Synthesis of a Highly Efficient Mesoporous Green Catalyst from Waste Avocado Peels for Biodiesel Production from Used Cooking–Baobab Hybrid Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. CAP Catalyst Characterization
FT-IR Analysis
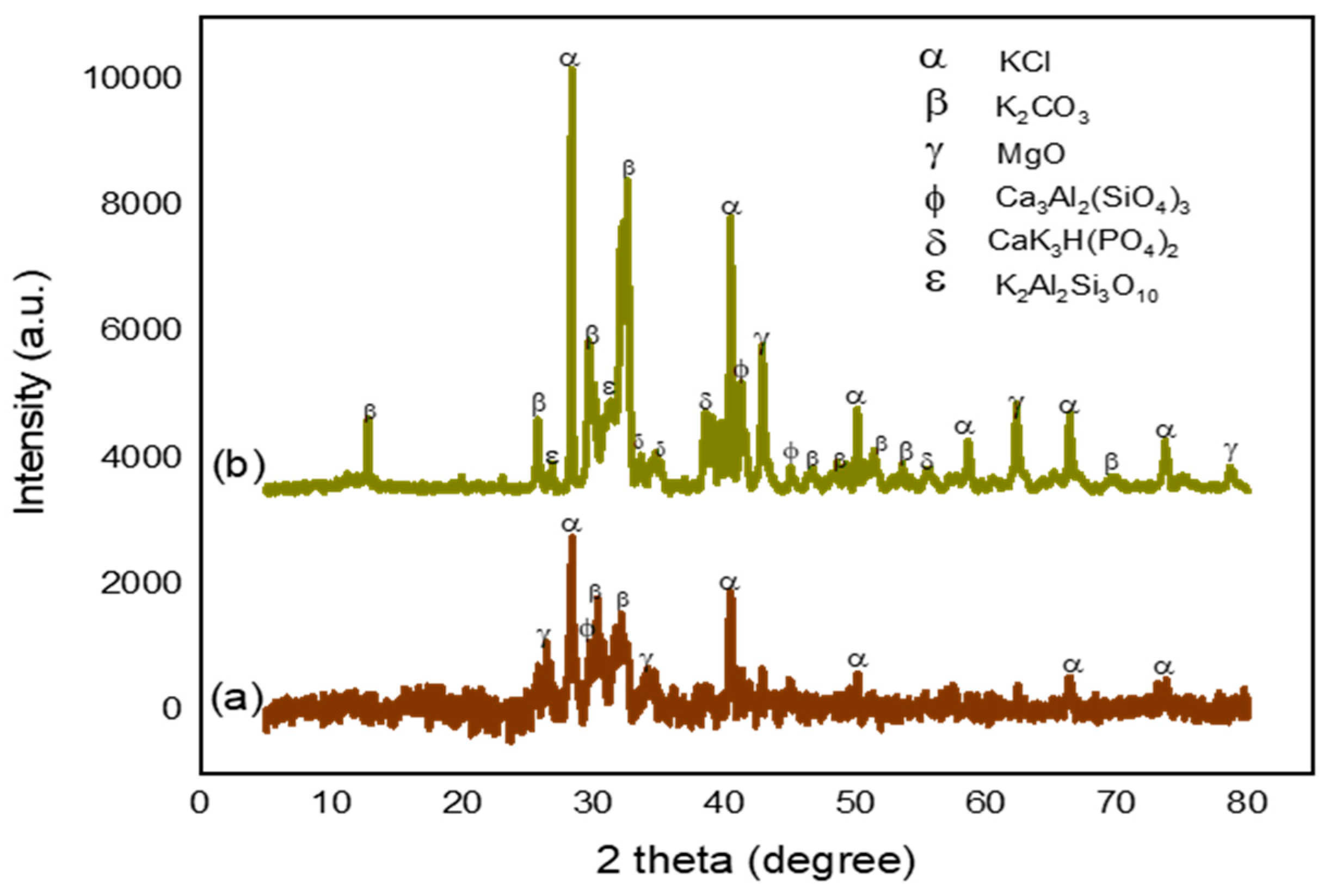
2.2. XRD Analysis
- K represents Scherrer’s constant denoted by (K = 0.9)
- λ represents X-ray wavelength equal to 0.15406 (nm)
- β represent the Radian for full width at half maximum (FWHM)
- θ represents the Bragg-diffraction angle (Peaks position) in radians.
EDS Analysis
2.3. SEM Analysis
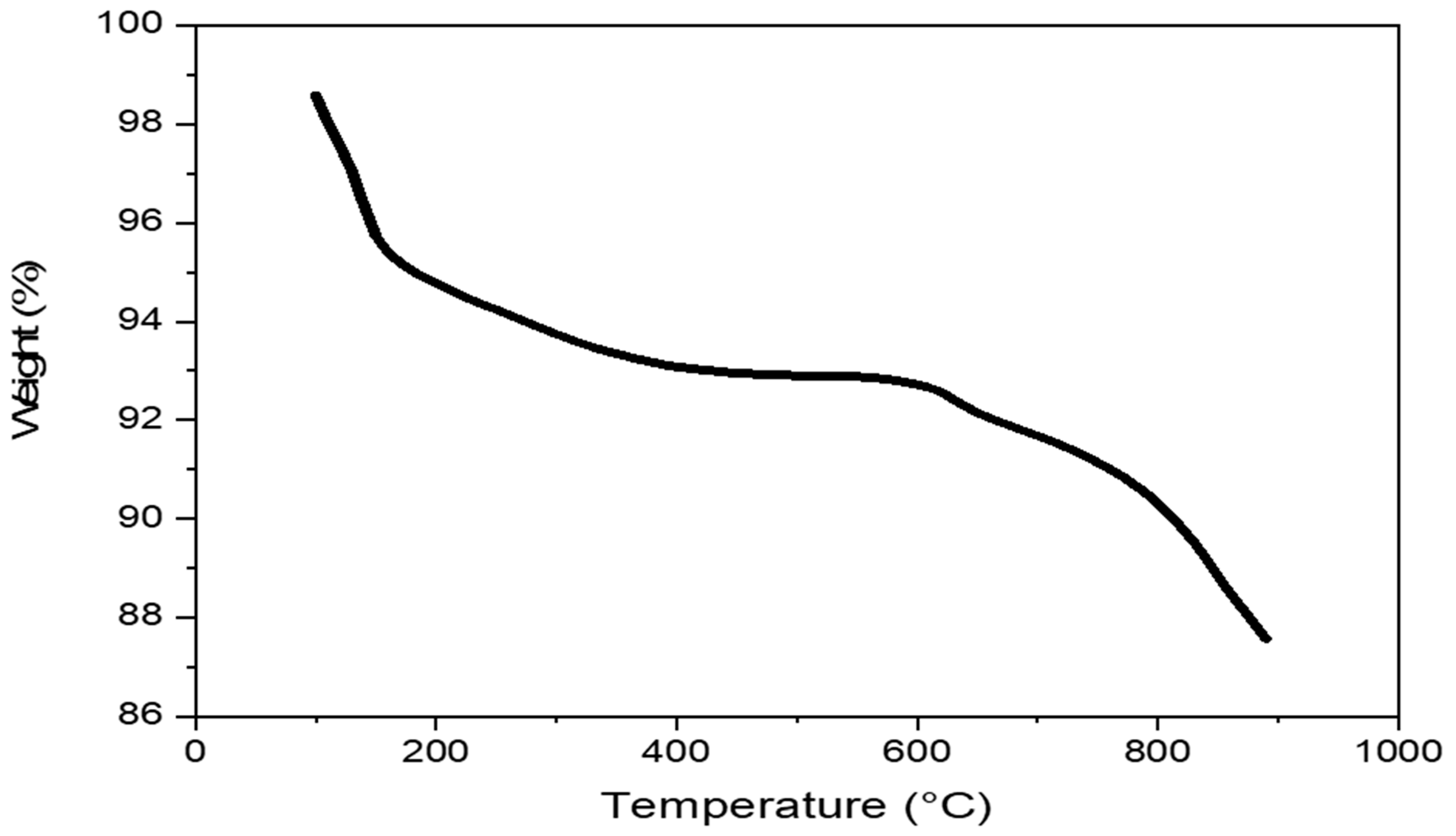
2.4. The Thermogravimetric Analysis (TGA)
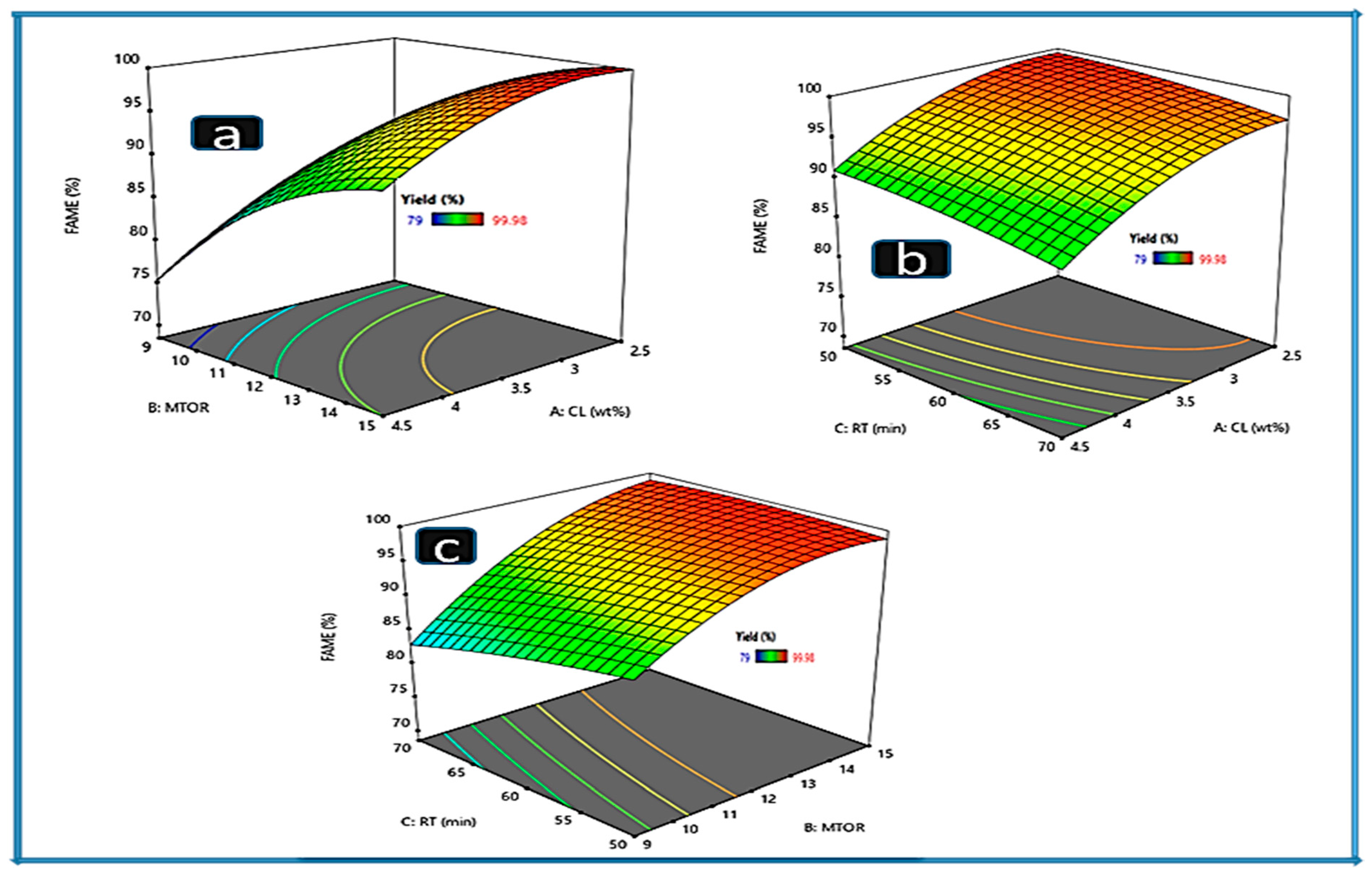
2.5. Modeling and Optimization Results
2.6. Effect of the Process Variables Interaction
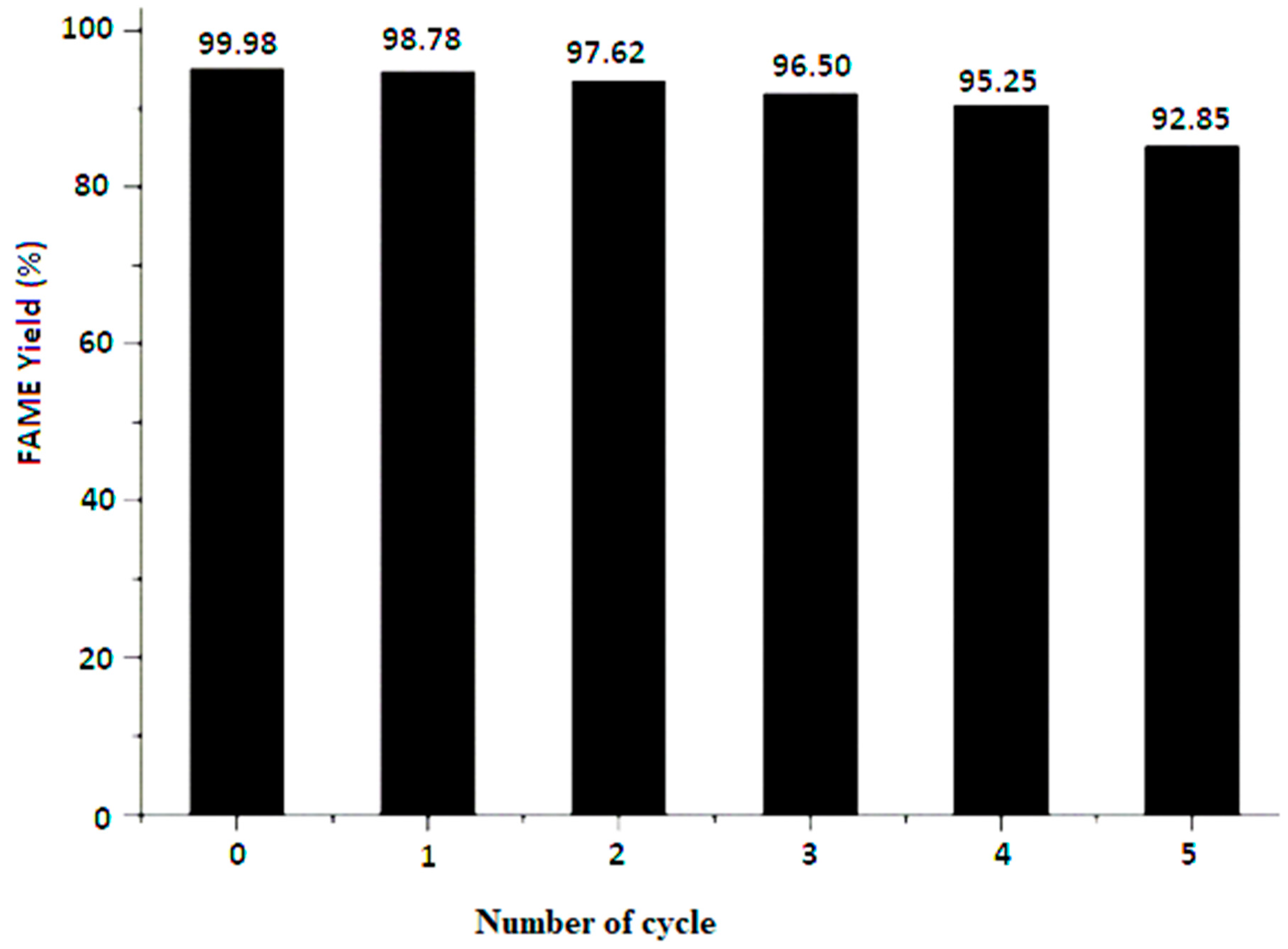
2.7. Reusability Study of CAP
2.8. Biodiesel Quality Characterization
3. Materials and Methodology
3.1. Materials
3.2. CAP Catalyst Preparation
Characterization of CAP Catalyst
3.3. Blend Preparation of Used Cooking Oil–Baobab Oil (UCO-BO) Hybrid
3.4. Statistical Analysis of the Transesterification Process
Model Performance Evaluation
| Correlation coefficient | (4) | |
| Coefficient of determination | (5) | |
| Average absolute deviation | (6) | |
| Mean absolute error | (7) |
3.5. Transesterification Reaction of UCO-BO Hybrid with CAP Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Mansir, N.; Teo, S.H.; Rashid, U.; Saiman, M.I.; Tan, Y.P.; Alsultan, G.A.; Taufiq-Yap, Y.H. Modified waste eggshell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renew. Sustain. Energy Rev. 2018, 82, 3645–3655. [Google Scholar] [CrossRef]
- Al-mawali, K.S.; Osman, A.I.; Al-muhtaseb, A.H.; Mehta, N.; Jamil, F.; Mjalli, F.; Vakili-nezhaad, G.R.; Rooney, D.W. Life cycle assessment of biodiesel production utilising waste date seed oil and a novel magnetic catalyst: A circular bioeconomy approach. Renew. Energy 2021, 170, 832–846. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Z.; Xuan, J.; Jiao, K. Crises and opportunities in terms of energy and AI technologies during the COVID-19 pandemic. Energy AI 2020, 1, 100–113. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Abdollahi Asl, M.; Tahvildari, K.; Bigdeli, T. Eco-friendly synthesis of biodiesel from WCO by using electrolysis technique with graphite electrodes. Fuel 2020, 270, 117–582. [Google Scholar] [CrossRef]
- Modiba, E.; Osifo, P.; Rutto, H. Biodiesel production from baobab (Adansonia digitata L.) seed kernel oil and its fuel properties. Ind. Crops Prod. 2014, 59, 50–54. [Google Scholar] [CrossRef]
- Msalilwa, U.L.; Makule, E.E.; Munishi, L.K.; Ndakidemi, P.A. Physicochemical Properties, Fatty Acid Composition, and the Effect of Heating on the Reduction of Cyclopropenoid Fatty Acids on Baobab (Adansonia digitata L.). Crude Seed Oil 2020, 2020, 669–1298. [Google Scholar] [CrossRef]
- Giwa, S.; Adekomaya, O.; Nwaokocha, C. Potential hybrid feedstock for biodiesel production in the tropics. Front. Energy 2016, 10, 329–336. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al, A.; Ala, H.; Al, H.; Rooney, D.W. Conversion of Biomass to Biofuels and Life Cycle Assessment: A Review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Basumatary, S.; Nath, B.; Das, B.; Kalita, P.; Basumatary, B. Utilization of renewable and sustainable basic heterogeneous catalyst from Heteropanax fragrans (Kesseru) for effective synthesis of biodiesel from Jatropha curcas oil. Fuel 2021, 286, 119–357. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Muthivhi, J.; Linganiso, L.Z.; Dziike, F. Recent improvements to ensure sustainability of biodiesel production. Biofuels 2024, 1–15. [Google Scholar] [CrossRef]
- Hashemzadeh Gargari, M.; Sadrameli, S.M. Investigating continuous biodiesel production from linseed oil in the presence of a Co-solvent and a heterogeneous based catalyst in a packed bed reactor. Energy 2018, 148, 888–895. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rajkumari, K.; Rokhum, L. Exploiting waste: Towards a sustainable production of biodiesel using: Musa acuminata peel ash as a heterogeneous catalyst. Green Chem. 2018, 20, 2365–2373. [Google Scholar] [CrossRef]
- Rajkumari, K.; Rokhum, L. A sustainable protocol for production of biodiesel by transesterification of soybean oil using banana trunk ash as a heterogeneous catalyst. Biomass Convers. Biorefinery 2020, 10, 839–848. [Google Scholar] [CrossRef]
- Eldiehy, K.S.H.; Gohain, M.; Daimary, N.; Borah, D.; Mandal, M.; Deka, D. Radish (Raphanus sativus L.) leaves: A novel source for a highly efficient heterogeneous base catalyst for biodiesel production using waste soybean cooking oil and Scenedesmus obliquus oil. Renew. Energy 2022, 191, 888–901. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.R.L.; Maia, P.J.S.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S.; de Freitas, F.A. New heterogeneous catalyst for biodiesel production from waste tucumã peels (Astrocaryum aculeatum Meyer): Parameters optimization study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Etim, A.; Betiku, E.; Ajala, S.; Olaniyi, P.; Ojumu, T. Potential of Ripe Plantain Fruit Peels as an Ecofriendly Catalyst for Biodiesel Synthesis: Optimization by Artificial Neural Network Integrated with Genetic Algorithm. Sustainability 2018, 10, 707. [Google Scholar] [CrossRef]
- Aleman-Ramirez, J.L.; Moreira, J.; Torres-Arellano, S.; Longoria, A.; Okoye, P.U.; Sebastian, P.J. Preparation of a heterogeneous catalyst from moringa leaves as a sustainable precursor for biodiesel production. Fuel 2021, 284, 118–983. [Google Scholar] [CrossRef]
- Nath, B.; Das, B.; Kalita, P.; Basumatary, S. Waste to value addition: Utilization of waste Brassica nigra plant derived novel green heterogeneous base catalyst for effective synthesis of biodiesel. J. Clean. Prod. 2019, 239, 118112. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. Banana peduncle—A green and renewable heterogeneous base catalyst for biodiesel production from Ceiba pentandra oil. Renew. Energy 2020, 146, 2255–2269. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Del Pino-García, R.; Curiel, J.A.; Lozano-Sánchez, J.; Segura-Carretero, A. Functional ingredient from avocado peel: Microwave-assisted extraction, characterization and potential applications for the food industry. Food Chem. 2021, 352, 129–300. [Google Scholar] [CrossRef]
- Nyakang’i, C.O.; Ebere, R.; Marete, E.; Arimi, J.M. Avocado production in Kenya in relation to the world, Avocado by-products (seeds and peels) functionality and utilization in food products. Appl. Food Res. 2023, 3, 100–275. [Google Scholar] [CrossRef]
- Ramos-Aguilar, A.L.; Ornelas-Paz, J.; Tapia-Vargas, L.M.; Gardea-Béjar, A.A.; Yahia, E.M.; Ornelas-Paz, J.d.J.; Ruiz-Cruz, S.; Rios-Velasco, C.; Escalante-Minakata, P. Effect of cultivar on the content of selected phytochemicals in avocado peels. Food Res. Int. 2021, 140, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Permal, R.; Chia, T.; Arena, G.; Fleming, C.; Chen, J.; Chen, T.; Chang, W.L.; Seale, B.; Hamid, N.; Kam, R. Converting avocado seeds into a ready to eat snack and analysing for persin and amygdalin. Food Chem. 2023, 399, 134011. [Google Scholar] [CrossRef] [PubMed]
- Betiku, E.; Etim, A.O.; Pereao, O.; Ojumu, T.V. Two-Step Conversion of Neem (Azadirachta indica) Seed Oil into Fatty Methyl Esters Using a Heterogeneous Biomass-Based Catalyst: An Example of Cocoa Pod Husk. Energy Fuels 2017, 31, 6182–6193. [Google Scholar] [CrossRef]
- Falowo, A.O.; Betiku, E. A novel heterogeneous catalyst synthesis from agrowastes mixture and application in transesterification of yellow oleander-rubber oil: Optimization by Taguchi approach. Fuel 2022, 312, 122–999. [Google Scholar] [CrossRef]
- Gohain, M.; Laskar, K.; Paul, A.K.; Daimary, N.; Maharana, M.; Goswami, I.K.; Hazarika, A.; Bora, U.; Deka, D. Carica papaya stem: A source of versatile heterogeneous catalyst for biodiesel production and C–C bond formation. Renew. Energy 2020, 147, 541–555. [Google Scholar] [CrossRef]
- Odude, V.O.; Adesina, A.J.; Oyetunde, O.O.; Adeyemi, O.O.; Ishola, N.B.; Etim, A.; Betiku, E. Application of Agricultural Waste-Based Catalysts to Transesterification of Esterified Palm Kernel Oil into Biodiesel: A Case of Banana Fruit Peel Versus Cocoa Pod Husk. Waste Biomass Valorization 2019, 10, 877–888. [Google Scholar] [CrossRef]
- Barros, S.D.S.; Pessoa, W.A.G.; Sá, I.S.C.; Takeno, M.L.; Nobre, F.X.; Pinheiro, W.; Manzato, L.; Iglauer, S. Bioresource Technology Pineapple (Ananás comosus) leaves ash as a solid base catalyst for biodiesel synthesis. Bioresour. Technol. 2020, 312, 123–569. [Google Scholar]
- Basumatary, S.; Nath, B.; Kalita, P. Application of agro-waste derived materials as heterogeneous base catalysts for biodiesel synthesis. J. Renew. Sustain. Energy 2018, 10, 043–105. [Google Scholar] [CrossRef]
- Betiku, E.; Okeleye, A.A.; Ishola, N.B.; Osunleke, A.S.; Ojumu, T.V. Development of a Novel Mesoporous Biocatalyst Derived from Kola Nut Pod Husk for Conversion of Kariya Seed Oil to Methyl Esters: A Case of Synthesis, Modeling and Optimization Studies. Catal. Lett. 2019, 149, 1772–1787. [Google Scholar] [CrossRef]
- Chizoo, E.; Chimamkpam, S.; Gabriel, E.; Gerald, U. Adaptive neuro-fuzzy inference system-genetic algorithm versus response surface methodology-desirability function algorithm modelling and optimization of biodiesel synthesis from waste chicken fat. J. Taiwan Inst. Chem. Eng. 2022, 136, 104–389. [Google Scholar]
- Adepoju, T.F.; Eyibio, U.; Emberru, E.R.; Balogun, T.A. Optimization conversion of beef tallow blend with waste used vegetable oil for fatty acid ethyl ester (FAEE) synthesis in the presence of bio-base derived from Theobroma cacao pod husks. Case Stud. Chem. Environ. Eng. 2022, 6, 100–218. [Google Scholar] [CrossRef]
- Hasni, K.; Ilham, Z.; Dharma, S.; Varman, M. Optimization of biodiesel production from Brucea javanica seeds oil as novel non-edible feedstock using response surface methodology. Energy Convers. Manag. 2017, 149, 392–400. [Google Scholar] [CrossRef]
- Gohain, M.; Devi, A.; Deka, D. Musa balbisiana Colla peel as highly effective renewable heterogeneous base catalyst for biodiesel production. Ind. Crops Prod. 2017, 109, 8–18. [Google Scholar] [CrossRef]
- Almeida, V.F.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Biodiesel production from mixtures of waste fish oil, palm oil and waste frying oil: Optimization of fuel properties. Fuel Process. Technol. 2015, 133, 152–160. [Google Scholar] [CrossRef]
- Salim, S.M.; Izriq, R.; Almaky, M.M.; Al-Abbassi, A.A. Synthesis and characterization of ZnO nanoparticles for the production of biodiesel by transesterification: Kinetic and thermodynamic studies. Fuel 2022, 321, 124–135. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Cesur, C.; Aslan, V.; Yilbasi, Z. The production of biodiesel from safflower (Carthamus tinctorius L.) oil as a potential feedstock and its usage in compression ignition engine: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 119, 109–574. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]





| Heat Treatment Condition | Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Na | Mg | Si | P | S | Cl | K | Ca | |
| CAP at 650 °C, 3 h | 7.20 | 36.71 | 0.4 | 2.54 | 0.41 | 4.17 | 0.46 | 1.84 | 45.0 | 1.65 |
| Burnt AP | 49.95 | 30.78 | - | 1.20 | 0.16 | 1.85 | 0.21 | 0.75 | 14.30 | 0.80 |
| Raw AP | 64.52 | 24.50 | - | 0.86 | - | 0.43 | 0.16 | - | 9.26 | 0.27 |
| Source | Coefficient Estimate | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Model | 95.72 | 591.97 | 9 | 65.77 | 405.06 | <0.0001 |
| K1-Catalyst concentration | −4.50 | 161.91 | 1 | 161.91 | 997.09 | <0.0001 |
| K2-Methanol/oil | 6.30 | 317.27 | 1 | 317.27 | 1953.84 | <0.0001 |
| K3-Reaction Time | −1.73 | 23.84 | 1 | 23.84 | 146.81 | <0.0001 |
| K1K2 | 0.5800 | 1.35 | 1 | 1.35 | 8.29 | 0.0237 |
| K1K3 | −0.2225 | 0.198 | 1 | 0.198 | 1.22 | 0.3060 |
| K2K3 | 1.72 | 11.90 | 1 | 11.90 | 73.30 | <0.0001 |
| K12 | −2.40 | 24.23 | 1 | 24.23 | 149.20 | <0.0001 |
| K22 | −3.25 | 44.51 | 1 | 44.51 | 274.09 | <0.0001 |
| K32 | −0.5437 | 1.24 | 1 | 1.24 | 7.67 | 0.0277 |
| Residual | 1.14 | 7 | 0.16 | |||
| Lack of Fit | 0.648 | 3 | 0.22 | 1.77 | 0.291 | |
| Pure Error | 0.488 | 4 | 0.12 | |||
| Cor Total | 593.11 | 16 | ||||
| Fit Statistic of the model | ||||||
| R2 | 0.9981 | R | 0.9990 | |||
| Adjusted R2 | 0.9963 | MAE | 0.020% | |||
| Predicted R2 | 0.9812 | AAD | 0.001% | |||
| Adeq Precision | 69.8648 | |||||
| Property | Unit | Test Method [38] | Present Study | ASTM D6751 | EN 14214 |
|---|---|---|---|---|---|
| Moisture content | % | ASTM D2709 | 0.01 | <0.05 | 0.050% max |
| Density | g/cm3 | ASTM D4052 | 0.886 | 0.85 | 0.86–0.90 |
| Kinematic viscosity at 40 °C | mm2/s | ASTM D 445 | 3.22 | 1.9–6.0 | 3.5–5.0 |
| Acid value | Mg KOH/g | ASTM D 664 | 0.38 | 0.5 max | 0.5 max |
| Iodine value | AOAC | 78 | 120 max | N/S | |
| Copper strip at 50 °C, 3 h | Rating | ASTM D130 | 1 | No. 3 max | Class 1 min |
| Calorific value | MJ/Kg | 40.5 | N/S | 35 min | |
| Cetane number | - | ASTM D613 | 60 | 47 min | 51 min |
| API | deg | 28.20 | 36.95 | N/S |
| Factors | Symbol | Unit | Levels and Coded Factor | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Catalyst loading | K1 | wt% | 2.5 | 3.5 | 4.5 |
| Methanol/oil molar ratio | K2 | w/w | 9:1 | 12:1 | 15:1 |
| Reaction time | K3 | min | 50 | 60 | 70 |
| Std Order | Run | K1 (wt%) | K2 (w/w) | K3 (min) | Yield (%) | Predicted Value (%) | Viscosity (mm2/s) |
|---|---|---|---|---|---|---|---|
| 3 | 1 | 2.5 | 15:1 | 60 | 99.98 | 99.97 | 2.99 |
| 12 | 2 | 3.5 | 15:1 | 70 | 98.30 | 98.22 | 2.84 |
| 1 | 3 | 2.5 | 9:1 | 60 | 88.60 | 88.85 | 2.52 |
| 14 | 4 | 3.5 | 12:1 | 60 | 95.50 | 95.72 | 3.11 |
| 9 | 5 | 3.5 | 9:1 | 50 | 89.00 | 89.08 | 3.27 |
| 15 | 6 | 3.5 | 12:1 | 60 | 96.10 | 95.72 | 3.10 |
| 2 | 7 | 4.5 | 9:1 | 60 | 79.00 | 78.69 | 3.50 |
| 11 | 8 | 3.5 | 9:1 | 70 | 82.20 | 82.18 | 2.94 |
| 10 | 9 | 3.5 | 15:1 | 50 | 98.20 | 98.22 | 2.85 |
| 6 | 10 | 4.5 | 12:1 | 50 | 90.00 | 90.23 | 3.72 |
| 8 | 11 | 4.5 | 12:1 | 70 | 86.00 | 86.33 | 3.15 |
| 13 | 12 | 3.5 | 12:1 | 60 | 95.40 | 95.72 | 3.01 |
| 5 | 13 | 2.5 | 12:1 | 50 | 99.11 | 98.78 | 3.18 |
| 17 | 14 | 3.5 | 12:1 | 60 | 96.10 | 95.72 | 3.29 |
| 4 | 15 | 4.5 | 15:1 | 60 | 92.70 | 92.45 | 3.16 |
| 7 | 16 | 2.5 | 12:1 | 70 | 96.00 | 95.77 | 2.86 |
| 16 | 17 | 3.5 | 12:1 | 60 | 95.50 | 95.72 | 2.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etim, A.O.; Musonge, P. Synthesis of a Highly Efficient Mesoporous Green Catalyst from Waste Avocado Peels for Biodiesel Production from Used Cooking–Baobab Hybrid Oil. Catalysts 2024, 14, 261. https://doi.org/10.3390/catal14040261
Etim AO, Musonge P. Synthesis of a Highly Efficient Mesoporous Green Catalyst from Waste Avocado Peels for Biodiesel Production from Used Cooking–Baobab Hybrid Oil. Catalysts. 2024; 14(4):261. https://doi.org/10.3390/catal14040261
Chicago/Turabian StyleEtim, Anietie O., and Paul Musonge. 2024. "Synthesis of a Highly Efficient Mesoporous Green Catalyst from Waste Avocado Peels for Biodiesel Production from Used Cooking–Baobab Hybrid Oil" Catalysts 14, no. 4: 261. https://doi.org/10.3390/catal14040261




