Investigating the Impact of Na2WO4 Doping in La2O3-Catalyzed OCM Reaction: A Structure–Activity Study via In Situ XRD-MS
Abstract
1. Introduction
2. Results and Discussion
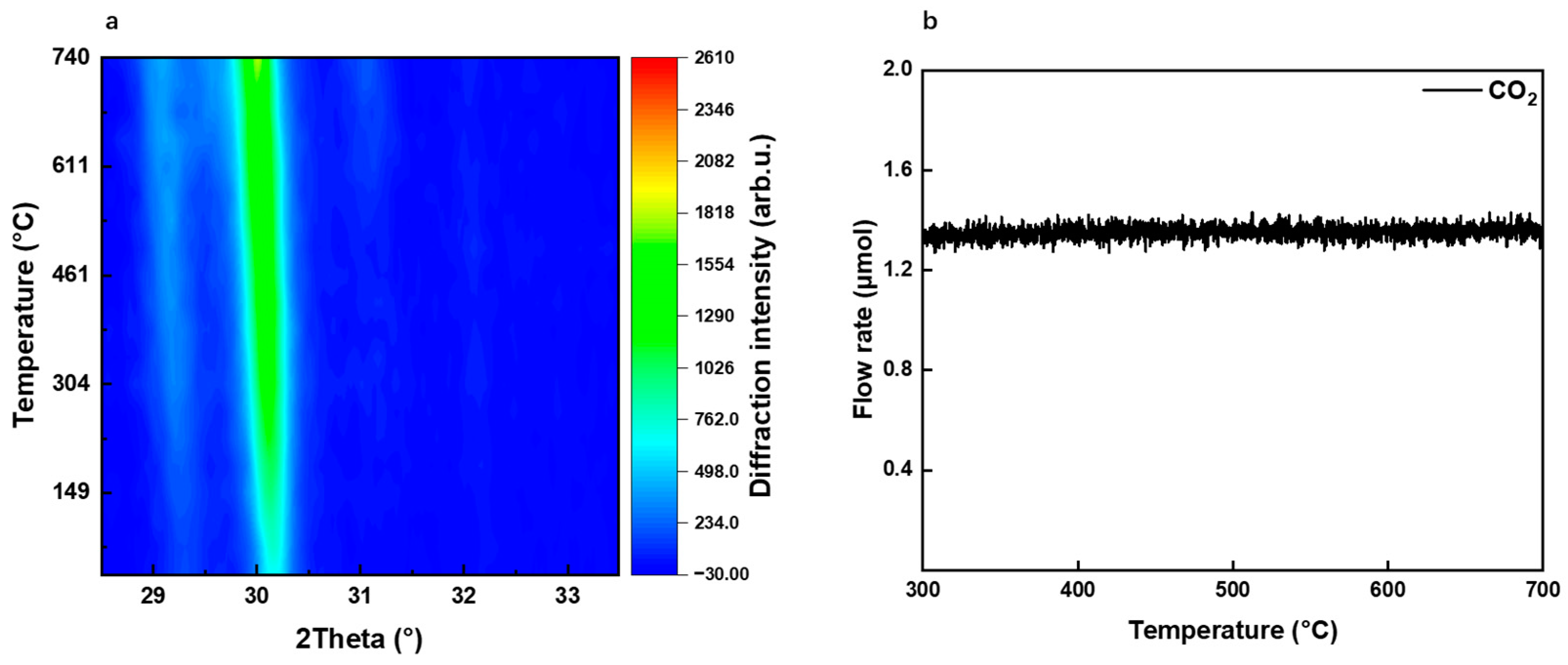
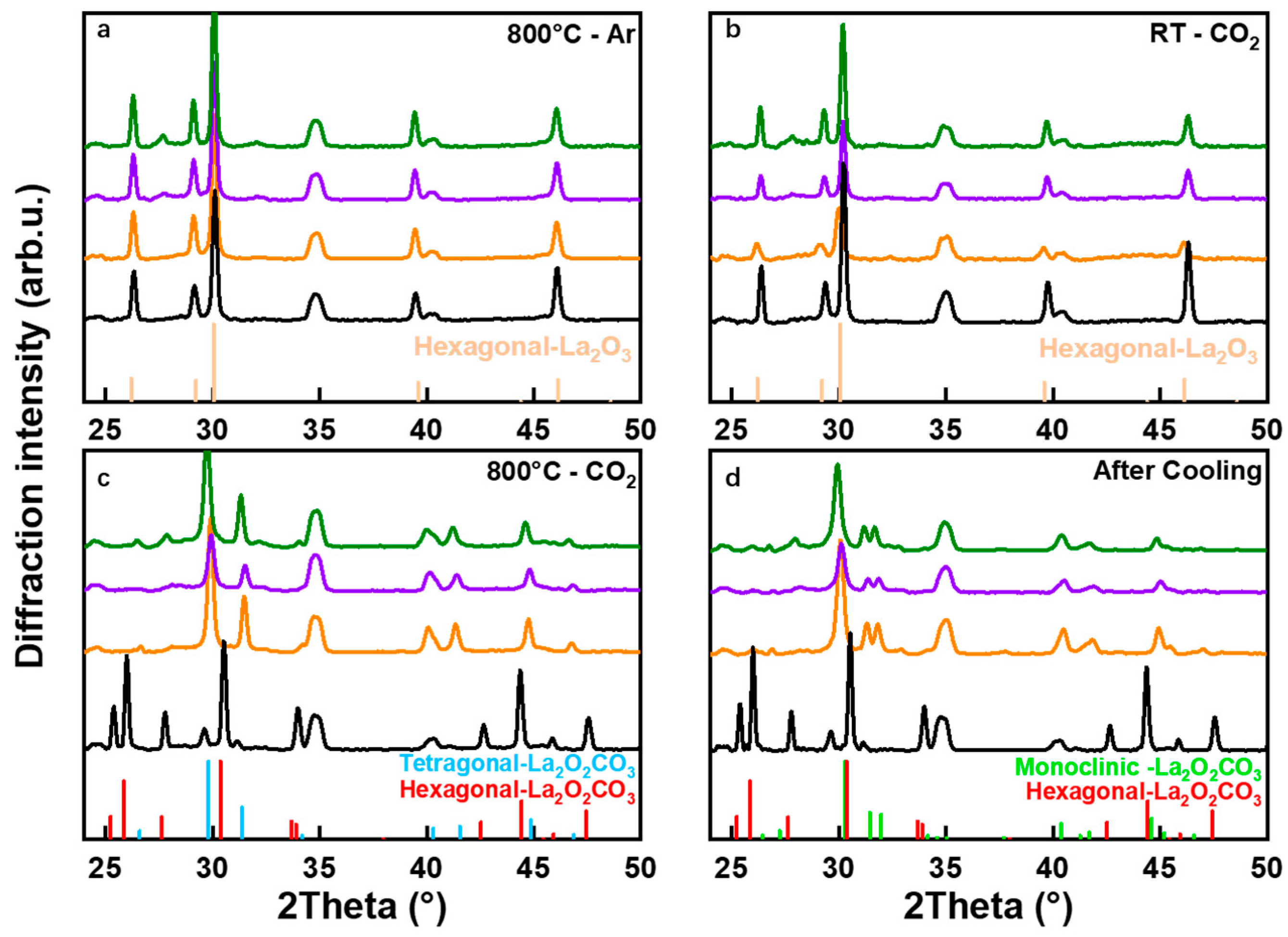
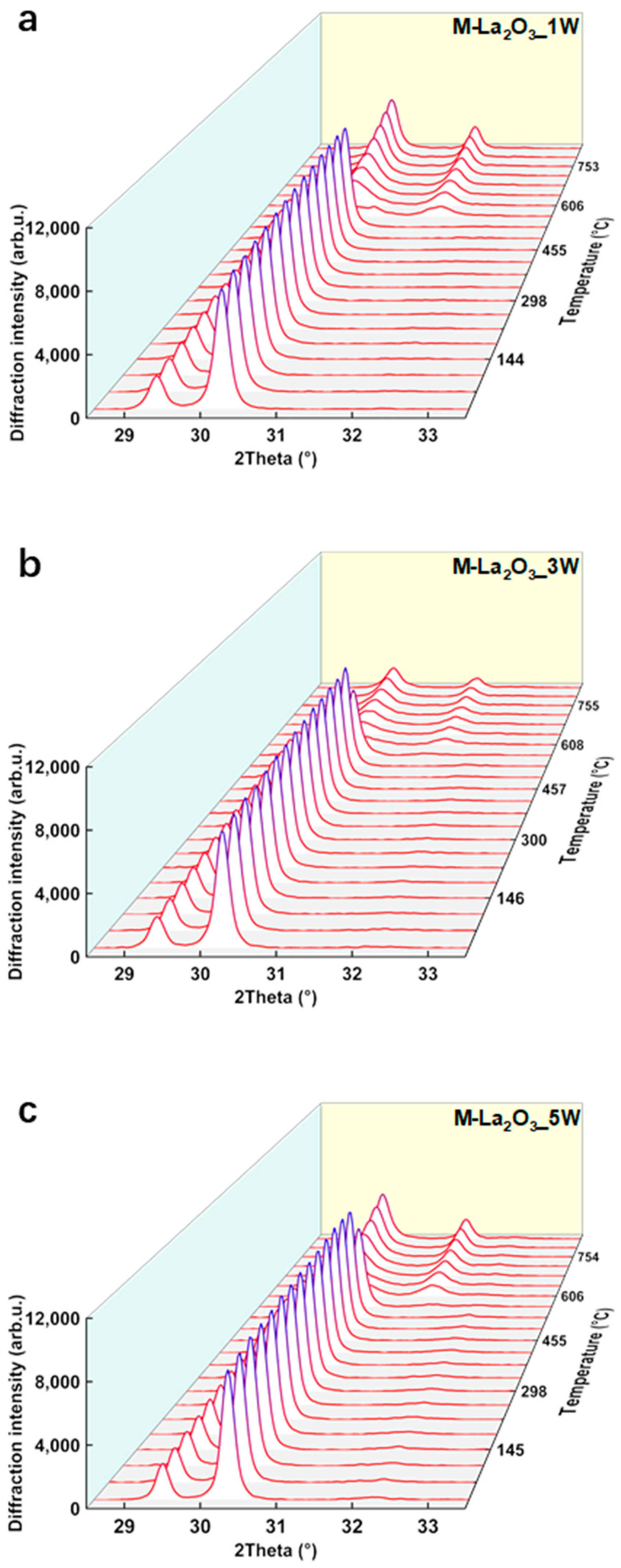
2.1. Differences in La2O3 and La2O2CO3 Phase Structure
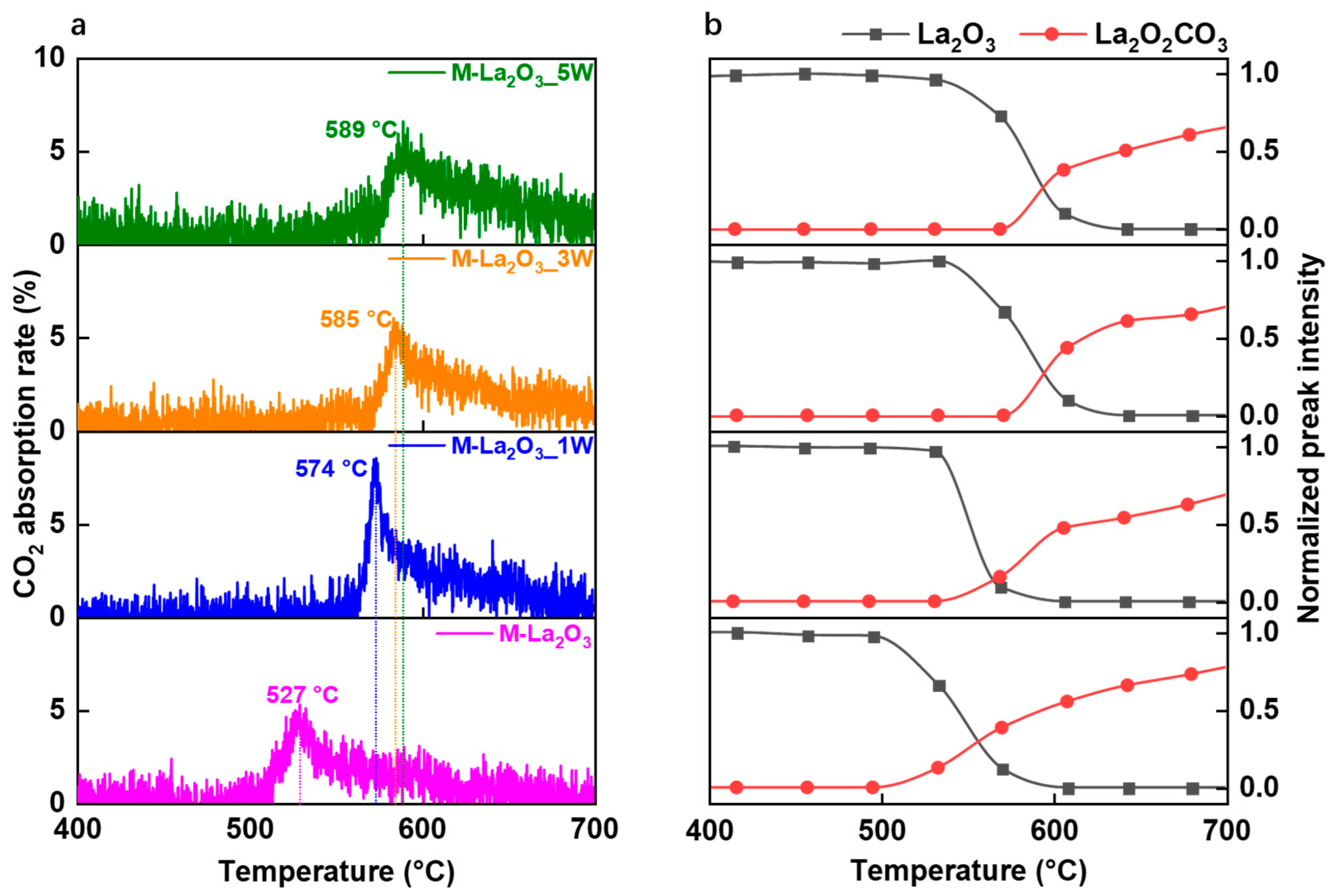
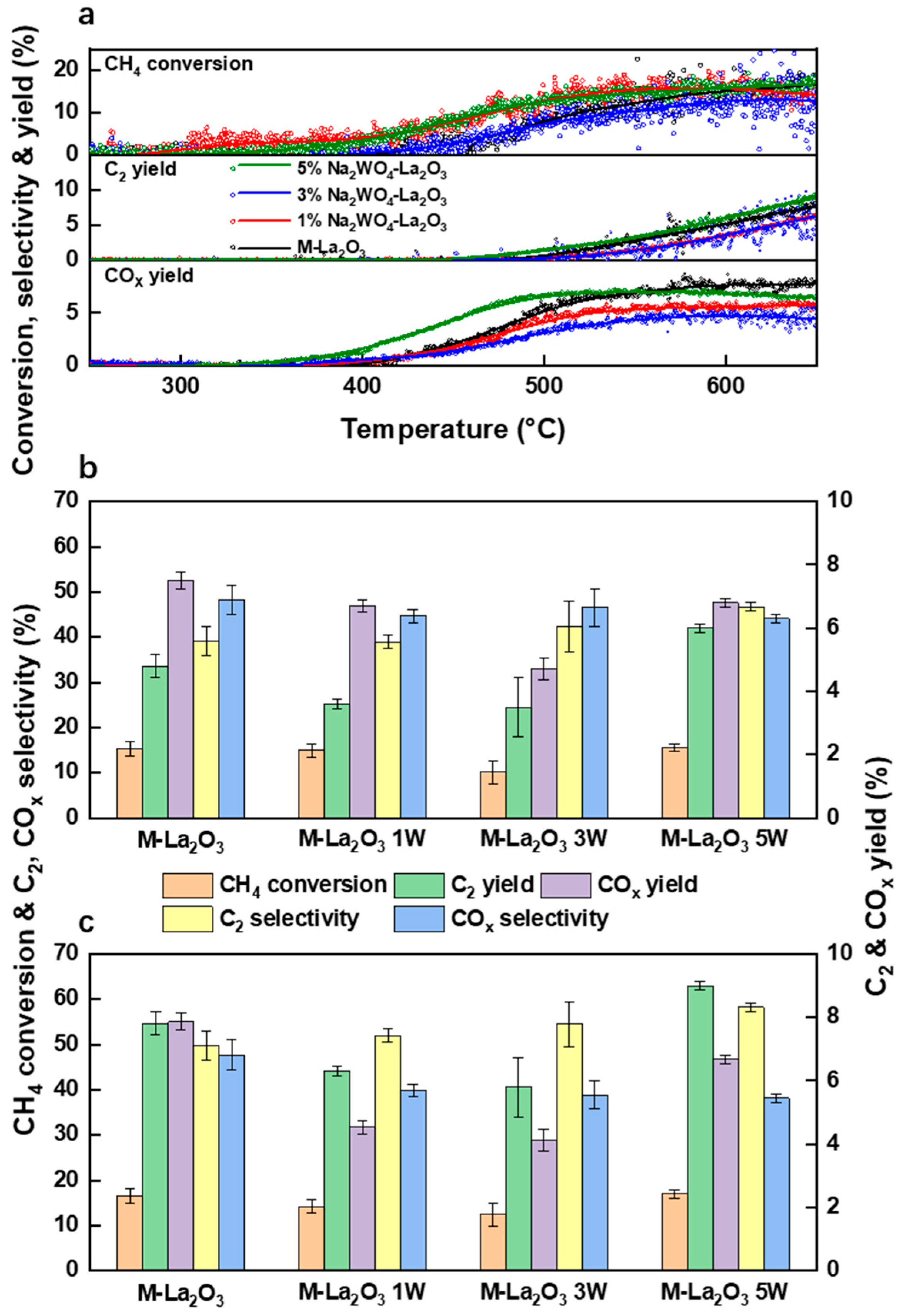
2.2. Adsorption of CO2 and OCM Reaction Performance
2.3. Differences in Carbonate Bulk Phase between Doped and Undoped Samples
↓
CO2 adsorption/poisoning resistance
↓
Low-temperature OCM activity
3. Materials and Methods
3.1. Catalyst Preparation
3.2. In Situ XRD-MS
3.3. Online MS Microreactor
3.4. XPS Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Lunsford, J.H. The catalytic oxidative coupling of methane. Angew. Chem. Int. Ed. 1995, 34, 970–980. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Schwach, P.; Pan, X.; Bao, X. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: Challenges and prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef] [PubMed]
- Godini, H.; Gili, A.; Görke, O.; Arndt, S.; Simon, U.; Thomas, A.; Schomäcker, R.; Wozny, G. Sol–gel method for synthesis of Mn–Na2WO4/SiO2 catalyst for methane oxidative coupling. Catal. Today 2014, 236, 12–22. [Google Scholar] [CrossRef]
- Kuo, J.; Kresge, C.; Palermo, R. Evaluation of direct methane conversion to higher hydrocarbons and oxygenates. Catal. Today 1989, 4, 463–470. [Google Scholar] [CrossRef]
- Pirro, L.; Mendes, P.S.; Paret, S.; Vandegehuchte, B.D.; Marin, G.B.; Thybaut, J.W. Descriptor–property relationships in heterogeneous catalysis: Exploiting synergies between statistics and fundamental kinetic modelling. Catal. Sci. Technol. 2019, 9, 3109–3125. [Google Scholar] [CrossRef]
- Zavyalova, U.; Holena, M.; Schlögl, R.; Baerns, M. Statistical analysis of past catalytic data on oxidative methane coupling for new insights into the composition of high-performance catalysts. ChemCatChem 2011, 3, 1935–1947. [Google Scholar] [CrossRef]
- Keller, G.; Bhasin, M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Peppel, T.; Seeburg, D.; Kondratenko, V.A.; Kalevaru, N.; Martin, A.; Wohlrab, S. Methane conversion into different hydrocarbons or oxygenates: Current status and future perspectives in catalyst development and reactor operation. Catal. Sci. Technol. 2017, 7, 366–381. [Google Scholar] [CrossRef]
- Guan, C.; Yang, Y.; Pang, Y.; Liu, Z.; Li, S.; Vovk, E.I.; Zhou, X.; Li, J.P.H.; Zhang, J.; Yu, N. How CO2 poisons La2O3 in an OCM catalytic reaction: A study by in situ XRD-MS and DFT. J. Catal. 2021, 396, 202–214. [Google Scholar] [CrossRef]
- Liu, Z.; Ho Li, J.P.; Vovk, E.; Zhu, Y.; Li, S.; Wang, S.; van Bavel, A.P.; Yang, Y. Online kinetics study of oxidative coupling of methane over La2O3 for methane activation: What is behind the distinguished light-off temperatures? ACS Catal. 2018, 8, 11761–11772. [Google Scholar] [CrossRef]
- Luo, L.; Tang, X.; Wang, W.; Wang, Y.; Sun, S.; Qi, F.; Huang, W. Methyl radicals in oxidative coupling of methane directly confirmed by synchrotron VUV photoionization mass spectroscopy. Sci. Rep. 2013, 3, 1625. [Google Scholar] [CrossRef]
- Bernal, S.; Diaz, J.; Garcia, R.; Rodriguez-Izquierdo, J. Study of some aspects of the reactivity of La2O3 with CO2 and H2O. J. Mater. Sci. 1985, 20, 537–541. [Google Scholar] [CrossRef]
- Hou, Y.-H.; Han, W.-C.; Xia, W.-S.; Wan, H.-L. Structure sensitivity of La2O2CO3 catalysts in the oxidative coupling of methane. ACS Catal. 2015, 5, 1663–1674. [Google Scholar] [CrossRef]
- Klingenberg, B.; Vannice, M.A. Influence of pretreatment on lanthanum nitrate, carbonate, and oxide powders. Chem. Mater. 1996, 8, 2755–2768. [Google Scholar] [CrossRef]
- Levan, T.; Che, M.; Tatibouet, J.; Kermarec, M. Infrared study of the formation and stability of La2O2CO3 during the oxidative coupling of methane on La2O3. J. Catal. 1993, 142, 18–26. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, Y. Synthesis, characterization, shape-preserved transformation, and optical properties of La(OH)3, La2O2CO3, and La2O3 nanorods. J. Alloys Compd. 2011, 509, 396–401. [Google Scholar] [CrossRef]
- Turcotte, R.P.; Sawyer, J.O.; Eyring, L. Rare earth dioxymonocarbonates and their decomposition. Inorg. Chem. 1969, 8, 238–246. [Google Scholar] [CrossRef]
- Chu, C.; Zhao, Y.; Li, S.; Sun, Y. CO2 chemisorption and its effect on methane activation in La2O3-catalyzed oxidative coupling of methane. J. Phys. Chem. C 2016, 120, 2737–2746. [Google Scholar] [CrossRef]
- Chu, C.; Zhao, Y.; Li, S.; Sun, Y. Correlation between the acid–base properties of the La2O3 catalyst and its methane reactivity. Phys. Chem. Chem. Phys. 2016, 18, 16509–16517. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.-J.; Zeng, L.; Zhao, J.; Tian, H.; Chen, S.; Li, K.; Sang, S.; Gong, J. On the role of Ce in CO2 adsorption and activation over lanthanum species. Chem. Sci. 2018, 9, 3426–3437. [Google Scholar] [CrossRef]
- Song, J.; Sun, Y.; Ba, R.; Huang, S.; Zhao, Y.; Zhang, J.; Sun, Y.; Zhu, Y. Monodisperse Sr–La2O3 hybrid nanofibers for oxidative coupling of methane to synthesize C2 hydrocarbons. Nanoscale 2015, 7, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Fierro, J. Catalysis in C 1 chemistry: Future and prospect. Catal. Lett. 1993, 22, 67–91. [Google Scholar] [CrossRef]
- Zhou, X.; Pang, Y.; Liu, Z.; Vovk, E.I.; van Bavel, A.P.; Li, S.; Yang, Y. Active oxygen center in oxidative coupling of methane on La2O3 catalyst. J. Energy Chem. 2021, 60, 649–659. [Google Scholar] [CrossRef]
- Taylor, R.P.; Schrader, G.L. Lanthanum catalysts for methane oxidative coupling: A comparison of the reactivity of phases. Ind. Eng. Chem. Res. 1991, 30, 1016–1023. [Google Scholar] [CrossRef]
- Schmack, R.; Friedrich, A.; Kondratenko, E.V.; Polte, J.; Werwatz, A.; Kraehnert, R. A meta-analysis of catalytic literature data reveals property-performance correlations for the OCM reaction. Nat. Commun. 2019, 10, 441. [Google Scholar] [CrossRef]
- Lacombe, S.; Holmena, A.; Wolf, E.; Ducarme, V.; Moral, P.; Mirodatos, C. Isotopic exchange and volumetric studies on methane activation over rare-earth oxides. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1994; Volume 81, pp. 211–216. [Google Scholar]
- Lacombe, S.; Zanthoff, H.; Mirodatos, C. Oxidative coupling of methane over lanthana catalysts: II. A mechanistic study using isotope transient kinetics. J. Catal. 1995, 155, 106–116. [Google Scholar] [CrossRef]
- Yide, X.; Lin, Y.; Xiexian, G. Effect of basicity and adding CO2 in the feed on the oxidative coupling of methane over K2O and SrO promoted La2O3/ZnO catalysts. Appl. Catal. A Gen. 1997, 164, 47–57. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mulla, S.A.; Uphade, B.S. Oxidative coupling of methane over supported La2O3 and La-promoted MgO catalysts: Influence of catalyst− support interactions. Ind. Eng. Chem. Res. 1997, 36, 2096–2100. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Uphade, B.S.; Mulla, S.A. Oxidative coupling of methane over a Sr-promoted La2O3 catalyst supported on a low surface area porous catalyst carrier. Ind. Eng. Chem. Res. 1997, 36, 3594–3601. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, Y.; Zhang, J.; Zhu, Y.; Sun, Y. Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction. Nanoscale 2013, 5, 10844–10848. [Google Scholar] [CrossRef]
- Jiang, T.; Song, J.; Huo, M.; Yang, N.; Liu, J.; Zhang, J.; Sun, Y.; Zhu, Y. La2O3 catalysts with diverse spatial dimensionality for oxidative coupling of methane to produce ethylene and ethane. RSC Adv. 2016, 6, 34872–34876. [Google Scholar] [CrossRef]
- Guan, C.; Liu, Z.; Wang, D.; Zhou, X.; Pang, Y.; Yu, N.; van Bavel, A.P.; Vovk, E.; Yang, Y. Exploring the formation of carbonates on La2O3 catalysts with OCM activity. Catal. Sci. Technol. 2021, 11, 6516–6528. [Google Scholar] [CrossRef]
- Arndt, S.; Otremba, T.; Simon, U.; Yildiz, M.; Schubert, H.; Schomäcker, R. Mn–Na2WO4/SiO2 as catalyst for the oxidative coupling of methane. What is really known? Appl. Catal. A Gen. 2012, 425, 53–61. [Google Scholar] [CrossRef]
- Beck, B.; Fleischer, V.; Arndt, S.; Hevia, M.G.; Urakawa, A.; Hugo, P.; Schomäcker, R. Oxidative coupling of methane—A complex surface/gas phase mechanism with strong impact on the reaction engineering. Catal. Today 2014, 228, 212–218. [Google Scholar] [CrossRef]
- Fang, X.; Li, S.; Gu, J.; Yang, D. Preparation and characterization of W-Mn catalyst for oxidative coupling of methane. J. Mol. Catal. 1992, 6, 255–261. [Google Scholar]
- Fang, X.; Li, S.; Lin, J.; Chu, Y. Oxidative coupling of methane on W-Mn catalysts. J. Mol. Catal. 1992, 6, 427–433. [Google Scholar]
- Fleischer, V.; Steuer, R.; Parishan, S.; Schomäcker, R. Investigation of the surface reaction network of the oxidative coupling of methane over Na2WO4/Mn/SiO2 catalyst by temperature programmed and dynamic experiments. J. Catal. 2016, 341, 91–103. [Google Scholar] [CrossRef]
- Si, J.; Zhao, G.; Sun, W.; Liu, J.; Guan, C.; Yang, Y.; Shi, X.R.; Lu, Y. Oxidative Coupling of Methane: Examining the Inactivity of the MnOx-Na2WO4/SiO2 Catalyst at Low Temperature. Angew. Chem. Int. Ed. 2022, 61, e202117201. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, G.; Wang, Y.; Lu, Y. MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3 − Na2WO4/SiO2 catalyst. Sci. Adv. 2017, 3, e1603180. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Li, Z.; Zhou, Q.; Pan, Y.; Yuan, W.; He, L.; Wang, S.; Wen, W.; Liu, J.; Wang, Y. Surface coupling of methyl radicals for efficient low-temperature oxidative coupling of methane. Chin. J. Catal. 2021, 42, 1117–1125. [Google Scholar] [CrossRef]
- Werny, M.J.; Wang, Y.; Girgsdies, F.; Schlögl, R.; Trunschke, A. Fluctuating storage of the active phase in a Mn − Na2WO4/SiO2 catalyst for the oxidative coupling of methane. Angew. Chem. Int. Ed. 2020, 59, 14921–14926. [Google Scholar] [CrossRef]
- Ho, S.-F.; Contarini, S.; Rabalais, J. Ion-beam-induced chemical changes in the oxyanions (MOyn−) and oxides (MOx) where M = chromium, molybdenum, tungsten, vanadium, niobium and tantalum. J. Phys. Chem. 1987, 91, 4779–4788. [Google Scholar] [CrossRef]
- Zhou, X.; Vovk, E.I.; Liu, Y.; Guan, C.; Yang, Y. An In Situ Temperature-Dependent Study of La2O3 Reactivation Process. Front. Chem. 2021, 9, 694559. [Google Scholar] [CrossRef]
- Li, J.P.H.; Zhou, X.; Pang, Y.; Zhu, L.; Vovk, E.I.; Cong, L.; van Bavel, A.P.; Li, S.; Yang, Y. Understanding of binding energy calibration in XPS of lanthanum oxide by in situ treatment. Phys. Chem. Chem. Phys. 2019, 21, 22351–22358. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zebang, L.; Li, J.P.H.; Evgeny, V. Reaction Control and Mass Spectrometry Workstation for Coupling an X-ray Spectroscopic Characterization Instrument with an In-Situ Reaction Cell. U.S. Patent No. 11,435,301, 6 September 2022. [Google Scholar]
- Li, J.P.H.; Liu, Z.; Wu, H.; Yang, Y. Investigation of CO oxidation over Au/TiO2 catalyst through detailed temperature programmed desorption study under low temperature and Operando conditions. Catal. Today 2018, 307, 84–92. [Google Scholar] [CrossRef]

| SAMPLES | XPS ATOMIC PERCENTAGES | XRD | |
|---|---|---|---|
| W | La | Grain Size (nm) | |
| M-La2O3_1W | 0.8 | 99.2 | 27.12 |
| M-La2O3_3W | 2.9 | 97.1 | 37.85 |
| M-La2O3_5W | 4.2 | 95.8 | 39.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lang, J.; Qiu, Z.; Ding, N.; Yang, Y. Investigating the Impact of Na2WO4 Doping in La2O3-Catalyzed OCM Reaction: A Structure–Activity Study via In Situ XRD-MS. Catalysts 2024, 14, 150. https://doi.org/10.3390/catal14020150
Wang D, Lang J, Qiu Z, Ding N, Yang Y. Investigating the Impact of Na2WO4 Doping in La2O3-Catalyzed OCM Reaction: A Structure–Activity Study via In Situ XRD-MS. Catalysts. 2024; 14(2):150. https://doi.org/10.3390/catal14020150
Chicago/Turabian StyleWang, Danyu, Junyu Lang, Zhehao Qiu, Ningxujin Ding, and Yong Yang. 2024. "Investigating the Impact of Na2WO4 Doping in La2O3-Catalyzed OCM Reaction: A Structure–Activity Study via In Situ XRD-MS" Catalysts 14, no. 2: 150. https://doi.org/10.3390/catal14020150
APA StyleWang, D., Lang, J., Qiu, Z., Ding, N., & Yang, Y. (2024). Investigating the Impact of Na2WO4 Doping in La2O3-Catalyzed OCM Reaction: A Structure–Activity Study via In Situ XRD-MS. Catalysts, 14(2), 150. https://doi.org/10.3390/catal14020150







