Visible-Light-Photocatalyzed C5-H Nitration of 8-Aminoquinoline Amides
Abstract
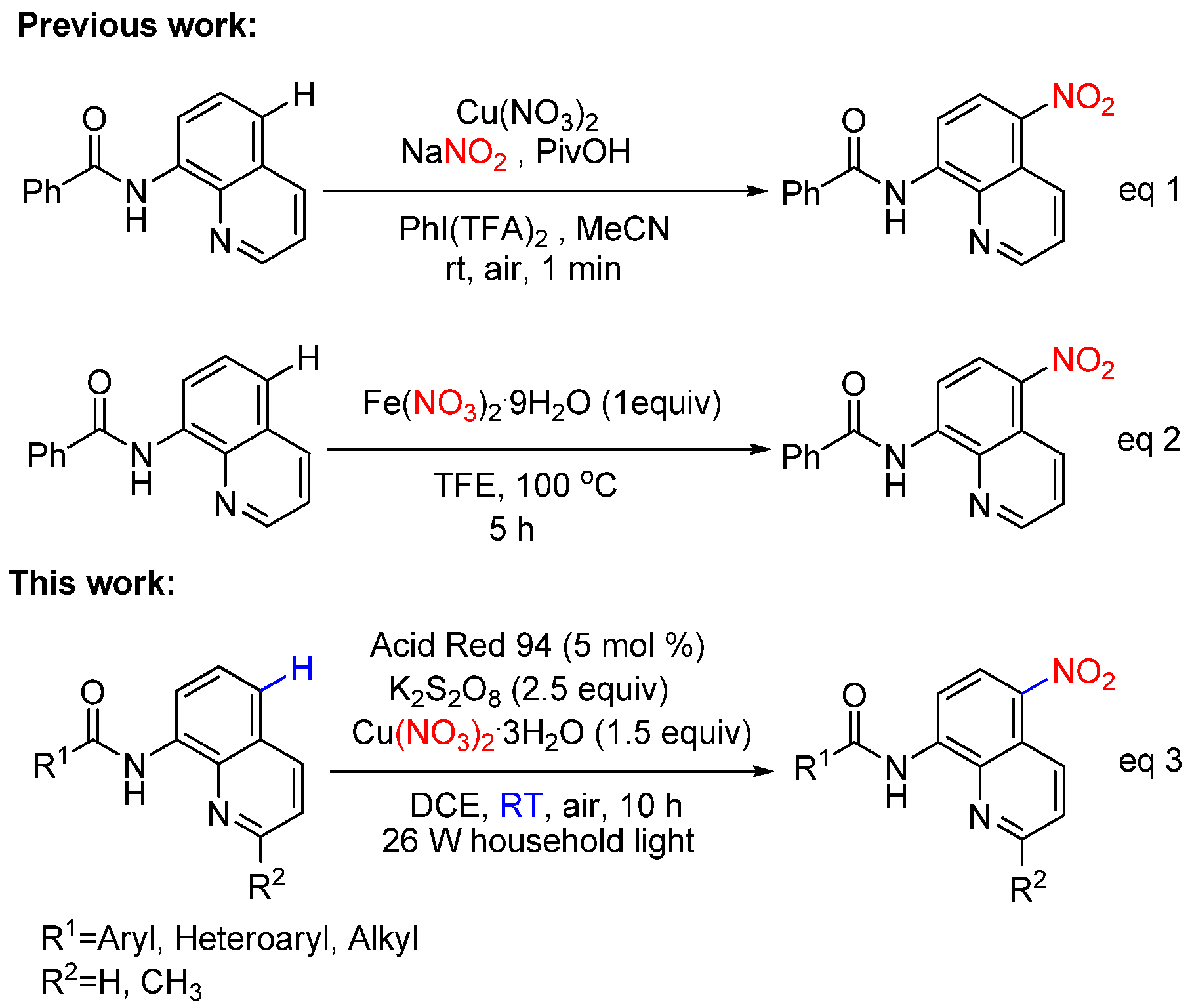
:1. Introduction
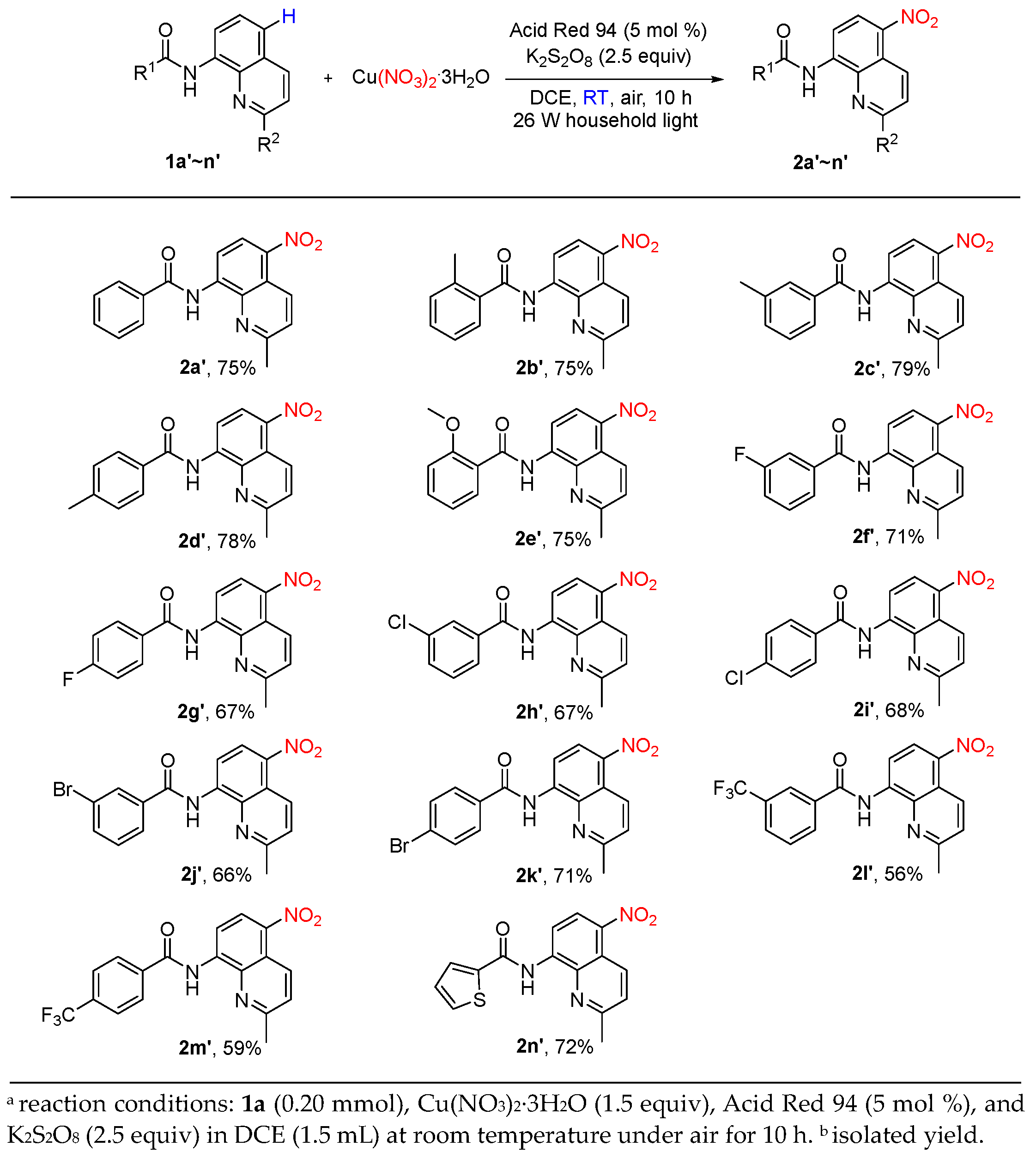
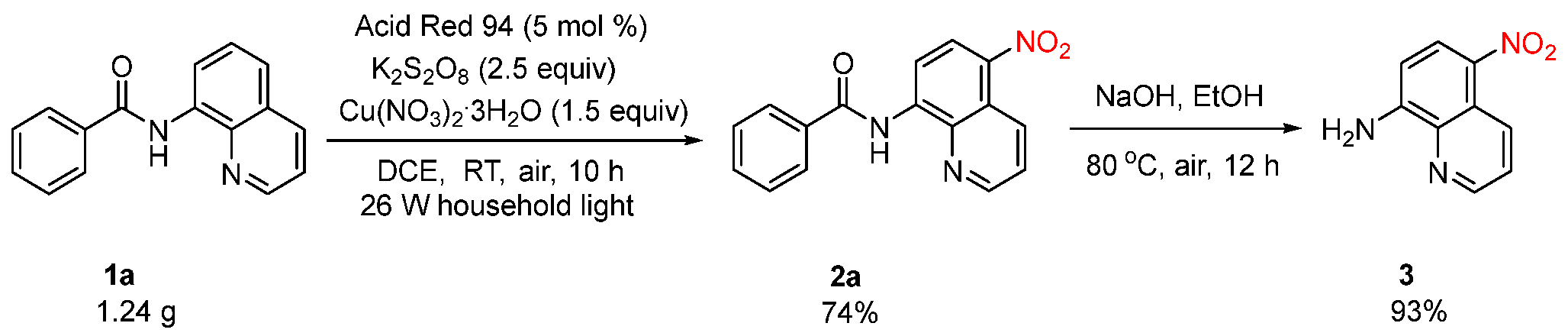
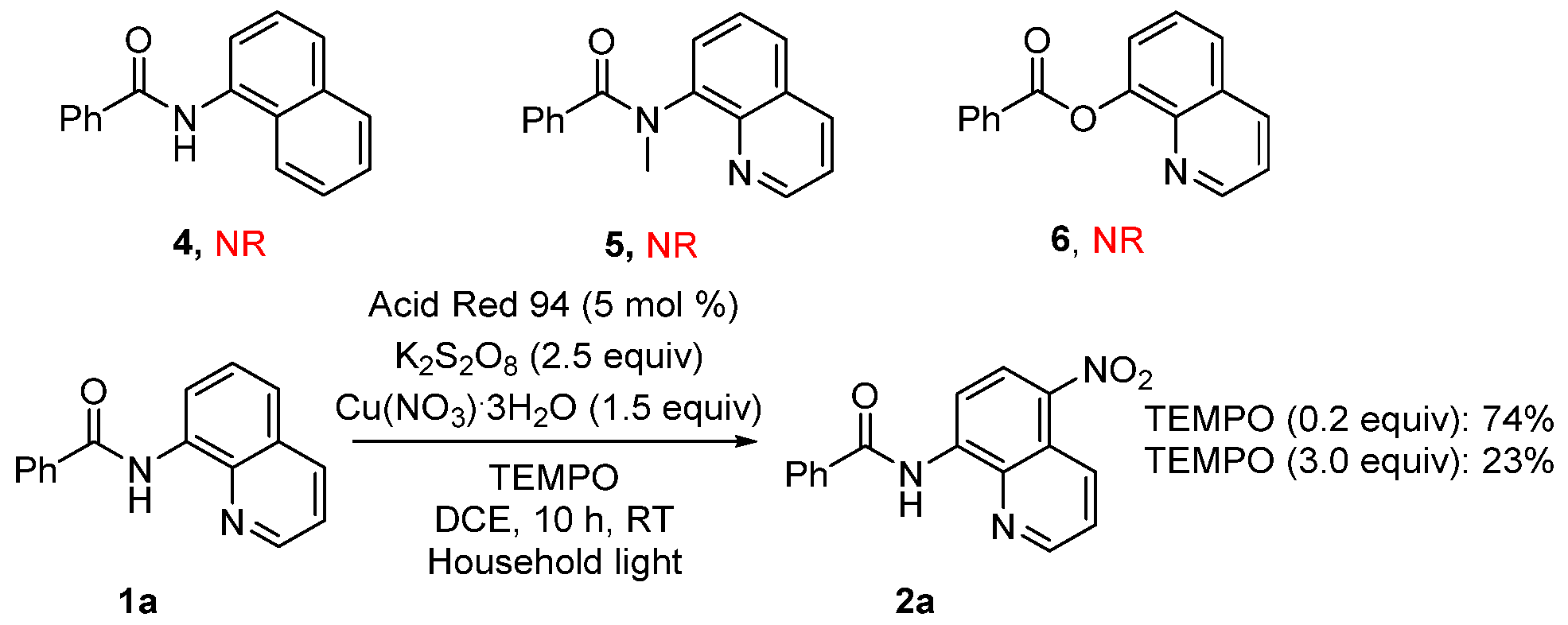
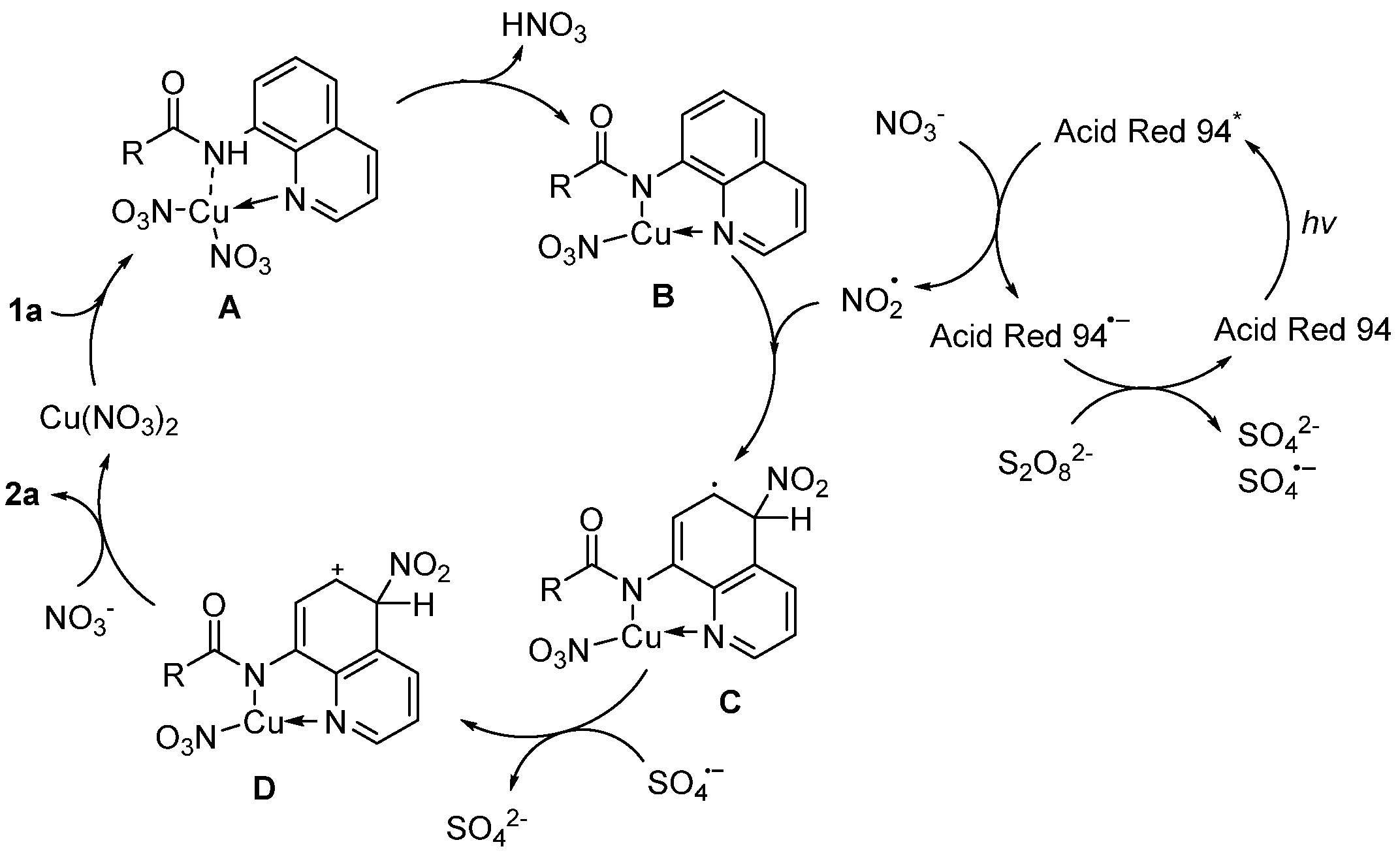
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. General Information
4.2. Typical Procedure for Synthesizing the Catalytic C5-H Nitration of 8-Aminoquinoline Amides
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feuer, H.; Nielsen, A.T. Nitro Compounds: Recent Advances in Synthesis and Chemistry; Wiley-VCH: Weinheim, Germany, 1990. [Google Scholar]
- Ono, N. The Nitro Group in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Feldman, M.; Wheeler, J.W.J. Nitration of benzoic acid: Determination of isomer distribution by the isotope dilution technique. Chem. Educ. 1967, 44, 464. [Google Scholar] [CrossRef]
- Olah, G.A.; Malhotra, R.; Narang, S.C. Nitration: Methods and Mechanisms; VCH: New York, NY, USA, 1989. [Google Scholar]
- Manna, S.; Maity, S.; Rana, S.; Agasti, S.; Maiti, D. ipso-Nitration of Arylboronic Acids with Bismuth Nitrate and Perdisulfate. Org. Lett. 2012, 14, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Panja, C.; Mathew, T.; Surampudi, V.; Petasis, N.A.; Olah, G.A. ipso-Nitration of Arylboronic Acids with Chlorotrimethylsilane-Nitrate Salts. Org. Lett. 2004, 6, 2205–2207. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Koizumi, Y. Copper-catalyzed coupling of aryl halides and nitrite salts: A mild Ullmann-type synthesis of aromatic nitro compounds. Tetrahedron Lett. 2005, 46, 4715–4717. [Google Scholar] [CrossRef]
- Fors, B.P.; Buchwald, S.L.J. Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics. Am. Chem. Soc. 2009, 131, 12898–12899. [Google Scholar] [CrossRef]
- Natarajan, P.; Chaudhary, R.; Venugopalan, P.J. Silver(I)-Promoted ipso-Nitration of Carboxylic Acids by Nitronium Tetrafluoroborate. Org. Chem. 2015, 80, 10498–10504. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Carmeli, M.J. From azides to nitro compounds in a few seconds using HOF·CH3CN. Am. Chem. Soc. 2003, 125, 8118–8119. [Google Scholar] [CrossRef]
- Reddy, K.R.; Maheswari, C.U.; Venkateshwar, M. Selective Oxidation of Aromatic Amines to Nitro Derivatives using Potassium Iodide-tert-Butyl Hydroperoxide Catalytic System. Adv. Synth. Catal. 2009, 351, 93–96. [Google Scholar] [CrossRef]
- Mkhalid, I.A.I.; Barnard, J.H.; Marder, T.B.; Murphy, J.M.; Hartwig, J.F. C–H Activation for the Construction of C–B Bonds. Chem. Rev. 2010, 110, 890–931. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.A.; Bergman, R.G.; Ellman, J.A. Rhodium-Catalyzed C–C Bond Formation via Heteroatom-Directed C–H Bond Activation. Chem. Rev. 2010, 110, 624–655. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, P.; Taylor, R.J.K.; Fairlamb, I.J.S. Emergence of Palladium(IV) Chemistry in Synthesis and Catalysis. Chem. Rev. 2010, 110, 824–889. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-M.; Li, B.-J.; Yang, Z.; Shi, Z.-J. Organopalladium(iv) chemistry. Chem. Soc. Rev. 2010, 39, 712–733. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, C.; Lei, A. Transition-metal catalyzed oxidative cross-coupling reactions to form C–C bonds involving organometallic reagents as nucleophiles. Chem. Soc. Rev. 2011, 40, 2761–2776. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Wang, F.; Li, X. C–C, C–O and C–N bond formation via rhodium(iii)-catalyzed oxidative C–H activation. Chem. Soc. Rev. 2012, 41, 3651–3678. [Google Scholar] [CrossRef] [PubMed]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-Catalyzed C–H Bond Activation and Functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L. Carboxylate-Assisted Ruthenium-Catalyzed Alkyne Annulations by C–H/Het–H Bond Functionalizations. Acc. Chem. Res. 2014, 47, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Yang, L.; Xia, C.-G.; Li, F.-W. Nickel-Catalyzed Alkynylation of a C(sp2)–H Bond Directed by an 8-Aminoquinoline Moiety. J. Org. Chem. 2015, 80, 6213–6221. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.-C.; Wei, Z.-J.; Shen, C.; Xu, J.; Yang, Y.; Su, W.-K.; Zhang, P.-F. Palladium-catalyzed direct ortho-sulfonylation of azobenzenes with arylsulfonyl chlorides via C–H activation. RSC Adv. 2015, 5, 52588–52594. [Google Scholar] [CrossRef]
- Pawar, G.G.; Brahmanandan, A.; Kapur, M. Palladium(II)-Catalyzed, Heteroatom-Directed, Regioselective C–H Nitration of Anilines Using Pyrimidine as a Removable Directing Group. Org. Lett. 2016, 18, 448–451. [Google Scholar] [CrossRef]
- Suess, A.M.; Ertem, M.Z.; Cramer, C.J.; Stahl, S.S.J. Divergence between Organometallic and Single-Electron-Transfer Mechanisms in Copper(II)-Mediated Aerobic C–H Oxidation. Am. Chem. Soc. 2013, 135, 9797–9804. [Google Scholar] [CrossRef]
- Wang, Z.; Kuninobu, Y.; Kanai, M. Copper-Mediated Direct C(sp3)–H and C(sp2)–H Acetoxylation. Org. Lett. 2014, 16, 4790–4793. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zeng, X. Iron-Catalyzed, Chelation-Induced Remote C–H Allylation of Quinolines via 8-Amido Assistance. Org. Lett. 2014, 16, 3716–3719. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-W.; Jiang, K.; Ding, W.; Yuan, Y.; Shuai, L.; Chen, Y.-C.; Wei, Y. Selective remote C–H sulfonylation of aminoquinolines with arylsulfonyl chlorides via copper catalysis. Chem. Commun. 2015, 51, 16928–16931. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-Z.; Qiu, R.-H.; Cao, X.; Xiao, S.; Xu, X.-H.; Au, C.-T.; Yin, S.-F. Copper-Mediated Remote C–H Bond Chalcogenation of Quinolines on the C5 Position. Org. Lett. 2015, 17, 5528–5531. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.-J.; Sun, S.-Y.; Yang, F.; Zhu, Y.; Zhu, W.-G.; Dong, Y.-X.; Wu, Y.-S.; Kong, X.-T.; Jiang, L.; Wu, Y.-J. Copper(I)-Catalyzed Sulfonylation of 8-Aminoquinoline Amides with Sulfonyl Chlorides in Air. Org. Lett. 2015, 17, 6086–6089. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-M.; Sun, S.-Y.; Qiao, H.-J.; Yang, F.; Zhu, Y.; Kang, J.-X.; Wu, Y.-S.; Wu, Y.-J. Silver (i)-promoted C5–H phosphonation of 8-aminoquinoline amides with H-phosphonates. Org. Chem. Front. 2016, 3, 1646–1650. [Google Scholar] [CrossRef]
- Wei, J.; Jiang, J.; Xiao, X.; Lin, D.; Deng, Y.; Ke, Z.; Jiang, H.; Zeng, W.J. Copper-Catalyzed Regioselective C–H Sulfonylation of 8-Aminoquinolines. Org. Chem. 2016, 81, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, P.; Wang, M.; Wang, L. Nickel-Catalyzed Site-Selective C–H Bond Difluoroalkylation of 8-Aminoquinolines on the C5-Position. Org. Lett. 2016, 18, 4794–4797. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H.; Reddy, M.K.; Ramakrishna, I.; Baidya, M. Copper-Catalyzed 8-Amido Chelation-Induced Remote C–H Amination of Quinolines. Chem. Eur. J. 2016, 22, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, X.-L.; Zhou, G.-B.; Ying, B.-B.; Ye, P.-P.; Su, L.-Y.; Shen, C.; Zhang, P.-F. Copper(ii)-catalyzed C5 and C7 halogenation of quinolines using sodium halides under mild conditions. Org. Biomol. Chem. 2016, 14, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.-C.; Wang, K.; Xu, J.; Wei, Z.-J.; Shen, C.; Duan, G.-Y.; Zhu, Q.; Zhang, P.-F. Copper(II)-catalyzed remote sulfonylation of aminoquinolines with sodium sulfinates via radical coupling. RSC Adv. 2016, 6, 37173–37179. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Planas, O.; Company, A.; Ribas, X. A First Example of Cobalt-Catalyzed Remote C–H Functionalization of 8-Aminoquinolines Operating through a Single Electron Transfer Mechanism. Adv. Synth. Catal. 2016, 358, 1679–1688. [Google Scholar] [CrossRef]
- Zhu, X.; Qiao, L.; Ye, P.; Ying, B.; Xu, J.; Shen, C.; Zhang, P. Copper-catalyzed rapid C–H nitration of 8-aminoquinolines by using sodium nitrite as the nitro source under mild conditions. RSC Adv. 2016, 6, 89979–89983. [Google Scholar] [CrossRef]
- He, Y.; Zhao, N.-N.; Qiu, L.-Q.; Zhang, X.-Y.; Fan, X.-S. Regio-and chemoselective mono-and bisnitration of 8-amino quinoline amides with Fe (NO3)3·9H2O as promoter and nitro source. Org. Lett. 2016, 18, 6054–6057. [Google Scholar] [CrossRef] [PubMed]
- Nicewicz, G.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xia, W. Photoredox functionalization of C–H bonds adjacent to a nitrogen atom. Chem. Soc. Rev. 2012, 41, 7687–7697. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Beatty, J.W.; Stephenson, C.R.J. Amine Functionalization via Oxidative Photoredox Catalysis: Methodology Development and Complex Molecule Synthesis. Acc. Chem. Res. 2015, 48, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Le, C.C.; MacMillan, D.W.C.J. Fragment Couplings via CO2 Extrusion–Recombination: Expansion of a Classic Bond-Forming Strategy via Metallaphotoredox. Am. Chem. Soc. 2015, 137, 11938–11941. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Koeller, J.; Ackermann, L. Photoinduced Copper-Catalyzed C–H Arylation at Room Temperature. Angew. Chem. Int. Ed. 2016, 55, 4759–4762. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mu, X.; Liu, W.; Wang, Y.; Mi, Z.; Li, C.-J. Simple and Clean Photoinduced Aromatic Trifluoromethylation Reaction. J. Am. Chem. Soc. 2016, 138, 5809–5812. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-X.; Sun, S.-Y.; Qiao, H.-J.; Yang, F.; Zhu, Y.; Kang, J.-X.; Wu, Y.-S.; Wu, Y.-J. Palladium-Catalyzed Regioselective C8–H Amination of 1-Naphthylamine Derivatives with Aliphatic Amines. Org. Lett. 2016, 18, 4594–4597. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Rickard, C.E.F.; Roper, W.R.; Wright, L.J. Electrophilic Substitution Reactions at the Phenyl Ring of the Chelated 2-(2′-Pyridyl)phenyl Ligand Bound to Ruthenium(II) or Osmium(II). Organometallics 1999, 18, 2813–2820. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, B.; Huang, X.-R.; Wang, C.; Yao, J.; Chen, H. Origins of Selective C(sp2)–H Activation Using Transition Metal Complexes with N,N-Bidentate Directing Groups: A Combined Theoretical–Experimental Study. ACS Catal. 2014, 4, 649–656. [Google Scholar] [CrossRef]
- Aihara, Y.; Chatani, N.J. Nickel-Catalyzed Direct Arylation of C(sp3)–H Bonds in Aliphatic Amides via Bidentate-Chelation Assistance. Am. Chem. Soc. 2014, 136, 898–901. [Google Scholar] [CrossRef] [PubMed]
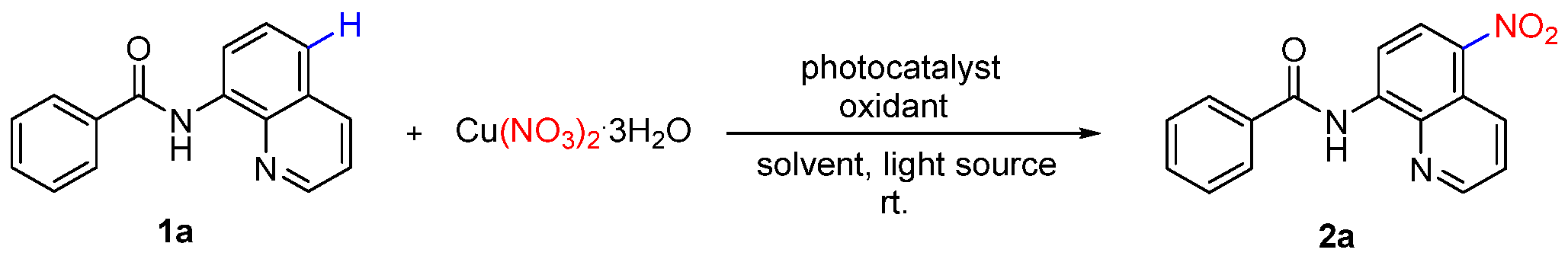

| Entry | Catalyst | Oxidant | Light Source | Yield (%) b |
|---|---|---|---|---|
| 1 | EosinB | K2S2O8 | household light | 68 |
| 2 | EosinY | K2S2O8 | household light | 65 |
| 3 | Ru(bpy)3(PF6)2 | K2S2O8 | household light | 69 |
| 4 | Ru(bpy)3Cl2 | K2S2O8 | household light | 75 |
| 5 | Ru(bpy)3Cl2·6H2O | K2S2O8 | household light | 76 |
| 6 | Alizarin Red S | K2S2O8 | household light | 72 |
| 7 | Acid Red 94 | K2S2O8 | household light | 82 |
| 8 c | Acid Red 94 | K2S2O8 | household light | 69 |
| 9 d | Acid Red 94 | K2S2O8 | household light | 76 |
| 10 e | Acid Red 94 | K2S2O8 | household light | 68 |
| 11 f | Acid Red 94 | K2S2O8 | household light | 73 |
| 12 | Acid Red 94 | K2S2O8 | blue | 66 |
| 13 | Acid Red 94 | K2S2O8 | green | 62 |
| 14 | Acid Red 94 | K2S2O8 | red | 43 |
| 15 | Acid Red 94 | K2S2O8 | dark | 23 |
| 16 | — | K2S2O8 | household light | 25 |
| 17 g | Acid Red 94 | K2S2O8 | household light | 51 |
| 18 h | Acid Red 94 | K2S2O8 | household light | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Qiao, H.; Su, X.; Bai, P.; Yang, F. Visible-Light-Photocatalyzed C5-H Nitration of 8-Aminoquinoline Amides. Catalysts 2024, 14, 263. https://doi.org/10.3390/catal14040263
Liu P, Qiao H, Su X, Bai P, Yang F. Visible-Light-Photocatalyzed C5-H Nitration of 8-Aminoquinoline Amides. Catalysts. 2024; 14(4):263. https://doi.org/10.3390/catal14040263
Chicago/Turabian StyleLiu, Pugen, Huijie Qiao, Xiaoxue Su, Peirong Bai, and Fan Yang. 2024. "Visible-Light-Photocatalyzed C5-H Nitration of 8-Aminoquinoline Amides" Catalysts 14, no. 4: 263. https://doi.org/10.3390/catal14040263




