Biodiesel Production via Transesterification Reaction over Mono- and Bimetallic Copper-Noble Metal (Pt, Ru) Catalysts Supported on BEA Zeolite
Abstract
1. Introduction
2. Results
2.1. Catalytic Activity
2.2. Specific Surface Area Measurements
2.3. Reducibility of Mono- and Bimetallic Cu Catalysts
2.4. The Acidity of the Investigated Catalysts
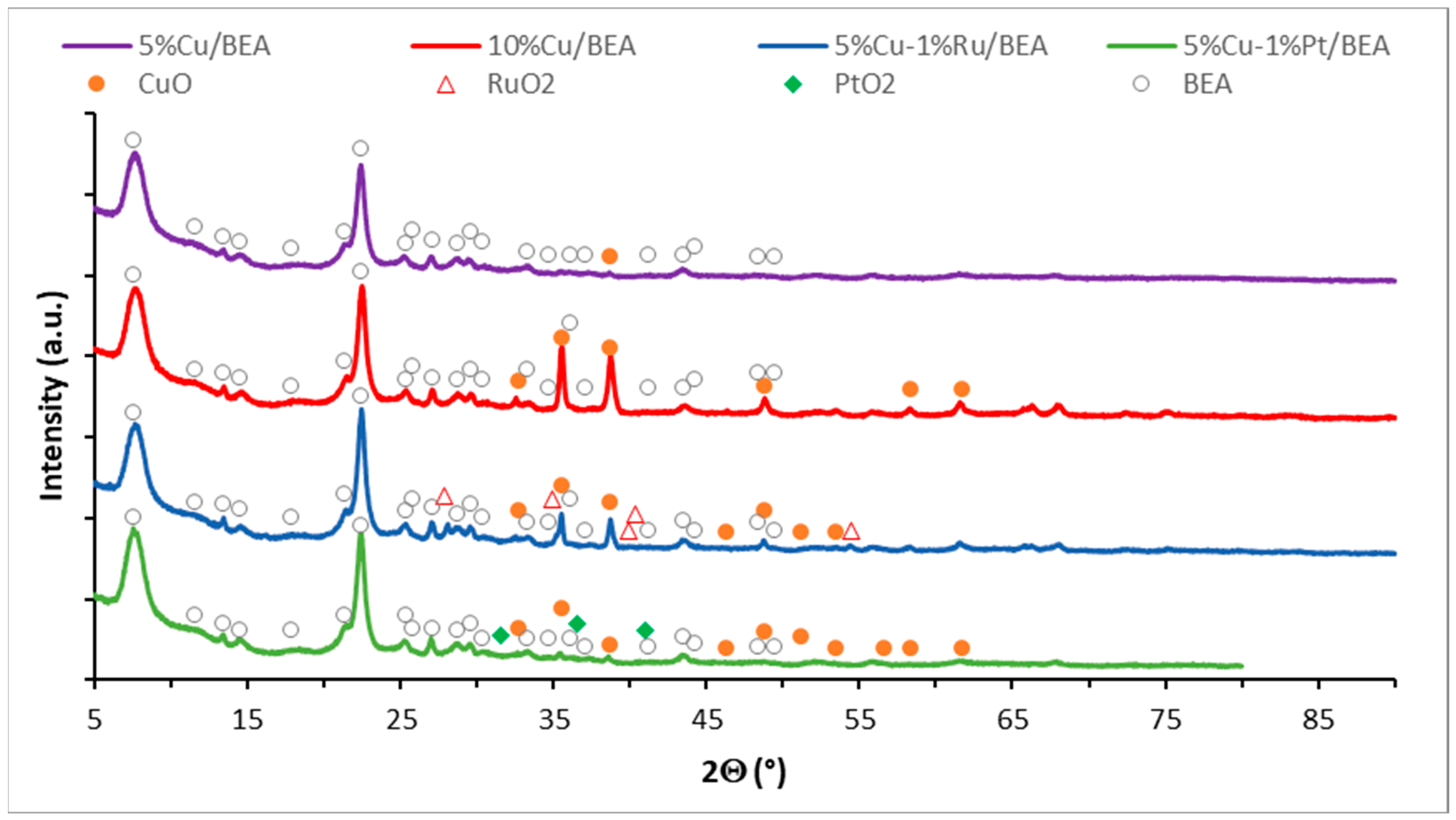
2.5. Phase Composition Studies of Mono and Bimetallic Catalysts
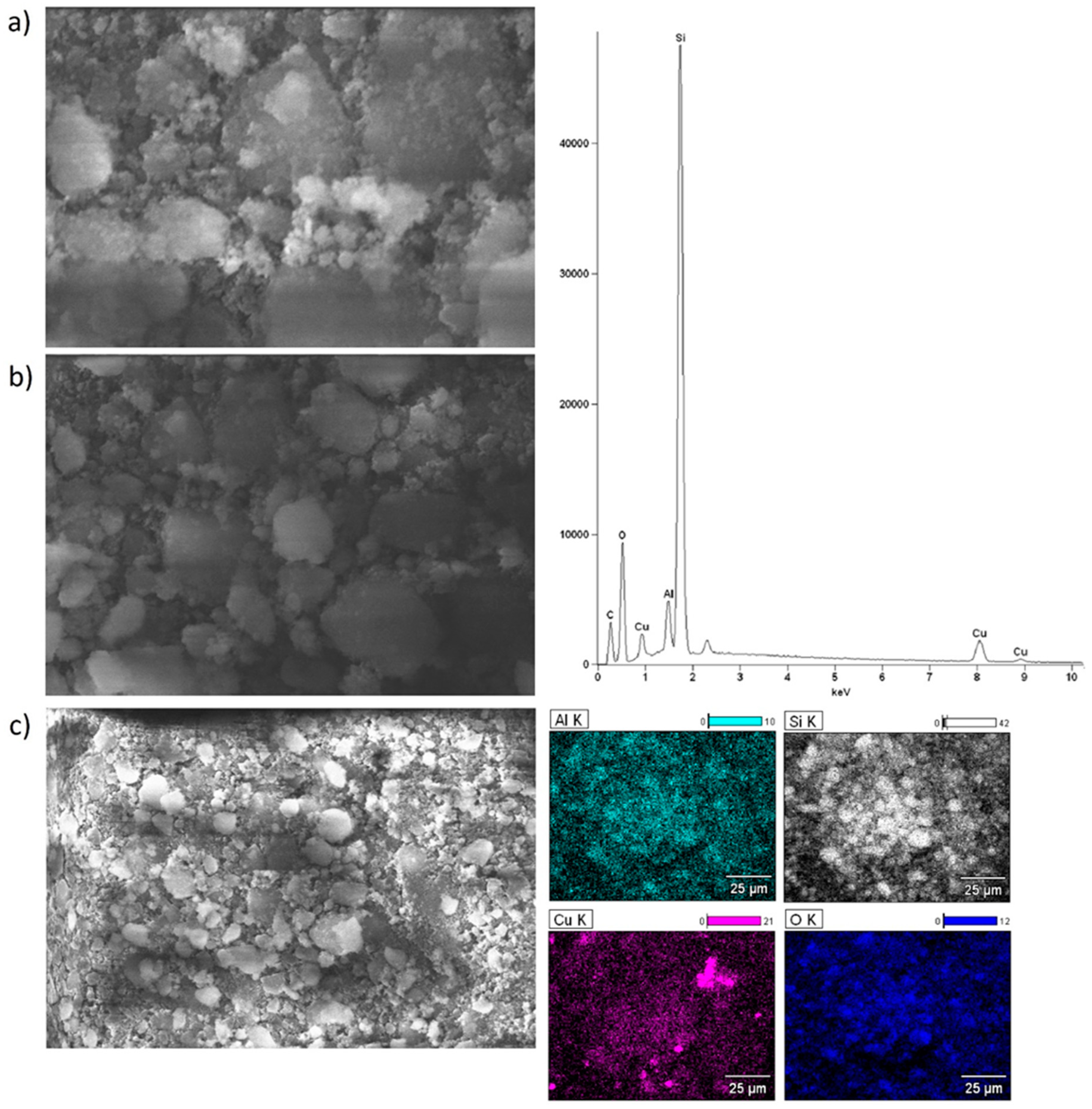
2.6. Morphology of the Mono and Bimetallic Catalysts
3. Materials and Methods
3.1. Materials
3.2. Preparation of Mono- and Bimetallic Cu–Zeolite Catalysts
3.3. Catalyst Characterization
3.4. Reaction Conditions
3.5. Analysis of Transesterification Products and Determination of TG Conversion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gardy, J.; Nourafkan, E.; Osatiashtiani, A.; Lee, A.F.; Wilson, K.; Hassanpour, A.; Lai, X. A core-shell SO4/Mg-Al-Fe3O4 catalyst for biodiesel production. Appl. Catal. B Environ. 2019, 259, 118093. [Google Scholar] [CrossRef]
- Behera, B.; Selvam, S.M.; Dey, B.; Balasubramanian, P. Algal biodiesel production with engineered biochar as a heterogeneous solid acid catalyst. Bioresour. Technol. 2020, 310, 123392. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, M.; Khorasheh, F.; Larimi, A. Biodiesel production via transesterification of canola oil in the presence of Na-K doped CaO derived from calcined eggshell. Renew. Energy 2021, 163, 1626–1636. [Google Scholar] [CrossRef]
- Alvarez Serafini, M.S.; Tonetto, G.M. Synthesis of Fatty Acid Methyl Esters from Pomace Oil Catalyzed by Zinc Stearate: A Kinetic Study of the Transesterification and Esterification Reactions. Catalysts 2019, 9, 978. [Google Scholar] [CrossRef]
- Chen, C.-L.; Huang, C.-C.; Ho, K.-C.; Hsiao, P.-X.; Wu, M.-S.; Chang, J.-S. Biodiesel production from wet microalgae feedstock using sequential wet extraction/transesterification and direct transesterification processes. Bioresour. Technol. 2015, 194, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- Xu, C.; Enache, D.I.; Lloyd, R.; Knight, D.W.; Bartley, J.K.; Hutchings, G.J. Mgo Catalysed Triglyceride Transesterification for Biodiesel Synthesis. Catal. Lett. 2010, 138, 1–7. [Google Scholar] [CrossRef]
- Szkudlarek, Ł.; Chałupka, K.; Maniukiewicz, W.; Albińska, J.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. The influence of si/al ratio on the physicochemical and catalytic properties of mgo/zsm-5 catalyst in transesterification reaction of rapeseed oil. Catalysts 2021, 11, 1260. [Google Scholar] [CrossRef]
- Szkudlarek, Ł.; Chałupka-Śpiewak, K.; Maniukiewicz, W.; Nowosielska, M.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. Biodiesel Production by Methanolysis of Rapeseed Oil—Influence of SiO2/Al2O3 Ratio in BEA Zeolite Structure on Physicochemical and Catalytic Properties of Zeolite Systems with Alkaline Earth Oxides (MgO, CaO, SrO). Int. J. Mol. Sci. 2024, 25, 3570. [Google Scholar] [CrossRef]
- Mierczynski, P.; Szkudlarek, L.; Chalupka, K.; Maniukiewicz, W.; Wahono, S.K.; Vasilev, K.; Szynkowska-Jozwik, M.I. The Effect of the Activation Process and Metal Oxide Addition (CaO, MgO, SrO) on the Catalytic and Physicochemical Properties of Natural Zeolite in Transesterification Reaction. Materials 2021, 14, 2415. [Google Scholar] [CrossRef]
- Mierczynski, P.; Mosińska, M.; Szkudlarek, L.; Chalupka, K.; Tatsuzawa, M.; Al Maskari, M.; Maniukiewicz, W.; Wahono, S.K.; Vasilev, K.; Szynkowska-Jozwik, M.I. Biodiesel Production on Monometallic Pt, Pd, Ru, and Ag Catalysts Supported on Natural Zeolite. Materials 2021, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Han, Y.; Wang, H. Magnetic Fe3O4/MCM-41 composite-supported sodium silicate as heterogeneous catalysts for biodiesel production. Renew. Energy 2018, 125, 675–681. [Google Scholar] [CrossRef]
- Xie, W.; Gao, C.; Li, J. Sustainable biodiesel production from low-quantity oils utilizing H6PV3MoW8O40 supported on magnetic Fe3O4/ZIF-8 composites. Renew. Energy 2021, 168, 927–937. [Google Scholar] [CrossRef]
- Xie, W.; Li, J. Magnetic solid catalysts for sustainable and cleaner biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2023, 171, 113017. [Google Scholar] [CrossRef]
- Sulaiman, N.F.; Ramly, N.I.; Abd Mubin, M.H.; Lee, S.L. Transition metal oxide (NiO, CuO, ZnO)-doped calcium oxide catalysts derived from eggshells for the transesterification of refined waste cooking oil. RSC Adv. 2021, 11, 21781–21795. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Shao, W.-L.; Zhou, W.-X.; Liu, Y.; Han, Y.-Y.; Zheng, Y.; Liu, Y.-J. Biodiesel Production by Esterification Reaction on K+ Modified MgAl-Hydrotalcites Catalysts. Catalysts 2019, 9, 742. [Google Scholar] [CrossRef]
- Machorro, J.J.; Lazaro, A.L.; Espejel-Ayala, F.; Coutiño-Gonzalez, E.; Chavarria-Hernandez, J.C.; Godínez, L.A.; Rodríguez-Valadez, F.J. The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel. Catalysts 2019, 9, 989. [Google Scholar] [CrossRef]
- Enguilo Gonzaga, V.; Romero, R.; Gómez-Espinosa, R.M.; Romero, A.; Martínez, S.L.; Natividad, R. Biodiesel Production from Waste Cooking Oil Catalyzed by a Bifunctional Catalyst. ACS Omega 2021, 6, 24092–24105. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.a.H. Utilisation of Non-Edible Source (Pongamia pinnata Seeds Shells) for Producing Methyl Esters as Cleaner Fuel in the Presence of a Novel Heterogeneous Catalyst Synthesized from Waste Eggshells. Molecules 2021, 26, 5772. [Google Scholar] [CrossRef]
- Pang, H.; Yang, G.; Li, L.; Yu, J. Efficient transesterification over two-dimensional zeolites for sustainable biodiesel production. Green Energy Environ. 2020, 5, 405–413. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Inuwa, I.M.; Opotu, L.A.; Zakaria, Z.Y.; Widayat, W. Influence of desilication route of ZSM-5 zeolite in mesoporous zeolite supported calcium oxide catalyst for biodiesel production. Microporous Mesoporous Mater. 2022, 343, 112153. [Google Scholar] [CrossRef]
- Orlyk, S.; Kyriienko, P.; Kapran, A.; Chedryk, V.; Balakin, D.; Gurgul, J.; Zimowska, M.; Millot, Y.; Dzwigaj, S. CO2-Assisted Dehydrogenation of Propane to Propene over Zn-BEA Zeolites: Impact of Acid–Base Characteristics on Catalytic Performance. Catalysts 2023, 13, 681. [Google Scholar] [CrossRef]
- Lee, K.; Kosaka, H.; Sato, S.; Yokoi, T.; Choi, B.; Kim, D. Effects of Cu loading and zeolite topology on the selective catalytic reduction with C3H6 over Cu/zeolite catalysts. J. Ind. Eng.Chem. 2019, 72, 73–86. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; De La Torre, U.; Illán-Gómez, M.J.; Bueno-López, A.; González-Velasco, J.R. Role of the different copper species on the activity of Cu/zeolite catalysts for SCR of NOx with NH3. Appl. Catal. B Environ. 2014, 147, 420–428. [Google Scholar] [CrossRef]
- Witsuthammakul, A.; Sooknoi, T. Selective hydrodeoxygenation of bio-oil derived products: Acetic acid to propylene over hybrid CeO2–Cu/zeolite catalysts. Catal. Sci. Technol. 2016, 6, 1737–1745. [Google Scholar] [CrossRef]
- Xing, X.; Li, N.; Sun, Y.; Wang, G.; Cheng, J.; Hao, Z. Selective catalytic oxidation of n-butylamine over Cu-zeolite catalysts. Catal. Today 2020, 339, 192–199. [Google Scholar] [CrossRef]
- Moreno-González, M.; Blasco, T.; Góra-Marek, K.; Palomares, A.E.; Corma, A. Study of propane oxidation on Cu-zeolite catalysts by in-situ EPR and IR spectroscopies. Catal. Today 2014, 227, 123–129. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Lovell, E.; Kan, T.; Weldekidan, H.; He, J.; Jahan, S.; Dastjerdi, B.; Scott, J. Enhanced bio-oil deoxygenation activity by Cu/zeolite and Ni/zeolite catalysts in combined in-situ and ex-situ biomass pyrolysis. J. Anal. Appl. Pyrolysis 2019, 140, 148–160. [Google Scholar] [CrossRef]
- Zhu, J.; Sushkevich, V.L.; Knorpp, A.J.; Newton, M.A.; Mizuno, S.C.M.; Wakihara, T.; Okubo, T.; Liu, Z.; van Bokhoven, J.A. Cu-Erionite Zeolite Achieves High Yield in Direct Oxidation of Methane to Methanol by Isothermal Chemical Looping. Chem. Mater. 2020, 32, 1448–1453. [Google Scholar] [CrossRef]
- Brezicki, G.; Zheng, J.; Paolucci, C.; Schlögl, R.; Davis, R.J. Effect of the Co-cation on Cu Speciation in Cu-Exchanged Mordenite and ZSM-5 Catalysts for the Oxidation of Methane to Methanol. ACS Catal. 2021, 11, 4973–4987. [Google Scholar] [CrossRef]
- Zhou, C.; Li, S.; He, S.; Zhao, Z.; Jiao, Y.; Zhang, H. Temperature-dependant active sites for methane continuous conversion to methanol over Cu-zeolite catalysts using water as the oxidant. Fuel 2022, 329, 125483. [Google Scholar] [CrossRef]
- Chang, F.; Yan, C.; Zhou, Q. Synthesis of a New Copper-Based Supramolecular Catalyst and Its Catalytic Performance for Biodiesel Production. Int. J. Chem. Eng. 2018, 2018, 7452817. [Google Scholar] [CrossRef]
- Pangestu, T.; Kurniawan, Y.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Yuliana, M.; Hartono, S.B.; Ismadji, S. The synthesis of biodiesel using copper based metal-organic framework as a catalyst. J. Environ. Chem. Eng. 2019, 7, 103277. [Google Scholar] [CrossRef]
- da Silva, R.B.; Lima Neto, A.F.; Soares dos Santos, L.S.; de Oliveira Lima, J.R.; Chaves, M.H.; dos Santos, J.R.; de Lima, G.M.; de Moura, E.M.; de Moura, C.V.R. Catalysts of Cu(II) and Co(II) ions adsorbed in chitosan used in transesterification of soy bean and babassu oils—A new route for biodiesel syntheses. Bioresour. Technol. 2008, 99, 6793–6798. [Google Scholar] [CrossRef]
- Chen, L.; Yin, P.; Liu, X.; Yang, L.; Yu, Z.; Guo, X.; Xin, X. Biodiesel production over copper vanadium phosphate. Energy 2011, 36, 175–180. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Zaborowski, M.; Maniukiewicz, W.; Nowosielska, M.; Szynkowska, M.I.; Maniecki, T.P. Novel Pd-Cu/ZnAl2O4-ZrO2 Catalysts for Methanol Synthesis. Catal. Lett. 2014, 144, 723–735. [Google Scholar] [CrossRef]
- Mierczynski, P.; Maniecki, T.; Maniukiewicz, W.; Jozwiak, W. Cu/Cr2O3 center dot 3Al2O3 and Au-Cu/Cr2O3 center dot 3Al2O3 catalysts for methanol synthesis and water gas shift reactions. React. Kinet. Mech. Catal. 2011, 104, 139–148. [Google Scholar] [CrossRef]
- Schaidle, J.A.; Ruddy, D.A.; Habas, S.E.; Pan, M.; Zhang, G.; Miller, J.T.; Hensley, J.E. Conversion of Dimethyl Ether to 2,2,3-Trimethylbutane over a Cu/BEA Catalyst: Role of Cu Sites in Hydrogen Incorporation. ACS Catal. 2015, 5, 1794–1803. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, J.; Liu, S.; Xu, S.; Lin, C.; Feng, X.; Wang, Y.; Xu, H.; Chen, Y. Barium-promoted hydrothermal stability of monolithic Cu/BEA catalyst for NH3-SCR. Dalton Trans. 2018, 47, 15038–15048. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Jin, Y.; Zhang, R. Zeolite structure effects on Cu active center, SCR performance and stability of Cu-zeolite catalysts. Catal. Today 2019, 327, 295–307. [Google Scholar] [CrossRef]
- Mierczynski, P.; Maniecki, T.P.; Chalupka, K.; Maniukiewicz, W.; Jozwiak, W.K. Cu/ZnxAlyOz supported catalysts (ZnO: Al2O3 = 1, 2, 4) for methanol synthesis. Catal. Today 2011, 176, 21–27. [Google Scholar] [CrossRef]
- Mierczynski, P.; Vasilev, K.; Mierczynska, A.; Maniukiewicz, W.; Maniecki, T.P. Highly selective Pd–Cu/ZnAl2O4 catalyst for hydrogen production. Appl. Catal. A Gen. 2014, 479, 26–34. [Google Scholar] [CrossRef]
- Mierczynski, P.; Maniukiewicz, W.; Maniecki, T.P. Comparative studies of Pd, Ru, Ni, Cu/ZnAl2O4 catalysts for the water gas shift reaction. Cent. Eur. J. Chem. 2013, 11, 912–919. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Murata, K.; Okabe, K.; Inaba, M.; Takahara, I. Effect of Mn addition on activity and resistance to catalyst deactivation for Fischer–Tropsch synthesis over Ru/Al2O3 and Ru/SiO2 catalysts. Catal. Commun. 2007, 8, 1531–1537. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Briones, L.; Medina, S.; Martínez, A. Hydroreforming of the oils from LDPE thermal cracking over Ni–Ru and Ru supported over hierarchical Beta zeolite. Fuel 2015, 144, 287–294. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Pimerzin, A.; Nedolivko, V.; Zasypalov, G.; Stytsenko, V.; Karakhanov, E.; Vinokurov, V. Ruthenium Catalysts Templated on Mesoporous MCM-41 Type Silica and Natural Clay Nanotubes for Hydrogenation of Benzene to Cyclohexane. Catalysts 2020, 10, 537. [Google Scholar] [CrossRef]
- Pérez-Bustos, H.F.; Lucio-Ortiz, C.J.; de la Rosa, J.R.; de Haro del Río, D.A.; Sandoval-Rangel, L.; Martínez-Vargas, D.X.; Maldonado, C.S.; Rodriguez-González, V.; Garza-Navarro, M.A.; Morales-Leal, F.J. Synthesis and characterization of bimetallic catalysts Pd-Ru and Pt-Ru supported on γ-alumina and zeolite FAU for the catalytic transformation of HMF. Fuel 2019, 239, 191–201. [Google Scholar] [CrossRef]
- Xie, J.; Sun, X.; Barrett, L.; Walker, B.R.; Karote, D.R.; Langemeier, J.M.; Leaym, X.; Kroh, F.; Traylor, W.; Feng, J.; et al. Autothermal reforming and partial oxidation of n-hexadecane via Pt/Ni bimetallic catalysts on ceria-based supports. Int. J. Hydrogen Energy 2015, 40, 8510–8521. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollár, M.; Szanyi, J.; Peden, C.H.F. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Lezcano-Gonzalez, I.; Deka, U.; Arstad, B.; Van Yperen-De Deyne, A.; Hemelsoet, K.; Waroquier, M.; Van Speybroeck, V.; Weckhuysen, B.M.; Beale, A.M. Determining the storage, availability and reactivity of NH3 within Cu-Chabazite-based Ammonia Selective Catalytic Reduction systems. Phys. Chem. Chem. Phys. 2014, 16, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Fan, C.; Sun, C.; Zhu, H.; Yi, W.; Chen, J.; Guo, L.; Niu, X.; Chen, J.; Peng, Y.; et al. New Insight into the In Situ SO2 Poisoning Mechanism over Cu-SSZ-13 for the Selective Catalytic Reduction of NOx with NH3. Catalysts 2020, 10, 1391. [Google Scholar] [CrossRef]
- Ribeiro, N.F.P.; Henriques, C.A.; Schmal, M. Copper-based Catalysts for Synthesis of Methylamines: The Effect of the Metal and the Role of the Support. Catal. Lett. 2005, 104, 111–119. [Google Scholar] [CrossRef]
- Shutkina, O.V.; Ponomareva, O.A.; Ivanova, I.I. Catalytic synthesis of cumene from benzene and acetone. Pet. Chem. 2013, 53, 20–26. [Google Scholar] [CrossRef]
- Tang, J.; Xu, M.; Yu, T.; Ma, H.; Shen, M.; Wang, J. Catalytic deactivation mechanism research over Cu/SAPO-34 catalysts for NH3-SCR (II): The impact of copper loading. Chem. Eng. Sci. 2017, 168, 414–422. [Google Scholar] [CrossRef]
- Feng, L.; Wang, R.; Zhang, Y.; Ji, S.; Chuan, Y.; Zhang, W.; Liu, B.; Yuan, C.; Du, C. In situ XRD observation of CuO anode phase conversion in lithium-ion batteries. J. Mater. Sci. 2019, 54, 1520–1528. [Google Scholar] [CrossRef]
- Devadas, A.; Baranton, S.; Napporn, T.W.; Coutanceau, C. Tailoring of RuO2 nanoparticles by microwave assisted “Instant method” for energy storage applications. J. Power Sour. 2011, 196, 4044–4053. [Google Scholar] [CrossRef]
- Cruz, J.C.; Baglio, V.; Siracusano, S.; Antonucci, V.; Aricò, A.S.; Ornelas, R.; Ortiz-Frade, L.; Osorio-Monreal, G.; Durón-Torres, S.M.; Arriaga, L.G. Preparation and characterization of RuO2 catalysts for oxygen evolution in a solid polymer electrolyte. Int. J. Electrochem. Sci. 2011, 6, 6607–6619. [Google Scholar] [CrossRef]
- Sivakami, R.; Dhanuskodi, S.; Karvembu, R. Estimation of lattice strain in nanocrystalline RuO2 by Williamson–Hall and size–strain plot methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 43–50. [Google Scholar] [CrossRef]
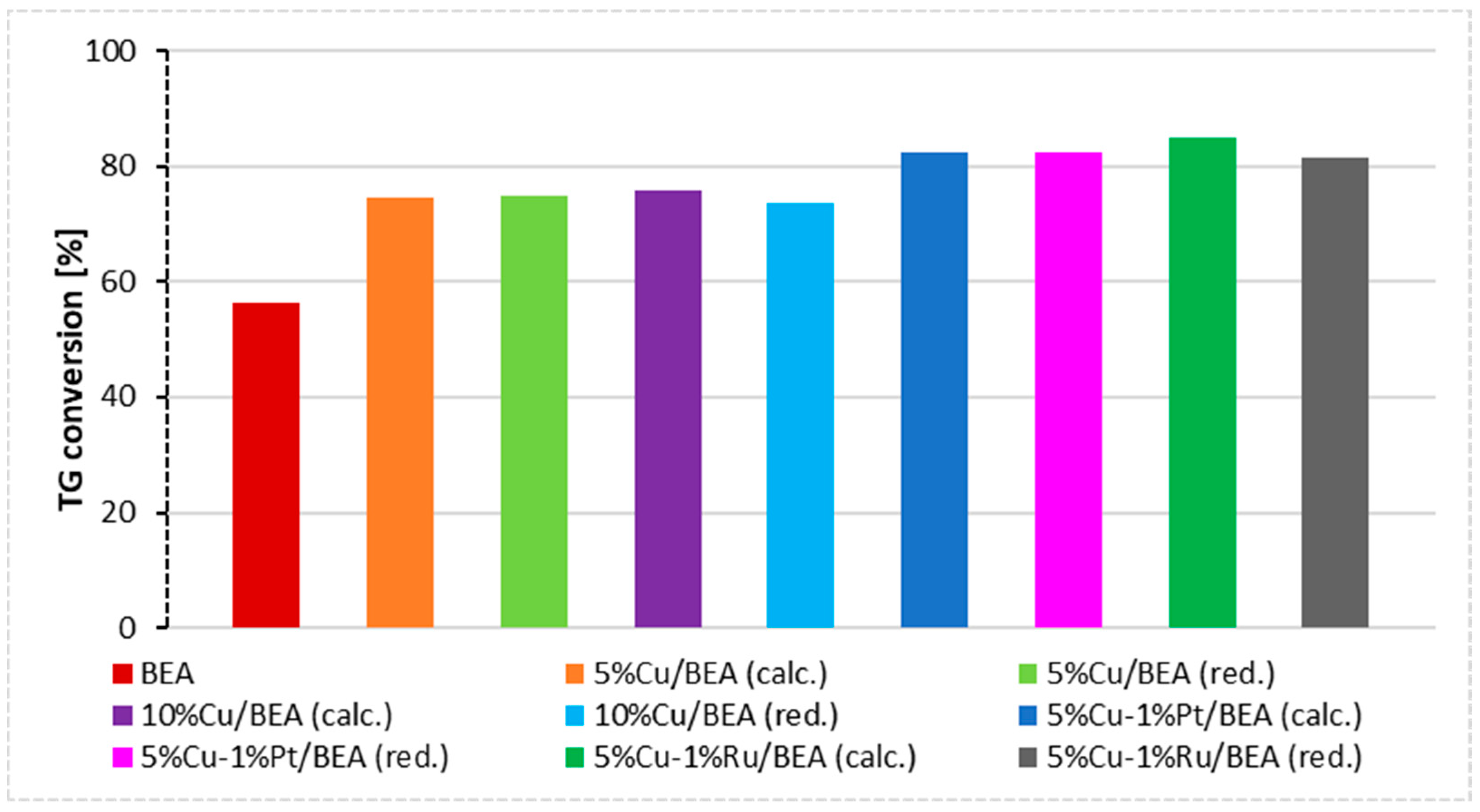
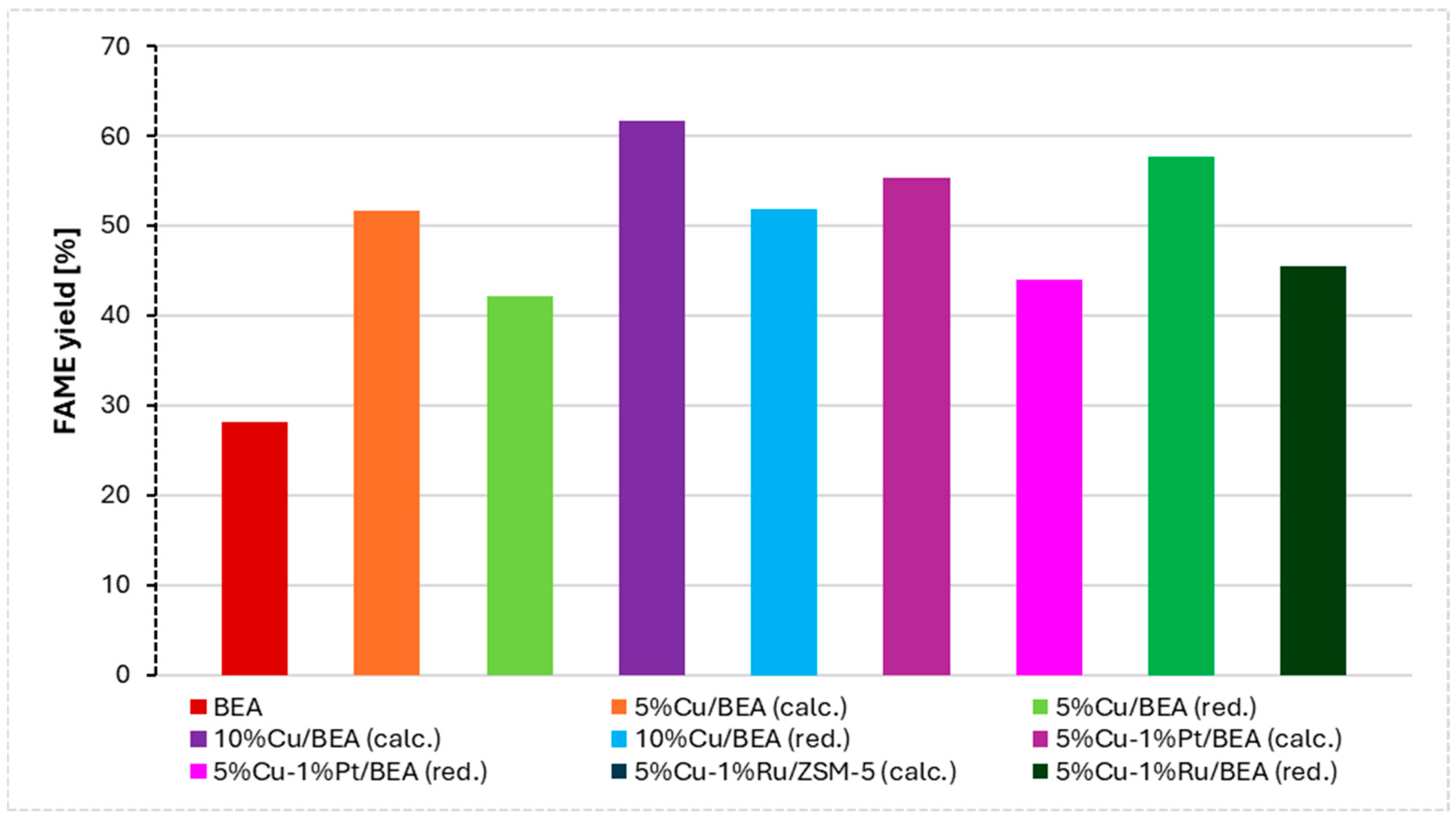


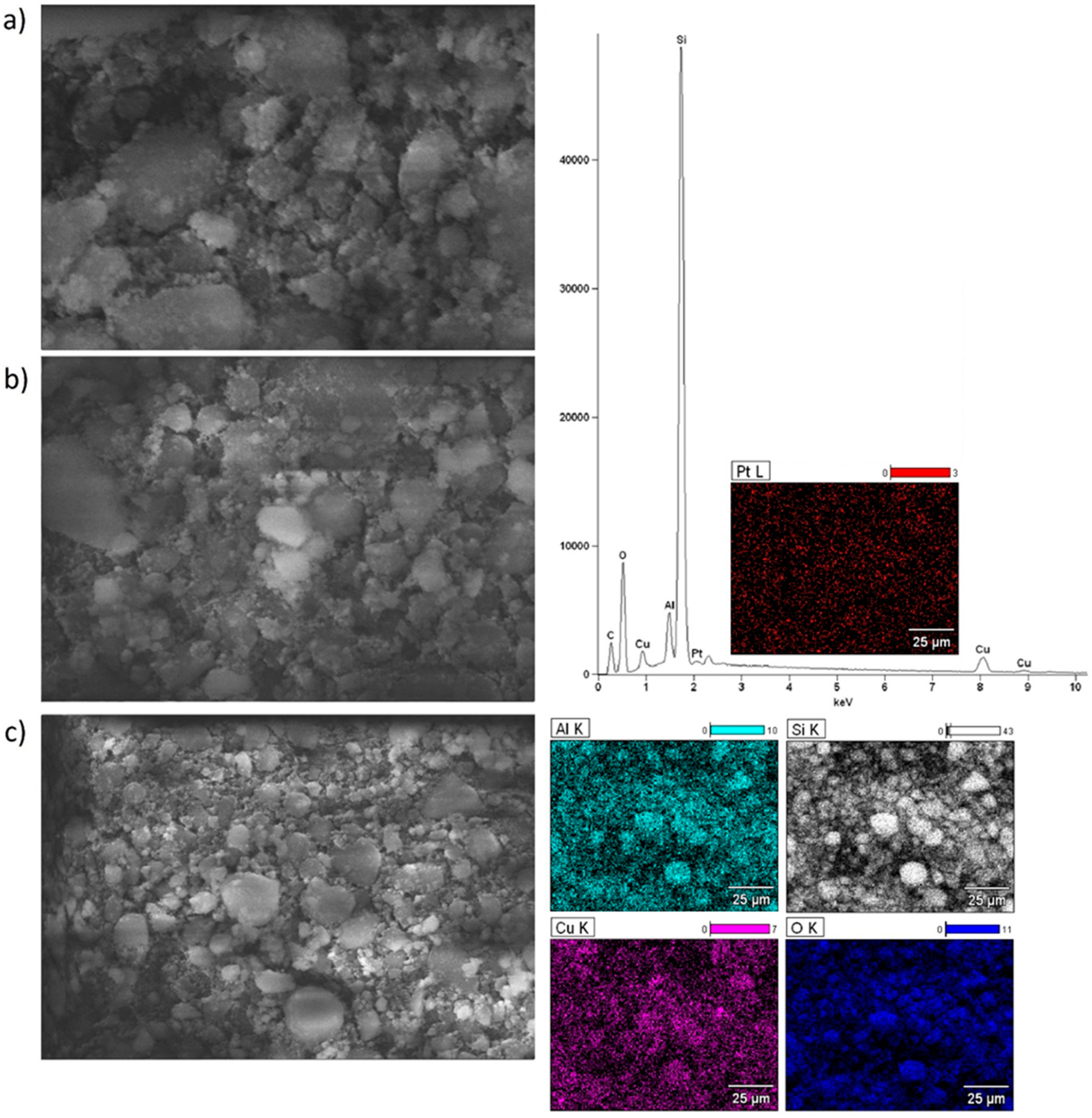
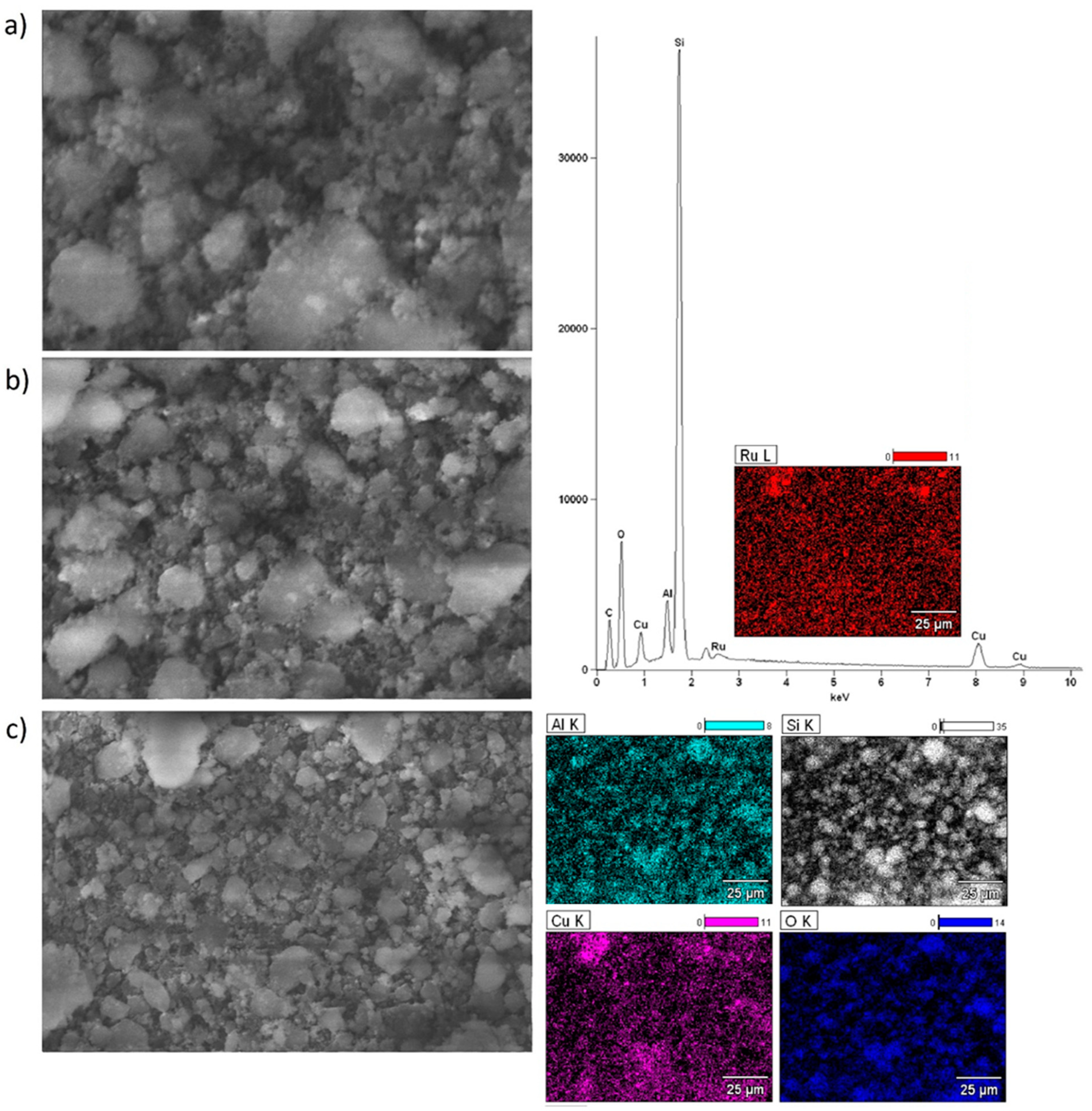
| Catalyst | Triglycerides Conversion [%] | FAME Yield [%] |
|---|---|---|
| BEA | 56.3 | 28.2 |
| 5%Cu/BEA (calc.) | 74.5 | 51.7 |
| 5%Cu/BEA (red.) | 74.8 | 42.1 |
| 10%Cu/BEA (calc.) | 75.7 | 61.6 |
| 10%Cu/BEA (red.) | 73.6 | 51.9 |
| 5%Cu–1%Pt/BEA (calc.) | 82.5 | 55.4 |
| 5%Cu–1%Pt/BEA (red.) | 82.4 | 44.0 |
| 5%Cu–1%Ru/BEA (calc.) | 85.1 | 58.4 |
| 5%Cu–1%Ru/BEA (red.) | 81.5 | 45.5 |
| Catalyst | BET Surface Area | t-Plot Micropore Area | t-Plot External Surface Area | Pore Volume | t-Plot Micropore Volume | Average Pore Size |
|---|---|---|---|---|---|---|
| (m²/g) | (m²/g) | (m²/g) | (cm³/g) | (cm³/g) | (nm) | |
| 5%Cu/BEA (calc.) | 467 | 299 | 168 | 0.706 | 0.155 | 7.80 |
| 5%Cu/BEA (red.) | 490 | 311 | 179 | 0.719 | 0.161 | 7.44 |
| 10%Cu/BEA (calc.) | 407 | 252 | 155 | 0.663 | 0.131 | 7.88 |
| 10%Cu/BEA (red.) | 443 | 280 | 163 | 0.703 | 0.145 | 8.01 |
| 5%Cu–1%Pt/BEA (calc.) | 467 | 297 | 170 | 0.689 | 0.154 | 7.59 |
| 5%Cu–1%Pt/BEA (red.) | 482 | 307 | 174 | 0.747 | 0.159 | 7.96 |
| 5%Cu–1%Ru/BEA (calc.) | 454 | 274 | 180 | 0.729 | 0.141 | 7.41 |
| 5%Cu–1%Ru/BEA (red.) | 482 | 300 | 181 | 0.739 | 0.155 | 7.45 |
| Catalytic Systems | Distribution of Acid Centers | Total Acidity (mmol/g) 100–600 °C | ||
|---|---|---|---|---|
| Weak (mmol/g) 100–300 °C | Moderate (mmol/g) 300–450 °C | Strong (mmol/g) 450–600 °C | ||
| 5%Cu/BEA (calc.) | 1.54 | 1.03 | 0.76 | 3.33 |
| 10%Cu/BEA (calc.) | 1.46 | 1.22 | 1.17 | 3.85 |
| 5%Cu–1%Ru/BEA (calc.) | 1.50 | 1.15 | 0.74 | 3.39 |
| 5%Cu–1%Pt/BEA (calc.) | 0.78 | 1.16 | 0.75 | 2.69 |
| 5%Cu/BEA (red. 300 °C/2 h) | 1.02 | 1.24 | 0.79 | 3.05 |
| 10%Cu/BEA (red. 300 °C/2 h) | 1.15 | 1.23 | 0.90 | 3.28 |
| 5%Cu–1%Ru/BEA (red. 400 °C/2 h) | 0.84 | 1.08 | 0.66 | 2.58 |
| 5%Cu–1%Pt/BEA (red. 300 °C/2 h) | 0.76 | 1.09 | 0.66 | 2.51 |
| Mobile Phase Gradient | Flow Rate [mL/min] | ||
|---|---|---|---|
| Time [min] | Solvent A (%) | Solvent B (%) | |
| 0 | 100 | 0 | 0.9 |
| 20 | 100 | 0 | 0.9 |
| 45 | 0 | 100 | 0.9 |
| 70 | 0 | 100 | 0.9 |
| 75 | 100 | 0 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudlarek, Ł.; Chałupka-Śpiewak, K.; Maniukiewicz, W.; Albińska, J.; Szynkowska-Jóźwik, M.I.; Mierczyński, P. Biodiesel Production via Transesterification Reaction over Mono- and Bimetallic Copper-Noble Metal (Pt, Ru) Catalysts Supported on BEA Zeolite. Catalysts 2024, 14, 260. https://doi.org/10.3390/catal14040260
Szkudlarek Ł, Chałupka-Śpiewak K, Maniukiewicz W, Albińska J, Szynkowska-Jóźwik MI, Mierczyński P. Biodiesel Production via Transesterification Reaction over Mono- and Bimetallic Copper-Noble Metal (Pt, Ru) Catalysts Supported on BEA Zeolite. Catalysts. 2024; 14(4):260. https://doi.org/10.3390/catal14040260
Chicago/Turabian StyleSzkudlarek, Łukasz, Karolina Chałupka-Śpiewak, Waldemar Maniukiewicz, Jadwiga Albińska, Małgorzata Iwona Szynkowska-Jóźwik, and Paweł Mierczyński. 2024. "Biodiesel Production via Transesterification Reaction over Mono- and Bimetallic Copper-Noble Metal (Pt, Ru) Catalysts Supported on BEA Zeolite" Catalysts 14, no. 4: 260. https://doi.org/10.3390/catal14040260
APA StyleSzkudlarek, Ł., Chałupka-Śpiewak, K., Maniukiewicz, W., Albińska, J., Szynkowska-Jóźwik, M. I., & Mierczyński, P. (2024). Biodiesel Production via Transesterification Reaction over Mono- and Bimetallic Copper-Noble Metal (Pt, Ru) Catalysts Supported on BEA Zeolite. Catalysts, 14(4), 260. https://doi.org/10.3390/catal14040260







