Three-Dimensional Electrode-Enhanced Ozone Catalytic Oxidation for Thiamethoxam Wastewater Treatment: Performance, Kinetics, and Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of 3DE-GAC-O3, GAC-O3, 3DE-GAC, and 2DE-O3
2.2. Effects of The Operating Parameters
2.2.1. Reaction Time
2.2.2. Current Density
2.2.3. Ozone Concentration
2.2.4. Initial Thiamethoxam Concentration
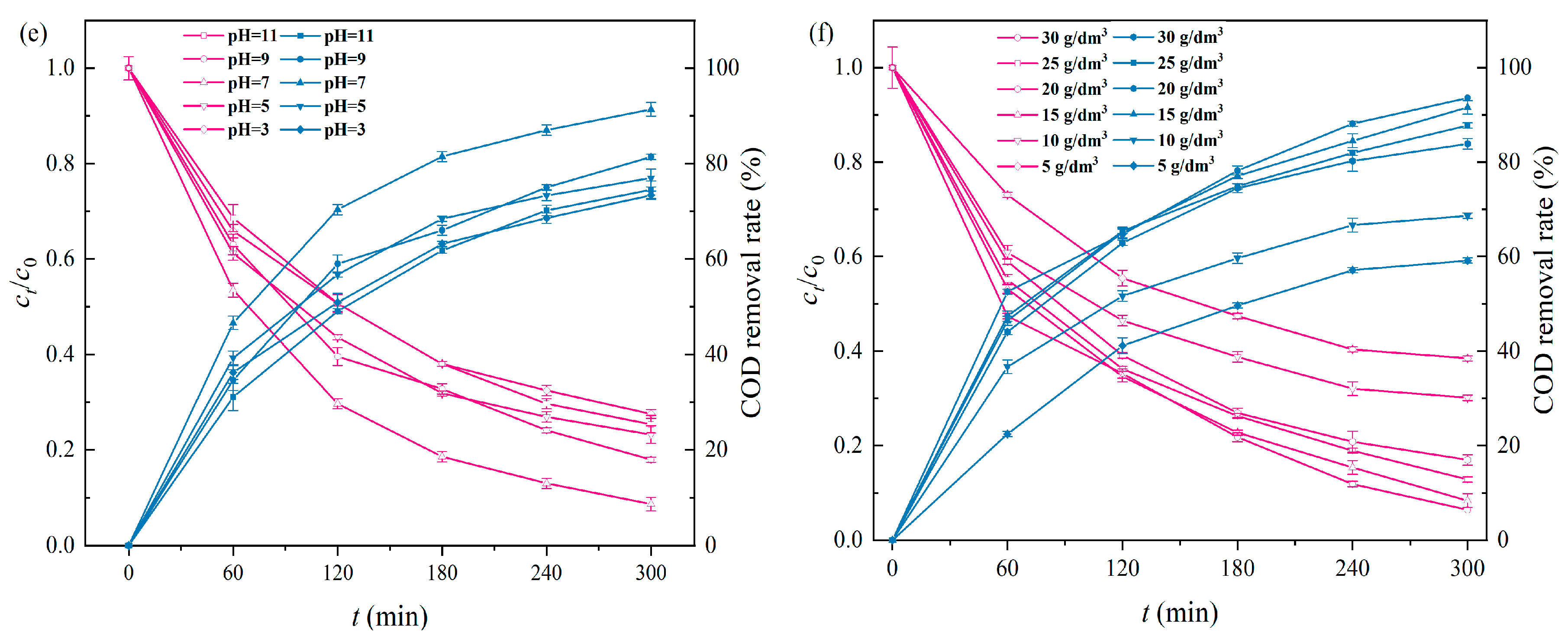
2.2.5. Initial pH
2.2.6. Particle Electrode Dosage
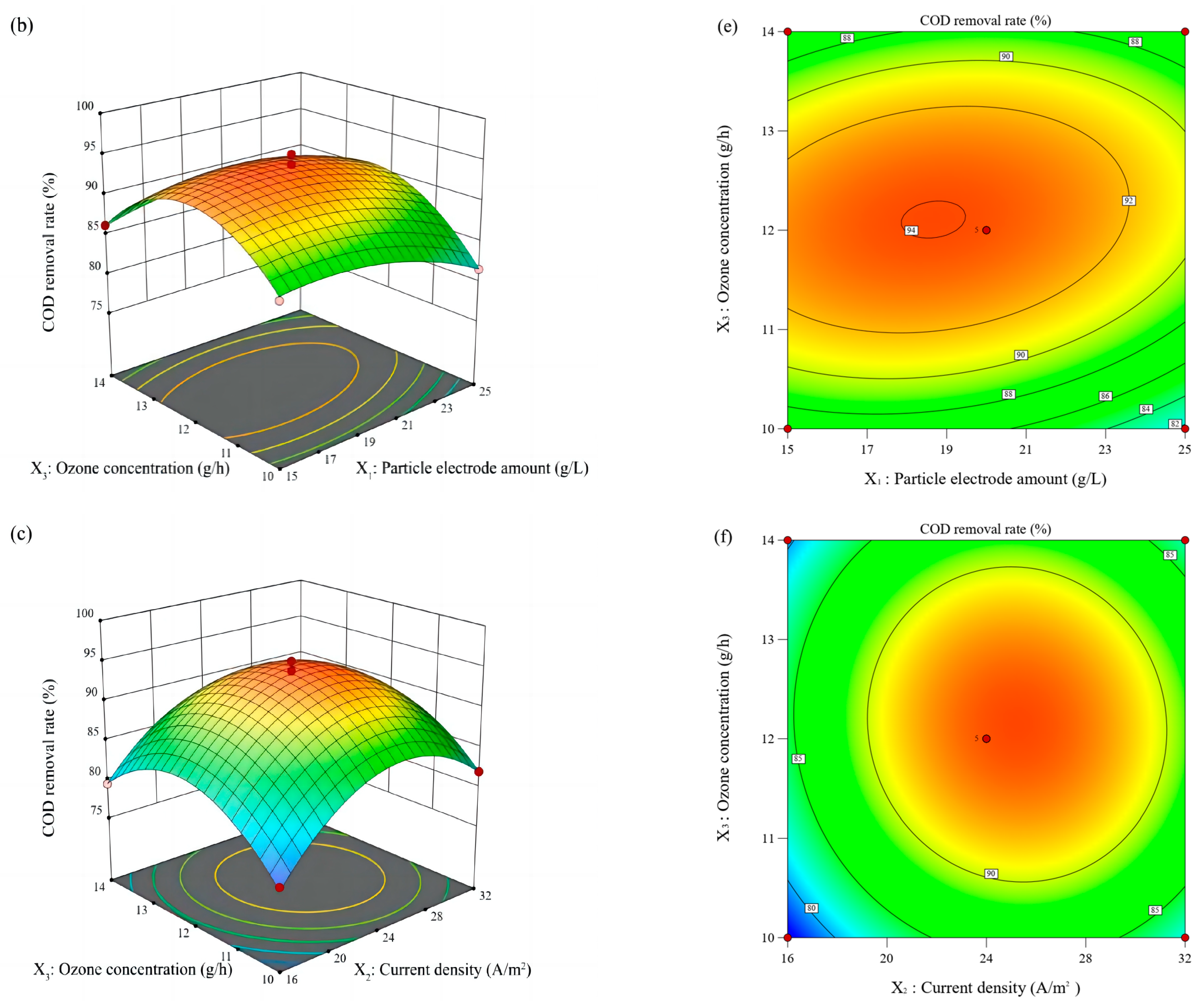
2.3. Response Surface Methodology Optimization
2.4. Reaction Kinetics
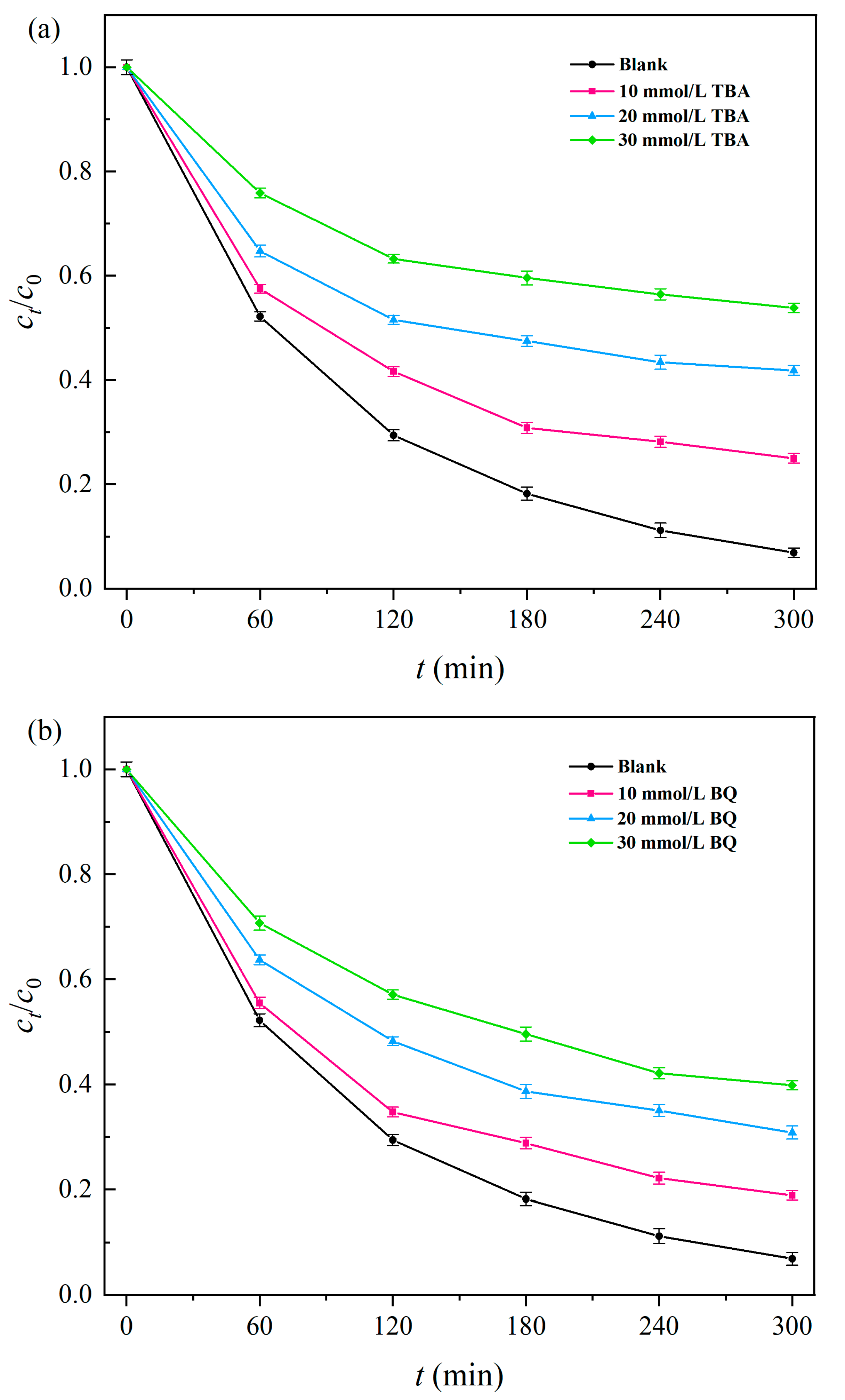
2.5. Free-Radical Quenching Experiment
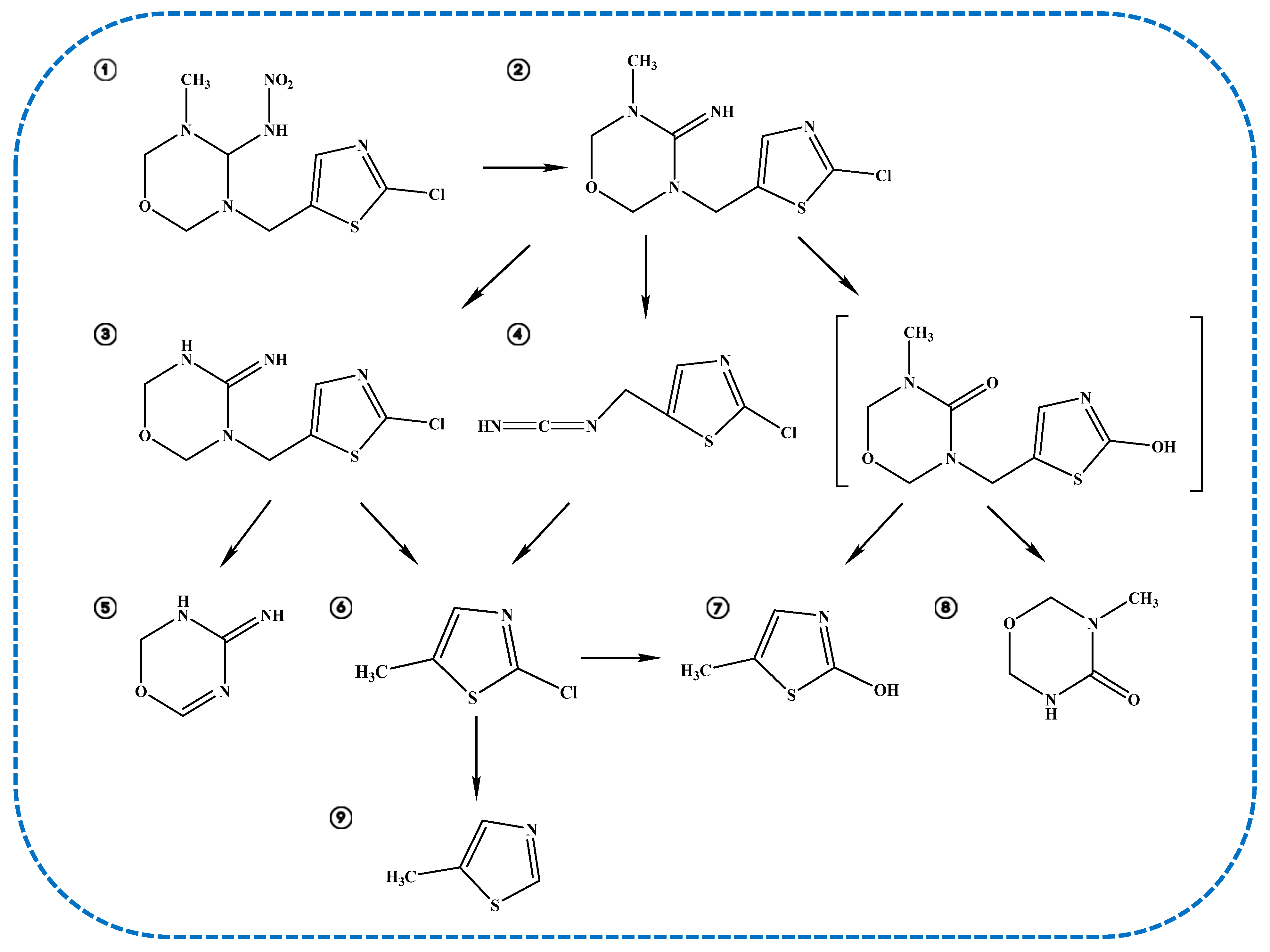
2.6. Degradation Products and Degradation Pathway
2.7. Economic Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Device and Method
3.3. Analytical Methods
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, Y.; Liu, R.; Zheng, M.; Wang, Z.; Yu, S.; Zhou, Y.; Zhou, Z.; Diao, J. From the first to third generation of neonicotinoids: Implication for saving the loss of fruit quality and flavor by pesticide applications. J. Agric. Food Chem. 2020, 70, 15415–15429. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Zhao, M.; Wang, P.; Huang, S.; Fu, W. Control efficacy and joint toxicity of thiamethoxam mixed with spirotetramat against the Asian citrus psyllid, Diaphorina citri Kuwayama. Pest Manag. Sci. 2020, 77, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Singh, B.; Bhardwaj, R.; Singh, R.; Alsahli, A.A.; Kaushik, P.; Ahmad, P. The pesticide thiamethoxam induced toxicity in Brassica juncea and its detoxification by Pseudomonas putida through biochemical and molecular modifications. Chemosphere 2023, 342, 140111. [Google Scholar] [CrossRef] [PubMed]
- Sadaria, A.M.; Supowit, S.D.; Halden, R.U. Mass balance assessment for six neonicotinoid insecticides during conventional wastewater and wetland treatment: Nationwide reconnaissance in united states wastewater. Environ. Sci. Technol. 2016, 50, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Singer, H.; Berg, M.; Müller, B.; Pernet-Coudrier, B.; Liu, H.; Qu, J. Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere 2015, 119, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Bojić, D.V.; Kostić, M.M.; Radović-Vučić, M.D.; Velinov, N.D.; Najdanović, S.M.; Petrović Milica, M.; Bojić, A.L. Removal of the herbicide 2,4-dichlorophenoxyacetic acid from water by using an ultrahighly efficient thermochemically activated carbon. Hem. Ind. 2019, 73, 223–237. [Google Scholar] [CrossRef]
- Petrović, M.; Rančev, S.; Đorđević, M.P.; Najdanović, S.; Velinov, N.; Vučić, M.R.; Bojić, A. Electrochemically synthesized Molybdenum oxides for enhancement of atmospheric pressure non-thermal pulsating corona plasma induced degradation of an organic compound. Chem. Eng. Sci. 2021, 230, 116209. [Google Scholar] [CrossRef]
- Li, Z.Y.; Song, W.; Zhang, L. The degradation of organophosphorous pesticide wastewater by sonophotocatalytic oxidation technology. Adv. Mater. Res. 2013, 781–784, 2184–2188. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Q.; Bai, Z.; Wang, S.; Chen, H.; Cao, Y. Catalytic wet air oxidation of wastewater of the herbicide fomesafen production with CeO2-TiO2 catalysts. Environ. Sci. Technol. 2015, 32, 389–396. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Pariente, M.I.; Rodríguez, I.; Petre, A.L.; Leton, P.; García, J. Treatment of a wastewater from a pesticide manufacture by combined coagulation and Fenton oxidation. Environ. Sci. Pollut. Res. 2014, 21, 12129–12134. [Google Scholar] [CrossRef]
- Tang, X.Y.; Wang, S.Z.; Qian, L.L.; Li, Y.H. Treatment of organic phosphorus pesticides manufacturing wastewater by supercritical water oxidation. Adv. Mater. Res. 2013, 788, 434–439. [Google Scholar] [CrossRef]
- Cao, S.; Chen, L.; Zhao, M.; Liu, A.; Wang, M.; Sun, Y. Advanced treatment of phosphorus pesticide wastewater using an integrated process of coagulation and ozone catalytic oxidation. Catalysts 2022, 12, 103. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, J.; Gao, L.; Yu, G.; Komarneni, S.; Wang, Y. Kinetics and mechanism of thiamethoxam abatement by ozonation and ozone-based advanced oxidation processes. J. Hazard. Mater. 2020, 390, 122180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, L.; Chen, F.; Zhao, S.; Zhu, J.; Yin, C. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Liu, S.; Chen, P.; Yang, T.; Wu, P.; Liu, C.; He, J.; Jiang, W. Intensification of gas-liquid mass-transfer efficiency by introducing a superaerophilic surface in the ozonation process. Ind. Eng. Chem. Res. 2022, 61, 10955–10968. [Google Scholar] [CrossRef]
- Meng, X.; Ren, Y.; Zhang, X.; Liu, J.; Ding, Y.; Gao, G.; Jiang, W. Study on 3D electrochemical degradation of tetracycline hydrochloride by using Fe/Cu/Mn trimetal-doped granular activated carbon: Reactor, mechanism and regeneration. Sep. Purif. Technol. 2023, 324, 124526. [Google Scholar] [CrossRef]
- Li, Y.L. Experimental investigation on ozone mass transfer coefficient enhanced by electric field in liquid phase. Adv. Mater. Res. 2013, 864–867, 2139–2144. [Google Scholar] [CrossRef]
- Wang, T.; Song, Y.; Ding, H.; Liu, Z.; Baldwin, A.; Wong, I.; Li, H.; Zhao, C. Insight into synergies between ozone and in-situ regenerated granular activated carbon particle electrodes in a three-dimensional electrochemical reactor for highly efficient nitrobenzene degradation. Chem. Eng. J. 2020, 394, 124852. [Google Scholar] [CrossRef]
- Zhan, J.; Li, Z.; Yu, G.; Pan, X.; Wang, J.; Zhu, W.; Han, X.; Wang, Y. Enhanced treatment of pharmaceutical wastewater by combining three-dimensional electrochemical process with ozonation to in situ regenerate granular activated carbon particle electrodes. Sep. Purif. Technol. 2019, 208, 12–18. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, N.; Feng, C.; Liu, Y.; Ma, N.; Deng, J.; Xing, L.; Gao, Y. Ozonation catalyzed by iron- and/or manganese-supported granular activated carbons for the treatment of phenol. Environ. Sci. Pollut. Res. 2019, 26, 21022–21033. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.; Zhu, S.; Sun, W.; Shah, K.J.; Zheng, H. Degradation of chloramphenicol using Ti-Sb/attapulgite ceramsite particle electrodes. Water Environ. Res. 2019, 91, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, C.; Hwang, I. Electrochemical degradation of ibuprofen using an activated-carbon-based continuous-flow three-dimensional electrode reactor (3DER). Chemosphere 2020, 259, 127382. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.; Chen, L.J.; Wu, Y.C.; Liu, R. Evaluation of the removal of antiestrogens and antiandrogens via ozone and granular activated carbon using bioassay and fluorescent. Chemosphere 2016, 153, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Cui, J.; Li, X.; Zhang, H.; Pei, Y. Electrochemical treatment of organic pollutants in landfill leachate using a three-dimensional electrode system. Chemosphere 2020, 243, 125438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Can, W.; Yao-Kun, H.; Qing, Z.; Min, J. Treatment of secondary effluent using a three-dimensional electrode system: COD removal, biotoxicity assessment, and disinfection effects. Chem. Eng. J. 2014, 243, 1–6. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Q.; Ni, J.; Xu, Y.; Song, N.; Gao, M. Highly efficient removal of amoxicillin from water by three-dimensional electrode system within granular activated carbon as particle electrode. J. Water Process. Eng. 2020, 38, 101656. [Google Scholar] [CrossRef]
- Real, F.J.; Acero, J.L.; Benitez, F.J.; Matamoros, E. Elimination of neonicotinoids by ozone-based advanced oxidation processes: Kinetics and performance in real water matrices. Sep. Purif. Technol. 2022, 301, 121975. [Google Scholar] [CrossRef]
- Fijołek, L.; Malaika, A.; Biernacki, W.; Nawrocki, J. H2O2 Generation during carbon ozonation-factors influencing the process and their implications. Ozone Sci. Eng. 2019, 41, 128–136. [Google Scholar] [CrossRef]
- Zhao, Q.; Ge, Y.; Zuo, P.; Shi, D.; Jia, S. Degradation of thiamethoxam in aqueous solution by ozonation: Influencing factors, intermediates, degradation mechanism and toxicity assessment. Chemosphere 2016, 146, 105–112. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Li, Y.; Xu, T.; Zhu, S. Continuous electrochemical oxidation of methyl orange waste water using a three-dimensional electrode reactor. J. Environ. Sci. 2011, 23, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.F.; Chedeville, O.; Cagnon, B.; Fauduet, H. Degradation kinetics of DEP in water by ozone/activated carbon process: Influence of pH. Desalination 2011, 269, 271–275. [Google Scholar] [CrossRef]
- Bhagawati, P.B.; Shivayogimath, C.B. Electrochemical technique for paper mill effluent degradation using concentric aluminum tube electrodes (CATE). J. Environ. Health Sci. Eng. 2021, 19, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Teng, Y.; Xu, Y.; Zheng, Y.; Zhang, Y.; Zhu, M.; Sun, Y. Ozone catalytic oxidation of biologically pretreated semi-coking wastewater (BPSCW) by spinel-type MnFe2O4 magnetic nanoparticles. Sep. Purif. Technol. 2021, 278, 118277. [Google Scholar] [CrossRef]
- Guan, Z.; Guo, Y.; Li, S.; Feng, S.; Deng, Y.; Ou, X.; Ren, J.; Sun, S.; Liang, J. Decomplexation of heterogeneous catalytic ozonation assisted with heavy metal chelation for advanced treatment of coordination complexes of Ni. Sci. Total Environ. 2020, 732, 139223. [Google Scholar] [CrossRef] [PubMed]
- Pourali, P.; Fazlzadeh, M.; Aaligadri, M.; Dargahi, A.; Poureshgh, Y.; Kakavandi, B. Enhanced three-dimensional electrochemical process using magnetic recoverable of Fe3O4@GAC towards furfural degradation and mineralization. Arab. J. Chem. 2022, 15, 103980. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Wang, B.; Cui, C.; Wang, S.; Wang, J.; Zhang, W. Enhanced degradation of quinoline in three-dimensional electro-Fenton system using catalytic Fe-Co-Ni-P/g-C3N4 particles. Int. J. Electrochem. Sci. 2022, 17, 221296. [Google Scholar] [CrossRef]
- Watanabe, E.; Baba, K.; Eun, H. Simultaneous determination of neonicotinoid insecticides in agricultural samples by solid-phase extraction cleanup and liquid chromatography equipped with diode-array detection. J. Agric. Food Chem. 2007, 55, 3798–3804. [Google Scholar] [CrossRef] [PubMed]
- Urzedo, A.P.F.M.; Diniz, M.E.R.; Nascentes, C.C.; Catharino, R.R.; Eberlin, M.N.; Augusti, R. Photolytic degradation of the insecticide thiamethoxam in aqueous medium monitored by direct infusion electrospray ionization mass spectrometry. J. Mass. Spectrom. 2007, 42, 1319–1325. [Google Scholar] [CrossRef]
- Watanabe, E. Review of sample preparation methods for chromatographic analysis of neonicotinoids in agricultural and environmental matrices: From classical to state-of-the-art methods. J. Chromatogr. A 2021, 1643, 462042. [Google Scholar] [CrossRef]
- Meijide, J.; Gómez, J.; Pazos, M.; Sanromán, M.A. Degradation of thiamethoxam by the synergetic effect between anodic oxidation and fenton reactions. J. Hazard. Mater. 2016, 319, 43–50. [Google Scholar] [CrossRef]
- Žabar, R.; Komel, T.; Fabjan, J.; Kralj, M.B.; Trebše, P. Photocatalytic degradation with immobilised TiO2 of three selected neonicotinoid insecticides: Imidacloprid, thiamethoxam and clothianidin. Chemosphere 2012, 89, 293–301. [Google Scholar] [CrossRef]
- Fenoll, J.; Garrido, I.; Hellín, P.; Flores, P.; Navarro, S. Photodegradation of neonicotinoid insecticides in water by semiconductor oxides. Environ. Sci. Pollut. Res. 2015, 22, 15055–15066. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V.; et al. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Mir, N.A.; Khan, A.; Muneer, M.; Vijayalakhsmi, S. Photocatalytic degradation of a widely used insecticide thiamethoxam in aqueous suspension of TiO2: Adsorption, kinetics, product analysis and toxicity assessment. Sci. Total Environ. 2013, 458–460, 388–398. [Google Scholar] [CrossRef]
- State Environmental Protection Administration of China. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 210–213. [Google Scholar]
- Najdanović, S.M.; Petrović, M.M.; Kostić, M.M.; Velinov, N.D.; Vučić, M.D.R.; Matović, B.Ž.; Bojić, A.L. New Way of Synthesis of Basic Bismuth Nitrate by Electrodeposition from Ethanol Solution: Characterization and Application for Removal of RB19 from Water. Arab. J. Sci. Eng. 2019, 44, 9939–9950. [Google Scholar] [CrossRef]




| Thiamethoxam Concentration (mg/dm3) | Order of Reaction | Reaction Equation | Rate Constant (k) | Correlation Coefficient | MRD (%) |
|---|---|---|---|---|---|
| 150 | Zero order | ct = −0.5492t + 161.2691 | 0.5492 | 0.6313 | 2.70 |
| First order | lnct = −0.0104t + 5.3555 | 0.0104 | 0.9295 | 0.47 | |
| Second order | 1/ct = 1.0834 × 10−4t + 0.0045 | 1.0834 × 10−4 | 0.9984 | 0.03 | |
| 250 | Zero order | ct = −0.9418t + 296.0871 | 0.9418 | 0.7898 | 4.20 |
| First order | lnct = −0.0073t + 5.8572 | 0.0073 | 0.9477 | 0.67 | |
| Second order | 1/ct =4.1482 × 10−5t + 0.0027 | 4.1482 × 10−5 | 0.9821 | 0.02 | |
| 500 | Zero order | ct = −1.9281t + 535.1329 | 1.9281 | 0.6943 | 4.48 |
| First order | lnct = −0.0111t + 6.5513 | 0.0111 | 0.9666 | 0.91 | |
| Second order | 1/ct = 3.5431 × 10−5t + 0.0014 | 3.5431 × 10−5 | 0.9925 | 0.15 | |
| 625 | Zero order | ct = −2.1841t + 752.8043 | 2.1841 | 0.8649 | 6.11 |
| First order | lnct = −0.0058t + 6.7424 | 0.0058 | 0.9767 | 1.05 | |
| Second order | 1/ct = 1.2178 × 10−5t + 0.0011 | 1.2178 × 10−5 | 0.9888 | 0.22 | |
| 750 | Zero order | ct = −2.6307t + 880.5286 | 2.6307 | 0.7759 | 7.37 |
| First order | lnct = −0.0066t + 6.9385 | 0.0066 | 0.9544 | 1.41 | |
| Second order | 1/ct = 1.2084 × 10−5t + 0.0009 | 1.2084 × 10−5 | 0.9987 | 0.36 |
| Number | Name | Chemical Formula | Structure | Mass Fragmentation Pattern (m/z) |
|---|---|---|---|---|
| 1 | Thiamethoxam | C8H12ClN5O3S |  | 247,212,182,132,99,73,58 |
| 2 | 3-((2-chlorothiazol-5-yl) methyl)-5-methyl-1,3,5- oxadiazinan-4-imine | C8H11CIN4OS | 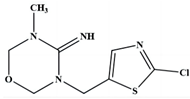 | 247,212,182,132,99,71,58 |
| 3 | 3-((2-chlorothiazol-5-yl) methyl)-1,3,5-oxadiazinan-4-imine | C7H9CIN4O2 |  | 232,175,147,141,132,97,71,58 |
| 4 | 1-(2-chlorothiazol-5-yl)-N-(iminomethylene) methanamine | C5H4CIN3S |  | 174,132,97,86,71,57 |
| 5 | 2,3-dihydro-4H-1,3,5- oxadiazin-4-imine | C3H5N3O | 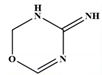 | 99,86,85,71,57,55 |
| 6 | 2-chloro-5-methylthiazole | C4H4CINS | 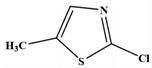 | 133,121,105,93,84,69 |
| 7 | 3-methyl-1,3,5- oxadiazinan-4-one | C4H8N2O2 | 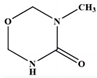 | 114,86,71,58,57 |
| 8 | 5-methylthiazol-2-ol | C4H5NOS |  | 115,84,71,69,57,51 |
| 9 | 5-methylthiazole | C4H5NS | 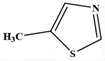 | 99,85,84,71,57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Xiao, J.; Jiang, X.; Su, J.; Chu, S.; Ma, X.; Li, J. Three-Dimensional Electrode-Enhanced Ozone Catalytic Oxidation for Thiamethoxam Wastewater Treatment: Performance, Kinetics, and Pathway. Catalysts 2024, 14, 245. https://doi.org/10.3390/catal14040245
Zhou W, Xiao J, Jiang X, Su J, Chu S, Ma X, Li J. Three-Dimensional Electrode-Enhanced Ozone Catalytic Oxidation for Thiamethoxam Wastewater Treatment: Performance, Kinetics, and Pathway. Catalysts. 2024; 14(4):245. https://doi.org/10.3390/catal14040245
Chicago/Turabian StyleZhou, Weijie, Jibo Xiao, Xiang Jiang, Jianchao Su, Shuyi Chu, Xiao Ma, and Jun Li. 2024. "Three-Dimensional Electrode-Enhanced Ozone Catalytic Oxidation for Thiamethoxam Wastewater Treatment: Performance, Kinetics, and Pathway" Catalysts 14, no. 4: 245. https://doi.org/10.3390/catal14040245








