Versatile Polyoxometalates of Different Structural Dimensionalities for Liquid Phase Catalytic Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Structures of the IPOM Catalysts
2.2. Catalytic Studies
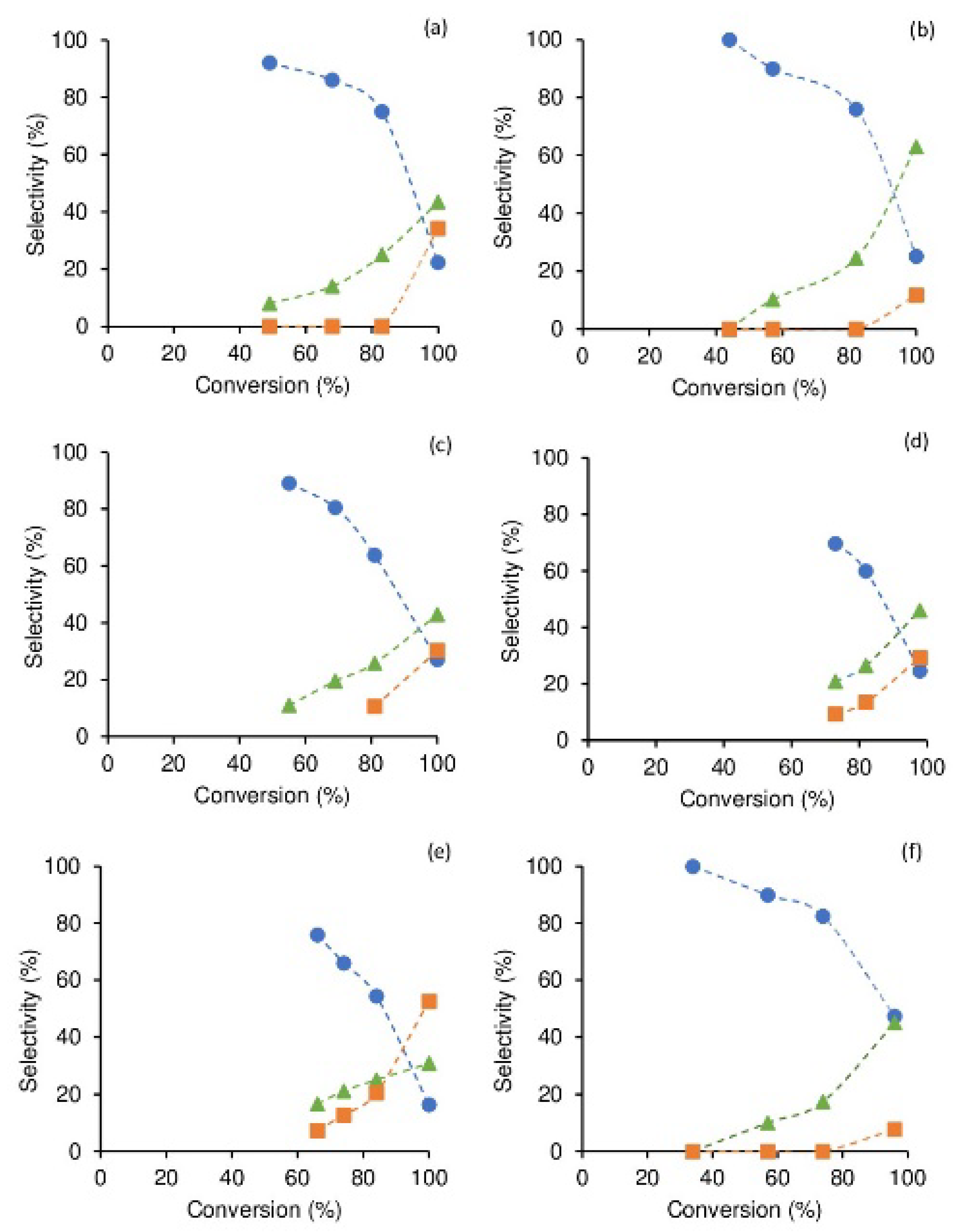
2.2.1. Olefin Epoxidation
Impact of IPOM Features
Biobased Olefin Epoxidation
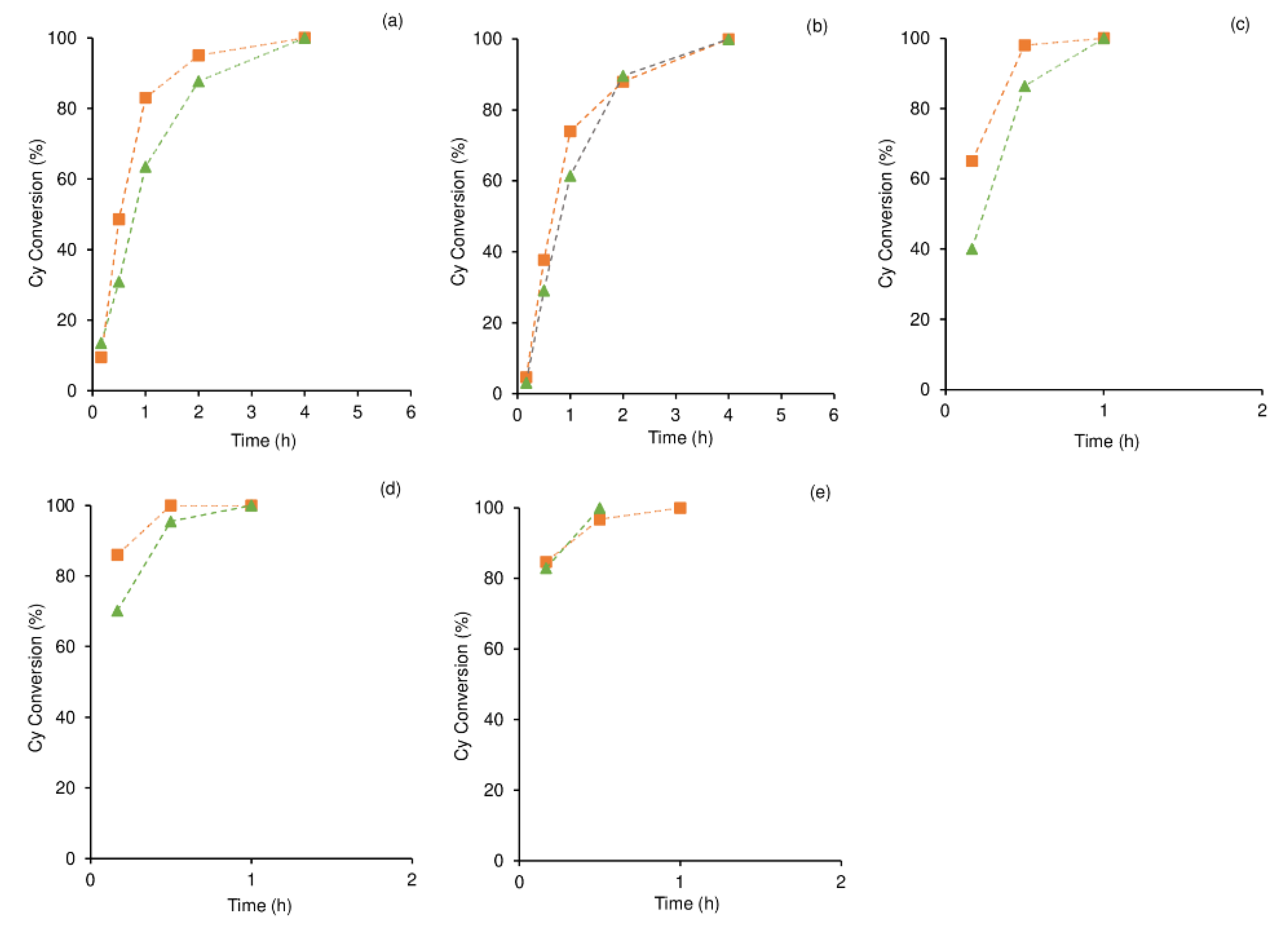
2.2.2. Cyclooctane Oxidation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the IPOM Compounds
3.3. Characterisation of the Compounds
3.4. Catalytic Tests
3.4.1. Olefin Epoxidation
3.4.2. Kinetic Modelling
3.4.3. Cyclooctane Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lligadas, G.; Ronda, J.C.; Galià, M.; Biermann, U.; Metzger, J.O. Synthesis and Characterization of Polyurethanes from Epoxidized Methyl Oleate Based Polyether Polyols as Renewable Resources. J. Polym. Sci. A Polym. Chem. 2006, 44, 634–645. [Google Scholar] [CrossRef]
- Musik, M.; Janus, E.; Pełech, R.; Sałaciński, Ł. Effective Epoxidation of Fatty Acid Methyl Esters with Hydrogen Peroxide by the Catalytic System H3PW12O40/Quaternary Phosphonium Salts. Catalysts 2021, 11, 1058. [Google Scholar] [CrossRef]
- Hájek, M.; Hájek, T.; Kocián, D.; Frolich, K.; Peller, A. Epoxidation of Methyl Esters as Valuable Biomolecules: Monitoring of Reaction. Molecules 2023, 28, 2819. [Google Scholar] [CrossRef]
- Sharma, B.K.; Doll, M.; Erhan, S.Z. Oxidation, Friction Reducing, and Low Temperature Properties of Epoxy Fatty Acid Methyl Esters. Green Chem. 2007, 9, 469–474. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Salley, S.O.; Ng, K.Y.S. Effects of Partial Hydrogenation, Epoxidation, and Hydroxylation on the Fuel Properties of Fatty Acid Methyl Esters. Eur. J. Lipid Sci. Technol. 2009, 90, 1292–1299. [Google Scholar] [CrossRef]
- Available online: https://polymer-additives.specialchem.com/selectors/tr-vikoflex (accessed on 21 December 2023).
- Turco, R.; Tesser, R.; Russo, V.; Vitiello, R.; Fagnano, M.; Di Serio, M. Comparison of Different Possible Technologies for Epoxidation of Cynara Cardunculus Seed Oil. Eur. J. Lipid Sci. Technol. 2020, 122, 1900100. [Google Scholar] [CrossRef]
- Available online: https://www.fortunebusinessinsights.com/epoxidized-soybean-oil-market-104343 (accessed on 21 December 2023).
- Meng, Y.; Taddeo, F.; Aguilera, A.F.; Cai, X.; Russo, V.; Tolvanen, P.; Leveneur, S. The Lord of the Chemical Rings: Catalytic Synthesis of Important Industrial Epoxide Compounds. Catalysts 2021, 11, 765. [Google Scholar] [CrossRef]
- Yan, W.; Wang, Z.; Luo, C.; Xia, X.; Liu, Z.; Zhao, Y.; Du, F.; Jin, X. Opportunities and Emerging Challenges of the Heterogeneous Metal-Based Catalysts for Vegetable Oil Epoxidation. ACS Sustain. Chem. Eng. 2022, 10, 7426–7446. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; Di Serio, M.; Esposito, R.; Melchiorre, M.; Ruffo, F.; Russo, V.; Tesser, R.; Turco, R.; Vitiello, R. Catalysis for Oleochemical Platforms. Eur. J. Inorg. Chem. 2023, 26, e202200783. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Noskov, A.S. Prospects for the Development of Catalysts for the Oxidation Processes of Advanced Propylene Processing. Catal. Ind. 2020, 12, 176–200. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Wai, P.T.; Gu, Q.; Zhang, W. Recent Progress in Application of Molybdenum-Based Catalysts for Epoxidation of Alkenes. Catalysts 2019, 9, 31. [Google Scholar] [CrossRef]
- Brégeault, J.M. Transition-Metal Complexes for Liquid-Phase Catalytic Oxidation: Some Aspects of Industrial Reactions and of Emerging Technologies. J. Chem. Soc. Dalton Trans. 2003, 3, 3289–3302. [Google Scholar] [CrossRef]
- Pisk, J.; Agustin, D. Molybdenum, Vanadium, and Tungsten-Based Catalysts for Sustainable (Ep)Oxidation. Molecules 2022, 27, 6011. [Google Scholar] [CrossRef]
- Bridgeman, A.J.; Cavigliasso, G. Electronic Structure of the α and β Isomers of [Mo8O26]4−. Inorg. Chem. 2002, 41, 3500–3507. [Google Scholar] [CrossRef]
- Available online: https://colapret.cm.utexas.edu/courses/320M%20Syllabus_Sp2019.pdf (accessed on 21 December 2023).
- Available online: https://openstax.org/books/organic-chemistry/pages/24-4-basicity-of-arylamines?query=cyclohexylammonium (accessed on 21 December 2023).
- Bożek, B.; Neves, P.; Valente, A.A. Ionic Ammonium and Anilinium Based Polymolybdate Hybrid Catalysts for Olefin Epoxidation. Appl. Catal. A Gen. 2018, 564, 13–25. [Google Scholar] [CrossRef]
- Zhou, M.-D.; Liu, M.-J.; Huang, L.-L.; Zhang, J.; Wang, J.-Y.; Li, X.-B.; Kühn, F.E.; Zang, S.-L. Olefin Epoxidation with Hydrogen Peroxide Using Octamolybdate-Based Self-Separating Catalysts. Green Chem. 2015, 17, 1186–1193. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.K.; Devi, M.; Gonsalves, K.E.; Pradeep, C.P. Engineering Multifunctionality in Hybrid Polyoxometalates: Aromatic Sulfonium Octamolybdates as Excellent Photochromic Materials and Self-Separating Catalysts for Epoxidation. Inorg. Chem. 2017, 56, 10325–10336. [Google Scholar] [CrossRef]
- Neves, P.; Amarante, T.R.; Valente, A.A.; Pillinger, M.; Gonçalves, I.S. Catalytic Application of an Octamolybdate Salt (H3biim)4[β-Mo8O26] in Olefin Epoxidation (H2biim = 2,2′-Biimidazole). Catal. Lett. 2016, 146, 841–850. [Google Scholar] [CrossRef]
- Gamelas, C.A.; Neves, P.; Gomes, A.C.; Valente, A.A.; Romão, C.C.; Gonçalves, I.S.; Pillinger, M. Molybdenum(II) Diiodo-Tricarbonyl Complexes Containing Nitrogen Donor Ligands as Catalyst Precursors for the Epoxidation of Methyl Oleate. Catal. Lett. 2012, 142, 1218–1224. [Google Scholar] [CrossRef]
- Available online: https://www.masterorganicchemistry.com/2017/04/18/basicity-of-amines-and-pkah/ (accessed on 21 December 2023).
- Kama, A.B.; Dessapt, R.; Serier-Brault, H.; Sidibe, M.; Diop, C.A.K.; Gautier, R. Stabilization of β-Octamolybdate with Large Counterions. J. Mol. Struct. 2017, 1141, 698–702. [Google Scholar] [CrossRef]
- Szyman, A.; Pamin, K.; Połtowicz, J. Molybdenum Complexes as Catalysts for the Oxidation of Cycloalkanes with Molecular Oxygen. Catal. Lett. 2016, 146, 998–1010. [Google Scholar] [CrossRef]
- Veiros, L.F.; Prazeres, A.; Costa, P.J.; Romão, C.C.; Kühn, F.E.; José Calhorda, M. Olefin Epoxidation with Tert-Butyl Hydroperoxide Catalyzed by MoO2X2L Complexes: A DFT Mechanistic Study. Dalton Trans. 2006, 11, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Comas-Vives, A.; Lledós, A.; Poli, R. A Computational Study of the Olefin Epoxidation Mechanism Catalyzed by Cyclopentadienyloxidomolybdenum(VI) Complexes. Chem. Eur. J. 2010, 16, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Al-Ajlouni, A.; Valerie, A.A.; Nunes, C.D.; Pillinger, M.; Santos, A.M.; Zhao, J.; Romão, C.C.; Gonçalves, I.S.; Kühn, F.E. Kinetics of Cyclooctene Epoxidation with tert-Butyl Hydroperoxide in the Presence of [MoO2X2L]-Type Catalysts (L = Bidentate Lewis Base). Eur. J. Inorg. Chem. 2005, 2005, 1716–1723. [Google Scholar] [CrossRef]
- Prazeres, A.; Santos, A.M.; Calhorda, M.J.; Roma, C.C.; Gonçalves, I.S. Octahedral Bipyridine and Bipyrimidine Dioxomolybdenum (VI) Complexes. Chem. Eur. J. 2002, 8, 2370–2383. [Google Scholar] [CrossRef]
- Garrido, G.; Rosés, M.; Ràfols, C.; Bosch, E. Acidity of Several Anilinium Derivatives in Pure Tetrahydrofuran. J. Solut. Chem. 2008, 37, 689–700. [Google Scholar] [CrossRef]
- Neves, P.; Gomes, A.C.; Cunha-Silva, L.; Valente, A.A.; Pillinger, M.; Gonçalves, I.S. Silicododecamolybdate/Pyridinium-Tetrazole Hybrid Molecular Salt as a Catalyst for the Epoxidation of Bio-Derived Olefins. Inorg. Chim. Acta A 2021, 516, 120129. [Google Scholar] [CrossRef]
- Nunes, M.S.; Gomes, A.C.; Neves, P.; Mendes, R.F.; Almeida Paz, F.A.; Lopes, A.D.; Pillinger, M.; Gonçalves, I.S.; Valente, A.A. Molybdenum(VI) Complexes with Ligands Derived from 5-(2-Pyridyl)-2H-Tetrazole as Catalysts for the Epoxidation of Olefins. Catal. Today 2023, 423, 114273. [Google Scholar] [CrossRef]
- Herman, K.; Foodtechn, J. Catalytic Epoxidation of Methyl Linoleate-Cyclisation Products of Epoxyacid Esters. Fat. Sci. Technol. 1995, 97, 269–273. [Google Scholar]
- Dorado, V.; Herrerías, C.I.; Fraile, J.M. Catalytic Hydrolysis of Epoxyfatty Esters with Solid Sulfonic Acids. Mol. Catal. 2023, 547, 113282. [Google Scholar] [CrossRef]
- Piazza, G.J.; Nuñz, A.; Foglia, T.A. Hydrolysis of Mono- and Diepoxyoctadecanoates by Alumina. J. Am. Oil Chem. Soc. 2003, 80, 901–904. [Google Scholar] [CrossRef]
- Bantchev, G.B.; Doll, K.M.; Biresaw, G.; Vermillion, K.E. Formation of Furan Fatty Alkyl Esters from Their Bis-Epoxide Fatty Esters. J. Am. Oil Chem. Soc. 2014, 91, 2117–2123. [Google Scholar] [CrossRef]
- Stolp, L.J.; Joseph, E.; Kodali, D.R. Synthesis and Evaluation of Soy Fatty Acid Ester Estolides as Bioplasticizers in Poly(Vinyl Chloride). J. Am. Oil Chem. Soc. 2019, 96, 1291–1302. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Hay, M.B. Recent Advances in the Stereoselective Synthesis of Tetrahydrofurans. Tetrahedron 2007, 63, 261–290. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Barrow, R.A. Acid-Mediated Conversion of Methylene-Interrupted Bisepoxides to Tetrahydrofurans: A Biomimetic Transformation. J. Org. Chem. 1998, 63, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Araji, N.; Chatel, G.; Moores, A.; Jérôme, F.; De Oliveira Vigier, K. Oxidative Cyclization of Linoleic Acid in the Presence of Hydrogen Peroxide and Phosphotungstic Acid. Mol. Catal. 2020, 493, 111084. [Google Scholar] [CrossRef]
- Oszajca, M.; Nitek, W.; Rafalska-Łasocha, A.; Pamin, K.; Połtowicz, J.; Łasocha, W. Synthesis, Crystal Structure and Selected Properties of Three New 4-Propylanilinium Polyoxomolybdates. J. Mol. Struct. 2023, 1273, 134292. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A Kit of Tools for Phasing Crystal Structures from Powder Data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Cerný, R. FOX, ‘free Objects for Crystallography’: A Modular Approach to Ab Initio Structure Determination from Powder Diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. The Crystallographic Computing System; Institute of Physics: Jana, Czech Republic, 2006. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond Version 3.2g, Crystal Impact GbR, Bonn, Germany, 1997–2001. Available online: http://www.crystalimpact.com/diamond (accessed on 4 January 2023).
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Bożek, B.; Neves, P.; Oszajca, M.; Valente, A.A.; Połtowicz, J.; Pamin, K.; Łasocha, W. Simple Hybrids Based on Mo or W Oxides and Diamines: Structure Determination and Catalytic Properties. Catal. Lett. 2020, 150, 713–727. [Google Scholar] [CrossRef]
- Pamin, K.; Jachimska, B.; Onik, K.; Połtowicz, J.; Grabowski, R. Electrostatic Self-Assembly of Polyoxometalates on Chitosan as Catalysts of Oxidation of Cyclic Hydrocarbons. Catal. Lett. 2009, 127, 167–174. [Google Scholar] [CrossRef]




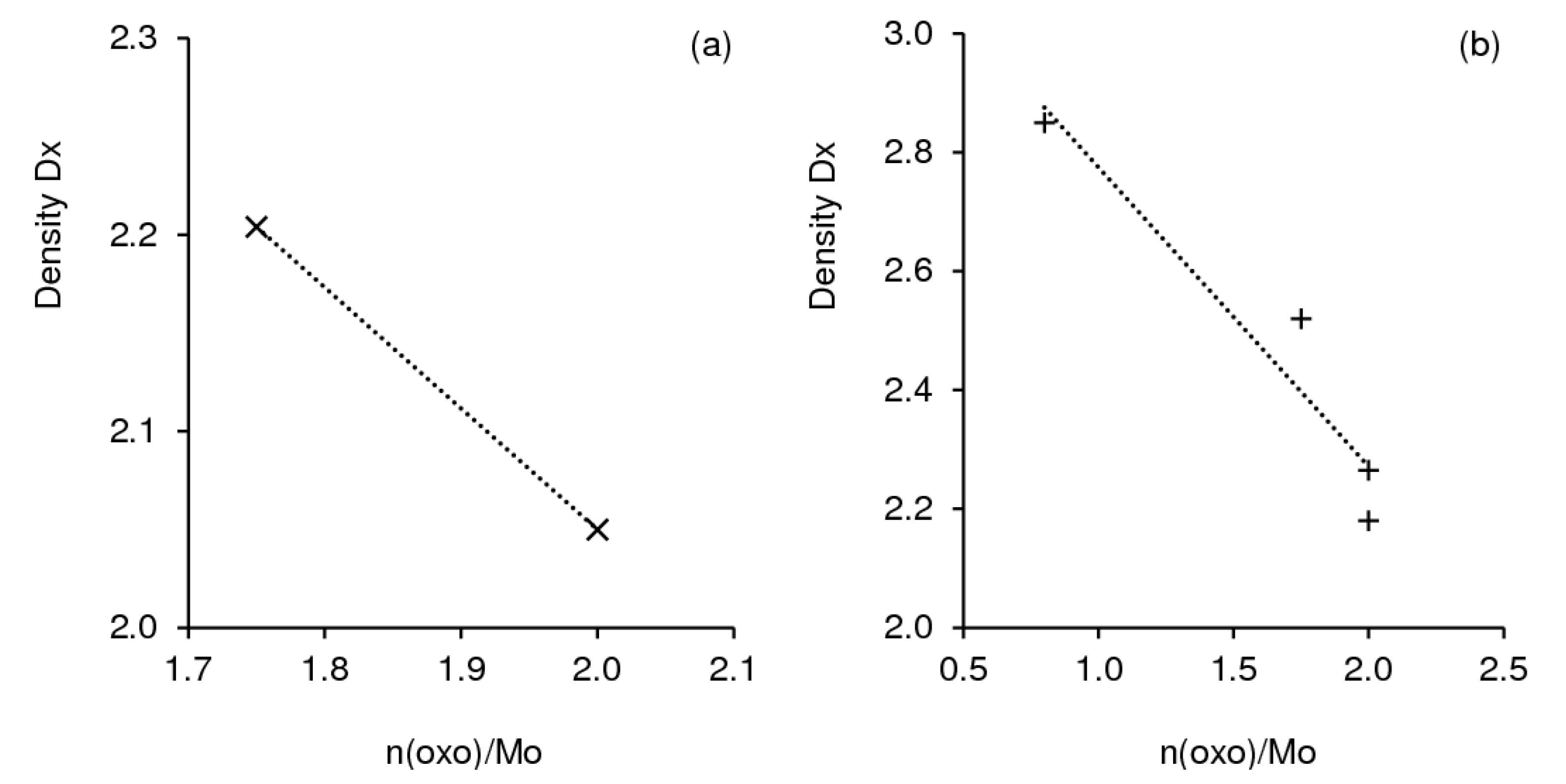




| Chemical Name | Chemical Formula | Structural Features | Dim a | Dx/ DC b | n(oxo)/Mo c | |
|---|---|---|---|---|---|---|
| 1 | Cyclohexylammonium β-octamolybdate dihydrate | (C6H14N)4[Mo8O26]∙2H2O | isolated clusters | 0-D | 2.264/ 4 | 1.75 |
| 2 | Cyclohexylammonium trimolybdate hydrate | (C6H14N)2[Mo3O10]∙H2O | polymeric | 1-D | 2.056/ 3 | 2 |
| 3 | Anilinium β-octamolybdate dihydrate | (C6H8N)4[Mo8O26]∙2H2O | isolated clusters | 0-D | 2.520/ 4 | 1.75 |
| 4 | Anilinium trimolybdate tetrahydrate | (C6H8N)2[Mo3O10]∙4H2O | polymeric | 1-D | 2.180/ 3 | 2 |
| 5 | Anilinium trimolybdate dihydrate | (C6H8N)2[Mo3O10]∙2H2O | polymeric | 1-D | 2.399/ 3 | 2 |
| 6 | Anilinium pentamolybdate | (C6H8N)2[Mo5O16] | layered | 2-D | 2.850/ 5 | 0.8 |
| Compound (XRD Technique) | 1 (Single Crystals) | 2 (Powder Data) | 5 (Powder Data) |
|---|---|---|---|
| Chemical formula | C24H60Mo8N4O28 | C12H30Mo3N2O11 | C12H20Mo3N2O12 |
| MW (g/mol) | 1620.27 | 666.20 | 672.12 |
| T(K) | 100 | 293(2) | 293(2) |
| Wavelength, [Å] | MoKα: 0.71073 | CuKα: 1.54187 | CuKα: 1.54187 |
| Crystal system, space group | triclinic, P-1 | Orthorhombic, P nma | Monoclinic, P 21/c |
| Cell parameters | |||
| a [Å] | 8.7255(2) | 8.763(3) | 14.711 |
| b [Å] | 10.5278(2) | 7.645(3) | 7.578 |
| c [Å] | 14.4778(4) | 32.114(4) | 17.852 |
| α [°] | 75.019(2) | 90 | 90 |
| β [°] | 76.273(2) | 90 | 110.81 |
| γ [°] | 69.550(2) | 90 | 90 |
| V (Å3) | 1187.79(5) | 2151.4(4) | 1860.31 |
| Z, calculated density (g/cm3) | 1, 2.264 | 4, 2.056 | 4, 2.399 |
| Absorption coefficient (mm−1) | 12.775 | 14.627 | 16.959 na |
| F(000) | 792 | 1200 | 996 |
| 2Theta range | 5.04–31.9363.86 | 3.5–80 | 3.5–70 |
| Limiting indices | −12 ≤ h ≤ 12; −15 ≤ k ≤ 15; −20 ≤ l ≤ 21 | 7; 6; 26 | 10; 5; 13 |
| Reflections collected | 7841 | 524 | 526 |
| Completeness to theta(max) | 95.5% | 100% (xrpd) | 100% (xrpd) |
| Absorption correction | Multi-scan | const. (Bragg-Brentano geom.) | const. (Bragg-Brentano geom.) |
| Maximum and minimum transmission | n.a. (powder sample) | ||
| Refinement method | wR2 | Rietveld | Rietveld |
| Data/restraints/parameters | 7841/0/321 | 3824/36/25 | 3325/30/20 |
| Goodness of fit on F2 | 1.23 | 8 | 11 |
| Final R indices wR2 (I > 2σ) or Rp/Rwp | 8.70 | 22.8/26.0 | 15.1/20.9 |
| RF (all data) | 3.15 | 14.29 | 19.11 |
| Largest difference peak and hole (eA−3) | 1.612/−1.447 | PD | PD |
| CCDC | 2,320,511 | PD−1 | PD−1 |
| ki (h−1) | Reaction | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| kCy | Cy-CyO | 1.3989 | 1.0774 | 6.3279 | 11.5946 | 10.9802 | 0.5565 |
| Fobj | 4.05 × 10−2 | 4.70 × 10−2 | 1.20 × 10−3 | 2.00 × 10−5 | 1.60 × 10−3 | 1.88 × 10−2 | |
| kOle | Ole-OleOx | 0.5356 | 0.3002 | 2.4035 | 3.1423 | 2.4031 | 0.3149 |
| Fobj | 5.61 × 10−2 | 2.27 × 10−2 | 4.26 × 10−4 | 3.33 × 10−6 | 6.24 × 10−4 | 2.76 × 10−2 | |
| kLin,1 | Lin-LinOx | 0.5827 | 0.4767 | 0.6392 | 0.7294 | 0.7607 | 0.3957 |
| kLin,2 | LinOx-LinDiOx | 0.1262 | 0.1087 | 0.1888 | 0.2248 | 0.2191 | 0.0893 |
| kLin,3 | LinDiOx-LinFur | 0.0594 | 0.0013 | 0.2527 | 0.2811 | 0.2594 | 0.0647 |
| Fobj | 5.20 × 10−2 | 7.20 × 10−2 | 1.36 × 10−2 | 7.48 × 10−2 | 6.70 × 10−2 | 7.00 × 10−2 |
| # | Catalyst | Dim. b | t (h) c | Conv., CyO yield (%) d | Ref. |
|---|---|---|---|---|---|
| 1 | 1 | 0-D | 1/2/4 | 83/95/100 | - |
| 2 | 2 | 1-D | 1/2/4 | 74/88/100 | - |
| 3 | 3 | 0-D | 0.5/1 | 98/100 | - |
| 4 | 4 | 1-D | 0.5 | 100 | - |
| 5 | 5 | 1-D | 0.5/1 | 97/100 | - |
| 6 | 6 | 2-D | 1/4 | 48/86 | [19] |
| 7 | (C8H12N)2[Mo3O10] | 1-D | 1/6 | 97/100 | [19] |
| 8 | (C7H10N)2[Mo3O10] | 1-D | 1 | 100 | [19] |
| 9 | (C8H12N)2[Mo5O16] | 2-D | 1/6 | 54/98 | [19] |
| 10 | (C7H10N)2[Mo5O16] | 2-D | 1/6 | 40/94 | [19] |
| 11 | (C8H12N)2[Mo5O16] | 2-D | 1/6 | 44/92 | [19] |
| # | Catalyst a | Dim. b | Reaction Conditions c | Conv. d (%) | OleOx Yield (%) | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| Solv. | T (°C) | Mo:S:ox | t (h) | ||||||
| 1 | 1 | 0-D | TFT | 70 | 1:100:300 | ½/4 | 22/76/98 | 22/74/96 | - |
| 2 | 2 | 1-D | TFT | 70 | 1:100:300 | ½/4 | 14/42/80 | 14/42/80 | - |
| 3 | 3 | 0-D | TFT | 70 | 1:100:300 | ½/4 | 91/97/100 | 91/97/100 | - |
| 4 | 4 | 1-D | TFT | 70 | 1:100:300 | ½ | 95/100 | 95/100 | - |
| 5 | 5 | 1-D | TFT | 70 | 1:100:300 | ½/4 | 92/97/98 | 92/97/98 | - |
| 6 | 6 | 2-D | TFT | 70 | 1:100:300 | ½/4/24 | 14/43/83/100 | 14/43/83/96 | - |
| 7 | (C8H12N)2[Mo3O10] | 1-D | TFT | 70 | 1:100:153 | 1/6/24 | 70/89/99 | 66/84/92 | [19] |
| 8 | (Hptz)4[SiMo12O40]∙nH2O | 0-D | TFT | 70 | 1:100:250 | 1/6/24 | 56/88/100 | 56/88/100 | [32] |
| 9 | (tBu-ptz)2[Mo6O19] | 0-D | TFT | 70 | 1:100:210 | 0.5/3 | 73/97 | 73/97 | [33] |
| 10 | (py)4[Mo8O26] | 0-D | DCE | 75 | 1:100:152 | 2/6 | ~97/100 | ~97/99 | [23] |
| 11 | (tBu-py)4[Mo8O26] | 0-D | DCE | 75 | 1:100:152 | 2/6 | ~97/100 | ~97/99 | [23] |
| Catalyst a | Reaction Conditions b | Conv. (%) c | Selectivity (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Solv.; T (°C) | Mo:S:ox | t (h) | LinOx | LinDiOx | LinFur | |||
| 1 | TFT; 70 | 1:100:300 | 1/4/24 | 49/83/100 | 92/75/23 | 8/25/43 | 0/0/34 | - |
| 2 | TFT; 70 | 1:100:300 | 1/4/24 | 44/82/100 | 100/76/25 | 0/24/63 | 0/0/12 | - |
| 3 | TFT; 70 | 1:100:300 | 1/4/24 | 55/81/100 | 89/64/27 | 11/25/43 | 0/11/30 | - |
| 4 | TFT; 70 | 1:100:300 | 1/4/24 | 64/82/98 | 77/60/25 | 18/27/46 | 5/13/29 | - |
| 5 | TFT; 70 | 1:100:300 | 1/4/24 | 66/84/100 | 76/54/16 | 17/25/31 | 7/21/53 | - |
| 6 | TFT; 70 | 1:100:300 | 1/4/24 | 34/74/96 | 100/82/47 | 0/18/45 | 0/0/8 | - |
| (C8H12N)2[Mo3O10] | TFT; 70 | 1:100:250 | 24 | 95 | 41 | 41 | 17 | [19] |
| [tBu-Hptz]2[Mo6O19] | TFT; 70 | 1:100:210 | 0.5/24 | 36/85 | 100/83 | 0/17 | 0 | [33] |
| [tBu-Hptz]2[Mo6O19] | TFT; 90 | 1:100:210 | 0.5/2 | 80/100 | 81/63 | 19/37 | 0 | [33] |
| (Hptz)4[SiMo12O40]∙nH2O | TFT; 70 | 1:100:250 | 1/6/24 | 45/87/100 | 100/60/28 | 0/31/33 | 0/9/40 | [32] |
| Catalyst | Dim | Ketone Yield (A) (%) | Alcohol Yield (B) (%) | Suberic Acid Yield (C) (%) | Conv. b (%) | A/B | (A + B)/(A + B + C) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 0-D | 35 | 13 | 8 | 56 | 2.29 | 0.86 | - |
| 2 | 1-D | 41 | 13 | 12 | 66 | 3.15 | 0.82 | - |
| 3 | 0-D | 37 | 14 | 13 | 64 | 2.64 | 0.80 | - |
| 4 | 1-D | 41 | 15 | 11 | 67 | 2.73 | 0.83 | - |
| 5 | 1-D | 39 | 14 | 11 | 64 | 2.79 | 0.83 | - |
| 6 | 2-D | 27 | 11 | 8 | 46 | 2.45 | 0.83 | - |
| (C7H10N)4[Mo8O26]∙2H2O | 0-D | 11 | 9 | 0 | 20 | 1.14 | 1.00 | [26] |
| (C8H12N)2[Mo3O10] | 1-D | 30 | 16 | 0 | 46 | 1.91 | 1.00 | [26] |
| (C7H10N)2[Mo3O10] | 1-D | 26 | 16 | 0 | 42 | 1.61 | 1.00 | [26] |
| (C7H10N)2[Mo5O16] | 2-D | 34 | 11 | ~5 | ~50 | 3.16 | ~0.90 | [26] |
| (C6H7NBr)2[Mo5O16] | 2-D | 34 | 11 | ~4 | ~49 | 3.21 | ~0.92 | [26] |
| (C8H12N)2[Mo5O16] | 2-D | 23 | 15 | ~2 | ~41 | 1.53 | ~0.95 | [26] |
| (C9H14N)2[Mo5O16] | 2-D | 29 | 15 | 5 | 49 | 1.97 | 0.91 | [42] |
| (C9H14N)4[Mo8O26]∙ H2O | 0-D | 30 | 18 | 0 | 48 | 1.69 | 1.00 | [42] |
| (C9H14N)6[Mo8O26(C2H3O2)2] | 0-D | 28 | 17 | 0 | 45 | 1.66 | 1.00 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, P.; Simões, G.; Napruszewska, B.D.; Pamin, K.; Serda, P.; Łasocha, W.; Valente, A.A. Versatile Polyoxometalates of Different Structural Dimensionalities for Liquid Phase Catalytic Oxidation. Catalysts 2024, 14, 251. https://doi.org/10.3390/catal14040251
Neves P, Simões G, Napruszewska BD, Pamin K, Serda P, Łasocha W, Valente AA. Versatile Polyoxometalates of Different Structural Dimensionalities for Liquid Phase Catalytic Oxidation. Catalysts. 2024; 14(4):251. https://doi.org/10.3390/catal14040251
Chicago/Turabian StyleNeves, Patrícia, Guilherme Simões, Bogna D. Napruszewska, Katarzyna Pamin, Paweł Serda, Wieslaw Łasocha, and Anabela A. Valente. 2024. "Versatile Polyoxometalates of Different Structural Dimensionalities for Liquid Phase Catalytic Oxidation" Catalysts 14, no. 4: 251. https://doi.org/10.3390/catal14040251
APA StyleNeves, P., Simões, G., Napruszewska, B. D., Pamin, K., Serda, P., Łasocha, W., & Valente, A. A. (2024). Versatile Polyoxometalates of Different Structural Dimensionalities for Liquid Phase Catalytic Oxidation. Catalysts, 14(4), 251. https://doi.org/10.3390/catal14040251