Biocatalytic Production of Solketal Esters from Used Oil Utilizing Treated Macauba Epicarp Particles as Lipase Immobilization Support: A Dual Valorization of Wastes for Sustainable Chemistry
Abstract
:1. Introduction
2. Results and Discussion
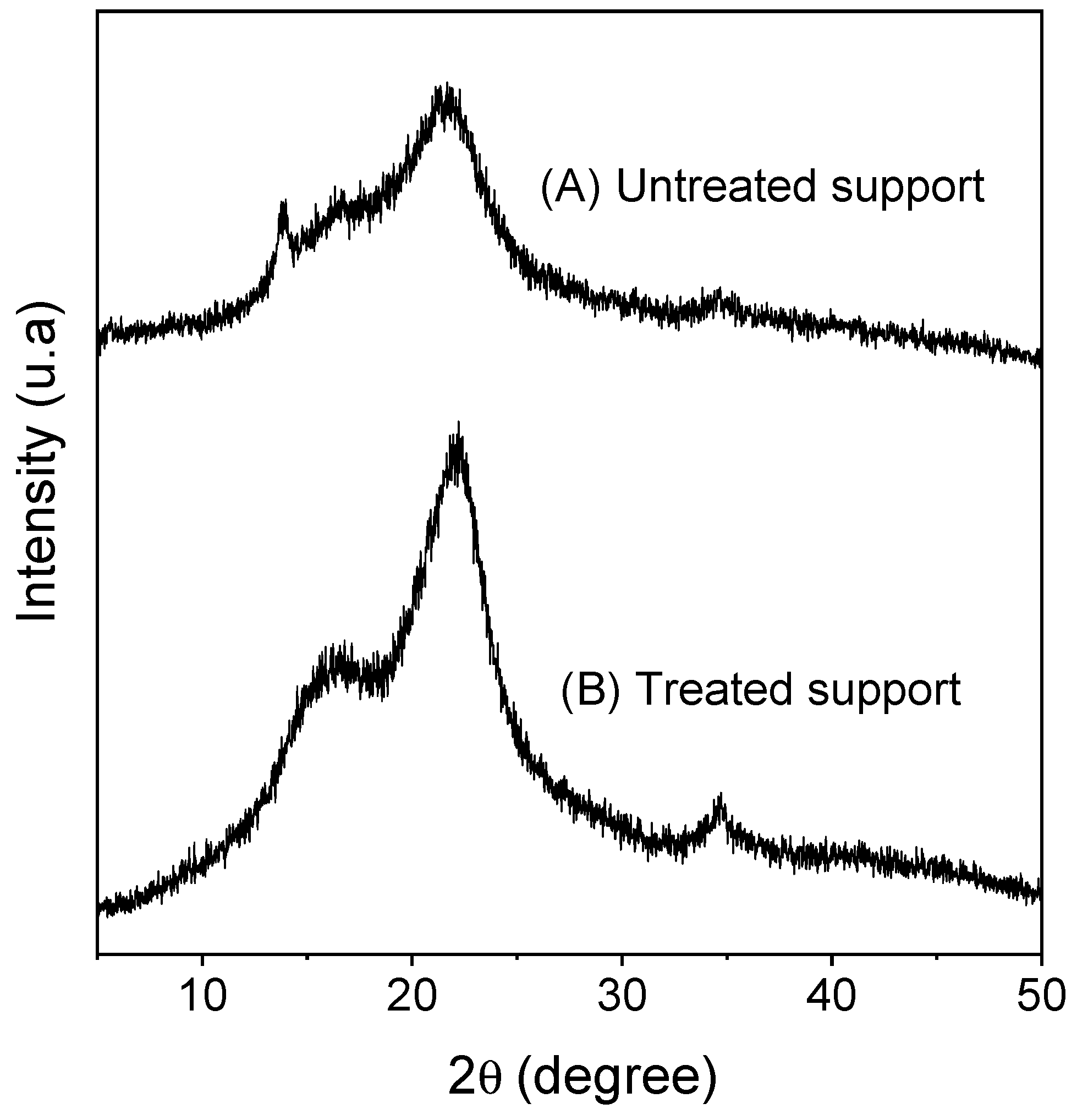
2.1. Macauba Epicarp Particles Characterization
2.2. Characterization of the Heterogeneous Biocatalyst Prepared via Physical Adsorption
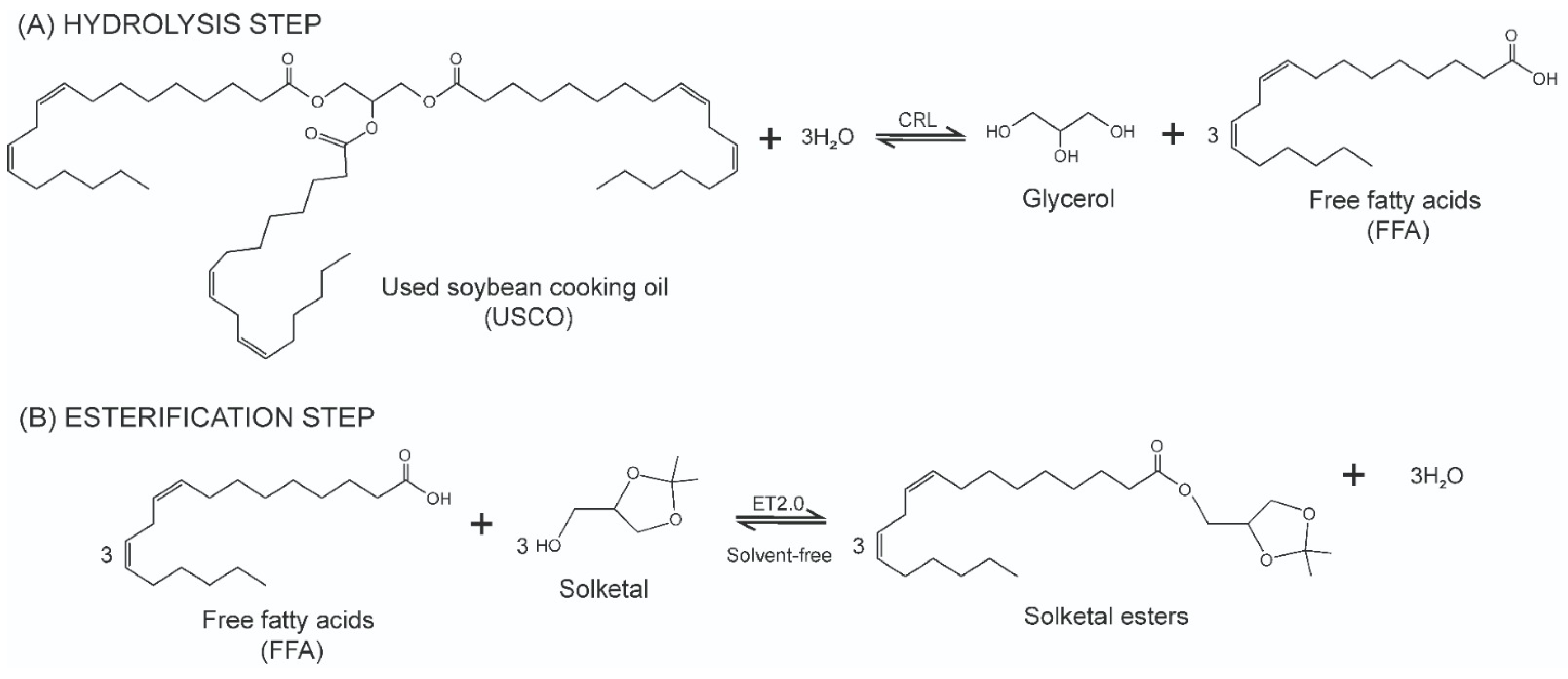
2.3. Optimization of the Enzymatic Production of Solketal Esters via Esterification Reaction
2.4. Reaction Course
2.5. Operational Stability Tests of the Immobilized Biocatalyst
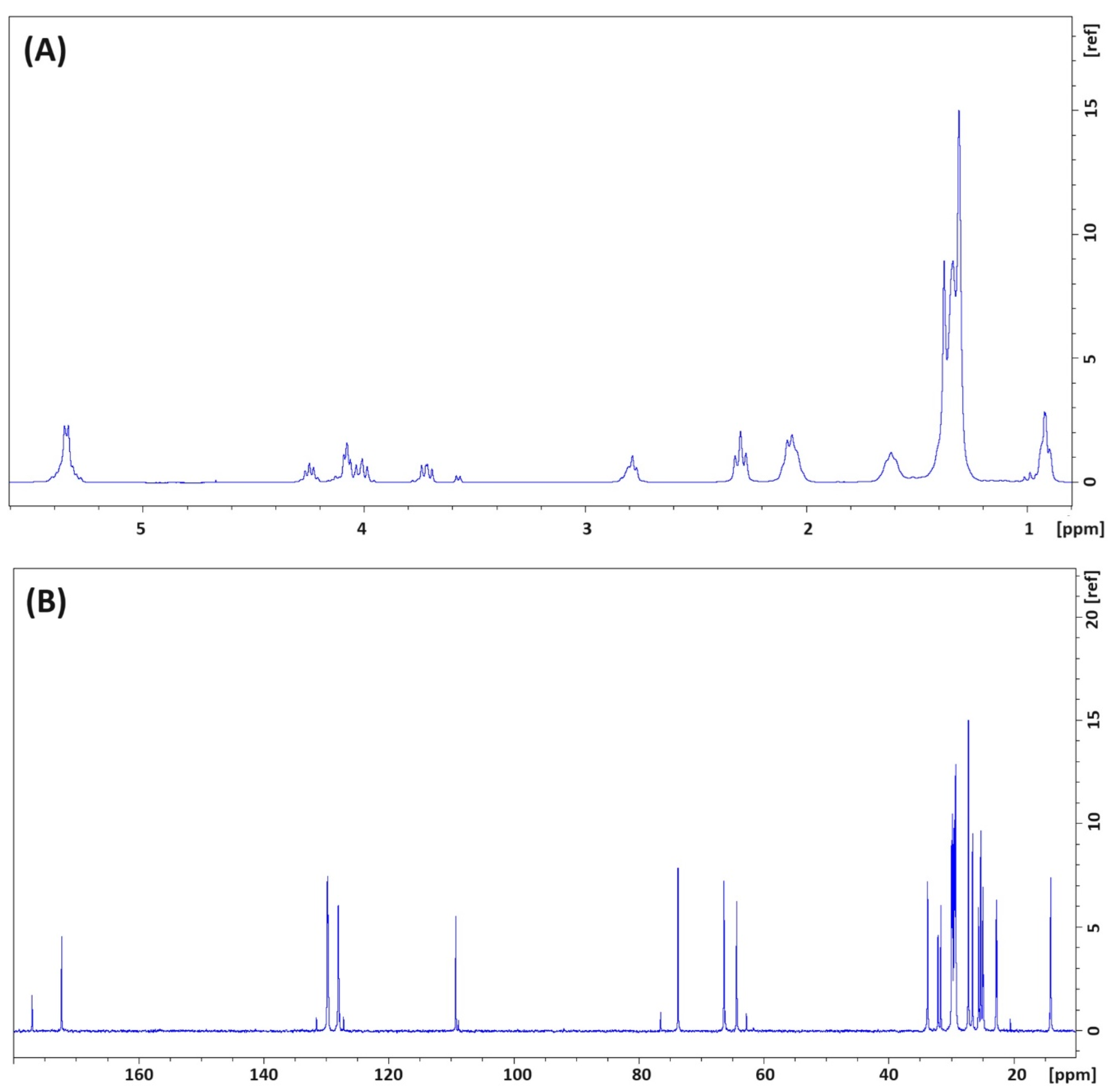
2.6. Identification of the Reaction Products via FT–IR and NMR Analysis
2.7. Comparing with Previous Reports
3. Materials and Methods
3.1. Materials
3.2. Preparation of Macauba Epicarp Particles
3.3. Support Characterization
3.4. General Immobilization Procedure of ET2.0 for Treated Macauba Epicarp Particles
3.5. Enzymatic Hydroesterification of USCO
3.6. CCRD Optimization of Solketal Esters’ Production via Esterification Reaction
3.7. Effect of Reaction Time on Enzymatic Solketal Esters’ Production
3.8. Operational Stability Tests of the Immobilized ET2.0
3.9. Separation of the Esters
3.10. Identification via FT-IR and NMR Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenar, J.A.; Knothe, G. 1,2-Isopropylidene Glycerol Carbonate: Preparation, Characterization, and Hydrolysis. J. Am. Oil Chem. Soc. 2008, 85, 365–372. [Google Scholar] [CrossRef]
- Mariam, N.M.D.N.S.; Hoong, S.S.; Arniza, M.Z.; Adnan, S.; Ismail, T.N.M.T.; Yeong, S.K.; Yusop, M.R. Optimisation of Reaction Parameters for the Synthesis of Solketal Levulinate as Potential Additive. J. Oil Palm Res. 2022, 34, 524–534. [Google Scholar] [CrossRef]
- Neamtu, C.; Stepan, E.; Plesu, V.; Bozga, G.; Tuluc, A. Synthesis and Characterization of New Solketal Alkylesters Usable as Diesel Biobased Fuel Additives. Rev. Chim. 2019, 70, 1167–1172. [Google Scholar] [CrossRef]
- Yang, J.; Ma, W.J.; Li, N.; Zhou, J.H.; Sun, H.Z. Synthesis of Tung Oil Monoglyceride. Adv. Mater. Res. 2014, 941–944, 1021–1025. [Google Scholar] [CrossRef]
- Perosa, A.; Moraschini, A.; Selva, M.; Noè, M. Synthesis of the Fatty Esters of Solketal and Glycerol-Formal: Biobased Specialty Chemicals. Molecules 2016, 21, 170. [Google Scholar] [CrossRef]
- Sultanova, R.M.; Khusnutdinova, N.S.; Borisova, Y.G.; Raskildina, G.Z.; Meshcheryakova, S.A.; Samorodov, A.V.; Zlotsky, S.S. Selective Synthesis of Some Carboxylic Acids Esters. Russ. J. Gen. Chem. 2023, 93, 1–7. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Lima, P.C.; dos Santos, W.T.P.; Klein, S.I.; Clososki, G.C.; Caires, F.J. Oxygenated Biofuels: Synthesis of Fatty Acid Solketal Esters with a Mixture of Sulfonated Silica and (Bu4N)(BF4) Catalyst. Catal. Commun. 2019, 120, 76–79. [Google Scholar] [CrossRef]
- Lozano, P.; Gomez, C.; Nicolas, A.; Polo, R.; Nieto, S.; Bernal, J.M.; García-Verdugo, E.; Luis, S.V. Clean Enzymatic Preparation of Oxygenated Biofuels from Vegetable and Waste Cooking Oils by Using Spongelike Ionic Liquids Technology. ACS Sustain. Chem. Eng. 2016, 4, 6125–6132. [Google Scholar] [CrossRef]
- Sousa, R.R.; Silva, A.S.; Fernandez-Lafuente, R.; Ferreira-Leitão, V.S. Solvent-Free Esterifications Mediated by Immobilized Lipases: A Review from Thermodynamic and Kinetic Perspectives. Catal. Sci. Technol. 2021, 11, 5696–5711. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in Lipase-Catalyzed Esterification Reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Verger, R. “Interfacial Activation” of Lipases: Facts and Artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial Enzymes with Attractive Applications. Angew. Chemie Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Mine, Y.; Zhang, L.; Fukunaga, K.; Sugimura, Y. Enhancement of Enzyme Activity and Enantioselectivity by Cyclopentyl Methyl Ether in the Transesterification Catalyzed by Pseudomonas cepacia Lipase Co-Lyophilized with Cyclodextrins. Biotechnol. Lett. 2005, 27, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Zniszczol, A.; Walczak, K. Lipases Aided Esterification of (2,2-Dimethyl-1,3-Dioxolan-4-Yl)Methanol. Lett. Org. Chem. 2014, 11, 6–12. [Google Scholar] [CrossRef]
- Da Silva Junior, V.A.; Shigueyuki Kanda, L.R.; Zandoná-Filho, A.; Corazza, M.L.; Sutile De Lima, C. Effect of Supercritical Carbon Dioxide over the Esterification of Levulinic Acid with Ethanol Using Montmorillonite K10 as Catalyst. J. CO2 Util. 2020, 39, 101158. [Google Scholar] [CrossRef]
- Romano, D.; Ferrario, V.; Molinari, F.; Gardossi, L.; Montero, J.M.S.; Torre, P.; Converti, A. Kinetic Resolution of (R, S)-1,2-O-Isopropylideneglycerol by Esterification with Dry Mycelia of Moulds. J. Mol. Catal. B Enzym. 2006, 41, 71–74. [Google Scholar] [CrossRef]
- Torregrosa, R.; Yara-Varón, E.; Balcells, M.; Torres, M.; Canela-Garayoa, R. Entirely Solvent-Free Biocatalytic Synthesis of Solketal Fatty Esters from Soybean Seeds. Comptes Rendus Chim. 2016, 19, 749–753. [Google Scholar] [CrossRef]
- Boncel, S.; Zniszczoł, A.; Szymańska, K.; Mrowiec-Białoń, J.; Jarzebski, A.; Walczak, K.Z. Alkaline Lipase from Pseudomonas Fluorescens Non-Covalently Immobilised on Pristine versus Oxidised Multi-Wall Carbon Nanotubes as Efficient and Recyclable Catalytic Systems in the Synthesis of Solketal Esters. Enzyme Microb. Technol. 2013, 53, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Itabaiana, I.; Gonçalves, K.M.; Zoumpanioti, M.; Leal, I.C.R.; Miranda, L.S.M.E.; Xenakis, A.; De Souza, R.O.M.A. Microemulsion-Based Organogels as an Efficient Support for Lipase-Catalyzed Reactions under Continuous-Flow Conditions. Org. Process Res. Dev. 2014, 18, 1372–1376. [Google Scholar] [CrossRef]
- Johny, J.; Jatla, A.; Eruva, V.K.; Misra, S.; Kaki, S.S. Synthesis, Characterization and Evaluation of 1-Monoacylglycerols of Unsaturated Fatty Acids as Potential Bioactive Lipids. Grasas Aceites 2019, 70, e325. [Google Scholar] [CrossRef]
- Junior, I.I.; Flores, M.C.; Sutili, F.K.; Leite, S.G.F.; Leandro, L.S.; Leal, I.C.R.; De Souza, R.O.M.A. Fatty Acids Residue from Palm Oil Refining Process as Feedstock for Lipase Catalyzed Monoacylglicerol Production under Batch and Continuous Flow Conditions. J. Mol. Catal. B Enzym. 2012, 77, 53–58. [Google Scholar] [CrossRef]
- Mendoza-Ortiz, P.A.; Gama, R.S.; Gómez, O.C.; Luiz, J.H.H.; Fernandez-Lafuente, R.; Cren, E.C.; Mendes, A.A. Sustainable Enzymatic Synthesis of a Solketal Ester—Process Optimization and Evaluation of Its Antimicrobial Activity. Catalysts 2020, 10, 218. [Google Scholar] [CrossRef]
- Rocha, K.S.C.; Queiroz, M.S.R.; Gomes, B.S.; Dallago, R.; de Souza, R.O.M.A.; Guimarães, D.O.; Itabaiana, I.; Leal, I.C.R. Lipases of Endophytic Fungi Stemphylium lycopersici and Sordaria Sp.: Application in the Synthesis of Solketal Derived Monoacylglycerols. Enzyme Microb. Technol. 2020, 142, 109664. [Google Scholar] [CrossRef]
- Villa, R.; Alvarez, E.; Nieto, S.; Donaire, A.; Garcia-Verdugo, E.; Luis, S.V.; Lozano, P. Chemo-Enzymatic Production of Omega-3 Monoacylglycerides Using Sponge-like Ionic Liquids and Supercritical Carbon Dioxide. Green Chem. 2020, 22, 5701–5710. [Google Scholar] [CrossRef]
- Mendes, A.A.; Soares, C.M.F.; Tardioli, P.W. Recent Advances and Future Prospects for Biolubricant Base Stocks Production Using Lipases as Environmentally Friendly Catalysts: A Mini-Review. World J. Microbiol. Biotechnol. 2023, 39, 25. [Google Scholar] [CrossRef] [PubMed]
- Pourzolfaghar, H.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A Review of the Enzymatic Hydroesterification Process for Biodiesel Production. Renew. Sustain. Energy Rev. 2016, 61, 245–257. [Google Scholar] [CrossRef]
- Gryglewicz, S.; Muszyński, M.; Nowicki, J. Enzymatic Synthesis of Rapeseed Oil-Based Lubricants. Ind. Crops Prod. 2013, 45, 25–29. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Jindapon, W.; Ngamcharussrivichai, C. Valorization of Biodiesel Plant-Derived Products via Preparation of Solketal Fatty Esters over Calcium-Rich Natural Materials Derived Oxides. J. Taiwan Inst. Chem. Eng. 2017, 81, 57–64. [Google Scholar] [CrossRef]
- Domínguez De María, P.; Sánchez-Montero, J.M.; Sinisterra, J.V.; Alcántara, A.R. Understanding Candida rugosa Lipases: An Overview. Biotechnol. Adv. 2006, 24, 180–196. [Google Scholar] [CrossRef]
- Carvalho, W.C.A.; Luiz, J.H.H.; Fernandez-Lafuente, R.; Hirata, D.B.; Mendes, A.A. Eco-Friendly Production of Trimethylolpropane Triesters from Refined and Used Soybean Cooking Oils Using an Immobilized Low-Cost Lipase (Eversa® Transform 2.0) as Heterogeneous Catalyst. Biomass Bioenergy 2021, 155, 106302. [Google Scholar] [CrossRef]
- Guedes Júnior, J.G.E.; Mattos, F.R.; Sabi, G.J.; Carvalho, W.C.A.; Luiz, J.H.H.; Cren, É.C.; Fernandez-Lafuente, R.; Mendes, A.A. Design of a Sustainable Process for Enzymatic Production of Ethylene Glycol Diesters via Hydroesterification of Used Soybean Cooking Oil. J. Environ. Chem. Eng. 2022, 10, 107062. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is Enzyme Immobilization a Mature Discipline? Some Critical Considerations to Capitalize on the Benefits of Immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.D.C.; Steidle Neto, A.J.; Mendes, A.A.; Pereira, D.T.V. Economic Feasibility of Biodiesel Production from Macauba in Brazil. Energy Econ. 2013, 40, 819–824. [Google Scholar] [CrossRef]
- Silva, L.N.; Fortes, I.C.P.; De Sousa, F.P.; Pasa, V.M.D. Biokerosene and Green Diesel from Macauba Oils via Catalytic Deoxygenation over Pd/C. Fuel 2016, 164, 329–338. [Google Scholar] [CrossRef]
- Bijoy, G.; Rajeev, R.; Benny, L.; Jose, S.; Varghese, A. Enzyme Immobilization on Biomass-Derived Carbon Materials as a Sustainable Approach towards Environmental Applications. Chemosphere 2022, 307, 135759. [Google Scholar] [CrossRef]
- Girelli, A.M.; Chiappini, V. Renewable, Sustainable, and Natural Lignocellulosic Carriers for Lipase Immobilization: A Review. J. Biotechnol. 2023, 365, 29–47. [Google Scholar] [CrossRef]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-Industrial Wastes as Potential Carriers for Enzyme Immobilization: A Review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef]
- Foo, W.H.; Chia, W.Y.; Tang, D.Y.Y.; Koay, S.S.N.; Lim, S.S.; Chew, K.W. The Conundrum of Waste Cooking Oil: Transforming Hazard into Energy. J. Hazard. Mater. 2021, 417, 126129. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Parchami, M.; Agnihotri, S.; Taherzadeh, M.J. Aqueous Ethanol Organosolv Process for the Valorization of Brewer’s Spent Grain (BSG). Bioresour. Technol. 2022, 362, 127764. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.; Mohamed, T.A.; Zheng, G.; Qu, F.; Wang, F.; Zhao, Y.; Song, C. Lignocellulose Biomass Bioconversion during Composting: Mechanism of Action of Lignocellulase, Pretreatment Methods and Future Perspectives. Chemosphere 2022, 286, 131635. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.K.; Goyal, D. Optimization of Pretreatment of Wheat Straw Using Response Surface Methodology for Production of Lactic Acid Using Bacillus sonorenesis Strain DGS15. Bioenergy Res. 2023, 16, 967–978. [Google Scholar] [CrossRef]
- Du, S.K.; Su, X.; Yang, W.; Wang, Y.; Kuang, M.; Ma, L.; Fang, D.; Zhou, D. Enzymatic Saccharification of High Pressure Assist-Alkali Pretreated Cotton Stalk and Structural Characterization. Carbohydr. Polym. 2016, 140, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.S.; Ji, X.; Khushk, I.; Mirjatt, A.N.; Tunio, A.A.; Huang, Y. Ionic Liquid and Diluted Sulfuric Acid Combinatorial Pretreatment for Efficient Sugarcane Bagasse Conversion to L-Lactic Acid. Ind. Crops Prod. 2023, 204, 117272. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, B.M.; Koo, D.H.; Kang, P.H.; Jeun, J.P. Preparation of Nanocellulose from a Kenaf Core Using E-Beam Irradiation and Acid Hydrolysis. Cellulose 2016, 23, 3039–3049. [Google Scholar] [CrossRef]
- Alves, M.D.; Aracri, F.M.; Cren, É.C.; Mendes, A.A. Isotherm, Kinetic, Mechanism and Thermodynamic Studies of Adsorption of a Microbial Lipase on a Mesoporous and Hydrophobic Resin. Chem. Eng. J. 2017, 311, 1–12. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Arana-Peña, S.; da Rocha, T.N.; Miranda, L.P.; Berenguer-Murcia, Á.; Tardioli, P.W.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Liquid Lipase Preparations Designed for Industrial Production of Biodiesel. Is It Really an Optimal Solution? Renew. Energy 2021, 164, 1566–1587. [Google Scholar] [CrossRef]
- Martínez-Sanchez, J.A.; Arana-Peña, S.; Carballares, D.; Yates, M.; Otero, C.; Fernandez-Lafuente, R. Immobilized Biocatalysts of Eversa® Transform 2.0 and Lipase from Thermomyces lanuginosus: Comparison of Some Properties and Performance in Biodiesel Production. Catalysts 2020, 10, 738. [Google Scholar] [CrossRef]
- Pedro, K.C.N.R.; da Silva, J.V.V.; Cipolatti, E.P.; Manoel, E.A.; Campisano, I.S.P.; Henriques, C.A.; Langone, M.A.P. Adsorption of Lipases on Porous Silica-Based Materials for Esterification in a Solvent-Free System. 3 Biotech 2023, 13, 380. [Google Scholar] [CrossRef]
- Sabi, G.J.; Gama, R.S.; Fernandez-Lafuente, R.; Cancino-Bernardi, J.; Mendes, A.A. Decyl Esters Production from Soybean-Based Oils Catalyzed by Lipase Immobilized on Differently Functionalized Rice Husk Silica and Their Characterization as Potential Biolubricants. Enzyme Microb. Technol. 2022, 157, 110019. [Google Scholar] [CrossRef] [PubMed]
- Shomal, R.; Du, W.; Al-Zuhair, S. Immobilization of Lipase on Metal-Organic Frameworks for Biodiesel Production. J. Environ. Chem. Eng. 2022, 10, 107265. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Evaluation of Different Commercial Hydrophobic Supports for the Immobilization of Lipases: Tuning Their Stability, Activity and Specificity. RSC Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Badgujar, V.C.; Badgujar, K.C.; Yeole, P.M.; Bhanage, B.M. Investigation of Effect of Ultrasound on Immobilized C. rugosa Lipase: Synthesis of Biomass Based Furfuryl Derivative and Green Metrics Evaluation Study. Enzyme Microb. Technol. 2021, 144, 109738. [Google Scholar] [CrossRef]
- Morales, A.H.; Hero, J.S.; Ledesma, A.E.; Martínez, M.A.; Navarro, M.C.; Gómez, M.I.; Romero, C.M. Tuning Surface Interactions on MgFe2O4 Nanoparticles to Induce Interfacial Hyperactivation in Candida rugosa Lipase Immobilization. Int. J. Biol. Macromol. 2023, 253, 126615. [Google Scholar] [CrossRef]
- Morales, A.H.; Hero, J.S.; Ledesma, A.E.; Perez, H.A.; Navarro, M.C.; Gómez, M.I.; Romero, C.M. Interfacial Hyperactivation of Candida rugosa Lipase onto Ca2Fe2O5 Nanoparticles: pH and Ionic Strength Fine-Tuning to Modulate Protein-Support Interactions. Langmuir 2023, 39, 12004–12019. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, K.C.; Bhanage, B.M. The Green Metric Evaluation and Synthesis of Diesel-Blend Compounds from Biomass Derived Levulinic Acid in Supercritical Carbon Dioxide. Biomass Bioenergy 2016, 84, 12–21. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, H.; Li, J.; Huang, Y.; Alsaedi, A.; Hayat, T.; Xu, X.; Wang, X. Rice Husks as a Sustainable Silica Source for Hierarchical Flower-like Metal Silicate Architectures Assembled into Ultrathin Nanosheets for Adsorption and Catalysis. J. Hazard. Mater. 2017, 321, 92–102. [Google Scholar] [CrossRef]
- Machado, N.B.; Miguez, J.P.; Bolina, I.C.A.; Salviano, A.B.; Gomes, R.A.B.; Tavano, O.L.; Luiz, J.H.H.; Tardioli, P.W.; Cren, É.C.; Mendes, A.A. Preparation, Functionalization and Characterization of Rice Husk Silica for Lipase Immobilization via Adsorption. Enzyme Microb. Technol. 2019, 128, 9–21. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters Necessary to Define an Immobilized Enzyme Preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Li, M.; Butka, E.; Wang, X. Comprehensive Quantification of Triacylglycerols in Soybean Seeds by Electrospray Ionization Mass Spectrometry with Multiple Neutral Loss Scans. Sci. Rep. 2014, 4, 6581. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Jeong, M.K.; Park, C.; Lee, J. Distribution of Triacylglycerols and Fatty Acids in Soybean Oil with Thermal Oxidation and Methylene Blue Photosensitization. J. Am. Oil Chem. Soc. 2011, 88, 373–380. [Google Scholar] [CrossRef]





| Runs | Independent Variables’ Coded (Actual) FFAs Conversion (%) | FFAs Conversion (%) a | ||
|---|---|---|---|---|
| FFAs–Solketal Molar Ratio | Temperature (°C) | Biocatalyst Concentration (% wt.) | ||
| 1 | −1 (1:1.6) | −1 (46) | −1 (8) | 6.2 ± 3.5 |
| 2 | +1 (1:3.4) | −1 (46) | −1 (8) | 30.2 ± 2.8 |
| 3 | −1 (1:1.6) | +1 (64) | −1 (8) | 23.4 ± 2.2 |
| 4 | +1 (1:3.4) | +1 (64) | −1 (8) | 24.6 ± 1.7 |
| 5 | −1 (1:1.6) | −1 (46) | +1 (17) | 44.5 ± 0.5 |
| 6 | +1 (1:3.4) | −1 (46) | +1 (17) | 40.5 ± 2.1 |
| 7 | −1 (1:1.6) | +1 (64) | +1 (17) | 43.0 ± 0.3 |
| 8 | +1 (1:3.4) | +1 (64) | +1 (17) | 30.1 ± 3.8 |
| 9 | −1.68 (1:1) | 0 (55) | 0 (12.5) | 21.7 ± 1.4 |
| 10 | +1.68 (1:4) | 0 (55) | 0 (12.5) | 22.2 ± 2.2 |
| 11 | 0 (1:2.5) | −1.68 (40) | 0 (12.5) | 28.9 ± 0.1 |
| 12 | 0 (1:2.5) | +1.68 (70) | 0 (12.5) | 39.8 ± 2.1 |
| 13 | 0 (1:2.5) | 0 (55) | −1.68 (5) | 19.3 ± 0.9 |
| 14 | 0 (1:2.5) | 0 (55) | +1.68 (20) | 55.7 ± 2.4 |
| 15 | 0 (1:2.5) | 0 (55) | 0 (12.5) | 35.7 ± 1.3 |
| 16 | 0 (1:2.5) | 0 (55) | 0 (12.5) | 30.2 ± 1.3 |
| 17 | 0 (1:2.5) | 0 (55) | 0 (12.5) | 31.5 ± 0.3 |
| 18 | 0 (1:2.5) | 0 (55) | 0 (12.5) | 33.0 ± 2.2 |
| Parameters | Regression Coefficients | Standard Errors | p-Values | |
| Mean | 32.64 | 1.68 | 0.0000 | |
| x1 | 0.66 | 0.91 | 0.4897 * | |
| x12 | −3.96 | 0.95 | 0.0031 | |
| x2 | 1.32 | 0.91 | 0.1875 * | |
| x22 | 0.43 | 0.95 | 0.6605 * | |
| x3 | 9.88 | 0.91 | 0.0000 | |
| x32 | 1.53 | 0.95 | 0.1457 * | |
| x1.x2 | −3.96 | 1.19 | 0.0106 | |
| x1.x3 | −5.27 | 1.19 | 0.0022 | |
| x2.x3 | −2.92 | 1.19 | 0.0400 | |
| ANOVA | ||||
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Test |
| Regression | 2058.6732 | 9 | 228.74 | 20.11 |
| Residual | 90.9850 | 8 | 11.37 | |
| Lack of fit | 74.0784 | 5 | 14.82 | 2.63 |
| Pure error | 16.9066 | 3 | 5.64 | |
| Total | 2149.6582 | 17 | ||
| R2 = 0.9577; F0.05;9;8 = 3.39 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel Júnior, J.; Dimas, J.V.B.; Barbosa, M.S.; Gomes, R.A.B.; Carvalho, A.K.F.; Soares, C.M.F.; Fernandez-Lafuente, R.; Mendes, A.A. Biocatalytic Production of Solketal Esters from Used Oil Utilizing Treated Macauba Epicarp Particles as Lipase Immobilization Support: A Dual Valorization of Wastes for Sustainable Chemistry. Catalysts 2024, 14, 693. https://doi.org/10.3390/catal14100693
Miguel Júnior J, Dimas JVB, Barbosa MS, Gomes RAB, Carvalho AKF, Soares CMF, Fernandez-Lafuente R, Mendes AA. Biocatalytic Production of Solketal Esters from Used Oil Utilizing Treated Macauba Epicarp Particles as Lipase Immobilization Support: A Dual Valorization of Wastes for Sustainable Chemistry. Catalysts. 2024; 14(10):693. https://doi.org/10.3390/catal14100693
Chicago/Turabian StyleMiguel Júnior, José, João V. B. Dimas, Milson S. Barbosa, Raphael A. B. Gomes, Ana K. F. Carvalho, Cleide M. F. Soares, Roberto Fernandez-Lafuente, and Adriano A. Mendes. 2024. "Biocatalytic Production of Solketal Esters from Used Oil Utilizing Treated Macauba Epicarp Particles as Lipase Immobilization Support: A Dual Valorization of Wastes for Sustainable Chemistry" Catalysts 14, no. 10: 693. https://doi.org/10.3390/catal14100693








