Ethylene Oligomerization Catalyzed by Different Homogeneous or Heterogeneous Catalysts
Abstract
:1. Introduction
2. Nickel-Based Catalysts
2.1. Nickel-Based Homogeneous Catalysts
2.2. Nickel-Based Heterogeneous Catalysts
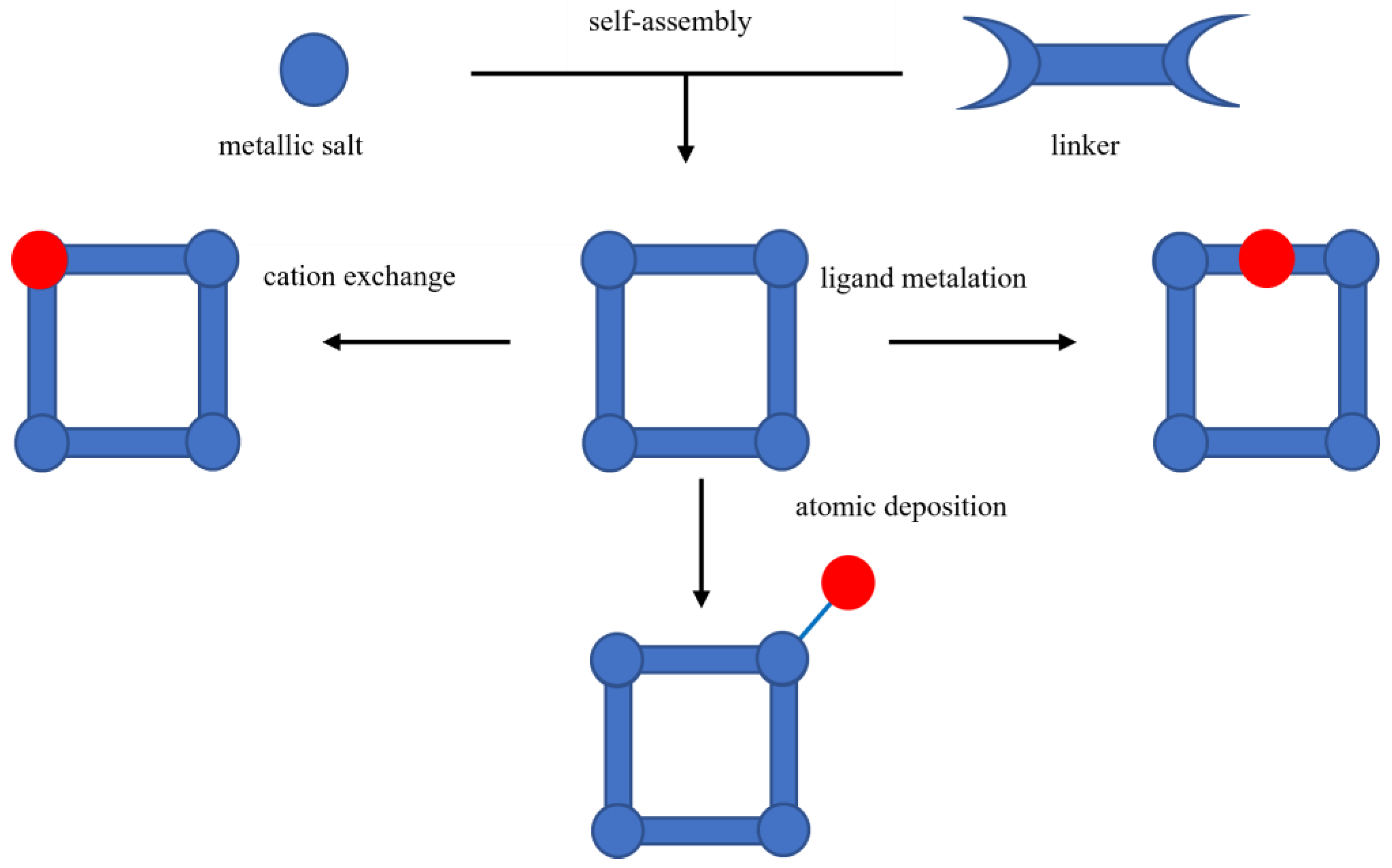
2.2.1. Metal–Organic Frameworks (MOFs)
2.2.2. Covalent Organic Frameworks (COFs)
2.2.3. Molecular Sieve Materials
| Catalyst | Temperature (°C) | Pressure (bar) | Reaction Time (h) | Co-Catalyst (Al:Ni) | Solvent | Activity or TOF | C4 (1-C4) | C6 | C8 | C8+ | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni-UMOFNS-190 1 | 25 | 10 | 1 | Et2AlCl (500) | toluene | 5536 h−1 | 75.6 | 0.4 | 22 | 2 | [70] |
| CPO-27(Ni) 1 | 21 | 10 | 1 | Et2AlCl (17) | toluene | 1 g/(mol·h) | 100 | [71] | |||
| DUT-8(Ni)-rigid 1 | 21 | 20 | 1 | Et2AlCl (17) | toluene | 182 g/(mol·h) | 76 | 24 | |||
| [Ni(bdc)(dabaco)0.5]n 1 | 21 | 10 | 1 | Et2AlCl (17) | toluene | 41 g/(mol·h) | 56 | 44 | |||
| [Ni(bdc)(dabaco)n]n 1 | 21 | 10 | 1 | Et2AlCl (17) | toluene | 41 g/(mol·h) | 49 | 51 | |||
| 1D-Ni-MIL-77 1 | 30 | 10 | 0.5 | Et2AlCl (100) | toluene | 5544 h−1 | 98 (93.3) | 0.14 | 1.84 | [72] | |
| 3D-Ni-MIL-77 1 | 30 | 15 | 0.5 | Et2AlCl (180) | toluene | 2226 h−1 | 99.6 (90) | 0.22 | 0.17 | ||
| Ni-ZIF-8 (0.4 wt%) 1 | 35 | 30 | 0.17 | MAO (4640) | toluene | 1,116,000 h−1 | 97 (87.7) | [53] | |||
| 15Ni-ZIF-L 1 | 40 | 30 | 0.17 | MAO | toluene | 342,030 h−1 | 96.9 (94.7) | [73] | |||
| Ni(1%)-MFU-4l 2 | 25 | 50 | 1 | MAO (500) | toluene | 41,500 h−1 | 97.4 (94.5) | 2.6 | [74] | ||
| Ni(7.5%)-CFA-1 2 | 22 | 50 | 1 | MMAO-12 (2000) | toluene | 37,100 h−1 | 95.5 (91.2) | [75] | |||
| 20Ni-MOF5 (5.32 wt%) 2 | 35 | 50 | 0.17 | MAO | toluene | 352,000 h−1 | 96.3 (84.2) | [76] | |||
| Ni-AIM-NU-1000 3 | 45 | 2 | 10 | Et2AlCl | 1080 h−1 | 42 | 8 | 46 | [77] | ||
| Ni-Facac-AIM-NU-1000 3 | 45 | 2 | 10 | Et2AlCl | 12.6 h−1 | 100 (78) | [49] | ||||
| Ni-acac-AIM-NU-1000 3 | 45 | 2 | 10 | Et2AlCl | 15.84 h−1 | 100 (82) | |||||
| (30)Ni@(Fe)MIL-101 4 | 10 | 15 | 1 | Et2AlCl (70) | heptane | 3215 h−1 | 94 | 5.5 | 0.5 | [78] | |
| MixMOFs-Ni-a 4 | 20 | 20 | 0.5 | Et2AlCl (100) | toluene | 2071 h−1 | 71.3 | 6.9 | 0 | 21.8 | [79] |
| MixMOFs-Ni-b 4 | 40 | 20 | 0.5 | Et2AlCl (100) | toluene | 16,428 h−1 | 92.7 | 6.1 | 0 | 1.2 | |
| MixMOFs-Ni-c 4 | 20 | 20 | 0.5 | Et2AlCl (100) | toluene | 2000 h−1 | 79.5 | 7.2 | 0 | 14.3 | |
| IRMOF-3-Ni-a 4 | 20 | 20 | 0.5 | Et2AlCl (100) | toluene | 2246 h−1 | 35 | 9.3 | 0 | 55.7 | |
| MIL-125(Ti)-NH2(Ni) 4 | 50 | 10 | 0.5 | MAO (800) | cyclohexane | 6464 h−1 | 19.6 (87.2) | 76.7 (92.5) | 1.5 | 2.2 | [54] |
| NU-1000-bpy-NiCl2 4 | 21 | 15 | 1 | Et2AlCl (70) | heptane | 1560 h−1 | 82 | 18 | [80] | ||
| [Al]-Ni-bpydc(MOF) 4 | 5 | 15 | 1 | Et2AlCl (70) | heptane | 20 g/(g·h) | 89.1 (26.3) | 8.9 | 2.1 | [81] | |
| [Ni]-Ni-bpydc(MOF)4 | 5 | 15 | 1 | Et2AlCl (70) | heptane | 20 g/(g·h) | 92.7 (71.3) | 7.3 | |||
| Zr6O4(OH)4 (bpydc)0.84(bpdc)5.16 (NiBr2)0.84 4 | 55 | 59 | 1 | Et2AlCl (70) | cyclohexane | 370 g/(g/h) | [82] | ||||
| NiCl@DUT-133 4 | 40 | 35 | 1 | Et2AlCl (51) | 1,2- dichlorobenzene | 42 mol/(mol·h) | 81 | 19 | [83] |
2.2.4. Other Materials
3. Iron-Based Catalysts
3.1. Iron-Based Homogeneous Catalysts
3.1.1. NNN Ligand
3.1.2. NN Ligand
3.1.3. NNOO Ligand
3.1.4. NNO Ligand
3.1.5. NNNNOO Ligand
3.2. Iron-Based Heterogeneous Catalyst
4. Cobalt-Based Catalysts
4.1. Cobalt-Based Homogeneous Catalysts
4.1.1. Tri-Dentate Cobalt Complex
4.1.2. Bi-Dentate Cobalt Complex
4.1.3. Other Types of Cobalt Complex
4.2. Cobalt-Based Heterogeneous Catalysts
5. Chromium-Based Catalysts
5.1. Chromium-Based Homogeneous Catalysts
5.1.1. Ethylene Trimerization
5.1.2. Ethylene Tetramerization
5.1.3. Unselective Ethylene Oligomerization
5.2. Chromium-Based Heterogeneous Catalysts
6. Other Metals-Based Heterogeneous Catalysts
7. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Speiser, F.; Braunstein, P.; Saussine, L. New Nickel Ethylene Oligomerization Catalysts Bearing Bidentate P,N-Phosphinopyridine Ligands with Different Substituents α to Phosphorus. Organometallics 2004, 23, 2625–2632. [Google Scholar] [CrossRef]
- Breuil, P.A.R.; Magna, L.; Olivier-Bourbigou, H. Role of Homogeneous Catalysis in Oligomerization of Olefins: Focus on Selected Examples Based on Group 4 to Group 10 Transition Metal Complexes. Catal. Lett. 2015, 145, 173–192. [Google Scholar] [CrossRef]
- de Klerk, A. Thermal cracking of Fischer-Tropsch waxes. Ind. Eng. Chem. Res. 2007, 46, 5516–5521. [Google Scholar] [CrossRef]
- Li, C.; Ying, W.; Cao, F.; Zhang, H.; Fang, D. Effects of Impregnation Solvents on Catalytic Performance of Co-Ru-ZrO2/γ-Al2O3 Catalyst for Fischer-Tropsch Synthesis. Pet. Sci. Technol. 2008, 26, 704–716. [Google Scholar] [CrossRef]
- Liu, X.H.; Linghu, W.S.; Li, X.H.; Asami, K.; Fujimoto, K. Effects of solvent on Fischer-Tropsch synthesis. Appl. Catal. A Gen. 2006, 303, 251–257. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, J.; Wu, T.J.; Wang, H.; Xie, S.H.; Pei, Y.; Yan, S.R.; Qiao, M.H.; Zong, B.N. Mg and K dual-decorated Fe-on-reduced graphene oxide for selective catalyzing CO hydrogenation to light olefins with mitigated CO2 emission and enhanced activity. Appl. Catal. B Environ. 2017, 204, 475–485. [Google Scholar] [CrossRef]
- Li, A.L.; Tian, D.X.; Zhao, Z.B. DFT studies on the reaction mechanism for the selective oxidative dehydrogenation of light alkanes by BN catalysts. N. J. Chem. 2020, 44, 11584–11592. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhang, S.; Zhu, X.L.; Li, C.Y.; Shan, H.H. Dehydrogenation versus hydrogenolysis in the reaction of light alkanes over Ni-based catalysts. J. Ind. Eng. Chem. 2020, 86, 1–12. [Google Scholar] [CrossRef]
- Yan, B.; Li, W.C.; Lu, A.H. Metal-free silicon boride catalyst for oxidative dehydrogenation of light alkanes to olefins with high selectivity and stability. J. Catal. 2019, 369, 296–301. [Google Scholar] [CrossRef]
- Rajapaksha, R.; Samanta, P.; Quadrelli, E.A.; Canivet, J. Heterogenization of molecular catalysts within porous solids: The case of Ni-catalyzed ethylene oligomerization from zeolites to metal-organic frameworks. Chem. Soc. Rev. 2023, 52, 8059–8076. [Google Scholar] [CrossRef]
- Wang, M.Z.; Wu, W.; Wang, X.; Huang, X.; Nai, Y.N.; Wei, X.Y.; Mao, G.L. Research progress of iron-based catalysts for selective oligomerization of ethylene. RSC Adv. 2020, 10, 43640–43652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Solan, G.A.; Zhang, W.J.; Sun, W.H. Carbocyclic-fused N,N,N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Hao, B.B.; Alam, F.; Jiang, Y.; Wang, L.B.; Fan, H.A.; Ma, J.; Chen, Y.H.; Wang, Y.T.; Jiang, T. Selective ethylene tetramerization: An overview. Inorg. Chem. Front. 2023, 10, 2860–2902. [Google Scholar] [CrossRef]
- Keim, W. Oligomerization of Ethylene to α-Olefins: Discovery and Development of the Shell Higher Olefin Process (SHOP). Angew. Chem.-Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, D.S. Olefin Oligomerization via Metallacycles: Dimerization, Trimerization, Tetramerization, and Beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef] [PubMed]
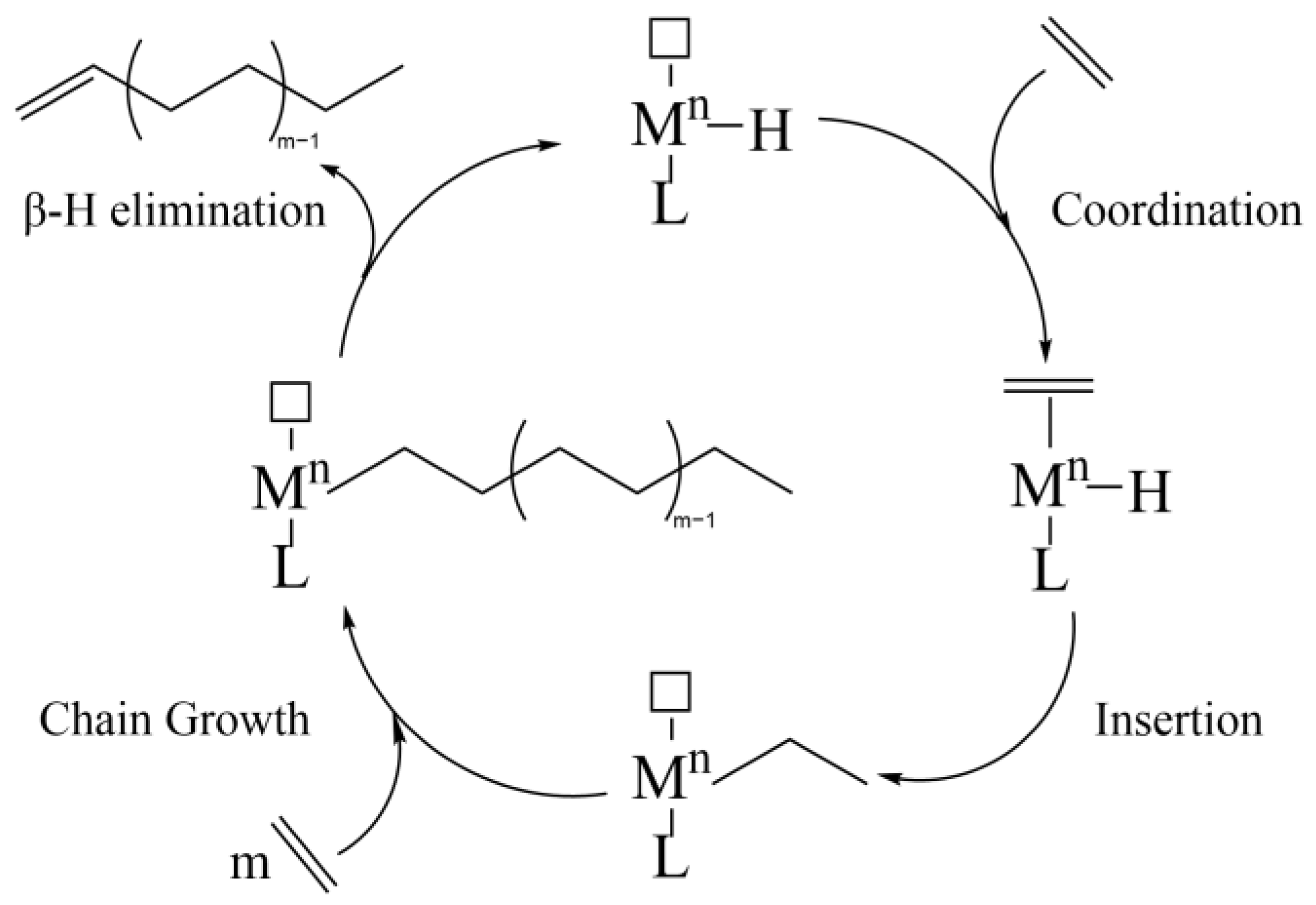
- Cossee, P. On the mechanism of cis-ligand insertion. Recl. Trav. Chim. Pays-Bas 1966, 85, 1151–1160. [Google Scholar] [CrossRef]
- Arlman, E.J.; Cossee, P. Ziegler-Natta catalysis III. Stereospecific polymerization of propene with the catalyst system TiCl3-AlEt3. J. Catal. 1964, 3, 99–104. [Google Scholar] [CrossRef]
- Briggs, J.R. The selective trimerization of ethylene to hex-1-ene. J. Chem. Soc.-Chem. Commun. 1989, 674–675. [Google Scholar] [CrossRef]
- Chabbra, S.; Smith, D.M.; Bell, N.L.; Watson, A.J.B.; Bühl, M.; Cole-Hamilton, D.J.; Bode, B.E. First experimental evidence for a bis-ethene chromium(I) complex forming from an activated ethene oligomerization catalyst. Sci. Adv. 2020, 6, eabd7057. [Google Scholar] [CrossRef]
- Fischer, K.; Jonas, K.; Misbach, P.; Stabba, R.; Wilke, G. The “Nickel Effect” Angew. Chem. Int. Ed. Engl. 1973, 12, 943–953. [Google Scholar] [CrossRef]
- Peuckert, M.; Keim, W. A new nickel complex for the oligomerization of ethylene. Organometallics 1983, 2, 594–597. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and .alpha.-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Zvukova, T.M.; Fedyanin, I.V.; Bulychev, B.M. Synthesis, characterization and ethylene oligomerization behavior of new nickel(II) octofluoro-α-diimine complex. Inorg. Chim. Acta 2016, 442, 167–171. [Google Scholar] [CrossRef]
- Rishina, L.A.; Kissin, Y.V.; Lalayan, S.S.; Gagieva, S.C.; Kurmaev, D.A.; Tuskaev, V.A.; Bulychev, B.M. New α-diimine nickel complexes-Synthesis and catalysis of alkene oligomerization reactions. J. Mol. Catal. A Chem. 2016, 423, 495–502. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Chen, L.; Zhang, N.; Lan, T.; Wang, L. Ethylene Oligomerization Study Bearing Nickel (II) and Cobalt (II) Complexes with N, O-Donor Schiff Bases as Ligands. Chemistryselect 2019, 4, 13959–13963. [Google Scholar] [CrossRef]
- Falivene, L.; Wiedemann, T.; Goettker-Schnetmann, I.; Caporaso, L.; Cavallo, L.; Mecking, S. Control of Chain Walking by Weak Neighboring Group Interactions in Unsymmetrical Catalysts. J. Am. Chem. Soc. 2018, 140, 1305–1312. [Google Scholar] [CrossRef]
- Wang, K.; Gao, R.; Hao, X.; Sun, W.-H. Nickel complexes bearing 2-(1H-benzo[d]imidazol-2-yl)-N-benzylidenequinolin-8-amines: Synthesis, structure and catalytic ethylene oligomerization. Catal. Commun. 2009, 10, 1730–1733. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, Y.L.; Huang, J.L.; Duan, J.J. Novel neutral arylnickel(II) phosphine catalysts containing 2-oxazolinylphenolato N-O chelate ligands for ethylene oligomerization and propylene dimerization. J. Organomet. Chem. 2004, 689, 2614–2623. [Google Scholar] [CrossRef]
- Wang, C.; Friedrich, S.; Younkin, T.R.; Li, R.T.; Grubbs, R.H.; Bansleben, D.A.; Day, M.W. Neutral Nickel(II)-Based Catalysts for Ethylene Polymerization. Organometallics 1998, 17, 3149–3151. [Google Scholar] [CrossRef]
- Zhang, S.; Pattacini, R.; Jie, S.; Braunstein, P. A phosphino-oxazoline ligand as a P,N-bridge in palladium/cobalt or P,N-chelate in nickel complexes: Catalytic ethylene oligomerization. Dalton Trans. 2012, 41, 379–386. [Google Scholar] [CrossRef]
- Ortiz de la Tabla, L.; Matas, I.; Palma, P.; Álvarez, E.; Cámpora, J. Nickel and Palladium Complexes with New Phosphinito-Imine Ligands and Their Application as Ethylene Oligomerization Catalysts. Organometallics 2012, 31, 1006–1016. [Google Scholar] [CrossRef]
- Zhang, S.; Pattacini, R.; Braunstein, P. Reactions of a Phosphinoimino-thiazoline-Based Metalloligand with Organic and Inorganic Electrophiles and Metal-Induced Ligand Rearrangements. Organometallics 2010, 29, 6660–6667. [Google Scholar] [CrossRef]
- Boulens, P.; Lutz, M.; Jeanneau, E.; Olivier-Bourbigou, H.; Reek, J.N.H.; Breuil, P.-A.R. Iminobisphosphines to (Non-)Symmetrical Diphosphinoamine Ligands: Metal-Induced Synthesis of Diphosphorus Nickel Complexes and Application in Ethylene Oligomerisation Reactions. Eur. J. Inorg. Chem. 2014, 2014, 3754–3762. [Google Scholar] [CrossRef]
- Song, K.; Gao, H.; Liu, F.; Pan, J.; Guo, L.; Zai, S.; Wu, Q. Syntheses, Structures, and Catalytic Ethylene Oligomerization Behaviors of Bis(phosphanyl)aminenickel(II) Complexes Containing N-Functionalized Pendant Groups. Eur. J. Inorg. Chem. 2009, 2009, 3016–3024. [Google Scholar] [CrossRef]
- Cooley, N.A.; Green, S.M.; Wass, D.F.; Heslop, K.; Orpen, A.G.; Pringle, P.G. Nickel Ethylene Polymerization Catalysts Based on Phosphorus Ligands. Organometallics 2001, 20, 4769–4771. [Google Scholar] [CrossRef]
- Ghisolfi, A.; Fliedel, C.; Rosa, V.; Monakhov, K.Y.; Braunstein, P. Combined Experimental and Theoretical Study of Bis(diphenylphosphino)(N-thioether)amine-Type Ligands in Nickel(II) Complexes for Catalytic Ethylene Oligomerization. Organometallics 2014, 33, 2523–2534. [Google Scholar] [CrossRef]
- Tayade, K.N.; Mane, M.V.; Sen, S.; Murthy, C.N.; Tembe, G.L.; Pillai, S.M.; Vanka, K.; Mukherjee, S. A catalytic and DFT study of selective ethylene oligomerization by nickel(II) oxime-based complexes. J. Mol. Catal. A Chem. 2013, 366, 238–246. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, M.; Gao, R.; Cao, X.; Sun, W.-H. 2-(1H-2-Benzimidazolyl)-6-(1-(arylimino)ethyl)pyridylnickel Complexes: Synthesis, Characterization, and Ethylene Oligomerization. Aust. J. Chem. 2010, 63, 109–115. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, P.; Zhang, C.; Li, G.; Yang, X.-J.; Wu, B.; Janiak, C. Synthesis, structure, and catalytic ethylene oligomerization of nickel complexes bearing 2-pyrazolyl substituted 1,10-phenanthroline ligands. J. Mol. Catal. A Chem. 2008, 296, 9–17. [Google Scholar] [CrossRef]
- Jie, S.; Zhang, S.; Sun, W.-H. 2-arylimino-9-phenyl-1,10-phenanthrolinyl-iron, -cobalt and -nickel complexes: Synthesis, characterization and ethylene oligomerization behavior. Eur. J. Inorg. Chem. 2007, 2007, 5584–5598. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Yu, J.; Redshaw, C.; Hao, X.; Sun, W.-H. 2-(N-Alkylcarboxamide)-6-iminopyridyl palladium and nickel complexes: Coordination chemistry and catalysis. Dalton Trans. 2011, 40, 12856–12865. [Google Scholar] [CrossRef]
- Chen, Y.; Hao, P.; Zuo, W.; Gao, K.; Sun, W.-H. 2-(1-Isopropyl-2-benzimidazolyl)-6-(1-aryliminoethyl)pyridyl transition metal (Fe, Co, and Ni) dichlorides: Syntheses, characterizations and their catalytic behaviors toward ethylene reactivity. J. Organomet. Chem. 2008, 693, 1829–1840. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Li, A.; Ye, H.; Li, Z. Nickel(II) complexes chelated by 2,6-pyridinedicarboxamide: Syntheses, characterization, and ethylene oligomerization. N. J. Chem. 2016, 40, 7027–7033. [Google Scholar] [CrossRef]
- Ulbrich, A.H.D.P.S.; Bergamo, A.L.; Casagrande, O.D.L., Jr. Oligomerization of ethylene using tridentate nickel catalysts bearing ether-pyrazol ligands with pendant O- and S-donor groups. Catal. Commun. 2011, 16, 245–249. [Google Scholar] [CrossRef]
- Bekmukhamedov, G.E.; Sukhov, A.V.; Kuchkaev, A.M.; Yakhvarov, D.G. Ni-Based Complexes in Selective Ethylene Oligomerization Processes. Catalysts 2020, 10, 498. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Breuil, P.A.R.; Magna, L.; Michel, T.; Pastor, M.F.E.; Delcroix, D. Nickel Catalyzed Olefin Oligomerization and Dimerization. Chem. Rev. 2020, 120, 7919–7983. [Google Scholar] [CrossRef]
- Crabtree, R.H. Deactivation in Homogeneous Transition Metal Catalysis: Causes, Avoidance, and Cure. Chem. Rev. 2015, 115, 127–150. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Reticular chemistry: Occurrence and taxonomy of nets and grammar for the design of frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef]
- Liu, J.; Ye, J.; Li, Z.; Otake, K.-I.; Liao, Y.; Peters, A.W.; Noh, H.; Truhlar, D.G.; Gagliardi, L.; Cramer, C.J.; et al. Beyond the Active Site: Tuning the Activity and Selectivity of a Metal-Organic Framework-Supported Ni Catalyst for Ethylene Dimerization. J. Am. Chem. Soc. 2018, 140, 11174–11178. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Bruce, M.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; Mastroianni, S.; McTavish, S.J.; Redshaw, C.; Solan, G.A.; Strömberg, S.; et al. Iron and cobalt ethylene polymerization catalysts bearing 2,6-bis(imino)pyridyl ligands: Synthesis, structures, and polymerization studies. J. Am. Chem. Soc. 1999, 121, 8728–8740. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly Active Iron and Cobalt Catalysts for the Polymerization of Ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Zhou, T.; Li, Y.-Q.; Lu, X.; Guan, Y.-B.; Cao, Y.-C.; Cao, G.-P. Microenvironment Modulation of Metal-Organic Frameworks (MOFs) for Coordination Olefin Oligomerization and (co)Polymerization. Small 2023, 19, 2205898. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Alalouni, M.R.; Dong, X.; Cao, Z.; Cheng, Q.; Zheng, L.; Meng, L.; Guan, C.; Liu, L.; Abou-Hamad, E.; et al. Highly Active Heterogeneous Catalyst for Ethylene Dimerization Prepared by Selectively Doping Ni on the Surface of a Zeolitic Imidazolate Framework. J. Am. Chem. Soc. 2021, 143, 7144–7153. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, Y.; Huo, H.; Liu, J.; Li, Y.; Li, C.; Zhang, N.; Wang, J. Metal-organic framework-based composite Ni@MOF as Heterogenous catalyst for ethylene trimerization. Appl. Catal. A Gen. 2020, 594, 117457. [Google Scholar] [CrossRef]
- Yeh, B.; Vicchio, S.P.; Chheda, S.; Zheng, J.; Schmid, J.; Löbbert, L.; Bermejo-Deval, R.; Gutiérrez, O.Y.; Lercher, J.A.; Lu, C.C.; et al. Site Densities, Rates, and Mechanism of Stable Ni/UiO-66 Ethylene Oligomerization Catalysts. J. Am. Chem. Soc. 2021, 143, 20274–20280. [Google Scholar] [CrossRef] [PubMed]
- Komurcu, M.; Lazzarini, A.; Kaur, G.; Borfecchia, E.; Oien-Odegaard, S.; Gianolio, D.; Bordiga, S.; Lillerud, K.P.; Olsbye, U. Co-catalyst free ethene dimerization over Zr-based metal-organic framework (UiO-67) functionalized with Ni and bipyridine. Catal. Today 2021, 369, 193–202. [Google Scholar] [CrossRef]
- Sharma, R.K.; Yadav, P.; Yadav, M.; Gupta, R.; Rana, P.; Srivastava, A.; Zboril, R.; Varma, R.S.; Antonietti, M.; Gawande, M.B. Recent development of covalent organic frameworks (COFs): Synthesis and catalytic (organic-electro-photo) applications. Mater. Horiz. 2020, 7, 411–454. [Google Scholar] [CrossRef]
- Rozhko, E.; Bavykina, A.; Osadchii, D.; Makkee, M.; Gascon, J. Covalent organic frameworks as supports for a molecular Ni based ethylene oligomerization catalyst for the synthesis of long chain olefins. J. Catal. 2017, 345, 270–280. [Google Scholar] [CrossRef]
- Li, D.; Guo, L.; Li, F.; Huang, J.; Li, J.; Li, M.; Li, C. Synthesis and catalytic behavior of nickel heterogenized in covalent organic frameworks as precatalysts in ethylene oligomerization. Microporous Mesoporous Mater. 2022, 338, 111979. [Google Scholar] [CrossRef]
- Guo, L.; Gao, Y.; Huang, J.; Xue, J.; Li, F.; Li, C. Imine-linked covalent organic frameworks coordinated with nickel for ethylene oligomerization. J. Appl. Polym. Sci. 2023, 140, e53320. [Google Scholar] [CrossRef]
- Lallemand, M.; Rusu, O.A.; Dumitriu, E.; Finiels, A.; Fajula, F.; Hulea, V. NiMCM-36 and NiMCM-22 catalysts for the ethylene oligomerization: Effect of zeolite texture and nickel cations/acid sites ratio. Appl. Catal. A Gen. 2008, 338, 37–43. [Google Scholar] [CrossRef]
- Andrei, R.D.; Popa, M.L.; Fajula, F.; Hulea, V. Heterogeneous oligomerization of ethylene over highly active and stable Ni-AlSBA-15 mesoporous catalysts. J. Catal. 2015, 323, 76–84. [Google Scholar] [CrossRef]
- Shin, D.Y.; Yoon, J.H.; Baik, H.; Lee, S.J. A way to avoid polymeric side products during the liquid-phase ethylene oligomerization with SBA-15 supported (bpy)Ni(II)Cl2 heterogeneous catalyst. Appl. Catal. A Gen. 2020, 590, 117363. [Google Scholar] [CrossRef]
- Lacarriere, A.; Robin, J.; Swierczynski, D.; Finiels, A.; Fajula, F.; Luck, F.; Hulea, V. Distillate-Range Products from Non-Oil-Based Sources by Catalytic Cascade Reactions. Chemsuschem 2012, 5, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.O.; Parsapur, R.K.; Hita, I.; Ramirez, A.; Huang, K.-W.; Gascon, J.; Castano, P. Stable and reusable hierarchical ZSM-5 zeolite with superior performance for olefin oligomerization when partially coked. Appl. Catal. B Environ. 2022, 316, 121582. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Velisoju, V.K.; Hita, I.; Abed, O.; Parsapur, R.K.; Zambrano, N.; Ben Hassine, M.; Morlanes, N.; Emwas, A.-H.; Huang, K.-W.; et al. Highly productive framework bounded Ni2+ on hierarchical zeolite for ethylene oligomerization. Chem. Eng. J. 2023, 475, 146077. [Google Scholar] [CrossRef]
- McCaig, J.; Lamb, H.H. Ni-H-Beta Catalysts for Ethylene Oligomerization: Impact of Parent Cation on Ni Loading, Speciation, and Siting. Catalysts 2022, 12, 824. [Google Scholar] [CrossRef]
- Seufitelli, G.V.S.; Park, J.J.W.; Tran, P.N.; Dichiara, A.; Resende, F.L.P.; Gustafson, R. The Role of Nickel and Bronsted Sites on Ethylene Oligomerization with Ni-H-Beta Catalysts. Catalysts 2022, 12, 565. [Google Scholar] [CrossRef]
- Lee, M.; Yoon, J.W.; Kim, Y.; Yoon, J.S.; Chae, H.-J.; Han, Y.-H.; Hwang, D.W. Ni/SIRAL-30 as a heterogeneous catalyst for ethylene oligomerization. Appl. Catal. A Gen. 2018, 562, 87–93. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Han, Y.; Sheng, D.; Shan, D.; Liu, X.; Cheng, A. Ultrathin Nickel-Based Metal–Organic Framework Nanosheets as Reusable Heterogeneous Catalyst for Ethylene Dimerization. ACS Appl. Nano Mater. 2019, 2, 136–142. [Google Scholar] [CrossRef]
- Arrozi, U.S.F.; Bon, V.; Kutzscher, C.; Senkovska, I.; Kaskel, S. Towards highly active and stable nickel-based metal-organic frameworks as ethylene oligomerization catalysts. Dalton Trans. 2019, 48, 3415–3421. [Google Scholar] [CrossRef]
- Wang, C.; Li, G.; Guo, H. Heterogeneous dimerization of ethylene by coordinatively unsaturated metal sites in two forms of Ni-MIL-77. Mol. Catal. 2022, 524, 112340. [Google Scholar] [CrossRef]
- Chen, C.L.; Alalouni, M.R.; Xiao, P.Y.; Li, G.X.; Pan, T.T.; Shen, J.; Cheng, Q.P.; Dong, X.L. Ni-Loaded 2D Zeolitic Imidazolate Framework as a Heterogeneous Catalyst with Highly Activity for Ethylene Dimerization. Ind. Eng. Chem. Res. 2022, 61, 14374–14381. [Google Scholar] [CrossRef]
- Metzger, E.D.; Brozek, C.K.; Comito, R.J.; Dincă, M. Selective Dimerization of Ethylene to 1-Butene with a Porous Catalyst. ACS Cent. Sci. 2016, 2, 148–153. [Google Scholar] [CrossRef]
- Metzger, E.D.; Comito, R.J.; Wu, Z.; Zhang, G.; Dubey, R.C.; Xu, W.; Miller, J.T.; Dinca, M. Highly Selective Heterogeneous Ethylene Dimerization with a Scalable and Chemically Robust MOF Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 6654–6661. [Google Scholar] [CrossRef]
- Chen, C.L.; Meng, L.K.; Alalouni, M.R.; Dong, X.L.; Wu, Z.P.; Zuo, S.W.; Zhang, H.B. Ultra-Highly Active Ni-Doped MOF-5 Heterogeneous Catalysts for Ethylene Dimerization. Small 2023, 19, e2301235. [Google Scholar] [CrossRef]
- Li, Z.; Schweitzer, N.M.; League, A.B.; Bernales, V.; Peters, A.W.; Getsoian, A.B.; Wang, T.C.; Miller, J.T.; Vjunov, A.; Fulton, J.L.; et al. Sintering-Resistant Single-Site Nickel Catalyst Supported by Metal Organic Framework. J. Am. Chem. Soc. 2016, 138, 1977–1982. [Google Scholar] [CrossRef]
- Canivet, J.; Aguado, S.; Schuurman, Y.; Farrusseng, D. MOF-Supported Selective Ethylene Dimerization Single-Site Catalysts through One-Pot Postsynthetic Modification. J. Am. Chem. Soc. 2013, 135, 4195–4198. [Google Scholar] [CrossRef]
- Liu, B.; Jie, S.; Bu, Z.; Li, B.-G. Postsynthetic modification of mixed-linker metal-organic frameworks for ethylene oligomerization. RSC Adv. 2014, 4, 62343–62346. [Google Scholar] [CrossRef]
- Madrahimov, S.T.; Gallagher, J.R.; Zhang, G.; Meinhart, Z.; Garibay, S.J.; Delferro, M.; Miller, J.T.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Gas-Phase Dimerization of Ethylene under Mild Conditions Catalyzed by MOF Materials Containing (bpy)NiII Complexes. ACS Catal. 2015, 5, 6713–6718. [Google Scholar] [CrossRef]
- Kyogoku, K.; Yamada, C.; Suzuki, Y.; Nishiyama, S.; Fukumoto, K.; Yamamoto, H.; Indo, S.; Sano, M.; Miyake, T. Syntheses of Metal-organic Framework Compounds Containing Ni-bipyridyl Complex for Oligomerization of Ethylene. J. Jpn. Pet. Inst. 2010, 53, 308–312. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Oktawiec, J.; Long, J.R. Ethylene oligomerization in metal-organic frameworks bearing nickel(II) 2,2′-bipyridine complexes. Faraday Discuss. 2017, 201, 351–367. [Google Scholar] [CrossRef]
- Arrozi, U.S.F.; Bon, V.; Krause, S.; Lübken, T.; Weiss, M.S.; Senkovska, I.; Kaskel, S. In Situ Imine-Based Linker Formation for the Synthesis of Zirconium MOFs: A Route to CO2 Capture Materials and Ethylene Oligomerization Catalysts. Inorg. Chem. 2020, 59, 350–359. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Ahamad, T.; Aldalbahi, A.; Alhokbany, N. Pyridylimine Cobalt(II) and Nickel(II) Complex Functionalized Multiwalled Carbon Nanotubes and Their Catalytic Activities for Ethylene Oligomerization. Adv. Polym. Technol. 2016, 35, 21528–21538. [Google Scholar] [CrossRef]
- Kohler, F.T.U.; Gaertner, K.; Hager, V.; Haumann, M.; Sternberg, M.; Wang, X.; Szesni, N.; Meyer, K.; Wasserscheid, P. Dimerization of ethene in a fluidized bed reactor using Ni-based Supported Ionic Liquid Phase (SILP) catalysts. Catal. Sci. Technol. 2014, 4, 936–947. [Google Scholar] [CrossRef]
- Aid, A.; Andrei, R.D.; Amokrane, S.; Cammarano, C.; Nibou, D.; Hulea, V. Ni-exchanged cationic clays as novel heterogeneous catalysts for selective ethylene oligomerization. Appl. Clay Sci. 2017, 146, 432–438. [Google Scholar] [CrossRef]
- Kim, M.J.; Ahn, S.; Yi, J.; Hupp, J.T.; Notestein, J.M.; Farha, O.K.; Lee, S.J. Ni(II) complex on a bispyridine-based porous organic polymer as a heterogeneous catalyst for ethylene oligomerization. Catal. Sci. Technol. 2017, 7, 4351–4354. [Google Scholar] [CrossRef]
- Yoon, J.S.; Park, M.B.; Kim, Y.; Hwang, D.W.; Chae, H.-J. Effect of Metal Oxide-Support Interactions on Ethylene Oligomerization over Nickel Oxide/Silica-Alumina Catalysts. Catalysts 2019, 9, 933. [Google Scholar] [CrossRef]
- Finiels, A.; Fajula, F.; Hulea, V. Nickel-based solid catalysts for ethylene oligomerization—A review. Catal. Sci. Technol. 2014, 4, 2412–2426. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M. Iron-Based Catalysts with Exceptionally High Activities and Selectivities for Oligomerization of Ethylene to Linear α-Olefins. J. Am. Chem. Soc. 1998, 120, 7143–7144. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Solan, G.A. Bis(imino)pyridines: Surprisingly reactive ligands and a gateway to new families of catalysts. Chem. Rev. 2007, 107, 1745–1776. [Google Scholar] [CrossRef]
- Britovsek, G.J.P.; Gibson, V.C.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J.; Britovsek, G.J.P.; Kimberley, B.S.; Maddox, P.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Ngcobo, M.; Nose, H.; Jayamani, A.; Ojwach, S.O. Structural and ethylene oligomerization studies of chelating (imino)phenol Fe(II), Co(II) and Ni(II) complexes: An experimental and theoretical approach. N. J. Chem. 2022, 46, 6219–6229. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Q.; Zhang, W.; Liang, T.; Sun, W.-H. The benzhydryl-modified 2-imino-1,10-phenanthrolyliron precatalyst in ethylene oligomerization. J. Organomet. Chem. 2021, 936, 121713. [Google Scholar] [CrossRef]
- Sheng, D.; Zhang, Y.; Wang, Z.; Xu, G.; Song, Q.; Shan, D.; Hou, H. Selective ethylene oligomerization based on Fe-containing metal-organic coordination polymer catalysts. J. Organomet. Chem. 2021, 956, 122128. [Google Scholar] [CrossRef]
- Maksakova, I.S.; Pervova, I.G.; Belov, G.P.; Khasbiullin, I.I.; Ivanova, N.A.; Frolova, E.N.; Lipunov, I.N. Synthesis, structure, and catalytic properties of iron complexes with mono- and bisformazans in sulfide oxidation and ethylene oligomerization reactions. Pet. Chem. 2015, 55, 217–223. [Google Scholar] [CrossRef]
- Appukuttan, V.K.; Liu, Y.; Son, B.C.; Ha, C.-S.; Suh, H.; Kim, I. Iron and Cobalt Complexes of 2,3,7,8-Tetrahydroacridine-4,5(1H,6H)-diimine Sterically Modulated by Substituted Aryl Rings for the Selective Oligomerization to Polymerization of Ethylene. Organometallics 2011, 30, 2285–2294. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, W.; Lai, J.; Sun, W.-H. Iron-oriented ethylene oligomerization and polymerization: The Iron Age or a flash in the pan. C. R. Chim. 2011, 14, 851–855. [Google Scholar] [CrossRef]
- Tomov, A.K.; Gibson, V.C.; Britovsek, G.J.P.; Long, R.J.; van Meurs, M.; Jones, D.J.; Tellmann, K.P.; Chirinos, J.J. Distinguishing Chain Growth Mechanisms in Metal-catalyzed Olefin Oligomerization and Polymerization Systems: C2H4/C2D4 Co-oligomerization/Polymerization Experiments Using Chromium, Iron, and Cobalt Catalysts. Organometallics 2009, 28, 7033–7040. [Google Scholar] [CrossRef]
- Görl, C.; Beck, N.; Kleiber, K.; Alt, H.G. Iron(III) complexes with meta-substituted bis(arylimino)pyridine ligands: Catalyst precursors for the selective oligomerization of ethylene. J. Mol. Catal. A Chem. 2012, 352, 110–127. [Google Scholar] [CrossRef]
- Li, G.; Ge, Y.; Xu, G.; Dai, S. The electronic effects on unsymmetrical Bis(imino)pyridyl iron(III) catalyzed ethylene polymerization. J. Organomet. Chem. 2020, 923, 121457. [Google Scholar] [CrossRef]
- Görl, C.; Alt, H.G. Influence of the para-substitution in bis(arylimino)pyridine iron complexes on the catalytic oligomerization and polymerization of ethylene. J. Organomet. Chem. 2007, 692, 4580–4592. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Qian, C.; Xie, G.; Chen, Y. Multi-center nature of ethylene polymerization catalysts based on 2,6-bis(imino)pyridyl complexes of iron and cobalt. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6159–6170. [Google Scholar] [CrossRef]
- Minaev, B.; Baryshnikova, A.; Sun, W.-H. Spin-dependent effects in ethylene polymerization with bis(imino)pyridine iron(II) complexes. J. Organomet. Chem. 2016, 811, 48–65. [Google Scholar] [CrossRef]
- Cartes, M.Á.; Rodríguez-Delgado, A.; Palma, P.; Sánchez, L.J.; Cámpora, J. Direct evidence for a coordination–insertion mechanism of ethylene oligomerization catalysed by neutral 2,6-bisiminopyridine iron monoalkyl complexes. Catal. Sci. Technol. 2014, 4, 2504–2507. [Google Scholar] [CrossRef]
- Li, H.-y.; Duan, B.-g.; Hu, Y.-l. The influence of substituent electronic effect on ethylene oligomerization activities of bis(imino)pyridyl Fe(II) catalysts: A combined molecular mechanics and charge equilibration method. Chin. J. Polym. Sci. 2009, 27, 711. [Google Scholar] [CrossRef]
- Guo, C.Y.; Xu, H.; Zhang, M.G.; Zhang, X.H.; Yan, F.W.; Yuan, G.Q. Immobilization of bis(imino)pyridine iron complexes onto mesoporous molecular sieves and their catalytic performance in ethylene oligomerization. Catal. Commun. 2009, 10, 1467–1471. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Bariashir, C.; Zhang, Q.-Y.; Ulambayar, B.; Solan, G.A.; Liang, T.-L.; Sun, W.-H. High Temperature Iron Ethylene Polymerization Catalysts Bearing N, N, N′-2-(1-(2,4-Dibenzhydryl-6-fluorophenylimino)ethyl)-6-(1-(arylphenylimino)ethyl)pyridines. Chin. J. Polym. Sci. 2024, 42, 188–201. [Google Scholar] [CrossRef]
- Zhao, W.; Yue, E.; Wang, X.; Yang, W.; Chen, Y.; Hao, X.; Cao, X.; Sun, W.-H. Activity and stability spontaneously enhanced toward ethylene polymerization by employing 2-(1-(2,4-dibenzhydrylnaphthylimino)ethyl)-6-(1-(arylimino)ethyl) pyridyliron(II) dichlorides. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 988–996. [Google Scholar] [CrossRef]
- Kaul, F.A.R.; Puchta, G.T.; Frey, G.D.; Herdtweck, E.; Herrmann, W.A. Iminopyridine Complexes of 3d Metals for Ethylene Polymerization: Comparative Structural Studies and Ligand Size Controlled Chain Termination. Organometallics 2007, 26, 988–999. [Google Scholar] [CrossRef]
- Xie, G.; Li, T.; Zhang, A. Highly active and selective ethylene oligomerization catalysts: Asymmetric 2,6-bis(imino)pyridyl iron (II) complexes with alkyl and halogen substitutients. Inorg. Chem. Commun. 2010, 13, 1199–1202. [Google Scholar] [CrossRef]
- Bianchini, C.; Mantovani, G.; Meli, A.; Migliacci, F.; Zanobini, F.; Laschi, F.; Sommazzi, A. Oligomerisation of Ethylene to Linear α-Olefins by new Cs- and C1-Symmetric [2,6-Bis(imino)pyridyl]iron and -cobalt Dichloride Complexes. Eur. J. Inorg. Chem. 2003, 2003, 1620–1631. [Google Scholar] [CrossRef]
- Sun, W.-H.; Xing, Q.; Yu, J.; Novikova, E.; Zhao, W.; Tang, X.; Liang, T.; Redshaw, C. Probing the Characteristics of Mono- or Bimetallic (Iron or Cobalt) Complexes Bearing 2,4-Bis(6-iminopyridin-2-yl)-3H-benzazepines: Synthesis, Characterization, and Ethylene Reactivity. Organometallics 2013, 32, 2309–2318. [Google Scholar] [CrossRef]
- Zhang, N.; Wu, Y.; Li, Y.; Chen, L.; Zhang, M.; Wang, J. Star iminopyridyl iron, cobalt and nickel complexes: Synthesis, molecular structures, and evaluation as ethylene oligomerization catalysts. Polym. Bull. 2022, 79, 4219–4231. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Huo, H.; Chen, L.; Shi, W.; Li, C.; Wang, J. Iron, cobalt and nickel complexes bearing hyperbranched iminopyridyl ligands: Synthesis, characterization and evaluation as ethylene oligomerization catalysts. Inorg. Chim. Acta 2018, 469, 209–216. [Google Scholar] [CrossRef]
- Li, C.-Q.; Wang, F.-F.; Gao, R.; Sun, P.; Zhang, N.; Wang, J. Bidentate iron complexes based on hyperbranched salicylaldimine ligands and their catalytic behavior toward ethylene oligomerization. Transit. Met. Chem. 2017, 42, 339–346. [Google Scholar] [CrossRef]
- Nyamato, G.S.; Alam, M.G.; Ojwach, S.O.; Akerman, M.P. (Pyrazolyl)-(phosphinoyl)pyridine iron(II), cobalt(II) and nickel(II) complexes: Synthesis, characterization and ethylene oligomerization studies. J. Organomet. Chem. 2015, 783, 64–72. [Google Scholar] [CrossRef]
- Rossetto, E.; Nicola, B.P.; de Souza, R.F.; Bernardo-Gusmão, K.; Pergher, S.B.C. Heterogeneous complexes of nickel MCM-41 with β-diimine ligands: Applications in olefin oligomerization. J. Catal. 2015, 323, 45–54. [Google Scholar] [CrossRef]
- Kim, I.; Han, B.H.; Kim, J.S.; Ha, C.-S. Bis(imino)pyridyl Co(II) and Fe(II) catalysts immobilized on SBA-15 mesoporous material: New highly active supported catalysts for the polymerization of ethylene. Catal. Lett. 2005, 101, 249–253. [Google Scholar] [CrossRef]
- Ionkin, A.S.; Marshall, W.J.; Adelman, D.J.; Bobik Fones, B.; Fish, B.M.; Schiffhauer, M.F.; Spence, R.E.; Xie, T. High-Temperature Catalysts for the Production of α-Olefins Based on Iron(II) and Cobalt(II) Tridentate Bis(imino)pyridine Complexes with a Double Pattern of Substitution: o-Methyl plus o-Fluorine in the Same Imino Arm. Organometallics 2008, 27, 1147–1156. [Google Scholar] [CrossRef]
- Ionkin, A.S.; Marshall, W.J.; Adelman, D.J.; Fones, B.B.; Fish, B.M.; Schiffhauer, M.F. High-Temperature Catalysts for the Production of α-Olefins Based on Iron(II) and Iron(III) Tridentate Bis(imino)pyridine Complexes with Double Pattern of Substitution: ortho-Methyl plus meta-Aryl. Organometallics 2006, 25, 2978–2992. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Li, Y. Ethylene polymerization with silica-supported bis(imino)pyridyl iron(II) catalysts. J. Catal. 2005, 234, 101–110. [Google Scholar] [CrossRef]
- Guo, C.-Y.; Xu, H.; Zhang, M.; Yang, H.-J.; Yan, F.; Yuan, G. Copolymerization of ethylene and in situ-generated α-olefins to high-performance linear low-density polyethylene with a two-catalyst system supported on mesoporous molecular sieves. Polym. Int. 2010, 59, 725–732. [Google Scholar] [CrossRef]
- Jayamani, A.; Nyamato, G.S.; Ojwach, S.O. Ethylene oligomerization reactions catalyzed by homogeneous and silica immobilized NO Fe(II) and Co(II) complexes. J. Organomet. Chem. 2019, 903, 120987. [Google Scholar] [CrossRef]
- Armspach, D.; Matt, D.; Peruch, F.; Lutz, P. Cyclodextrin-Encapsulated Iron Catalysts for the Polymerization of Ethylene. Eur. J. Inorg. Chem. 2003, 2003, 805–809. [Google Scholar] [CrossRef]
- Bouilhac, C.; Cloutet, E.; Cramail, H.; Deffieux, A.; Taton, D. Functionalized Star-Like Polystyrenes as Organic Supports of a Tridentate Bis(imino)pyridinyliron/Aluminic Derivative Catalytic System for Ethylene Polymerization. Macromol. Rapid Commun. 2005, 26, 1619–1625. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Zhang, Y.L.; Cheng, A.C.; Hu, Y.P.; Wang, Z.H. Selective ethylene tetramerization with iron-based metal-organic framework MIL-100 to obtain C8 alkanes. Appl. Catal. A Gen. 2018, 564, 183–189. [Google Scholar] [CrossRef]
- Kaul, F.A.R.; Puchta, G.T.; Schneider, H.; Bielert, F.; Mihalios, D.; Herrmann, W.A. Immobilization of bis(imino)pyridyliron(II) complexes on silica. Organometallics 2002, 21, 74–82. [Google Scholar] [CrossRef]
- Ngcobo, M.; Ouissa, A.; Kleist, W.; Thiel, W.R.; Ojwach, S.O. Regulating the physical properties of silica immobilized Fe(II), Ni(II) and Co(II) catalysts towards ethylene oligomerization reactions. Mol. Catal. 2023, 549, 113465. [Google Scholar] [CrossRef]
- Ngcobo, M.; Ojwach, S.O. Ethylene oligomerization reactions catalyzed by recyclable Fe(II), Ni(II) and Co(II) complexes immobilized on Fe3O4 magnetic nanoparticles. Mol. Catal. 2021, 508, 111583. [Google Scholar] [CrossRef]
- Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. Constrained formation of 2-(1-(arylimino)ethyl)-7-arylimino-6,6-dimethylcyclopentapyridines and their cobalt(II) chloride complexes: Synthesis, characterization and ethylene polymerization. RSC Adv. 2015, 5, 32720–32729. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, R.; Liang, T.; Hu, X.; Solan, G.A.; Sun, W.-H. Selectivity Effects on N,N,N′-Cobalt Catalyzed Ethylene Dimerization/Trimerization Dictated through Choice of Aluminoxane Cocatalyst. Organometallics 2019, 38, 1143–1150. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, W.; Liang, T.; Sun, W.-H. Revisiting the 2-imino-1,10-phenanthrolylmetal precatalyst in ethylene oligomerization: Benzhydryl-modified cobalt(II) complexes and their dimerization of ethylene. Polyhedron 2021, 193, 114865. [Google Scholar] [CrossRef]
- Antonov, A.A.; Semikolenova, N.V.; Talsi, E.P.; Bryliakov, K.P. Catalytic ethylene oligomerization on cobalt(II) bis(imino)pyridine complexes bearing electron-withdrawing groups. J. Organomet. Chem. 2019, 884, 55–58. [Google Scholar] [CrossRef]
- Caovilla, M.; Thiele, D.; de Souza, R.F.; Gregorio, J.R.; Bernardo-Gusmao, K. Cobalt-β-diimine complexes for ethylene oligomerization. Catal. Commun. 2017, 101, 85–88. [Google Scholar] [CrossRef]
- Chen, L.; Ma, L.; Jiang, Y.; Liu, J.; Li, C.; Zhang, N.; Wang, J. Synthesis and characterization of iron, cobalt and nickel complexes bearing para-phenylene-linked pyridine imine ligand and their catalytic properties for ethylene oligomerization. Polym. Bull. 2021, 78, 415–432. [Google Scholar] [CrossRef]
- Khoshsefat, M.; Ahmadjo, S.; Zohuri, G.H.; Ma, Y.; Sun, W.-H. From tetramerization to oligomerization/polymerization of ethylene by dinuclear pyridyl-imine Co- and Ni-based catalysts. Appl. Organomet. Chem. 2023, 37, e7226. [Google Scholar] [CrossRef]
- Wei, W.; Yu, B.; Alam, F.; Huang, Y.; Cheng, S.; Jiang, T. Cobalt(II)-based ethylene dimerization catalysts with silicon-bridged diphosphine ligands. Appl. Organomet. Chem. 2018, 32, e4604. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, X.J.; Chen, Y.H.; Cao, C.G.; Jiang, T. Chromium-Based Ethylene Tetramerization Catalysts Supported by Silicon-Bridged Diphosphine Ligands: Further Combination of High Activity and Selectivity. Chemcatchem 2017, 9, 76–79. [Google Scholar] [CrossRef]
- Härzschel, S.; Kühn, F.E.; Wöhl, A.; Müller, W.; Al-Hazmi, M.H.; Alqahtani, A.M.; Müller, B.H.; Peulecke, N.; Rosenthal, U. Comparative study of new chromium-based catalysts for the selective tri- and tetramerization of ethylene. Catal. Sci. Technol. 2015, 5, 1678–1682. [Google Scholar] [CrossRef]
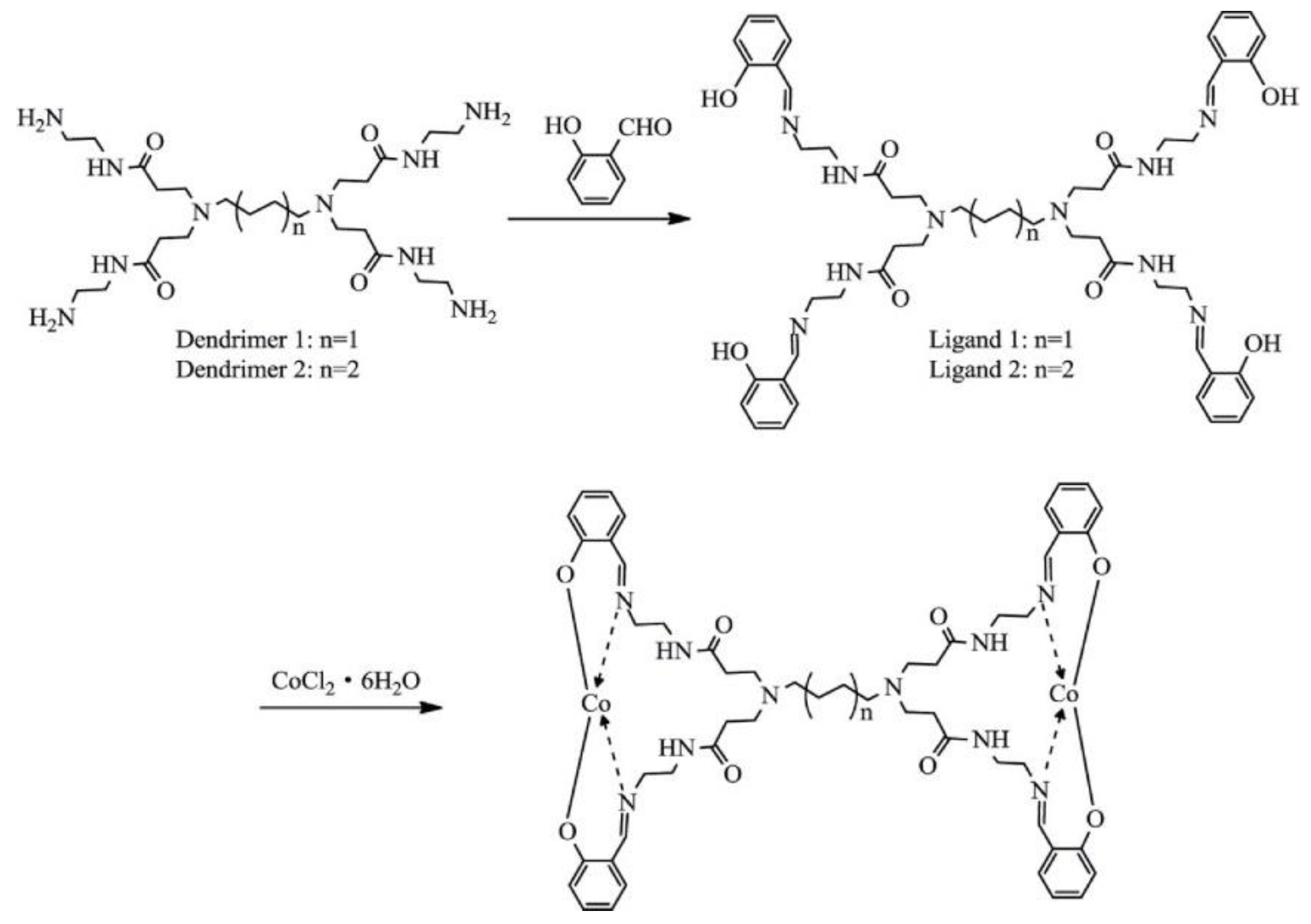
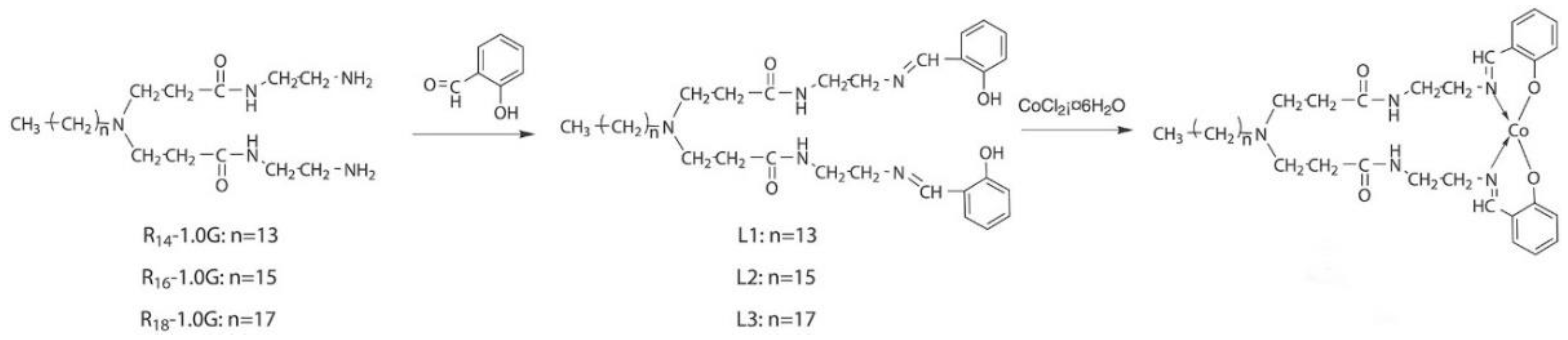
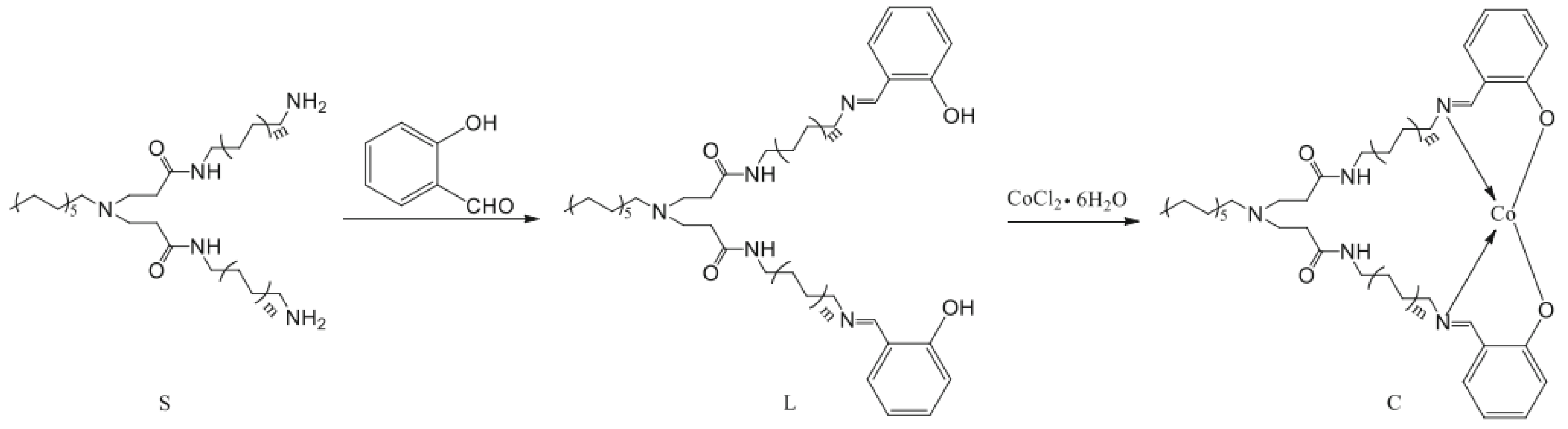
- Wang, J.; Li, H.-Y.; Song, L.; Shi, W.-G.; Zhang, N.; Li, C.-Q. Cobalt complexes based on dendritic PAMAM bridged salicylaldimine ligands: Synthesis, characterization and performance in ethylene oligomerization. J. Macromol. Sci. Part A Pure Appl. Chem. 2016, 53, 709–715. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Lin, Z.; Zhang, N.; Wang, J. Cobalt complexes based on hyperbranched salicylaldimine ligands as catalyst precursors for ethylene oligomerization. Appl. Organomet. Chem. 2017, 31, e3756. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, S.-h.; Song, L.; Li, C.-q.; Wang, J. Hyperbranched macromolecules bridged salicylaldimine cobalt complexes: Synthesis, characterization and ethylene oligomerization studies. Chem. Pap. 2017, 71, 1037–1046. [Google Scholar] [CrossRef]
- Ngcobo, M.; Nyamato, G.S.; Ojwach, S.O. Structural elucidation of N^O (ethylimino-methyl)phenol Fe(II) and Co(II) complexes and their applications in ethylene oligomerization catalysis. Mol. Catal. 2019, 478, 110590. [Google Scholar] [CrossRef]
- Zubkevich, S.V.; Tuskaev, V.A.; Gagieva, S.C.; Kayda, A.S.; Khrustalev, V.N.; Pavlov, A.A.; Zarubin, D.N.; Bulychev, B.M. NNNO-Heteroscorpionate nickel (II) and cobalt (II) complexes for ethylene oligomerization: The unprecedented formation of odd carbon number olefins. Appl. Organomet. Chem. 2020, 34, e5873. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, D.; Chada, J.P.; Rosenfeld, D.C.; Rogers, J.L.; Hermans, L.; Huber, G.W. Olefin conversion on nitrogen-doped carbon-supported cobalt catalyst: Effect of feedstock. J. Catal. 2017, 354, 213–222. [Google Scholar] [CrossRef]
- Xu, Z.; Chada, J.P.; Xu, L.; Zhao, D.; Rosenfeld, D.C.; Rogers, J.L.; Hermans, I.; Mavrikakis, M.; Huber, G.W. Ethylene Dimerization and Oligomerization to 1-Butene and Higher Olefins with Chromium-Promoted Cobalt on Carbon Catalyst. ACS Catal. 2018, 8, 2488–2497. [Google Scholar] [CrossRef]
- Jonathan, A.; Eagan, N.M.; Bruns, D.L.; Stahl, S.S.; Lanci, M.P.; Dumesic, J.A.; Huber, G.W. Ethylene oligomerization into linear olefins over cobalt oxide on carbon catalyst. Catal. Sci. Technol. 2021, 11, 3599–3608. [Google Scholar] [CrossRef]
- Jonathan, A.; Tomashek, E.G.; Lanci, M.P.; Dumesic, J.A.; Huber, G.W. Reaction kinetics study of ethylene oligomerization into linear olefins over carbon-supported cobalt catalysts. J. Catal. 2021, 404, 954–963. [Google Scholar] [CrossRef]
- Jonathan, A.; Dastidar, R.G.; Wang, C.; Dumesic, J.A.; Huber, G.W. Effect of catalyst support on cobalt catalysts for ethylene oligomerization into linear olefins. Catal. Sci. Technol. 2022, 12, 3639–3649. [Google Scholar] [CrossRef]
- Zhu, K.; An, Y.; Yu, F.; Liu, L.; Zhong, L. Structure-Performance Evolution of Cobalt-Ammonia Activated Carbon Catalyst for Ethylene Oligomerization. Catal. Lett. 2022, 152, 2131–2140. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, N.; Shi, D.; Zhang, F.; Tang, J. Synthesis of UiO-66-NH2 Grafted Pyridineimine Cobalt Catalyst and Its Catalytic Performance in Ethylene Oligomerization. Chin. J. Appl. Chem. 2022, 39, 258–265. [Google Scholar] [CrossRef]
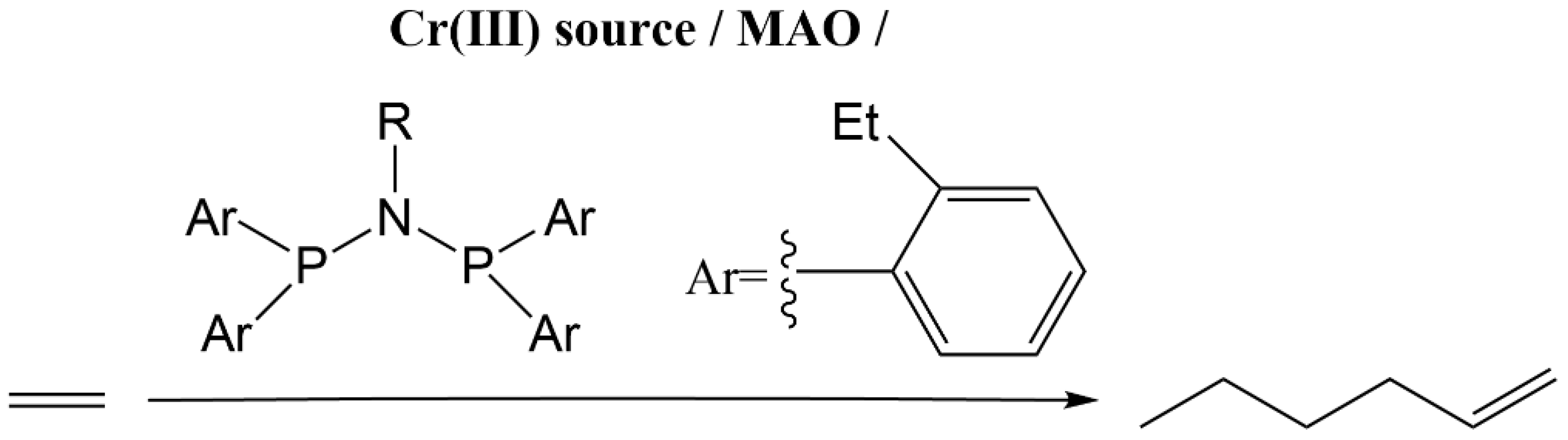
- Weng, Z.; Teo, S.; Hor, T.S.A. Chromium(III) catalysed ethylene tetramerization promoted by bis(phosphino)amines with an N-functionalized pendant. Dalton Trans. 2007, 3493–3498. [Google Scholar] [CrossRef]
- Mao, G.; Ning, Y.; Hu, W.; Li, S.; Song, X.; Niu, B.; Jiang, T. Synthesis of a novel triple-site diphosphinoamine (PNP) ligand and its applications in ethylene tetramerization. Chin. Sci. Bull. 2008, 53, 3511–3515. [Google Scholar] [CrossRef]
- Carter, A.; Cohen, S.A.; Cooley, N.A.; Murphy, A.; Scutt, J.; Wass, D.F. High activity ethylene trimerisation catalysts based on diphosphine ligands. Chem. Commun. 2002, 858–859. [Google Scholar] [CrossRef]
- Sydora, O.L.; Jones, T.C.; Small, B.L.; Nett, A.J.; Fischer, A.A.; Carney, M.J. Selective Ethylene Tri-/Tetramerization Catalysts. ACS Catal. 2012, 2, 2452–2455. [Google Scholar] [CrossRef]
- Zhang, J.; Braunstein, P.; Hor, T.S.A. Highly Selective Chromium(III) Ethylene Trimerization Catalysts with [NON] and [NSN] Heteroscorpionate Ligands. Organometallics 2008, 27, 4277–4279. [Google Scholar] [CrossRef]
- Peng, J.; Zhu, M.; Chen, L.; Liu, Z. Unravelling the chain growth mechanism in Cr/NNN-catalysed ethylene oligomerization. Catal. Sci. Technol. 2023, 13, 6137–6145. [Google Scholar] [CrossRef]
- Ogawa, T.; Lindeperg, F.; Stradiotto, M.; Turculet, L.; Sydora, O.L. Chromium N-phosphinoamidine ethylene tri-/tetramerization catalysts: Designing a step change in 1-octene selectivity. J. Catal. 2021, 394, 444–450. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, H.; Xu, S.; Zhang, X.; Shi, M.; Zhang, J. Highly active chromium-based selective ethylene tri-/tetramerization catalysts supported by PNPO phosphazane ligands. Dalton Trans. 2015, 44, 9545–9550. [Google Scholar] [CrossRef]
- Manyik, R.M.; Walker, W.E.; Wilson, T.P. A soluble chromium-based catalyst for ethylene trimerization and polymerization. J. Catal. 1977, 47, 197–209. [Google Scholar] [CrossRef]
- Zhang, J.; Alam, F.; Fan, H.; Ma, J.; Jiang, T. Chromium catalysts based on PNP(NR2)2 ligands for selective ethylene oligomerization. Appl. Organomet. Chem. 2022, 36, e6454. [Google Scholar] [CrossRef]
- Kong, W.; Ma, X.; Zuo, J.; Zhao, X.; Zhang, J. Ethylene Tri-/Tetramerization Catalysts Supported by Phenylene-Bridged Mixed Alkyl/Aryl Diphosphine Ligands. Organometallics 2023, 42, 651–659. [Google Scholar] [CrossRef]
- Blann, K.; Bollmann, A.; Dixon, J.T.; Hess, F.M.; Killian, E.; Maumela, H.; Morgan, D.H.; Neveling, A.; Otto, S.; Overett, M.J. Highly selective chromium-based ethylene trimerisation catalysts with bulky diphosphinoamine ligands. Chem. Commun. 2005, 620–621. [Google Scholar] [CrossRef]
- Temple, C.; Jabri, A.; Crewdson, P.; Gambarotta, S.; Korobkov, I.; Duchateau, R. The question of the Cr oxidation state in the {Cr(SNS)} catalyst for selective ethylene trimerization: An unanticipated re-oxidation pathway. Angew. Chem.-Int. Ed. 2006, 45, 7050–7053. [Google Scholar] [CrossRef]
- Soheili, M.; Mohamadnia, Z.; Karimi, B. Switching from Ethylene Trimerization to Ethylene Polymerization by Chromium Catalysts Bearing SNS Tridentate Ligands: Process Optimization Using Response Surface Methodology. Catal. Lett. 2018, 148, 3685–3700. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Brown, D.B.; Tooze, R.P.; Hess, F.M.; Dixon, J.T.; Slawin, A.M.Z. Ethylene trimerization with Cr-PNP and Cr-SNS complexes: Effect of ligand structure, metal oxidation state, and role of activator on catalysis. Organometallics 2006, 25, 3605–3610. [Google Scholar] [CrossRef]
- Zhang, J.; Li, A.; Hor, T.S.A. Crystallographic revelation of the role of AIMe3 (in MAO) in Cr [NNN] pyrazolyl catalyzed ethylene trimerization. Organometallics 2009, 28, 2935–2937. [Google Scholar] [CrossRef]
- Alferov, K.A.; Belov, G.P.; Meng, Y. Chromium catalysts for selective ethylene oligomerization to 1-hexene and 1-octene: Recent results. Appl. Catal. A Gen. 2017, 542, 71–124. [Google Scholar] [CrossRef]
- Alam, F.; Zhang, L.; Zhai, Y.; Wang, J.; Tang, H.; Chen, Y.; Jiang, T. In situ formed Cr(III) based silicon-bridged PNS systems for selective ethylene tri-/tetramerization. J. Catal. 2019, 378, 312–319. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Liu, D.; Kong, W.; Zhang, J. Chromium Ethylene Tri-/Tetramerization Catalysts Supported by Iminophosphine Ligands. ACS Omega 2023, 8, 34549–34556. [Google Scholar] [CrossRef]
- Kuhlmann, S.; Blann, K.; Bollmann, A.; Dixon, J.T.; Killian, E.; Maumela, M.C.; Maumela, H.; Morgan, D.H.; Prétorius, M.; Taccardi, N.; et al. N-substituted diphosphinoamines: Toward rational ligand design for the efficient tetramerization of ethylene. J. Catal. 2007, 245, 279–284. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Wasserscheid, P.; Morgan, D.H.; Dixon, J.T. Ethylene trimerization with mixed-donor ligand (N,P,S) chromium complexes: Effect of ligand structure on activity and selectivity. Organometallics 2005, 24, 552–556. [Google Scholar] [CrossRef]
- Boelter, S.D.; Davies, D.R.; Margl, P.; Milbrandt, K.A.; Mort, D.; Vanchura, B.A.I.; Wilson, D.R.; Wiltzius, M.; Rosen, M.S.; Klosin, J. Phospholane-Based Ligands for Chromium-Catalyzed Ethylene Tri- and Tetramerization. Organometallics 2020, 39, 976–987. [Google Scholar] [CrossRef]
- Alam, F.; Zhang, L.; Wei, W.; Wang, J.; Chen, Y.; Dong, C.; Jiang, T. Catalytic Systems Based on Chromium(III) Silylated-Diphosphinoamines for Selective Ethylene Tri-/Tetramerization. ACS Catal. 2018, 8, 10836–10845. [Google Scholar] [CrossRef]
- Zhang, C.; Song, L.; Wu, H.; Ji, X.; Jiao, J.; Zhang, J. Ethylene tri-/tetramerization catalysts supported by diphosphinothiophene ligands. Dalton Trans. 2017, 46, 8399–8404. [Google Scholar] [CrossRef]
- Radcliffe, J.E.; Batsanov, A.S.; Smith, D.M.; Scott, J.A.; Dyer, P.W.; Hanton, M.J. Phosphanyl Methanimine (PCN) Ligands for the Selective Trimerization/Tetramerization of Ethylene with Chromium. ACS Catal. 2015, 5, 7095–7098. [Google Scholar] [CrossRef]
- Rosenthal, U. PNPN-H in Comparison to other PNP, PNPN and NPNPN Ligands for the Chromium Catalyzed Selective Ethylene Oligomerization. ChemCatChem 2020, 12, 41–52. [Google Scholar] [CrossRef]
- Lifschitz, A.M.; Hirscher, N.A.; Lee, H.B.; Buss, J.A.; Agapie, T. Ethylene Tetramerization Catalysis: Effects of Aluminum-Induced Isomerization of PNP to PPN Ligands. Organometallics 2017, 36, 1640–1648. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, X.; Kong, W.; Zhang, J. Switchable ethylene tri-/tetramerization with high catalytic performance: Subtle effect presented by P-alkyl substituent of PCCP ligands. J. Catal. 2022, 415, 95–101. [Google Scholar] [CrossRef]
- Choi, E.M.; Park, J.E.; Park, G.S.; Shong, B.; Kang, S.K.; Son, K.s. Selective ethylene oligomerization with in-situ -generated chromium catalysts supported by trifluoromethyl-containing ligands. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 444–450. [Google Scholar] [CrossRef]
- Wang, J.; Gao, R.; Zhang, N.; Tan, X.; Wang, L.; Ma, L.; Shi, W. Novel Dendritic PNP Chromium Complexes: Synthesis, Characterization, and Performance on Ethylene Oligomerization. Helv. Chim. Acta 2017, 100, e1700162. [Google Scholar] [CrossRef]
- Stennett, T.E.; Haddow, M.F.; Wass, D.F. Avoiding MAO: Alternative Activation Methods in Selective Ethylene Oligomerization. Organometallics 2012, 31, 6960–6965. [Google Scholar] [CrossRef]
- Fan, H.; Yang, X.; Ma, J.; Hao, B.; Alam, F.; Huang, X.; Wang, A.; Jiang, T. Computer-assisted design of asymmetric PNP ligands for ethylene tri-/tetramerization: A combined DFT and artificial neural network approach. J. Catal. 2023, 418, 121–129. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, L.; Guo, X.; Sun, L.; Liu, B.; Liu, Z. Cr/PCCP-catalysed selective ethylene oligomerization: Analysis of various conformations and the hemilabile methoxy group. Catal. Sci. Technol. 2022, 12, 5586–5596. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Ma, X.; Liu, Y.; Mi, P.; Liu, Z.; Zhang, J. Effect of an additional donor on decene formation in ethylene oligomerization catalyzed by a Cr/PCCP system: A combined experimental and DFT study. Catal. Sci. Technol. 2021, 11, 4596–4604. [Google Scholar] [CrossRef]
- Yu, Z.-X.; Houk, K.N. Why Trimerization? Computational Elucidation of the Origin of Selective Trimerization of Ethene Catalyzed by [TaCl3(CH3)2] and An Agostic-Assisted Hydride Transfer Mechanism. Angew. Chem. Int. Ed. 2003, 42, 808–811. [Google Scholar] [CrossRef]
- Bollmann, A.; Blann, K.; Dixon, J.T.; Hess, F.M.; Killian, E.; Maumela, H.; McGuinness, D.S.; Morgan, D.H.; Neveling, A.; Otto, S.; et al. Ethylene tetramerization: A new route to produce 1-octene in exceptionally high selectivities. J. Am. Chem. Soc. 2004, 126, 14712–14713. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, K.; Sun, W.-H. Chromium(III) complexes bearing 2-benzazole-1,10-phenanthrolines: Synthesis, molecular structures and ethylene oligomerization and polymerization. Dalton Trans. 2009, 6354–6363. [Google Scholar] [CrossRef]
- Hao, Z.; Xu, B.; Gao, W.; Han, Y.; Zeng, G.; Zhang, J.; Li, G.; Mu, Y. Chromium Complexes with N,N,N-Tridentate Quinolinyl Anilido-Imine Ligand: Synthesis, Characterization, and Catalysis in Ethylene Polymerization. Organometallics 2015, 34, 2783–2790. [Google Scholar] [CrossRef]
- Luo, W.; Li, A.; Liu, S.; Ye, H.; Li, Z. 2-Benzimidazol-6-pyrazol-pyridine Chromium(III) Trichlorides: Synthesis, Characterization, and Application for Ethylene Oligomerization and Polymerization. Organometallics 2016, 35, 3045–3050. [Google Scholar] [CrossRef]
- Wang, D.; Liu, S.; Zeng, Y.; Sun, W.-H.; Redshaw, C. 2-Benzimidazolyl-N-phenylquinoline-8-carboxamide Chromium(III) Trichlorides: Synthesis and Application for Ethylene Oligomerization and Polymerization. Organometallics 2011, 30, 3001–3009. [Google Scholar] [CrossRef]
- Junges, F.; Kuhn, M.C.A.; Dos Santos, A.H.D.P.; Rabello, C.R.K.; Thomas, C.M.; Carpentier, J.-F.; Casagrande, O.L., Jr. Chromium catalysts based on tridentate pyrazolyl ligands for ethylene oligomerization. Organometallics 2007, 26, 4010–4014. [Google Scholar] [CrossRef]
- Gibson, V.C.; Newton, C.; Redshaw, C.; Solan, G.A.; White, A.J.P.; Williams, D.J. Chromium complexes bearing pyrrolide-imine N,N-chelate ligands: Synthesis, structures and ethylene polymerisation behaviour. J. Chem. Soc. Dalton Trans. 2002, 4017–4023. [Google Scholar] [CrossRef]
- De Oliveira, L.L.; Batista, F.I.; Oliboni, R.S.; Casagrande, A.C.A.; Stieler, R.; Casagrande, O.L. Chromium(III) complexes based on phenoxy-imine ligands with pendant N- and O-donor groups as precatalysts for ethylene oligomerization: Synthesis, characterization, and DFT studies. J. Organomet. Chem. 2021, 936, 121710. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Chen, L.; Lan, T.; Wang, L. Preparation of chromium catalysts bearing bispyridylamine and its performance in ethylene oligomerization. Transit. Met. Chem. 2019, 44, 681–688. [Google Scholar] [CrossRef]
- Klemps, C.; Buchard, A.; Houdard, R.; Auffrant, A.; Mézailles, N.; Le Goff, X.F.; Ricard, L.; Saussine, L.; Magna, L.; Le Floch, P. Chromium (III)-bis(iminophosphoranyl)methanido complexes: Synthesis, X-ray crystal structures and catalytic ethylene oligomerization. N. J. Chem. 2009, 33, 1748–1752. [Google Scholar] [CrossRef]
- Milani, J.L.S.; Biajoli, A.F.P.; Batista, F.I.; Oliboni, R.S.; Casagrande, O.L. Chromium complexes supported by bidentate thioether-imine [N,S] ligands: Synthesis and ethylene oligomerization studies. N. J. Chem. 2021, 45, 1814–1821. [Google Scholar] [CrossRef]
- Grauke, R.; Schepper, R.; Rabeah, J.; Schoch, R.; Bentrup, U.; Bauer, M.; Brückner, A. Impact of Al Activators on Structure and Catalytic Performance of Cr Catalysts in Homogeneous Ethylene Oligomerization—A Multitechnique in situ/operando Study. ChemCatChem 2020, 12, 1025–1035. [Google Scholar] [CrossRef]
- Carney, M.J.; Robertson, N.J.; Halfen, J.A.; Zakharov, L.N.; Rheingold, A.L. Octahedral chromium(III) complexes supported by bis(2-pyridylmethyl)amines: Ligand influence on coordination geometry and ethylene polymerization activity. Organometallics 2004, 23, 6184–6190. [Google Scholar] [CrossRef]
- Lamb, M.J.; Apperley, D.C.; Watson, M.J.; Dyer, P.W. The Role of Catalyst Support, Diluent and Co-Catalyst in Chromium-Mediated Heterogeneous Ethylene Trimerisation. Top. Catal. 2018, 61, 213–224. [Google Scholar] [CrossRef]
- Fallahi, M.; Ahmadi, E.; Ramazani, A.; Mohamadnia, Z. Trimerization of ethylene catalyzed by Cr-based catalyst immobilized on the supported ionic liquid phase. J. Organomet. Chem. 2017, 848, 149–158. [Google Scholar] [CrossRef]
- Müller, T.; Dixon, J.T.; Haumann, M.; Wasserscheid, P. Trimerization and tetramerization of ethylene in continuous gas-phase reaction using a Cr-based supported liquid phase catalyst. React. Chem. Eng. 2018, 4, 131–140. [Google Scholar] [CrossRef]
- Gao, X.; Wang, M.; Chen, Y.; Sun, Y.; Dong, B.; Jiang, T. Biphasic trimerization of ethylene with diphosphinoamine/chromium(III)/methylaluminoxane immobilized in organochloroaluminate ionic liquid. React. Kinet. Mech. Catal. 2014, 113, 159–167. [Google Scholar] [CrossRef]
- Goetjen, T.A.; Zhang, X.; Liu, J.; Hupp, J.T.; Farha, O.K. Metal–Organic Framework Supported Single Site Chromium(III) Catalyst for Ethylene Oligomerization at Low Pressure and Temperature. ACS Sustain. Chem. Eng. 2019, 7, 2553–2557. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zhang, Y.; Han, Y.; Feng, G.L.; Gao, F.; Wang, H.; Qiu, P. Selective Ethylene Oligomerization with Chromium-Based Metal Organic Framework MIL-100 Evacuated under Different Temperatures. Organometallics 2017, 36, 632–638. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Luz, I.; Ortuno, M.A.; Oregui-Bengoechea, M.; Gandarias, I.; Lopez, N.; Lail, M.A.; Soukri, M. Gas reactions under intrapore condensation regime within tailored metal-organic framework catalysts. Nat. Commun. 2019, 10, 2076. [Google Scholar] [CrossRef]
- LiBretto, N.J.; Xu, Y.; Quigley, A.; Edwards, E.; Nargund, R.; Vega-Vila, J.C.; Caulkins, R.; Saxena, A.; Gounder, R.; Greeley, J.; et al. Olefin oligomerization by main group Ga3+ and Zn2+ single site catalysts on SiO2. Nat. Commun. 2021, 12, 2322. [Google Scholar] [CrossRef]
- Keim, W. Nickel—An element with application in industrial homogeneous catalysis. Angew. Chem.-Int. Ed. Engl. 1990, 29, 235–244. [Google Scholar] [CrossRef]
- Trofymchuk, O.S.; Gutsulyak, D.V.; Quintero, C.; Parvez, M.; Daniliuc, C.G.; Piers, W.E.; Rojas, R.S. N-Arylcyano-β-diketiminate Methallyl Nickel Complexes: Synthesis, Adduct Formation, and Reactivity toward Ethylene. Organometallics 2013, 32, 7323–7333. [Google Scholar] [CrossRef]
- Wang, S.D.; Zhang, W.J.; Du, S.Z.; Asuha, S.; Flisak, Z.; Sun, W.H. Propyl substituted 4-arylimino-1,2,3-trihydroacridylnickel complexes: Their synthesis, characterization and catalytic behavior toward ethylene. J. Organomet. Chem. 2015, 798, 408–413. [Google Scholar] [CrossRef]
- Fan, H.A.; Zhang, Y.; Alam, F.; Ma, J.; Hao, B.B.; Chen, Y.H.; Wang, Y.T.; Huang, J.M.; Jiang, T. Highly efficient PCCP/Cr(III) ethylene tetramerization catalyst designed using artificial neural network approach. J. Catal. 2024, 429, 115237. [Google Scholar] [CrossRef]
- Fan, H.N.; Hao, B.B.; Jiang, Y.; Yang, X.D.; Wang, Y.T.; Jiang, T. Selective ethylene tri-/tetramerization chromium catalysts based on binuclear PCCP ligands. Mol. Catal. 2023, 549, 113545. [Google Scholar] [CrossRef]
- Boelter, S.D.; Davies, D.R.; Milbrandt, K.A.; Wilson, D.R.; Wiltzius, M.; Rosen, M.S.; Klosin, J. Evaluation of Bis(phosphine) Ligands for Ethylene Oligomerization: Discovery of Alkyl Phosphines as Effective Ligands for Ethylene Tri- and Tetramerization. Organometallics 2020, 39, 967–975. [Google Scholar] [CrossRef]
- Ji, X.Y.; Song, L.B.; Zhang, C.Y.; Jiao, J.J.; Zhang, J. Highly active chromium-based selective ethylene tri-/tetramerization catalysts supported by N,N-diphospholylamines. Inorg. Chim. Acta 2017, 466, 117–121. [Google Scholar] [CrossRef]

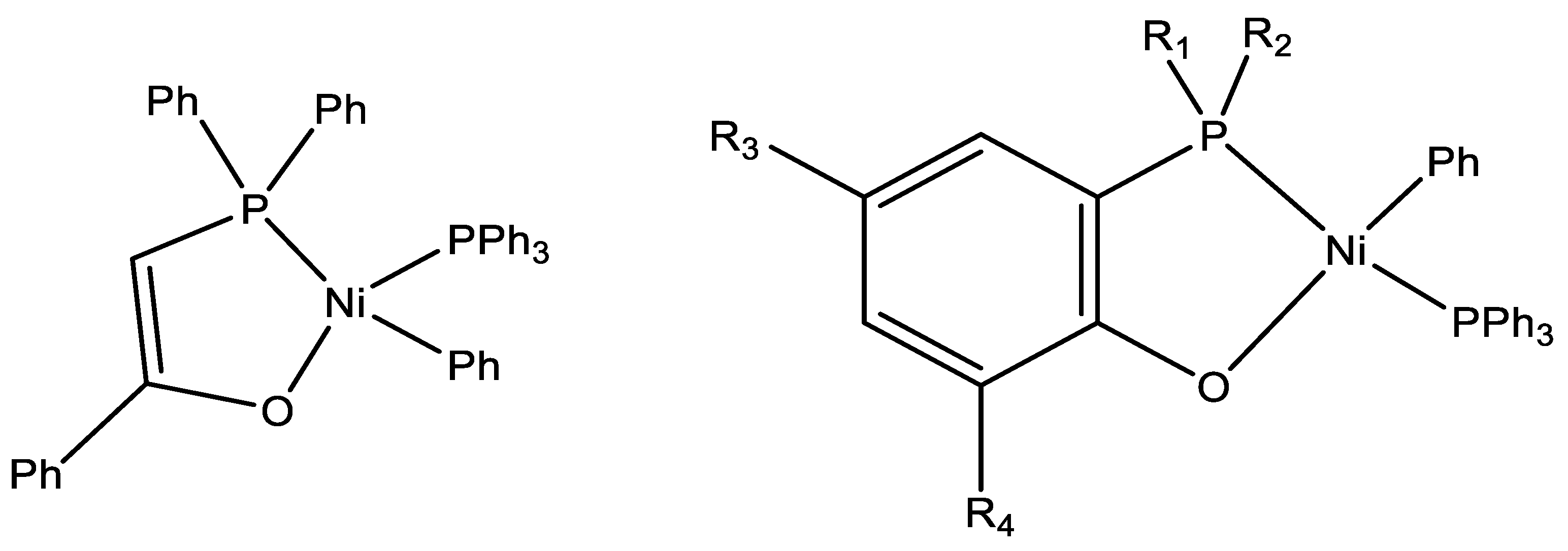
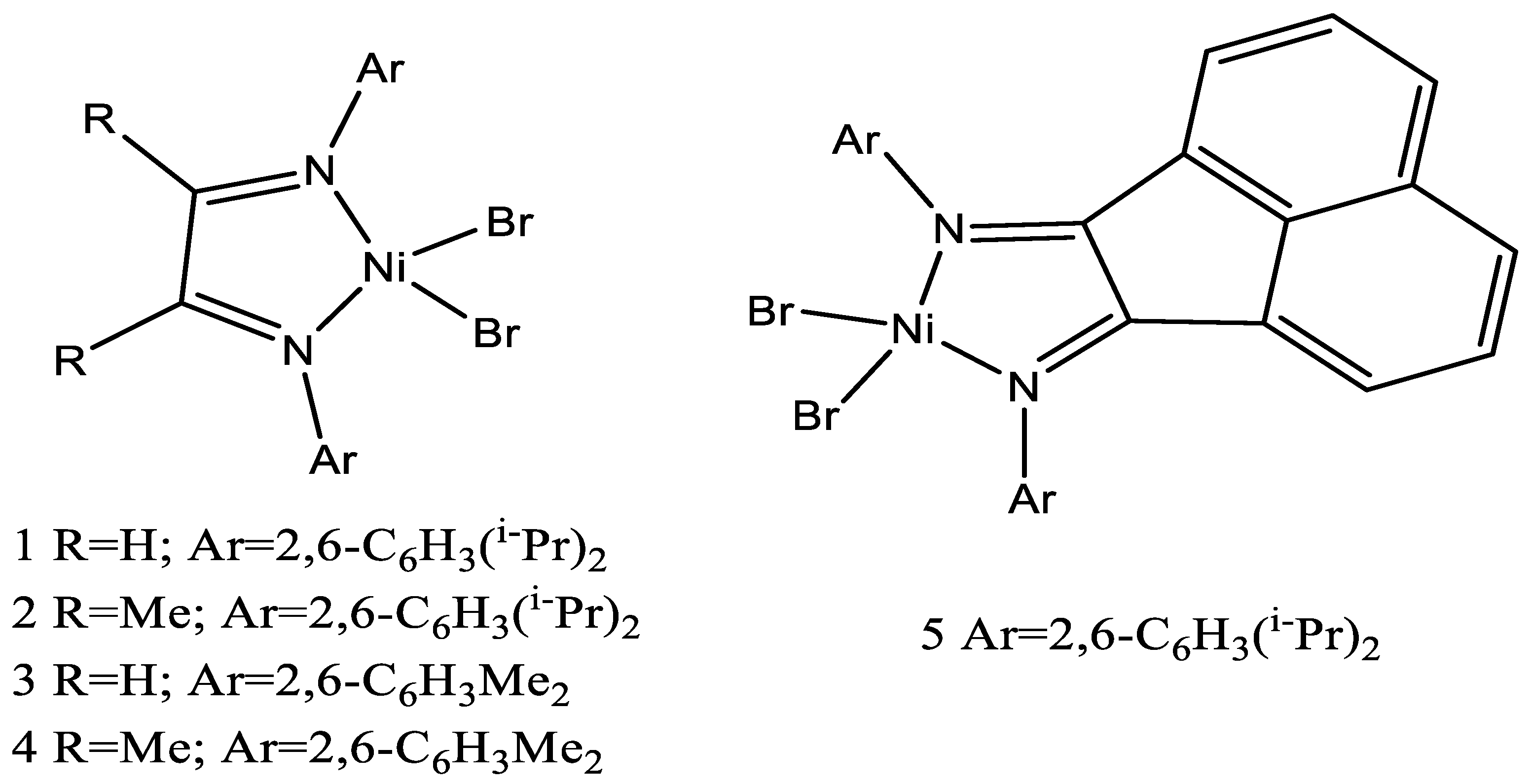
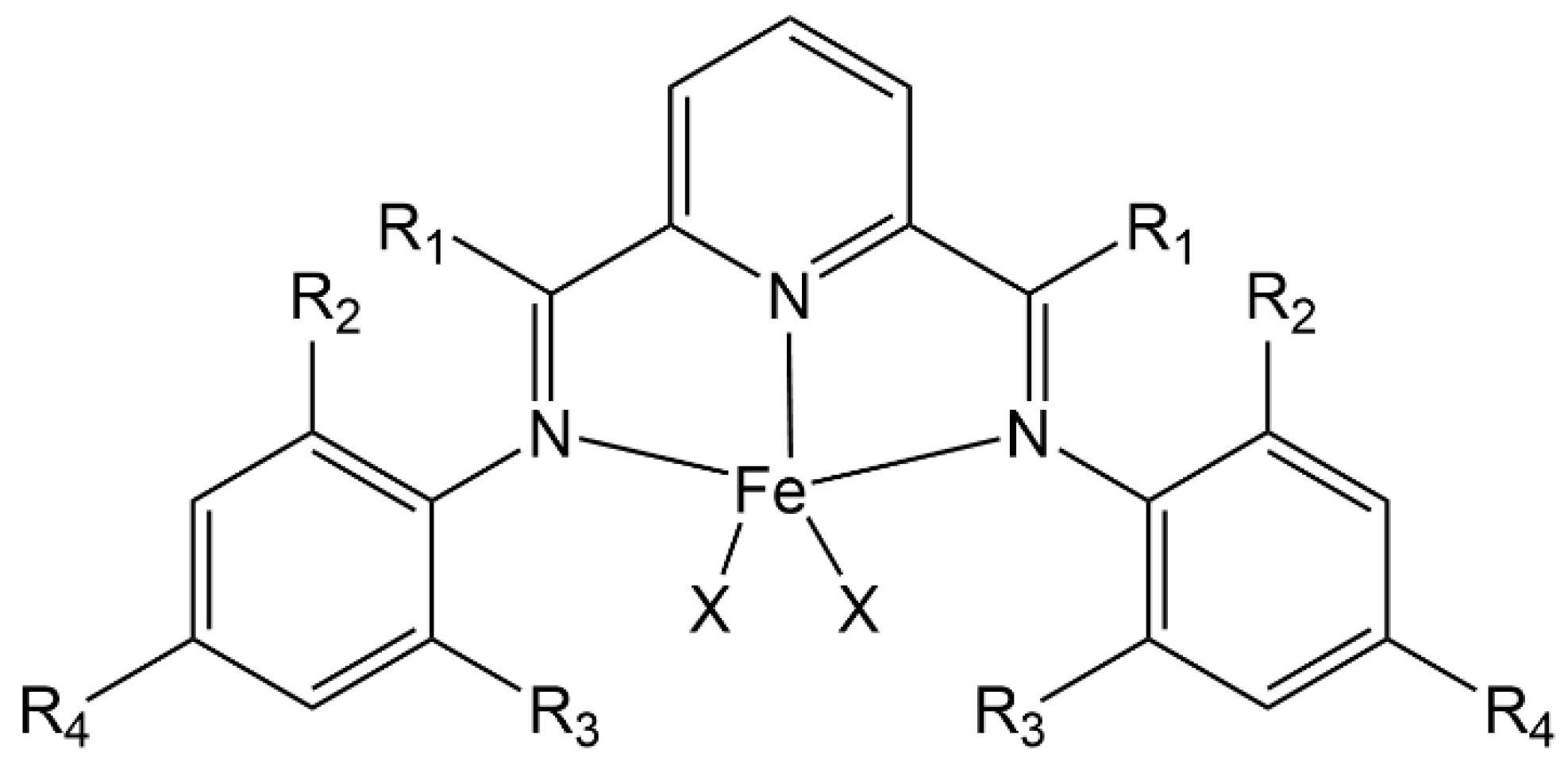
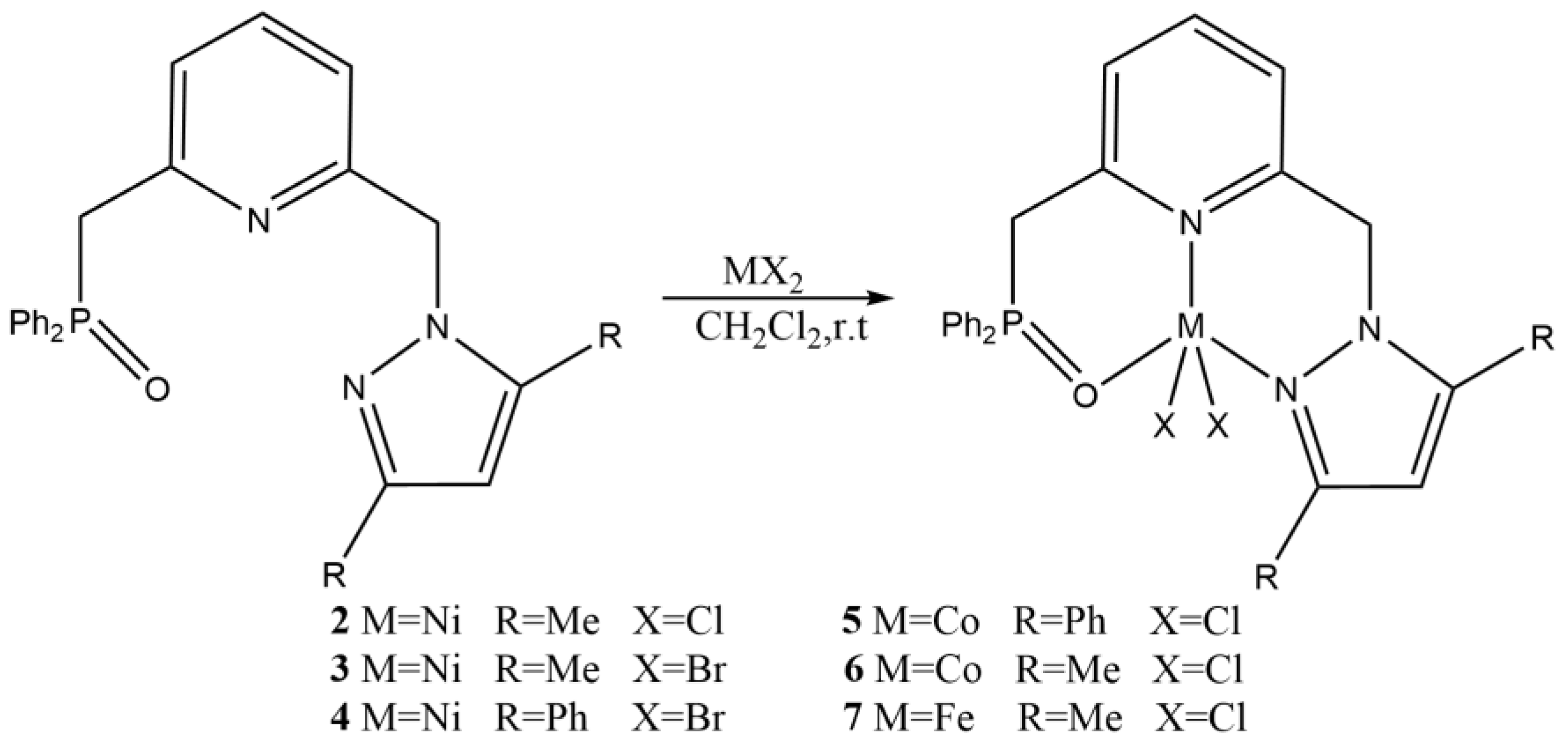
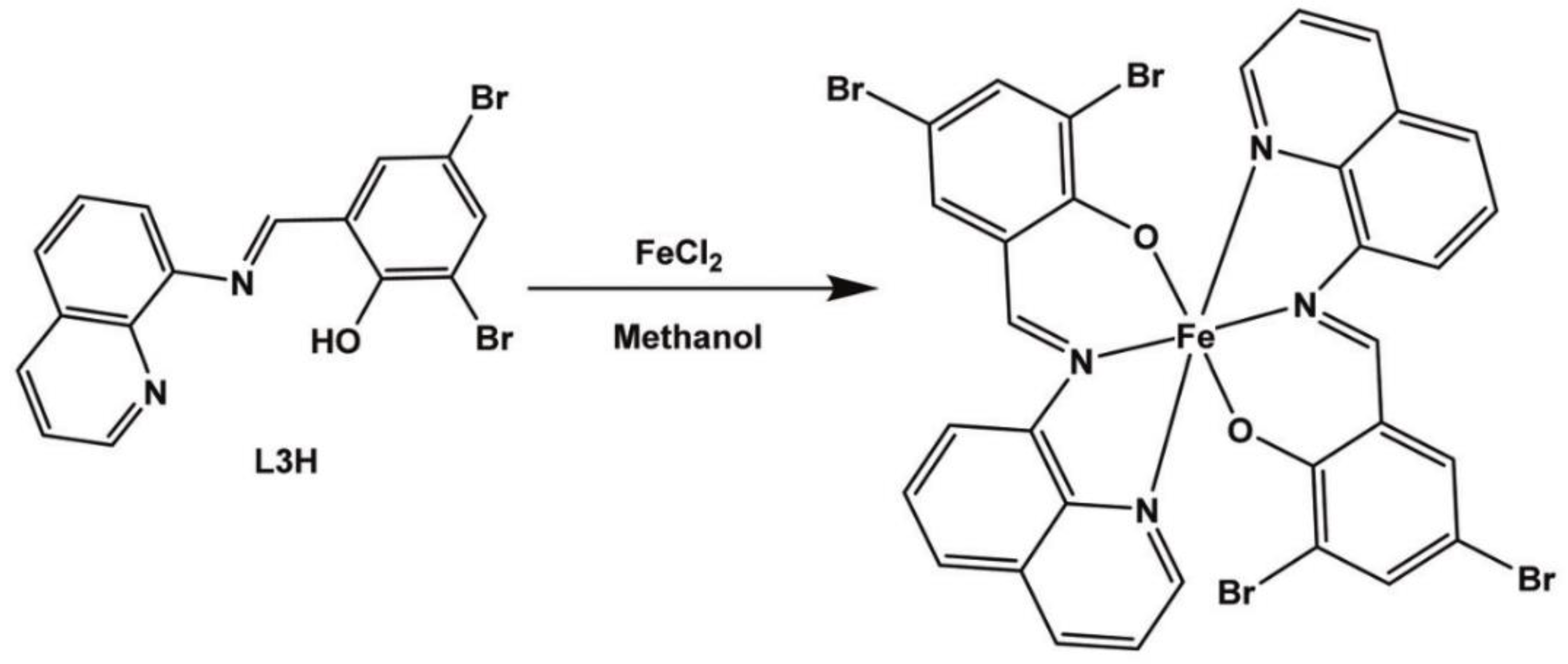
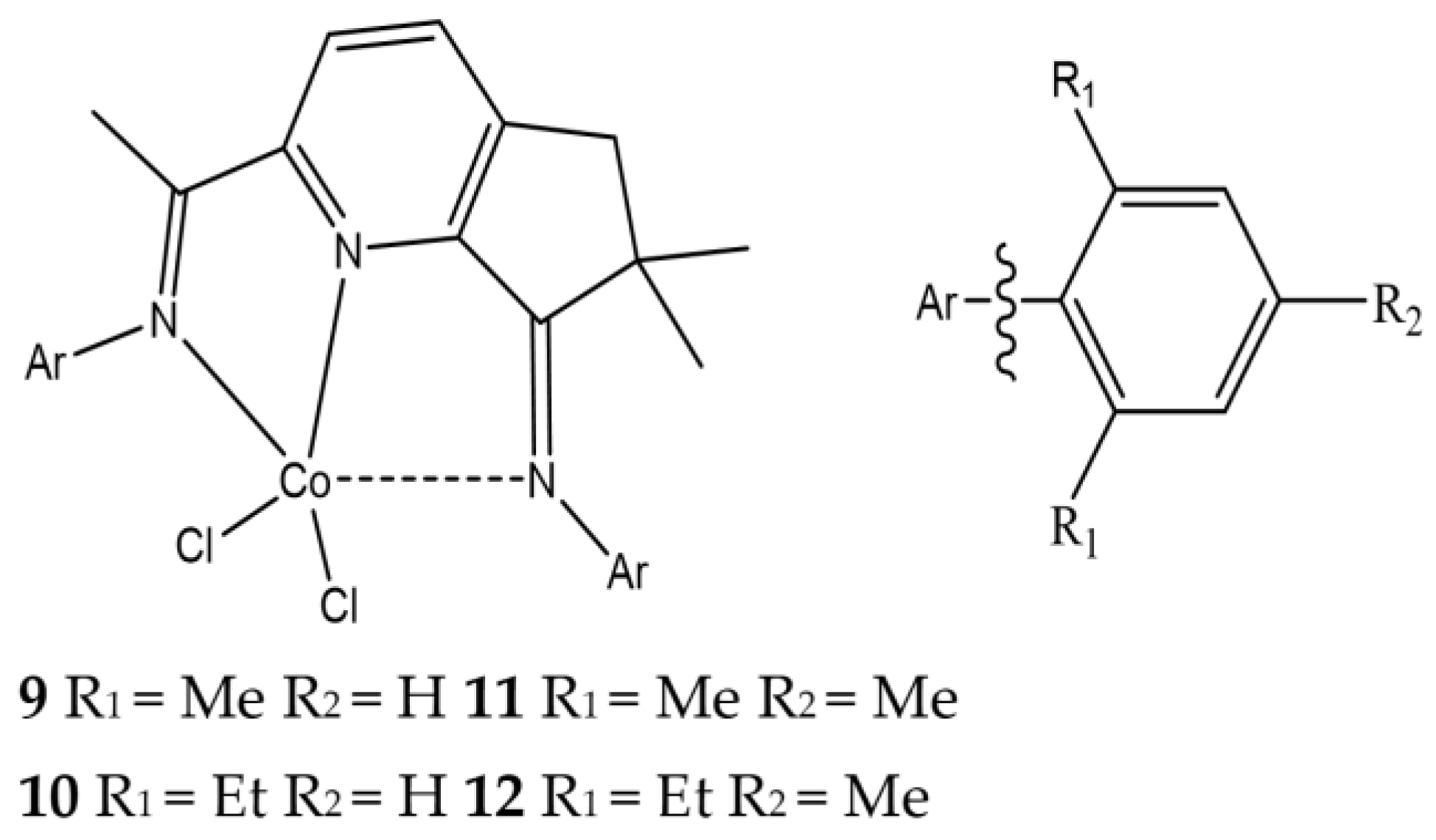
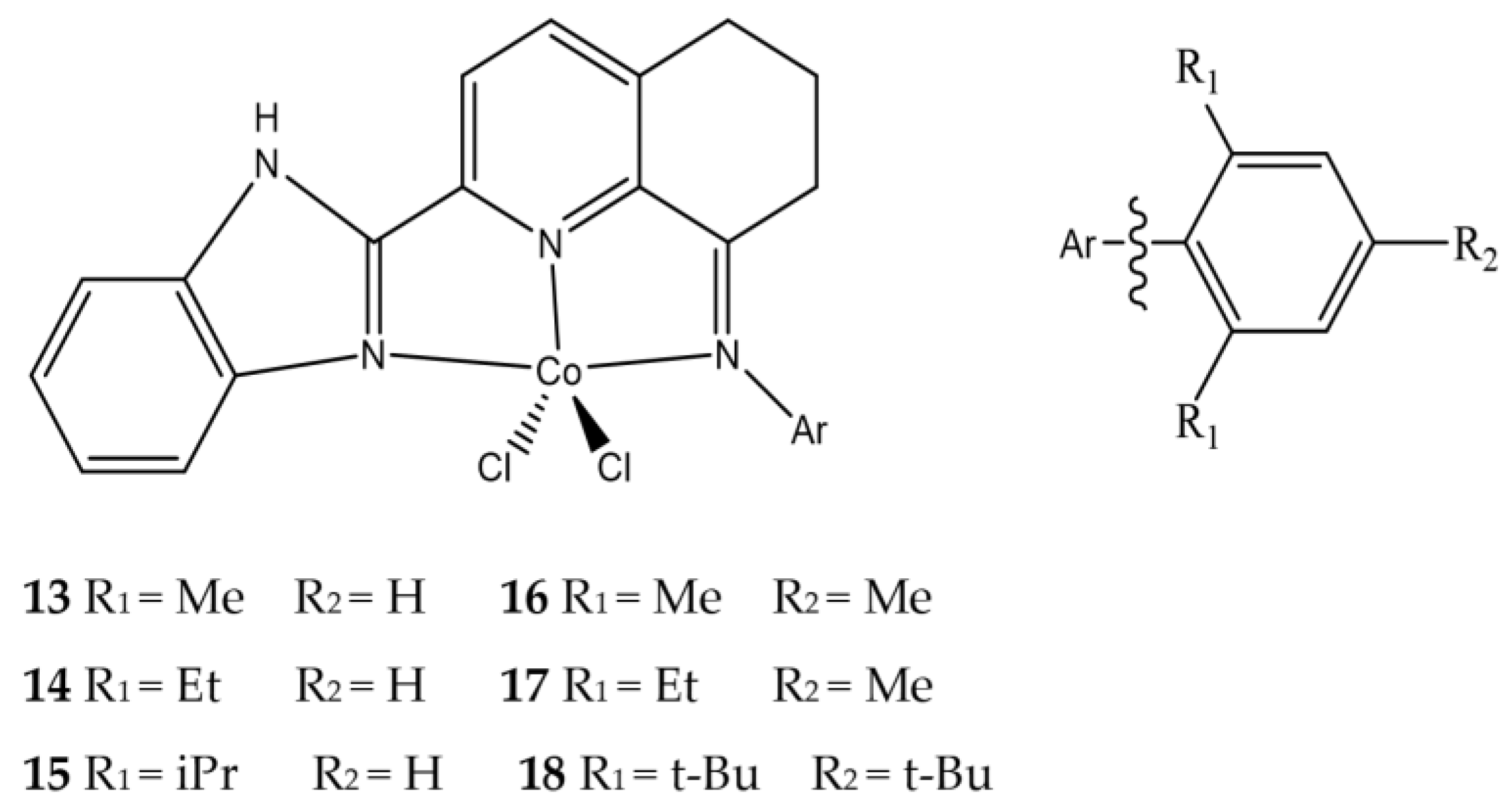
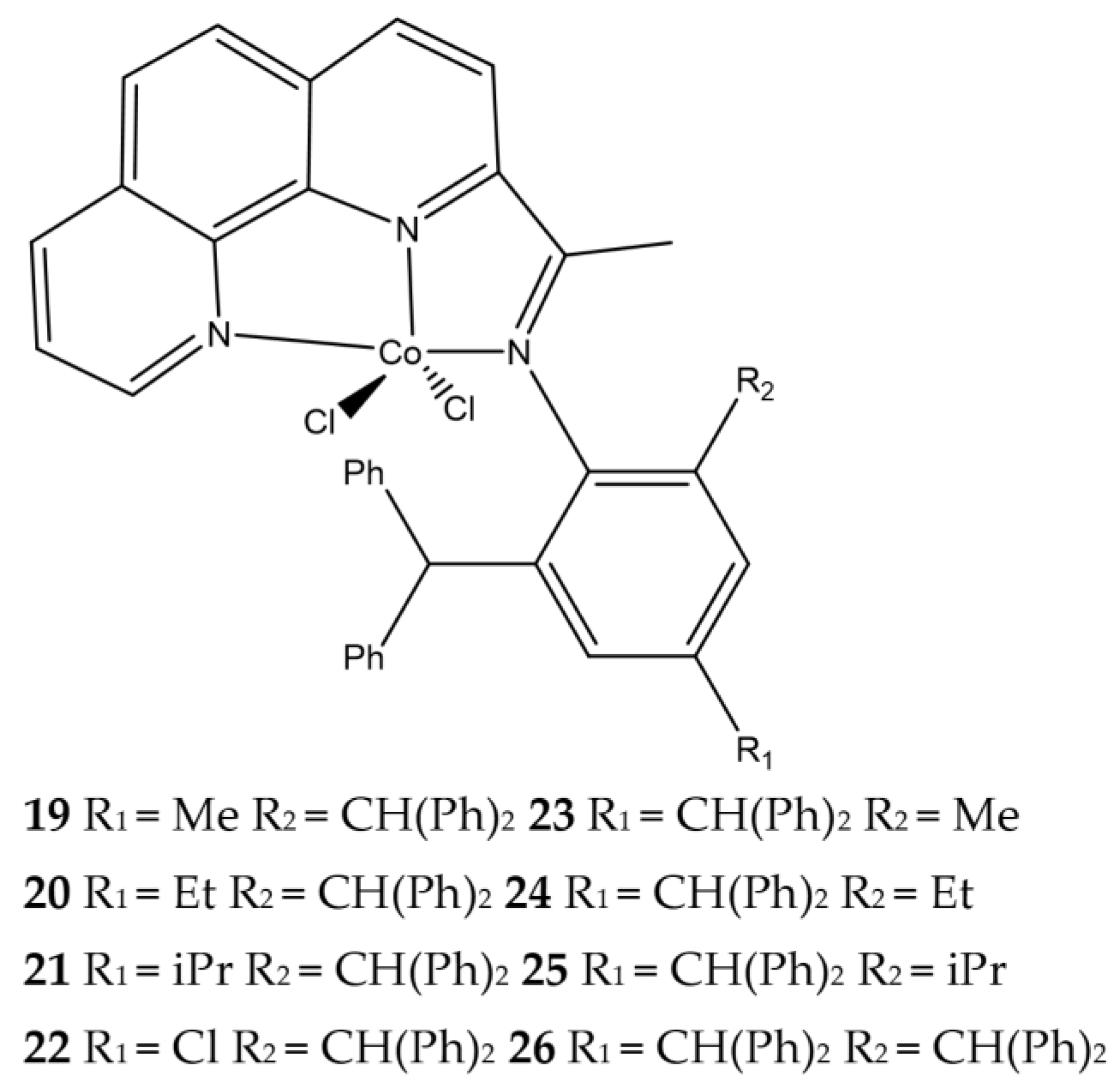
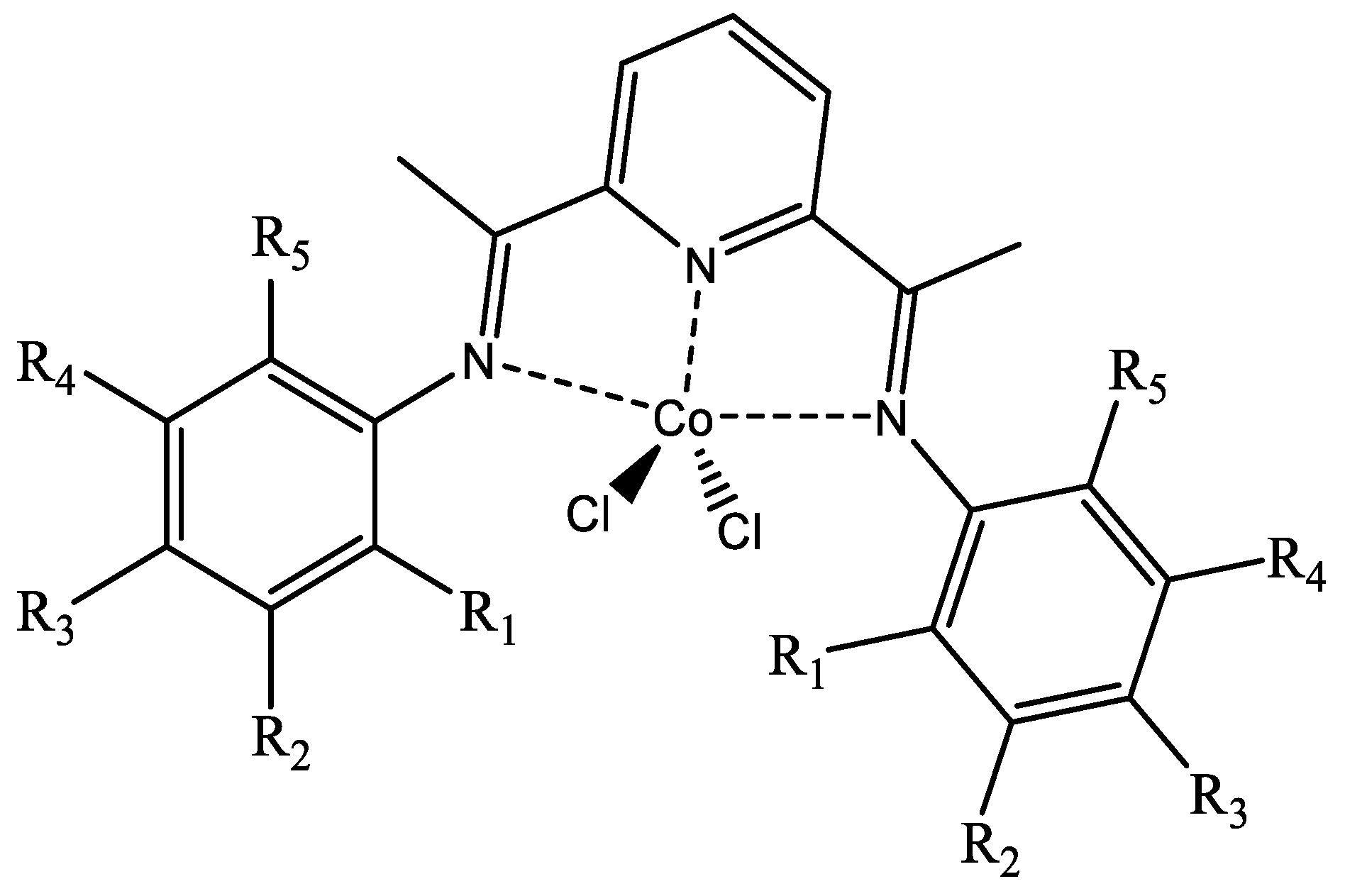

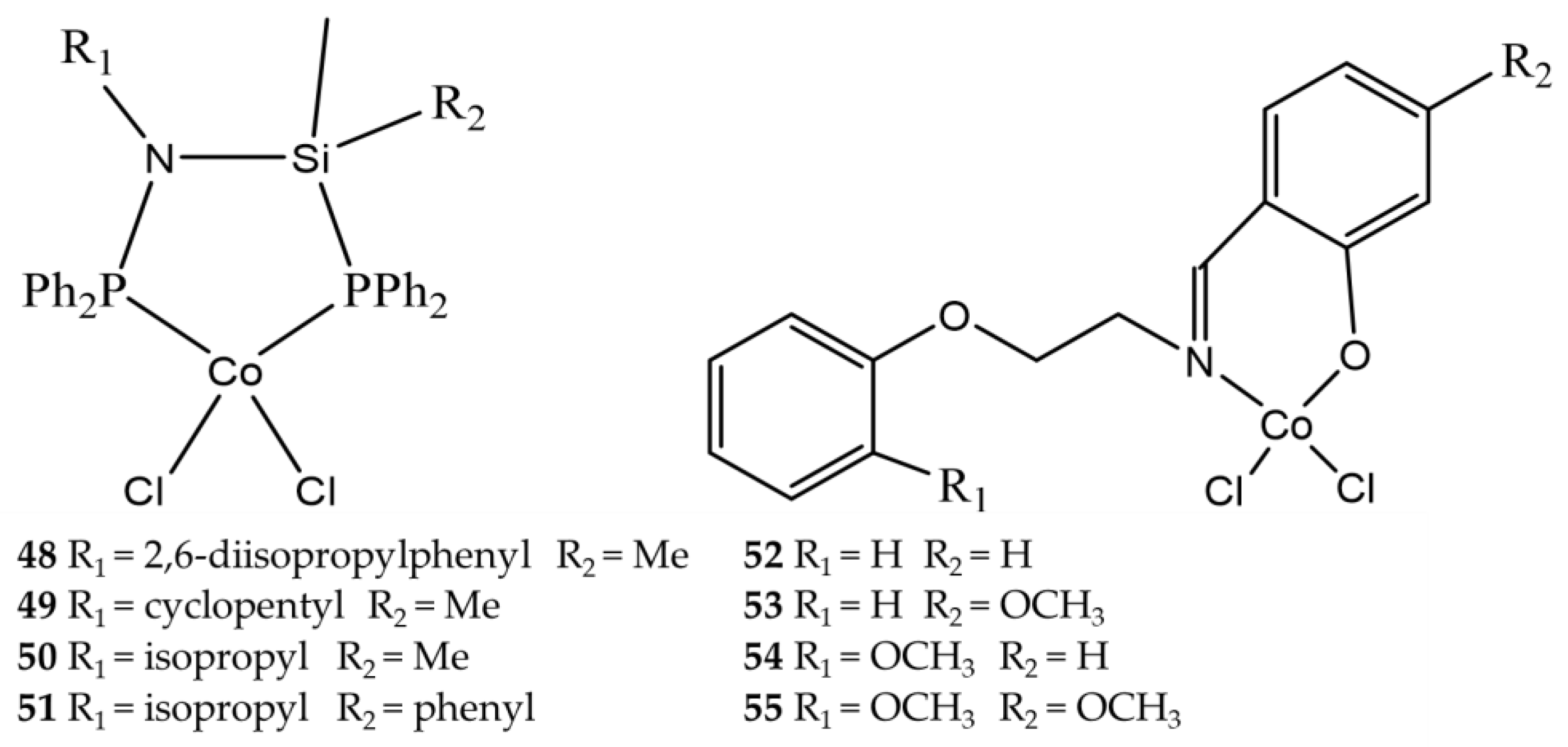

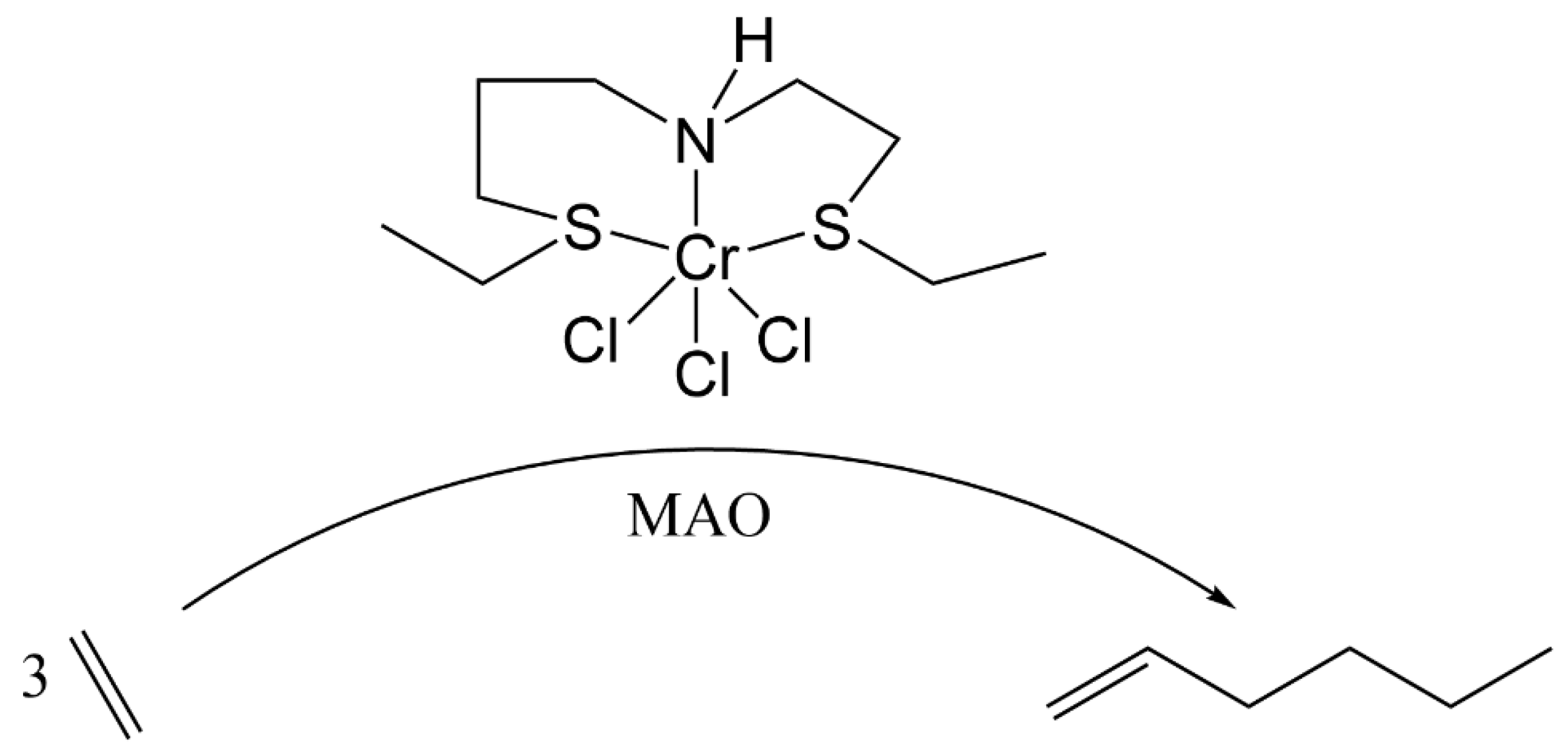
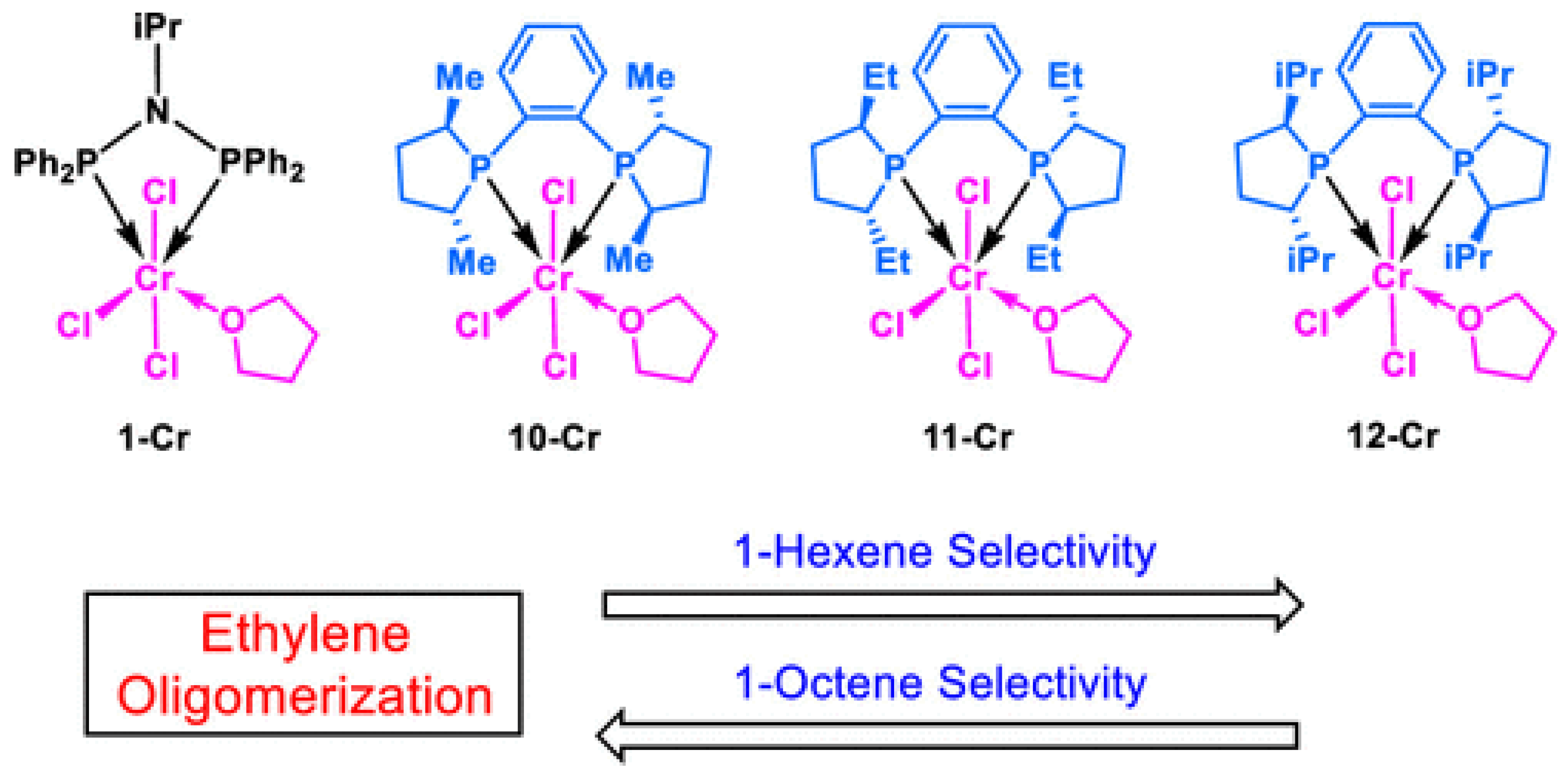
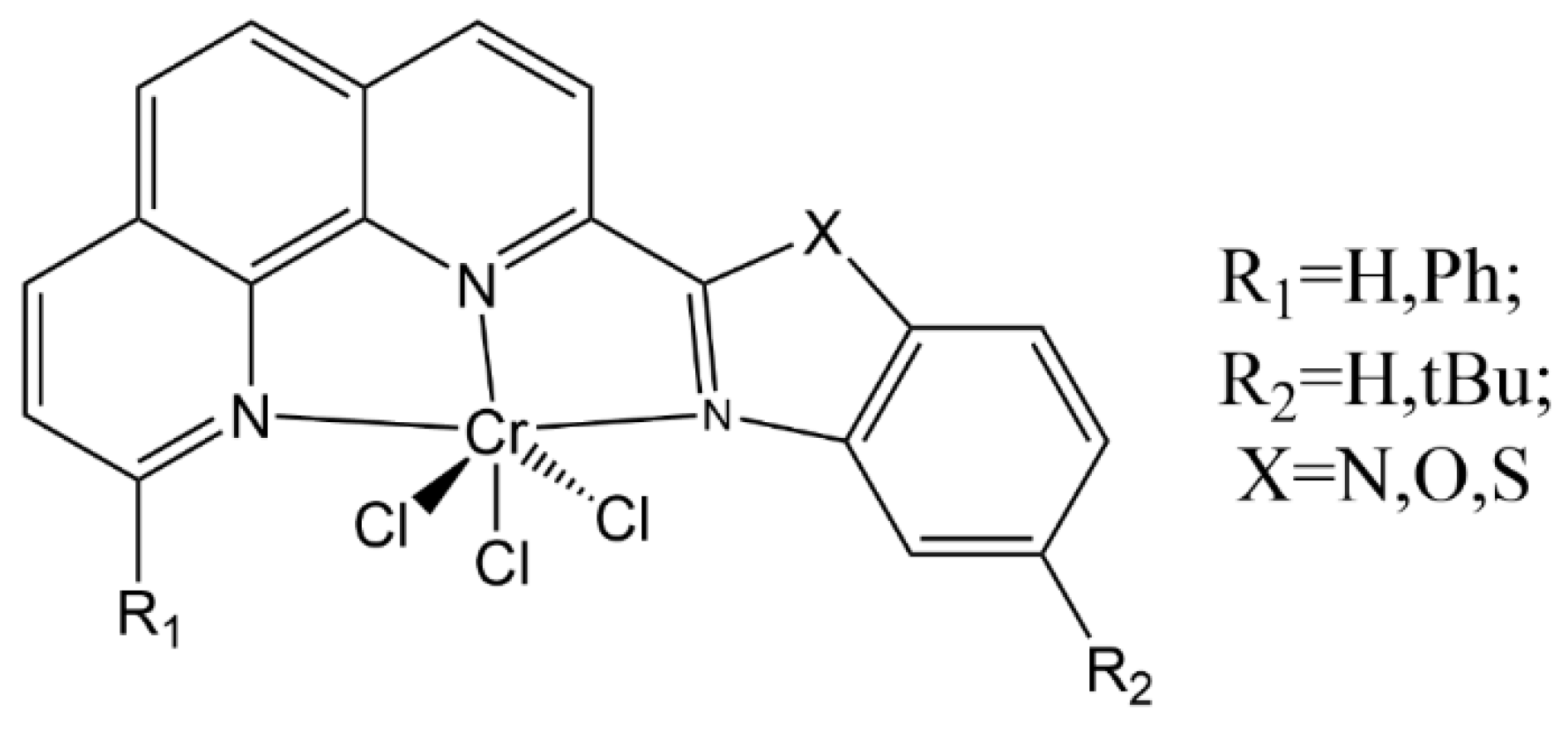
| Complex | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| 27 | H | H | F | H | H |
| 28 | H | H | Br | H | H |
| 29 | Cl | H | F | H | H |
| 30 | F | H | H | H | H |
| 31 | H | Cl | H | Cl | H |
| 32 | CF3 | H | H | H | H |
| 33 | H | F | H | F | H |
| 34 | CF3 | H | F | H | H |
| 35 | H | CF3 | F | H | H |
| 36 | F | H | F | H | F |
| 37 | Cl | H | H | H | Cl |
| 38 | H | Cl | H | H | H |
| 39 | Br | H | H | H | H |
| 40 | Me | H | H | H | Me |
| Catalyst | Pressure (atm) | Conversion(%) 1 | Selectivity (%) | ||||
|---|---|---|---|---|---|---|---|
| Butenes (C4=) | Hexenes (C6=) | Octenes (C8=) | C10+ | Rate (mol C4H8 molM−1 s−1) 2 | |||
| Ni/SiO2 | 1 | 3 | 86.1 | 11.8 | 0.4 | 0 | |
| Ga/SiO2 | 3 | 75.9 | 16.8 | 6.4 | 0 | ||
| Zn/SiO2 | 3 | 85.5 | 2.0 | 0.0 | 0 | ||
| Ni/SiO2 | 30.6 | 20.7 | 86.2 | 11.1 | 2.9 | 0 | |
| Ga/SiO2 | 20.6 | 74.2 | 16.1 | 4.9 | 4.2 | ||
| Zn/SiO2 | 15.2 | 96.0 | 0.8 | 0 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, A.; Huang, Z.; Li, G. Ethylene Oligomerization Catalyzed by Different Homogeneous or Heterogeneous Catalysts. Catalysts 2024, 14, 268. https://doi.org/10.3390/catal14040268
Peng A, Huang Z, Li G. Ethylene Oligomerization Catalyzed by Different Homogeneous or Heterogeneous Catalysts. Catalysts. 2024; 14(4):268. https://doi.org/10.3390/catal14040268
Chicago/Turabian StylePeng, Anfeng, Zheng Huang, and Gang Li. 2024. "Ethylene Oligomerization Catalyzed by Different Homogeneous or Heterogeneous Catalysts" Catalysts 14, no. 4: 268. https://doi.org/10.3390/catal14040268
APA StylePeng, A., Huang, Z., & Li, G. (2024). Ethylene Oligomerization Catalyzed by Different Homogeneous or Heterogeneous Catalysts. Catalysts, 14(4), 268. https://doi.org/10.3390/catal14040268









