Magnetic Metallic Nanoparticles Coated with Carbon for the Catalytic Removal of Bromate from Water
Abstract
1. Introduction
2. Results
2.1. Materials Characterization
2.2. Catalytic Tests
2.2.1. Bromate Reduction over Magnetic Nanoparticles
2.2.2. Bromate Reduction over Magnetic Nanoparticles Coated with Carbon
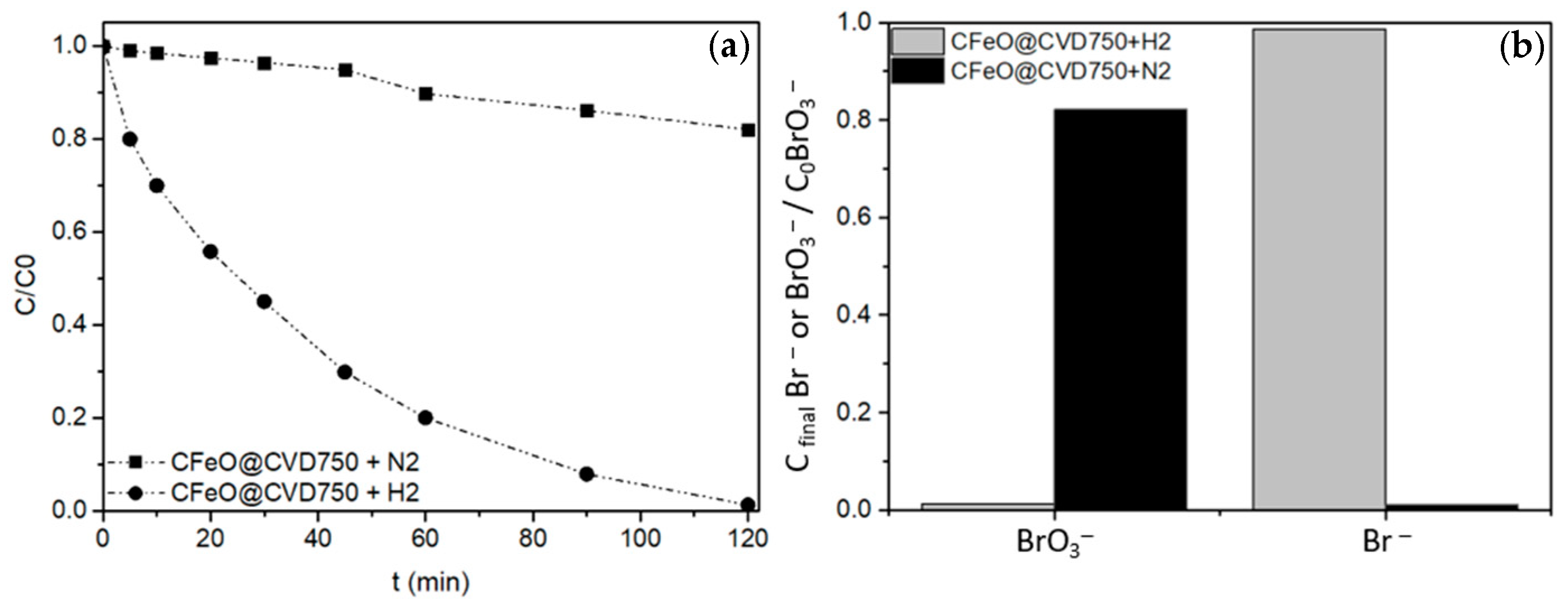
2.2.3. Hydrogen-Free Experiment
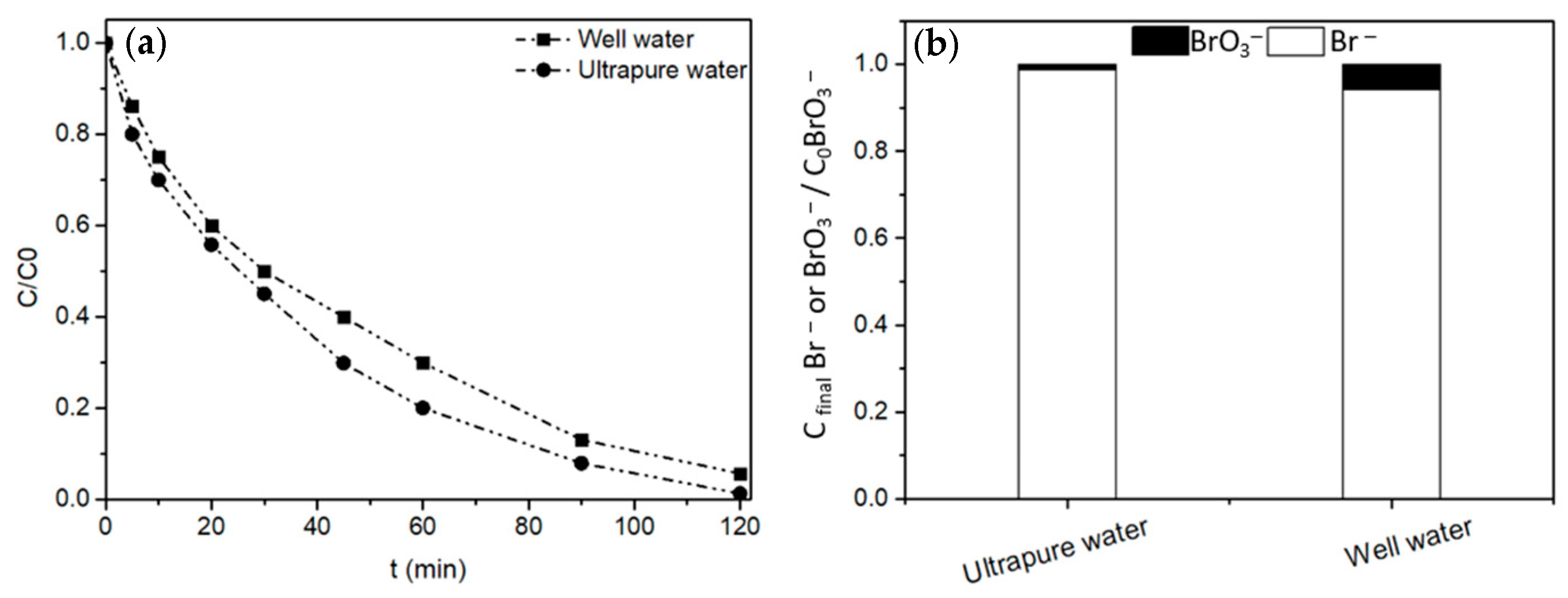
2.2.4. Application to Real Water
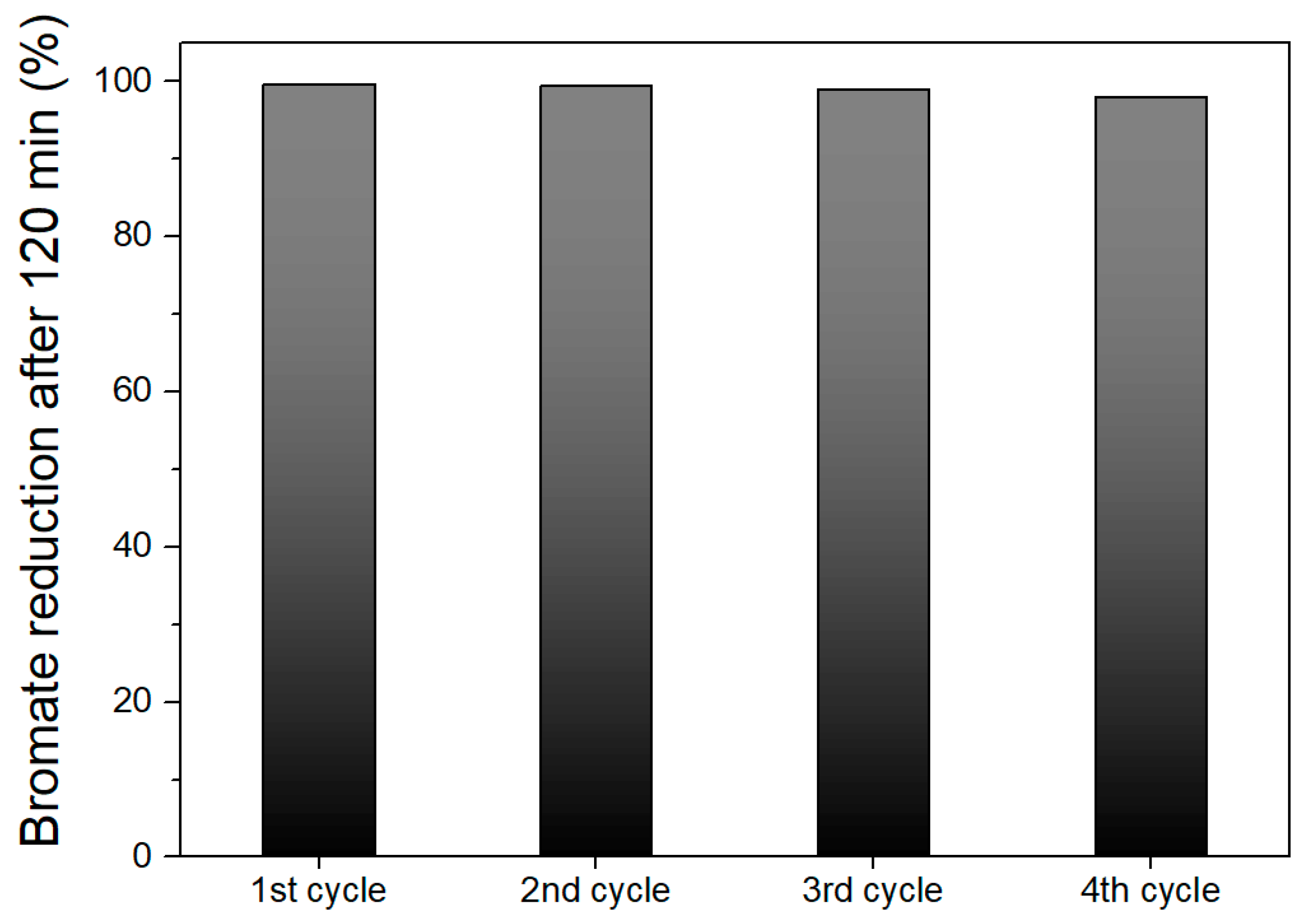
2.2.5. Reutilization Experiments
3. Materials and Methods
3.1. Preparation of Materials
3.2. Characterization of Materials
3.3. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.-H.; Gao, N.-Y.; Zhao, D.-Y.; Zhang, W.-X.; Xu, Q.-K.; Xiao, A.-H. Efficient reduction of bromate in water by nano-iron hydroxide impregnated granular activated carbon (Fe-GAC). Chem. Eng. J. 2015, 275, 189–197. [Google Scholar] [CrossRef]
- Krasner, S.W.; Glaze, W.H.; Weinberg, H.S.; Daniel, P.A.; Najm, I.N. Formation and Control of Bromate During Ozonation of Waters Containing Bromide. J. AWWA 1993, 85, 73–81. [Google Scholar] [CrossRef]
- Restivo, J.; Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Metal assessment for the catalytic reduction of bromate in water under hydrogen. Chem. Eng. J. 2015, 263, 119–126. [Google Scholar] [CrossRef]
- Costa, J.M.C.B.D.; Barbosa, J.R.M.; Restivo, J.; Orge, C.A.; Nogueira, A.; Castro-Silva, S.; Pereira, M.F.R.; Soares, O.S.G.P. Engineering of Nanostructured Carbon Catalyst Supports for the Continuous Reduction of Bromate in Drinking Water. C 2022, 8, 21. [Google Scholar] [CrossRef]
- Freitas, C.M.A.S.; Soares, O.S.G.P.; Órfão, J.J.M.; Fonseca, A.M.; Pereira, M.F.R.; Neves, I.C. Highly efficient reduction of bromate to bromide over mono and bimetallic ZSM5 catalysts. Green Chem. 2015, 17, 4247–4254. [Google Scholar] [CrossRef]
- Shen, W.; Lin, F.; Jiang, X.; Li, H.; Ai, Z.; Zhang, L. Efficient removal of bromate with core-shell Fe@Fe2O3 nanowires. Chem. Eng. J. 2017, 308, 880–888. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Ramalho, P.S.F.; Fernandes, A.; Órfão, J.J.M.; Pereira, M.F.R. Catalytic bromate reduction in water: Influence of carbon support. J. Environ. Chem. Eng. 2019, 7, 103015. [Google Scholar] [CrossRef]
- World Health Organization. Bromide in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Wiśniewski, J.A.; Kabsch-Korbutowicz, M.; Łakomska, S. Removal of bromate ions from water in the processes with ion-exchange membranes. Sep. Purif. Technol. 2015, 145, 75–82. [Google Scholar] [CrossRef]
- Wiśniewski, J.A.; Kabsch-Korbutowicz, M. Bromate removal in the ion-exchange process. Desalination 2010, 261, 197–201. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Zhou, J.; Gao, N. Photocatalytic decomposition of bromate ion by the UV/P25-Graphene processes. Water Res. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; You, Y.; Huang, S.; Cheng, F.; Chen, P.; Li, H.; Zhang, Y. Facile construction of reduced graphene oxide supported three-dimensional polyaniline/WO2.72 nanobelt-flower as a full solar spectrum light response catalyst for efficient photocatalytic conversion of bromate. Chemosphere 2019, 222, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Cheng, Y.-L. Effect of characteristics of activated carbon on removal of bromate. Sep. Purif. Technol. 2008, 59, 101–107. [Google Scholar] [CrossRef]
- Ji, H.; Wu, W.; Li, F.; Yu, X.; Fu, J.; Jia, L. Enhanced adsorption of bromate from aqueous solutions on ordered mesoporous Mg-Al layered double hydroxides (LDHs). J. Hazard. Mater. 2017, 334, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Matsuda, N. Bromate Ion Removal by Electrochemical Reduction Using an Activated Carbon Felt Electrode. Environ. Sci. Technol. 2009, 43, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Yang, Q.; Sun, J.; Chen, F.; Zhong, Y.; Yin, H.; He, L.; Tao, Z.; Pi, Z.; Wang, D.; et al. Electrochemical reduction of bromate using noble metal-free nanoscale zero-valent iron immobilized activated carbon fiber electrode. Chem. Eng. J. 2019, 123588. [Google Scholar] [CrossRef]
- Peldszus, S.; Andrews, S.A.; Souza, R.; Smith, F.; Douglas, I.; Bolton, J.; Huck, P.M. Effect of medium-pressure UV irradiation on bromate concentrations in drinking water, a pilot-scale study. Water Res. 2004, 38, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bensalah, N.; Liu, X.; Abdel-Wahab, A. Bromate reduction by ultraviolet light irradiation using medium pressure lamp. Int. J. Environ. Stud. 2013, 70, 566–582. [Google Scholar] [CrossRef]
- Nurlan, N.; Akmanova, A.; Lee, W. The Use of H2 in Catalytic Bromate Reduction by Nanoscale Heterogeneous Catalysts. Nanomaterials 2022, 12, 1212. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Wan, H.; Zheng, J.; Yin, D.; Zheng, S. Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl. Catal. B 2010, 96, 307–313. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Y.; Guo, Y.; Shi, W.; Wang, W.; Zhang, B.; Zhang, R.; Bao, X.; Wu, S.; Cui, F. Pd and Pt nanoparticles supported on the mesoporous silica molecular sieve SBA-15 with enhanced activity and stability in catalytic bromate reduction. Chem. Eng. J. 2018, 344, 114–123. [Google Scholar] [CrossRef]
- Chen, X.; Huo, X.; Liu, J.; Wang, Y.; Werth, C.J.; Strathmann, T.J. Exploring beyond palladium: Catalytic reduction of aqueous oxyanion pollutants with alternative platinum group metals and new mechanistic implications. Chem. Eng. J. 2017, 313, 745–752. [Google Scholar] [CrossRef]
- Franch, C.; Rodríguez-Castellón, E.; Reyes-Carmona, Á.; Palomares, A.E. Characterization of (Sn and Cu)/Pd catalysts for the nitrate reduction in natural water. Appl Catal A-Gen 2012, 425–426, 145–152. [Google Scholar] [CrossRef]
- Barrabés, N.; Just, J.; Dafinov, A.; Medina, F.; Fierro, J.L.G.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Catalytic reduction of nitrate on Pt-Cu and Pd-Cu on active carbon using continuous reactor: The effect of copper nanoparticles. Appl. Catal. B Environ. 2006, 62, 77–85. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A.; Carlos, F.; Mariana, R.; Ricardo, M.; Garcia, M.; Guedes, M.; Pedro, B.T.; Jean Marc, G.; Araújo, J.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route. Chem. Mater. 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, P.; Soares, O.S.G.P.; Ramalho, P.S.F.; Pereira, M.F.R.; Alves, M.M. Synthesis, characterization and application of magnetic carbon materials as electron shuttles for the biological and chemical reduction of the azo dye Acid Orange 10. Appl. Catal. B 2017, 212, 175–184. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gambhir, S.S.; Cheon, J. Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 841. [Google Scholar] [CrossRef] [PubMed]
- Suber, L.; Marchegiani, G.; Olivetti, E.S.; Celegato, F.; Coïsson, M.; Tiberto, P.; Allia, P.; Barrera, G.; Pilloni, L.; Barba, L.; et al. Pure magnetic hard fct FePt nanoparticles: Chemical synthesis, structural and magnetic properties correlations. Mater. Chem. Phys. 2014, 144, 186–193. [Google Scholar] [CrossRef]
- Long, Y.; Xie, M.; Niu, J.; Wang, P.; Ma, J. Preparation of acid–base bifunctional core–shell structured Fe3O4@SiO2 nanoparticles and their cooperative catalytic activity. Appl. Surf. Sci. 2013, 277, 288–292. [Google Scholar] [CrossRef]
- Xu, X.; Wu, H.; Li, Z.; Sun, X.; Wang, Z. Iron oxide-silver magnetic nanoparticles as simple heterogeneous catalysts for the direct inter/intramolecular nucleophilic substitution of π-activated alcohols with electron-deficient amines. Tetrahedron 2015, 71, 5254–5259. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Heidari, F.; Hekmati, M.; Veisi, H. Magnetically separable and recyclable Fe3O4@SiO2/isoniazide/Pd nanocatalyst for highly efficient synthesis of biaryls by Suzuki coupling reactions. J. Colloid Interface Sci. 2017, 501, 175–184. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Selective Removal of Heavy Metals from Industrial Wastewater Using Maghemite Nanoparticle: Performance and Mechanisms. J. Environ. Eng. 2006, 132, 709–715. [Google Scholar] [CrossRef]
- Ali, Q.; Ahmed, W.; Lal, S.; Sen, T. Novel Multifunctional Carbon Nanotube Containing Silver and Iron Oxide Nanoparticles for Antimicrobial Applications in Water Treatment. Mater. Today Proc. 2017, 4, 57–64. [Google Scholar] [CrossRef]
- Funari, V.; Mantovani, L.; Vigliotti, L.; Tribaudino, M.; Dinelli, E.; Braga, R. Superparamagnetic iron oxides nanoparticles from municipal solid waste incinerators. Sci. Total Environ. 2018, 621, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Orge, C.; Soares, O.; Ramalho, P.; Pereira, M.; Faria, J. Magnetic Nanoparticles for Photocatalytic Ozonation of Organic Pollutants. Catalysts 2019, 9, 703. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Sun, J.; Fu, H.; Qu, X.; Xu, Z.; Zheng, S. Highly effective bromate reduction by liquid phase catalytic hydrogenation over Pd catalysts supported on core-shell structured magnetites: Impact of shell properties. Sci. Total Environ. 2019, 663, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, S.; Ji, S.; Shi, D.; Yan, J.; Huo, Y.; Zhang, Q. Magnetically Recyclable Cu-BTC@SiO2@Fe3O4 Catalysts and Their Catalytic Performance for the Pechmann Reaction. Ind. Eng. Chem. Res. 2014, 53, 14948–14955. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Tan, W.; Jiang, F.; Tang, R. Palladium supported on amino functionalized magnetic MCM-41 for catalytic hydrogenation of aqueous bromate. RSC Adv. 2014, 4, 38743–38749. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chen, S.-Y. Catalytic Reduction of Bromate Using ZIF-Derived Nanoscale Cobalt/Carbon Cages in the Presence of Sodium Borohydride. ACS Sustain. Chem. Eng. 2015, 3, 3096–3103. [Google Scholar] [CrossRef]
- Kameoka, S.; Tanabe, T.; Tsai, A.P. Spinel CuFe2O4: A precursor for copper catalyst with high thermal stability and activity. Catal. Lett. 2005, 100, 89–93. [Google Scholar] [CrossRef]
- Pinto, M.; Ramalho, P.S.F.; Moreira, N.F.F.; Gonçalves, A.G.; Nunes, O.C.; Pereira, M.F.R.; Soares, O.S.G.P. Application of magnetic nanoparticles for water purification. Environ. Adv. 2020, 2, 100010. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Al-Abed, S.R.; Dionysiou, D.D. Iron–cobalt mixed oxide nanocatalysts: Heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications. Appl. Catal. B 2009, 88, 462–469. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y. Sol-Gel Synthesized Magnetic MnFe2O4 Spinel Ferrite Nanoparticles as Novel Catalyst for Oxidative Degradation of Methyl Orange. J. Nanomater. 2013, 2013, 640940. [Google Scholar] [CrossRef]
- Hagen, J. Heterogeneous Catalysis: Fundamentals. In Industrial Catalysis: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 99–217. [Google Scholar]
- Xie, L.; Shang, C.; Zhou, Q. Effect of Fe(III) on the bromate reduction by humic substances in aqueous solution. J. Environ. Sci. 2008, 20, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, Q.; Luo, K.; Wu, X.; Li, X.; Liu, Y.; Tang, W.; Zeng, G.; Peng, B. Fe(II)–Al(III) layered double hydroxides prepared by ultrasound-assisted co-precipitation method for the reduction of bromate. J. Hazard. Mater. 2013, 250–251, 345–353. [Google Scholar] [CrossRef]
- Perez-Coronado, A.M.; Soares, O.S.G.P.; Calvo, L.; Rodriguez, J.J.; Gilarranz, M.A.; Pereira, M.F.R. Catalytic reduction of bromate over catalysts based on Pd nanoparticles synthesized via water-in-oil microemulsion. Appl. Catal. B 2018, 237, 206–213. [Google Scholar] [CrossRef]
- Soares, O.S.G.P.; Órfão, J.J.M.; Gallegos-Suarez, E.; Castillejos, E.; Rodríguez-Ramos, I.; Pereira, M.F.R. Nitrate reduction over a Pd-Cu/MWCNT catalyst: Application to a polluted groundwater. Environ. Technol. 2012, 33, 2353–2358. [Google Scholar] [CrossRef]
- Cerrillo, J.L.; Palomares, A.E. A Review on the Catalytic Hydrogenation of Bromate in Water Phase. Catalysts 2021, 11, 365. [Google Scholar] [CrossRef]
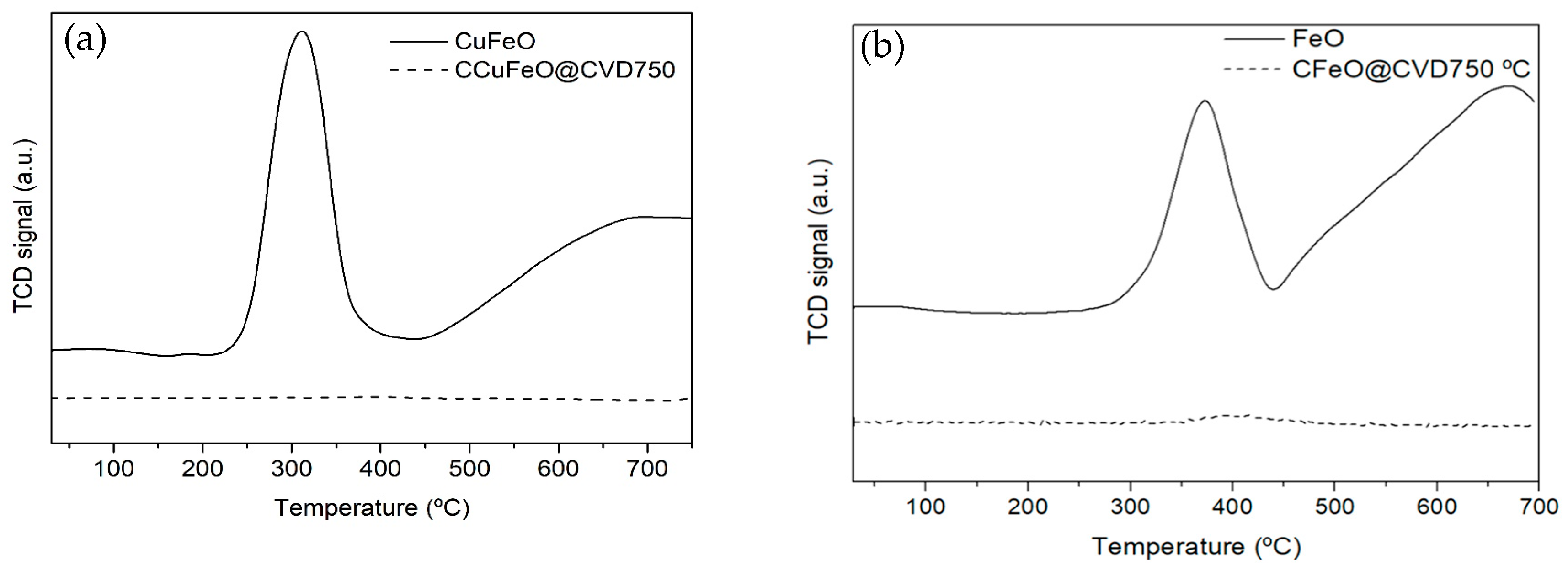

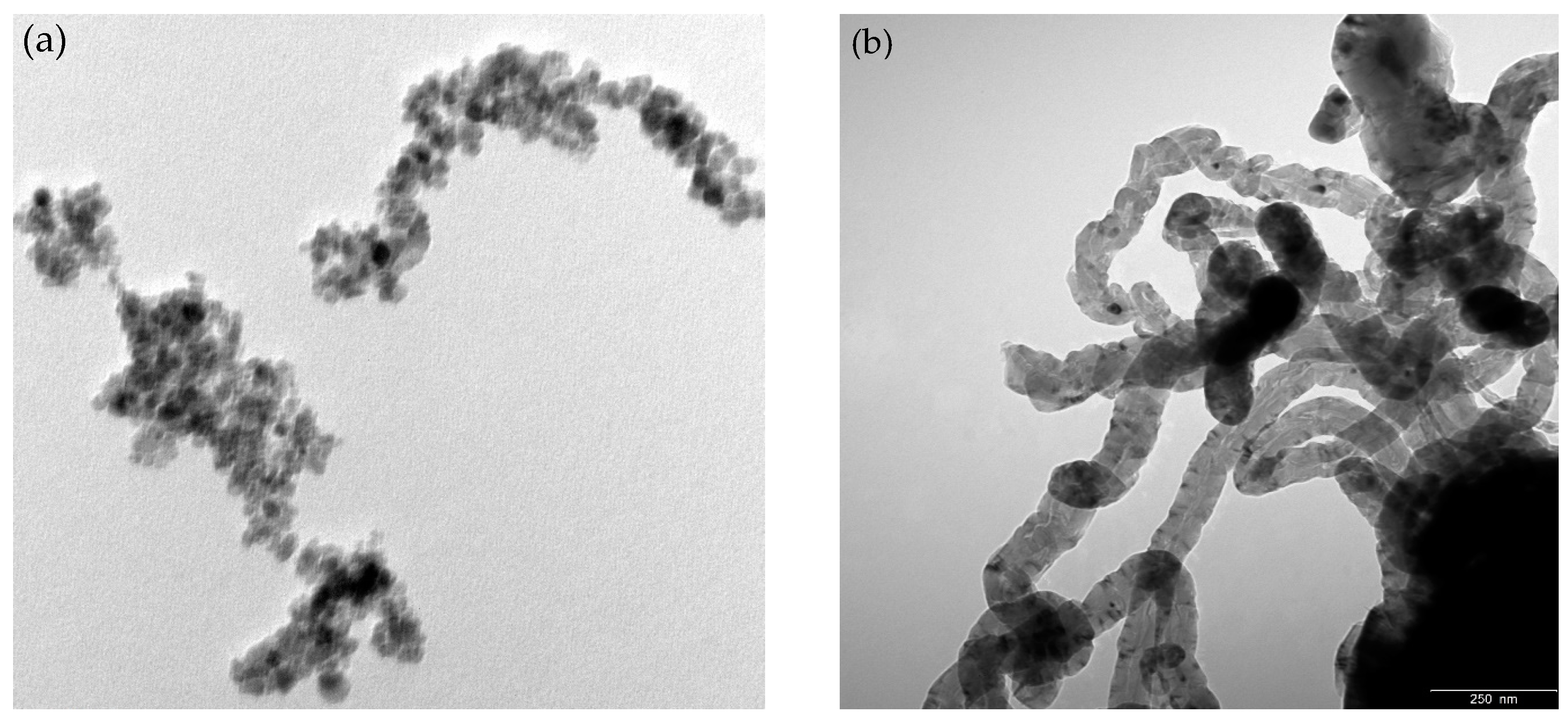
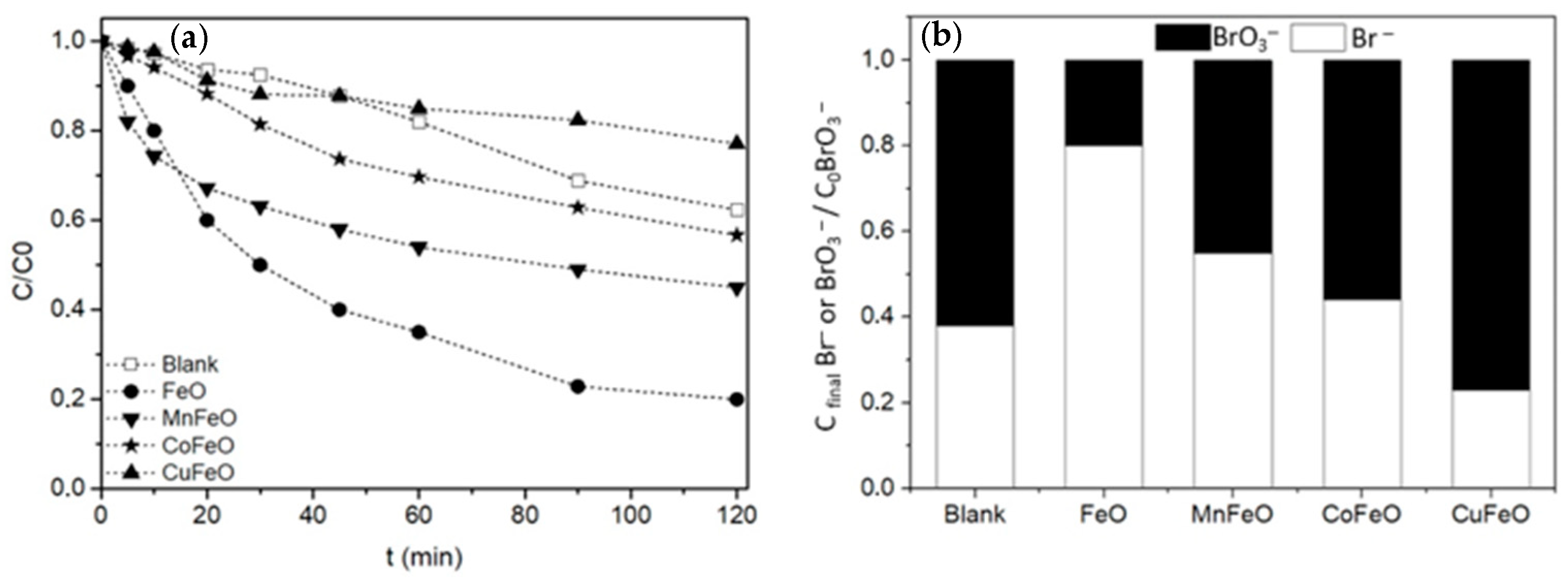
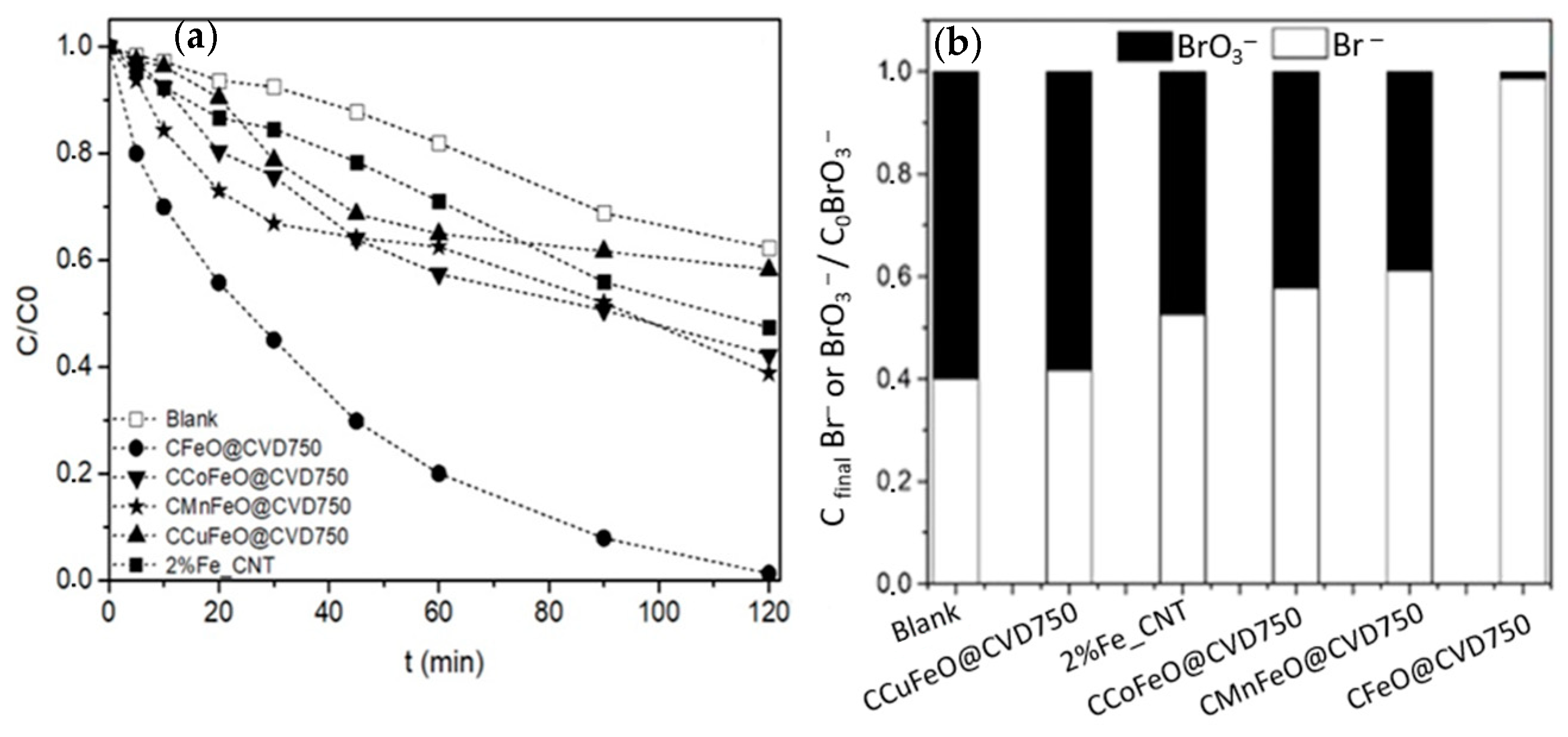

| Sample | SBET (m2 g−1) | % Carbon | % Metal |
|---|---|---|---|
| FeO | 154 | - | 98 |
| CFeO@CVD750 | 63· | 16 | 84 |
| 2%Fe_CNT | 266 | 98 | 2 |
| MnFeO | 101 | - | 98 |
| CMnFeO@CVD750 | 45· | 22 | 78 |
| CoFeO | 184 | - | 96 |
| CCoFeO@CVD750 | 29· | 34 | 66 |
| CuFeO | 235 | - | 96 |
| CCuFeO@CVD750 | 27· | 33 | 67 |
| Sample | Phase (%V/V) | Crystallite Size (nm) |
|---|---|---|
| FeO | Magnetite = 100 | 20.5 ± 0.5 |
| CFeO@CVD750 | Cementite (Fe3C) = 3.0 | 84 ± 10 |
| Fe α = 1.6 | 72 ± 5 | |
| Grafite = 95.4 | 16 ± 1 |
| Before Reaction | After Reaction | |
|---|---|---|
| pH | 7.17 | 7.25 |
| Conductivity (µS cm−1) | 238 | 237 |
| TOC (mg L−1) | 2.08 | 3.03 |
| TC (mg L−1) | 12.86 | 11.14 |
| IC (mg L−1) | 10.78 | 8.11 |
| Br− (mg L−1) | 0.15 | 3.01 |
| NO3− (mg L−1) | 0.65 | 0.33 |
| NO2− (mg L−1) | 0.11 | 0.09 |
| Cl− (mg L−1) | 0.38 | 0.01 |
| SO42− (mg L−1) | 16.16 | 14.86 |
| Na+ (mg L−1) | 12.28 | 11.54 |
| NH4+ (mg L−1) | 1.03 | 0.63 |
| Ca2+ (mg L−1) | 14.94 | 12.35 |
| Mg2+ (mg L−1) | 3.35 | 2.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramalho, P.S.F.; Soares, O.S.G.P.; Órfão, J.J.M.; Pereira, M.F.R. Magnetic Metallic Nanoparticles Coated with Carbon for the Catalytic Removal of Bromate from Water. Catalysts 2024, 14, 149. https://doi.org/10.3390/catal14020149
Ramalho PSF, Soares OSGP, Órfão JJM, Pereira MFR. Magnetic Metallic Nanoparticles Coated with Carbon for the Catalytic Removal of Bromate from Water. Catalysts. 2024; 14(2):149. https://doi.org/10.3390/catal14020149
Chicago/Turabian StyleRamalho, Patrícia S. F., Olívia Salomé G. P. Soares, José J. M. Órfão, and Manuel Fernando R. Pereira. 2024. "Magnetic Metallic Nanoparticles Coated with Carbon for the Catalytic Removal of Bromate from Water" Catalysts 14, no. 2: 149. https://doi.org/10.3390/catal14020149
APA StyleRamalho, P. S. F., Soares, O. S. G. P., Órfão, J. J. M., & Pereira, M. F. R. (2024). Magnetic Metallic Nanoparticles Coated with Carbon for the Catalytic Removal of Bromate from Water. Catalysts, 14(2), 149. https://doi.org/10.3390/catal14020149








