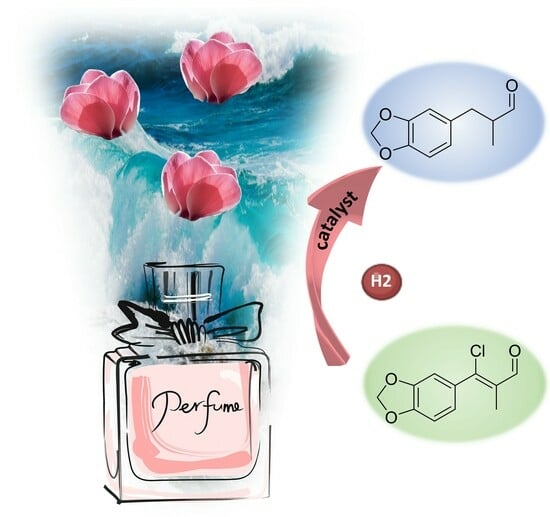
Synthesis of Helional by Hydrodechlorination Reaction in the Presence of Mono- and Bimetallic Catalysts Supported on Alumina
Abstract
1. Introduction
2. Results
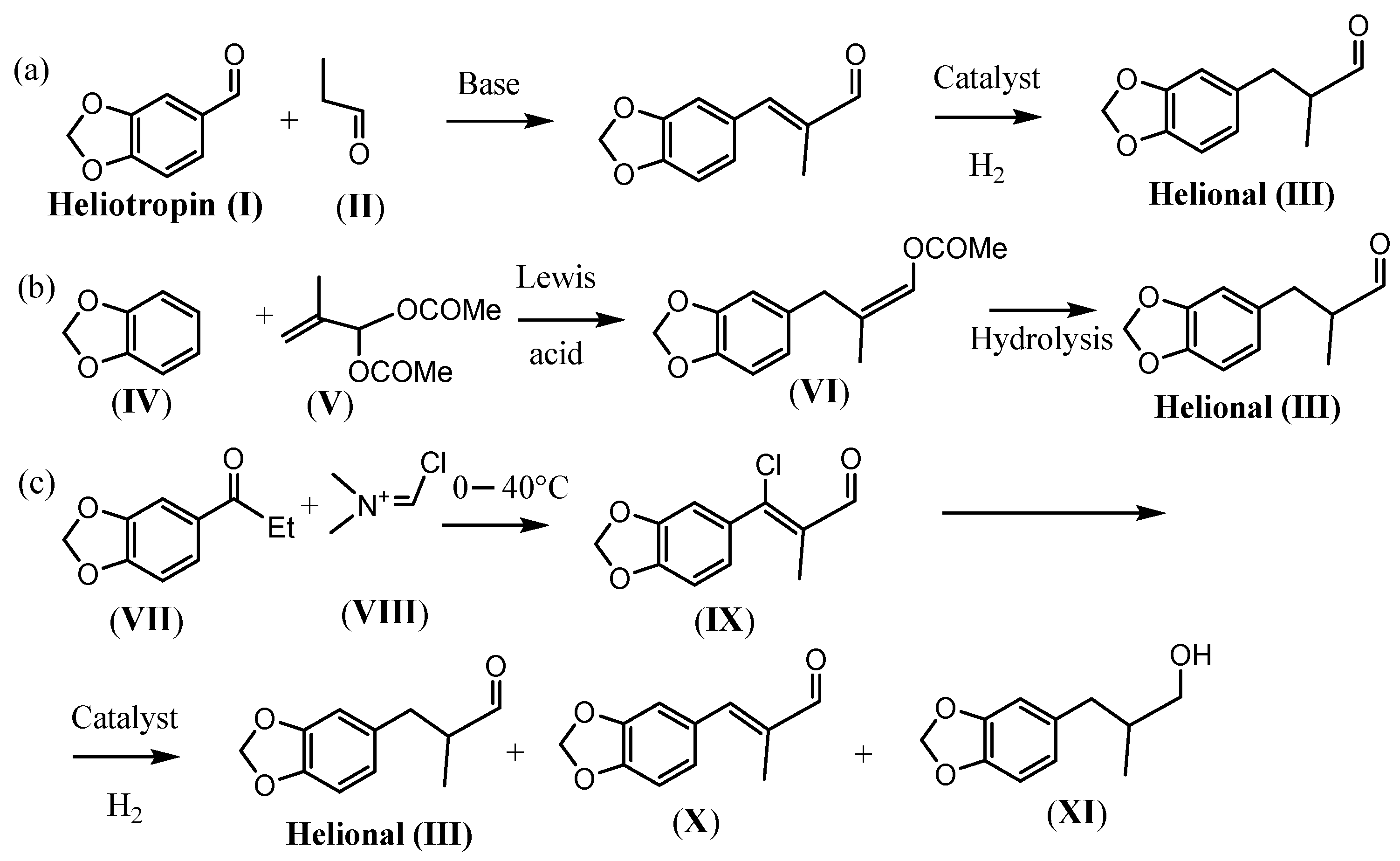
Catalytic Hydrodechlorination of 3-(Benzo1,3-dioxol-5-yl)-3-chloro-2-methylacrylaldehyde (IX)
3. Discussion
4. Materials and Methods
4.1. Preparation of Pd/Al2O3
4.2. Preparation of Rh/Al2O3
4.3. Preparation of Pd-Cu/Al2O3
4.4. Preparation of Rh-Cu/Al2O3
4.5. Synthesis of 3-(Benzo1,3-dioxol-5-yl)-3-chloro-2-methylacrylaldehyde (IX)
4.6. General Procedure for the Hydrodechlorination of 3-(Benzo-1,3-dioxol-5-yl)-3-chloro-2-methylacrylaldehyde (IX)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartolomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Piccolo, O.; Verrazzani, A. Preparation and Use of a Heterogeneous Rhodium Catalyst for the Hydrogenation of a Double Bond of an Alpha-Beta-Unsaturated Carbonyl Compound. U.S. Patent 7087548, 8 August 2006. [Google Scholar]
- Paganelli, S.; Tassini, R.; Rathod, V.D.; Onida, B.; Fiorilli, S.; Piccolo, O. A low rhodium content smart catalyst for hydrogenation and hydroformylation reactions. Catal. Lett. 2021, 151, 1508–1521. [Google Scholar] [CrossRef]
- Paganelli, S.; Angi, A.; Pajer, N.; Piccolo, O. A smart heterogeneous catalyst for efficient, chemo- and stereoselective hydrogenation of 3-hexyn-1-ol. Catalysts 2021, 11, 14. [Google Scholar] [CrossRef]
- Kraft, P.; Bajgrowicz, J.A.; Denis, C.; Frater, G. Odds and Trends: Recent Developments in the Chemistry of Odorants. Angew. Chem. Int. Ed. 2000, 39, 2980–3010. [Google Scholar] [CrossRef]
- Wenxiang, L. Synthesis of helional from piperonylenepropanal. Huaxue Yanjiu Yu Yingyong 2004, 16, 417. [Google Scholar]
- Beets Muus Gerrit, J.; van Essen, H. 2-Piperonyl-Propanal, a New Perfume, and Process for Making Same. GB841921 20 July 1960. [Google Scholar]
- Takashi, D.; Yoshida, Y.; Eiji, S.; Satoru, F. 2-Methyl-3-(3,4-methylenedioxyphenyl)propanal, and Method for Production Thereof. EP2119713A1, 18 November 2009. [Google Scholar]
- Yoshida, Y.; Doi, T. Development of new environmentally benign process for marine fragrance (Heliofresh). Fain Kemikaru 2013, 42, 14–19. [Google Scholar]
- Doi, T.; Yoshida, Y.; Sajiki, E.; Fujitsu, S. Method of Retaining the Quality of 2-methyl-3-(3,4-methylenedioxyphenyl)propanal and Process for Producing the Same. WO2008108429A1, 12 September 2008. [Google Scholar]
- Borzatta, V.; Capparella, E.; Poluzzi, E. Process for the Preparation for 3-(3,4-methylenedioxyphenyl)-2-methylpropanal. WO2005/105774 A1 10 November 2005. [Google Scholar]
- Fang, D.; Li, W.; Zhao, J.; Liu, S.; Ma, X.; Xu, J.; Xia, C. Catalytic hydrodechlorination of 4-chlorophenol over a series of Pd–Cu/γ-Al2O3 bimetallic catalysts. RSC Adv. 2014, 4, 59204–59210. [Google Scholar] [CrossRef]
- Bosello, N.; Di Michele, A.; Piccolo, O.; Paganelli, S. CO and H2 gas-free efficient reductive carbonylation of aryl iodides. Use of smart recyclable metal-based catalysts. Appl. Catal. A Gen. 2023, 657, 119145. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Athena, Artemis, Hephaestus: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Rehr, J.J.; Albers, R.C.; Zabinsky, S.I. High-order multiple-scattering calculations of X-ray absorption fine structure. Phys. Rev. Lett. 1992, 69, 3397–3400. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.V.; Frenkel, A.I.; Crooks, R.M. X-ray Absorption Study of PdCu bimetallic alloy nanoparticles containing an average of ∼64 atoms. Chem. Mater. 2009, 21, 4824–4829. [Google Scholar] [CrossRef]
- Kroner, A.B.; Newton, M.A.; Tromp, M.; Russell, A.E.; Dent, A.J.; Evans, J. Structural characterization of alumina-supported Rh catalysts: Effects of ceriation and zirconiation by using metal-organic precursors. ChemPhysChem 2013, 14, 3606–3617. [Google Scholar] [CrossRef] [PubMed]
- Waser, J.; Levy, H.A.; Peterson, S.W. The structure of PdO. Acta Crystallogr. 1953, 6, 661–663. [Google Scholar] [CrossRef]
- Di Cicco, A.; Aquilanti, G.; Minicucci, M.; Principi, E.; Novello, N.; Cognigni, A.; Olivi, L. Novel XAFS capabilities at Elettra synchrotron light source. J. Phys. Conf. Ser. 2009, 190, 012043. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Arčon, I.; Djinović, P.; Tchernychova, E.; Pintar, A. In-situ XAS study of catalytic N2O decomposition over CuO/CeO2 catalysts. ChemCatChem 2021, 13, 1814–1823. [Google Scholar] [CrossRef]
- Gogate, M.R.; Davis, R.J. X-ray Absorption Spectroscopy of a Fe-promoted Rh/TiO2 catalyst for synthesis of ethanol from synthesis gas. ChemCatChem 2009, 1, 295–303. [Google Scholar] [CrossRef]
- Arčon, I.; Paganelli, S.; Piccolo, O.; Gallo, M.; Vogel-Mikuš, K.; Baldi, F. XAS analysis of iron and palladium bonded to a polysaccharide produced anaerobically by a strain of Klebsiella Oxytoca. fACS Appl. Mater. Interfaces 2023, 22, 1215. [Google Scholar]
- Žumbar, T.; Arčon, I.; Djinović, P.; Aquilanti, G.; Žerjav, G.; Pintar, A.; Ristić, A.; Dražić, G.; Volavšek, J.; Mali, G.; et al. Winning combination of Cu and Fe Oxide clusters with an alumina support for low-temperature catalytic oxidation of volatile organic compounds. ACS Appl. Mater. Interfaces 2023, 15, 28747–28762. [Google Scholar] [CrossRef] [PubMed]
- Christy, A.G.; Clark, S.M. Structural behavior of palladium (II) oxide and a palladium suboxide at high pressure: An energy-dispersive X-ray diffraction study. Phys. Rev. B 1995, 52, 9259–9265. [Google Scholar] [CrossRef] [PubMed]
- Saraev, A.A.; Yashnik, S.A.; Gerasimov, E.Y.; Kremneva, A.M.; Vinokurov, Z.S.; Kaichev, V.V. Atomic structure of Pd-, Pt-, and PdPt-Based catalysts of total oxidation of methane: In situ EXAFS study. Catalysts 2021, 11, 1446. [Google Scholar] [CrossRef]
- Muravev, V.; Simons, J.F.M.; Parastaev, A.; Verheijen, M.A.; Struijs, J.J.C.; Kosinov, N.; Hensen, E.J.M. Operando spectroscopy unveils the catalytic role of different palladium oxidation states in CO oxidation on Pd/CeO2 catalysts. Angew. Chem. Int. Ed. 2022, 61, e202200434. [Google Scholar] [CrossRef] [PubMed]
| Run | Catalyst | Base | t (h) | Conv. (%) a | (X) (%) a | (III) (%) a | (XI) (%) a |
|---|---|---|---|---|---|---|---|
| 1 | Pd/Al2O3 | Na2CO3 | 6 | 65 | 15 | 49 | 1 |
| 2 | Na2CO3 | 24 | 98 | 3 | 74 | 21 | |
| 3 | TOA | 6 | 81 | 6 | 56 | 19 | |
| 4 | TOA | 24 | 95 | 3 | 62 | 30 | |
| 5 | TEA | 6 | 88 | 11 | 72 | 5 | |
| 6 | TEA | 24 | 100 | 1 | 83 | 16 | |
| 7 | Pd-Cu/Al2O3 | TEA | 6 | 7 | 3 | 4 | n.d. |
| 8 | TEA | 24 | 12 | 5 | 6 | 1 | |
| 9 b | TEA | 6 | 29 | 6 | 20 | 3 | |
| 10 b | TEA | 24 | 50 | 5 | 40 | 5 |
| Run | Catalyst | Base | p(H2) (MPa) | t (h) | Conv. a (%) | (X) (%) a | (III) (%) a | (XI) (%) a |
|---|---|---|---|---|---|---|---|---|
| 1 | Rh/Al2O3 | Na2CO3 | 0.5 | 6 | 67 | 25 | 41 | 1 |
| 2 | Na2CO3 | 0.5 | 24 | 85 | 3 | 72 | 10 | |
| 3 | Na2CO3 | 1.0 | 6 | 75 | 18 | 57 | n.d. | |
| 4 | TEA | 1.0 | 24 | 100 | 1 | 99 | n.d. | |
| 5 | Rh-Cu/Al2O3 | TEA | 1.0 | 4 | 23 | 14 | 9 | n.d. |
| 6 | TEA | 1.0 | 16 | 43 | 12 | 28 | 3 | |
| 7 | TEA | 1.0 | 24 | 68 | 9 | 51 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccolo, O.; Arčon, I.; Das, G.; Aquilanti, G.; Prai, A.; Paganelli, S.; Facchin, M.; Beghetto, V. Synthesis of Helional by Hydrodechlorination Reaction in the Presence of Mono- and Bimetallic Catalysts Supported on Alumina. Catalysts 2024, 14, 255. https://doi.org/10.3390/catal14040255
Piccolo O, Arčon I, Das G, Aquilanti G, Prai A, Paganelli S, Facchin M, Beghetto V. Synthesis of Helional by Hydrodechlorination Reaction in the Presence of Mono- and Bimetallic Catalysts Supported on Alumina. Catalysts. 2024; 14(4):255. https://doi.org/10.3390/catal14040255
Chicago/Turabian StylePiccolo, Oreste, Iztok Arčon, Gangadhar Das, Giuliana Aquilanti, Andrea Prai, Stefano Paganelli, Manuela Facchin, and Valentina Beghetto. 2024. "Synthesis of Helional by Hydrodechlorination Reaction in the Presence of Mono- and Bimetallic Catalysts Supported on Alumina" Catalysts 14, no. 4: 255. https://doi.org/10.3390/catal14040255
APA StylePiccolo, O., Arčon, I., Das, G., Aquilanti, G., Prai, A., Paganelli, S., Facchin, M., & Beghetto, V. (2024). Synthesis of Helional by Hydrodechlorination Reaction in the Presence of Mono- and Bimetallic Catalysts Supported on Alumina. Catalysts, 14(4), 255. https://doi.org/10.3390/catal14040255