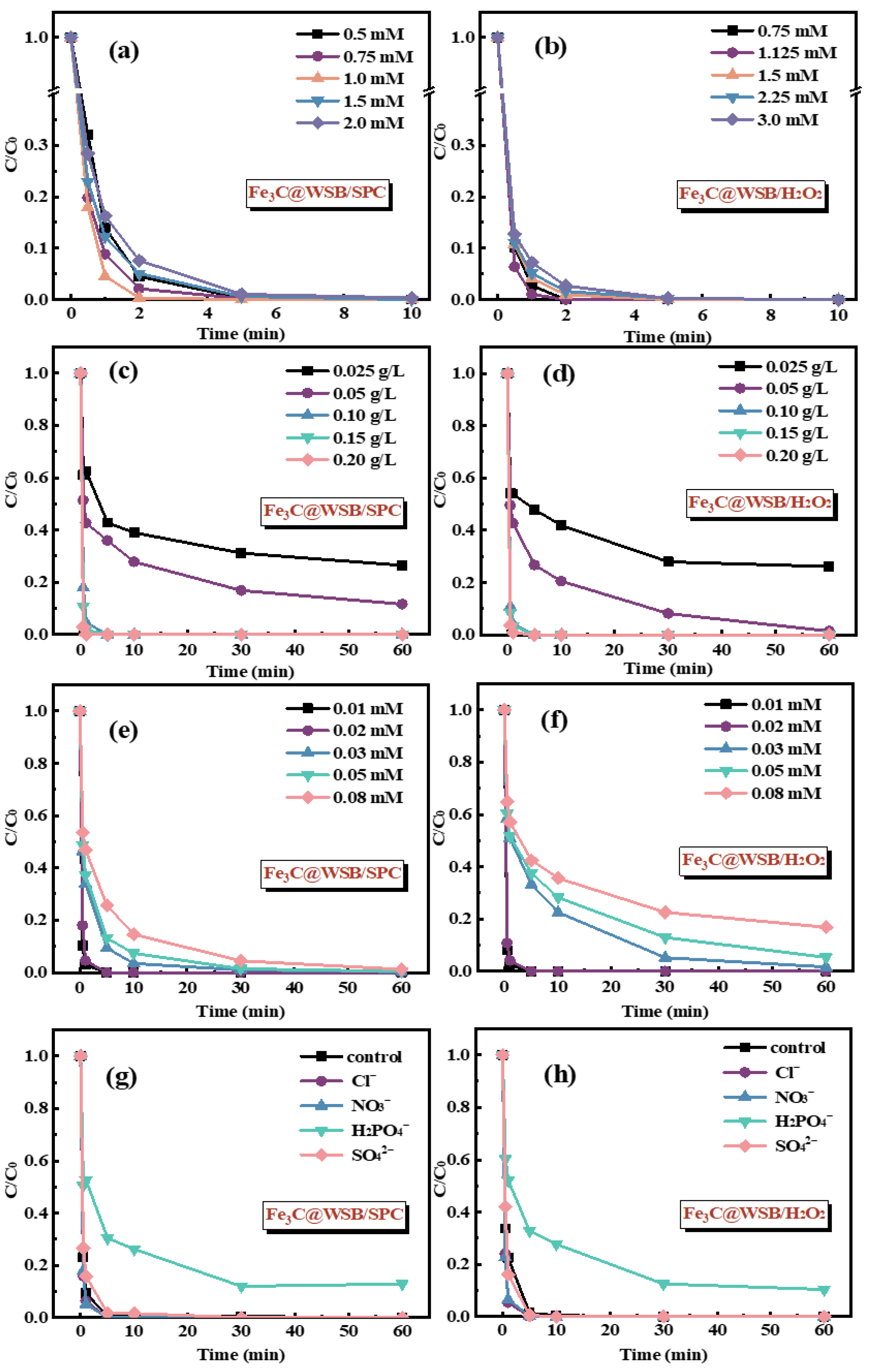
2.3. Influence of Different Parameters
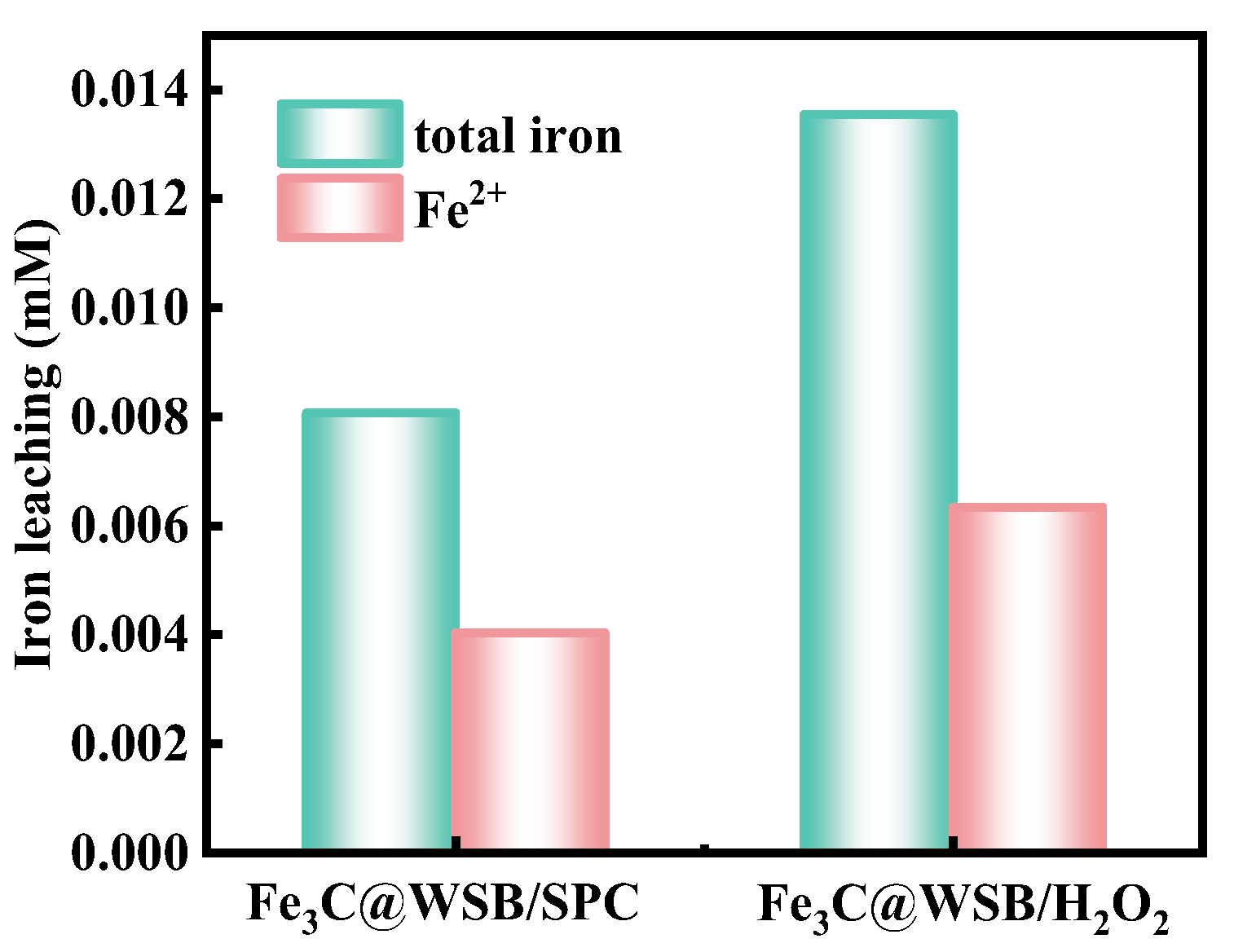
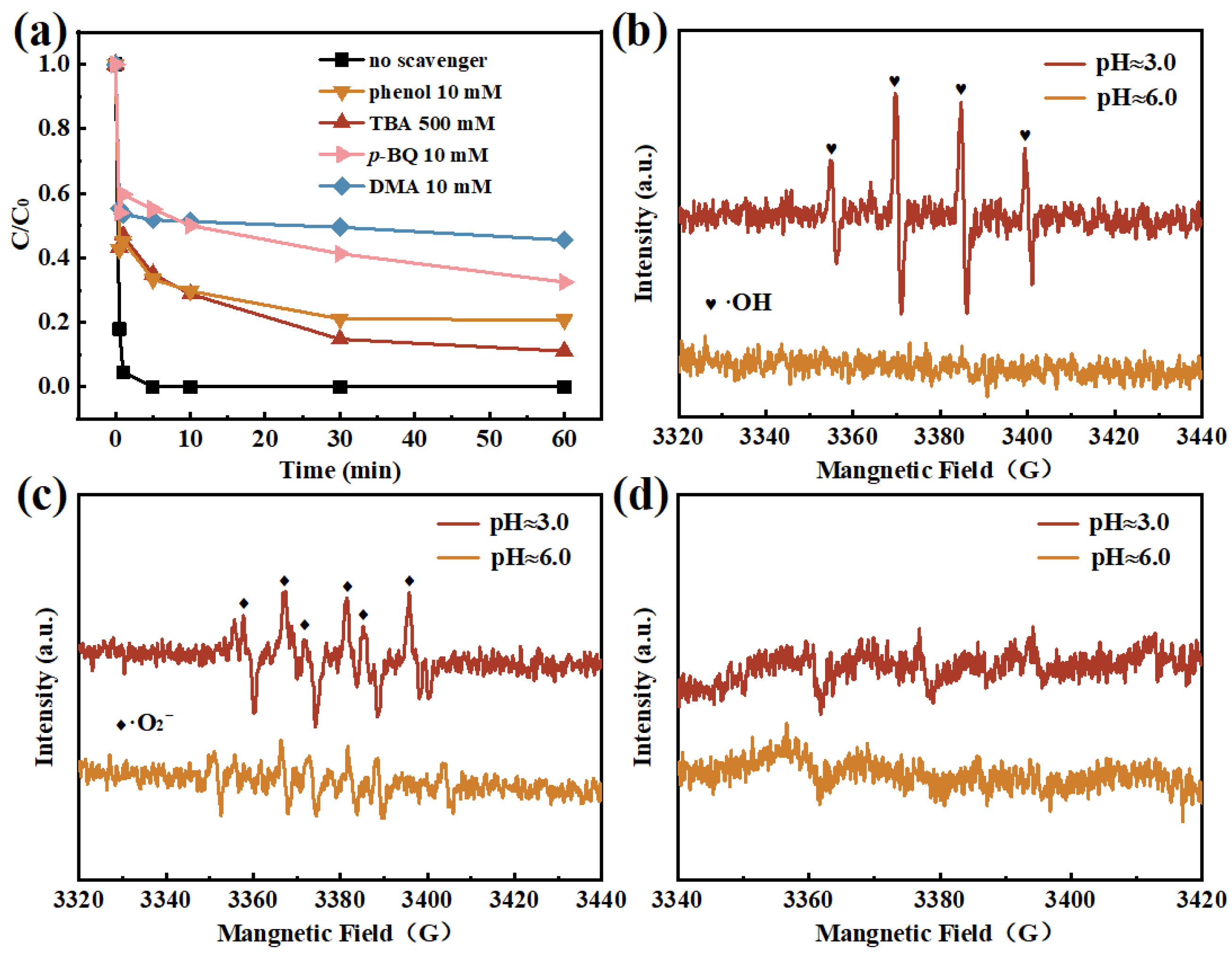
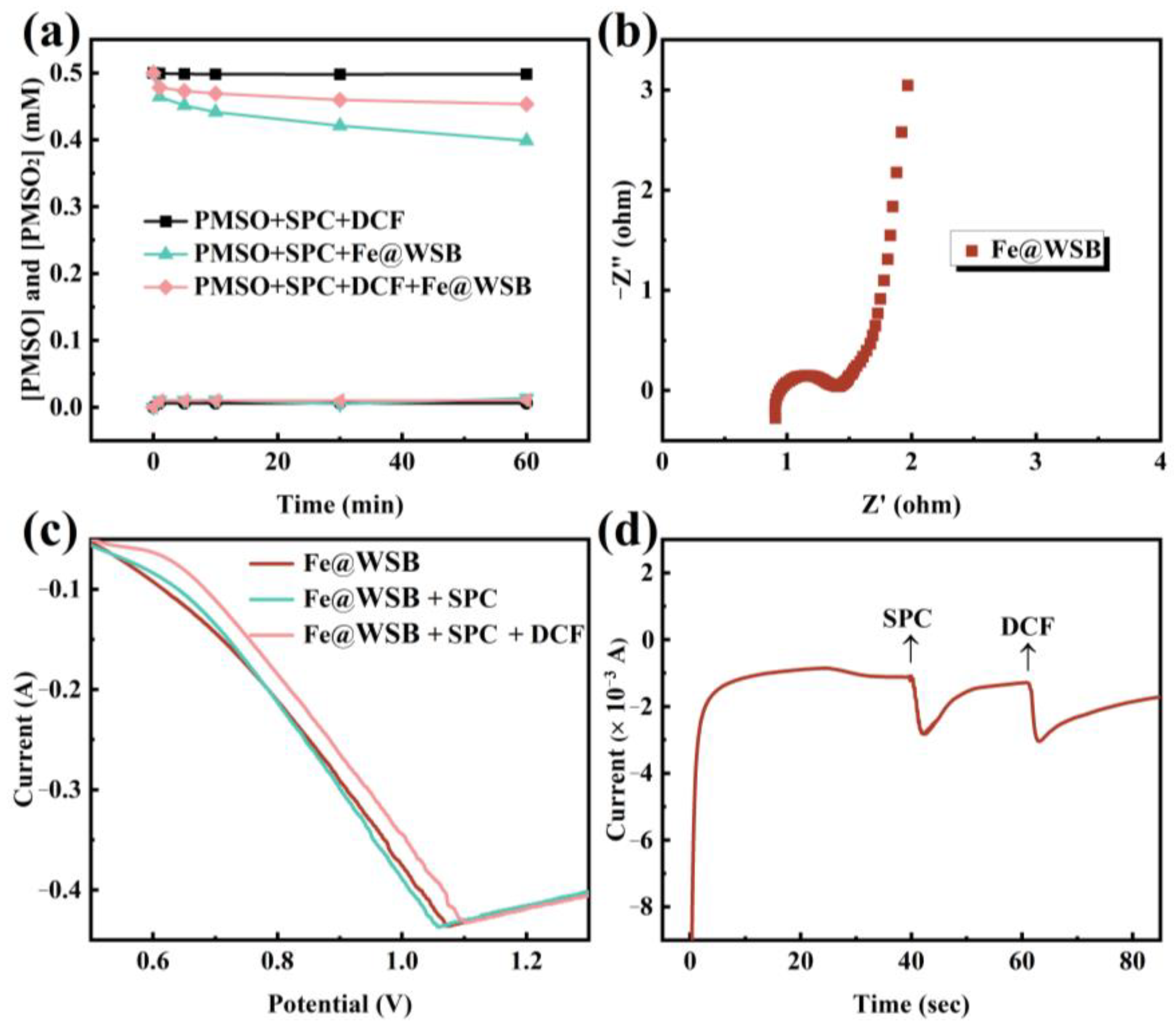
2.4. Activation Mechanism of SPC
2.5. The Reuse Performance of Fe3C@WSB
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of the Catalyst
3.3. Characterization Methods
3.4. Experimental Conditions
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Chen, C.; Guo, K.; Wu, Z.; Wang, L.; Hua, Z.; Fang, J. Kinetics and pathways of the degradation of PPCPs by carbonate radicals in advanced oxidation processes. Water Res. 2020, 185, 116231. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Duan, X.; O’Shea, K.; Dionysiou, D.D. Degradation and transformation of bisphenol A in UV/Sodium percarbonate: Dual role of carbonate radical anion. Water Res. 2020, 171, 115394. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tang, P.; Lu, S.; Zhang, X.; Qiu, Z.; Sui, Q. Enhanced reductive degradation of carbon tetrachloride by carbon dioxide radical anion-based sodium percarbonate/Fe(II)/formic acid system in aqueous solution. Front. Environ. Sci. Eng. 2018, 12, 6. [Google Scholar] [CrossRef]
- Cui, H.; Gu, X.; Lu, S.; Fu, X.; Zhang, X.; Fu, G.Y.; Qiu, Z.; Sui, Q. Degradation of ethylbenzene in aqueous solution by sodium percarbonate activated with EDDS-Fe(III) complex. Chem. Eng. J. 2017, 309, 80–88. [Google Scholar] [CrossRef]
- Che, M.; Xiao, J.; Shan, C.; Chen, S.; Huang, R.; Zhou, Y.; Cui, M.; Qi, W.; Su, R. Efficient removal of chloroform from groundwater using activated percarbonate by cellulose nanofiber-supported Fe/Cu nanocomposites. Water Res. 2023, 243, 120420. [Google Scholar] [CrossRef]
- Shi, J.; Dai, B.; Shen, X.; Xu, L.; Zhang, Y.; Gan, L. Wood induced preparation of Fe3C decorated biochar for peroxymonosulfate activation towards bisphenol a degradation with low ion leaching. J. Environ. Manag. 2023, 340, 117978. [Google Scholar] [CrossRef]
- Gan, L.; Wang, L.; Xu, L.; Fang, X.; Pei, C.; Wu, Y.; Lu, H.; Han, S.; Cui, J.; Shi, J.; et al. Fe3C-porous carbon derived from Fe2O3 loaded MOF-74(Zn) for the removal of high concentration BPA: The integrations of adsorptive/catalytic synergies and radical/non-radical mechanisms. J. Hazard. Mater. 2021, 413, 125305. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of sulfamethoxazole from water via activation of persulfate by Fe3C@NCNTs including mechanism of radical and nonradical process. Chem. Eng. J. 2019, 375, 122004. [Google Scholar] [CrossRef]
- Huang, X.; Niv, Y.; Hu, W. Fe/Fe3C nanoparticles loaded on Fe/N-doped graphene as an efficient heterogeneous Fenton catalyst for degradation of organic pollutants. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 518, 145–150. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, D.; Hu, Z.; Xiao, Y.; He, C.; Jiang, F.; Zhao, N.; Zhao, C.; Zhang, W.; Qiu, R. The adsorption and reduction of anionic Cr(VI) in groundwater by novel iron carbide loaded on N-doped carbon nanotubes: Effects of Fe-confinement. Chem. Eng. J. 2023, 452, 139357. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, H.; Xia, Y.; Liu, X.; Yao, Y.; Wang, W.; Chen, H. Carbon fiber-assisted iron carbide nanoparticles as an efficient catalyst via peroxymonosulfate activation for organic contaminant removal. Catal. Sci. Technol. 2019, 9, 4365–4373. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, Y.; Kim, S.; Min, J.; Kim, D.-G.; Lee, S.-H.; Lee, S.; Jung, S.; Lee, K.-S.; Kim, P.; et al. Insight on the treatment of pig blood as biomass derived electrocatalyst precursor for high performance in the oxygen reduction reaction. Appl. Surf. Sci. 2021, 545, 148940. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Jiang, S.; Li, H.; Zhang, R.; Qu, J.; Zhang, S.; Han, W. One-step synthesis of biochar supported nZVI composites for highly efficient activating persulfate to oxidatively degrade atrazine. Chem. Eng. J. 2021, 420, 129868. [Google Scholar] [CrossRef]
- Ma, M.; You, S.; Wang, W.; Liu, G.; Qi, D.; Chen, X.; Qu, J.; Ren, N. Biomass-Derived Porous Fe3C/Tungsten Carbide/Graphitic Carbon Nanocomposite for Efficient Electrocatalysis of Oxygen Reduction. ACS Appl. Mater. Interfaces 2016, 8, 32307–32316. [Google Scholar] [CrossRef]
- Bo, L.; Feng, L.; Fu, J.; Li, X.; Li, P.; Zhang, Y. The fate of typical pharmaceuticals in wastewater treatment plants of Xi’an city in China. J. Environ. Chem. Eng. 2015, 3, 2203–2211. [Google Scholar] [CrossRef]
- Zhao, N.; Chang, F.; Hao, B.; Yu, L.; Morel, J.L.; Zhang, J. Removal of organic dye by biomass-based iron carbide composite with an improved stability and efficiency. J. Hazard. Mater. 2019, 369, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Catalytic degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) by nano-Fe2O3 activated peroxymonosulfate: Influential factors and mechanism determination. Chemosphere 2017, 169, 568–576. [Google Scholar] [CrossRef]
- Zhuo, S.-N.; Sun, H.; Wang, Z.-Y.; Ren, H.-Y.; Xing, D.-F.; Ren, N.-Q.; Liu, B.-F. A magnetic biochar catalyst with dual active sites of Fe3C and Fe4N derived from floc: The activation mechanism for persulfate on degrading organic pollutant. Chem. Eng. J. 2023, 455, 140702. [Google Scholar] [CrossRef]
- Yu, J.; Afzal, S.; Zeng, T.; Wang, H.; Fu, H. Degradation of bisphenol A by peroxymonosulfate activated with MIL-88B(Fe) derived CC-Fe/C catalysts: Effect of annealing temperature, performance and mechanism. Catal. Commun. 2023, 177, 106660. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, W.; Zhang, Y.; Fan, Q.; Zhang, Y.; Ling, C.; Pan, Y. Boron/Fe2+/H2O2 combined with resins process cost-effectively remove Ni(II) in wastewater containing Ni-EDTA: Performance and mechanism. J. Environ. Chem. Eng. 2024, 12, 114112. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, W.; Jiang, N.; Yu, G.; Guo, H.; Li, J. Plasma photo-Fenton-like catalysis with iron-doped g-C3N4 enhances chloramphenicol degradation in an atmospheric dielectric barrier discharge system. Sep. Purif. Technol. 2024, 346, 127347. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Lu, W.; Xu, L.; Shen, X.; Meng, L.; Pan, Y.; Zhang, Y.; Han, J.; Mei, X.; Qiao, W.; Gan, L. Highly efficient activation of sulfite by p-type S-doped g-C3N4 under visible light for emerging contaminants degradation. Chem. Eng. J. 2023, 472, 144708. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Xiao, J.; Li, L.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Zhang, H. Advanced oxidation processes for water purification using percarbonate: Insights into oxidation mechanisms, challenges, and enhancing strategies. J. Hazard. Mater. 2023, 442, 130014. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, P.; Boyer, T.H.; Zhao, L.; Huang, C.-H. Degradation of Pharmaceuticals and Metabolite in Synthetic Human Urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015, 49, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Thi Minh Tam, N.; Liu, Y.; Bashir, H.; Yin, Z.; He, Y.; Zhou, X. Efficient Removal of Diclofenac from Aqueous Solution by Potassium Ferrate-Activated Porous Graphitic Biochar: Ambient Condition Influences and Adsorption Mechanism. Int. J. Environ. Res. Public Health 2019, 17, 291. [Google Scholar] [CrossRef]
- N.Al-Rimawi, L.; Al-Jabari, M.H.; Sulaiman, S.M.; Nazal, M.K.; Idrees, A.S. Pencil graphite synergistic improvement of zero-valent iron composite for the removal of diclofenac sodium in aqueous solutions: Kinetics and comparative study. Adv. Powder Technol. 2022, 33, 103610. [Google Scholar] [CrossRef]
- Tong, M.; Liu, F.; Dong, Q.; Ma, Z.; Liu, W. Magnetic Fe3O4-deposited flower-like MoS2 nanocomposites for the Fenton-like Escherichia coli disinfection and diclofenac degradation. J. Hazard. Mater. 2020, 385, 121604. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, N.; Huang, X.; Kang, N.; Wu, P.; Dang, Z. Efficient degradation of sodium diclofenac via heterogeneous Fenton reaction boosted by Pd/Fe@Fe3O4 nanoparticles derived from bio-recovered palladium. J. Environ. Manag. 2020, 260, 110072. [Google Scholar] [CrossRef]
- Rao, Y.; Zhang, Y.; Han, F.; Guo, H.; Huang, Y.; Li, R.; Qi, F.; Ma, J. Heterogeneous activation of peroxymonosulfate by LaFeO3 for diclofenac degradation: DFT-assisted mechanistic study and degradation pathways. Chem. Eng. J. 2018, 352, 601–611. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, M.; Xiong, Z.; Guo, Y.; Lai, B. Highly efficient degradation of emerging contaminants by magnetic CuO@FexOy derived from natural mackinawite (FeS) in the presence of peroxymonosulfate. Chin. Chem. Lett. 2022, 33, 948–952. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals. J. Phys. Chem. Ref. Data 1988, 17, 513–887. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, H.; Wang, W.; Li, W.; Ren, Y.; Li, X. Synthesis of Fe0/Fe3O4@porous carbon through a facile heat treatment of iron-containing candle soots for peroxymonosulfate activation and efficient degradation of sulfamethoxazole. J. Hazard. Mater. 2021, 411, 124952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Jiang, W.; Guo, H. Efficient degradation of bisphenol A by a novel ternary synergistic dielectric barrier discharge plasma advanced oxidation process: The role of peracetic acid and ferrous ions. Sep. Purif. Technol. 2025, 354, 129568. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Yang, Y.; Xu, F.; Yang, C.; Guo, H.; Hou, J.; Han, J. Simultaneous removal of organic matters and phosphorus by a novel dielectric barrier discharge coupled with CaO2 system: Feasible and mechanism. Sep. Purif. Technol. 2025, 354, 129519. [Google Scholar] [CrossRef]
- Huang, J.-W.; Han, J.-G.; Guo, H. Fe2+/Fe3+ cycle promoting hydroxylamine activation in dielectric barrier discharge system for efficient degradation of antibiotics: Insight into performance and activation mechanism. Sep. Purif. Technol. 2025, 355, 129709. [Google Scholar] [CrossRef]
- Dong, Z.; Dong, Z.; Xue, Y.; Lu, H.; Yu, M.; Duan, Y.; Li, X.; Li, Z.; Zhang, W.; Ren, G.; et al. Amino polycarboxylic acid complex agent modified Fe0 enhanced activation of H2O2 double decomposition pathways for tetracycline removal under neutral condition. Chem. Eng. J. 2024, 497, 155026. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, Y.; Wan, J.; Ma, Y.; Yan, Z.; Huang, S.; Zeng, C. Fe-N-C catalyst with Fe-NX sites anchored nano carboncubes derived from Fe-Zn-MOFs activate peroxymonosulfate for high-effective degradation of ciprofloxacin: Thermal activation and catalytic mechanism. J. Hazard. Mater. 2022, 424, 127380. [Google Scholar] [CrossRef]
- Fu, L.; Jin, T.; Hu, R.; Pan, Y.; Zhao, Y. Hydroxylamine enhanced Fe3O4/H2O2 system for removing tartrazine. Desalination Water Treat. 2024, 318, 100362. [Google Scholar] [CrossRef]
- Han, Y.; Gan, L.; Gong, H.; Han, J.; Qiao, W.; Xu, L. Photoactivation of peroxymonosulfate by wood pulp cellulose biochar/g-C3N4 composite for diclofenac degradation: The radical and nonradical pathways. Biochar 2022, 4, 35. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Li, S.; Lin, Y.; Zhang, S.; Guan, Q. Activation of sodium percarbonate with Fe3O4 @MXene composite for the efficient removal of bisphenol A. J. Environ. Chem. Eng. 2022, 10, 108702. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Q.; Lian, L.; Li, Y.; Wang, S.; Li, C.; Guan, X. Degradation of Organic Contaminants in the Fe(II)/Peroxymonosulfate Process under Acidic Conditions: The Overlooked Rapid Oxidation Stage. Environ. Sci. Technol. 2021, 55, 15390–15399. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Choi, Y.-K.; Kan, E. Iron-activated bermudagrass-derived biochar for adsorption of aqueous sulfamethoxazole: Effects of iron impregnation ratio on biochar properties, adsorption, and regeneration. Sci. Total Environ. 2021, 750, 141691. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zheng, F.; Xiao, Y.; Fang, J.; Zhao, C.; Zhao, N.; Zhao, C.; Zhang, W.; Zhang, W.; Qiu, R. High Fe utilization efficiency and low toxicity of Fe3C@Fe0 loaded biochar for removing of tetracycline hydrochloride in wastewater. J. Clean. Prod. 2022, 353, 131630. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, J.-M.; Huang, M.; Park, S.-J.; Lee, C.-G. Degradation of imidacloprid via the activation of peroxymonosulfate and peroxydisulfate using a Fenton-sludge-derived Fe0/Fe3C composite. J. Water Process Eng. 2023, 56, 104347. [Google Scholar] [CrossRef]
- Xu, L.; Qi, L.; Han, Y.; Lu, W.; Han, J.; Qiao, W.; Mei, X.; Pan, Y.; Song, K.; Ling, C.; et al. Improvement of Fe2+/peroxymonosulfate oxidation of organic pollutants by promoting Fe2+ regeneration with visible light driven g-C3N4 photocatalysis. Chem. Eng. J. 2022, 430, 132828. [Google Scholar] [CrossRef]



| Composite | Pore Feature | DCF Removal Rate (mM∙min−1) | Preparation Conditions | Ref. |
|---|---|---|---|---|
| Fe3C@WSB | SSA = 184.04 m2 g−1, mesoporous | ca. 0.0250 | walnut shell, Fe(NO3)3∙9H2O, 900 °C, 3 h | this study |
| Fe@biochar | SSA = 175.8 m2 g−1, mesoporous | ca. 3.47 × 10−5 | pitch pine sawdust, K2FeO4, 900 °C, 2 h | [26] |
| Fe0-pencil graphite | SSA = 88.56 m2 g−1, mesoporous | ca. 0.0005 | pencil lead graphite, FeCl3∙6H2O, NaBH4 | [27] |
| Fe3O4/MoS2 | SSA = 77.1 m2 g−1, mesoporous | ca. 0.0031 | (NH4)6Mo7O24·4H2O, CH4N2S, 220 °C, 18 h | [28] |
| bio-Pd/Fe@Fe3O4 | -- | ca. 0.0016 | Na2PdCl, FeCl3∙6H2O, E. faecalis living cell, FeSO4·7H2O, NaBH4, −50 °C, 48 h | [29] |
| LaFeO3 | SSA = 27.4 m2 g−1, mesoporous | ca. 0.0008 | La(NO3)3∙6H2O, Fe(NO3)3∙9H2O, C6H8O7∙H2O, 500 °C, 4 h | [30] |
| CuO@FexOy | SSA = 8.68 m2 g−1, mesoporous | ca. 0.0156 | CuSO4·5H2O, EDTA-2Na, FeS, 450 °C, 3 h | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Huo, Y.; Pei, C.; Zhang, Y.; Xu, L.; Gan, L. Walnut Shell Biomass Triggered Formation of Fe3C-Biochar Composite for Removal of Diclofenac by Activating Percarbonate. Catalysts 2024, 14, 687. https://doi.org/10.3390/catal14100687
Zhang N, Huo Y, Pei C, Zhang Y, Xu L, Gan L. Walnut Shell Biomass Triggered Formation of Fe3C-Biochar Composite for Removal of Diclofenac by Activating Percarbonate. Catalysts. 2024; 14(10):687. https://doi.org/10.3390/catal14100687
Chicago/Turabian StyleZhang, Na, Yudong Huo, Chun Pei, Ying Zhang, Lijie Xu, and Lu Gan. 2024. "Walnut Shell Biomass Triggered Formation of Fe3C-Biochar Composite for Removal of Diclofenac by Activating Percarbonate" Catalysts 14, no. 10: 687. https://doi.org/10.3390/catal14100687









