Abstract
Dimethyl carbonate (DMC) is widely used as an intermediate and solvent in the organic chemical industry. In recent years, compared with the traditional DMC production methods (phosgene method, transesterification method), methanol oxidation carbonylation method, gas-phase methyl nitrite method, and the direct synthesis of CO2 and methanol method have made much progress in the synthesis process and development of catalysts. The key to the industrial application of DMC synthesis technology is the design and development of high-performance catalysts. Therefore, this paper reviews the research status of the methanol oxidative carbonylation method, gas-phase methyl nitrite method, and direct synthesis method of CO2 and methanol in the aspects of new catalyst design, catalyst preparation, and catalytic mechanism, and puts forward the problems to be solved and the future development direction of DMC catalysts.
1. Introduction
Dimethyl carbonate (DMC for short), with the molecular structure formula (CH3O)2CO, is an important organic chemical intermediate with low toxicity, excellent environmental performance, safe use, less pollution, and easy transportation in production, so it is widely used as a raw chemical material [1,2,3,4,5,6]. The molecular structure of DMC contains four different functional groups, namely carbonyl (–CO–), methoxy (–OCH3), methyl (–CH3), and methoxy carbonyl (CH3–CO–), which have good reactivity and are used in the production of polycarbonate, isocyanate, polyurethane, and other chemicals [3,4,5]. At the same time, as a solvent with excellent solubility, DMC not only has a good compatibility with other solvents, but also has the characteristics of a fast evaporation rate and high evaporation temperature, and can be used as low-toxic solvents, raw materials for pesticide production, pharmaceutical industry supplies, coating solvents, etc. [4,5,6,7]. In recent years, with the rapid development of the downstream polycarbonate (PC) and lithium battery industries, the demand for DMC has increased year by year, and the development of DMC production technology has been promoted. DMC synthesis methods include the phosgene method, oxidative carbonylation method, nitrite carbonylation method, transesterification method, direct synthesis of methanol and CO2, and the urea alcoholysis method [8,9,10,11,12,13,14,15,16,17]. The phosgene method uses the phosgene and methanol (or sodium methanol) reaction in the preparation of DMC; however, this has been gradually eliminated as the technical route of the raw material phosgene is highly toxic, the process is complex, the operation cycle is long, there is a large amount of hydrochloric acid as a by-product, there is serious corrosion of equipment, the environmental pollution is serious, and at the same time, because the product contains a large number of halogens, the product quality is poor. In addition, the relevant literature includes detailed reports on the research progress of the transesterification method and urea alcoholysis method, which will not be repeated here. In this review, we summarize the research status of DMC catalysts from the aspects of new catalyst design, catalyst preparation, and catalytic mechanism including the oxidative carbonylation of methanol, gaseous methyl nitrite, and the direct synthesis of CO2 and methanol. We also identify some problems to be solved and the future development direction of DMC catalysts.
2. Synthesis of DMC by Methanol Oxidative Carbonylation
Methanol oxidation and carbonylation to DMC is an important product route in the modern coal chemical industry, especially in China, where coal is the main energy resource. The acquisition of DMC from coal establishes a new connection between coal, bulk chemicals, and even fine chemicals. The methanol oxidative carbonylation method takes methanol, CO, and O2 as raw materials and direct oxidative carbonylation to obtain DMC, where the reaction equation is as follows:
This method has the advantages of the easy availability of raw materials, low production cost, simple process, and good product quality. It is mainly divided into the gas-phase method and liquid-phase method, and there are significant differences in the catalyst type, catalytic mechanism, and product yield between the two methods.
2.1. Catalyst for Methanol Liquid-Phase Oxidative Carbonylation Method
The liquid-phase oxidative carbonylation of methanol (for short, liquid-phase carbonylation of methanol) uses CO, O2, and methanol as raw materials to produce DMC. It has the characteristics of low production cost, mild reaction conditions, favorable thermodynamics, and high product selectivity, and the by-product is water, which has little impact on the environment. It is the main method for producing DMC in developed countries, for example, GE in the United States, Enichem in Italy, and Big Lu and Ube in Japan. In the methanol liquid-phase carbonylation process, Cu-based catalysts including chlorine-containing and chlorine-free catalysts show better catalytic performance. A typical chlorine-containing catalyst is CuCl. DMC is obtained through two steps: (1) methanol and O2 interact with the CuCl catalyst to form Cu(OCH3)Cl, and (2) DMC is synthesized by the carbonylation of CO and CuCl is reduced.
The production cost of methanol by liquid-phase carbonylation is low, the process is simple, and there is no environmental pollution. However, the conversion rate of methanol is low, as is the one-way yield of DMC. Chlorine-containing catalysts have problems such as the rapid inactivation of catalysts and equipment corrosion caused by chloride ion loss [18]. At present, the development of chlorine-free copper based catalysts has become the focus of research.
The synthesis of DMC by the liquid-phase carbonylation of methanol without a chlorine catalyst has the advantages of cheap raw materials, low toxicity, and low cost. Under the condition of good catalyst activity and stability, the development of Cu supported on activated carbon (AC), Cu/carbon nanotubes, and other catalysts can eliminate the problems of equipment corrosion and catalyst deactivation caused by chloride ion loss from the source. Among them, Cu/AC prepared with AC as a carrier can make full use of the advantages of the high specific surface area of AC, the existence of multi-level pores, rich surface functional groups, and stable physical and chemical properties [19,20].
2.2. Catalyst for Methanol Gas-Phase Oxidative Carbonylation
Although the process of the oxidation and carbonylation of liquid methanol to DMC by Enichem has been industrialized, there are still many problems in the process. For example, the use of a large amount of CuCl will produce a large amount of hydrochloric acid in the system, which means that the corrosion resistance problem has to be considered when designing and building production equipment. The DOW Chemical Company in the United States developed the gas-phase oxidation carbonylation method. The reactant methanol was first gasified in the gasification chamber and then mixed with CO and O2 to pass into the fixed bed reactor [21]. The reaction temperature is 100–130 °C, the reaction pressure is 10–30 bar, and the catalyst is generally a supported Cu-based catalyst.
For the CuCl2 catalyst supported by AC, Cu species were highly dispersed on the surface of the AC when the loading of CuCl2 was less than 10%. When the loading was 12.3% and 19.7%, the corresponding CuCl2 particles could be observed [22]. In addition, as the Cl/Cu ratio in the catalyst system is lower than the theoretical value of 2, the Cl/Cu ratio will gradually increase and approach the theoretical value of 2 with the increase in CuCl2 loading [23,24]. Combined with EXAFS analysis, it has been shown [25] that when the active component CuCl2 is loaded on the surface of AC, the oxygen-containing groups (such as AC–COOH, AC–OH, etc.) on the surface of the carrier will undergo a displacement reaction with CuCl2 to form HCl, which results in the loss of Cl during the preparation of the catalyst. When the load capacity of Cu is lower than 5% (wt), Cu mainly binds to the oxygen-containing groups on the surface of AC and exists on the catalyst surface in the form of single-layer adsorption; when the load capacity of Cu is higher than 5%, a multi-layer adsorption form will form [26]. Han et al. [27] found that when the Cu loading was between 5% and 10%, the catalyst activity reached the highest and did not increase with the increase in Cu loading. At this time, the methanol conversion rate was 21% and the DMC selectivity was 80%.
As a chlorine-free catalyst, Cu-exchange zeolite catalysts have attracted wide attention due to their high catalytic activity, especially the CuY zeolite catalyst [28,29,30]. CuY zeolites prepared by solid ion exchange, liquid-phase ion exchange, and initial wet impregnation showed significant differences in catalytic performance, which could be attributed to the differences in the presence of Cu in the zeolite (Cu+, Cu2+, Cu2O, and CuO) [29,30,31]. Although the influence of the Cu occurrence state on DMC yield was obtained through experimental preparation and characterization, the theoretical calculation provided a reasonable explanation. Through DFT calculation [32], Zheng’s research group clarified the formation mechanism of DMC. CH3OH is adsorbed and oxidized into CH3O, then reacts with CO to form CH3OCO, before reacting with CH3O to form DMC. On this basis, it has been found that when Cu exists in the form of Cu+, Cu2+, and Cu2O, CH3OH is oxidized to CH3O in the presence of oxygen, and when Cu exists in the form of CuO, the lattice oxygen of copper oxide oxidizes CH3OH. It is the oxidation of lattice oxygen that reduces the activation energy of the rate-limiting step to only 60.01 kJ·mol−1. These reports provide new ideas for the construction of Cu catalytic active sites in subsequent Cu-based zeolite catalysts.
3. Gas-Phase Carbonylation of Methyl Nitrite to Synthesize DMC
Ube Industries, Japan, proposed the process route for preparation of DMC through the carbonylation of methyl nitrite in the gas phase and successfully realized its industrialization. The synthesis of DMC by the nitrite carbonylation method originates from the process route of dimethyl oxalate synthesis by the oxidative carbonylation gas phase. In this synthesis process, the oxygen carrier nitrite is introduced to react with CO to synthesize DMC [2]. The reaction equation is as follows:
In this process route, first, NO, O2, and methanol react to form methyl nitrite (MN) and water, then under the action of a Pd-based catalyst, CO and MN undergo the esterification reaction to form DMC. At the same time, the reduced NO continues to participate in the previous step as a recycling medium to achieve MN regeneration.
In the preparation of DMC by the carbonylation of methyl nitrite in the gas phase, O2 in the MN regenerator does not make contact with the CO in the DMC reactor, which reduces the production of CO2 as a by-product and reduces the risk of system explosion. At the same time, since the MN regenerator does not require a catalyst and is independent of the DMC reactor, the H2O generated in it avoids affecting the stability of the catalyst in the DMC reactor [2]. In addition, CO and methanol are used as the reaction raw materials to realize the recycling of NO, which reduces the production cost. The production process of this method is pollution-free, environmentally friendly, and has no subsequent separation problems, which make it one of the most advanced production methods for the synthesis of DMC, and has attracted the attention of many researchers. However, the key to its industrialization lies in the preparation of efficient catalysts.
In the process of preparing DMC by the carbonylation of methyl nitrite in the gas phase, Pd(II) species are gradually reduced to Pd(0) due to the presence of the reactant CO and agglomeration occurs, resulting in a decline in the catalytic activity of the catalyst [33]. In the presence of nitrite, the reduced Pd(0) can be oxidized by adding hydrogen chloride gas to maintain the high catalytic activity of the catalyst, thus improving the DMC yield. Therefore, the catalysts used to catalyze the synthesis of DMC by gaseous carbonyl nitrite are mainly divided into two categories: one is the Pd-Cu bimetallic catalyst containing chlorine, and the other is the chlorine-free Pd-based supported catalyst.
3.1. Chlorine Containing Catalyst
In the low-pressure gas-phase synthesis of DMC from CO and MN, Pd(II) in the Pd-Cu bimetal catalyst provides the active site for the catalytic reaction. However, due to the presence of CO in the feed, Pd(II) is easily reduced to Pd(0), causing the deactivation of the catalyst. At this time, Cu(II) acts as a catalyst promoter to oxidize the reduced Pd(0) during the catalytic reaction, thereby maintaining the catalytic activity of Pd(II). In the catalytic reaction process of the PdCl2-CuCl2 type catalyst [33,34,35,36], a Pd-Cl-Cu structural form will form on the surface of the catalyst, where chloride ions play a role in electron transfer. As the catalytic reaction proceeds, chloride ions will be lost to a certain extent, thereby affecting the redox cycle between palladium and copper, and ultimately leading to a decrease in catalyst activity. For the application of the Pd-Cu bimetal catalyst in the MN carbonyl synthesis of DMC, researchers have carried out in-depth studies on the determination of the active species, carrier effect, and assistant effect.
Matsuzaki et al. [34,35,36], in comparing different supported solid catalysts used in the reaction of the MN carbonyl synthesis of DMC, found that the order of catalyst activity was as follows: Pd-Cu-Cl ˃ PdCl2 ˃ Pd(0) ˃ Cu-Cl. The Pd-Cu bimetal chloride catalyst showed the best catalytic activity, while the Cu-Cl catalyst showed almost no activity. This preliminarily indicates that the Pd species is the active component in the synthesis of DMC by the MN carbonyl groups, while Cu acts only as an auxiliary agent. Furthermore, Matsuzaki et al. [37,38] performed H2 reduction on the AC supported Pd-based catalyst PdCl2/AC and found a significant change in the distribution of products, with dimethyl oxalate (DMO) as the main product instead of DMC. Next, the catalyst reduced by hydrogen to Pd(0) was treated with MN and HCl, and a catalyst with high selectivity to DMC was obtained. Lv et al. reported on a Pd-CuCl2/γ-Al2O3 catalyst [39] that showed a high catalytic activity of the CO esterylation reaction and excellent DMC selectivity. The catalysts containing chloride ions were found to have high DMC selectivity, while the catalysts without chloride ions showed very low DMC selectivity. In addition, the addition of Pd could increase the conversion rate of CO, which also proved that Pd(II) was the active site of the catalysis, and the Lewis acidity of the carrier could also enhance the catalytic activity.
X-ray photoelectron spectroscopy (XPS) was used to characterize the PdCl2/AC catalyst before and after treatment [40,41]. The results showed that the valence of the Pd species had a great influence on the catalytic activity of the catalyst. The Pd(II) species was the active species in the synthesis of DMC by the MN carbonyl group, while the Pd(0) species was more conducive to the synthesis of DMO. Cl ions can maintain the oxidation state of Pd(II), so HCl gas needs to be added to replenish the lost Cl ions in the reaction process to maintain the stability of the catalyst.
The carrier effect of the MN gas-phase carbonyl synthesis of the DMC system is very significant. From the comparison of the catalytic activity of conventional catalyst carriers [37,38,39], it can be seen that the catalyst with silicon oxide as the carrier had the worst catalytic activity, the catalyst with activated alumina as the carrier was slightly better, and the catalyst with AC as the carrier showed the best catalytic efficiency; the space–time yield (STY for short) of DMC reached 553 g·Lcat−1·h−1. Studies have reported [40,41,42,43,44] that the acidity of the support plays an important role in the yield and selectivity of DMC, and that the acidic site will cause the decomposition of the reactant MN. The excessive decomposition of MN produces more MF, resulting in the decreased selectivity of the main product, DMC. Therefore, it is the support with no acid site or low acidity on the surface that is the best choice for the synthesis of DMC by MN carbonyl. Japan UBE [45] invented a lithium aluminum spanner oxide carrier with a large specific surface area and weak acidity. The Wacker-type catalyst prepared with it as a carrier shows excellent catalytic performance in the reaction of the MN carbonyl synthesis of DMC, and the time and space yield of DMC reached 670 g·Lcat−1·h−1. The selectivity of both CO and the MN-based products was greater than 95%. The few acidic sites on the catalyst surface, spinel structure, and electron conduction performance of Li ions are the reasons for its high activity [46], but its catalytic activity still decreases with the extension of reaction time.
The reasons for the deactivation of the catalyst for the MN gas-phase carbonyl synthesis of DMC are as follows. First, due to the presence of the reactant CO, the active component Pd(II) species is gradually reduced to Pd(0) at the reaction temperature, followed by agglomeration, forming large particles of Pd clusters. Second, due to the loss of chloride ions during the reaction, Pd(0) cannot be timely and completely converted to Pd(II), and the catalytic activity gradually decreases. Therefore, it is necessary to supplement a certain amount of chloride ions in the feedstock gas to maintain its catalytic activity. In general, the reduced Pd(0) can be re-oxidized to Pd(II) by entraining trace amounts of methyl chloroformate or hydrogen chloride in the feedstock gas [47,48].
3.2. Chlorine-Free Catalyst
The loss of the chlorine-containing catalyst in the reaction process easily leads to the reduction of Pd(II) active components, thus making the catalyst inactive. In addition, the introduction of chloride ions can also lead to corrosion of the reaction equipment, affecting production safety and product purity. Therefore, researchers have carried out a lot of work on the exploration of chlorine-free catalysts. Japan UBE [49] developed a chlorine-free catalyst with Pd as the active metal and molecular sieve as the carrier for the first time. The Pd/NaY catalyst showed excellent catalytic activity, even in the absence of chlorine. After 700 h of reaction, the spatial and temporal yield of DMC could still reach 200 g·Lcat−1·h−1, and the selectivity of DMC based on MN was stable at about 80%. For Pd-supported molecular sieve catalysts, the metal position, size, and cluster shape can be accurately controlled by ion exchange, roasting, and the reduction and oxidation conditions [50,51,52,53,54]. The unique pore cage structure of molecular sieves enables Pd to form a stable Pd cluster structure during the catalytic synthesis of DMC by MN carbonyl such as the Pd13(CO)x cluster. This species can stably exist in the pore structure of molecular sieves and show stable catalytic performance [49,55,56].
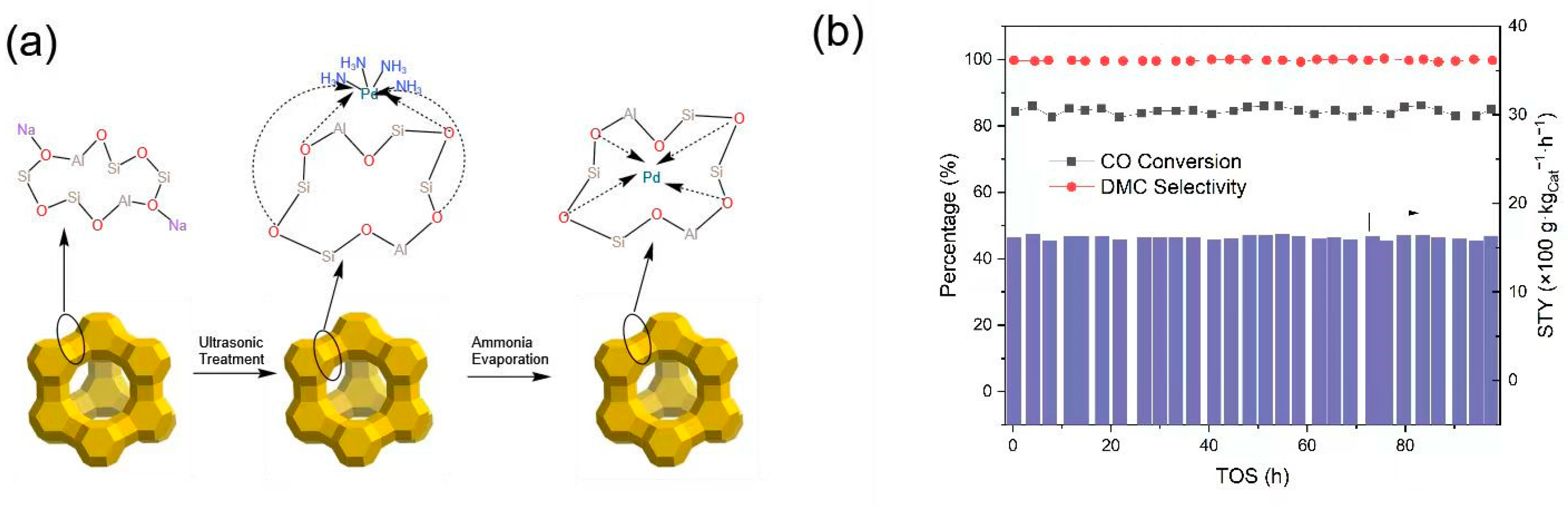
The excellent catalytic activity [57,58,59,60] of the chloride-free zeolite catalyst system can be attributed to the following: Pd can be well-embedded in the unique pore-cage structure of the zeolite under appropriate calcination conditions; Pd is reduced during the reaction, and the pore structure of the zeolite prevents further growth of the Pd cluster, thus maintaining the activity of the DMC synthesis reaction, as shown in Figure 1. In addition, the molecular sieve skeleton oxygen anion can also play a role in oxidizing the reduced Pd.
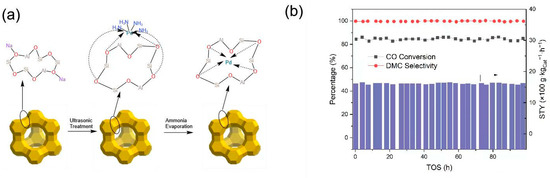
Figure 1.
(a) Diagram of the formation process of the Pd(II)/NaY catalyst. (b) CO conversion, product selectivity, and WTY of DMC in the long-term CO direct catalytic conversion reaction on the Pd(II)/NaY catalyst. Reaction conditions: 0.2 g of catalyst, 120 °C, 0.1 MPa, weight hour space velocity (WHSV) = 2500 L·kgcat−1·h−1, CO/MN/Ar/N2 = 19:45:3:33 [57].
With the in-depth study of molecular sieve-supported Pd catalysts, the types of molecular sieves have been expanded from 12-element ring macroporous Y-type molecular sieves to Silicalite-1 molecular sieves [61], HCUST-1 molecular sieves [62], and EMT molecular sieves [63] with a 10-element ring pore structure that show good catalytic selectivity and stability. At the same time, by controlling the distribution of Al atoms in the molecular sieve skeleton [59] and introducing heteroatomic metals [64] to adjust the acidity of the molecular sieve, the catalytic performance is also significantly improved.
The influence of the catalyst forming process on the catalyst structure is obvious, but it also has a significant influence on the existing form of metal species containing metal zeolite catalysts [65]. The ratio of Pd(2 + δ)+ and Pdδ+ species in Pd/NaY catalysts (0 < δ ≤ 2) can be regulated by changing the mechanical forming pressure. Monodisperse Pd clusters (1.3 nm) were obtained in the Pd/NaY catalyst under mechanical treatment at 300 MPa. The catalyst showed a high CO conversion of 89% and a DMC selectivity of 83%, which was maintained for at least 150 h. Combined with experiments and density functional theory, it was further found that Pdδ+ species enhanced the adsorption of CO and CH3ONO reactants and inhibited the decomposition of CH3ONO reactants into by-products, thus improving the selectivity of DMC.
In the chlorine-free catalyst system, Pd(II) and Pd(0) are still the only catalytically active species to selectively obtain DMC and DMO, respectively [66]. Therefore, in the chlorine-free catalyst system, there is still the issue of Pd(II) reduction by CO, leading to a decline in selectivity. Pd(II) active sites can be effectively stabilized by selecting the appropriate support, so the nature of the catalyst support is particularly important. In the design and synthesis of catalysts, the support plays a very important role, which can not only effectively disperse the metal active center, but can also improve the stability of the catalyst by enhancing the interaction between the metal support. Metal–organic framework materials (MOFs), which are crystalline porous materials composed of metal ions or metal clusters and organic ligands, have attracted extensive attention in recent years. In particular, as a catalyst carrier, it is expected to obtain a stable catalyst for the preparation of DMC with high selectivity by stabilizing the Pd active site through MOFs [66,67,68,69,70,71].
Xu et al. [66] used different MOF materials as carriers to stabilize the Pd(II) active center and obtained three catalysts: Pd(II)/UiO-66, Pd(II)/MIL-101, and Pd(II)/MOF-5, among which Pd(II)/UiO-66 showed the best catalytic performance with 87.9% CO conversion and 98.5% DMC selectivity. Characterization analysis showed that UiO-66 with more defects had a large number of Lewis acid sites, and its catalytic performance was positively correlated with the number of Lewis acid sites in the MOF carrier.
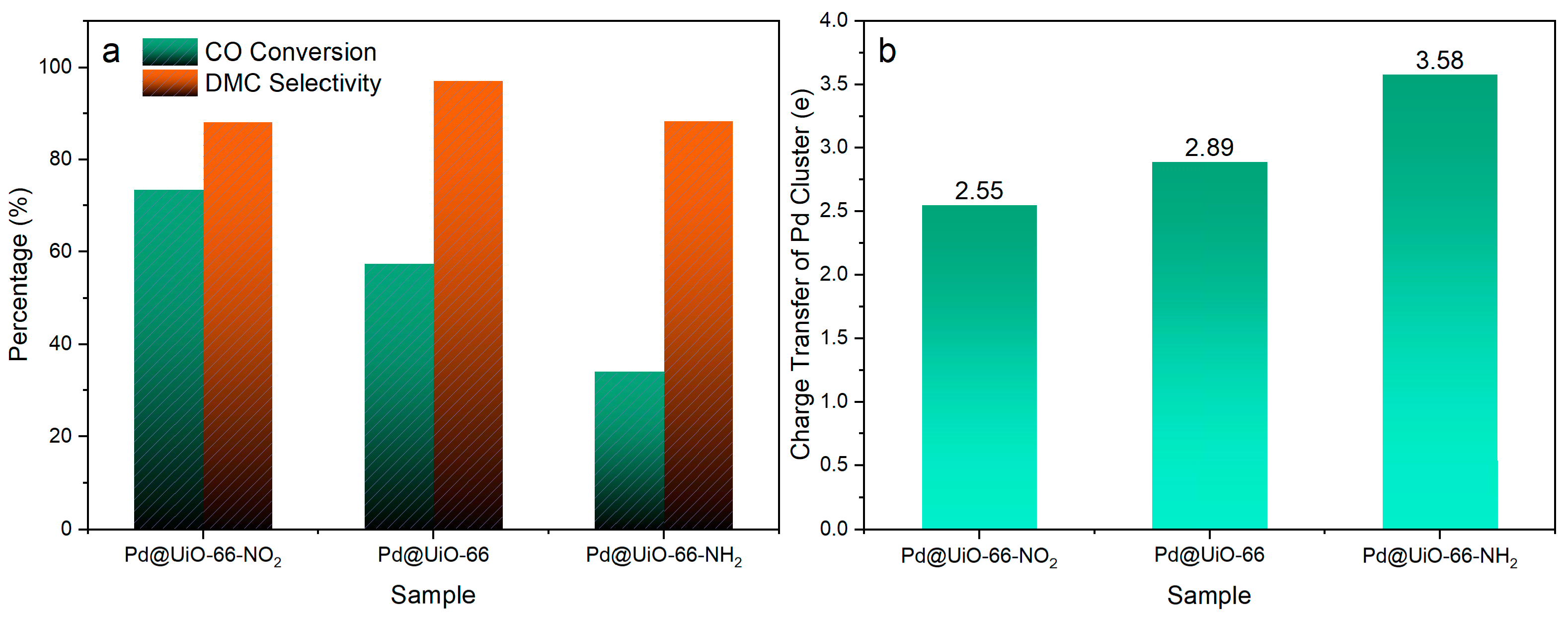
By changing the functional groups of MOFs, the Pd electron density in Pd@UiO-66-X (X = −H, −NO2, −NH2) could be reasonably adjusted based on microenvironment regulation, thus optimizing the activity [67]. Both experimental and DFT calculations showed that the microenvironment of Lewis acid sites around the Pd NPs was related to the level of the interface between Pd and Zr-oxygen clusters and played a key role in the resulting selectivity, with abundant Lewis acid sites in Pd@UiO-66 favoring high DMC selectivity. However, Pd/UiO-66, which had fewer interfaces and fewer Lewis acid sites, was around the Pd NPs and followed a different reaction pathway to provide DMO products, as shown in Figure 2. Tuning the microenvironment of metal nanoparticles provides important insights into their optimized electronic states and activity; moreover, it opens up a new avenue for selective modulation by controlling the position of the active metal site relative to the porous support in heterogeneous catalysis [66,67,71].
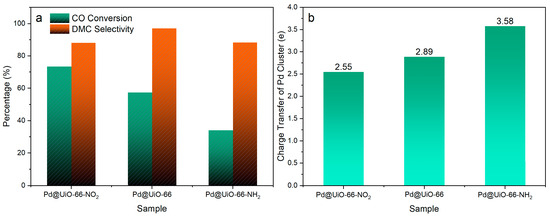
Figure 2.
(a) The conversion and selectivity to DMC over Pd@UiO-66-X (X = −H, −NO2, −NH2) in direct CO esterification under the reaction condition tended to produce DMC: 180 mg catalyst, 120 °C, 0.1 MPa, WHSV = 2500 L·kgcat−1·h−1; reactant gases composed of 19% CO, 45% MN, 3% Ar, and 33% N2. The selectivity and conversion were calculated based on CO. (b) The calculated number of electron transfer from Pd clusters to the MOF in Pd@UiO-66-X [67].
3.3. Mechanism of Gas-Phase Carbonylation Reaction of Methyl Nitrite
According to the literature reports, both chlorine-containing and chlorine-free systems have confirmed that Pd(II) species are more conducive to the catalytic reaction synthesis of DMC, while Pd(0) species are more conducive to the catalytic reaction synthesis of DMO. The difference between the two catalytic systems is whether the chlorine species affect the catalytic mechanism, and how the chlorine species affect the catalytic mechanism is the focus of research.
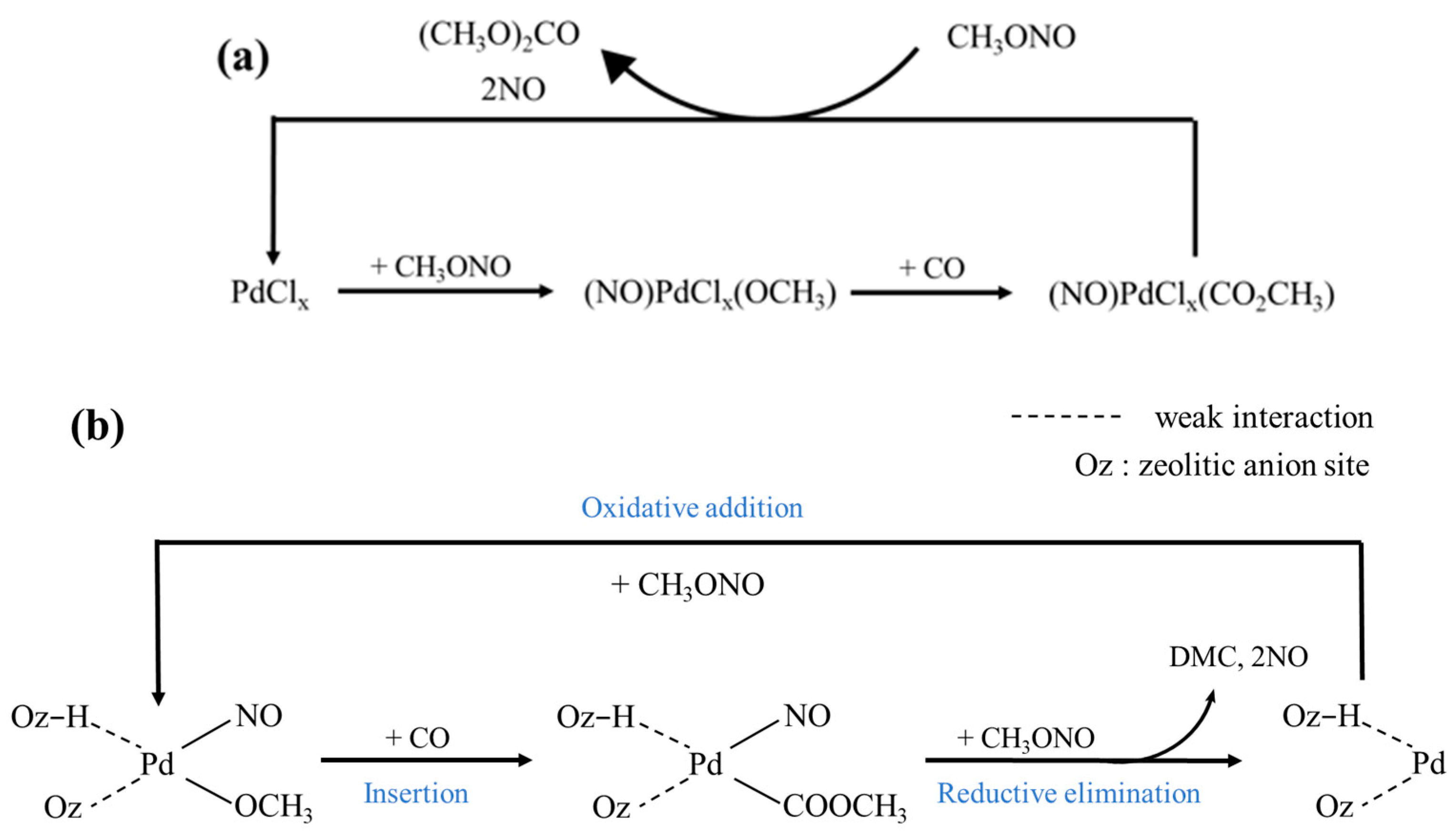
In the Cu-Pd bimetallic catalytic system containing chlorine, researchers [33] believe that chloride ions may be included in the intermediate of the palladium complex formed during the reaction, as shown in Figure 3. First, MN molecules adsorb on PdCl2 to form PdC12(OCH3)(NO) species, and then Mn molecules adsorb on PdCl2 to form PDC12(OCH3)(NO) species. The intermediate species Pd(COOCH3)(NO)Cl2 is formed by CO insertion, and then the intermediate species Pd(COOCH3)(NO)Cl2 is attacked and interacted with by MN, finally removing DMC and NO. However, some researchers [49] believe that although the experimental data concluded that Pd(II) catalyzed the synthesis of DMC and Pd(0) catalyzed the synthesis of DMO, the possibility that the reaction to synthesize DMC originated from Pd(0) species could not be ruled out due to the circulation of Pd species in the reaction process.
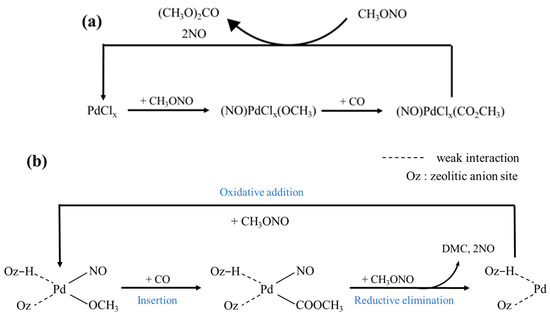
Figure 3.
(a) Catalytic mechanism for DMC formation in the presence of chloride ions [33]. (b) Reaction mechanism for DMC synthesis from MN and CO over Pd/NaY [49].
In the chlorine-free Pd-based zeolite catalytic system, the active component Pd binds to the zeolite skeleton oxygen anion in the form of a cation. Japan UBE [49] proposed the following mechanism: first, MN is adsorbed on the Pd species to form an intermediate species (I), followed by CO insertion to form an intermediate species (II), and then collides with another molecule of MN to remove DMC and NO. The reaction mechanism was modeled after that of the chlorine-containing catalyst, but it was not clearly indicated in what state the initiating Pd species participated in the reaction.
4. Direct Synthesis of DMC from CO2 and Methanol
The direct carbon dioxide synthesis method uses methanol and carbon dioxide as raw materials to directly synthesize DMC in one step, and the reaction equation is as follows.
The direct synthesis of carbon dioxide is a green and environmentally friendly process technology with wide sources of raw materials and low price, and can solve the problems of greenhouse gas and carbon dioxide emissions [72,73,74]. Catalysts for the direct CO2 synthesis of DMC mainly include homogeneous catalysts and heterogeneous catalysts.
4.1. Homogeneous Catalyst
Homogeneous catalysts for the direct synthesis of DMC by CO2 mainly include alkoxy-based organic compound catalysts, acetate metal catalysts, alkali metal catalysts and so on. Danielle et al. [75] used the metal stannoxy group as the catalyst and found through in situ infrared characterization that the metal site on the surface of the catalyst could adsorb molecular CH3OH and form a bond connected with metal and oxygen. CO2 inserted into Sn-OCH3 formed a weak active intermediate, which could not only adsorb on the catalyst surface, but also react on the catalyst surface. Thus DMC and water are generated. Sakakura et al. [76] developed a catalyst that used methanol, carbon dioxide and dimethyl ketone as raw materials, Bu2SnO-or Ti(O-i-Pr)4 as a catalyst, and added a small amount of (Ph2NH2)OTf to significantly improve the activity of DMC. This catalyst can be used for multiple cycle experiments without reducing the catalytic performance.
In recent years, the mechanism analysis by means of theoretical chemical calculation has provided help in the research of alkoxy organic compound catalysts. DFT calculations showed in [77] that in the process of catalyzing CO2 and methanol to form DMC with the organotin [Me2SnO]2 dimer as a catalyst, the tin oxide dimer promotes the activation of four methanol molecules to form the [Me2Sn(OMe)2]2 dimer, which acts as a CO2 trapping active agent. Two CO2 molecules are inserted into the Sn-OMe bond outside the ring. Although DMC can be obtained directly from the reconstruction of the intermediate carbonate, the calculation showed that the path of DMC produced by the reaction of the dimer formed by oligomerization with methanol has a lower energy barrier, which is the optimal reaction path. Therefore, for dialkyl tin(IV) organometallic compounds, on the basis of capturing and activating CO2, the degree of polymerization or coordination configuration is regulated to make it easier to react with methanol to obtain DMC [77,78].
Fang et al. [79] investigated the effect of a series of alkali metal catalysts on the catalytic performance of the direct synthesis of DMC from CO2 and found that K2CO3 had the best catalytic effect when CH3I was used as a cocatalyst. The catalytic mechanism of alkali metal was speculated as follows: first, methanol interacts with alkali to form methoxy anion (CH3O–), and then forms intermediate products with CO2. The intermediate and CH3I are methylated to form target products DMC and HI, and the regeneration of CH3I is produced by the interaction between HI and methanol. It is worth noting that the reaction must use CH3I as an assistant to provide CH3 in the reaction process—by combining with the reaction produced H to generate HI (without CH3I, the reaction will not have DMC), the HI and CH3OH reaction reduction to CH3I takes place, thus completing a catalytic reaction cycle.
Inspired by the basic catalytic mechanism of the direct conversion of CO2 to DMC by CH3I and alkali catalysts containing potassium, Santosh et al. [80] proposed a new catalytic system for a switchable ionic liquid (SIL). In this new reaction, an organic superbase such as 1,8-Diazabicyclo-[5.4.0]-Undec-7-ene, DBU is mixed with methanol to capture and activate carbon dioxide to obtain SIL [DBUH][CH3CO3], which can then be switched to further react with an equal amount of CH3I to produce DMC. According to the experimental results, when methanol was used as the solvent, the yield of DMC reached 89%. In this study, CO2 was captured at room temperature and catalyzed into valuable chemicals. This idea of catalyst design with SIL provides a new research idea for the capture and utilization of carbon dioxide [80,81].
The acetate metal catalyst also showed good catalytic activity in the direct synthesis of DMC from CO2 [82]. In the supercritical CO2 state, the DMC synthesis reaction was carried out at low temperature with nickel acetate as the catalyst, and the DMC yield was significantly higher than that in the noncritical state. FTIR characterization was used to speculate the reaction mechanism of the system: under alkaline conditions, methanol combines with metal nickel atoms to form methoxy nickel, followed by CO2 insertion between the nickel and oxygen bonds, and the products DMC and water form under the action of the cocatalyst. The yield and selectivity of DMC depend on the supercritical pressure and phase composition. The supercritical pressure has a higher catalytic performance on the reaction and pushes the equilibrium to a positive shift, which is mainly due to the higher pressure promoting the production of nickel methyxyl species and inhibiting the formation of CH3COOCH3 species, thus increasing the DMC content.
4.2. Heterogeneous Catalyst
Heterogeneous catalysts overcome the disadvantages of difficult separation and recycling of the product, and have the advantages of easy separation of the product and good stability of the catalyst. At present, the heterogeneous catalysts mainly studied include supported catalysts, heteropoly acid catalysts, metal oxide catalysts, new photocatalysts, and new electrocatalysts.
4.2.1. Supported Catalysts
The supported catalysts for this reaction mainly involve the catalytic active centers of organotin compounds and Cu-based bimetals, and the corresponding catalyst carriers are zeolites, mesoporous silica, diatomite, carbon materials (AC, graphite, carbon nanotubes), and metal–organic framework materials (MOFs for short).
The initial design idea of supported catalysts was to load or graft homogeneous catalysts such as organotin compounds onto a carrier (SBA-15) with a large specific surface area and mesopore structure to improve the separation and recovery of homogeneous catalysts [83,84,85,86]. Fan et al. [83] used SBA-15 with a mesoporous pore size and large specific surface area as a carrier to graft organotin compounds in situ to obtain a stable heterogeneous organotin catalyst. It has been shown that the six-coordinated organotin cluster has a higher catalytic activity than the four-coordinated species, and the catalytic activity increases significantly with increasing reaction temperature and CO2 pressure. In addition, the grafting of organotin can also be promoted by modifying the silicon hydroxyl group on the surface of SBA-15. However, although the homogeneous catalyst has been heterogeneous, there is still a gap in catalytic performance.
With an in-depth understanding of the reaction, the rational design of the supported catalyst has mainly been carried out from the following aspects. (1) The formation of Lewis sites by metal sites to promote the carbonylation reaction. (2) The introduction of metal Cu and Cu-based bimetals that are easy to activate CO2 to promote the conversion of CO2 and improve the reaction performance.
The Cu-Ni bimetal was loaded on a graphite support by the impregnation method to study the catalytic activity of the composite catalyst on the direct synthesis of DMC from CO2 [87,88,89]. The results showed that the layered structure of the composite catalyst graphite was still well-maintained, and the active metal particles were evenly dispersed on the surface of the graphite support. The average grain size was 15.8 nm. In this type of composite catalyst, the interaction between metal particles and the unique structure of graphite forms smaller metal particles with high dispersion, which is conducive to the improvement in catalytic performance. The above findings indicate that by loading active metal species on the carrier surface, catalysts with acid–base bifunctionality are obtained, which can adsorb both activated methanol and CO2, thus generating more of the target product DMC. An in-situ dehydration system for the direct synthesis of dimethyl carbonate (DMC) was formed by combining a supported catalyst with a 3A molecular sieve [90]. Compared with the conventional reaction, the selectivity of DMC remained above 88% at 13 h and decreased to 78% at 22 h under the condition of in-situ dehydration. Although the catalyst gradually deactivated in the reaction, the in situ dehydration catalytic system provides a new idea for the design and development of supported catalysts.
MOF materials have attracted much attention due to their high specific surface area and porosity, and their metal framework sites and defect sites show potential for the catalytic synthesis of DMC. At present, zirconium-based MOF materials such as UiO-66 [91,92,93], MOF-808 [94], and ZIF-67 [95] have good catalytic DMC selectivity. Xuan et al. [91] used trifluoroacetic acid to adjust the framework structure, specific surface area, Lewis acid site, Lewis base site, and the number of hydroxyl groups of the metal–organic skeleton UiO-66, and these changes enhanced the activity of UIO-66-X. At the same time, they also proposed a possible reaction mechanism for the synthesis of DMC by CO2 and methanol on UiO-66-X combined with in-situ infrared. In addition, after the introduction of ZIF-67 into CeO2, the number of oxygen vacancies and surface acid–base sites of the catalyst increased significantly, and the catalyst had a high CO2 adsorption capacity, showing the best catalytic performance [95]. With MOF-808-4 as the catalyst [94], the methanol conversion rate reached 21.5% and the DMC selectivity was 100% at 140 °C and 12 MPa for 48 h.
4.2.2. Heteropoly Acid Catalyst
Heteropoly acids are oxometallic clusters containing oxygen bridges. Their special cage structure and Brønsted acidity bring potential applications for the catalytic direct synthesis of DMC from CO2. At present, H3PW12O40 with the Keggin structure shows more suitable acid content and acid strength for the catalytic synthesis of DMC, and commonly used carriers are ZrO2, Ce-Ti oxide, and Ce-Zr oxide.
The H3PW12O40/ZrO2 catalyst prepared by Jiang et al. [96] using the sol–gel method had Brønsted acid sites and Lewis acid sites, while the carrier ZrO2 had Lewis acid sites. The activity of H3PW12O40/ZrO2 on DMC synthesis was significantly higher than that of the pure ZrO2 carrier, and the DMC yield was linearly related to the amount of H3PW12O40. It can be seen that compared with the Lewis acid site, the Brønsted acid site on the catalyst could activate CH3OH more effectively, thus improving the catalytic performance. Under the optimized reaction conditions for the direct synthesis of DMC (reaction temperature 170 °C, reaction pressure 5.0 MPa), the H3PW12O40/ZrO2 catalyst had the best catalytic performance, the methanol conversion reached 4.45%, and the DMC selectivity was 89.93% [97]. When Co1.5PW12O40 was used as the catalyst, the methanol conversion rate was 7.6%, and the DMC selectivity was 86.5% at 200 °C and atmospheric pressure [98].
Similarly, the acid–base bifocal catalysts formed on CexTi1−xO2 [99] and CexZr1−xO2 [100] using H3PW12O40 as the heteropoly acid also showed that the activity of the catalysts increased with the increase in the acidity of the catalyst surface. The results show that the acidity of the catalyst plays an important role in the direct synthesis of DMC from CO2. Chiang et al. [101] used the sol–gel method with the catalyst H3PW12O40/Ce0.1Ti0.9O2 at a reaction temperature of 170 °C and a reaction pressure of 5.0 MPa; the methanol conversion reached 5.5% and the DMC selectivity was 91.4%. Combined with the carrier oxygen vacancy and the crystal structure defect, they believe that the oxygen vacancy on the catalyst surface provides the Lewis acid site, an oxygen of CO2 fills the oxygen vacancy in the reaction, then the hydrogen in methanol falls in the adjacent oxygen vacancy, and the carbon in carbon dioxide interacts with the oxygen in methanol. It is then further reacted with another molecule of methanol to form a large intermediate, which is cleaved to produce DMC and water.
4.2.3. Metal Oxide Catalyst
Lewis acid–base active sites of transition metal oxides provide suitable catalytic active sites for the direct synthesis of DMC from ethanol and CO2. In this reaction process, CH3OH is adsorbed and activated under the joint action of the Lewis acid–base active site of the transition metal oxide, CO2 is then inserted and reacted to obtain monocarbonate, which is then reacted with CH3OH activated at the acid active site to produce DMC. ZrO2 and CeO2 metal oxides with both acid–base active sites show good catalytic performance [102,103,104,105,106].
Tomishige et al. [105] obtained ZrO2 catalysts by the direct calcination of ZrO2·xH2O and investigated the influence of different calcination temperatures on catalytic performance. The study found that the formation rate of DMC was closely related to the crystalline structure of zirconia. When the calcination temperature was 673 K, the catalyst exhibited a metastable tetragonal phase and showed the optimal DMC formation rate. Characterization analysis indicated that the formation of DMC was related to the acid–base active sites on the surface of zirconia. Jung et al. [106] further studied the synthesis mechanism of CO2 and methanol catalyzed by ZrO2 by the in-situ infrared method and speculated on the activation mechanism of acid–base sites. Density functional theory calculation showed that in [107], the acid–base and electronic structure of the ZrO2 surface determined its catalytic performance, and the stronger the Lewis acid, the higher the catalytic activity. At the same time, it was also found that in different ZrO2 cubic (c), tequad (t), and monoclinic (m) ZrO2 phases, the optimal path of DMC formation was the CH3OCO intermediate path, and the catalytic activity sequence was t-ZrO2, m-ZrO2, and c-ZrO2.
The acid–base properties and oxygen vacancies of metal oxide catalysts play an important role in promoting the formation of DMC. In order to improve the acid–base properties and the number of oxygen vacancies on the catalyst surface, Liu et al. [88] modified CeO2 with CaO. Raman results showed that the interaction between CaO and CeO2 created more oxygen vacancies. More oxygen vacancies were conducive to CO2 adsorption and improved the catalytic performance of the CO2 synthesis of DMC, where the DMC yield was 2.47 mmol·g−1. Wu et al. [108] used H3PO4 to modify V2O5, and the results showed that the crystalline phase of H3PO4/V2O5 changed from a single orthorhombic phase to a mixed phase of orthorhombic and tetragonal phase, and when the ratio of P/V substances was in an appropriate range, H3PO4/V2O5 had the best catalytic performance. This is mainly because the interaction between V and P on the surface of H3PO4/V2O5 forms a weak Brønsted acid site, which is more conducive to the activation of CH3OH than the Lewis acid site on the bulk phase V2O5, thus increasing the DMC yield.
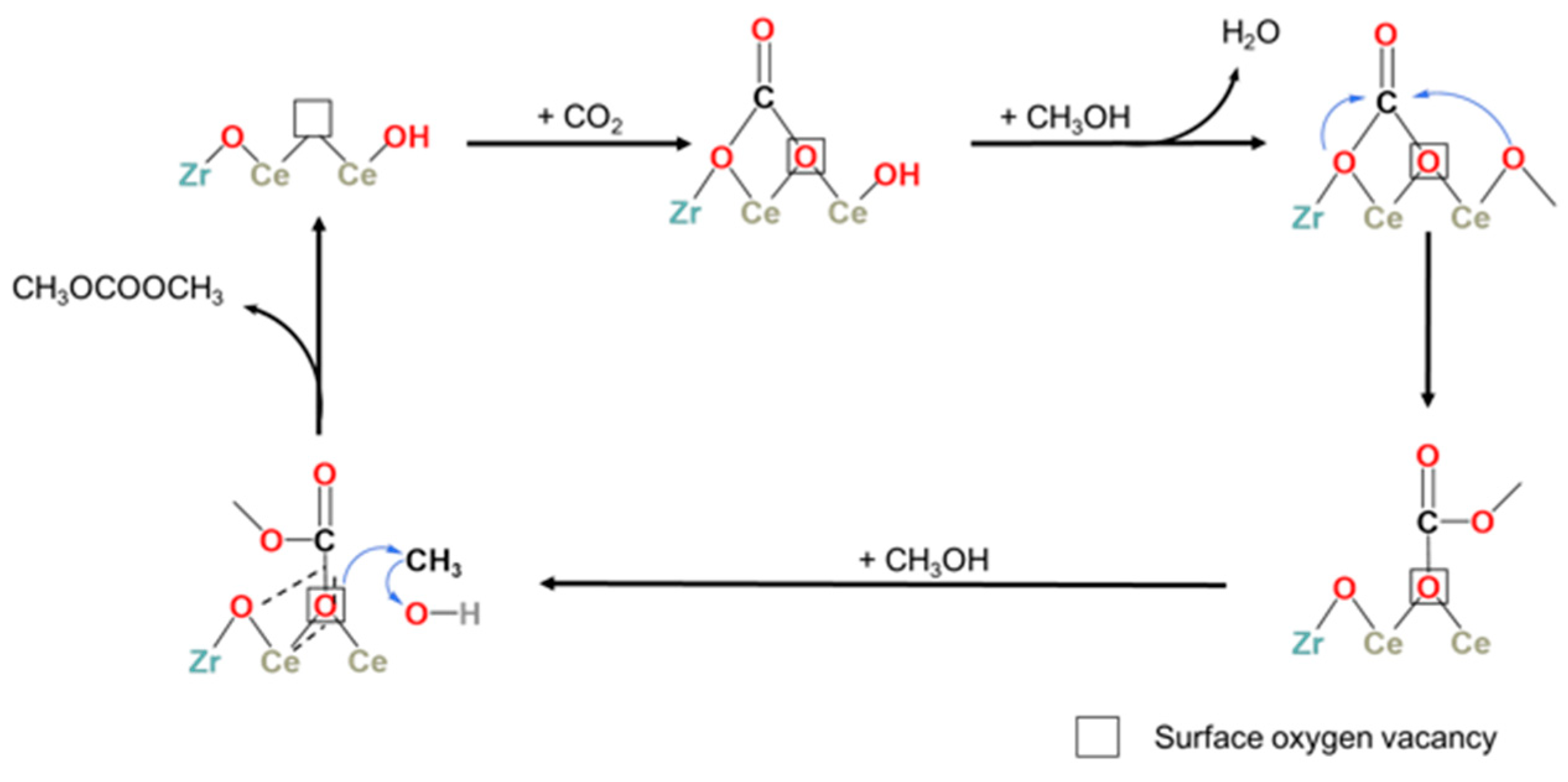
In addition, it has also been reported that the small number of acid–base sites and oxygen vacancies on the catalyst surface can be solved by doping heteroatoms into metal oxides. For example, Liu et al. [109] prepared Zr-doped CeO2 to form a Zr-Ce solid solution that increased the number of acid–base active sites and oxygen vacancies on the catalyst surface and then improved the catalytic performance. Based on the XPS, CO2-TPD, and FTIR results, it was found that CO2 and methanol interacted with the surface oxygen vacancies and surface hydroxyl groups, respectively. This is consistent with the LH mechanism. In addition, there is a linear relationship between the DMC yield of the catalyst and the CO2 adsorption capacity. The adsorption and activation steps of CO2 are the rate-determining steps for the direct synthesis of DMC by CO2 and methanol. Methanol can be adsorbed on adjacent oxygen vacancies to produce an intermediate, which can then adsorb CO2 to form DMC in further reactions, and then regenerate oxygen vacancies, as shown in Figure 4. At the same time, some researchers are doping Cu, Ca, Mn, Al, Ti, and other metals into ZrO2 or CeO2 to form a solid solution catalyst and increase the number of acid–base active sites and oxygen vacancies on its surface, thus significantly improving the catalytic performance.
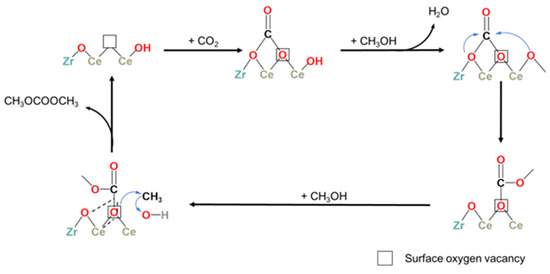
Figure 4.
Proposed mechanism of DMC synthesis on ZrxCe nanorods [109].
Another focus of research in the synthesis of DMC from CO2 and methanol by metal oxide catalysts is the source of oxygen atoms in the reaction product DMC. Identifying the source of oxygen in DMC plays a key role in understanding the reaction mechanism. The oxygen in this reaction may come from the catalyst, carbon dioxide, or methanol. For the CexZr1−xO2 catalyst, studies using the partial 18O labeled catalyst or 18O labeled methanol showed that the oxygen atoms in DMC came from methanol or carbon dioxide, while the oxygen atoms in the catalyst did not participate in the reaction [110]. In fact, the rate determining step in this reaction is the nucleophilic attack of methanol/methoxy species to form DMC. However, the above studies have led to a new understanding that the migration and transformation process of oxygen species (the reaction between methanol and surface hydroxyl groups to form water and methoxy, and the reaction between carbon dioxide and surface methoxy substances to form MMC) are also important steps in the whole reaction.
In recent years, high entropy oxides based on rare earth oxide materials have shown specific catalytic properties. Gu et al. [111] anchored rare earth metal ions to graphene oxide by electrostatic action and used calcination to obtain the high-entropy fluorite oxide Cex(LaPrSmY)1−x O2−y. Under the same reaction conditions, compared with CeO2, the time to reach the equilibrium reaction was reduced by half due to the abundant oxygen vacancy and excellent electron transfer ability on the surface of the high-entropy fluorite oxide, and the DMC yield was as high as 7.6 mmol/g at 140 °C. This new research progress provides a new idea for the design and development of DMC oxide catalysts, especially in the regulation of the oxide structure, metal content, and distribution, on the basis of high entropy oxides, which will have a significant impact on the formation of oxygen vacancies and catalytic performance.
4.3. Novel Photocatalysts and Electrocatalysts
The direct conversion of CO2 to DMC reaction is limited by thermodynamic equilibrium, and the introduction of photocatalysis technology can break the restriction of thermodynamic equilibrium and promote the reaction to the direction of product under the synergistic photothermal effect to improve the reaction efficiency. Wang et al. [112] studied the photocatalytic effect of a copper-modified (Ni, V, O) semiconductor complex catalyst on the direct synthesis of DMC by CO2. The results showed that under the reaction conditions of a temperature of 120~140 °C and pressure of 1 atm, the catalytic activity of the catalyst under UV radiation with a particle size of 4~8 nm was greatly improved compared with the ordinary surface catalytic reaction, and the reaction used a fixed bed reactor to separate the water in the product from the system in time. The reaction process is guaranteed, and it is also one of the most important reasons for the increase in yield.
In a special electrocatalytic reactor, under the action of an electric field, the catalyst can transfer electrons to CO2 molecules through electrodes and break through the thermodynamic limitation of CO2 and the shackle of kinetic inertia. The CO2 and methanol reactants can be activated through the interaction of the external voltage, electrolyte solution, and electrode material to achieve the purpose of activating CO2, the process of making it into a DMC. Yuan et al. [113] used Pt as the electrode, CH3OK as the cocatalyst, and 1-butyl-3-methylimidazolium bromide [Bmim][Br] as the electrolyte to initiate electrocatalytic activation and transformation to form DMC, and the yield of DMC could reach up to 3.9%. In this electrochemical catalytic process system, there is no CH3I or other organic additives in the system, which makes the process more environmentally friendly than the system reported in the previous study. Therefore, the DMC yield can be effectively improved by using a new type of electrocatalyst.
The direct synthesis of DMC from CO2 and methanol is an attractive route to convert CO2 into high value-added chemicals, and the key question is how to activate CO2 more efficiently. Furthermore, the difficulty of the photocatalyzed CO2 preparation of DMC lies in how to overcome the high negative reduction potential in the process of forming carbonate free radicals. Chen et al. [114] found that oxygen vacancies in Bi2O3 nanosheets could reduce the adsorption energy of CO2 at the active site, thus activating CO2 through single electron transfer under mild conditions to form ·CO2− species. Jin et al. [115] proposed introducing light energy into the thermal catalytic system and using CeO2 nanorods with oxygen vacancies as catalysts to promote the direct conversion of CO2 and methanol into DMC in low-pressure photothermal catalysis. This study showed that oxygen vacancy can promote CO2 adsorption and activation through Lewis acid–base interaction while acting as a photogenerated charge capture center to prevent the recombination of photogenerated electrons and holes. At the same time, Bai et al. [116] successfully prepared a tetrapyramidal octahedral CeO2 catalyst by the hydrothermal method, showing the highest DMC yield of 1.58%. The activity of the catalyst was superior to that of thermal catalysis alone due to the synergistic effect of photocatalysis and thermal catalysis. The active site was found to be a CeO2 angle defect combined with DFT. These works provide a new way to realize the conversion of CO2 into high value-added chemicals. However, the regulation and synergistic effect of oxygen vacancy need more in-depth research to further enhance the possibility of practical application.
5. Conclusions
In recent years, the research and development of DMC synthesis technology have been hot research directions in the chemical industry. With the continuous development and maturity of DMC synthesis technology, DMC production methods will also develop from traditional transesterification and methanol oxidation carbonylation toward a diversified direction. In particular, gaseous methyl nitrite carbonylation, CO2, and methanol direct synthesis of DMC have shown great application potential.
The DMC process of MN carbonyl synthesis based on the carbon-chemical industry is a relatively economical synthesis route, which has the advantages of abundant raw material sources, mild reaction conditions, environmental friendliness, and a relatively simple subsequent separation of products as well as broad development prospects. The research into chlorine-free PD-based catalysts is expected to promote the technical progress of this process route. As for the catalytic reaction of precious metal nanoparticles as active species, their catalytic activity depends to a large extent on the size and morphology of the nanoparticles, and more in-depth and fine regulation of the interaction between metal nanoparticles and carriers is an important direction to improve the synthesis performance of DMC in the future. New catalysts such as special pore structure, acidic molecular sieve materials, and metal–organic framework materials provide new ideas for the efficient synthesis of DMC while ensuring the stability of metal nanoparticles. In addition, on the basis of clarifying the adsorption of reactants and reaction intermediates, the reaction mechanism of the MN carbonyl synthesis of DMC should be clarified in combination with in-situ reactions and theoretical chemical calculations to provide help in the design and development of catalysts.
A large number of studies have been conducted on the synthesis of DMC from CO2. However, the results are not ideal due to the difficulties in CO2 activation and the easy deactivation of catalysts. Electrochemical technology provides an effective solution to the problem of CO2 activation and conversion. The synthesis of DMC by reducing CO2 by the electrochemical technique is not only easy to operate, but is also mild, and no new CO2 source will appear in the reaction process. However, the current research is still limited to the influence of the synthesis conditions and electrode materials on the DMC yield and lacks an in-depth understanding of the reduction mechanism of CO2, especially the adsorption process of CO2 to the electrode, the structural change of the organic solution, and the combination between reaction intermediates. At the same time, clarifying the relationship between the microstructure and performance of the catalyst and the synergistic effect between the catalyst and the electrolyte will promote the design of new electroreduction CO2 catalysts and become the key to improving their catalytic performance.
Author Contributions
Conceptualization: F.S. and L.W.; methodology, F.S.; resources, F.S.; writing—original draft preparation, D.W.; writing—review and editing, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is available on request from the authors.
Conflicts of Interest
Author Dong Wang was employed by the company Tianjin Jiuyuan Chemical Engineering Co., Ltd. and Technical Quality Department, Rongsheng New Materials (Zhoushan) Co., Ltd. Author Feng Shi was employed by the company Tianjin Jiuyuan Chemical Engineering Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tan, H.Z.; Wang, Z.Q.; Xu, Z.N.; Sun, J.; Xu, Y.P.; Chen, Q.S.; Chen, Y.; Guo, G.C. Review on the synthesis of dimethyl carbonate. Catal. Today 2018, 316, 2–12. [Google Scholar] [CrossRef]
- Keller, N.; Rebmann, G.; Keller, V. Catalysts, mechanisms and industrial processes for the dimethylcarbonate synthesis. J. Mol. Catal. A Chem. 2010, 317, 1–18. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Hong, S.T.; Park, H.S.; Lim, J.S.; Lee, Y.-W.; Anpo, M.; Kim, J.D. Synthesis of dimethyl carbonate from methanol and supercritical carbon dioxide. Res. Chem. Intermed. 2006, 32, 737–747. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Park, J.H.; Chang, T.-S.; Hatti-Kaul, R. Dimethyl carbonate as a green chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Tundo, P.; Musolino, M.; Aricò, F. The reactions of dimethyl carbonate and its derivatives. Green Chem. 2018, 20, 28–85. [Google Scholar] [CrossRef]
- Deng, W.; Shi, L.; Yao, J.; Zhang, Z. A review on transesterification of propylene carbonate and methanol for dimethyl carbonate synthesis. Carbon Resour. Convers. 2019, 2, 198–212. [Google Scholar] [CrossRef]
- Kohli, K.; Sharma, B.K.; Panchal, C.B. Dimethyl carbonate: Review of synthesis routes and catalysts Used. Energies 2022, 15, 5133. [Google Scholar] [CrossRef]
- Pacheco, M.A.; Marshall, C.L. Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive. Energy Fuels 1997, 11, 2–29. [Google Scholar] [CrossRef]
- Ono, Y. Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block. Appl. Catal. A Gen. 1997, 155, 133–166. [Google Scholar] [CrossRef]
- Shaikh, A.-A.G.; Sivaram, S. Organic carbonates. Chem. Rev. 1996, 96, 951–976. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y. Dimethyl carbonate for environmentally benign reactions. Catal. Today 1997, 35, 15–25. [Google Scholar] [CrossRef]
- Delledonne, D.; Rivetti, F.; Romano, U. Oxidative carbonylation of methanol to dimethyl carbonate (DMC): A new catalytic system. J. Organomet. Chem. 1995, 488, C15–C19. [Google Scholar] [CrossRef]
- Tundo, P.; Trotta, F.; Moragliob, G. Selective and continuous-flow mono-methylation of arylacetonitriles with dimethyl carbonate under gas-liquid phase-transfer catalysis conditions. J. Chem. Soc. Perkin Trans. 1 1989, 5, 1070–1071. [Google Scholar] [CrossRef]
- Dahiya, S.; Srivastava, V.C.; Kumar, V. Dimethyl carbonate synthesis via transesterification of propylene carbonate using a titanium–praseodymium-based catalyst. Energy Fuels 2022, 36, 13148–13158. [Google Scholar] [CrossRef]
- Shukla, K.; Srivastava, V.C. Diethyl carbonate: Critical review of synthesis routes, catalysts used and engineering aspects. RSC Adv. 2016, 6, 32624–32645. [Google Scholar] [CrossRef]
- Santos, B.A.; Silva, V.M.; Loureiro, J.M.; Rodrigues, A.E. Review for the direct synthesis of dimethyl carbonate. ChemBioEng Rev. 2014, 1, 214–229. [Google Scholar] [CrossRef]
- Romano, U.; Tesel, R.; Mauri, M.M.; Rebora, P. Synthesis of dimethyl carbonate from methanol, carbon monoxide, and oxygen catalyzed by copper compounds. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 396–403. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. The role of surface chemistry in catalysis with carbons. Catal. Today 2010, 150, 2–7. [Google Scholar] [CrossRef]
- Qi, W.; Su, D. Metal-free carbon catalysts for oxidative dehydrogenation reactions. ACS Catal. 2014, 4, 3212–3218. [Google Scholar] [CrossRef]
- Lee Curnutt, G.; Dale Harley, A. Copper catalyzed oxidative carbonylation of methanol to dimethyl carbonate. In Oxygen Complexes and Oxygen Activation by Transition Metals; Springer: Berlin/Heidelberg, Germany, 1988; pp. 215–232. [Google Scholar]
- Han, M.S.; Lee, B.G.; Ahn, B.S.; Moon, D.J.; Hong, S.I. Surface properties of CuCl2/AC catalysts with various Cu contents: XRD, SEM, TG/DSC and CO-TPD analyses. Appl. Surf. Sci. 2003, 211, 76–81. [Google Scholar] [CrossRef]
- Tomishige, K.; Sakaihori, T.; Sakai, S.-i.; Fujimoto, K. Dimethyl carbonate synthesis by oxidative carbonylation on activated carbon supported CuCl2 catalysts: Catalytic properties and structural change. Appl. Catal. A Gen. 1999, 181, 95–102. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsuzaki, T.; Ohdan, K.; Okamoto, Y. Structure and electronic state of PdCl2-CuCl2 catalysts supported on activated carbon. J. Catal. 1996, 161, 577–586. [Google Scholar] [CrossRef]
- Kriventsov, V.; Klimov, O.; Kikhtyanin, O.; Ione, K.; Kochubey, D. EXAFS study of Cu/C catalysts. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2000, 448, 318–322. [Google Scholar] [CrossRef]
- Punnoose, A.; Seehra, M.; Dunn, B.; Eyring, E. Characterization of CuCl2/PdCl2/activated carbon catalysts for the synthesis of diethyl carbonate. Energy Fuels 2002, 16, 182–188. [Google Scholar] [CrossRef]
- Han, M.S.; Lee, B.G.; Suh, I.; Kim, H.S.; Ahn, B.S.; Hong, S.I. Synthesis of dimethyl carbonate by vapor phase oxidative carbonylation of methanol over Cu-based catalysts. J. Mol. Catal. A Chem. 2001, 170, 225–234. [Google Scholar] [CrossRef]
- Zhang, G.; Liang, J.; Yin, J.; Yan, L.; Narkhede, N.; Zheng, H.; Li, Z. An efficient strategy to improve the catalytic activity of CuY for oxidative carbonylation of methanol: Modification of NaY by H4EDTA-NaOH sequential treatment. Microporous Mesoporous Mater. 2020, 307, 110500. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Wang, J.-Z.; Li, Z.; Yan, L.-F.; Wen, J.Z. Characterization and assessment of an enhanced CuY catalyst for oxidative carbonylation of methanol prepared by consecutive liquid-phase ion exchange and incipient wetness impregnation. Fuel Process. Technol. 2016, 152, 367–374. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Tan, C.; Sun, H.; Li, Z. High catalytic activity of CuY catalysts prepared by high temperature anhydrous interaction for the oxidative carbonylation of methanol. RSC Adv. 2020, 10, 3293–3300. [Google Scholar] [CrossRef]
- Zheng, H.; Narkhede, N.; Zhang, G.; Li, Z. Role of metal co-cations in improving CuY zeolite performance for DMC synthesis: A theoretical study. Appl. Organomet. Chem. 2020, 34, e5832. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, G.; Song, Y.; Yu, S.; Zhao, J.; Zheng, H. DFT investigations of the reaction mechanism of dimethyl carbonate synthesis from methanol and CO on various Cu species in Y zeolites. Catalysts 2023, 13, 477. [Google Scholar] [CrossRef]
- Yamamoto, Y. Vapor phase carbonylation reactions using methyl nitrite over Pd catalysts. Catal. Surv. Asia 2010, 14, 103–110. [Google Scholar] [CrossRef]
- Nakamura, A.; Matsuzaki, T. A new oxidation system using nitrite oxidants. Res. Chem. Intermed. 1998, 24, 213–225. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Nakamura, A. Dimethyl carbonate synthesis and other oxidative reactions using alkyl nitrites. Catal. Surv. Asia 1997, 1, 77–88. [Google Scholar] [CrossRef]
- Uchiumi, S.-I.; Ataka, K.; Matsuzaki, T. Oxidative reactions by a palladium–alkyl nitrite system. J. Organomet. Chem. 1999, 576, 279–289. [Google Scholar] [CrossRef]
- Lv, D.M.; Xu, Z.N.; Peng, S.Y.; Wang, Z.Q.; Chen, Q.S.; Chen, Y.; Guo, G.C. (Pd-CuCl2)/γ-Al2O3: A high-performance catalyst for carbonylation of methyl nitrite to dimethyl carbonate. Catal. Sci. Technol. 2015, 5, 3333–3339. [Google Scholar] [CrossRef]
- Matuzaki, T.; Simamura, T.; Fujitsu, S.; Toriyahara, Y. Method of Producing Carbonic Acid Diester. U.S. Patent No. EP0503618, 8 March 1992. [Google Scholar]
- Herman, F.L.; Savoca, A.C.; Listemann, M.L. Preparation of Metallated and Substituted Alkynes. U.S. Patent No. 5062998, 10 November 1992. [Google Scholar]
- Cassady, C.; Freiser, B. Gas-phase reactions of transition-metal ions with methyl nitrite and nitromethane. J. Am. Chem. Soc. 1985, 107, 1566–1573. [Google Scholar] [CrossRef]
- Choudhury, T.; He, Y.; Sanders, W.; Lin, M. Carbon monoxide formation in the thermal decomposition of methyl nitrite at high temperatures: Kinetic modeling of the methoxy decomposition rate. J. Phys. Chem. 1990, 94, 2394–2398. [Google Scholar] [CrossRef]
- Zhuo, G.L.; Jiang, X.Z. An attractive synthetic approach to methyl formate from methanol via methyl nitrite. Catal. Lett. 2002, 80, 171–174. [Google Scholar] [CrossRef]
- Zhuo, G.L.; Jiang, X.Z. Catalytic decompostiton of methyl nitrite over supported palladium catalysts in vapor phase. React. Kinet. Catal. Lett. 2002, 77, 219–226. [Google Scholar] [CrossRef]
- Zhuo, G.L.; Jiang, X.Z. Catalytic decomposition of ethyl nitrite over supported palladium catalyst. Chin. J. Catal. 2003, 24, 509–512. [Google Scholar]
- Matsuzaki, T.; Hitaka, M.; Tanaka, S.; Nishihira, K. Vapor Phase Dimethyl Carbonate Synthesis from Methyl Nitrite and Carbon Monoxide over Porous Lithium Aluminate Spinel Supported Palladium Catalyst. Nippon Kagaku Kaishi J. Chem. Soc. Jpn. Chem. Ind. Chem. 1999, 5, 347–354. [Google Scholar]
- Ge, Y.; Dong, Y.; Wang, S.; Zhao, Y.; Lv, J.; Ma, X. Influence of crystalline phase of Li-Al-O oxides on the activity of Wacker-type catalysts in dimethyl carbonate synthesis. Front. Chem. Sci. Eng. 2012, 6, 415–422. [Google Scholar] [CrossRef]
- Manada, N.; Murakami, M.; Yamamoto, Y.; Kurafuji, T. Preparation of dimethyl carbonate in the gas-phase reaction-release of Cl-compound from PdCl2 catalyst and effect of methyl chloroformate. Nippon Kagaku Kaishi 1994, 11, 985–991. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Ohdan, K.; Asano, M.; Tanaka, S.; Nishihira, K.; Chiba, Y. Preparation method of dimethyl carbonate using methyl nitrite. Nippon Kagaku Kaishi 1999, 1, 15–24. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, Y.; Matsuzaki, T.; Tanaka, S.; Nishihira, K.; Ohdan, K.; Nakamura, A.; Okamoto, Y. Catalysis and characterization of Pd/NaY for dimethyl carbonate synthesis from methyl nitrite and CO. J. Chem. Soc. Faraday Trans. 1997, 93, 3721–3727. [Google Scholar] [CrossRef]
- Zhang, Z.; Mestl, G.; Knözinger, H.; Sachtler, W. Effects of calcination program and rehydration on palladium dispersion in zeolites NaY and 5A. Appl. Catal. A Gen. 1992, 89, 155–168. [Google Scholar] [CrossRef]
- Bergeret, G.; Tri, T.M.; Gallezot, P. X-ray study of palladium location in Y zeolite during in situ hydrogen reduction, benzene adsorption and benzene hydrogenation. J. Phys. Chem. 1983, 87, 1160–1165. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P.; Imelik, B. X-ray study of the activation, reduction, and reoxidation of palladium in Y-type zeolites. J. Phys. Chem. 1981, 85, 411–416. [Google Scholar] [CrossRef]
- Zhang, Z.; Sachtler, W.M.; Chen, H. Identification by diffuse reflectance and EXAFS of the changes in coordination of NaY entrapped Pd (NH3)x2+ ion during calcination. Zeolites 1990, 10, 784–789. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, H.; Sachtler, W.M. Migration and coalescence of Pd carbonyl clusters in zeolite Y. J. Chem. Soc. Faraday Trans. 1991, 87, 1413–1418. [Google Scholar] [CrossRef]
- Sheu, L.L.; Knoezinger, H.; Sachtler, W.M. Palladium carbonyl clusters entrapped in NaY zeolite cages: Ligand dissociation and cluster-wall interactions. J. Am. Chem. Soc. 1989, 111, 8125–8131. [Google Scholar] [CrossRef]
- Beutel, T.; Zhang, Z.; Sachtler, W.; Knözinger, H. Temperature dependence of palladium cluster formation in NaY and 5A zeolites. J. Phys. Chem. 1993, 97, 3579–3583. [Google Scholar] [CrossRef]
- Tan, H.Z.; Chen, Z.N.; Xu, Z.N.; Sun, J.; Wang, Z.Q.; Si, R.; Zhuang, W.; Guo, G.-C. Synthesis of high-performance and high-stability Pd(II)/nay catalyst for co direct selective conversion to dimethyl carbonate by rational design. ACS Catal. 2019, 9, 3595–3603. [Google Scholar] [CrossRef]
- Dong, Y.; Huang, S.; Wang, S.; Zhao, Y.; Gong, J.; Ma, X. Synthesis of dimethyl carbonate through vapor-phase carbonylation catalyzed by Pd-doped zeolites: Interaction of lewis acidic sites and Pd species. Communications 2013, 1, 1. [Google Scholar] [CrossRef]
- Guo, R.; Hou, Z.; Chen, J.; Qin, Y.; Chai, G.; Yao, Y. Improved catalytic performance of Pd-Cu/NaY zeolite by tunning Al distribution for the synthesis of dimethyl carbonate. Fuel 2022, 330, 125484. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Liu, P.; Huang, K.; Xu, N.; Guo, H.; Bai, P.; Ling, L.; Liu, X.; Mintova, S. Elucidation of the reaction mechanism of indirect oxidative carbonylation of methanol to dimethyl carbonate on Pd/NaY catalyst: Direct identification of reaction intermediates. J. Catal. 2022, 412, 30–41. [Google Scholar] [CrossRef]
- Wang, C.; Xu, N.; Huang, K.; Liu, B.; Zhang, P.; Yang, G.; Guo, H.; Bai, P.; Mintova, S. Emerging co-synthesis of dimethyl oxalate and dimethyl carbonate using Pd/silicalite-1 catalyst with synergistic interactions of Pd and silanols. Chem. Eng. J. 2023, 466, 143136. [Google Scholar] [CrossRef]
- Tan, H.Z.; Chen, Z.N.; Jing, K.Q.; Sun, J.; Xu, Y.P.; Zhang, N.N.; Xu, Z.N.; Guo, G.C. Paired-Pd(II) centers embedded in HKUST-1 framework: Tuning the selectivity from dimethyl carbonate to dimethyl oxalate. J. Energy Chem. 2022, 67, 233–240. [Google Scholar] [CrossRef]
- Wang, C.; Xu, W.; Qin, Z.; Guo, H.; Liu, X.; Mintova, S. Highly active Pd containing EMT zeolite catalyst for indirect oxidative carbonylation of methanol to dimethyl carbonate. J. Energy Chem. 2021, 52, 191–201. [Google Scholar] [CrossRef]
- Wu, S.; Guo, R.; Chen, J.; Ye, R.; Qin, Y.; Wu, H.; Zong, S.; Liu, Y.; Yao, Y. Rational design of Ga-substituted NaY zeolites with controllable acidity for remarkable carbonylation of methyl nitrite to dimethyl carbonate. Fuel 2023, 342, 127756. [Google Scholar] [CrossRef]
- Wang, C.; Xu, N.; Liu, T.-T.; Xu, W.; Guo, H.; Li, Y.; Bai, P.; Wu, X.-P.; Gong, X.-Q.; Liu, X. Mechanical pressure-mediated Pd active sites formation in NaY zeolite catalysts for indirect oxidative carbonylation of methanol to dimethyl carbonate. J. Catal. 2021, 396, 269–280. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Wang, Z.-Q.; Tan, H.-Z.; Jing, K.-Q.; Xu, Z.-N.; Guo, G.-C. Lewis acid sites in MOFs supports promoting the catalytic activity and selectivity for CO esterification to dimethyl carbonate. Catal. Sci. Technol. 2020, 10, 1699–1707. [Google Scholar] [CrossRef]
- Hu, S.; Xie, C.; Xu, Y.P.; Chen, X.; Gao, M.L.; Wang, H.; Yang, W.; Xu, Z.N.; Guo, G.C.; Jiang, H.L. Selectivity control in the direct CO esterification over Pd@UiO-66: The Pd location matters. Angew. Chem. 2023, 135, e202311625. [Google Scholar] [CrossRef]
- Ji, S.; Chen, Y.; Zhao, G.; Wang, Y.; Sun, W.; Zhang, Z.; Lu, Y.; Wang, D. Atomic-level insights into the steric hindrance effect of single-atom Pd catalyst to boost the synthesis of dimethyl carbonate. Appl. Catal. B Environ. 2022, 304, 120922. [Google Scholar] [CrossRef]
- Xie, C.; Xu, Y.-P.; Gao, M.-L.; Xu, Z.-N.; Jiang, H.-L. MOF-stabilized Pd single sites for CO esterification to dimethyl carbonate. Acta Chim. Sin. 2022, 80, 867–873. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Qin, Y.-Y.; Xiao, Y.-H.; Chen, J.-S.; Guo, R.; Wu, S.-Q.; Zhang, L.; Zhang, J.; Yao, Y.-G. Synergistic Lewis acid and Pd active sites of metal–organic frameworks for highly efficient carbonylation of methyl nitrite to dimethyl carbonate. Inorg. Chem. Front. 2022, 9, 2379–2388. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Qin, Y.-Y.; Xiao, Y.-H.; Chen, J.-S.; Ye, R.; Guo, R.; Yao, Y.-G. Boosting activity and selectivity of UiO-66 through acidity/alkalinity functionalization in dimethyl carbonate catalysis. Small 2023, 19, 2208238. [Google Scholar] [CrossRef]
- Zhang, Y.; Khalid, M.S.; Wang, M.; Li, G. New strategies on green synthesis of dimethyl carbonate from carbon dioxide and methanol over oxide composites. Molecules 2022, 27, 5417. [Google Scholar] [CrossRef]
- Kumar, P.; Srivastava, V.C.; Štangar, U.L.; Mušič, B.; Mishra, I.M.; Meng, Y. Recent progress in dimethyl carbonate synthesis using different feedstock and techniques in the presence of heterogeneous catalysts. Catal. Rev. 2021, 63, 363–421. [Google Scholar] [CrossRef]
- Shi, D.; Heyte, S.; Capron, M.; Paul, S. Catalytic processes for the direct synthesis of dimethyl carbonate from CO2 and methanol: A review. Green Chem. 2022, 24, 1067–1089. [Google Scholar] [CrossRef]
- Ballivet-Tkatchenko, D.; Jerphagnon, T.; Ligabue, R.; Plasseraud, L.; Poinsot, D. The role of distannoxanes in the synthesis of dimethyl carbonate from carbon dioxide. Appl. Catal. A Gen. 2003, 255, 93–99. [Google Scholar] [CrossRef]
- Choi, J.-C.; Kohno, K.; Ohshima, Y.; Yasuda, H.; Sakakura, T. Tin-or titanium-catalyzed dimethyl carbonate synthesis from carbon dioxide and methanol: Large promotion by a small amount of triflate salts. Catal. Commun. 2008, 9, 1630–1633. [Google Scholar] [CrossRef]
- Poor Kalhor, M.; Chermette, H.; Ballivet-Tkatchenko, D. Dimethyl carbonate synthesis from CO2 and dimethoxytin (IV) complexes: The anatomy of the alkylation step viewed from DFT modeling. Ind. Eng. Chem. Res. 2019, 59, 6867–6873. [Google Scholar] [CrossRef]
- De Andrade, K.N.; da Costa, L.M.; Carneiro, J.W.D.M. Formation of dimethyl carbonate from CO2 and methanol catalyzed by Me2SnO: A density functional theory approach. J. Phys. Chem. A 2021, 125, 2413–2424. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Fujimoto, K. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol catalyzed by base. Appl. Catal. A Gen. 1996, 142, L1–L3. [Google Scholar] [CrossRef]
- Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P. One-pot, metal-free synthesis of dimethyl carbonate from CO2 at room temperature. Sustain. Chem. 2020, 1, 298–314. [Google Scholar] [CrossRef]
- Khokarale, S.; Shelke, G.; Mikkola, J.-P. Integrated and metal free synthesis of dimethyl carbonate and glycidol from glycerol derived 1,3-dichloro-2-propanol via CO2 capture. Clean Technol. 2021, 3, 685–698. [Google Scholar] [CrossRef]
- Zhao, T.; Han, Y.; Sun, Y. Novel reaction route for dimethyl carbonate synthesis from CO2 and methanol. Fuel Process. Technol. 2000, 62, 187–194. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, J.; Li, R.; Fan, W. In situ preparation of functional heterogeneous organotin catalyst tethered on SBA-15. Catal. Lett. 2008, 121, 297–302. [Google Scholar] [CrossRef]
- Fan, B.; Li, H.; Fan, W.; Zhang, J.; Li, R. Organotin compounds immobilized on mesoporous silicas as heterogeneous catalysts for direct synthesis of dimethyl carbonate from methanol and carbon dioxide. Appl. Catal. A Gen. 2010, 372, 94–102. [Google Scholar] [CrossRef]
- Ballivet-Tkatchenko, D.; Bernard, F.; Demoisson, F.; Plasseraud, L.; Sanapureddy, S.R. Tin-based mesoporous silica for the conversion of CO2 into dimethyl carbonate. ChemSusChem 2011, 4, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Li, H.; Fan, W.; Qin, Z.; Li, R. Direct synthesis of dimethyl carbonate from methanol and carbon dioxide over organotin-functionalized mesoporous benzene-silica. Pure Appl. Chem. 2011, 84, 663–673. [Google Scholar] [CrossRef]
- Wu, X.; Meng, Y.; Xiao, M.; Lu, Y. Direct synthesis of dimethyl carbonate (DMC) using Cu-Ni/VSO as catalyst. J. Mol. Catal. A Chem. 2006, 249, 93–97. [Google Scholar] [CrossRef]
- Bian, J.; Xiao, M.; Wang, S.; Wang, X.; Lu, Y.; Meng, Y. Highly effective synthesis of dimethyl carbonate from methanol and carbon dioxide using a novel copper–nickel/graphite bimetallic nanocomposite catalyst. Chem. Eng. J. 2009, 147, 287–296. [Google Scholar] [CrossRef]
- Zhang, M.; Alferov, K.A.; Xiao, M.; Han, D.M.; Wang, S.J.; Meng, Y.Z. Continuous dimethyl carbonate synthesis from CO2 and methanol using Cu-Ni@VSiO as catalyst synthesized by a novel sulfuration method. Catalysts 2018, 8, 142. [Google Scholar] [CrossRef]
- Han, D.M.; Chen, Y.; Wang, S.J.; Xiao, M.; Lu, Y.X.; Meng, Y.Z. Effect of in-situ dehydration on activity and stability of Cu-Ni-K2O/diatomite as catalyst for direct synthesis of dimethyl carbonate. Catalysts 2018, 8, 343. [Google Scholar] [CrossRef]
- Xuan, K.; Pu, Y.F.; Li, F.; Li, A.X.; Luo, J.; Li, L.; Wang, F.; Zhao, N.; Xiao, F.K. Direct synthesis of dimethyl carbonate from CO2 and methanol over trifluoroacetic acid modulated UiO-66. J. CO2 Util. 2018, 27, 272–282. [Google Scholar] [CrossRef]
- Xu, W.; Xu, Z.H.; Yao, W.X.; Hu, L.H.; Ding, K.Q.; Wu, G.D.; Xiao, G.M.; Gao, L.J. Directly synthesis of dimethyl carbonate from CO2 and methanol over UiO-66@CeO2 Catalyst. Appl. Catal. A Gen. 2023, 662, 119262. [Google Scholar] [CrossRef]
- Huo, L.; Wang, L.; Li, J.; Pu, Y.; Xuan, K.; Qiao, C.; Yang, H. Cerium doped Zr-based metal-organic framework as catalyst for direct synthesis of dimethyl carbonate from CO2 and methanol. J. CO2 Util. 2023, 68. [Google Scholar] [CrossRef]
- Xuan, K.; Pu, Y.F.; Li, F.; Luo, J.; Zhao, N.; Xiao, F.K. Metal-organic frameworks MOF-808-X as highly efficient catalysts for direct synthesis of dimethyl carbonate from CO2 and methanol. Chin. J. Catal. 2019, 40, 553–566. [Google Scholar] [CrossRef]
- Hu, L.H.; Wnag, X.; Hu, K.R.; Chen, C.; Xu, Z.H.; Xu, W. Direct synthesis of dimethyl carbonate from CO2 and methanol over ZIF-67/CeO2. Chin. J. Inorg. Chem. 2023, 39, 1315–1324. [Google Scholar]
- Jiang, C.; Guo, Y.; Wang, C.; Hu, C.; Wu, Y.; Wang, E. Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions. Appl. Catal. A Gen. 2003, 256, 203–212. [Google Scholar] [CrossRef]
- Chiang, C.L.; Lin, K.S.; Yu, S.H. Preparation and characterization of H3PW12O40/ZrO2 catalyst for carbonation of methanol into dimethyl carbonate. Res. Chem. Intermed. 2018, 44, 3797–3811. [Google Scholar] [CrossRef]
- Aouissi, A.; Al-Deyab, S.S. Comparative study between gas phase and liquid phase for the production of DMC from methanol and CO2. J. Nat. Gas Chem. 2012, 21, 189–193. [Google Scholar] [CrossRef]
- La, K.W.; Jung, J.C.; Kim, H.; Baeck, S.-H.; Song, I.K. Effect of acid–base properties of H3PW12O40/CexTi1−xO2 catalysts on the direct synthesis of dimethyl carbonate from methanol and carbon dioxide: A TPD study of H3PW12O40/CexTi1−xO2 catalysts. J. Mol. Catal. A Chem. 2007, 269, 41–45. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, S.; Jung, J.C.; Song, I.K. Direct synthesis of dimethyl carbonate from methanol and carbon dioxide over H3PW12O40/CeXZr1−XO2 catalysts: Effect of acidity of the catalysts. Korean J. Chem. Eng. 2011, 28, 1518–1522. [Google Scholar] [CrossRef]
- Chiang, C.L.; Lin, K.S.; Yu, S.H.; Lin, Y.G. Synthesis and characterization of H3PW12O40/Ce0.1Ti0.9O2 for dimethyl carbonate formation via Methanol carbonation. Int. J. Hydrogen Energy 2017, 42, 22108–22122. [Google Scholar] [CrossRef]
- Akune, T.; Morita, Y.; Shirakawa, S.; Katagiri, K.; Inumaru, K. ZrO2 nanocrystals as catalyst for synthesis of dimethylcarbonate from methanol and carbon dioxide: Catalytic activity and elucidation of active sites. Langmuir 2018, 34, 23–29. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Continuous DMC synthesis from CO2 and methanol over a CeO2 catalyst in a fixed bed reactor in the presence of a dehydrating agent. ACS Catal. 2014, 4, 3877–3880. [Google Scholar] [CrossRef]
- Tomishige, K.; Furusawa, Y.; Ikeda, Y.; Asadullah, M.; Fujimoto, K. CeO2–ZrO2 solid solution catalyst for selective synthesis of dimethyl carbonate from methanol and carbon dioxide. Catal. Lett. 2001, 76, 71–74. [Google Scholar] [CrossRef]
- Tomishige, K.; Sakaihori, T.; Ikeda, Y.; Fujimoto, K. A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia. Catal. Lett. 1999, 58, 225–229. [Google Scholar] [CrossRef]
- Jung, K.T.; Bell, A.T. An in situ infrared study of dimethyl carbonate synthesis from carbon dioxide and methanol over zirconia. J. Catal. 2001, 204, 339–347. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Sun, W.; Pei, Y.; An, J.; Li, Z.; Ren, J. A DFT study of dimethyl carbonate synthesis from methanol and CO2 on zirconia: Effect of crystalline phases. Comput. Mater. Sci. 2019, 159, 210–221. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, M.; Meng, Y.; Lu, Y. Direct synthesis of dimethyl carbonate on H3PO4 modified V2O5. J. Mol. Catal. A Chem. 2005, 238, 158–162. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yao, X.; Chuang, S.S.; Li, Z. Oxygen vacancy promoting dimethyl carbonate synthesis from CO2 and methanol over Zr-doped CeO2 nanorods. ACS Catal. 2018, 8, 10446–10456. [Google Scholar] [CrossRef]
- Daniel, C.; Farrusseng, D.; Schuurman, Y. Investigating the reaction mechanism of dimethyl carbonate synthesis through isotopic labeling experiments. Catal. Commun. 2023, 179, 106697. [Google Scholar] [CrossRef]
- Gu, Y.; Cheng, Q.; Li, X.; Zhang, S.; Wang, Z.; Wang, Y. Direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by cerium-based high-entropy oxides. Catal. Lett. 2024, 154, 513–523. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, M.; Wang, S.; Lu, Y.; Meng, Y. Direct synthesis of dimethyl carbonate from carbon dioxide and methanol using supported copper (Ni, V, O) catalyst with photo-assistance. J. Mol. Catal. A Chem. 2007, 278, 92–96. [Google Scholar] [CrossRef]
- Yuan, D.; Yan, C.; Lu, B.; Wang, H.; Zhong, C.; Cai, Q. Electrochemical activation of carbon dioxide for synthesis of dimethyl carbonate in an ionic liquid. Electrochim. Acta 2009, 54, 2912–2915. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Kang, Z.; Jin, S.; Zhang, X.; Zheng, X.; Qi, Z.; Zhu, J.; Pan, B.; Xie, Y. Oxygen vacancy associated single-electron transfer for photofixation of CO2 to long-chain chemicals. Nat. Commun. 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guan, X.; Zhang, X.; Zhang, C.; Liu, J.; Wang, Y.; Wang, Y.; Li, R.; Li, Z.; Fan, C. CeO2 nanorods with bifunctional oxygen vacancies for promoting low-pressure photothermocatalytic CO2 conversion with CH3OH to dimethyl carbonate. J. Environ. Chem. Eng. 2023, 11, 111374. [Google Scholar] [CrossRef]
- Bai, J.Q.; Lv, L.; Liu, J.; Wang, Q.; Cheng, Q.; Cai, M.; Sun, S. Control of CeO2 defect sites for photo-and thermal-synergistic catalysis of CO2 and methanol to DMC. Catal. Lett. 2023, 153, 3209–3218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).