Bubbles Management for Enhanced Catalytic Water Splitting Performance
Abstract
1. Introduction
2. Bubble-Behavior Monitoring
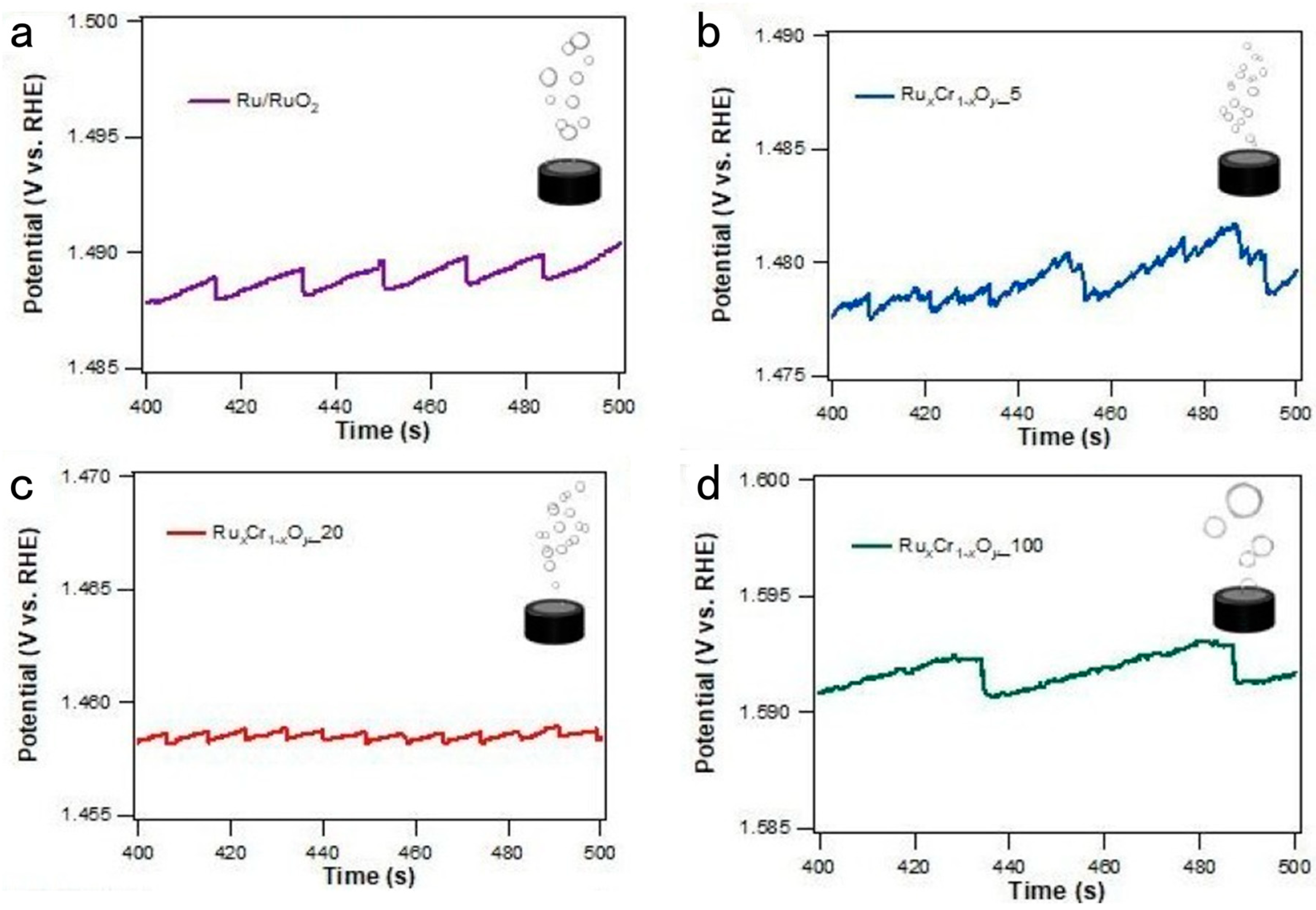
2.1. Operando Monitoring by Single High-Frequency Impedance
2.2. Operando Monitoring by Chronopotentiometric
2.3. Operando Monitoring by a Microfluidic Reactor
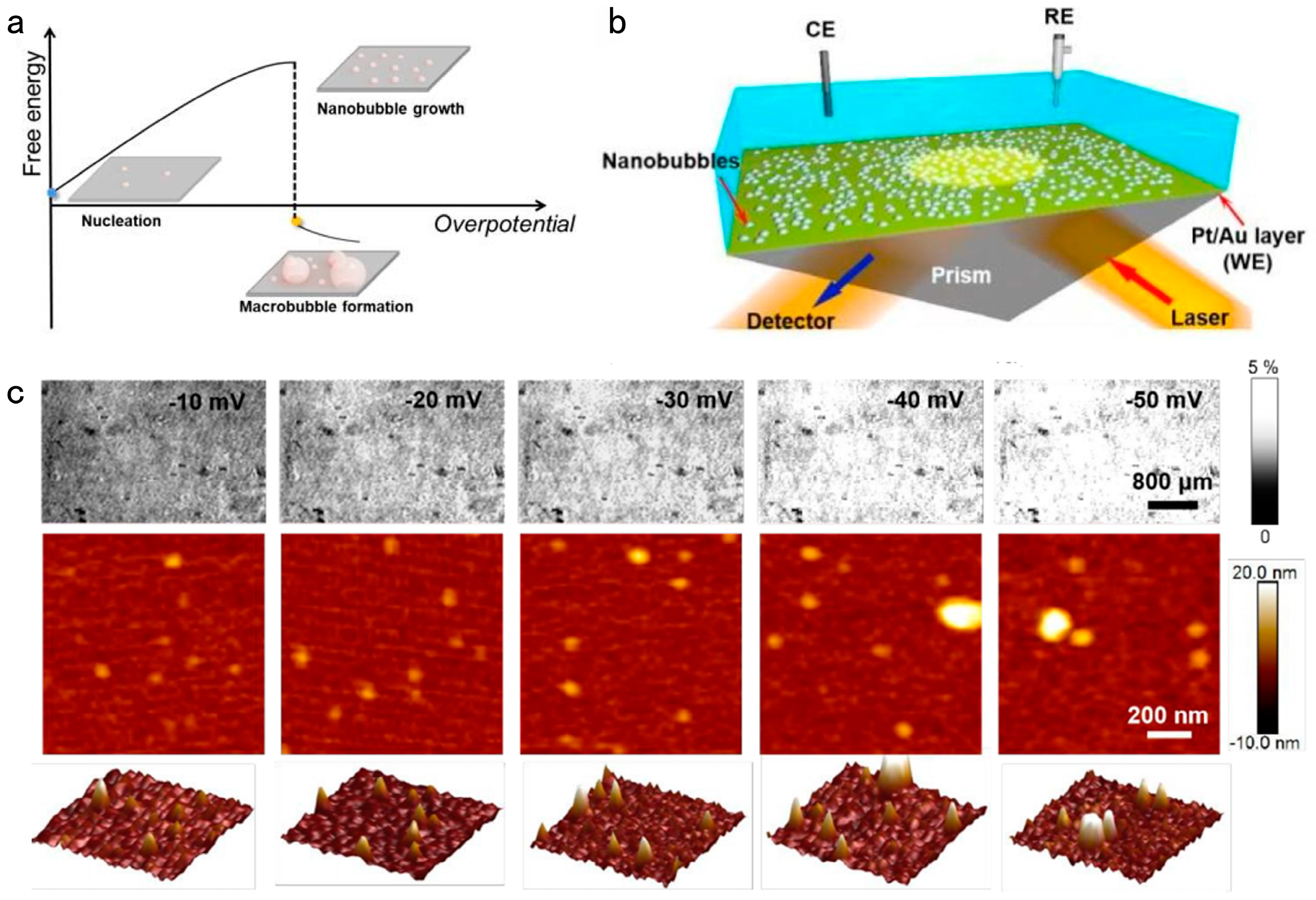
2.4. Operando Monitoring by Electrochemical Surface Plasmon Resonance Imaging
3. Factors Influencing Bubble Detachment
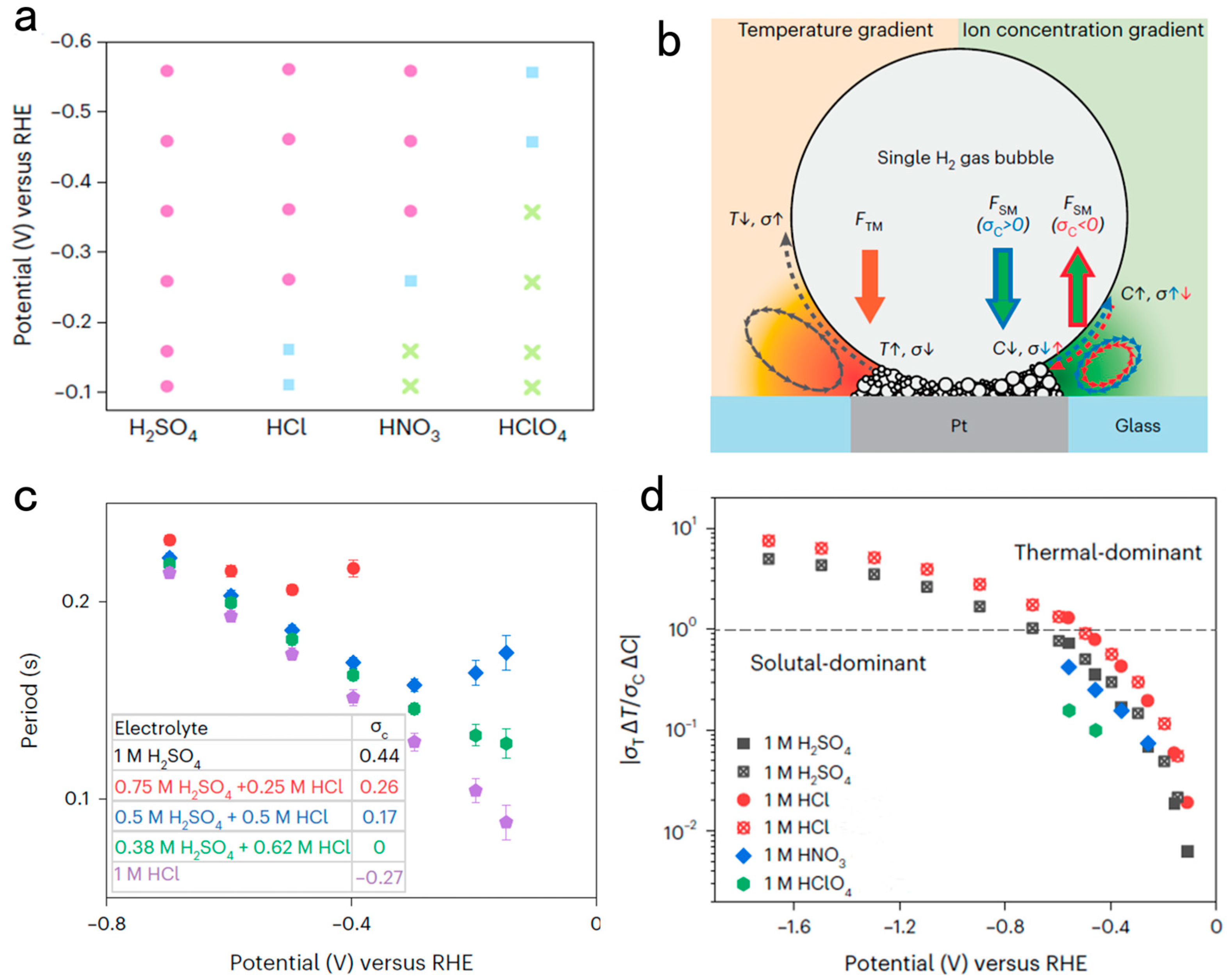
3.1. Solutal Marangoni Effect Determines Bubble Dynamics during Electrocatalytic Hydrogen Evolution
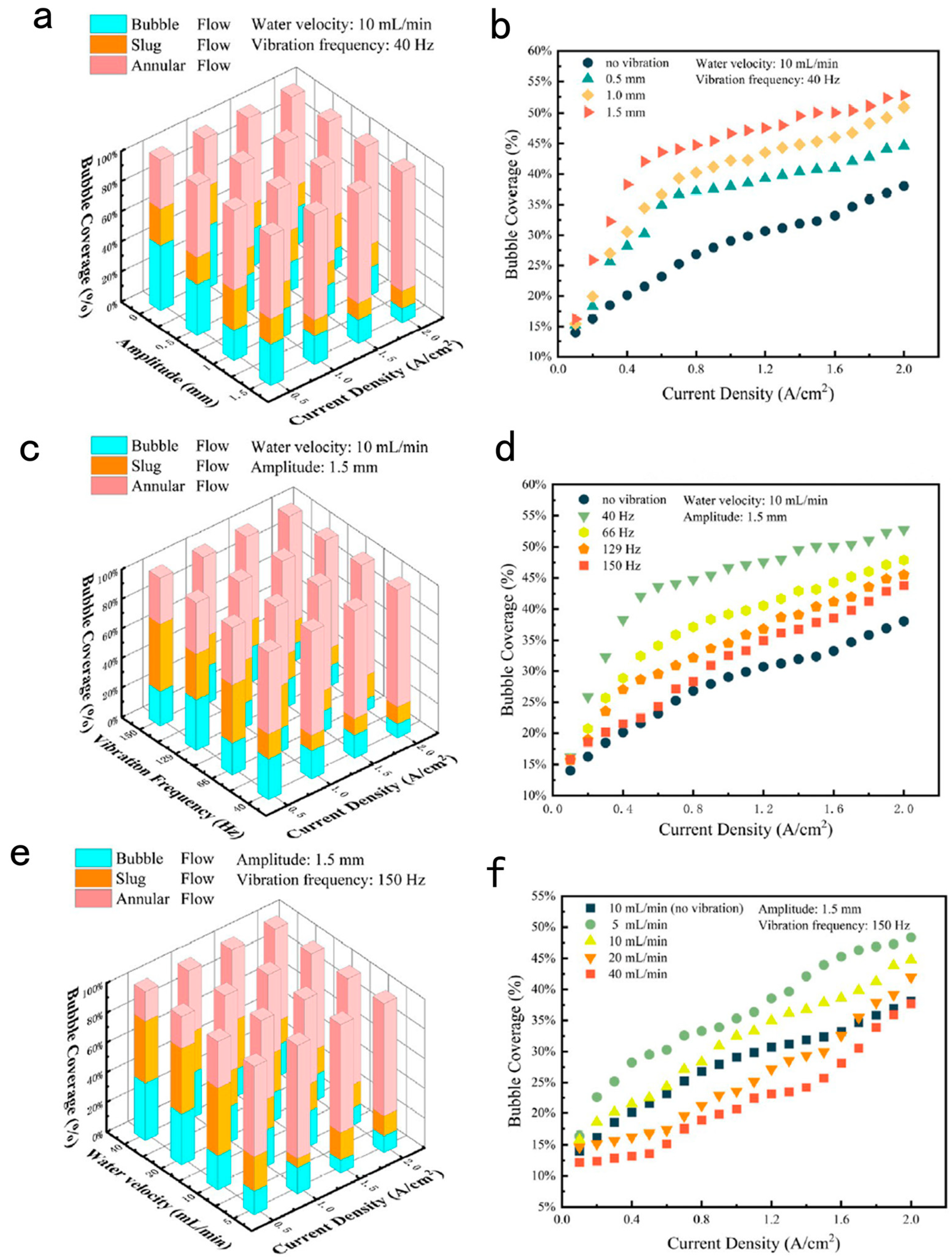
3.2. Effect of Mechanical Vibration and Water Velocity on Bubble Management in PEM Electrolysis Cell
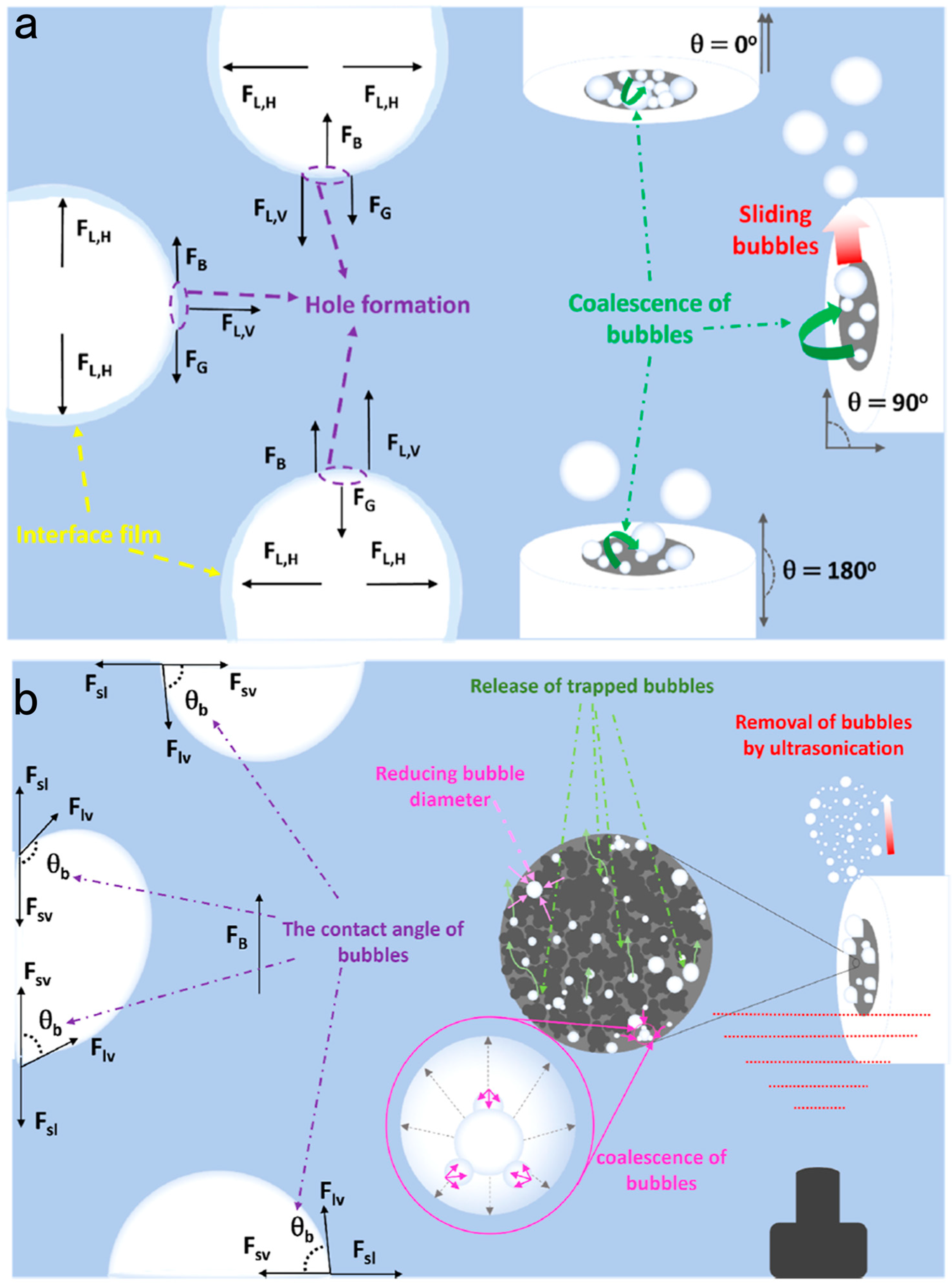
3.3. Effect of Orientation, Rotation, and Sonication on Bubble Management
4. Design of Catalysts to Promote Bubble Detachment
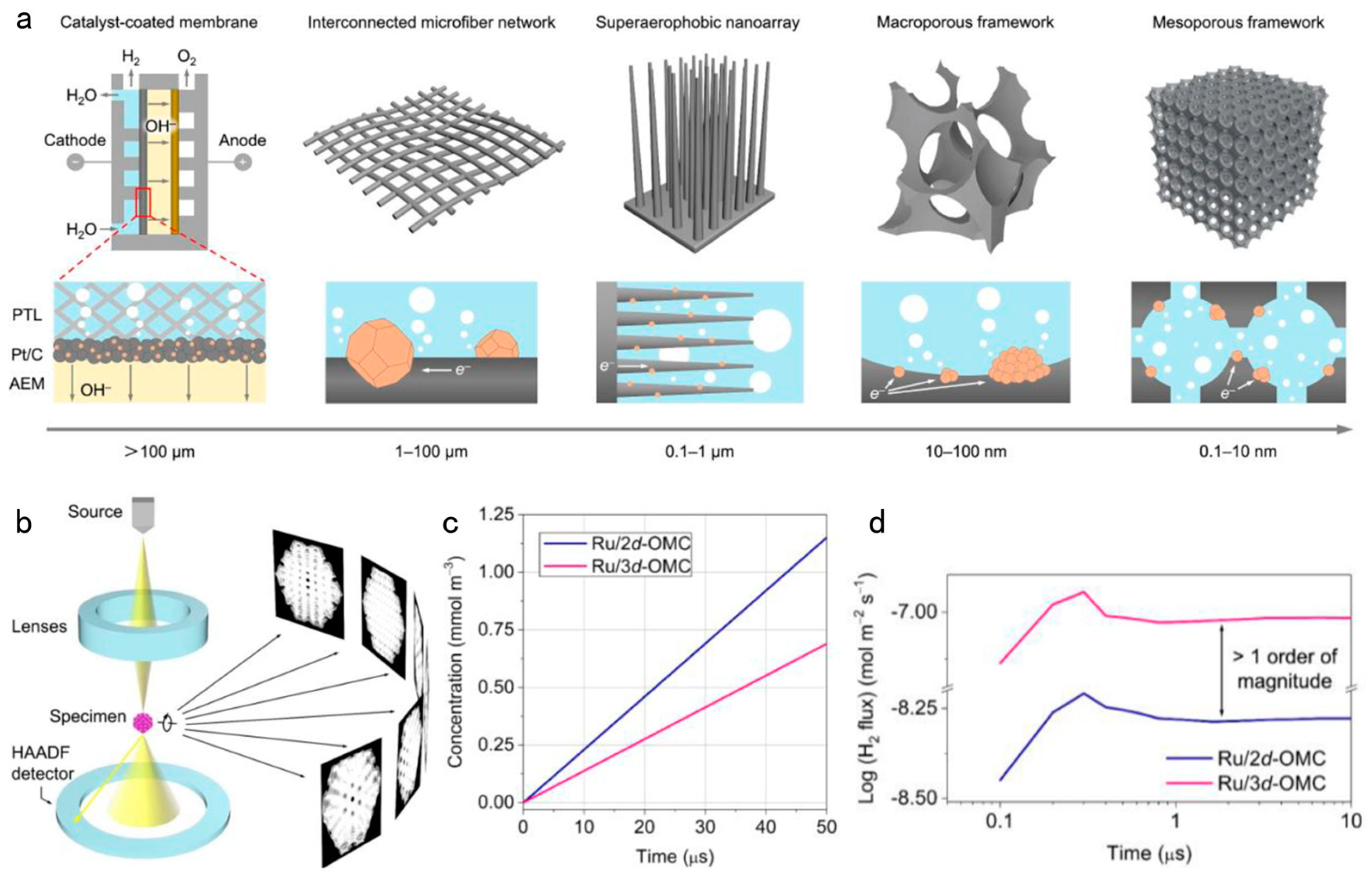
4.1. Design of Nanoarrays toward Efficient Electrochemical Water Splitting
4.2. Enhancing Catalyst-Carrier Interface Binding
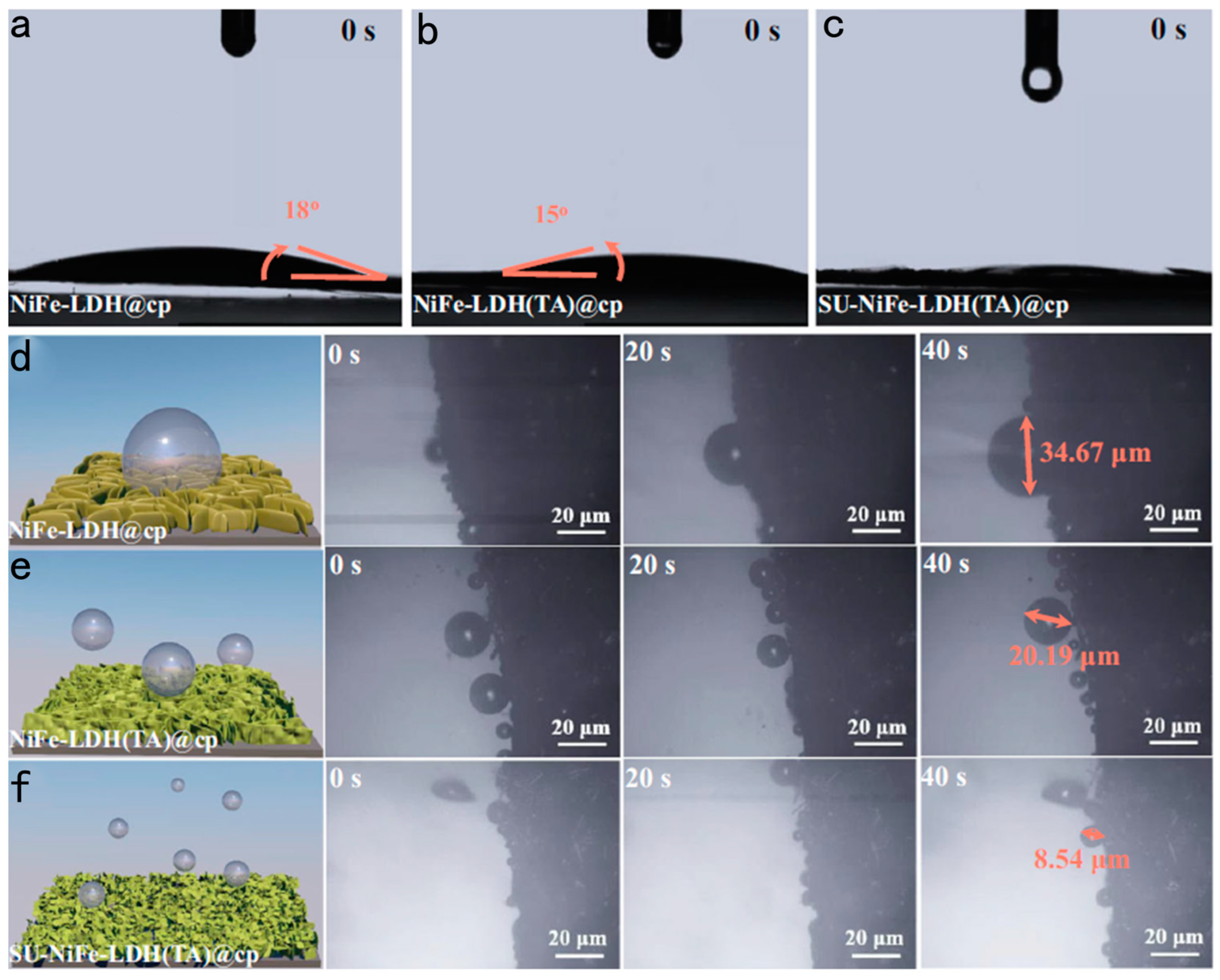
4.3. Bioinspired Trimesic Acid Anchored Electrocatalysts
4.4. Design of 3D Nano Graded Catalyst Materials
5. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Ren, H.; Luo, L. Gas Bubbles in Electrochemical Gas Evolution Reactions. Langmuir 2019, 35, 5392–5408. [Google Scholar] [CrossRef] [PubMed]
- Leal Pérez, B.J.; Medrano Jiménez, J.A.; Bhardwaj, R.; Goetheer, E.; van Sint Annaland, M.; Gallucci, F. Methane pyrolysis in a molten gallium bubble column reactor for sustainable hydrogen production: Proof of concept & techno-economic assessment. Int. J. Hydrogen Energy 2021, 46, 4917–4935. [Google Scholar] [CrossRef]
- Ketzer, M.; Praeg, D.; Rodrigues, L.F.; Augustin, A.; Pivel, M.A.G.; Rahmati-Abkenar, M.; Miller, D.J.; Viana, A.R.; Cupertino, J.A. Gas hydrate dissociation linked to contemporary ocean warming in the southern hemisphere. Nat. Commun. 2020, 11, 3788. [Google Scholar] [CrossRef] [PubMed]
- Rest, J.; Cooper, M.W.D.; Spino, J.; Turnbull, J.A.; Van Uffelen, P.; Walker, C.T. Fission gas release from UO2 nuclear fuel: A review. J. Nucl. Mater. 2019, 513, 310–345. [Google Scholar] [CrossRef]
- Frinking, P.; Segers, T.; Luan, Y.; Tranquart, F. Three Decades of Ultrasound Contrast Agents: A Review of the Past, Present and Future Improvements. Ultrasound Med. Biol. 2020, 46, 892–908. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Zhang, C.; Chen, X.; Liu, W.; Zheng, Y.; Xie, Y.; Huang, Y.; Li, J. Roll-to-roll prelithiation of Sn foil anode suppresses gassing and enables stable full-cell cycling of lithium ion batteries. Energy Environ. Sci. 2019, 12, 2991–3000. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, Y.; Lei, W.; Shao, H. Unraveling the Fundamental Concepts of Superaerophobic/Superhydrophilic Electrocatalysts for Highly Efficient Water Electrolysis: Implications for Future Research. ChemElectroChem 2023, 11, e202300465. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Mebrahtu, C.; Wang, S.; Palkovits, R. Innovative Electrochemical Strategies for Hydrogen Production: From Electricity Input to Electricity Output. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214333. [Google Scholar] [CrossRef]
- Cho, H.J.; Wang, E.N. Bubble nucleation, growth, and departure: A new, dynamic understanding. Int. J. Heat Mass Transf. 2019, 145, 118803. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Zhang, Y.; Qi, B.; Wei, J. A molecular dynamics study of surface wettability effects on heterogeneous bubble nucleation. Int. Commun. Heat Mass Transf. 2020, 119, 104991. [Google Scholar] [CrossRef]
- Pereiro, I.; Fomitcheva Khartchenko, A.; Petrini, L.; Kaigala, G.V. Nip the bubble in the bud: A guide to avoid gas nucleation in microfluidics. Lab Chip 2019, 19, 2296–2314. [Google Scholar] [CrossRef]
- Zhou, T.-T.; He, A.-M.; Wang, P. Dynamic evolution of He bubble and its effects on void nucleation-growth and thermomechanical properties in the spallation of aluminum. J. Nucl. Mater. 2020, 542, 152496. [Google Scholar] [CrossRef]
- Sarker, D.; Ding, W.; Hampel, U. Bubble growth during subcooled nucleate boiling on a vertical heater: A mechanistic attempt to evaluate the role of surface characteristics on microlayer evaporation. Appl. Therm. Eng. 2019, 153, 565–574. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Yu, B.; Zou, Y.; Chen, B.-N.; Tao, W.-Q. Molecular dynamics studies of bubble nucleation on a grooved substrate. Int. J. Heat Mass Transf. 2020, 158, 119850. [Google Scholar] [CrossRef]
- Ren, T.; Zhu, Z.; Yan, M.; Shi, J.; Yan, C. Experimental study on bubble nucleation and departure for subcooled flow boiling in a narrow rectangular channel. Int. J. Heat Mass Transf. 2019, 144, 118670. [Google Scholar] [CrossRef]
- Li, M.; Xie, P.; Yu, L.; Luo, L.; Sun, X. Bubble Engineering on Micro-/Nanostructured Electrodes for Water Splitting. ACS Nano 2023, 17, 23299–23316. [Google Scholar] [CrossRef]
- Shih, A.J.; Monteiro, M.C.O.; Dattila, F.; Pavesi, D.; Philips, M.; da Silva, A.H.M.; Vos, R.E.; Ojha, K.; Park, S.; van der Heijden, O.; et al. Water electrolysis. Nat. Rev. Methods Primers 2022, 2, 84. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Z.; Sun, X.; Jiang, L.; Duan, X. Superwetting Electrodes for Gas-Involving Electrocatalysis. Acc. Chem. Res. 2018, 51, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Han, Y.; Jia, C.; Wu, J.; Dong, S.; Wu, C. Impeding effect of bubbles on metal transfer in underwater wet FCAW. J. Manuf. Process. 2019, 45, 682–689. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, F.; Wang, C.; Wang, J.; Jiang, L.; Yu, C. Integrated Bundle Electrode with Wettability-Gradient Copper Cones Inducing Continuous Generation, Directional Transport, and Efficient Collection of H2 Bubbles. ACS Appl. Mater. Interfaces 2021, 13, 32435–32441. [Google Scholar] [CrossRef] [PubMed]
- Angulo, A.; van der Linde, P.; Gardeniers, H.; Modestino, M.; Fernández Rivas, D. Influence of Bubbles on the Energy Conversion Efficiency of Electrochemical Reactors. Joule 2020, 4, 555–579. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Shanmugam, S. Recent advances in methods and technologies for enhancing bubble detachment during electrochemical water splitting. Renew. Sustain. Energy Rev. 2019, 114, 109300. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, M.J.; Kim, J.J. Impact of Surface Hydrophilicity on Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 11940–11947. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zhao, Y.; Zhang, C.; Zhang, Y.; Yu, C.; Wu, Y.; Ma, J.; Cao, M.; Jiang, L. A Multi-Bioinspired Dual-Gradient Electrode for Microbubble Manipulation toward Controllable Water Splitting. Adv. Mater. 2020, 32, e1908099. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Li, D.; Yang, N.; Wan, J.; Zhang, C.; Zhang, X.; Jiang, H.; Zhang, Q.; Gu, L.; Wang, D. Delicate Control on the Shell Structure of Hollow Spheres Enables Tunable Mass Transport in Water Splitting. Angew. Chem. Int. Ed. Engl. 2021, 60, 6926–6931. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, R.; Wang, Y.; Han, L.; Gu, J.; Niu, Z.; Yuan, Y.; Qu, N.; Meng, J.; Wang, D. Superwettable Surface-Dependent efficiently electrocatalytic water splitting based on their excellent liquid adsorption and gas desorption. Chem. Eng. J. 2023, 452, 139513. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, J.; Yin, W.; Yu, H.; Wang, F.; Hu, W.; Wang, L.; Yan, D. Effective strategies to promote Z(S)-scheme photocatalytic water splitting. Chin. Chem. Lett. 2024, 35, 108724. [Google Scholar] [CrossRef]
- Hegner, K.I.; Wong, W.S.Y.; Vollmer, D. Ultrafast Bubble Bursting by Superamphiphobic Coatings. Adv. Mater. 2021, 33, e2101855. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Wang, X.; Lv, D.; Cao, C.; Ai, L.; Yao, X. Bio-inspired spontaneous splitting of underwater bubbles along a superhydrophobic open pathway without perturbation. Droplet 2022, 1, 65–75. [Google Scholar] [CrossRef]
- Qiao, S.; Cai, C.; Chen, W.; Pan, C.; Liu, Y. Control of the shape of bubble growth on underwater substrates with different sizes of superhydrophobic circles. Phys. Fluids 2022, 34, 067110. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Huo, J.; Hou, X.; Chen, F. Underwater gas self-transportation along femtosecond laser-written open superhydrophobic surface microchannels (<100 µm) for bubble/gas manipulation. Int. J. Extreme Manuf. 2022, 4, 015002. [Google Scholar] [CrossRef]
- Shen, J.; Li, B.; Zheng, Y.; Dai, Z.; Li, J.; Bao, X.; Guo, J.; Yu, X.; Guo, Y.; Ge, M.; et al. Engineering the composition and structure of superaerophobic nanosheet array for efficient hydrogen evolution. Chem. Eng. J. 2022, 433, 133517. [Google Scholar] [CrossRef]
- Jiao, Y.; Lv, X.; Zhang, Y.; Li, C.; Li, J.; Wu, H.; Xiao, Y.; Wu, S.; Hu, Y.; Wu, D.; et al. Pitcher plant-bioinspired bubble slippery surface fabricated by femtosecond laser for buoyancy-driven bubble self-transport and efficient gas capture. Nanoscale 2019, 11, 1370–1378. [Google Scholar] [CrossRef]
- Zhang, Q.; Prokhorenko, S.; Nahas, Y.; Xie, L.; Bellaiche, L.; Gruverman, A.; Valanoor, N. Deterministic Switching of Ferroelectric Bubble Nanodomains. Adv. Funct. Mater. 2019, 29, 1808573. [Google Scholar] [CrossRef]
- Giacomello, A.; Roth, R. Bubble formation in nanopores: A matter of hydrophobicity, geometry, and size. Adv. Phys. X 2020, 5, 1817780. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Y.; Kong, L.; Wang, W.; Chen, Y.; Wang, Y.; Soh, Y.; Xiong, Y.; Tian, M.; Du, H. Two-dimensional characterization of three-dimensional magnetic bubbles in Fe3Sn2 nanostructures. Natl. Sci. Rev. 2021, 8, nwaa200. [Google Scholar] [CrossRef]
- Yin, K.; Yang, S.; Dong, X.; Chu, D.; Gong, X.; Duan, J.-A. Femtosecond laser fabrication of shape-gradient platform: Underwater bubbles continuous self-driven and unidirectional transportation. Appl. Surf. Sci. 2019, 471, 999–1004. [Google Scholar] [CrossRef]
- Jeon, D.; Park, J.; Shin, C.; Kim, H.; Jang, J.W.; Lee, D.W.; Ryu, J. Superaerophobic hydrogels for enhanced electrochemical and photoelectrochemical hydrogen production. Sci. Adv. 2020, 6, eaaz3944. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Han, N.; Tian, Y.; Kallio, T.; Yu, C.; Jiang, L. Superaerophilic/superaerophobic cooperative electrode for efficient hydrogen evolution reaction via enhanced mass transfer. Sci. Adv. 2023, 9, eadd6978. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, W.; Pan, J.; Zhang, Y.; Wang, F.; Wang, L.; Zhao, Q. External field assisted hydrogen evolution reaction. Nano Res. 2023, 16, 8638–8654. [Google Scholar] [CrossRef]
- Dastafkan, K.; Meyer, Q.; Chen, X.; Zhao, C. Efficient Oxygen Evolution and Gas Bubble Release Achieved by a Low Gas Bubble Adhesive Iron-Nickel Vanadate Electrocatalyst. Small 2020, 16, e2002412. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, H.; Huo, D.; Yu, S.; Su, J.; Xie, Y.; Li, W.; Ma, L.; Chen, H.; Sun, Y. An Ultrasensitive Microsensor Based on Impedance Analysis for Oil Condition Monitoring. IEEE Trans. Ind. Electron. 2022, 69, 7441–7450. [Google Scholar] [CrossRef]
- Lu, S.M.; Li, Y.J.; Zhang, J.F.; Wang, Y.; Ying, Y.L.; Long, Y.T. Monitoring Hydrogen Evolution Reaction Catalyzed by MoS2 Quantum Dots on a Single Nanoparticle Electrode. Anal. Chem. 2019, 91, 10361–10365. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, C.; Liu, Y.; Ruiz, K.H.; Ren, H.; Fan, Y.; White, H.S.; Chen, Q. Visualization and Quantification of Electrochemical H2 Bubble Nucleation at Pt, Au, and MoS2 Substrates. ACS Sens. 2021, 6, 355–363. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, M.; Lin, Y.; Xu, J. Trapping and control of bubbles in various microfluidic applications. Lab Chip 2020, 20, 4512–4527. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Huang, Q.; Ohta, A.T.; Arai, T. Bubbles in microfluidics: An all-purpose tool for micromanipulation. Lab Chip 2021, 21, 1016–1035. [Google Scholar] [CrossRef]
- Viejo, C.G.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Bubbles, Foam Formation, Stability and Consumer Perception of Carbonated Drinks: A Review of Current, New and Emerging Technologies for Rapid Assessment and Control. Foods 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, Z.; Lu, L.; Cai, S.; Maxey, M.; Karniadakis, G.E. Operator learning for predicting multiscale bubble growth dynamics. J. Chem. Phys. 2021, 154, 104118. [Google Scholar] [CrossRef]
- Dastafkan, K.; Wang, S.; Rong, C.; Meyer, Q.; Li, Y.; Zhang, Q.; Zhao, C. Cosynergistic Molybdate Oxo-Anionic Modification of FeNi-Based Electrocatalysts for Efficient Oxygen Evolution Reaction. Adv. Funct. Mater. 2021, 32, 2107342. [Google Scholar] [CrossRef]
- Dastafkan, K.; Wang, S.; Song, S.; Meyer, Q.; Zhang, Q.; Shen, Y.; Zhao, C. Operando monitoring of gas bubble evolution in water electrolysis by single high-frequency impedance. EES Catal. 2023, 1, 998–1008. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Liu, H.; Chen, J.; Ma, S.; An, T. Micro/nano-bubble assisted synthesis of Au/TiO2@CNTs composite photocatalyst for photocatalytic degradation of gaseous styrene and its enhanced catalytic mechanism. Environ. Sci. Nano 2019, 6, 948–958. [Google Scholar] [CrossRef]
- Wang, Y.; Gordon, E.; Ren, H. Mapping the Nucleation of H2 Bubbles on Polycrystalline Pt via Scanning Electrochemical Cell Microscopy. J. Phys. Chem. Lett. 2019, 10, 3887–3892. [Google Scholar] [CrossRef]
- Zhong, X.; Eshraghi, J.; Vlachos, P.; Dabiri, S.; Ardekani, A.M. A model for a laser-induced cavitation bubble. Int. J. Multiph. Flow 2020, 132, 103433. [Google Scholar] [CrossRef]
- Song, C.; Jin, D.; Choi, S.; Lee, Y. Fiber-in-tube RuxCr1−xOy as highly efficient electrocatalysts for pH-universal water oxidation via facile bubble desorption. J. Mater. Chem. A 2023, 11, 26626–26635. [Google Scholar] [CrossRef]
- Wang, X.; Shuai, Y.; Zhang, H.; Sun, J.; Yang, Y.; Huang, Z.; Jiang, B.; Liao, Z.; Wang, J.; Yang, Y. Bubble breakup in a swirl-venturi microbubble generator. Chem. Eng. J. 2021, 403, 126397. [Google Scholar] [CrossRef]
- Mosavi, A.; Shamshirband, S.; Salwana, E.; Chau, K.-w.; Tah, J.H.M. Prediction of multi-inputs bubble column reactor using a novel hybrid model of computational fluid dynamics and machine learning. Eng. Appl. Comput. Fluid Mech. 2019, 13, 482–492. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; Cheng, X.; Sun, H.; Ma, H.; Li, Y. Elucidation of bubble evolution and defect formation in directed energy deposition based on direct observation. Addit. Manuf. 2020, 32, 101026. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, P.; Luan, B.; Zhang, X.; Fang, Z.; Xian, Y.; Lu, X.; Ostrikov, K.K.; Bazaka, K. Microplasma Bubbles: Reactive Vehicles for Biofilm Dispersal. ACS Appl. Mater. Interfaces 2019, 11, 20660–20669. [Google Scholar] [CrossRef]
- Sattari, A.; Hanafizadeh, P.; Hoorfar, M. Multiphase flow in microfluidics: From droplets and bubbles to the encapsulated structures. Adv. Colloid Interface Sci. 2020, 282, 102208. [Google Scholar] [CrossRef]
- Cherdantsev, A.V.; An, J.S.; Charogiannis, A.; Markides, C.N. Simultaneous application of two laser-induced fluorescence approaches for film thickness measurements in annular gas-liquid flows. Int. J. Multiph. Flow 2019, 119, 237–258. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, H.; Zhang, J.; Zhang, H.-Q. A numerical study on flow patterns inside an electrical submersible pump (ESP) and comparison with visualization experiments. J. Pet. Sci. Eng 2019, 173, 339–350. [Google Scholar] [CrossRef]
- Gallo, M.; Magaletti, F.; Casciola, C.M. Heterogeneous bubble nucleation dynamics. J. Fluid Mech. 2020, 906, A20. [Google Scholar] [CrossRef]
- Gallo, M.; Magaletti, F.; Cocco, D.; Casciola, C.M. Nucleation and growth dynamics of vapour bubbles. J. Fluid Mech. 2019, 883, A14. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Sabatino, S. Electro-generation of H2O2 and abatement of organic pollutant in water by an electro-Fenton process in a microfluidic reactor. Electrochem. Commun. 2013, 26, 45–47. [Google Scholar] [CrossRef]
- Lu, Y.; Fu, T.; Zhu, C.; Ma, Y.; Li, H.Z. Pinch-off mechanism for Taylor bubble formation in a microfluidic flow-focusing device. Microfluid. Nanofluid 2013, 16, 1047–1055. [Google Scholar] [CrossRef]
- Swiegers, G.F.; Terrett, R.N.L.; Tsekouras, G.; Tsuzuki, T.; Pace, R.J.; Stranger, R. The prospects of developing a highly energy-efficient water electrolyser by eliminating or mitigating bubble effects. Sustain. Energy Fuels 2021, 5, 1280–1310. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, D.; Zhu, X.; Wang, Y.; Yang, Y.; Chen, R.; Li, J.; Liao, Q. Bubble dynamic behaviors in the anode porous transport layer of proton exchange membrane electrolyzers using a microfluidic reactor. J. Power Sources 2023, 582, 233532. [Google Scholar] [CrossRef]
- Perez Sirkin, Y.A.; Gadea, E.D.; Scherlis, D.A.; Molinero, V. Mechanisms of Nucleation and Stationary States of Electrochemically Generated Nanobubbles. J. Am. Chem. Soc. 2019, 141, 10801–10811. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zion, A.; Nourmahnad, A.; Mittelstein, D.R.; Shivaei, S.; Yoo, S.; Buss, M.T.; Hurt, R.C.; Malounda, D.; Abedi, M.H.; Lee-Gosselin, A.; et al. Acoustically triggered mechanotherapy using genetically encoded gas vesicles. Nat. Nanotechnol. 2021, 16, 1403–1412. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, D.; Yang, B.; Yin, W.; Ardakani, M.S.; Yao, J.; Drelich, J.W. Effect of nano-sized roughness on the flotation of magnesite particles and particle-bubble interactions. Miner. Eng. 2020, 151, 106340. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, S.; Zhang, L.; Hu, J. Generation and stability of bulk nanobubbles: A review and perspective. Curr. Opin. Colloid Interface Sci. 2021, 53, 101439. [Google Scholar] [CrossRef]
- Yu, J.; Hu, K.; Zhang, Z.; Luo, L.; Liu, Y.; Zhou, D.; Wang, F.; Kuang, Y.; Xu, H.; Li, H.; et al. Interfacial nanobubbles’ growth at the initial stage of electrocatalytic hydrogen evolution. Energy Environ. Sci. 2023, 16, 2068–2079. [Google Scholar] [CrossRef]
- Morasch, M.; Liu, J.; Dirscherl, C.F.; Ianeselli, A.; Kuhnlein, A.; Le Vay, K.; Schwintek, P.; Islam, S.; Corpinot, M.K.; Scheu, B.; et al. Heated gas bubbles enrich, crystallize, dry, phosphorylate and encapsulate prebiotic molecules. Nat. Chem. 2019, 11, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhang, C.; Ma, H.; Zhang, Y.; Liu, G.; Cao, M.; Yu, C.; Jiang, L. Bioinspired Slippery Cone for Controllable Manipulation of Gas Bubbles in Low-Surface-Tension Environment. ACS Nano 2019, 13, 4083–4090. [Google Scholar] [CrossRef]
- Liu, W.; Li, N.; Weng, C.-s.; Huang, X.-l.; Kang, Y. Bubble dynamics and pressure field characteristics of underwater detonation gas jet generated by a detonation tube. Phys. Fluids 2021, 33, 023302. [Google Scholar] [CrossRef]
- Cheng, H.; Long, X.; Ji, B.; Peng, X.; Farhat, M. A new Euler-Lagrangian cavitation model for tip-vortex cavitation with the effect of non-condensable gas. Int. J. Multiph. Flow 2021, 134, 103441. [Google Scholar] [CrossRef]
- Rahimi, N.; Kang, D.; Gelinas, J.; Menon, A.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Solid carbon production and recovery from high temperature methane pyrolysis in bubble columns containing molten metals and molten salts. Carbon 2019, 151, 181–191. [Google Scholar] [CrossRef]
- Visser, C.W.; Amato, D.N.; Mueller, J.; Lewis, J.A. Architected Polymer Foams via Direct Bubble Writing. Adv. Mater. 2019, 31, e1904668. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Khater, M.M.A.; Attia, R.A.M.; Lu, D. The plethora of explicit solutions of the fractional KS equation through liquid–gas bubbles mix under the thermodynamic conditions via Atangana–Baleanu derivative operator. Adv. Differ. Equ. 2020, 2020, 62. [Google Scholar] [CrossRef]
- Wang, J. Continuum theory for dense gas-solid flow: A state-of-the-art review. Chem. Eng. Sci. 2020, 215, 115428. [Google Scholar] [CrossRef]
- Gordillo, J.M.; Rodríguez-Rodríguez, J. Capillary waves control the ejection of bubble bursting jets. J. Fluid Mech. 2019, 867, 556–571. [Google Scholar] [CrossRef]
- Yang, H.Y.K.; Gaspari, M.; Marlow, C. The Impact of Radio AGN Bubble Composition on the Dynamics and Thermal Balance of the Intracluster Medium. Astrophys. J. 2019, 871, 6. [Google Scholar] [CrossRef]
- Lechner, C.; Lauterborn, W.; Koch, M.; Mettin, R. Jet formation from bubbles near a solid boundary in a compressible liquid: Numerical study of distance dependence. Phys. Rev. Fluids 2020, 5, 093604. [Google Scholar] [CrossRef]
- Park, S.; Liu, L.; Demirkir, C.; van der Heijden, O.; Lohse, D.; Krug, D.; Koper, M.T.M. Solutal Marangoni effect determines bubble dynamics during electrocatalytic hydrogen evolution. Nat. Chem. 2023, 15, 1532–1540. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Penkov, N.V.; Baimler, I.V.; Lyakhov, G.A.; Pustovoy, V.I.; Simakin, A.V.; Sarimov, R.M.; Scherbakov, I.A. Effect of Mechanical Shaking on the Physicochemical Properties of Aqueous Solutions. Int. J. Mol. Sci. 2020, 21, 8033. [Google Scholar] [CrossRef]
- Ulseth, A.J.; Hall, R.O.; Boix Canadell, M.; Madinger, H.L.; Niayifar, A.; Battin, T.J. Distinct air–water gas exchange regimes in low- and high-energy streams. Nat. Geosci. 2019, 12, 259–263. [Google Scholar] [CrossRef]
- Zhu, D.; Xu, L.; Su, X.; Hu, B.; Jia, T.; Mi, L. Experimental study of the effect of mechanical vibration and water velocity on bubble management in PEM electrolysis cell. Int. J. Hydrogen Energy 2023, 49, 390–403. [Google Scholar] [CrossRef]
- Maier, M.; Meyer, Q.; Majasan, J.; Owen, R.E.; Robinson, J.B.; Dodwell, J.; Wu, Y.; Castanheira, L.; Hinds, G.; Shearing, P.R.; et al. Diagnosing Stagnant Gas Bubbles in a Polymer Electrolyte Membrane Water Electrolyser Using Acoustic Emission. Front. Energy Res. 2020, 8, 582919. [Google Scholar] [CrossRef]
- Al Ramadan, M.; Salehi, S.; Kwatia, G.; Ezeakacha, C.; Teodoriu, C. Experimental investigation of well integrity: Annular gas migration in cement column. J. Pet. Sci. Eng. 2019, 179, 126–135. [Google Scholar] [CrossRef]
- Ye, L.; Zhu, X.; Liu, Y. Numerical study on dual-frequency ultrasonic enhancing cavitation effect based on bubble dynamic evolution. Ultrason. Sonochem. 2019, 59, 104744. [Google Scholar] [CrossRef]
- Chu, H.; Yu, X.; Jiang, H.; Wang, D.; Xu, N. Progress in enhanced pool boiling heat transfer on macro- and micro-structured surfaces. Int. J. Heat Mass Transf. 2023, 200, 123530. [Google Scholar] [CrossRef]
- Karimi, V.; Sharma, R.; Morgen, P.; Andersen, S.M. Multiple Bubble Removal Strategies to Promote Oxygen Evolution Reaction: Mechanistic Understandings from Orientation, Rotation, and Sonication Perspectives. ACS Appl. Mater. Interfaces 2023, 15, 49233–49245. [Google Scholar] [CrossRef]
- Bae, M.; Kang, Y.; Lee, D.W.; Jeon, D.; Ryu, J. Superaerophobic Polyethyleneimine Hydrogels for Improving Electrochemical Hydrogen Production by Promoting Bubble Detachment. Adv. Energy Mater. 2022, 12, 2201452. [Google Scholar] [CrossRef]
- Shan, X.; Liu, J.; Mu, H.; Xiao, Y.; Mei, B.; Liu, W.; Lin, G.; Jiang, Z.; Wen, L.; Jiang, L. An Engineered Superhydrophilic/Superaerophobic Electrocatalyst Composed of the Supported CoMoS(x) Chalcogel for Overall Water Splitting. Angew. Chem. Int. Ed. Engl. 2020, 59, 1659–1665. [Google Scholar] [CrossRef]
- Andaveh, R.; Barati Darband, G.; Maleki, M.; Sabour Rouhaghdam, A. Superaerophobic/superhydrophilic surfaces as advanced electrocatalysts for the hydrogen evolution reaction: A comprehensive review. J. Mater. Chem. A 2022, 10, 5147–5173. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, e1806326. [Google Scholar] [CrossRef]
- Dastafkan, K.; Li, Y.; Zeng, Y.; Han, L.; Zhao, C. Enhanced surface wettability and innate activity of an iron borate catalyst for efficient oxygen evolution and gas bubble detachment. J. Mater. Chem. A 2019, 7, 15252–15261. [Google Scholar] [CrossRef]
- Xia, C.; Back, S.; Ringe, S.; Jiang, K.; Chen, F.; Sun, X.; Siahrostami, S.; Chan, K.; Wang, H. Confined local oxygen gas promotes electrochemical water oxidation to hydrogen peroxide. Nat. Catal. 2020, 3, 125–134. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, Y.; Kong, X.-Y.; Shi, R.; Waterhouse, G.I.N.; Wen, L.; Zhang, T. Underwater superaerophobic Ni nanoparticle-decorated nickel–molybdenum nitride nanowire arrays for hydrogen evolution in neutral media. Nano Energy 2020, 78, 105375. [Google Scholar] [CrossRef]
- Hashemi, L.; Glerum, W.; Farajzadeh, R.; Hajibeygi, H. Contact angle measurement for hydrogen/brine/sandstone system using captive-bubble method relevant for underground hydrogen storage. Adv. Water Resour. 2021, 154, 103964. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, D.; Pan, S.; Zhu, M. Microporosity formation and dendrite growth during solidification of aluminum alloys: Modeling and experiment. Int. J. Heat Mass Transf. 2020, 146, 118838. [Google Scholar] [CrossRef]
- Iwata, R.; Zhang, L.; Wilke, K.L.; Gong, S.; He, M.; Gallant, B.M.; Wang, E.N. Bubble growth and departure modes on wettable/non-wettable porous foams in alkaline water splitting. Joule 2021, 5, 887–900. [Google Scholar] [CrossRef]
- Shi, W.; Yang, X.; Sommerfeld, M.; Yang, J.; Cai, X.; Li, G.; Zong, Y. Modelling of mass transfer for gas-liquid two-phase flow in bubble column reactor with a bubble breakage model considering bubble-induced turbulence. Chem. Eng. J. 2019, 371, 470–485. [Google Scholar] [CrossRef]
- Liu, R.; Gong, Z.; Liu, J.; Dong, J.; Liao, J.; Liu, H.; Huang, H.; Liu, J.; Yan, M.; Huang, K.; et al. Design of Aligned Porous Carbon Films with Single-Atom Co-N-C Sites for High-Current-Density Hydrogen Generation. Adv. Mater. 2021, 33, e2103533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ranjan, B.; Tanaka, T.; Sugioka, K. Underwater persistent bubble-assisted femtosecond laser ablation for hierarchical micro/nanostructuring. Int. J. Extreme Manuf. 2020, 2, 015001. [Google Scholar] [CrossRef]
- Yin, W.; Cai, Y.; Xie, L.; Huang, H.; Zhu, E.; Pan, J.; Bu, J.; Chen, H.; Yuan, Y.; Zhuang, Z.; et al. Revisited electrochemical gas evolution reactions from the perspective of gas bubbles. Nano Res. 2022, 16, 4381–4398. [Google Scholar] [CrossRef]
- Li, M.; Yin, W.; Pan, J.; Zhu, Y.; Sun, N.; Zhang, X.; Wan, Y.; Luo, Z.; Yi, L.; Wang, L. Hydrogen spillover as a promising strategy for boosting heterogeneous catalysis and hydrogen storage. Chem. Eng. J. 2023, 471, 144691. [Google Scholar] [CrossRef]
- He, S.; Wang, K.; Li, B.; Du, H.; Du, Z.; Wang, T.; Li, S.; Ai, W.; Huang, W. The Secret of Nanoarrays toward Efficient Electrochemical Water Splitting: A Vision of Self-Dynamic Electrolyte. Adv. Mater. 2023, 35, e2307017. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Feng, L.; Ye, C.; Xue, X.; Lin, D.; Eisenberg, S.; Kou, T.; Duoss, E.B.; Zhu, C.; Li, Y. Nanocone-Modified Surface Facilitates Gas Bubble Detachment for High-Rate Alkaline Water Splitting. Adv. Energy Mater. 2023, 13, 2302073. [Google Scholar] [CrossRef]
- Wang, J.; Liang, C.; Ma, X.; Liu, P.; Pan, W.; Zhu, H.; Guo, Z.; Sui, Y.; Liu, H.; Liu, L.; et al. Dynamically Adaptive Bubbling for Upgrading Oxygen Evolution Reaction Using Lamellar Fern-Like Alloy Aerogel Self-Standing Electrodes. Adv. Mater. 2023, 36, e2307925. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, T.; He, S.; Li, B.; Wang, K.; Chen, Q.; Du, Z.; Ai, W.; Huang, W. Mountain-Shaped Nickel Nanostripes Enabled by Facet Engineering of Nickel Foam: A New Platform for High-Current-Density Water Splitting. Adv. Funct. Mater. 2023, 34, 2311854. [Google Scholar] [CrossRef]
- Kim, J.; Jung, S.M.; Lee, N.; Kim, K.S.; Kim, Y.T.; Kim, J.K. Efficient Alkaline Hydrogen Evolution Reaction Using Superaerophobic Ni Nanoarrays with Accelerated H2 Bubble Release. Adv. Mater. 2023, 35, e2305844. [Google Scholar] [CrossRef]
- Yu, X.; Yu, Z.Y.; Zhang, X.L.; Zheng, Y.R.; Duan, Y.; Gao, Q.; Wu, R.; Sun, B.; Gao, M.R.; Wang, G.; et al. “Superaerophobic” Nickel Phosphide Nanoarray Catalyst for Efficient Hydrogen Evolution at Ultrahigh Current Densities. J. Am. Chem. Soc. 2019, 141, 7537–7543. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yuan, L.; Huang, H.; Cai, Y.; Pan, J.; Sun, N.; Zhang, Q.; Shu, Q.; Gu, C.; Zhuang, Z.; et al. Strategies to accelerate bubble detachment for efficient hydrogen evolution. Chin. Chem. Lett. 2024, 35, 108351. [Google Scholar] [CrossRef]
- Tan, B.H.; An, H.; Ohl, C.-D. Stability of surface and bulk nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101428. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Liu, X.; Zhao, W.; Liu, S.; Huang, X.; Zhao, Q. Tetra-Coordinated W2S3 for Efficient Dual-pH Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2024, 63, e202316306. [Google Scholar] [CrossRef]
- Liu, H.; Xie, R.; Luo, Y.; Cui, Z.; Yu, Q.; Gao, Z.; Zhang, Z.; Yang, F.; Kang, X.; Ge, S.; et al. Dual interfacial engineering of a Chevrel phase electrode material for stable hydrogen evolution at 2500 mA cm−2. Nat. Commun. 2022, 13, 6382. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Sun, N.; Xie, L.; Zhi, T.; Zhang, Q.; Luo, Z.; Liu, X.; Liu, S.; Zhao, Q. Boosting hydrogen evolution on MoS2 via synergistic regulation of interlayer dislocations and interlayer spacing. Chem. Eng. J. 2023, 474, 145792. [Google Scholar] [CrossRef]
- Obayashi, I.; Nakamura, T.; Hiraoka, Y. Persistent Homology Analysis for Materials Research and Persistent Homology Software: HomCloud. J. Phys. Soc. Jpn. 2022, 91, 091013. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, C.; Tian, Q.; Yu, D. Liquid metals dealloying as a general approach for the selective extraction of metals and the fabrication of nanoporous metals: A review. Mater. Today Commun. 2021, 26, 102007. [Google Scholar] [CrossRef]
- He, L.; Wang, H.; Chen, L.; Wang, X.; Xie, H.; Jiang, C.; Li, C.; Elibol, K.; Meyer, J.; Watanabe, K.; et al. Isolating hydrogen in hexagonal boron nitride bubbles by a plasma treatment. Nat. Commun. 2019, 10, 2815. [Google Scholar] [CrossRef]
- Hu, S.; Setyawan, W.; Beeler, B.W.; Gan, J.; Burkes, D.E. Defect cluster and nonequilibrium gas bubble associated growth in irradiated UMo fuels—A cluster dynamics and phase field model. J. Nucl. Mater. 2020, 542, 152441. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Z.; Cao, S.; Hu, Y.; Liu, S.; Chen, X.; Chen, H.; Zhang, X.; Wei, S.; Xu, H.; et al. Bioinspired trimesic acid anchored electrocatalysts with unique static and dynamic compatibility for enhanced water oxidation. Nat. Commun. 2023, 14, 6714. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Zhao, W.; Liu, S.; Huang, W.; Zhao, Q. WS2 moire superlattices derived from mechanical flexibility for hydrogen evolution reaction. Nat. Commun. 2021, 12, 5070. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J. Design and construction of on-surface molecular nanoarchitectures: Lessons and trends from trimesic acid and other small carboxlyated building blocks. J. Phys. D Appl. Phys. 2020, 53, 043002. [Google Scholar] [CrossRef]
- Xin, L.; Hu, J.; Wu, X.; Huang, K.; Huang, X. Fenton-like degradation of carmine dyes based on artificial intelligence modeling and optimization of reduced graphene oxide loaded iron-cobalt-nickel trimetallic nanocomposites. Mater. Today Commun. 2022, 31, 103463. [Google Scholar] [CrossRef]
- Kou, T.; Wang, S.; Shi, R.; Zhang, T.; Chiovoloni, S.; Lu, J.Q.; Chen, W.; Worsley, M.A.; Wood, B.C.; Baker, S.E.; et al. Periodic Porous 3D Electrodes Mitigate Gas Bubble Traffic during Alkaline Water Electrolysis at High Current Densities. Adv. Energy Mater. 2020, 10, 2002955. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lim, A.; Kim, J.M.; Lim, D.; Chae, K.H.; Cho, E.N.; Han, H.J.; Jeon, K.U.; Kim, M.; Lee, G.H.; et al. Highly efficient oxygen evolution reaction via facile bubble transport realized by three-dimensionally stack-printed catalysts. Nat. Commun. 2020, 11, 4921. [Google Scholar] [CrossRef]
- Liu, Z.; Du, Y.; Yu, R.; Zheng, M.; Hu, R.; Wu, J.; Xia, Y.; Zhuang, Z.; Wang, D. Tuning Mass Transport in Electrocatalysis Down to Sub-5 nm through Nanoscale Grade Separation. Angew. Chem. Int. Ed. Engl. 2023, 62, e202212653. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, H.; Lu, D.; Lin, Y.; Liu, J.; Liu, Q.; Nie, Z.; Jiang, G. Mass spectrometry for multi-dimensional characterization of natural and synthetic materials at the nanoscale. Chem. Soc. Rev. 2021, 50, 5243–5280. [Google Scholar] [CrossRef] [PubMed]




| Catalyst | η@10 mA cm–2 (mV) | η@100 mA cm–2 (mV) | η@500 mA cm–2 (mV) | η@1000 mA cm–2 (mV) | Tafel Slope (mV dec–1) | Ref. |
|---|---|---|---|---|---|---|
| sFNSNA | 191 | 255 | - | - | - | [109] |
| LFA | 192 | - | 238 | 242 | 29.2 | [111] |
| NFMN-FeOOH | - | - | 308 | - | 36 | [112] |
| Ni-80 | 135 | 271 | - | - | - | [113] |
| CuMo6S8/Cu | - | - | - | - | 32.5 | [118] |
| SU-NiFe-LDH(TA) | 219 | - | - | - | 31.1 | [124] |
| Ru/3d-OMC | 20 | - | - | - | 46.68 | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Gu, C.; Wang, K.; Yu, H.; Qiu, J.; Wang, S.; Wang, L.; Yan, D. Bubbles Management for Enhanced Catalytic Water Splitting Performance. Catalysts 2024, 14, 254. https://doi.org/10.3390/catal14040254
Zhang Z, Gu C, Wang K, Yu H, Qiu J, Wang S, Wang L, Yan D. Bubbles Management for Enhanced Catalytic Water Splitting Performance. Catalysts. 2024; 14(4):254. https://doi.org/10.3390/catal14040254
Chicago/Turabian StyleZhang, Zheng, Chen Gu, Kun Wang, Haoxuan Yu, Jiaxuan Qiu, Shiyan Wang, Longlu Wang, and Dafeng Yan. 2024. "Bubbles Management for Enhanced Catalytic Water Splitting Performance" Catalysts 14, no. 4: 254. https://doi.org/10.3390/catal14040254
APA StyleZhang, Z., Gu, C., Wang, K., Yu, H., Qiu, J., Wang, S., Wang, L., & Yan, D. (2024). Bubbles Management for Enhanced Catalytic Water Splitting Performance. Catalysts, 14(4), 254. https://doi.org/10.3390/catal14040254







