Role of Vanadium in Thermal and Hydrothermal Aging of a Commercial V2O5-WO3/TiO2 Monolith for Selective Catalytic Reduction of NOx: A Case Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity
2.2. X-ray Diffraction
2.3. Specific Surface Area and Pores
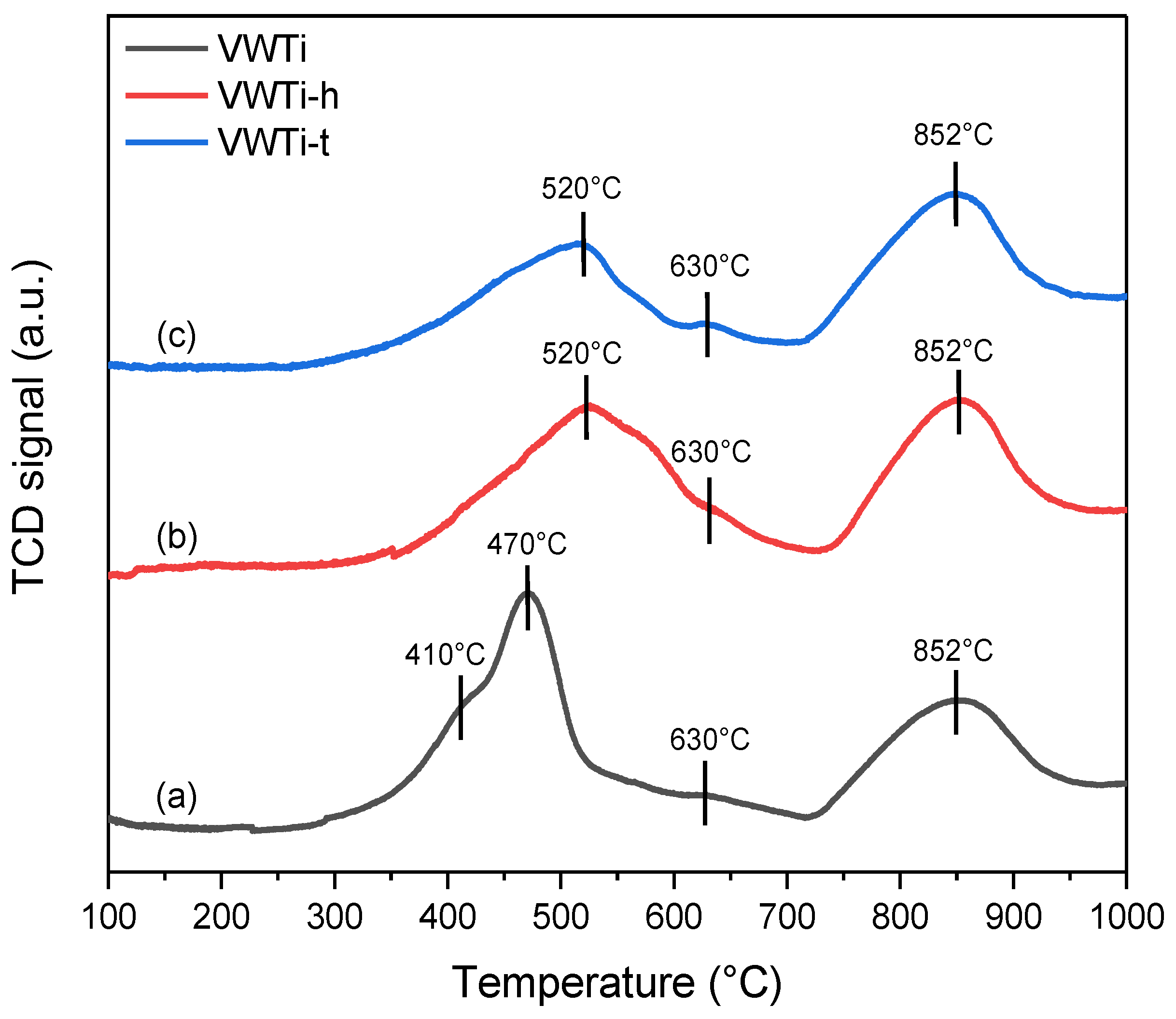
2.4. H2-TPR
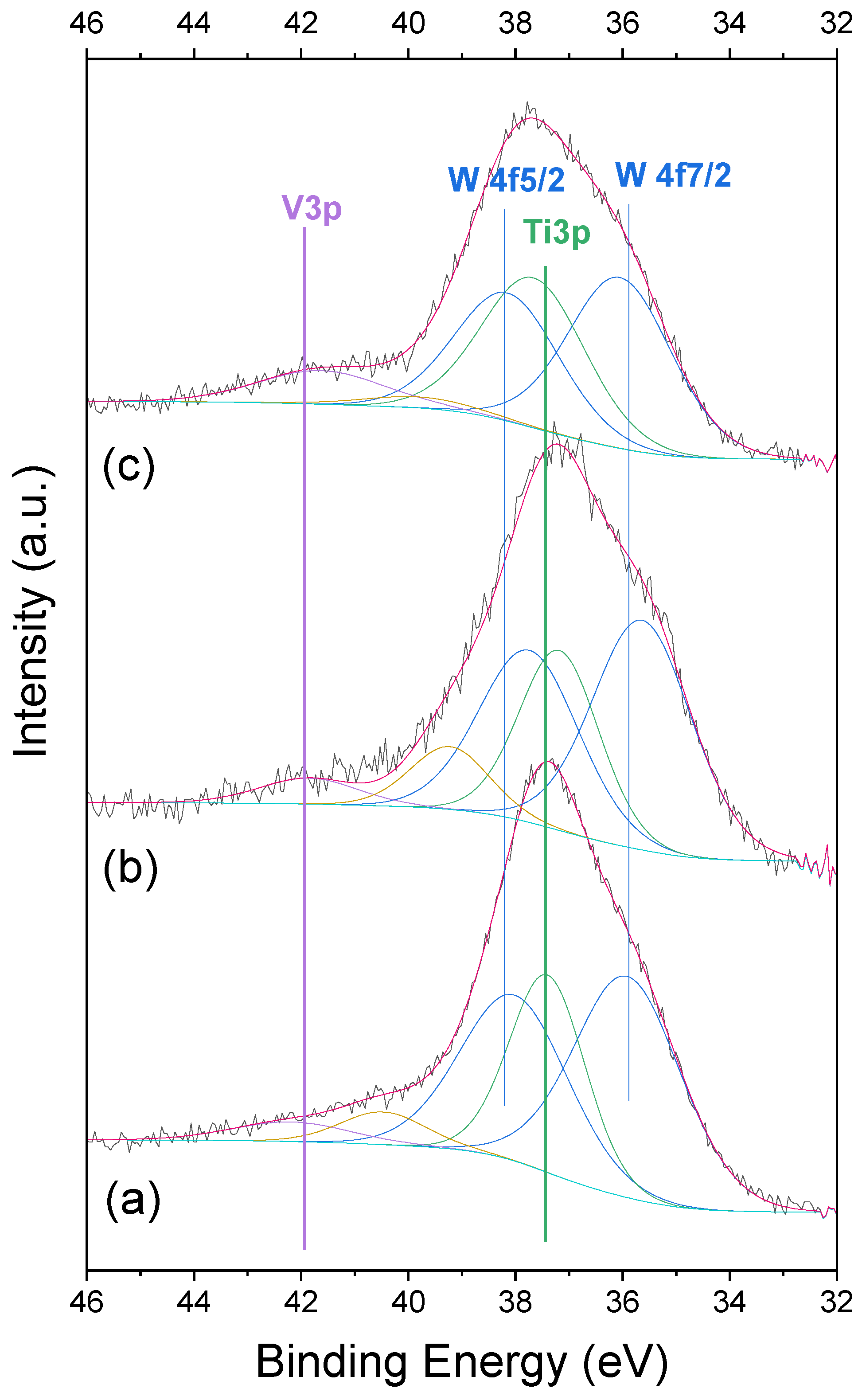
2.5. XPS
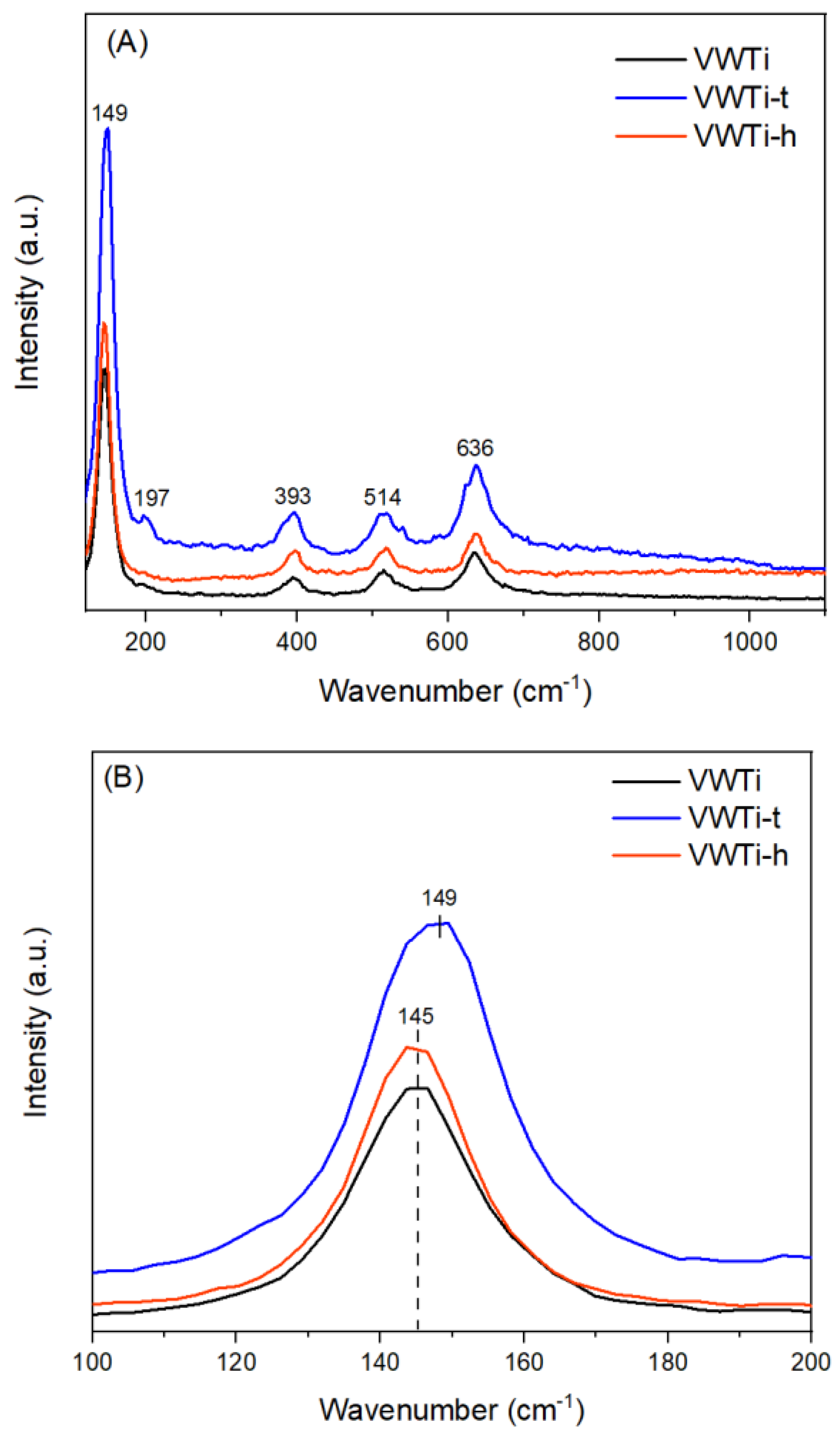
2.6. Raman Analysis
2.7. TGA Analysis
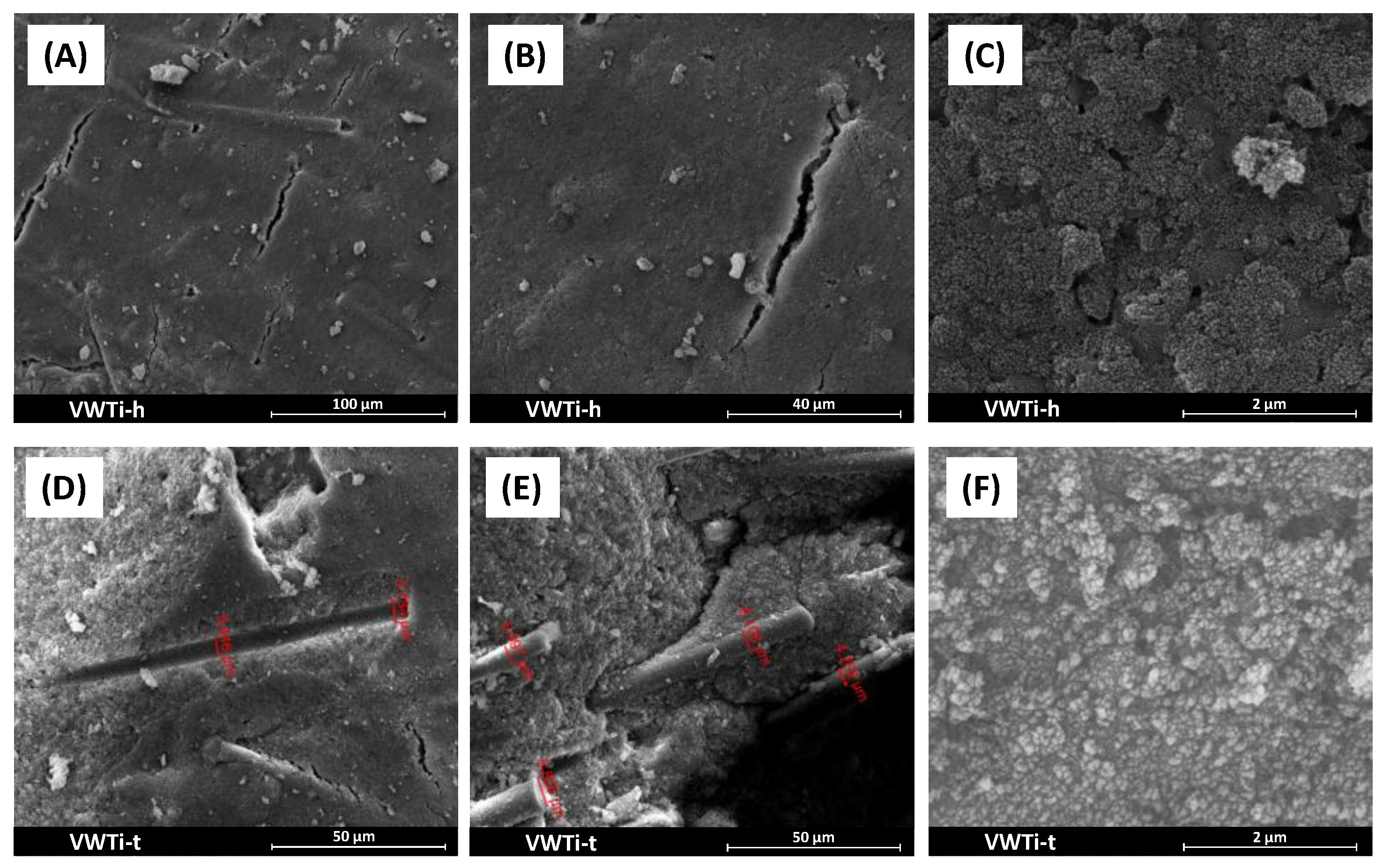
2.8. SEM/EDS
3. Materials and Methods
3.1. Monolithic Catalyst
3.2. Characterization of Monoliths
3.3. Catalyst Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, B.; Ma, Q.; Liu, J.; Ma, J.; Zhang, P.; Chen, T.; Feng, Q.; Wang, C.; Yang, N.; Ma, H.; et al. Air Pollutant Correlations in China: Secondary Air Pollutant Responses to NOx and SO2 Control. Environ. Sci. Technol. Lett. 2020, 7, 695–700. [Google Scholar] [CrossRef]
- Napolitano, P.; Liotta, L.F.; Guido, C.; Tornatore, C.; Pantaleo, G.; La Parola, V.; Beatrice, C. Insights of Selective Catalytic Reduction Technology for Nitrogen Oxides Control in Marine Engine Applications. Catalysts 2022, 12, 1191. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A Promising Technique to Reduce NOx Emissions from Automotive Diesel Engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Selleri, T.; Gramigni, F.; Nova, I.; Tronconi, E. NO Oxidation on Fe- and Cu-Zeolites Mixed with BaO/Al2O3: Free Oxidation Regime and Relevance for the NH3-SCR Chemistry at Low Temperature. Appl. Catal. B Environ. 2018, 225, 324–331. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. A Comparative Study of the NH3-SCR Reactions over a Cu-Zeolite and a Fe-Zeolite Catalyst. Catal. Today 2010, 151, 223–230. [Google Scholar] [CrossRef]
- Sitshebo, S.; Tsolakis, A.; Theinnoi, K.; Rodríguez-Fernández, J.; Leung, P. Improving the Low Temperature NOx Reduction Activity over a Ag-Al2O3 Catalyst. Chem. Eng. J. 2010, 158, 402–410. [Google Scholar] [CrossRef]
- Liu, W.; Long, Y.; Liu, S.; Zhou, Y.; Tong, X.; Yin, Y.; Li, X.; Hu, K.; Hu, J. Commercial SCR Catalyst Modified with Different Noble Metals (Ag, Pt, Pd) to Efficiently Remove Slip Ammonia and NOx in the Flue Gas. J. Taiwan Inst. Chem. Eng. 2022, 138, 104472. [Google Scholar] [CrossRef]
- Lietti, L.; Nova, I.; Ramis, G.; Dall’Acqua, L.; Busca, G.; Giamello, E.; Forzatti, P.; Bregani, F. Characterization and Reactivity of V2O5-MoO3/TiO2 De-NOx SCR Catalysts. J. Catal. 1999, 187, 419–435. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Zhang, R.; Wei, Y. Synergistic Effect of Niobium and Ceria on Anatase for Low-Temperature NH3-SCR of NO Process. Mol. Catal. 2019, 478, 110563. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z.; Xue, J.; Xu, Y.; Wang, C.; Wang, L. NH3-SCR Performance and the Resistance to SO2 for Nb Doped Vanadium Based Catalyst at Low Temperatures. J. Environ. Sci. 2018, 65, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bi, Z.; Liang, Y. Development of a Nutrient Recipe for Enhancing Methane Release from Coal in the Illinois Basin. Int. J. Coal Geol. 2018, 187, 11–19. [Google Scholar] [CrossRef]
- Forzatti, P. Present Status and Perspectives in De-NOx SCR Catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Kompio, P.G.W.A.; Brückner, A.; Hipler, F.; Auer, G.; Löffler, E.; Grünert, W. A New View on the Relations between Tungsten and Vanadium in V2O5WO3/TiO2 Catalysts for the Selective Reduction of NO with NH3. J. Catal. 2012, 286, 237–247. [Google Scholar] [CrossRef]
- Kompio, P.G.W.A.; Brückner, A.; Hipler, F.; Manoylova, O.; Auer, G.; Mestl, G.; Grünert, W. V2O5-WO3/TiO2 Catalysts under Thermal Stress: Responses of Structure and Catalytic Behavior in the Selective Catalytic Reduction of NO by NH3. Appl. Catal. B Environ. 2017, 217, 365–377. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Duevel, R.V.; Wachs, I.E. The Effect of Metal Oxide Additives on the Activity of V2O5/TiO2 Catalysts for the Selective Catalytic Reduction of Nitric Oxide by Ammonia. Appl. Catal. B Environ. 1999, 20, 111–122. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Metal Oxides: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-1-4200-2812-6. [Google Scholar]
- Wachs, I.E. Recent Conceptual Advances in the Catalysis Science of Mixed Metal Oxide Catalytic Materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Recent advances in de-NOxing catalysis for stationary applications. Heterog. Chem. Rev. 1996, 3, 33–51. [Google Scholar] [CrossRef]
- Li, J.; Fu, H.; Fu, L.; Hao, J. Preparation of Metallic Ion-Doped TiO2 Thin Films and Their Photocatalytic Performance for Toluene Degradation. Chin. J. Catal. 2005, 26, 503–507. [Google Scholar]
- Chapman, D.M. Behavior of Titania-Supported Vanadia and Tungsta SCR Catalysts at High Temperatures in Reactant Streams: Tungsten and Vanadium Oxide and Hydroxide Vapor Pressure Reduction by Surficial Stabilization. Appl. Catal. A Gen. 2011, 392, 143–150. [Google Scholar] [CrossRef]
- Nunney, M.J. Light and Heavy Vehicle Technology, 4th ed.; Routledge: London, UK, 2008; ISBN 9780080465753. [Google Scholar]
- Liu, X.; Chen, H.; Wu, X.; Cao, L.; Jiang, P.; Yu, Q.; Ma, Y. Effects of SiO2 Modification on the Hydrothermal Stability of the V2O5/WO3-TiO2 NH3-SCR Catalyst: TiO2 Structure and Vanadia Species. Catal. Sci. Technol. 2019, 9, 3711–3720. [Google Scholar] [CrossRef]
- Liu, J.; He, G.; Shan, W.; Yu, Y.; Huo, Y.; Zhang, Y.; Wang, M.; Yu, R.; Liu, S.; He, H. Introducing Tin to Develop Ternary Metal Oxides with Excellent Hydrothermal Stability for NH3 Selective Catalytic Reduction of NOx. Appl. Catal. B Environ. 2021, 291, 120125. [Google Scholar] [CrossRef]
- Species, S.; Joint, S.K.; Control, P. DRIFT Study on Cerium—Tungsten/Titiania Catalyst for Selective Catalytic Reduction of NOx with NH3. Environ. Sci. Technol. 2010, 44, 9590–9596. [Google Scholar]
- Bartholomew, C.H. Mechanisms of Catalyst Deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Djerad, S.; Tifouti, L.; Crocoll, M.; Weisweiler, W. Effect of vanadia and tungsten loadings on the physical and chemical characteristics of V2O5-WO3/TiO2 catalysts. J. Mol. Catal. A Chem. 2004, 208, 257–265. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Park, J.S.; Shafer, M.M.; Deminter, J.T.; Weinstein, J.P. Emissions of Metals Associated with Motor Vehicle Roadways. Environ. Sci. Technol. 2005, 39, 826–836. [Google Scholar] [CrossRef]
- Barceloux, D.G. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef]
- Costigan, M.; Cary, R.; Dobson, S. Concise International Chemical Assessment Document 29: Vanadium Pentoxide and Other Inorganic Vanadium Compounds; Geneva, 2001; ISBN 92 4 153029 4.
- Madia, G.; Elsener, M.; Koebel, M.; Raimondi, F.; Wokaun, A. Thermal Stability of Vanadia-Tungsta-Titania Catalysts in the SCR Process. Appl. Catal. B Environ. 2002, 39, 181–190. [Google Scholar] [CrossRef]
- Nova, I.; Dall’Acqua, L.; Lietti, L.; Giamello, E.; Forzatti, P. Study of Thermal Deactivation of a De-NOx Commercial Catalyst. Appl. Catal. B Environ. 2001, 35, 31–42. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Kröcher, O. VOx Surface Coverage Optimization of V2O5/WO3-TiO2 SCR Catalysts by Variation of the V Loading and by Aging. Catalysts 2015, 5, 1704–1720. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Solar, J.P. Selective Catalytic Reduction of Nitric Oxide by Ammonia over V2O5/TiO2, V2O5/TiO2/SiO2, and V2O5-WO3/TiO2 Catalysts: Effect of Vanadia Content on the Activation Energy. Ind. Eng. Chem. Res. 1996, 35, 978–981. [Google Scholar] [CrossRef]
- Wachs, I.E.; Deo, G.; Weckhuysen, B.M.; Andreini, A.; Vuurman, M.A.; De Boer, M.; Amiridis, M.D. Selective Catalytic Reduction of NO with NH3 over Supported Vanadia Catalysts. J. Catal. 1996, 161, 211–221. [Google Scholar] [CrossRef]
- Girard, J.W.; Montreuil, C.; Kim, J.; Cavataio, G.; Lambert, C. Technical Advantages of Vanadium SCR Systems for Diesel NOx Control in Emerging Markets. SAE Int. J. Fuels Lubr. 2008, 1, 488–494. [Google Scholar] [CrossRef]
- Asako, T.; Kai, R.; Toyoshima, T.; Vogt, C.; Hirose, S.; Nakao, S. Evaluation of Hydrothermally Aged Vanadia SCR on High-Porosity Substrate; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Abrams, B.L. Fundamental Chemistry of V-SCR Catalysts at Elevated Temperatures. Catal. Today 2017, 297, 60–63. [Google Scholar] [CrossRef]
- Maunula, T.; Kinnunen, T.; Kanniainen, K.; Viitanen, A.; Savimaki, A. Thermally Durable Vanadium-SCR Catalysts for Diesel Applications; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013; Volume 2. [Google Scholar] [CrossRef]
- Lin, C.H.; Bai, H. Adsorption Behavior of Moisture over a Vanadia/Titania Catalyst: A Study for the Selective Catalytic Reduction Process. Ind. Eng. Chem. Res. 2004, 43, 5983–5988. [Google Scholar] [CrossRef]
- Wang, C.; Yang, S.; Chang, H.; Peng, Y.; Li, J. Dispersion of Tungsten Oxide on SCR Performance of V2O5WO3/TiO2: Acidity, Surface Species and Catalytic Activity. Chem. Eng. J. 2013, 225, 520–527. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Metal Oxide and Zeolite Catalysts—A Review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Beale, A.M.; Lezcano-Gonzalez, I.; Maunula, T.; Palgrave, R.G. Development and Characterization of Thermally Stable Supported V–W–TiO2 Catalysts for Mobile NH3–SCR Applications. Catal. Struct. React. 2015, 1, 25–34. [Google Scholar] [CrossRef]
- Besselmann, S.; Freitag, C.; Hinrichsen, O.; Muhler, M. Temperature-Programmed Reduction and Oxidation Experiments with V2O5/TiO2 Catalysts. Phys. Chem. Chem. Phys. 2001, 3, 4633–4638. [Google Scholar] [CrossRef]
- Ousmane, M.; Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G.; Retailleau, L.; Boreave, A.; Giroir-Fendler, A. Supported Au Catalysts for Low-Temperature Abatement of Propene and Toluene, as Model VOCs: Support Effect. Appl. Catal. B Environ. 2011, 101, 629–637. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Yang, N.C.; Lin, K.S.; Chang, C.J.; Dinh, K.T.; Lin, Y.G. Formulation and Characterization of W-Doped Titania Nanotubes for Adsorption/Photodegradation of Methylene Blue and Basic Violet 3 Dyes. Catal. Today 2022, 388–389, 36–46. [Google Scholar] [CrossRef]
- Komornicki, S.; Radecka, M.; Sobaś, P. Structural Properties of TiO2-WO3 Thin Films Prepared by r.f. Sputtering. J. Mater. Sci. Mater. Electron. 2004, 15, 527–531. [Google Scholar] [CrossRef]
- Hu, J.; Lei, Y.; Yuan, M.; Lin, Y.; Jiang, Z.; Ouyang, Z.; Du, P.; Wu, Y. Enhanced Photoelectric Performance of GQDs Anchored WO3 with a “dot-on-Nanoparticle” Structure. Mater. Res. Express 2020, 7, 75602. [Google Scholar] [CrossRef]
- Zakharova, G.S.; Podval’Naya, N.V.; Kuznetsov, M.V. XPS Study of Nanorods of Doped Vanadium Oxide MxV2O5·nH2O (M = Na, K, Rb, Cs). Russ. J. Inorg. Chem. 2011, 56, 267–272. [Google Scholar] [CrossRef]
- Chenakin, S.P.; Silvy, R.P.; Kruse, N. X-ray Induced Surface Modification of Aluminovanadate Oxide. Catal. Lett. 2005, 102, 39–43. [Google Scholar] [CrossRef]
- Schuhl, Y.; Baussart, H.; Delobel, R.; Le Bras, M.; Leroy, J.M.; Gengembre, L.; Grimblot, J. Study of Mixed-Oxide Catalysts Containing Bismuth, Vanadium and Antimony. Preparation, Phase Composition, Spectroscopic Characterization and Catalytic Oxidation of Propene. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1983, 79, 2055–2069. [Google Scholar] [CrossRef]
- Palcheva, R.; Dimitrov, L.; Tyuliev, G.; Spojakina, A.; Jiratova, K. TiO2 Nanotubes Supported NiW Hydrodesulphurization Catalysts: Characterization and Activity. Appl. Surf. Sci. 2013, 265, 309–316. [Google Scholar] [CrossRef]
- Cheng, P.; Deng, C.S.; Liu, D.N.; Dai, X.M. Titania Surface Modification and Photovoltaic Characteristics with Tungsten Oxide. Appl. Surf. Sci. 2008, 254, 3391–3396. [Google Scholar] [CrossRef]
- Caravaca, M.; Morales, J.J.; Abad, J. Interaction of NO with SiOx/TiO2 (1 1 0)-(1 × 2). Appl. Surf. Sci. 2021, 551, 2–6. [Google Scholar] [CrossRef]
- Liu, B.; Du, J.; Lv, X.; Qiu, Y.; Tao, C. Washcoating of Cordierite Honeycomb with Vanadia-Tungsta-Titania Mixed Oxides for Selective Catalytic Reduction of NO with NH3. Catal. Sci. Technol. 2015, 5, 1241–1250. [Google Scholar] [CrossRef]
- Wachs, I.E. Raman and IR Studies of Surface Metal Oxide Species on Oxide Supports: Supported Metal Oxide Catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Boerio-Goates, J.; Woodfield, B.F. High Purity Anatase TiO2 Nanocrystals: Near Room-Temperature Synthesis, Grain Growth Kinetics, and Surface Hydration Chemistry. J. Am. Chem. Soc. 2005, 127, 8659–8666. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; D’Urso, L.; Compagnini, G.; Palmisano, L.; Scirè, S. Au/TiO2-CeO2 Catalysts for Photocatalytic Water Splitting and VOCs Oxidation Reactions. Catalysts 2016, 6, 121. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Gao, Y.; Lin, Q.; Hu, J.; Weng, D. Comparative Study on Sulfur Poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 Monolithic Catalysts for Low-Temperature NH3-SCR. Catal. Commun. 2017, 93, 33–36. [Google Scholar] [CrossRef]
- Cimino, S.; Ferone, C.; Cioffi, R.; Perillo, G.; Lisi, L. A Case Study for the Deactivation and Regeneration of a V2O5-WO3/TiO2 Catalyst in a Tail-End SCR Unit of a Municipal Waste Incineration Plant. Catalysts 2019, 9, 464. [Google Scholar] [CrossRef]
- Liu, H.; Fan, Z.; Sun, C.; Yu, S.; Feng, S.; Chen, W.; Chen, D.; Tang, C.; Gao, F.; Dong, L. Improved Activity and Significant SO2 Tolerance of Samarium Modified CeO2-TiO2 Catalyst for NO Selective Catalytic Reduction with NH3. Appl. Catal. B Environ. 2019, 244, 671–683. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Liu, X.; Cao, L.; Lin, Q.; Weng, D. Effect of Barium Sulfate Modification on the SO2 Tolerance of V2O5/TiO2 Catalyst for NH3-SCR Reaction. J. Environ. Sci. 2017, 57, 110–117. [Google Scholar] [CrossRef]
- Lisi, L.; Cimino, S. Catalysts Deactivation, Poisoning and Regeneration; MDPI: Basel, Switzerland, 2019; ISBN 9783039215461. [Google Scholar]
- Liu, W.; Long, Y.; Zhou, Y.; Liu, S.; Tong, X.; Yin, Y.; Li, X.; Hu, K.; Hu, J. Excellent Low Temperature NH3-SCR and NH3-SCO Performance over Ag-Mn/Ce-Ti Catalyst: Evaluation and Characterization. Mol. Catal. 2022, 528, 112510. [Google Scholar] [CrossRef]
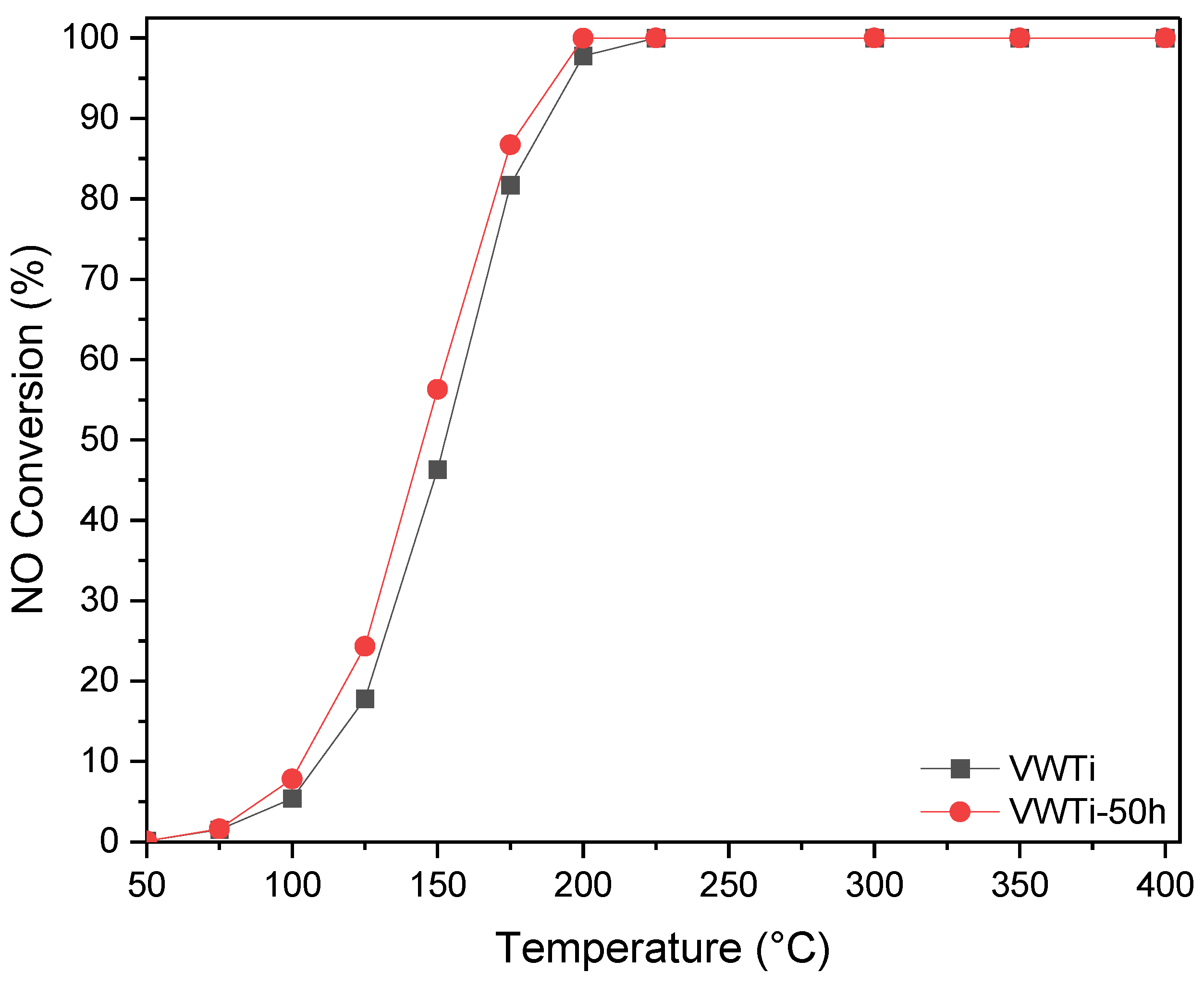


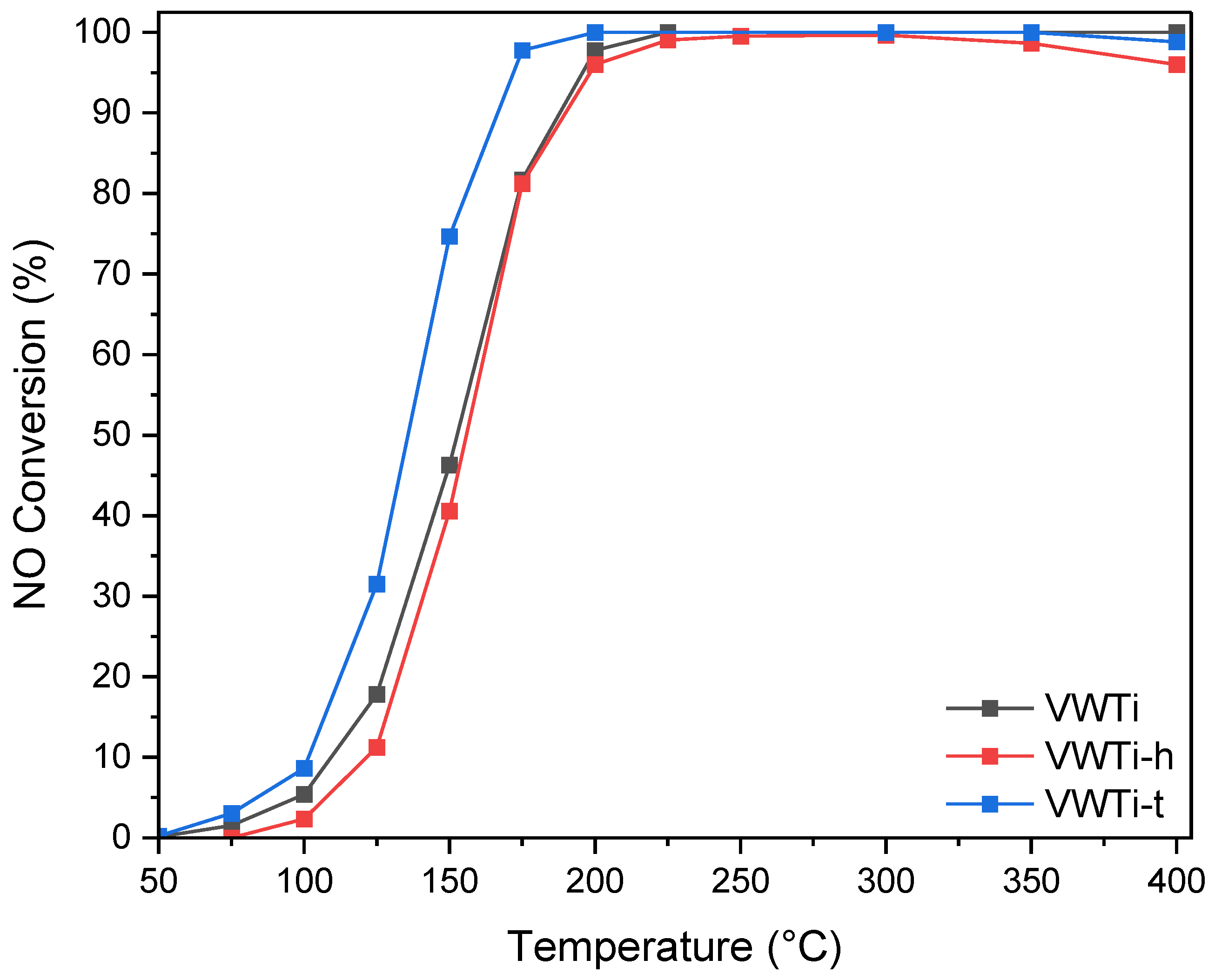
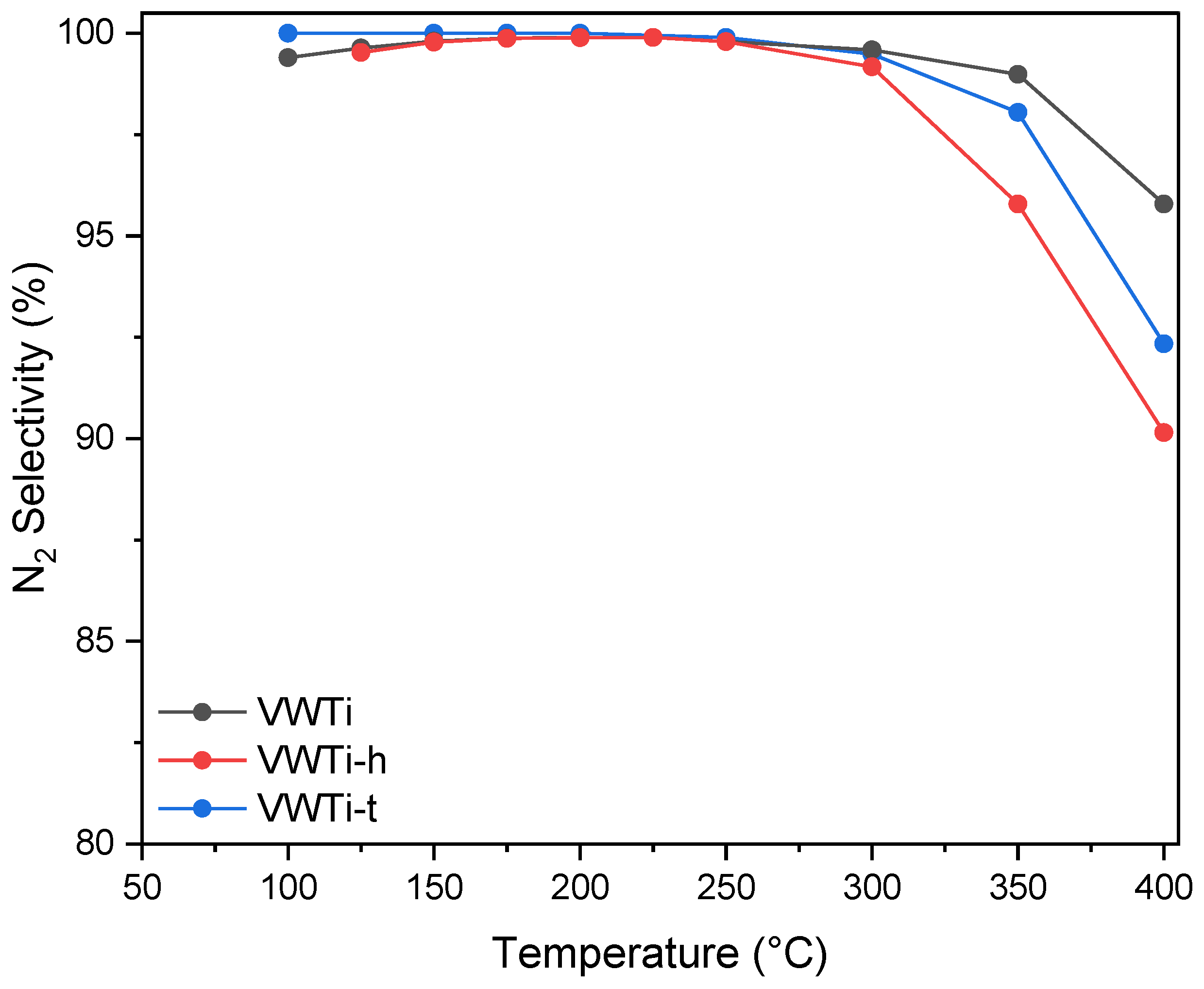
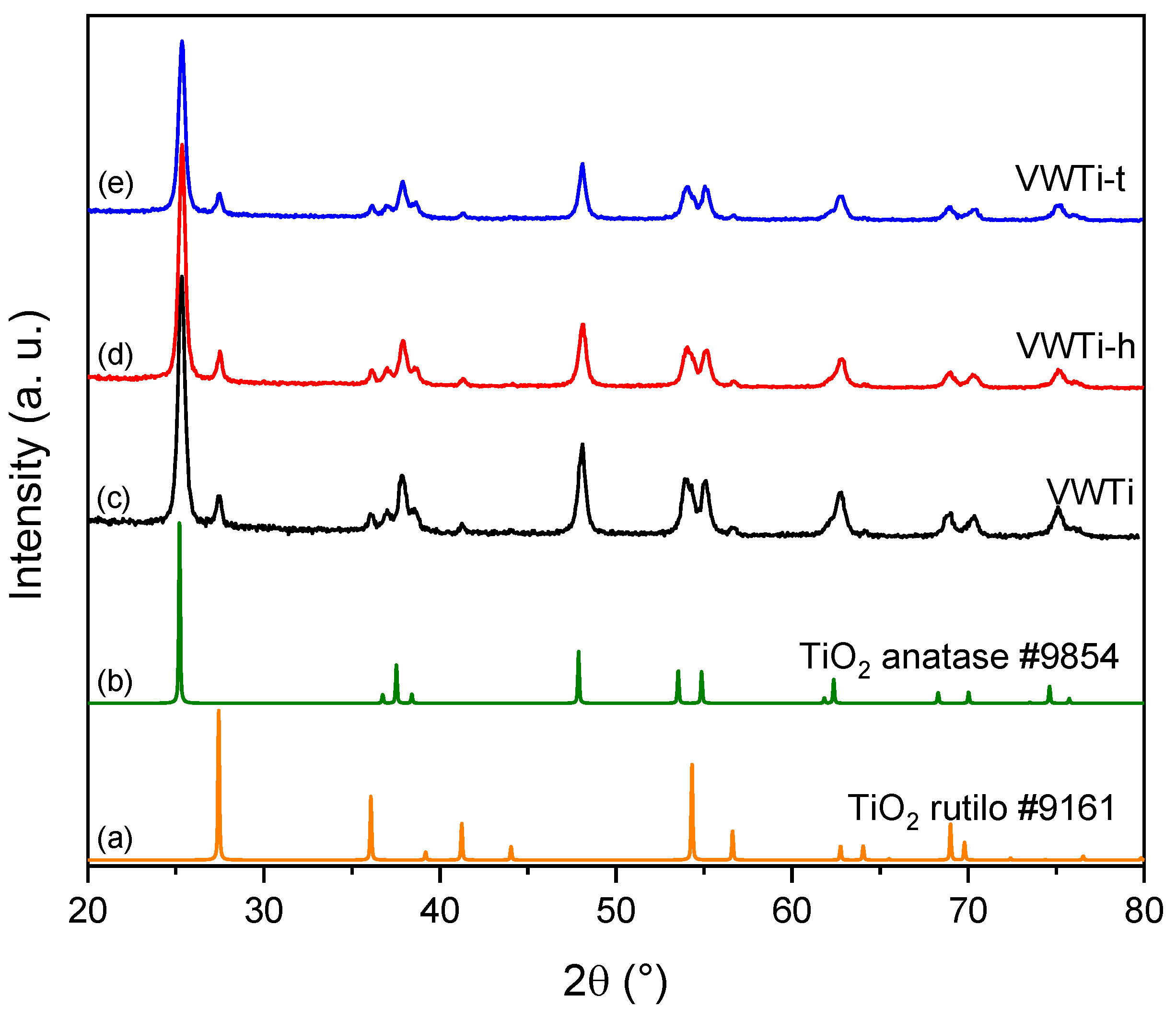




| Samples | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Mean Pore Size (nm) |
|---|---|---|---|
| VWTi | 53.0 | 0.26 | 15.8 |
| VWTi-h | 60.0 | 0.34 | 20.6 |
| VWTi-t | 37.0 | 0.25 | 24.9 |
| Sample | Temperature Range (°C) | Experimental H2 Consumption (mL g−1) | Theoretical H2 Consumption (mL g−1) |
|---|---|---|---|
| VWTi | 300–700 | 14.0 | 9.6 (V2O5 → V2O3) |
| 700–1000 | 12.5 | 10.6 (WO3 → WO2) | |
| Total | 26.5 | 20.2 | |
| VWTi-h | 400–700 | 10.9 | 9.6 (V2O5 → V2O3) |
| 700–1000 | 14.7 | 10.6 (WO3 → WO2) | |
| Total | 25.6 | 20.2 | |
| VWTi-t | 400–750 | 8.1 | 9.6 (V2O5 → V2O3) |
| 700–1000 | 14.7 | 10.6 (WO3 → WO2) | |
| Total | 22.8 | 20.2 |
| Sample | W 4f7/2 (eV) | V3p (eV) | O1s | W/Ti (0.03) * | V/Ti (0.03) * |
|---|---|---|---|---|---|
| VWTi | 35.8 | 41.0 | 529.3 (58%) 531.8 (42%) | 0.23 | 0.13 |
| VWTi-h | 35.6 | 41.7 | 529.6 (64%) 531.6 (36%) | 0.23 | 0.15 |
| VWTi-t | 36.0 | 41.4 | 529.4 (56%) 531.7 (44%) | 0.17 | 0.22 |
| Phase | Wt% |
|---|---|
| TiO2 | 75.0 |
| WO3 | 7.0 |
| V2O5 | 3.0 |
| bentonite | 6.0 |
| glass fiber | 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consentino, L.; Pantaleo, G.; La Parola, V.; La Greca, E.; Gallì, N.; Marcì, G.; Fiorenza, R.; Scirè, S.; Liotta, L.F. Role of Vanadium in Thermal and Hydrothermal Aging of a Commercial V2O5-WO3/TiO2 Monolith for Selective Catalytic Reduction of NOx: A Case Study. Catalysts 2024, 14, 241. https://doi.org/10.3390/catal14040241
Consentino L, Pantaleo G, La Parola V, La Greca E, Gallì N, Marcì G, Fiorenza R, Scirè S, Liotta LF. Role of Vanadium in Thermal and Hydrothermal Aging of a Commercial V2O5-WO3/TiO2 Monolith for Selective Catalytic Reduction of NOx: A Case Study. Catalysts. 2024; 14(4):241. https://doi.org/10.3390/catal14040241
Chicago/Turabian StyleConsentino, Luca, Giuseppe Pantaleo, Valeria La Parola, Eleonora La Greca, Nunzio Gallì, Giuseppe Marcì, Roberto Fiorenza, Salvatore Scirè, and Leonarda Francesca Liotta. 2024. "Role of Vanadium in Thermal and Hydrothermal Aging of a Commercial V2O5-WO3/TiO2 Monolith for Selective Catalytic Reduction of NOx: A Case Study" Catalysts 14, no. 4: 241. https://doi.org/10.3390/catal14040241
APA StyleConsentino, L., Pantaleo, G., La Parola, V., La Greca, E., Gallì, N., Marcì, G., Fiorenza, R., Scirè, S., & Liotta, L. F. (2024). Role of Vanadium in Thermal and Hydrothermal Aging of a Commercial V2O5-WO3/TiO2 Monolith for Selective Catalytic Reduction of NOx: A Case Study. Catalysts, 14(4), 241. https://doi.org/10.3390/catal14040241













