A Review of Ozone Decomposition by a Copper-Based Catalyst
Abstract
1. Introduction
2. The Powder Copper Catalyst: Mechanism and Key Factors
2.1. Ozone Decomposition Mechanism
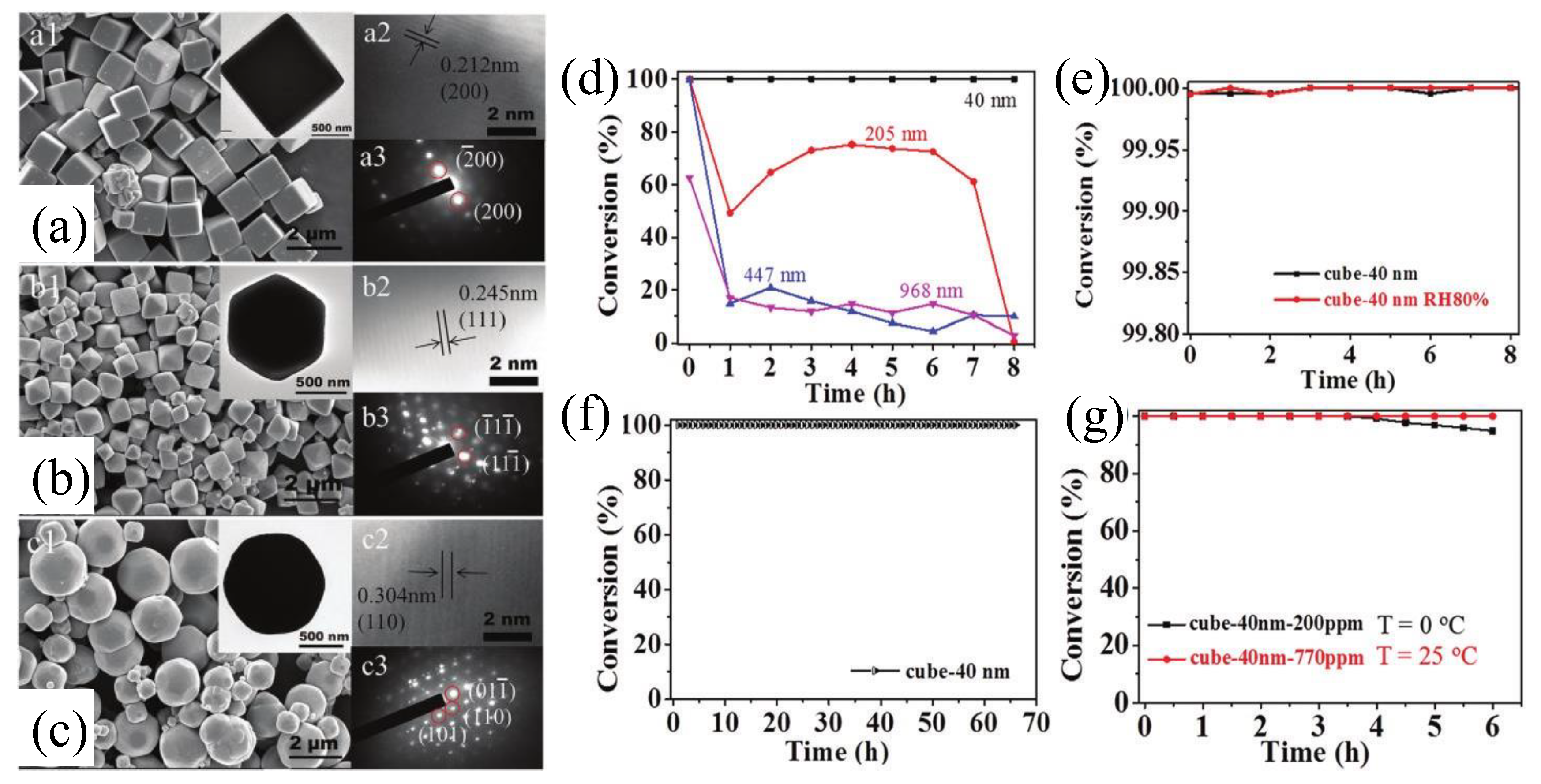
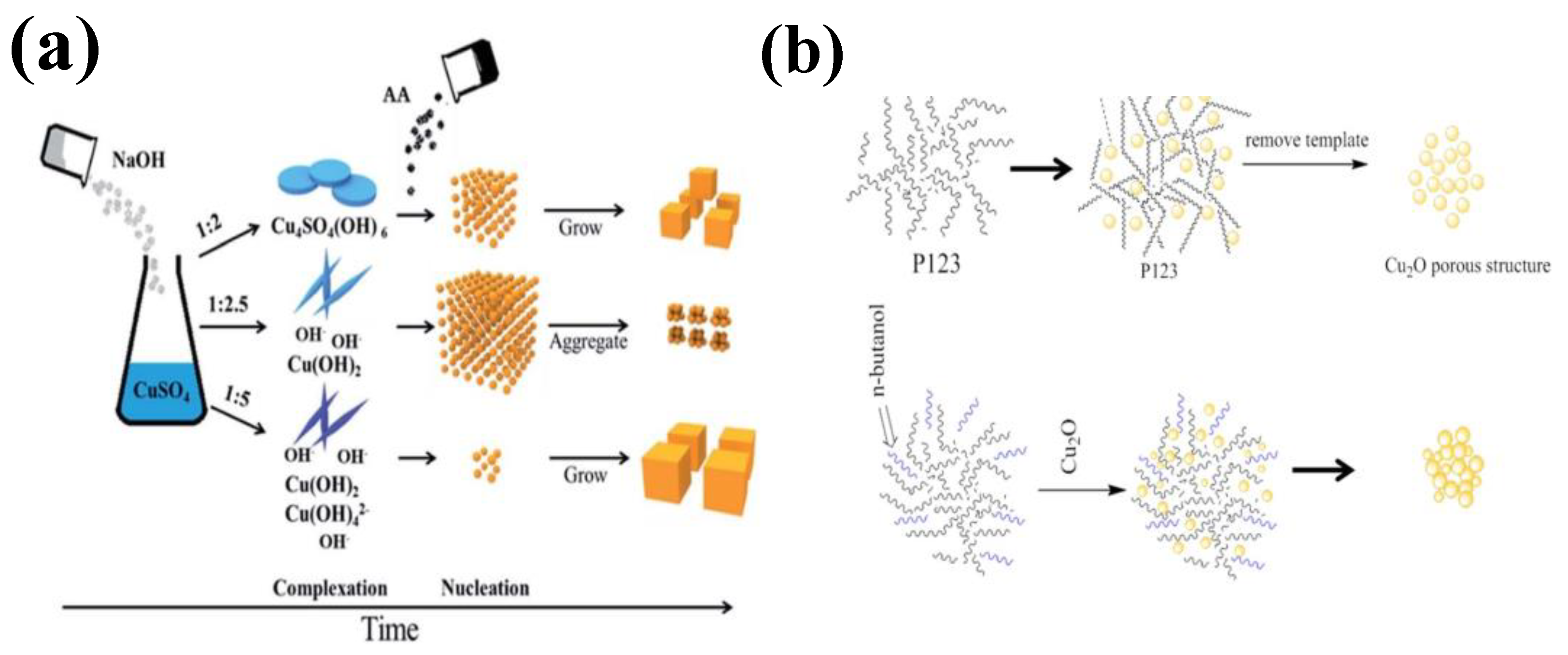
2.2. Morphology of Copper-Based Material
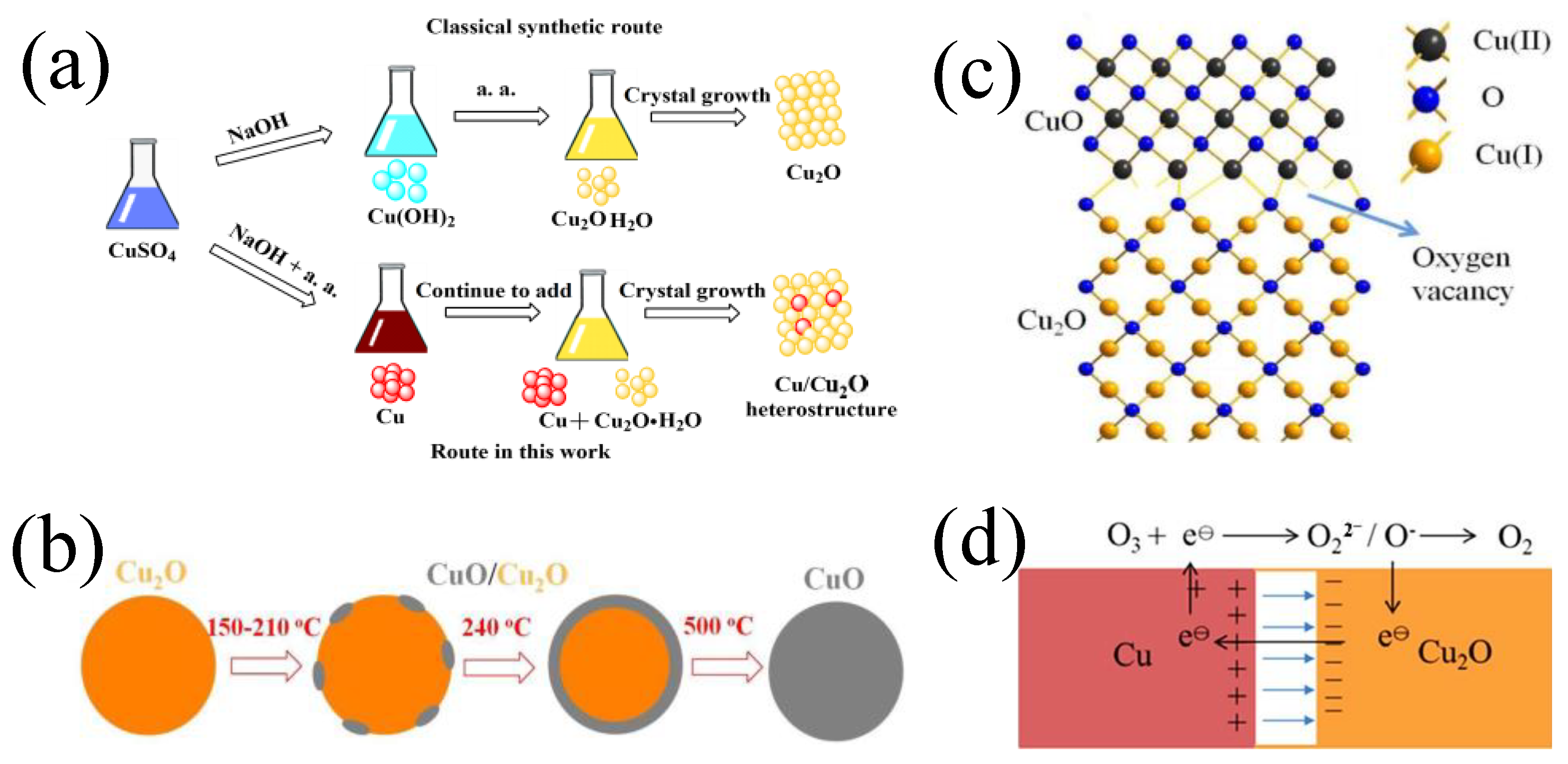
2.3. Heterostructure of Copper-Based Material
3. The Monolithic Copper-Based Catalyst
4. Copper-Based Catalyst for Water Treatment
5. Comparison of Copper-Based Catalytic Materials with Other Semiconductor Oxides
6. Conclusions
- Although the cost of copper-based material is lower than that of precious metal catalysts and meets the demand for substitution, its cost needs to be further reduced, such as using lower-cost activated carbon and copper oxide in combination to reduce its usage cost.
- Under high-humidity and corrosive conditions, all transition metal oxide catalysts, including cuprous oxide, need to improve their stability.
- The performance of a copper powder catalyst is excellent, but the catalytic performance of more practical Cu–monolithic catalysts still needs to be further improved.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, W.S.; Graedel, T.E. Photochemical Air Pollution in the Northeast United States. Science 1979, 204, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Ma, J.; Wu, H.; Liu, Y.; He, H. Photocatalytic Removal of NOx over Visible Light Responsive Oxygen-Deficient TiO2. J. Phys. Chem. C 2014, 118, 7434–7441. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Liu, C.; Ma, J.; He, H. A case study of Asian dust storm particles: Chemical composition, reactivity to SO2 and hygroscopic properties. J. Environ. Sci. 2012, 24, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Y.; He, H. Heterogeneous reactions between NO2 and anthracene adsorbed on SiO2 and MgO. Atmos. Environ. 2011, 45, 917–924. [Google Scholar] [CrossRef]
- Hudman, R.C.; Jacob, D.J.; Cooper, O.R.; Evans, M.J.; Heald, C.L.; Park, R.J.; Fehsenfeld, F.; Flocke, F.; Holloway, J.; Hubler, G.; et al. Ozone production in transpacific Asian pollution plumes and implications for ozone air quality in California. J. Geophys. Res. -Atmos. 2004, 109, 19. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Lo Meo, P.; Aprile, C.; Noto, R. Oxidative degradation properties of Co-based catalysts in the presence of ozone. Appl. Catal. B Environ. 2007, 75, 281–289. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Mehandjiev, D.; Naydenov, A.; Ivanov, G. Ozone decomposition, benzene and CO oxidation over NiMnO3-ilmenite and NiMn2O4-spinel catalysts. Appl. Catal. A Gen. 2001, 206, 13–18. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Ma, J.; Ma, Q.; He, H. Structural and hygroscopic changes of soot during heterogeneous reaction with O3. Phys. Chem. Chem. Phys. 2010, 12, 10896–10903. [Google Scholar] [CrossRef]
- Benson, S.W.; Axworthy, A.E., Jr. Mechanism of the Gas Phase, Thermal Decomposition of Ozone. J. Chem. Phys. 1957, 26, 1718–1726. [Google Scholar] [CrossRef]
- Ogata, A.; Saito, K.; Kim, H.-H.; Sugasawa, M.; Aritani, H.; Einaga, H. Performance of an Ozone Decomposition Catalyst in Hybrid Plasma Reactors for Volatile Organic Compound Removal. Plasma Chem. Plasma Process. 2010, 30, 33–42. [Google Scholar] [CrossRef]
- Cho, K.-C.; Hwang, K.-C.; Sano, T.; Takeuchi, K.; Matsuzawa, S. Photocatalytic performance of Pt-loaded TiO2 in the decomposition of gaseous ozone. J. Photochem. Photobiol. A Chem. 2004, 161, 155–161. [Google Scholar] [CrossRef]
- Wang, X.; Tan, X.; Yu, T. Kinetic Study of Ozone Photocatalytic Decomposition Using a Thin Film of TiO2 Coated on a Glass Plate and the CFD Modeling Approach. Ind. Eng. Chem. Res. 2014, 53, 7902–7909. [Google Scholar] [CrossRef]
- Li, W.; Gibbs, G.V.; Oyama, S.T. Mechanism of ozone decomposition on a manganese oxide catalyst. I. In situ Raman spectroscopy and ab initio molecular orbital calculations. J. Am. Chem. Soc. 1998, 120, 9041–9046. [Google Scholar] [CrossRef]
- Hao, Z.; Cheng, D.; Guo, Y.; Liang, Y. Supported gold catalysts used for ozone decomposition and simultaneous elimination of ozone and carbon monoxide at ambient temperature. Appl. Catal. B Environ. 2001, 33, 217–222. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, H.; Hibino, M.; Honma, I.; Ichihara, M. Synthesis of MnO2 Nanoparticles Confined in Ordered Mesoporous Carbon Using a Sonochemical Method. Adv. Funct. Mater. 2005, 15, 381–386. [Google Scholar] [CrossRef]
- Wei, L.; Chen, H.; Wei, Y.; Jia, J.; Zhang, R. Ce-promoted Mn/ZSM-5 catalysts for highly efficient decomposition of ozone. J. Environ. Sci. 2021, 103, 219–228. [Google Scholar] [CrossRef]
- Tao, L.; Zhang, Z.; Chen, P.; Zhao, G.; Liu, Y.; Lu, Y. Thin-felt Al-fiber-structured Pd-Co-MnOx/Al2O3 catalyst with high moisture resistance for high-throughput O3 decomposition. Appl. Surf. Sci. 2019, 481, 802–810. [Google Scholar] [CrossRef]
- Ji, J.; Fang, Y.; He, L.; Huang, H. Efficient catalytic removal of airborne ozone under ambient conditions over manganese oxides immobilized on carbon nanotubes. Catal. Sci. Technol. 2019, 9, 4036–4046. [Google Scholar] [CrossRef]
- Zhang, L.; Huo, F.; Wang, A.; Chai, S.; Guan, J.; Fan, G.; Yang, W.; Ma, G.; Han, N.; Chen, Y. Coordination-Controlled Catalytic Activity of Cobalt Oxides for Ozone Decomposition. Inorg. Chem. 2023, 62, 9178–9189. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; Zhang, C.; Zhang, R.; He, H. Detrimental role of residual surface acid ions on ozone decomposition over Ce-modified γ-MnO2 under humid conditions. J. Environ. Sci. 2020, 91, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Wang, A.; Wang, Y.; Liu, H.; Han, N.; Chen, Y. Heterostructured Ni/NiO Nanocatalysts for Ozone Decomposition. ACS Appl. Nano Mater. 2020, 3, 597–607. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; He, G.; Wang, Z.; He, H. In-situ formation of hydroxylated Ag active sites over Ag/MnO2 modified by alkali metals for stable decomposition of ozone under humid conditions. Appl. Catal. B Environ. Energy 2024, 346, 123736. [Google Scholar] [CrossRef]
- Li, X.; Shao, X.; Wang, Z.; Ma, J.; He, H. Regulating the chemical state of silver via surface hydroxyl groups to enhance ozone decomposition performance of Ag/Fe2O3 catalyst. Catal. Today 2023, 410, 117–126. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Li, X.; Ma, J.; He, G.; He, H. A superior catalyst for ozone decomposition: NiFe layered double hydroxide. J. Environ. Sci. 2023, 134, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Deng, H.; Lu, Y.; Ma, J.; Shan, W.; He, H. Facile Method to Create PdCe–MnOx Catalyst for Decomposing O3 under Humidity Conditions. Ind. Eng. Chem. Res. 2023, 62, 8665–8672. [Google Scholar] [CrossRef]
- Mohamed, E.F.; Awad, G.; Zaitan, H.; Andriantsiferana, C.; Manero, M.H. Transition metals-incorporated zeolites as environmental catalysts for indoor air ozone decomposition. Environ. Technol. 2018, 39, 878–886. [Google Scholar] [CrossRef]
- Chernykh, M.; Grabchenko, M.; Knyazev, A.; Mamontov, G. Cordierite-Supported Transition-Metal-Oxide-Based Catalysts for Ozone Decomposition. Crystals 2023, 13, 1674. [Google Scholar] [CrossRef]
- Lian, Z.; Ma, J.; He, H. Decomposition of high-level ozone under high humidity over Mn–Fe catalyst: The influence of iron precursors. Catal. Commun. 2015, 59, 156–160. [Google Scholar] [CrossRef]
- Spasova, I.; Nikolov, P.; Mehandjiev, D. Ozone Decomposition over Alumina-Supported Copper, Manganese and Copper-Manganese Catalysts. Ozone Sci. Eng. 2007, 29, 41–45. [Google Scholar] [CrossRef]
- Dhandapani, B.; Oyama, S.T. Gas phase ozone decomposition catalysts. Appl. Catal. B Environ. 1997, 11, 129–166. [Google Scholar] [CrossRef]
- Heisig, C.; Zhang, W.; Oyama, S.T. Decomposition of ozone using carbon-supported metal oxide catalysts. Appl. Catal. B Environ. 1997, 14, 117–129. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, M.H. Facile Synthesis of Cu2O Nanocrystals with Systematic Shape Evolution from Cubic to Octahedral Structures. J. Phys. Chem. C 2008, 112, 18355–18360. [Google Scholar] [CrossRef]
- Mallik, M.; Monia, S.; Gupta, M.; Ghosh, A.; Toppo, M.P.; Roy, H. Synthesis and characterization of Cu2O nanoparticles. J. Alloys Compd. 2020, 82, 154623. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, H.; Kong, T.; Wang, L. Synthesis and thermal conductivity of Cu2O nanofluids. Int. J. Heat Mass Transf. 2009, 52, 4371–4374. [Google Scholar] [CrossRef]
- Ahmed, A.; Gajbhiye, N.S.; Joshi, A.G. Low cost, surfactant-less, one pot synthesis of Cu2O nano-octahedra at room temperature. J. Solid State Chem. 2011, 184, 2209–2214. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Liu, S.; Mann, S. Template-free Hydrothermal Synthesis of CuO/Cu2O Composite Hollow Microspheres. Chem. Mater. 2007, 19, 4327–4334. [Google Scholar] [CrossRef]
- Gong, S.; Li, W.; Xie, Z.; Ma, X.; Liu, H.; Han, N.; Chen, Y. Low temperature decomposition of ozone by facilely synthesized cuprous oxide catalyst. New J. Chem. 2017, 41, 4828–4834. [Google Scholar] [CrossRef]
- Gong, S.; Wang, A.; Zhang, J.; Guan, J.; Han, N.; Chen, Y. Gram-scale synthesis of ultra-fine Cu2O for highly efficient ozone decomposition. RSC Adv. 2020, 10, 5212–5219. [Google Scholar] [CrossRef]
- Jiang, Y.S.; Chen, J.N.; Zhao, X.; Ma, G.J. Synthesis of Highly Porous Cu2O Catalysts for Efficient Ozone Decomposition. Catalysts 2021, 11, 600. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Zhao, P.; Zhang, L.; Dai, B.; Xu, J.; Huang, H.; He, Y.; Leung, D.Y.C. Novel Z-scheme Ag-C3N4/SnS2 plasmonic heterojunction photocatalyst for degradation of tetracycline and H2 production. Chem. Eng. J. 2021, 405, 126555. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, Y.; Cheng, L.; Chen, J.; Zhao, Y. A Stable Plasmonic Cu@Cu2O/ZnO Heterojunction for Enhanced Photocatalytic Hydrogen Generation. ChemSusChem 2018, 11, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Wang, D.; Gao, Y.; Shen, J. Cu2O/Cu/TiO2 nanotube Ohmic heterojunction arrays with enhanced photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2012, 37, 6431–6437. [Google Scholar] [CrossRef]
- Tagliabue, G.; DuChene, J.S.; Habib, A.; Sundararaman, R.; Atwater, H.A. Hot-Hole versus Hot-Electron Transport at Cu/GaN Heterojunction Interfaces. ACS Nano 2020, 14, 5788–5797. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Varghese, O.K.; Wilke, R.H.T.; Sharma, S.; Shankar, K.; Latempa, T.J.; Choi, K.-S.; Grimes, C.A. p-Type Cu−Ti−O Nanotube Arrays and Their Use in Self-Biased Heterojunction Photoelectrochemical Diodes for Hydrogen Generation. Nano Lett. 2008, 8, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Ma, G.; Wang, A.; Guan, J.; Zhang, L.; Wang, H.; Fan, G.; Tang, W.; Han, N.; Chen, Y. Controllable Synthesis of a Cu/Cu2O Mott–Schottky Heterojunctioned Catalyst for Highly Efficient Ozone Decomposition. J. Phys. Chem. C 2022, 126, 17520–17527. [Google Scholar] [CrossRef]
- Ma, G.; Tang, W.; Wang, A.; Zhang, L.; Guan, J.; Han, N.; Chen, Y. Heterojunctioned CuO/Cu2O catalyst for highly efficient ozone removal. J. Environ. Sci. 2023, 125, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Lu, X.; Chen, C.; He, M.; Huang, H. Potassium-modulated δ-MnO2 as robust catalysts for formaldehyde oxidation at room temperature. Appl. Catal. B Environ. 2020, 260, 118210. [Google Scholar] [CrossRef]
- Gong, S.Y.; Chen, J.Y.; Wu, X.F.; Han, N.; Chen, Y.F. In-situ synthesis of Cu2O/reduced graphene oxide composite as effective catalyst for ozone decomposition. Catal. Commun. 2018, 106, 25–29. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, L.; Guan, J.; Wang, X.; Ma, G.; Fan, G.; Wang, H.; Han, N.; Chen, Y. Highly efficient ozone elimination by metal doped ultra-fine Cu2O nanoparticles. J. Environ. Sci. 2023, 134, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Wu, X.; Zhang, J.; Han, N.; Chen, Y. Facile solution synthesis of Cu2O–CuO–Cu(OH)2 hierarchical nanostructures for effective catalytic ozone decomposition. Cryst. Eng. Comm. 2018, 20, 3096–3104. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Zhang, Q.; Zhao, X.; Xiao, F.; Wang, X.; Ma, G. Templated Synthesis of Cu2S Hollow Structures for Highly Active Ozone Decomposition. Catalysts 2024, 14, 153. [Google Scholar] [CrossRef]
- Dutta, A.; Rahaman, M.; Luedi, N.C.; Mohos, M.; Broekmann, P. Morphology Matters: Tuning the Product Distribution of CO2 Electroreduction on Oxide-Derived Cu Foam Catalysts. ACS Catal. 2016, 6, 3804–3814. [Google Scholar] [CrossRef]
- Chen, Q.; An, X.; Liu, Q.; Wu, X.; Xie, L.; Zhang, J.; Yao, W.; Hamdy, M.S.; Kong, Q.; Sun, X. Boosting electrochemical nitrite–ammonia conversion properties by a Cu foam@ Cu2O catalyst. Chem. Commun. 2022, 58, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C. Advanced Cu-Sn foam for selectively converting CO2 to CO in aqueous solution. Appl. Catal. B Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Xu, H.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Cu2O–Cu Hybrid Foams as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Catal. 2017, 7, 986–991. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.; Liu, X.; Huang, J.; Yin, J.; Wang, G.; Cao, D. Nanostructured CuO directly grown on copper foam and their supercapacitance performance. Electrochim. Acta 2012, 85, 393–398. [Google Scholar] [CrossRef]
- Jiang, X.; Herricks, T.; Xia, Y. CuO Nanowires Can Be Synthesized by Heating Copper Substrates in Air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Košiček, M.; Zavašnik, J.; Baranov, O.; Šetina Batič, B.; Cvelbar, U. Understanding the Growth of Copper Oxide Nanowires and Layers by Thermal Oxidation over a Broad Temperature Range at Atmospheric Pressure. Cryst. Growth Des. 2022, 22, 6656–6666. [Google Scholar] [CrossRef]
- Wang, A.; Guan, J.; Zhang, L.; Wang, H.; Ma, G.; Fan, G.; Tang, W.; Han, N.; Chen, Y. In Situ Synthesis of Monolithic Cu2O–CuO/Cu Catalysts for Effective Ozone Decomposition. J. Phys. Chem. C 2022, 126, 317–325. [Google Scholar] [CrossRef]
- Guan, J.; Guo, Y.; Ma, G.; Zhang, L.; Fan, G.; Yu, H.; Han, N.; Chen, Y. Monolithic Cu2O-CuO/Cu Mesh Catalyst for Ambient Ozone Removal. ACS Appl. Eng. Mater. 2023, 1, 2782–2790. [Google Scholar] [CrossRef]
- Rahimi, M.G.; Wang, A.; Ma, G.; Han, N.; Chen, Y. A one-pot synthesis of a monolithic Cu2O/Cu catalyst for efficient ozone decomposition. RSC Adv. 2020, 10, 40916–40922. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhang, F.; Hu, Y.; Feng, C.; Wu, H. Ozonation in water treatment: The generation, basic properties of ozone and its practical application. Rev. Chem. Eng. 2017, 33, 49–89. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Faghihinezhad, M.; Baghdadi, M.; Shahin, M.S.; Torabian, A. Catalytic ozonation of real textile wastewater by magnetic oxidized g-C3N4 modified with Al2O3 nanoparticles as a novel catalyst. Sep. Purif. Technol. 2022, 283, 120208. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y.C. Complete Oxidation of Formaldehyde at Room Temperature Using TiO2 Supported Metallic Pd Nanoparticles. ACS Catal. 2011, 1, 348–354. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rey, A.; Gimeno, O. The Role of Catalytic Ozonation Processes on the Elimination of DBPs and Their Precursors in Drinking Water Treatment. Catalysts 2021, 11, 521. [Google Scholar] [CrossRef]
- Chu, W.; Ma, C.-W. Quantitative prediction of direct and indirect dye ozonation kinetics. Water Res. 2000, 34, 3153–3160. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xie, R.; Huang, H.; Leung, M.K.H.; Li, J.; Leung, D.Y.C. Photocatalytic Oxidation for Volatile Organic Compounds Elimination: From Fundamental Research to Practical Applications. Environ. Sci. Technol. 2022, 56, 16582–16601. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Frasson, D.; Quinta-Ferreira, R.M.; Matos, A.; Martins, R.C. Removal of Enteric Pathogens from Real Wastewater Using Single and Catalytic Ozonation. Water 2019, 11, 127. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, Y.; Huang, H.; Zhou, P.; Li, J.; Zhang, L.; Dai, B.; Xu, J.; Zhu, F.; Sheng, N.; et al. A novel Z-scheme Ag3VO4/BiVO4 heterojunction photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. Appl. Catal. B Environ. 2019, 245, 448–458. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, F.J.; Acedo, B. Direct, radical and competitive reactions in the ozonation of water micropollutants. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1993, 28, 1947–1976. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Chen, C.-M.; Gu, H.-T.; Sun, Z.-Q.; Li, X.-Y.; Chen, S.-X.; Ma, J. Deep investigation on different effects of Cl− in transformation of reactive species in Fe(II)/NH2OH/PDS and Fe(II)/NH2OH/H2O2 systems. Water Res. 2022, 216, 118315. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Liu, C.; Nie, C.; Wang, T.; Xie, X.; Cao, J.; Zhou, J.; Huang, H.; Li, D.; et al. Removal of Gaseous Volatile Organic Compounds by a Multiwalled Carbon Nanotubes/Peroxymonosulfate Wet Scrubber. Environ. Sci. Technol. 2022, 56, 13996–14007. [Google Scholar] [CrossRef] [PubMed]
- Saeid, S.; Kråkström, M.; Tolvanen, P.; Kumar, N.; Eränen, K.; Mikkola, J.-P.; Kronberg, L.; Eklund, P.; Peurla, M.; Aho, A.; et al. Advanced Oxidation Process for Degradation of Carbamazepine from Aqueous Solution: Influence of Metal Modified Microporous, Mesoporous Catalysts on the Ozonation Process. Catalysts 2020, 10, 90. [Google Scholar] [CrossRef]
- Zilberman, A.; Gozlan, I.; Avisar, D. Pharmaceutical Transformation Products Formed by Ozonation—Does Degradation Occur? Molecules 2023, 28, 1227. [Google Scholar] [CrossRef]
- Feng, C.; Qiu, S.; Diao, P. Copper Foam-Supported CuxO@Fe2O3 Core–Shell Nanotubes: An Efficient Ozonation Catalyst for Degradation of Organic Pollutants. ACS EST Water 2023, 3, 465–474. [Google Scholar] [CrossRef]
- Wantala, K.; Suwannaruang, T.; Palalerd, J.; Chirawatkul, P.; Chanlek, N.; Wannapaiboon, S.; Saiyasombat, C.; Khunphonoi, R. Influence of in-situ and ex-situ Cu-Fe doping in K-OMS-2 catalysts on dye degradation via Fenton-like reaction with focus on catalytic properties and performances. Surf. Interfaces 2021, 23, 101030. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Zhang, C.; Zhang, R.; He, H. Facile synthesis of Ag-modified manganese oxide for effective catalytic ozone decomposition. J. Environ. Sci. 2019, 80, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P. Removing Surface Hydroxyl Groups of Ce-Modified MnO2 To Significantly Improve Its Stability for Gaseous Ozone Decomposition. J. Phys. Chem. C 2017, 121, 23488–23497. [Google Scholar] [CrossRef]
- Mathew, T.; Suzuki, K.; Ikuta, Y.; Nagai, Y.; Takahashi, N.; Shinjoh, H. Mesoporous Ferrihydrite-Based Iron Oxide Nanoparticles as Highly Promising Materials for Ozone Removal. Angew. Chem. Int. Ed. 2011, 50, 7381–7384. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Zeng, D.; Cao, X.; Qin, G.; Li, S. Synthesis of doped MnOx/diatomite composites for catalyzing ozone decomposition. Ceram. Int. 2019, 45, 6966–6971. [Google Scholar] [CrossRef]
- Kwon, D.W.; Kim, G.J.; Won, J.M.; Hong, S.C. Influence of Mn valence state and characteristic of TiO2 on the performance of Mn–Ti catalysts in ozone decomposition. Environ. Technol. 2017, 38, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, C.; Wang, Y.; Luo, M. Ozone Decomposition over NiO/Mn3O4 Monolithic Catalysts. Chanese J. Appl. Chem. 2019, 36, 698–703. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, M.; Yu, D.; Yaseen, M.; Duan, L.; Jiang, W.; Shi, L. In-situ fabrication and catalytic performance of Co-Mn@CuO core-shell nanowires on copper meshes/foams. Mater. Des. 2018, 147, 182–190. [Google Scholar] [CrossRef]

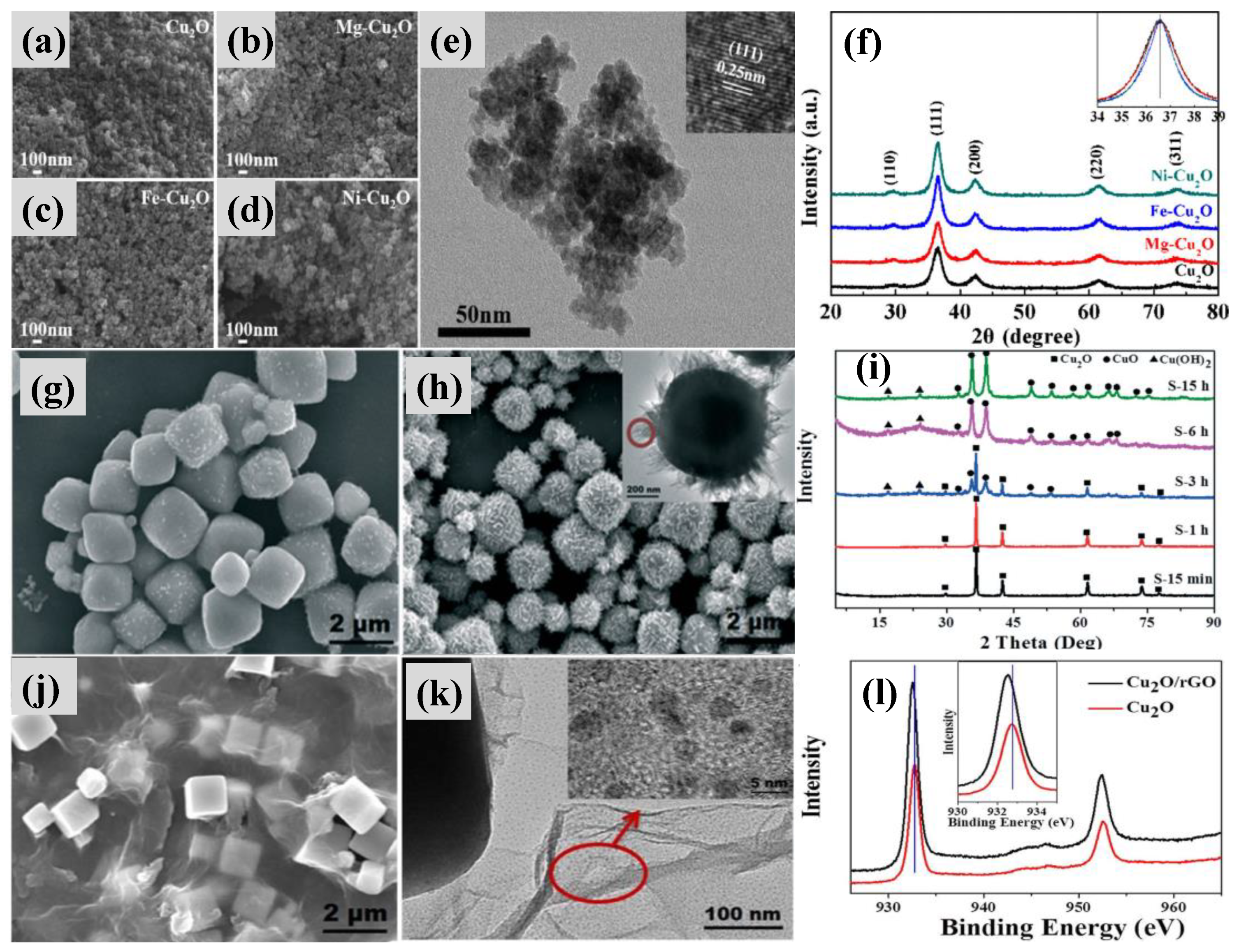
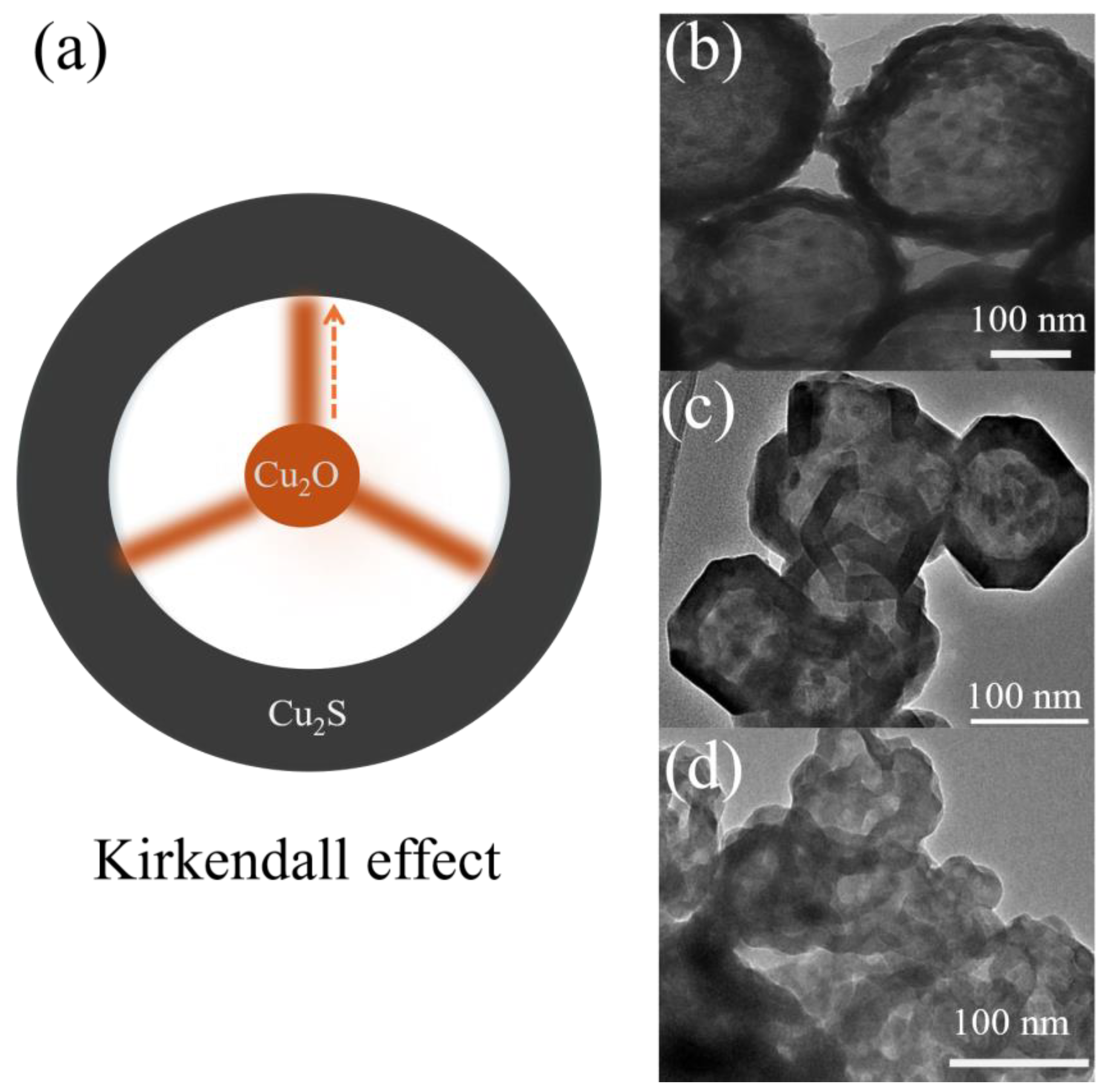

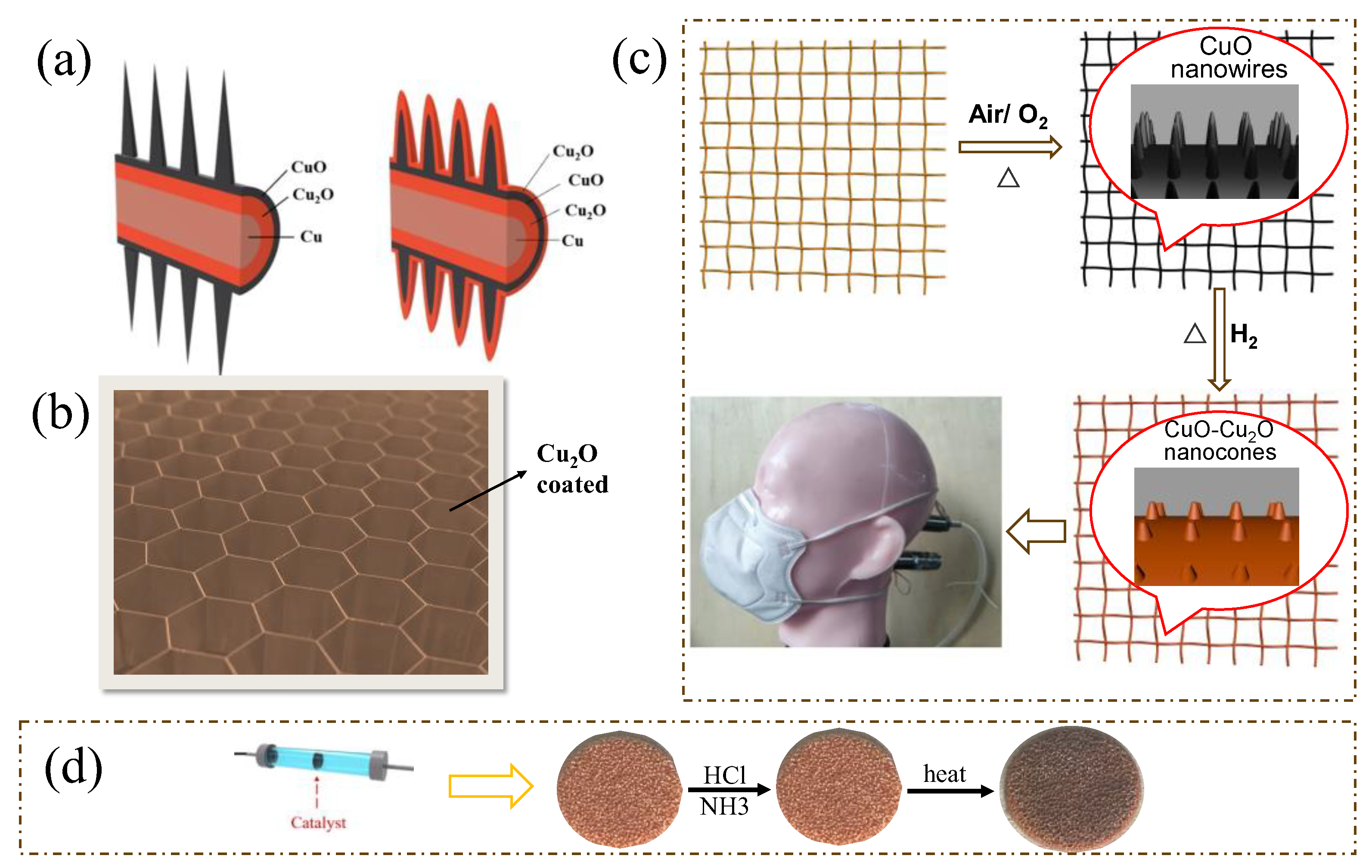
| Catalyst | O3 Conc. (ppm) | GHSV (h−1) | RH (%) | O3 (%) | Rate (μmol/g min−1) | Ref. |
|---|---|---|---|---|---|---|
| Ag-MnOx | 40 | 840,000 | 65 | 81 | 20.25 | [83] |
| Mn3O4 CNTs | 50 | 1,200,000 | 50 | 70 | 31.25 | [22] |
| Ce-MnO2 | 110–120 | 1,200,000 | dry | 98 | 99.60 | [84] |
| FeO-M2LFh | 600 | 1,500,000 | dry | 95 | 636.16 | [85] |
| Cu2Ocube-40 | 770 | 60,000 | dry | 100 | 300.59 | [41] |
| Cu2Oultra-fine | 3000 | 240,000 | 90 | 95 | 111.6 | [42] |
| Cu2O/rGO | 20 | 60,000 | 90 | 96 | 7.87 | [53] |
| Cu2O-Mg | 3000 | 240,000 | 90 | 99.97 | 32.0 | [52] |
| Cu2O-CuO-Cu(OH)2 | 20 | 240,000 | 90 | 82 | 6.67 | [55] |
| Cu/Cu2O | 800 | 1,920,000 | dry | 89.5 | 1022.85 | [50] |
| Cu2O/CuO | 1000 ± 50 | 1,920,000 | 90 | 55 | 785.71 | [51] |
| Cu2S | 400 | 480,000 | 90 | 85 | 254.7 | [56] |
| Catalyst | Substrate | O3 Conc. (ppm) | GHSV (h−1) | RH (%) | O3 Conv. (%) | Ref. |
|---|---|---|---|---|---|---|
| Ni-MnOx | diatomite | 16 | 25,000 | 1 | 80 | [86] |
| Mn | TiO2 | 21 | 82,000 | 60 | 83 | [87] |
| NiO/Mn3O4 | cordierite | 50 | 20,000 | 50 | 98 | [88] |
| Mn-Co | Al mesh | 400 | 2000 | dry | 100 | [89] |
| Cu2O | Cu foam | 20 | 12,500 | 90 | 80 | [64] |
| CuO-Cu2O | Cu mesh | 0.3 | 140,000 | 45 | 100 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, G.; Guan, J.; Zhu, Q.; Jiang, Y.; Han, N.; Chen, Y. A Review of Ozone Decomposition by a Copper-Based Catalyst. Catalysts 2024, 14, 264. https://doi.org/10.3390/catal14040264
Ma G, Guan J, Zhu Q, Jiang Y, Han N, Chen Y. A Review of Ozone Decomposition by a Copper-Based Catalyst. Catalysts. 2024; 14(4):264. https://doi.org/10.3390/catal14040264
Chicago/Turabian StyleMa, Guojun, Jian Guan, Qiuyi Zhu, Yishan Jiang, Ning Han, and Yunfa Chen. 2024. "A Review of Ozone Decomposition by a Copper-Based Catalyst" Catalysts 14, no. 4: 264. https://doi.org/10.3390/catal14040264
APA StyleMa, G., Guan, J., Zhu, Q., Jiang, Y., Han, N., & Chen, Y. (2024). A Review of Ozone Decomposition by a Copper-Based Catalyst. Catalysts, 14(4), 264. https://doi.org/10.3390/catal14040264