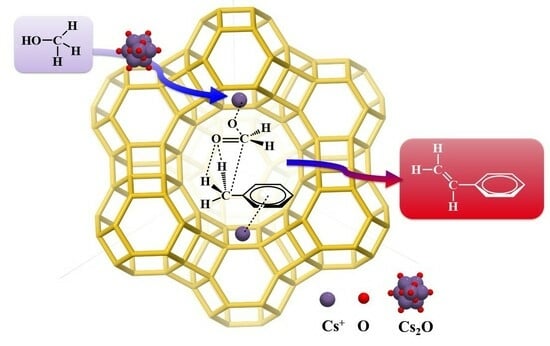
Investigating the Role of Cs Species in the Toluene–Methanol Side Chain Alkylation Catalyzed by CsX Catalysts
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Structure and Acid–Base Properties of CsX
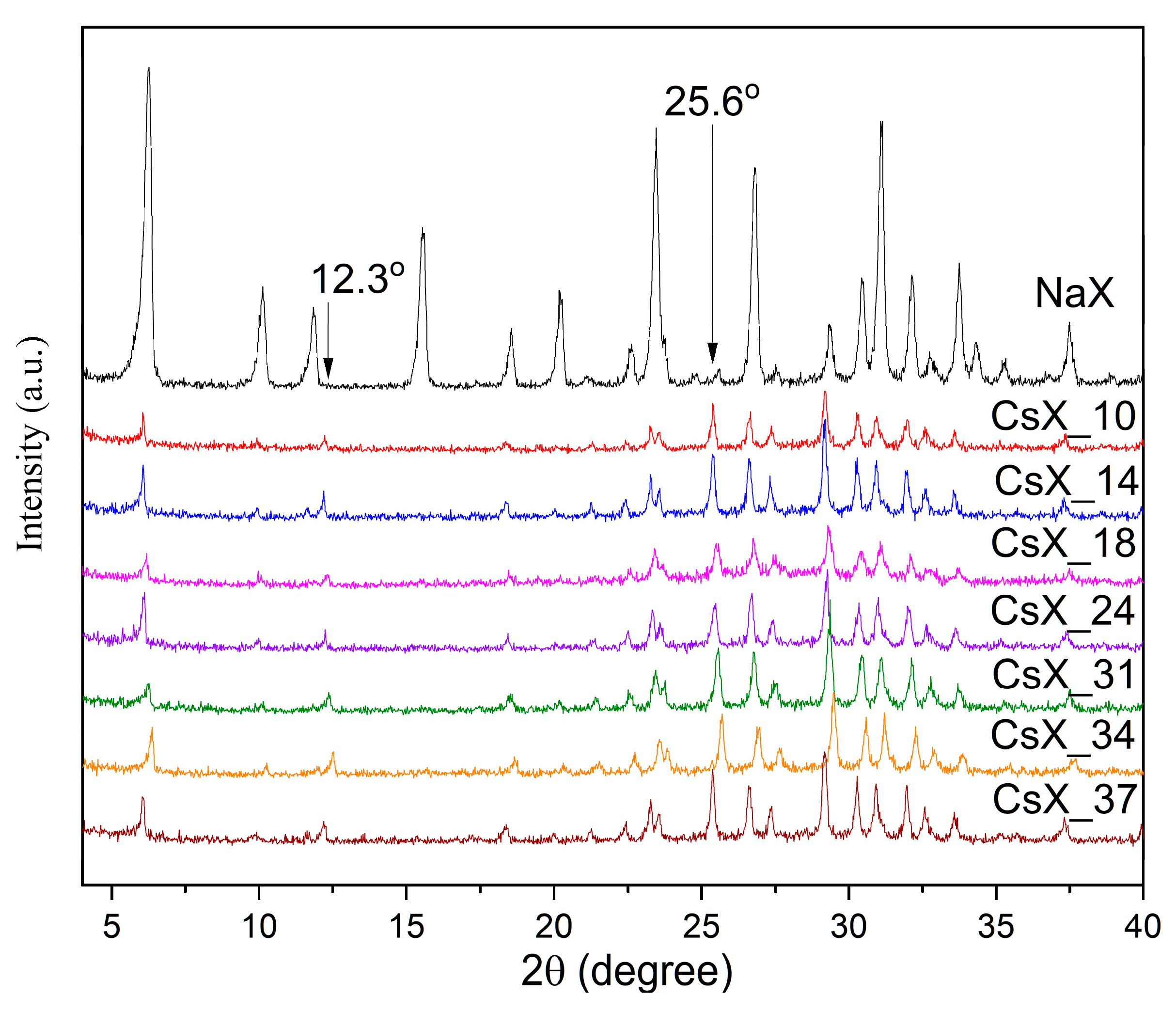
2.1.1. X-ray Diffraction (XRD) Characterization
2.1.2. Analysis of Cs Content and Specific Surface Areas in CsX
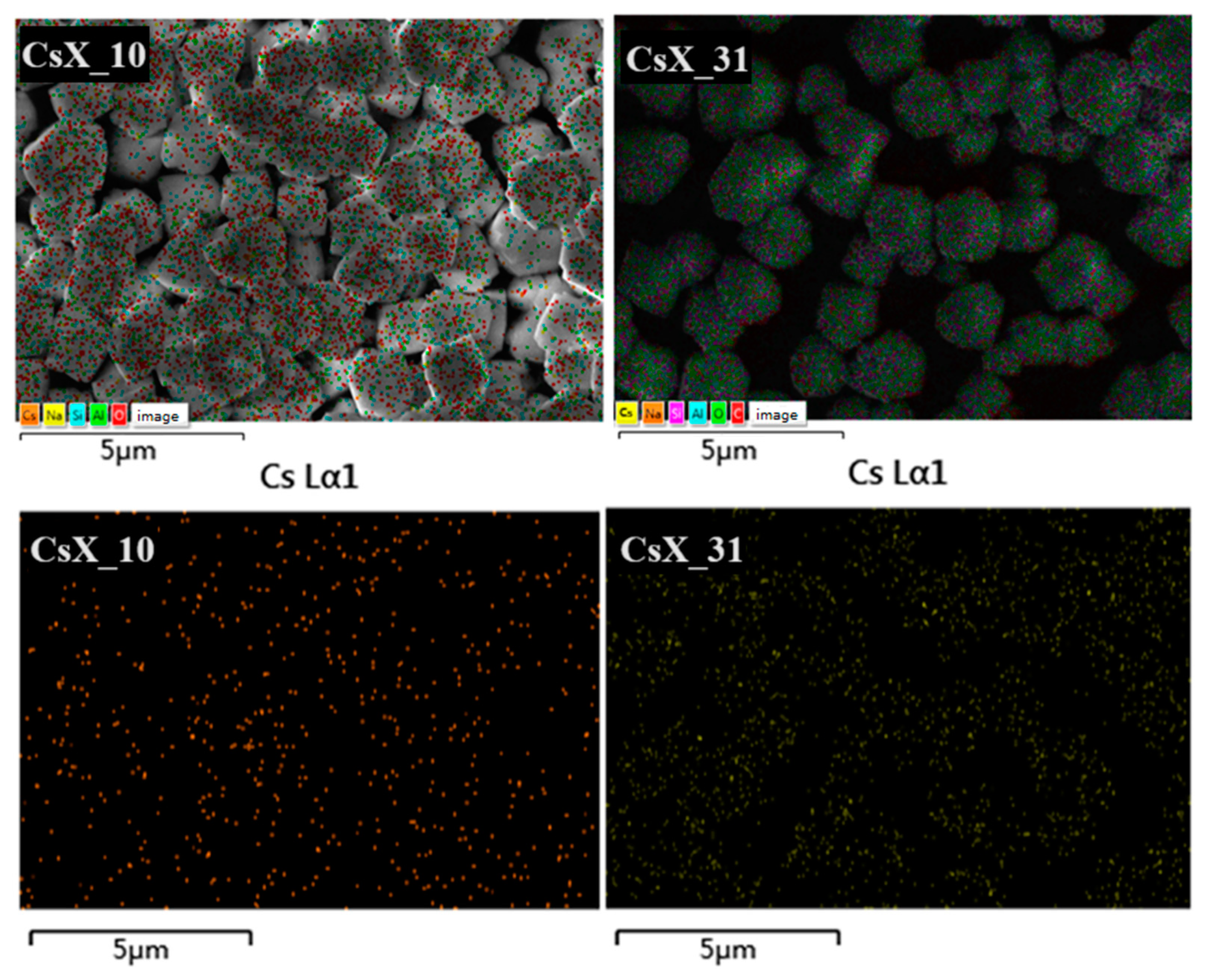
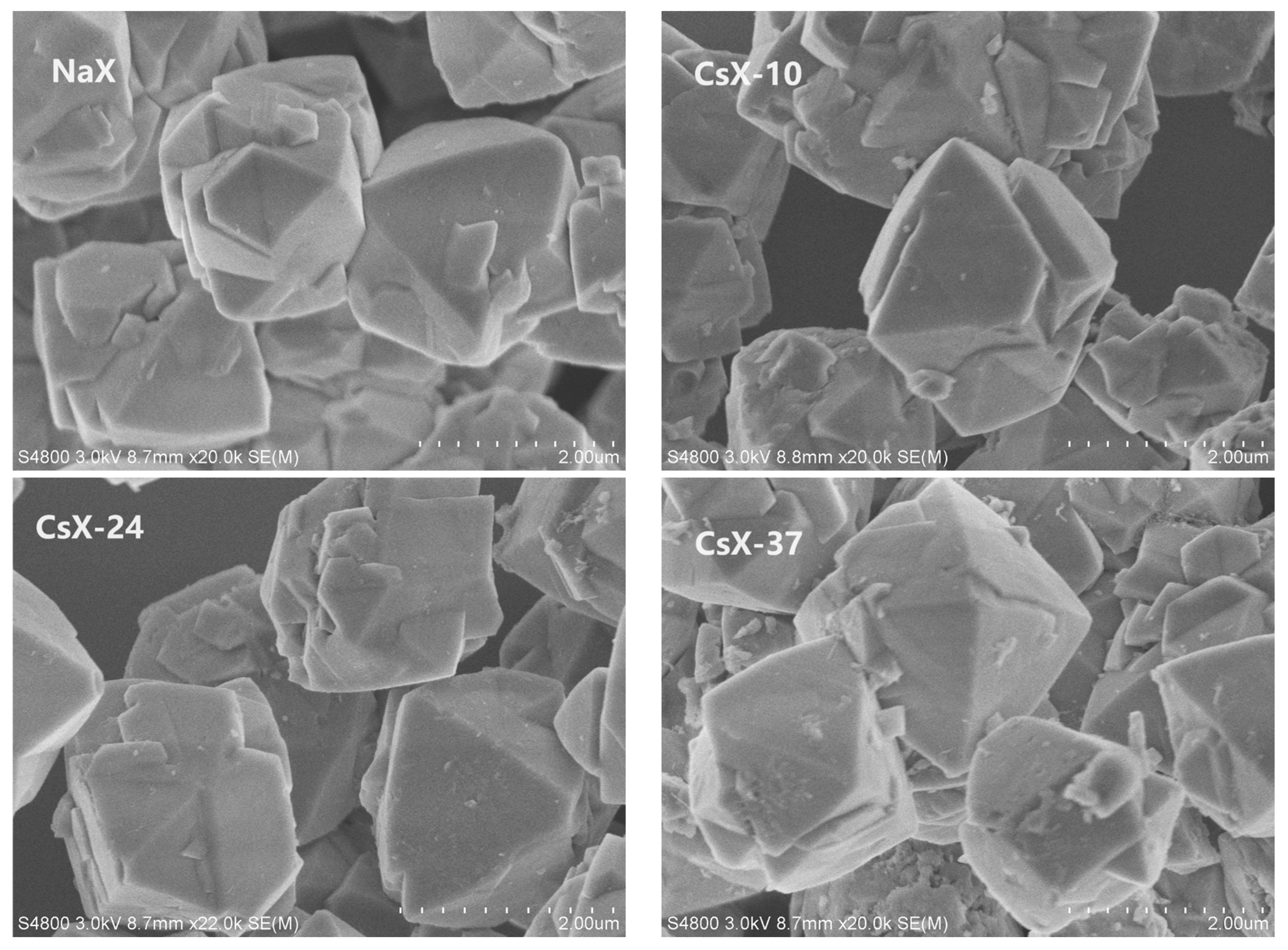
2.1.3. Scanning Electron Microscopy (SEM) Characterization
2.1.4. Characterization of Acid–Base Center Properties
2.1.5. States of Cs Species and Oxygen Species
2.2. Adsorption and Activation Properties of Methanol on CsX
2.2.1. Adsorption of Methanol on CsX (In Situ Infrared Characterization)
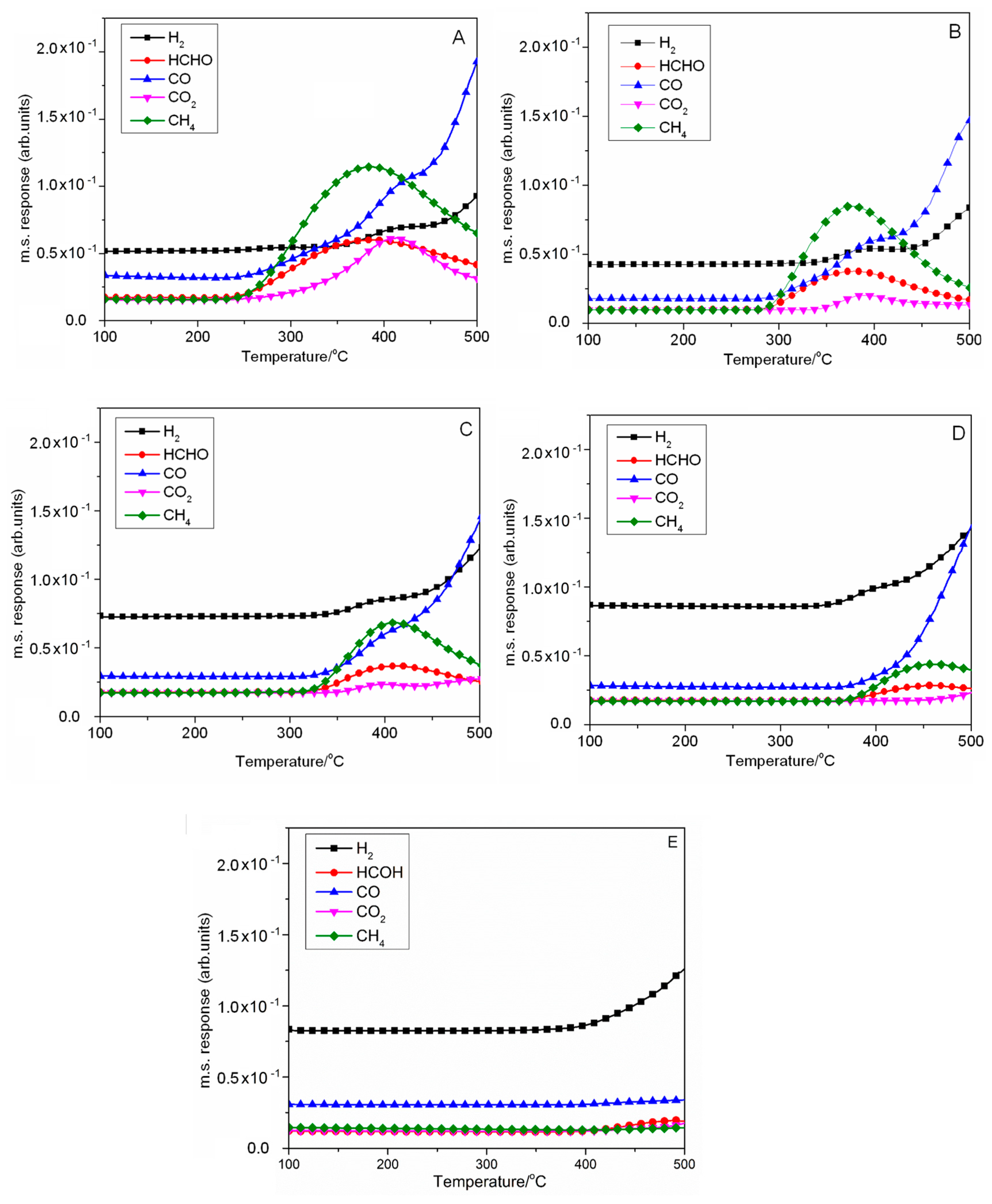
2.2.2. Decomposition of Methanol on CsX (TPD-MS Analysis)
2.3. The Effect of the Ion Exchange Temperature on Catalytic Performance
2.4. Properties of Active Centers in CsX Catalysts
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalytic Experiments
4.3. Catalyst Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kovacevic, M.; Agarwal, S.; Mojet, B.L.; Ommen, J.G.V.; Lefferts, L. The effects of morphology of cerium oxide catalysts for dehydrogenation of ethylbenzene to styrene. Appl. Catal. A Gen. 2015, 505, 354–364. [Google Scholar] [CrossRef]
- Jin, M.; Feng, X.; Feng, L.; Sun, T.; Zhai, J.; Li, T.; Jiang, L. Superhydrophobic Aligned Polystyrene Nanotube Films with High Adhesive Force. Adv. Mater. 2005, 17, 1977–1981. [Google Scholar] [CrossRef]
- Arrighi, V.; McEwen, I.J.; Qian, H.; Serrano Prieto, M.B. The glass transition and interfacial layer in styrene-butadiene rubber containing silica nanofiller. Polymer 2003, 44, 6259–6266. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Borgna, A.; Magni, S.; Sepulveda, J.; Padro, C.L.; Apesteguia, C.R. Side-chain alkylation of toluene with methanol on Cs-exchanged NaY zeolites: Effect of Cs loading. Catal. Lett. 2005, 102, 15–21. [Google Scholar] [CrossRef]
- Song, L.; Li, Z.; Zhang, R.; Zhao, L.; Li, W. Alkylation of toluene with methanol: The effect of K exchange degree on the direction to ring or side-chain alkylation. Catal. Commun. 2012, 19, 90–95. [Google Scholar] [CrossRef]
- Han, H.; Liu, M.; Ding, F.; Wang, Y.; Guo, X.; Song, C. Effects of Cesium Ions and Cesium Oxide in Side-Chain Alkylation of Toluene with Methanol over Cesium-Modified Zeolite X. Ind. Eng. Chem. Res. 2016, 55, 1849–1858. [Google Scholar] [CrossRef]
- Hunger, M.; Schenk, U.; Weitkamp, J. Mechanistic studies of the side-chain alkylation of toluene with methanol on basic zeolites Y by multi-nuclear NMR spectroscopy1Dedicated to Professor Herman van Bekkum on the occasion of his 65th birthday 1. J. Mol. Catal. A Chem. 1998, 134, 97–109. [Google Scholar] [CrossRef]
- Alabi, W.O.; Tope, B.B.; Jermy, R.B.; Aitani, A.M.; Hattori, H.; Al-Khattaf, S.S. Modification of Cs-X for styrene production by side-chain alkylation of toluene with methanol. Catal. Today 2014, 226, 117–123. [Google Scholar] [CrossRef]
- Palomares, A.E.; Eder-Mirth, G.; Rep, M.; Lercher, J.A. Alkylation of Toluene over Basic Catalysts—Key Requirements for Side Chain Alkylation. J. Catal. 1998, 180, 56–65. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, G.; Miao, C.; Wu, X.; Wu, W.; Sun, Q. Catalytic performance of X molecular sieve modified by alkali metal ions for the side-chain alkylation of toluene with methanol. Microporous Mesoporous Mater. 2013, 167, 213–220. [Google Scholar] [CrossRef]
- Hong, Z.; Xiong, C.; Zhao, G.; Zhu, Z. Side-chain alkylation of toluene with methanol to produce styrene: An overview. Catal. Sci. Technol. 2019, 9, 6828–6840. [Google Scholar] [CrossRef]
- Vadhri, V.M.; Hickey, T.; Mukherjee, M. Exelus Styrene Monomer (ExSyM) Process (Case Study). In Industrial Arene Chemistry; Wiley-VCH GmbH: Hoboken, NJ, USA, 2023; pp. 673–713. [Google Scholar]
- Diaz, E.; Munoz, E.; Vega, A.; Ordonez, S. Enhancement of the CO2 retention capacity of Y zeolites by Na and Cs treatments: Effect of adsorption temperature and water treatment. Ind. Eng. Chem. Res. 2008, 47, 412–418. [Google Scholar] [CrossRef]
- Liu, H.; Oakland, N.J. Modified Zeolite Catalyst Composition and Process for Alkylating Toluene with Methanol to Form Styrene. U.S. Patent 4,499,318 A, 12 February 1985. [Google Scholar]
- Unland, M.L. Infrared study of methanol decomposition on alkali metal X-type zeolites. J. Phys. Chem. 1978, 82, 580–583. [Google Scholar] [CrossRef]
- Hong, Z.; Zhao, G.; Huang, F.; Wang, X.; Zhu, Z. Enhancing the side-chain alkylation of toluene with methanol to styrene over the Cs-modified X zeolite by the assistance of basic picoline as a co-catalyst. Green Energy Environ. 2022, 7, 1241–1252. [Google Scholar] [CrossRef]
- Hong, Z.; Xiong, C.; Wang, X.; Huang, F.; Li, L.; Zhu, Z. Ammonia pools effect in Cs modified X zeolites for side-chain alkylation of toluene with methanol. Chem. Eng. J. 2023, 474, 145650. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, W.; Li, H.; Zhu, W.; Zhang, N.; Tang, Y.; Yu, J.; Jia, M.; Zhang, W.; Zhang, C. Side-chain alkylation of toluene with methanol over boron phosphate modified cesium ion-exchanged zeolite X catalysts. J. Porous Mater. 2015, 22, 1179–1186. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Yuan, X.; Miao, S.; Li, H.; Shan, W.; Jia, M. Effect of Fe Additives on the Catalytic Performance of Ion-Exchanged CsX Zeolites for Side-Chain Alkylation of Toluene with Methanol. Catalysts 2019, 9, 829. [Google Scholar] [CrossRef]
- Engelhardt, J.; Szanyi, J.; Valyon, J. Alkylation of toluene with methanol on commercial X zeolite in different alkali cation forms. J. Catal. 1987, 107, 296–306. [Google Scholar] [CrossRef]
- Romero, M.D.; Ovejero, G.; Uguina, M.A.; Rodrı’guez, A.; Gómez, J.M. Fast tailoring of the acid–base properties in the NaX zeolite by cesium-exchange under microwave heating. Microporous Mesoporous Mater. 2007, 98, 317–322. [Google Scholar] [CrossRef]
- Hathaway, P.E.; Davis, M.E. Base catalysis by alkali modified zeolites: III. Alkylation with methanol. J. Catal. 1989, 119, 497–507. [Google Scholar] [CrossRef]
- Nováková, J.; Kubelková, L.; Habersberger, K.; Dolejšek, Z. Catalytic Activity of Dealuminated Y and HZSM-5 Zeolites Measured by the Temperature-programmed Desorption of Small Amounts of Preadsorbed Methanol and by the Low-pressure Flow Reaction of Methanol. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1984, 80, 1457–1465. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Zhao, G.; Gu, H.; Zhu, Z. Free radical mechanism investigation of the side-chain alkylation of toluene with methanol on basic zeolites X. Chin. J. Catal. 2015, 36, 1726–1732. [Google Scholar] [CrossRef]
- Yashima, T.; Sato, K.; Hayasaka, T.; Hara, N. Alkylation on synthetic zeolites: III. Alkylation of toluene with methanol and formaldehyde on alkali cation exchanged zeolites. J. Catal. 1972, 26, 303–312. [Google Scholar] [CrossRef]
- Wieland, W.S.; Davis, R.J.; Garces, J.M. Side-Chain Alkylation of Toluene with Methanol over Alkali-Exchanged Zeolites X, Y, L, and β. J. Catal. 1998, 173, 490–500. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, H.M.; Zhang, X.Y.; Lan, D.X.; Chun, Y. Formation and Function of Formate in the Side-Chain Alkylation of Toluene with Methanol. Chin. J. Catal. 2012, 33, 1041–1047. [Google Scholar] [CrossRef]
- Mielczarski, E.; Davis, M.E. Infrared investigations of the alkylation of toluene with methanol by alkali-modified zeolites. Ind. Eng. Chem. Res. 1991, 29, 288–291. [Google Scholar] [CrossRef]
- Palomares, A.E.; Eder-Mirth, G.; Lercher, J.A. Selective Alkylation of Toluene over Basic Zeolites: Anin SituInfrared Spectroscopic Investigation. J. Catal. 1997, 168, 442–449. [Google Scholar] [CrossRef]
- King, S.T.; Garces, J.M. In situ infrared study of alkylation of toluene with methanol on alkali cation-exchanged zeolites. J. Catal. 1987, 104, 59–70. [Google Scholar] [CrossRef]
- Borowiak, M.A.; Jamróz, M.H.; Larsson, R. Catalytic decomposition of formic acid on oxide catalysts—An impulse-oscillation model approach to the unimolecular mechanism. J. Mol. Catal. A Chem. 1999, 139, 97–104. [Google Scholar] [CrossRef]
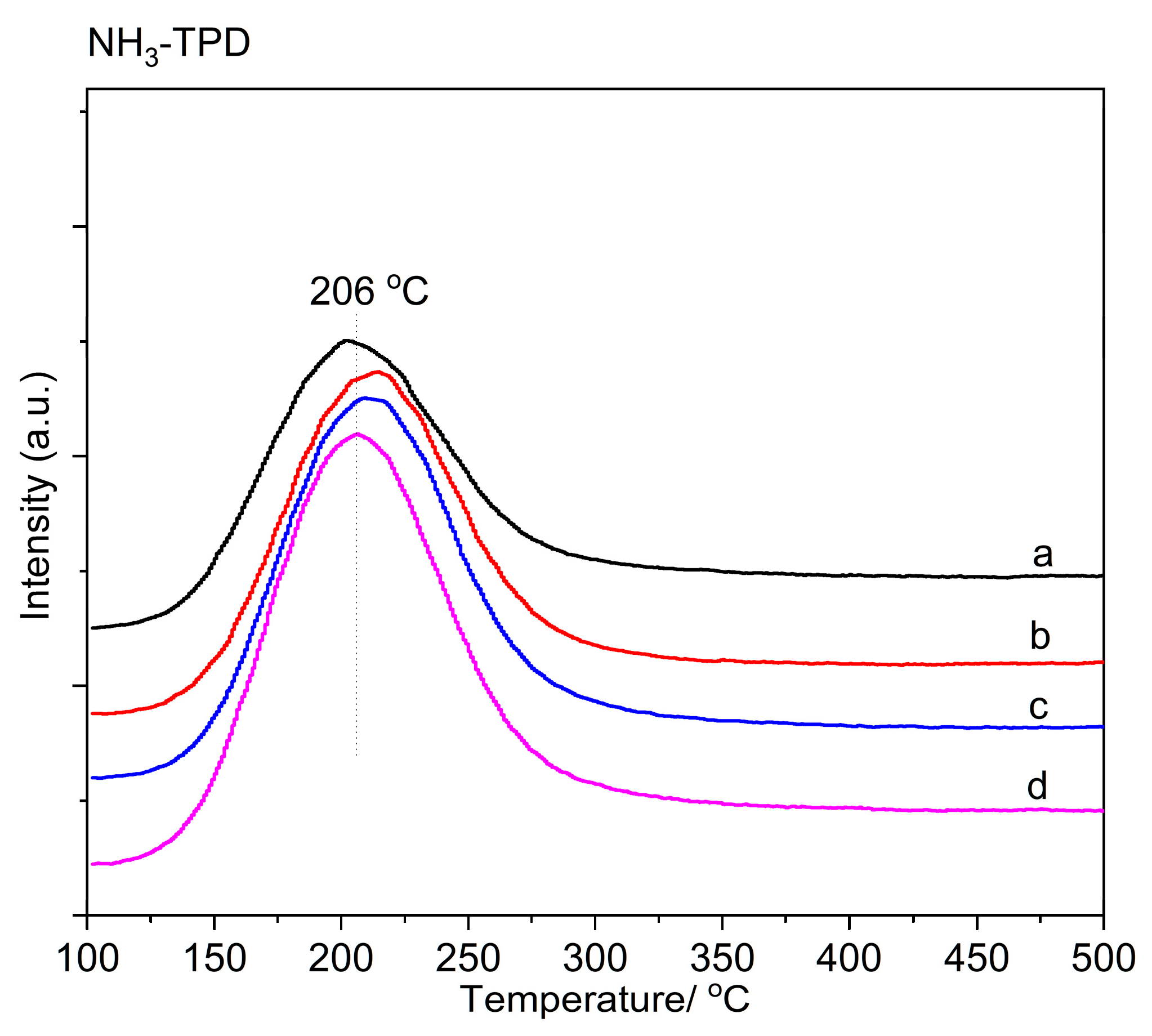
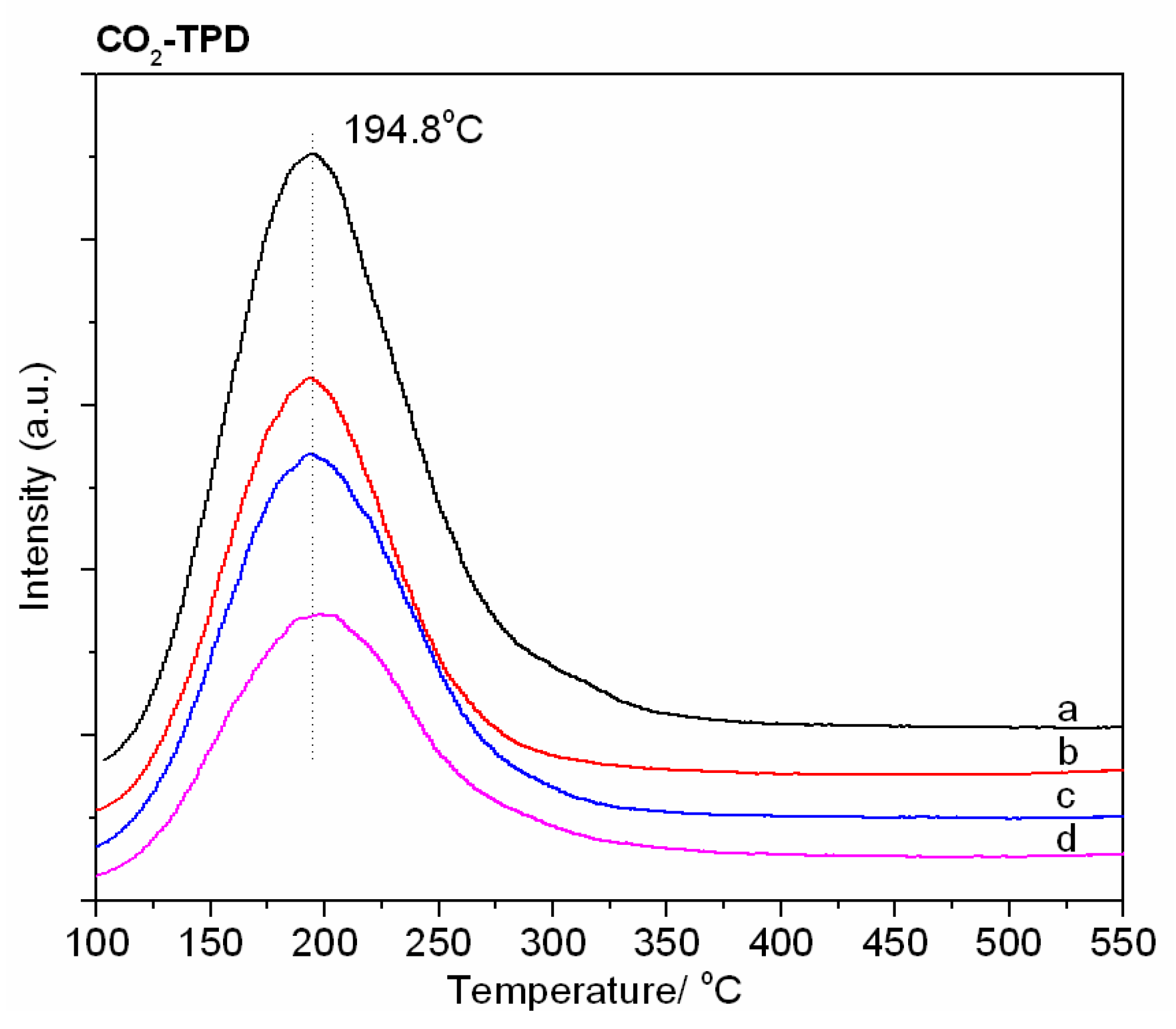
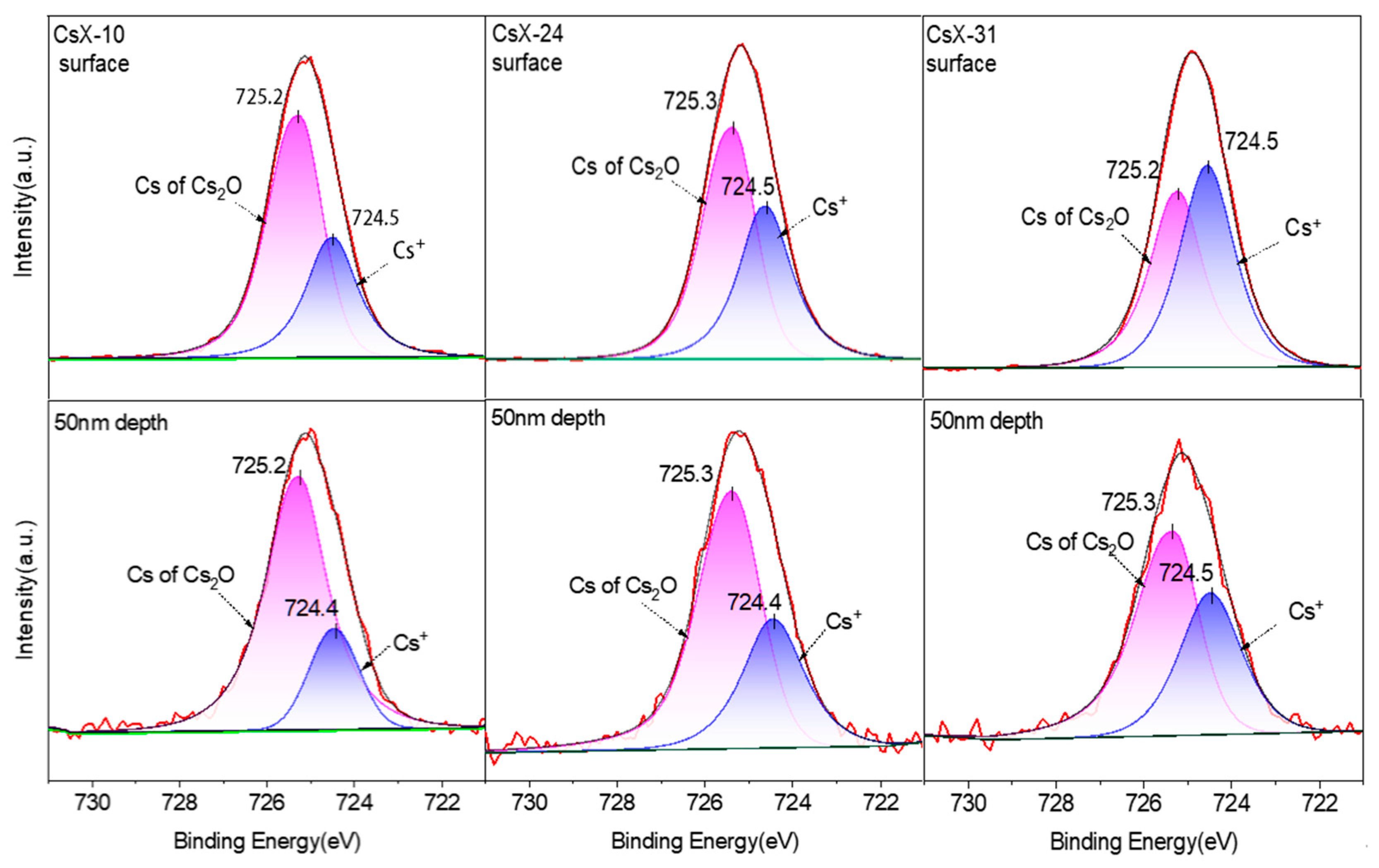

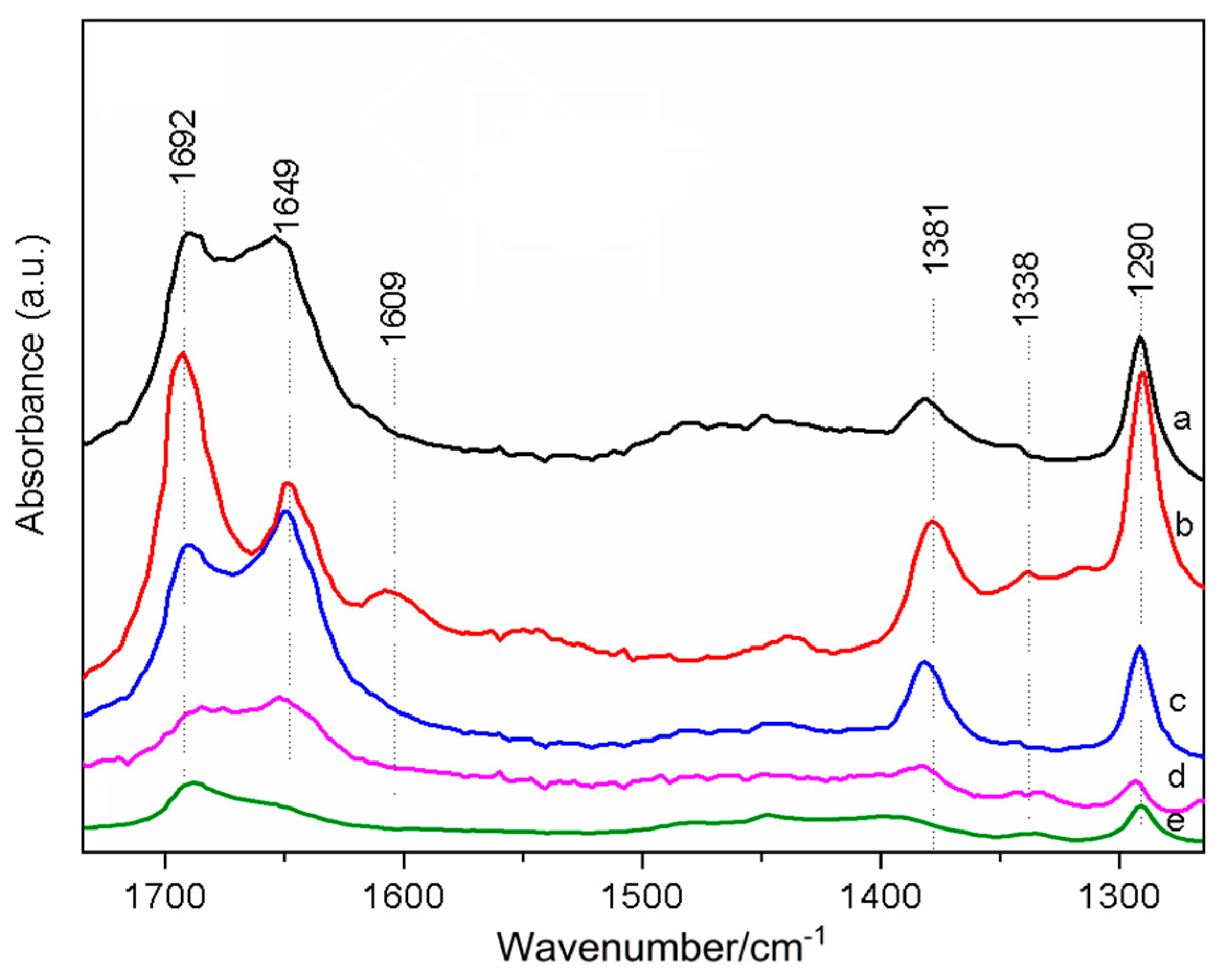

| Zeolite | Alkali Metal Content (wt%) | Exchange Degree b (%) | BET Surface Area (m2·g−1) | DCs2O c | |
|---|---|---|---|---|---|
| Na a | Cs a | ||||
| NaX | 14.09 | -- | -- | 677 | -- |
| CsX_10 | 11.02 | 10.12 | 13.7 | 351 | 51 |
| CsX_14 | 9.96 | 14.23 | 19.8 | 351 | 52 |
| CsX_18 | 8.90 | 18.19 | 26.1 | 352 | 55 |
| CsX_24 | 7.65 | 24.04 | 35.2 | 328 | 60 |
| CsX_31 | 5.63 | 31.78 | 49.4 | 341 | 65 |
| CsX_34 | 4.65 | 34.21 | 56.0 | 349 | 69 |
| CsX_37 | 3.91 | 37.83 | 62.6 | 386 | 76 |
| Zeolite | Chemical Composition (wt%) a | ||||
|---|---|---|---|---|---|
| Cs | Si | Na | Al | O | |
| NaX | -- | 23.5 | 10.6 | 18.8 | 47.1 |
| CsX_10 | 13.3 | 12.5 | 10.1 | 24.1 | 40.0 |
| CsX_14 | 14.5 | 12.5 | 9.2 | 24.1 | 39.7 |
| CsX_18 | 17.6 | 12.1 | 8.6 | 23.2 | 38.5 |
| CsX_24 | 23.3 | 11.3 | 7.5 | 21.7 | 36.2 |
| CsX_31 | 31.9 | 10.2 | 5.3 | 19.7 | 32.9 |
| CsX_34 | 35.3 | 9.9 | 4.2 | 19.0 | 31.7 |
| CsX_37 | 35.2 | 10.0 | 3.7 | 19.2 | 31.9 |
| XPS Spectra | Cs 3d5/2 | O 1s | |||
|---|---|---|---|---|---|
| Binding Energy (eV) | 724.4 | 725.2 | 531.7–532.2 | 530.9 | |
| Assignment | Cs of Cs+ | Cs of Cs2O | O of Framework | O of Cs2O | |
| Catalyst | Depth | ηCsion (%) | ηCsoxi (%) | ηOfra (%) | ηOoxi (%) |
| CsX-10 | Surface | 33.11 | 66.89 | 44.84 | 55.16 |
| 50 nm | 17.94 | 78.52 | 76.75 | 23.25 | |
| CsX-24 | Surface | 41.08 | 58.92 | 48.50 | 51.50 |
| 50 nm | 33.33 | 66.67 | 70.91 | 29.09 | |
| CsX-31 | Surface | 52.29 | 47.71 | 62.62 | 37.38 |
| 50 nm | 39.17 | 60.83 | 66.04 | 33.96 | |
| Catalyst | Temperature of Ion Exchange/°C | Cs Content /wt% | CTol 5 | SSt 5 | Sothers 2 | SSt+E 5 | YSt 5 | YSt+E 5 | UMe 5 |
|---|---|---|---|---|---|---|---|---|---|
| CsX_10 3 | 60 | 10.1 | 2.0 | 63.7 | 4.7 | 95.3 | 1.3 | 1.9 | 7.2 |
| CsX_14 3 | 70 | 14.2 | 2.5 | 58.0 | 4.9 | 95.1 | 1.4 | 2.3 | 8.1 |
| CsX_18 3 | 90 | 18.2 | 3.0 | 55.5 | 2.0 | 98.0 | 1.7 | 3.0 | 10.4 |
| CsX_24 4 | 60 | 24.0 | 4.6 | 24.2 | 2.3 | 97.7 | 1.1 | 4.5 | 14.2 |
| CsX_31 4 | 70 | 31.8 | 6.5 | 20.6 | 2.1 | 97.9 | 1.3 | 6.4 | 19.2 |
| CsX_34 4 | 80 | 34.2 | 6.3 | 22.9 | 2.3 | 97.7 | 1.4 | 6.2 | 18.9 |
| CsX_37 4 | 90 | 37.8 | 2.3 | 31.8 | 1.7 | 98.3 | 0.7 | 2.3 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, Q.; Gao, W.; Ma, C.; Yang, M. Investigating the Role of Cs Species in the Toluene–Methanol Side Chain Alkylation Catalyzed by CsX Catalysts. Catalysts 2024, 14, 256. https://doi.org/10.3390/catal14040256
Zhang Z, Wang Q, Gao W, Ma C, Yang M. Investigating the Role of Cs Species in the Toluene–Methanol Side Chain Alkylation Catalyzed by CsX Catalysts. Catalysts. 2024; 14(4):256. https://doi.org/10.3390/catal14040256
Chicago/Turabian StyleZhang, Zhihui, Qingwei Wang, Wenxiu Gao, Chunxiang Ma, and Miaomiao Yang. 2024. "Investigating the Role of Cs Species in the Toluene–Methanol Side Chain Alkylation Catalyzed by CsX Catalysts" Catalysts 14, no. 4: 256. https://doi.org/10.3390/catal14040256
APA StyleZhang, Z., Wang, Q., Gao, W., Ma, C., & Yang, M. (2024). Investigating the Role of Cs Species in the Toluene–Methanol Side Chain Alkylation Catalyzed by CsX Catalysts. Catalysts, 14(4), 256. https://doi.org/10.3390/catal14040256