Fungal Consortia Mediated Bio-Treatment of Organic Matter and Metals Uptake from Sewage Water: Maize Agro-Physiological Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sewage Water Analysis
2.2. Screening of Single and Consortia Fungal Culture for COD Removal Efficiency
2.3. Effect of Fungal Consortia Treatment on the Physicochemical Properties of SW
2.4. Impact of Sewage Water on Maize Plant Growth
2.4.1. Morphological Plant Growth Traits
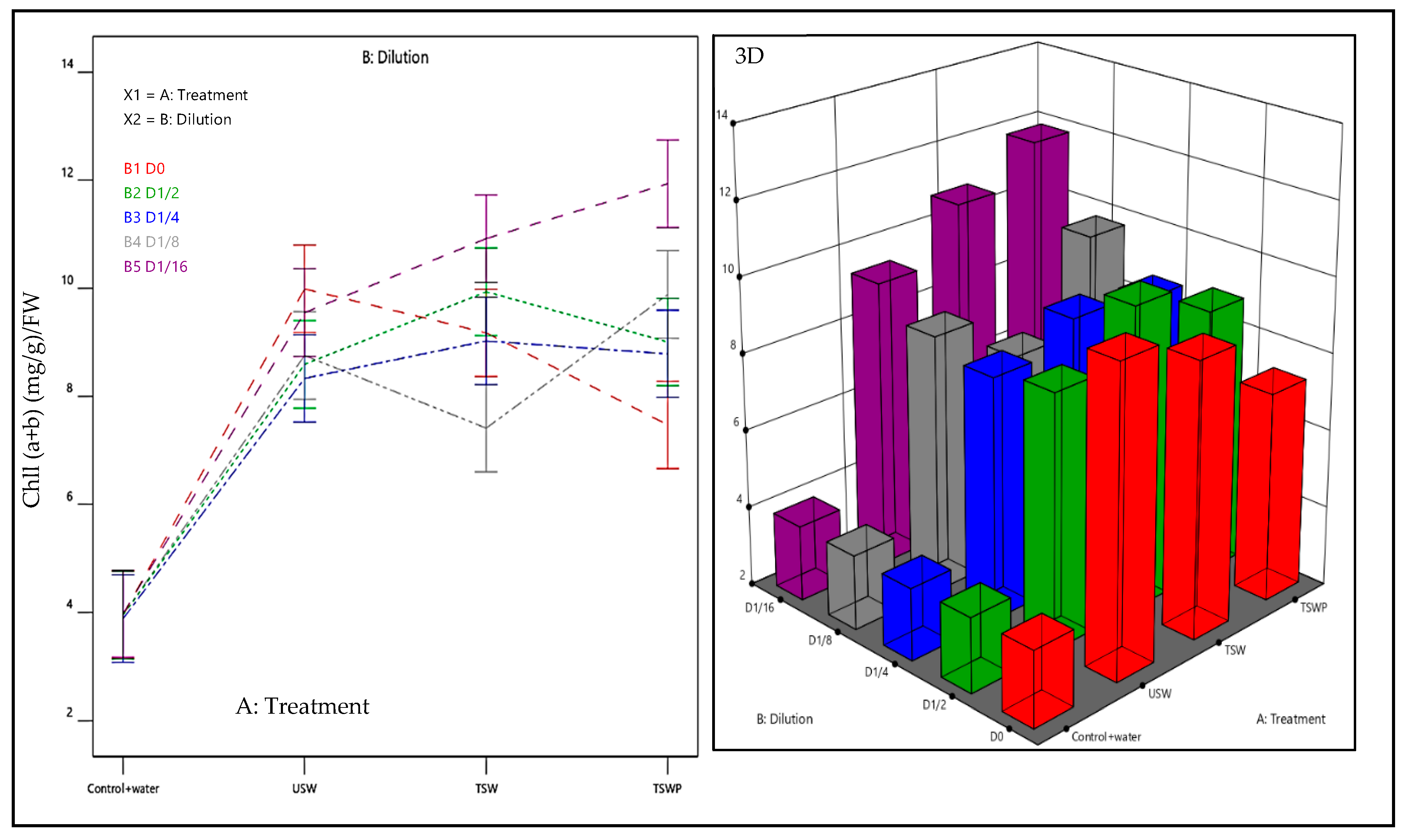
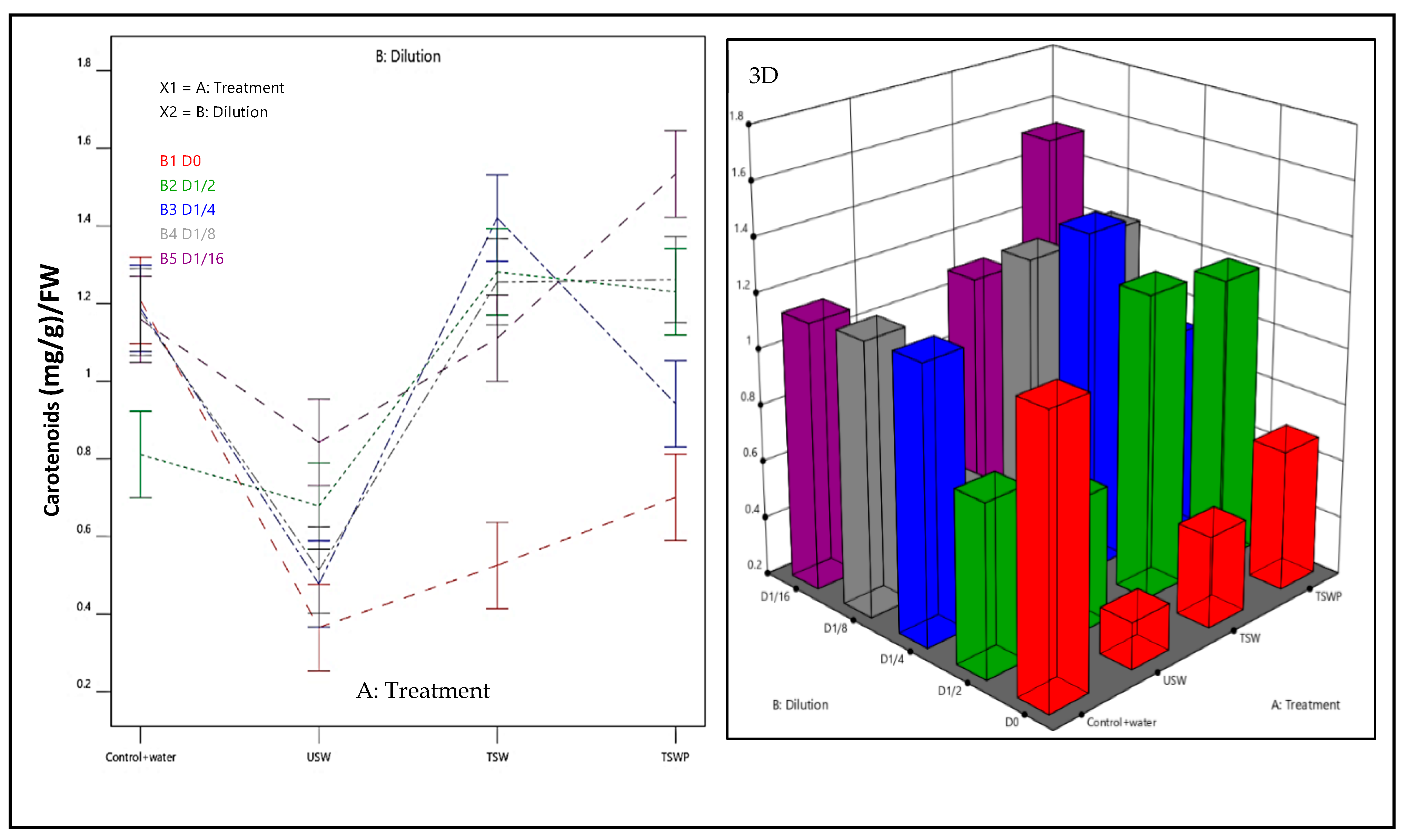
2.4.2. Physiological Traits
3. Materials and Methods
3.1. Chemicals
3.2. Fungal Strains
3.3. Irrigation Water Resources and Properties
3.4. Fungal Cultivation in Shake Flasks
3.5. Screening of Single and Consortia Fungal Culture for COD Removal Efficiency
3.6. Pot Experiment Set-Up and Plant Growth
3.7. Plant Sample Collection and Preparation
3.8. Growth Traits and Yield Parameters
3.8.1. Morphological Traits
3.8.2. Photosynthetic Pigments: Chlorophyll (a + b) and Carotenoids
3.8.3. Antioxidative Response to the SW-Irrigation
- Estimation of total phenolic content (TPC)
- b.
- Antioxidant enzymes: Peroxidase and catalase activities
3.9. Statistical Analysis
3.9.1. Graphical Interpretation
- Response R1: Total Phenols Content (TPC)
- b.
- Response R2 and R3: Total Chlorophyll Chll (a + b) and carotenoids
- c.
- Responses R4 and R5: Peroxidase POD and Catalase CAT Enzymes Activities
3.9.2. Desirability Function (DF)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-Díaz, M.; Gil-Serna, J.; Vázquez, C.; Botia, M.N.; Patiño, B. A comprehensive study on the occurrence of mycotoxins and their producing fungi during the maize production cycle in Spain. Microorganisms 2020, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations FAO. FAOSTAT: Land Use; FAO: Rome, Italy. Available online: http://www.fao.org/faostat/en/#data/RL (accessed on 1 October 2022).
- International Water Assocoation IWA. The reuse opportunity. Atmos. Chem. Phys. Discuss. 2018, 1–26. [Google Scholar]
- UN-Water. Sustainable Development Goal 6 Synthesis Report on Water and Sanitation; UN: New York, NY, USA, 2018. [Google Scholar]
- Bijl, D.L.; Biemans, H.; Bogaart, P.W.; Dekker, S.C.; Doelman, J.C.; Stehfest, E.; van Vuuren, D.P. A global analysis of future water deficit based on different allocation mechanisms. Water Resour. Res. 2018, 54, 58035824. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Offices of Water and Wastewater and Compliance (Ed.) Guidelines for Water Reuse. U.S. EPA, Washington. WA State Water Strategy. 1992. Available online: http://fao.org/nr/water/aquastat/water_use/index.stm (accessed on 14 April 2018).
- United Nations World Water Assessment Programme. The United Nations World Water Development Report 2017: Wastewater: The Untapped Resource; UNESCO: Paris, France, 2017. [Google Scholar]
- Khaskhoussy, K.; Kahlaoui, B.; Misle, E.; Hachich, M. Accumulation of trace elements by corn (Zea mays L.) under irrigation with treated wastewater using different irrigation methods. Ecotoxicol. Environ. Saf. 2019, 170, 530–537. [Google Scholar] [CrossRef]
- Yadav, G.; Mishra, A.; Ghosh, P.; Sindhu, R.; Vinayak, V.; Pugazhendhi, A. Technical, economic and environmental feasibility of resource recovery technologies from wastewater. Sci. Total Environ. 2021, 796, 149022. [Google Scholar] [CrossRef] [PubMed]
- Natasha; Shahid, M.; Khalid, S.; Niazi, N.K.; Murtaza, B.; Ahmad, N.; Farooq, A.; Zakir, A.; Imran, M.; Abbas, G. Health risks of arsenic buildup in soil and food crops after wastewater irrigation. Sci. Total Environ. 2021, 772, 145266. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Deshbhratar, P.B.; Ramteke, D.S. Effects of sewage wastewater irrigation on soil properties, crop yield, and environment. Agric. Water Manag. 2012, 103, 100–104. [Google Scholar] [CrossRef]
- Gurjar, O.P.; Meena, R.; Latare, A.M.; Rai, S.; Kant, S.; Kumar, A.; Kumar, A.; Sheshama, M.K. Effects of sewage wastewater irrigation compare to ground water irrigation on soil physicochemical properties. Int. J. Chem. Stud. 2017, 5, 265–267. [Google Scholar]
- Boudjabi, S.; Chenchouni, H. On the sustainability of land applications of sewage sludge: How to apply the sewage biosolid in order to improve soil fertility and increase crop yield. Chemosphere 2021, 282, 131122. [Google Scholar] [CrossRef]
- Saxena, P.; Hiwrale, I.; Das, S.; Shukla, V.; Tyagi, L.; Dafale, N.; Pal, S.; Dhodapkar, R. Profiling of emerging contaminants and antibiotic resistance in sewage treatment plants: An Indian Perspective. J. Hazard. Mater. 2021, 408, 124877. [Google Scholar] [CrossRef]
- Rajasulochan, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour.-Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, B.; Guo, Z.; Tang, S.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Fungal mycelium modified hierarchical porous carbon with enhanced performance and its application for removal of organic pollutants. J. Environ. Chem. Eng. 2022, 10, 108699. [Google Scholar] [CrossRef]
- Ramya, D.; Jennifer Michellin Kiruba, N.; Joseph Thatheyus, A. Chapter 15—Biosorption of heavy metals using fungal biosorbents—A review. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Volume 2: Extremophilic Fungi and Myco-Mediated Environmental Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 331–352. [Google Scholar]
- Muys, M.; Cámara, S.J.G.; Derese, S.; Spiller, M.; Verliefde, A.; Vlaeminck, S.E. Dissolution rate and growth performance reveal struvite as a sustainable nutrient source to produce a diverse set of microbial protein. Sci. Total Environ. 2023, 866, 161172. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, F.; Kalavrouziotis, I.; José, J. Use of treated municipal wastewater in irrigated agriculture—Review of some practices in Spain and Greece. Agric. Water Manag. 2010, 97, 1233–1241. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water scarcity and wastewater reuse in crop irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Kama, R.; Liu, Y.; Song, J.; Hamani, A.K.M.; Zhao, S.; Li, S.; Diatta, S.; Yang, F.; Li, Z. Treated LivestockWastewater Irrigation Is Safe for Maize (Zea mays) and Soybean (Glycine max) Intercropping System Considering Heavy Metals Migration in Soil–Plant System. Int. J. Environ. Res. Public Health 2023, 20, 3345. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Natasha, N.; ·ALOthman, Z.A.; Al-Kahtani, A.A.; Murtaza, B. Plant Physiological Responses After Fresh and Sewage Water Irrigation: Plant Health Perspectives. Gesunde Pflanzen 2023, 75, 1289–1296. [Google Scholar] [CrossRef]
- Shen, L.; Wang, X.; Yang, T.; Teng, Y.; Liu, T.; Li, L.; Zhang, W. Effects of Different Planting Patterns on the Growth and Yield of Maize and Soybean in Northwest China. J. Agric. Sci. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- MWE. Technical Guidelines for the Use of Treated Sanitary Wastewater in Irrigation for Landscaping and Agricultural Irrigation; Ministry of Water and Electricity: Riyadh, Saudi Arabia, 2006. [Google Scholar]
- Jawad, R.; Nawaz, A.; Ejaz, S.; Ali, S.; Saleem, M.S.; Hammad, H.M. Zeolite amendment reduces lead accumulation and improves growth and yield in tomato plants irrigated with sewage water. Environ. Sci. Pollut. Res. 2023, 30, 41970–41982. [Google Scholar] [CrossRef]
- KAUST (King Abdullah University of Science & Technology). KAUST industry and Collaboration Program (kicp), the kicp Annual Strategy Study: Promoting Wastewater Reclamation and Reuse in the Kingdom of Saudi Arabia: Technology Trend, Innovation Needs, and Business Opportunities; KAUST: Jeddah, Saudi Arabia, 2011. [Google Scholar]
- Alawsy, W.S.A.; Alabadi, L.A.S.; Khaeim, H.M. Effect of Sewage water irrigation on growth performance, Biomass and nutrient accumulation in Maize and Barley. Int. J. Agricult. Stat. Sci. 2018, 14, 519–524. [Google Scholar]
- Zidan, K.; Mandi, L.; Hejjaj, A.; Ouazzani, N.; Assabbane, A. Soil fertility and agro-physiological responses of maize (Zea mays) irrigated by treated domestic wastewater by hybrid multi-soil-layering technology. J. Environ. Manag. 2024, 351, 119802. [Google Scholar] [CrossRef] [PubMed]
- Daâssi, D.; Almaghrabi, F.Q. Petroleum-Degrading Fungal Isolates for the Treatment of Soil Microcosms. Microorganisms 2023, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, L.; Abebe, M.; Gizeyatu, A.; Berihun, G.; Teshome, D.; Walle, Z. Evaluation of the Effect of Wastewater Irrigation on the Microbiological Quality of Vegetables in Northeast Ethiopia: Implication for Food-Borne Infection and Intoxications. Environ. Health Insights 2022, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Coord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Fourmentin, M.; Ribeiro, A.R.L.; Noutsopoulos, C.; Mapelli, F.; Fenyvesi, É.; Vieira, M.G.A.; Picos-Corrales, L.A.; Moreno-Piraján, J.C.; et al. Removal of emerging contaminants from wastewater using advanced treatments. A review. Environ. Chem. Lett. 2022, 20, 1333–1375. [Google Scholar] [CrossRef]
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal degradation of wood: Emerging data, new insights, and changing perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- González-González, R.B.; Flores-Contreras, E.A.; Parra-Saldívar, R.; Iqbal, H.M.N. Bio-removal of emerging pollutants by advanced bioremediation techniques. Environ. Res. 2022, 214, 113936. [Google Scholar] [CrossRef]
- Bai, Y.; Huo, Y.; Liao, K.; Qu, J. Influence of microbial community diversity and function on pollutant removal in ecological wastewater treatment. Appl. Microbiol. Biotechnol. 2017, 101, 7293–7302. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Jawed, K.; Yazdani, S.S.; Koffas, M.A.G. Advances in the development and application of microbial consortia for metabolic engineering. Metab. Eng. Commun. 2019, 9, e00095. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological Treatment of Real Textile Effluent Using Aspergillus flavus and Fusarium oxysporium and Their Consortium along with the Evaluation of Their Phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Ouma, K.O. Recent Advances in Biosorption of Copper and Cobalt by Filamentous Fungi. Front. Microbiol. 2020, 11, 582016. [Google Scholar] [CrossRef] [PubMed]
- Hawrot-Paw, M.; Mikiciuk, M.; Koniuszy, A.; Meller, E. Influence of Organomineral Fertiliser from Sewage Sludge on Soil Microbiome and Physiological Parameters of Maize (Zea mays L.). Agronomy 2022, 12, 1114. [Google Scholar] [CrossRef]
- Incrocci, L.; Massa, D.; Pardossi, A. New Trends in the Fertigation Management of Irrigated Vegetable Crops. Horticulturae 2017, 3, 37. [Google Scholar] [CrossRef]
- Abd-Elwahed, M.S. Influence of long-term wastewater irrigation on soil quality and its spatial distribution. Ann. Agric. Sci. 2018, 63, 191–199. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Zheng, S.; Wang, L.; Cai, J.; Wang, W. Effect of rural domestic sewage regeneration irrigation on paddy soil properties and water and nitrogen utilization in southern China. Irrig. Drain. 2022, 72, 515–529. [Google Scholar] [CrossRef]
- EWW 2012. Examination of Water and Wastewater, 22nd ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Tandukar, M.; Uemura, S.; Machdar, I.; Ohashi, A.; Harada, H. A low-cost municipal sewage treatment system with a combination of UASB and the “fourth-generation” downflow hanging sponge reactors. Water Sci. Technol. 2005, 52, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, T.; Ruegner, H.; Sirdari, Z.Z.; Schwientek, M.; Grathwohl, P. Using total suspended solids (TSS) and turbidity as proxies for evaluation of metal transport in river water. Appl. Geochem. A 2016, 68, 1–9. [Google Scholar] [CrossRef]
- Dutta, S.D.; Hyder, S. Mycoremediation—A potential tool for sustainable management. J. Mycopathol. Res. 2017, 57, 25–34. [Google Scholar]
- Pace, J.; Lee, N.; Naik, H.S.; Ganapathysubramanian, B.; Lübberstedt, T. Analysis of maize (Zea mays L.) seedling roots with the high-throughput image analysis too ARIA (Automatic Root Image Analysis). PLoS ONE 2014, 9, 108255. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Nascimento, E.C.S.; do Nascimento, R.; da Silva, A.A.R.; de Castro Bezerra, C.V.; Batista, M.C.; Veloso, L.L.S.A.; de Araújo Pereira, M.C.; Oliveira, H. Growth and photosynthetic pigments of cotton cultivars irrigated with saline water. Agric. Sci. 2019, 10, 81–91. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Hakiman, M.; Maziah, M. Nonenzymatic and enzymatic antioxidant activities in aqueous extract of different Ficus deltoidea accessions. J. Med. Plants Res. 2009, 3, 120–131. [Google Scholar]
- Hadwan, M.H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lahori, A.H.; Mierzwa-Hersztek, M.; Demiraj, E.; Sajjad, R.U.; Ali, I.; Shehnaz, H.; Aziz, A.; Zuberi, M.H.; Pirzada, A.M.; Hassan, K.; et al. Direct and residual impacts of zeolite on the remediation of harmful elements in multiple contaminated soils using cabbage in rotation with corn. Chemosphere 2020, 250, 126317. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Tan, D.K.Y.; Afridi, M.Z.; Luo, H.; Tung, S.A.; Ajab, M.; Fahad, S. Nitrogen fertility and abiotic stresses management in cotton crop: A review. Environ. Sci. Pollut. Res. 2017, 24, 14551–14566. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Tavanti, T.R.; Rodrigues de Melo, A.A.; Moreira, D.K.; Sanchez, D.E.J.; dos Santos Silva, R.; Messias da Silva, R.; Rodrigues dos Reis, A. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Yavuz, D.; Kılıç, E.; Seymen, M.; Dal, Y.; Kayak, N.; Kal, Ü.; Yavuz, N. The effect of irrigation water salinity on the morph-physiological and biochemical properties of spinach under deficit irrigation conditions. Sci. Hortic. 2022, 304, 111272. [Google Scholar] [CrossRef]
- Rosalie, R.; Joas, J.; Deytieux-Belleau, C.; Vulcain, E.; Payet, B.; Dufossé, L.; Léchaudel, M. Antioxidant and enzymatic responses to oxidative stress induced by pre-harvest water supply reduction and ripening on mango (Mangifera indica L. cv. ‘Cogshall’) in relation to carotenoid content. J. Plant Physiol. 2015, 184, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Sallam, M.M. Changes in Growth and Some Biochemical Parameters of Maize Plants Irrigated with Sewage Water. Austin J. Plant Biol. 2015, 1, 1004. [Google Scholar]
- Sun, J.; Jia, Q.; Li, Y.; Zhang, T.; Chen, J.; Ren, Y.; Dong, K.; Xu, S.; Shi, N.-N.; Fu, S. Effects of Arbuscular Mycorrhizal Fungi and Biochar on Growth, Nutrient Absorption, and Physiological Properties of Maize (Zea mays L.). J. Fungi 2022, 8, 1275. [Google Scholar] [CrossRef] [PubMed]
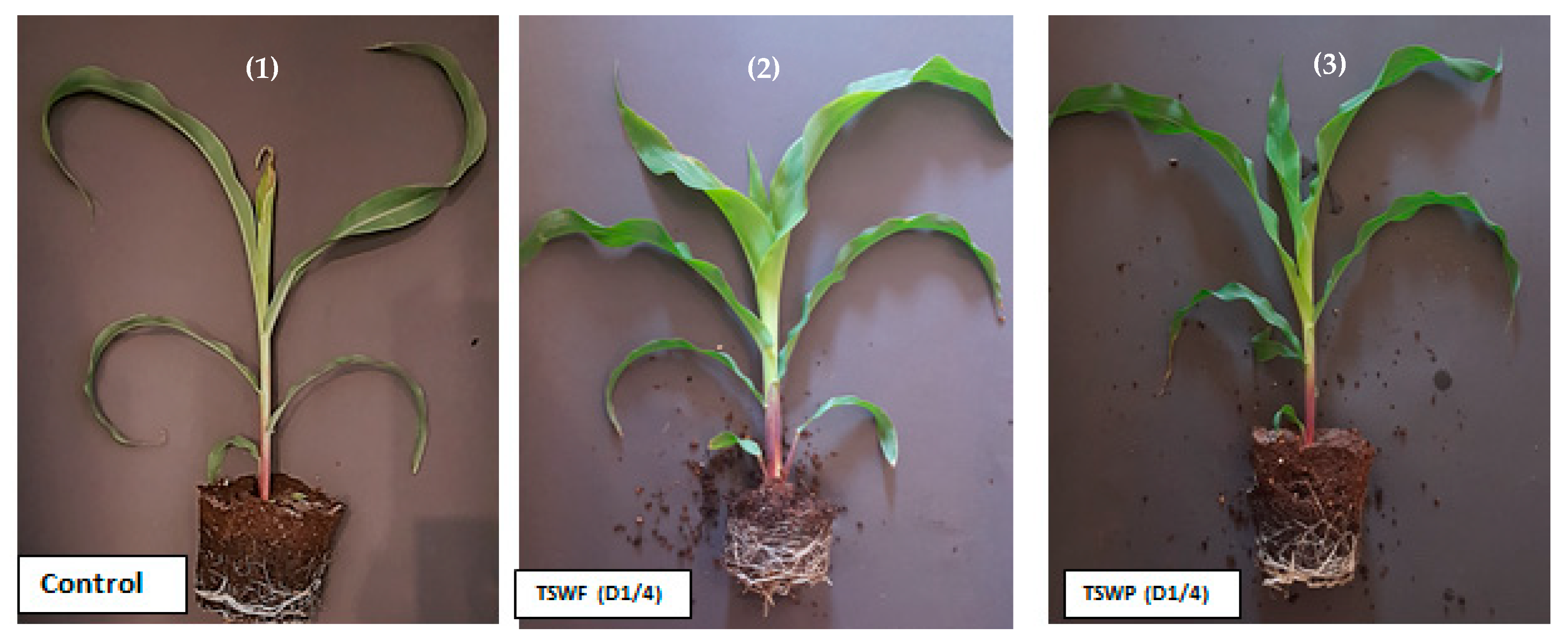



| Property | Unit | Untreated SW (USW) | Treated SW by Fungal Biomass (TSW) | SW Treated by Station Treatment Plant STP | Freshwater | 2006-MWE Standards for Unrestricted Irrigation |
|---|---|---|---|---|---|---|
| Physical parameters | ||||||
| Temperature | °C | 20 ± 0.5 | 20 ± 0.2 | 21 ± 0.1 | 21 ± 0.1 | 40 |
| pH | - | 7.8 ± 0.2 | 6.5 ± 0.1 | 7 ± 0.1 | 8.14 | 6.0–8.4 |
| TSS | mg/L | 43 ± 0.5 | 38 ± 1.4 | 2 ± 0.05 | ND | 40 |
| EC | uS/cm | 2650 ± 10.4 | 2103 | 1150 ± 11.6 | 350 ± 0.01 | <700 |
| Organic chemicals parameters | ||||||
| COD | mg/L | 2315 ± 4.2 | 276 ± 1.06 | 33 ± 2.15 | ND | ND |
| BOD5 | mg/L | 1065 ± 8.8 | 108 ± 2.5 | 12 ± 1.9 | ND | 40 |
| TKN | mg/L | 39 ± 1.3 | 18.5 | 2 ± 0.3 | ND | 5–40 |
| Inorganic chemicals parameters | ||||||
| Heavy metals | ||||||
| Arsenic (As) | mg/L | 0.088 | ND | ND | 0.1 | |
| Cobalt (Co) | mg/L | 0.007 ± 0.01 | ND | 0.005 | ND | 0.05 |
| Cadmium (Cd) | mg/L | 0.0035 ± 0.02 | 0.0007 ± 0.025 | 0.002 ± 0.0052 | ND | 0.01 |
| Nickel (Ni) | mg/L | 0.012 ± 0.29 | 0.001 ± 0.05 | 0.02 ± 0.001 | ND | 0.2 |
| Lead (Pb) | mg/L | 0.21 ± 0.01 | 0.11 ± 0.01 | 0.1 ± 0.06 | ND | 0.1 |
| Copper (Cu) | mg/L | 0.5 ± 0.04 | 0.02 ± 0.02 | 0.03 ± 0.1 | ND | 0.4 |
| Zinc (Zn) | mg/L | 0.05 ± 0.8 | 0.015 ± 0.08 | 2 ± 0.8 | ND | 4.0 |
| Iron (Fe) | mg/L | <4.988 ± 0.5 | ND | <0.4 ± 0.3 | 0.05 ± 0.01 | 5.0 |
| Calcium (Ca) | mg/L | 93 ± 1.94 | 21.57 ± 2.75 | 66.99 ± 4.5 | 0.98 ± 0.03 | 230 |
| Magnesium (Mg) | mg/L | 93 ± 1.51 | 7.64 ± 1.8 | 32 ± 2.32 | 1.34 ± 0.06 | 100 |
| Sodium (Na) | mg/L | 472 ± 5.54 | 175 ± 8.026 | 128 ± 4.15 | 0.85 ± 0.02 | 230 |
| Potassium (K) | mg/L | 147 ± 2.06 | 19 ± 0.036 | 19.36 ± 1.3 | 0.14 ± 0.02 | ND |
| Phosphorus (P) | mg/L | 15 ± 1.5 | 7 ± 0.025 | 3.95 ± 0.25 | ND | |
| * SAR | 0.1 ± 0.05 | 2.51 ± 0.34 | 0.83 ± 0.03 | |||
| Chemical compounds | ||||||
| Total Dissolved Solids (TDS) | mg/L | 1350 ± 10.5 | 129 ± 8.32 | ND | 2500 | |
| Chloride (Cl2) | mg/L | 215 ± 8.5 | 100 | ND | 100 | |
| Sulfate (SO4) | mg/L | 235 ± 8.24 | 165 ± 7.42 | 40 ± 2.28 | 0.81 ± 0.01 | 600 |
| Nitrate (NO3-N) | mg/L | 25.5 ± 0.89 | 28.8 ± 0.76 | 9 ± 0.5 | 0 | 10 |
| PO43-P | mg/L | 26.8 ± 1.4 | 20 ±1.02 | 4 ± 0.1 | 0 | |
| Biological parameters | ||||||
| Fecal coliforms per 100 mL | /100 mL | 1.05 × 105 ± 465 | 1.58 × 104 ± 132 | 2.1 ± 0.05 | ND | 2.2 |
| Strain Numbers | GenBank Accession Number(s) | Identification | COD (mg/L) |
|---|---|---|---|
| Strain 1 (S1) | MZ817960.1 | Aspergillus niger KB5 | 933 ± 7.35 |
| Strain 2 (S2) | MZ817957.1 | Fusarium chlamydosporum KB2 | 1054 ± 2.88 |
| Strain 3 (S3) | OK668265.1 | Paecilomyces formosus KW3 | 1512 ± 8.07 |
| Strain 4 (S4) | MW699898.1 | Sordariomycetes sp. D10 | 1206 ± 5.77 |
| Strain 5 (S5) | MW699893.1 | Coniochaetaceae sp. LB3 | 1795 ± 10.95 |
| Consortium 1 | S1 + S2 | KB5 + KB2 | 650 ± 2.82 |
| Consortium 2 | S1 + S3 | KB5 + KW3 | 517 ± 3.95 |
| Consortium 3 | S1 + S4 | KB5 + D10 | 709 ± 2.14 |
| Consortium 4 | S1 + S5 | KB5 + LB3 | 435 ± 1.55 |
| Consortium 5 | S2 + S3 | KB2 +KW3 | 893 ± 1.07 |
| Consortium 6 | S2 + S4 | KB2 +D10 | 1007 ± 2.33 |
| Consortium 7 | S2 + S5 | KB2 + LB3 | 553 ± 3.11 |
| Consortium 8 | S3 + S4 | KW3 + D10 | 965 ± 2.54 |
| Consortium 9 | S3 + S5 | KW3 +LB3 | 352 ± 1.76 |
| Consortium 10 | S4 + S5 | D10 + LB3 | 599 ± 1.88 |
| Consortium 11 | S1 + S2 + S3 | KB5 + KB2 + KW3 | 375 ± 3.07 |
| Consortium 12 | S1 + S3 + S4 | KB5 + KW3 + D10 | 288 ± 2.63 |
| Consortium 13 | S1 + S4 + S5 | KB5 + D10 + LB3 | 276 ± 1.06 |
| Consortium 14 | S2 + S3 + S4 | KB2 + KW3 + D10 | 375 ± 4.09 |
| Consortium 15 | S2 + S4 + S5 | KB2 + D10 + LB3 | 396 ± 5.94 |
| Consortium 16 | S3 + S4 + S5 | KW3 + D10 + LB3 | 498 ± 6.05 |
| Source | Sum of Squares | Df * (υ) | Mean Square | F-Value | p-Value | Sign ** | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Responses | R1 | R2 | R3 | R4 | R5 | R a | R1 | R2 | R3 | R4 | R5 | R1 | R2 | R3 | R4 | R5 | R a | R a |
| Model | 6.315 × 105 | 376.79 | 6.79 | 105.91 | 14.07 | 19 | 33,238.04 | 19.83 | 0.3576 | 5.57 | 0.7407 | 100.65 | 20.62 | 19.66 | 532.07 | 361.20 | <0.0001 | b |
| A-Treatment | 1.118 × 105 | 318.14 | 3.34 | 102.23 | 9.67 | 3 | 37,267.55 | 106.05 | 1.11 | 34.08 | 3.22 | 112.85 | 110.2 | 61.31 | 3252.5 | 1571.02 | ||
| B-Dilution | 2.078 × 105 | 21.65 | 1.41 | 1.62 | 1.16 | 4 | 51,947.84 | 5.41 | 0.3529 | 0.4046 | 0.2907 | 157.30 | 5.63 | 19.41 | 38.62 | 141.76 | ||
| AB | 3.119 × 105 | 37.00 | 2.04 | 2.07 | 3.25 | 12 | 25,994.06 | 3.08 | 0.1698 | 0.1722 | 0.2705 | 78.71 | 3.21 | 9.34 | 16.44 | 131.90 | ||
| Pure Error | 13,209.91 | 38.46 | 0.72 | 0.419 | 0.082 | 40 | 330.25 | 0.9616 | 0.0182 | 0.0105 | 0.0021 | |||||||
| Cor Total | 6.447 × 105 | 415.26 | 7.52 | 106.33 | 14.16 | 59 | ||||||||||||
| Fit Statistics | ||||||||||||||||||
| R1 | R2 | R3 | R4 | R5 | R1 | R2 | R3 | R4 | R5 | |||||||||
| Std. Dev. | 0.9806 | 0.9806 | 0.1348 | 0.1024 | 0.1024 | R² | 0.9795 | 0.9074 | 0.9033 | 0.9961 | 0.9942 | |||||||
| Mean | 7.92 | 7.92 | 0.9842 | 2.78 | 2.78 | Adjusted R² | 0.9698 | 0.8634 | 0.8574 | 0.9942 | 0.9915 | |||||||
| C.V. % | 12.37 | 12.37 | 13.70 | 3.68 | 3.65 | Predicted R² | 0.9539 | 0.7916 | 0.7824 | 0.9911 | 0.9870 | |||||||
| Adeq Precision | 33.7729 | 14.2247 | 15.0090 | 61.5673 | 63.8280 | |||||||||||||
| Block | A | B | Run | Block | A | B | Run | Block | A | B |
|---|---|---|---|---|---|---|---|---|---|---|
| B1 | USW | D1/8 | 21 | B2 | USW | D1/4 | 41 | B3 | TSWP | D1/8 |
| B1 | Cont | D1/4 | 22 | B2 | Cont | D1/8 | 42 | B3 | TSW | D1/8 |
| B1 | TSWP | D1/4 | 23 | B2 | TSWP | D1/4 | 43 | B3 | USW | D1/4 |
| B1 | TSW | D1/4 | 24 | B2 | TSW | D1/4 | 44 | B3 | Cont | D1/2 |
| B1 | Cont | D1/8 | 25 | B2 | TSWP | D1/8 | 45 | B3 | Cont | D1/8 |
| B1 | Cont | D1/2 | 26 | B2 | TSW | D1/8 | 46 | B3 | TSWP | D1/16 |
| B1 | TSWP | D0 | 27 | B2 | Cont | D1/16 | 47 | B3 | TSW | D1/16 |
| B1 | TSW | D0 | 28 | B2 | Cont | D0 | 48 | B3 | TSWP | D1/2 |
| B1 | TSWP | D1/16 | 29 | B2 | USW | D0 | 49 | B3 | TSW | D1/2 |
| B1 | TSW | D1/16 | 30 | B2 | USW | D1/8 | 50 | B3 | Cont | D1/16 |
| B1 | TSWP | D1/2 | 31 | B2 | Cont | D1/2 | 51 | B3 | USW | D0 |
| B1 | TSW | D1/2 | 32 | B2 | Cont | D1/4 | 52 | B3 | USW | D1/2 |
| B1 | USW | D1/2 | 33 | B2 | TSWP | D0 | 53 | B3 | USW | D1/8 |
| B1 | Cont | D0 | 34 | B2 | TSW | D0 | 54 | B3 | Cont | D0 |
| B1 | TSWP | D1/8 | 35 | B2 | USW | D1/2 | 55 | B3 | TSWP | D0 |
| B1 | TSW | D1/8 | 36 | B2 | TSWP | D1/2 | 56 | B3 | TSW | D0 |
| B1 | USW | D0 | 37 | B2 | TSW | D1/2 | 57 | B3 | USW | D1/16 |
| B1 | Cont | D1/16 | 38 | B2 | TSWP | D1/16 | 58 | B3 | Cont | D1/4 |
| B1 | USW | D1/4 | 39 | B2 | TSW | D1/16 | 59 | B3 | TSWP | D1/4 |
| B1 | USW | D1/16 | 40 | B2 | USW | D1/16 | 60 | B3 | TSW | D1/4 |
| N | Treatment | Dilution | Total Phenol/FW | Chll(a+b)/FW | Carotenoids/FW | POD/FW | CAT/FW | Desirability |
|---|---|---|---|---|---|---|---|---|
| 1 | USW | D1/16 | 333.358 | 9.549 | 0.843 | 4.619 | 1.537 | 0.531 |
| 2 | USW | D1/2 | 328.216 | 8.592 | 0.678 | 4.879 | 1.438 | 0.513 |
| 3 | USW | D1/8 | 344.954 | 8.757 | 0.514 | 4.993 | 1.757 | 0.498 |
| 4 | USW | D1/4 | 304.390 | 8.332 | 0.478 | 5.062 | 1.818 | 0.463 |
| 5 | TSW | D0 | 376.954 | 9.176 | 0.526 | 3.210 | 1.817 | 0.445 |
| 6 | TSW | D1/2 | 123.679 | 9.936 | 1.282 | 2.752 | 2.431 | 0.442 |
| 7 | USW | D0 | 304.634 | 9.989 | 0.365 | 5.377 | 1.913 | 0.387 |
| 8 | TSW | D1/4 | 331.786 | 9.025 | 1.420 | 2.033 | 1.213 | 0.373 |
| 9 | TSWP | D0 | 433.632 | 7.472 | 0.701 | 1.947 | 1.193 | 0.317 |
| 10 | TSWP | D1/2 | 319.966 | 9.008 | 1.230 | 1.890 | 0.882 | 0.280 |
| 11 | TSW | D1/8 | 107.529 | 7.411 | 1.256 | 2.237 | 1.099 | 0.260 |
| 12 | TSW | D1/16 | 79.285 | 10.920 | 1.111 | 2.313 | 1.387 | 0.224 |
| 13 | TSWP | D1/8 | 102.077 | 9.885 | 1.261 | 1.885 | 0.821 | 0.182 |
| 14 | Control | D1/4 | 278.386 | 3.883 | 1.188 | 1.800 | 0.810 | 0.143 |
| 15 | Control | D0 | 277.396 | 3.956 | 1.208 | 1.767 | 0.821 | 0.138 |
| 16 | Control | D1/8 | 278.526 | 3.954 | 1.178 | 1.775 | 0.793 | 0.129 |
| 17 | TSWP | D1/4 | 323.205 | 8.787 | 0.942 | 1.752 | 0.764 | 0.127 |
| 18 | Control | D1/16 | 277.745 | 3.977 | 1.159 | 1.777 | 0.797 | 0.125 |
| 19 | Control | D1/2 | 278.101 | 3.944 | 0.811 | 1.791 | 0.794 | 0.124 |
| 20 | TSWP | D1/16 | 81.038 | 11.937 | 1.534 | 1.738 | 0.762 | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daâssi, D.; Hajaji, A.N.; Alssulime, L.J.H.; Alkhatib, S.N.; Hamouda, R.A. Fungal Consortia Mediated Bio-Treatment of Organic Matter and Metals Uptake from Sewage Water: Maize Agro-Physiological Assessment. Catalysts 2024, 14, 257. https://doi.org/10.3390/catal14040257
Daâssi D, Hajaji AN, Alssulime LJH, Alkhatib SN, Hamouda RA. Fungal Consortia Mediated Bio-Treatment of Organic Matter and Metals Uptake from Sewage Water: Maize Agro-Physiological Assessment. Catalysts. 2024; 14(4):257. https://doi.org/10.3390/catal14040257
Chicago/Turabian StyleDaâssi, Dalel, Afef Nasraoui Hajaji, Lama J. H. Alssulime, Shaza N. Alkhatib, and Ragaa A. Hamouda. 2024. "Fungal Consortia Mediated Bio-Treatment of Organic Matter and Metals Uptake from Sewage Water: Maize Agro-Physiological Assessment" Catalysts 14, no. 4: 257. https://doi.org/10.3390/catal14040257







