Performance of Traditional and Emerging Water-Treatment Technologies in the Removal of Tetracycline Antibiotics
Abstract
1. Introduction
2. Harm of Tetracycline Antibiotic Residues in Water
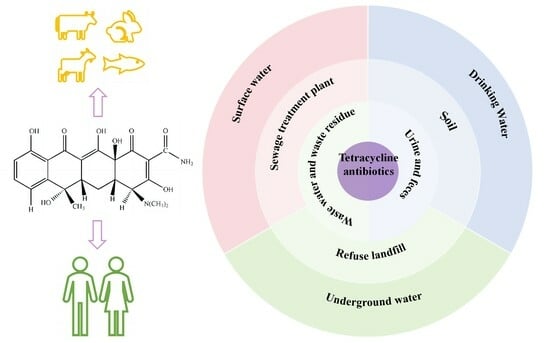
3. Pollution Status of Tetracycline Antibiotics in Water
4. Research Status of Treatment Technology Used for the Removal of Tetracycline Antibiotics from Water Environments
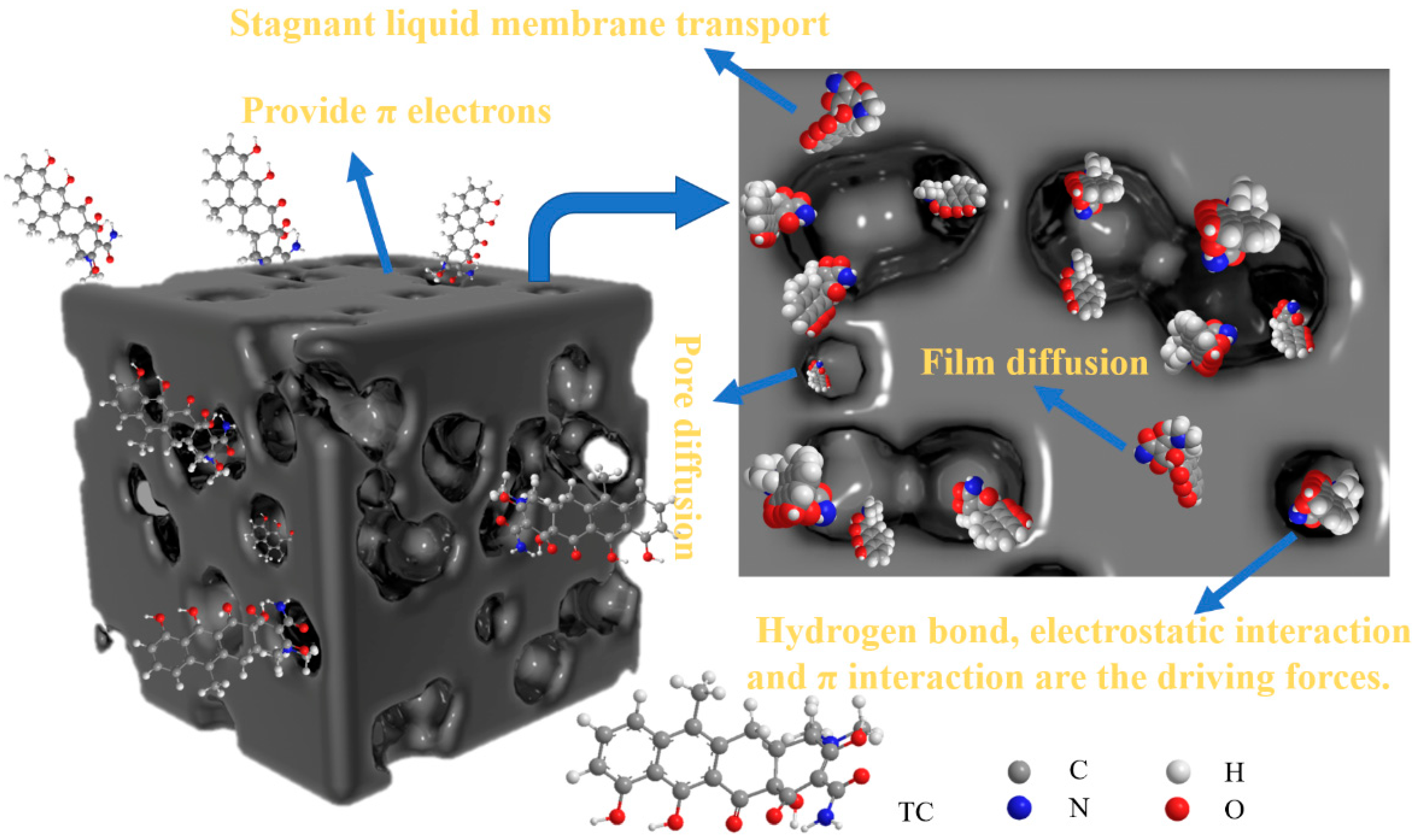
4.1. Adsorption Method
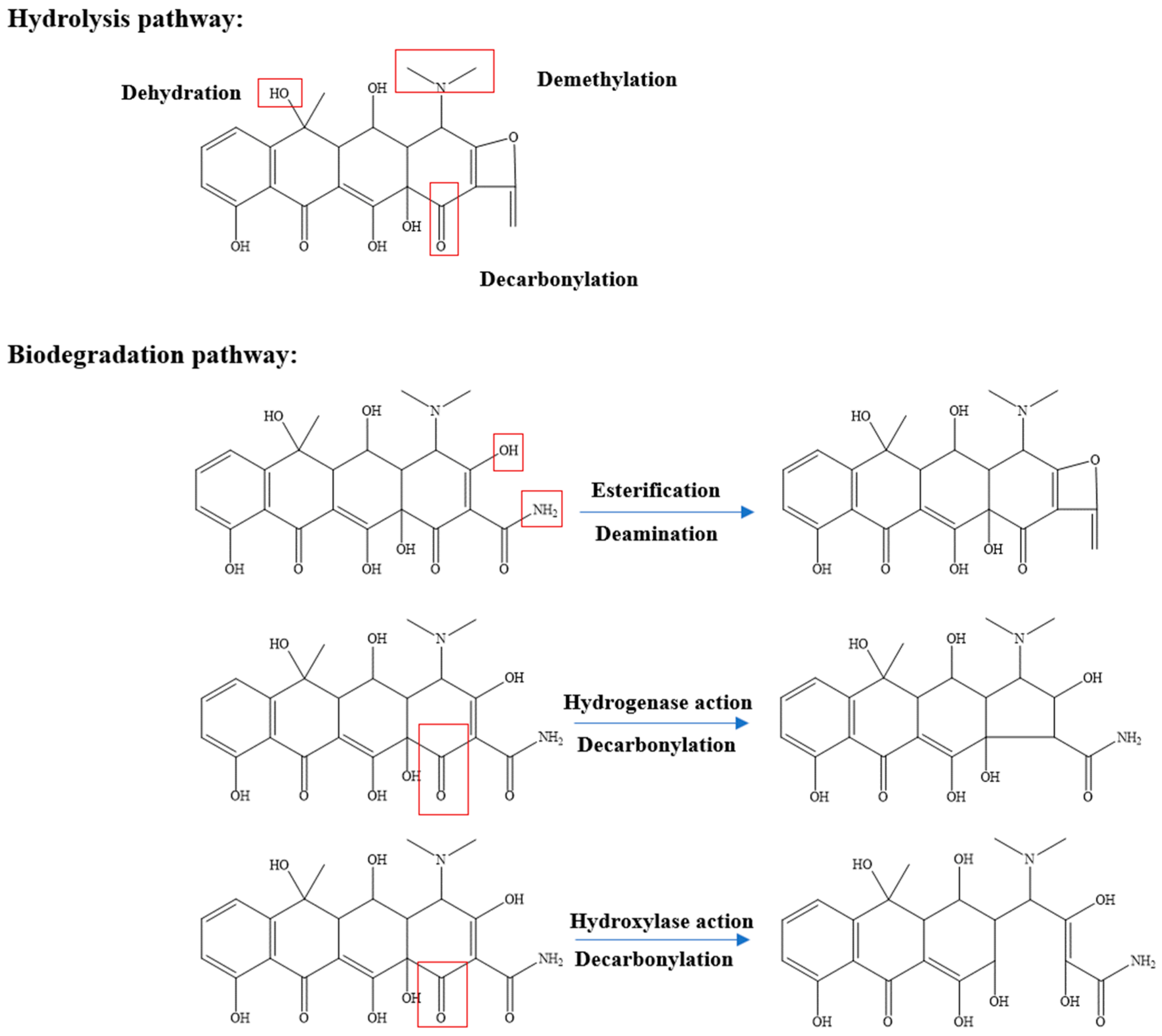
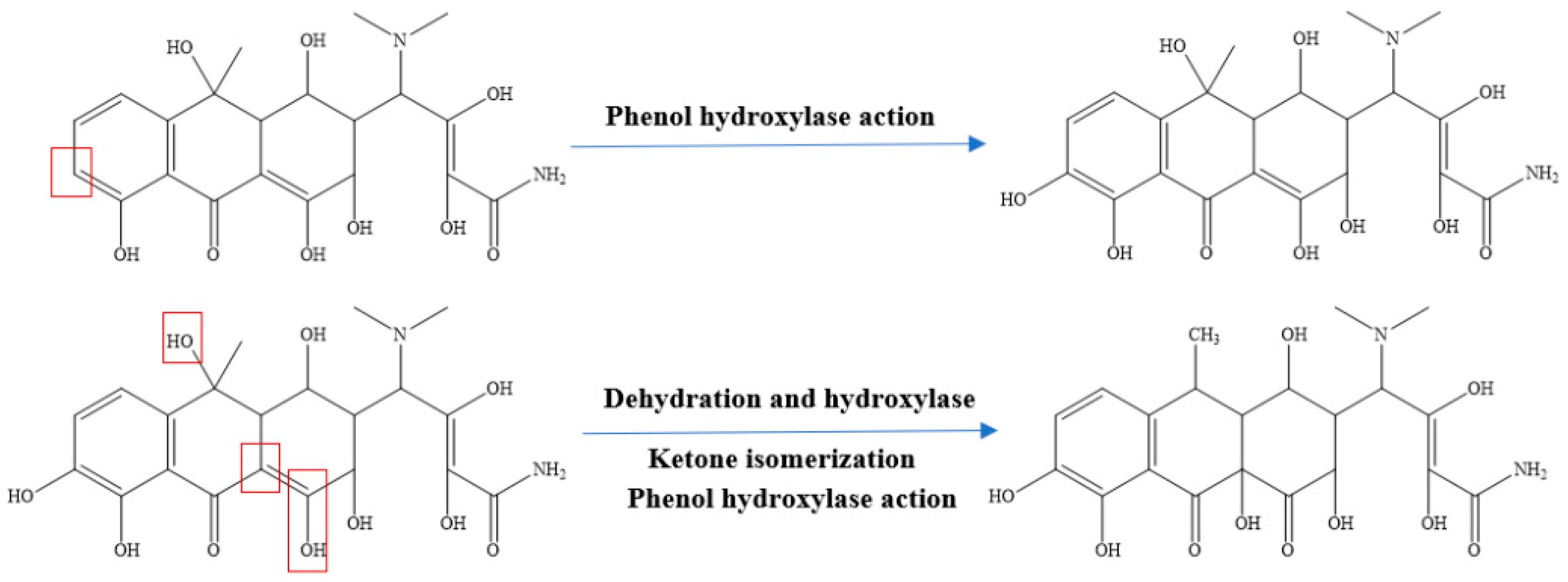
4.2. Biological Method
4.3. Physical and Chemical Method
4.4. Advanced Oxidation Processes
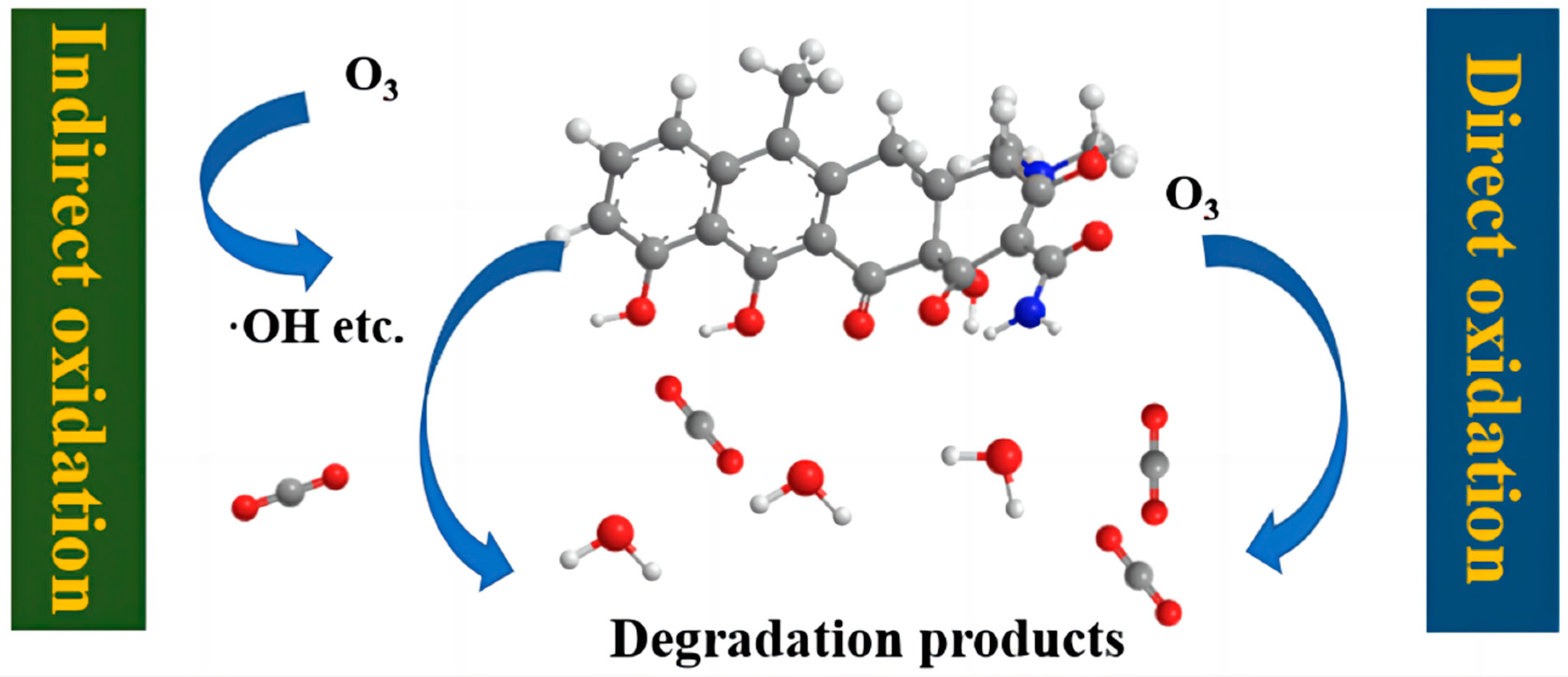
4.4.1. Ozone Oxidation Method
4.4.2. Fenton Oxidation Method
4.4.3. Photocatalytic Method
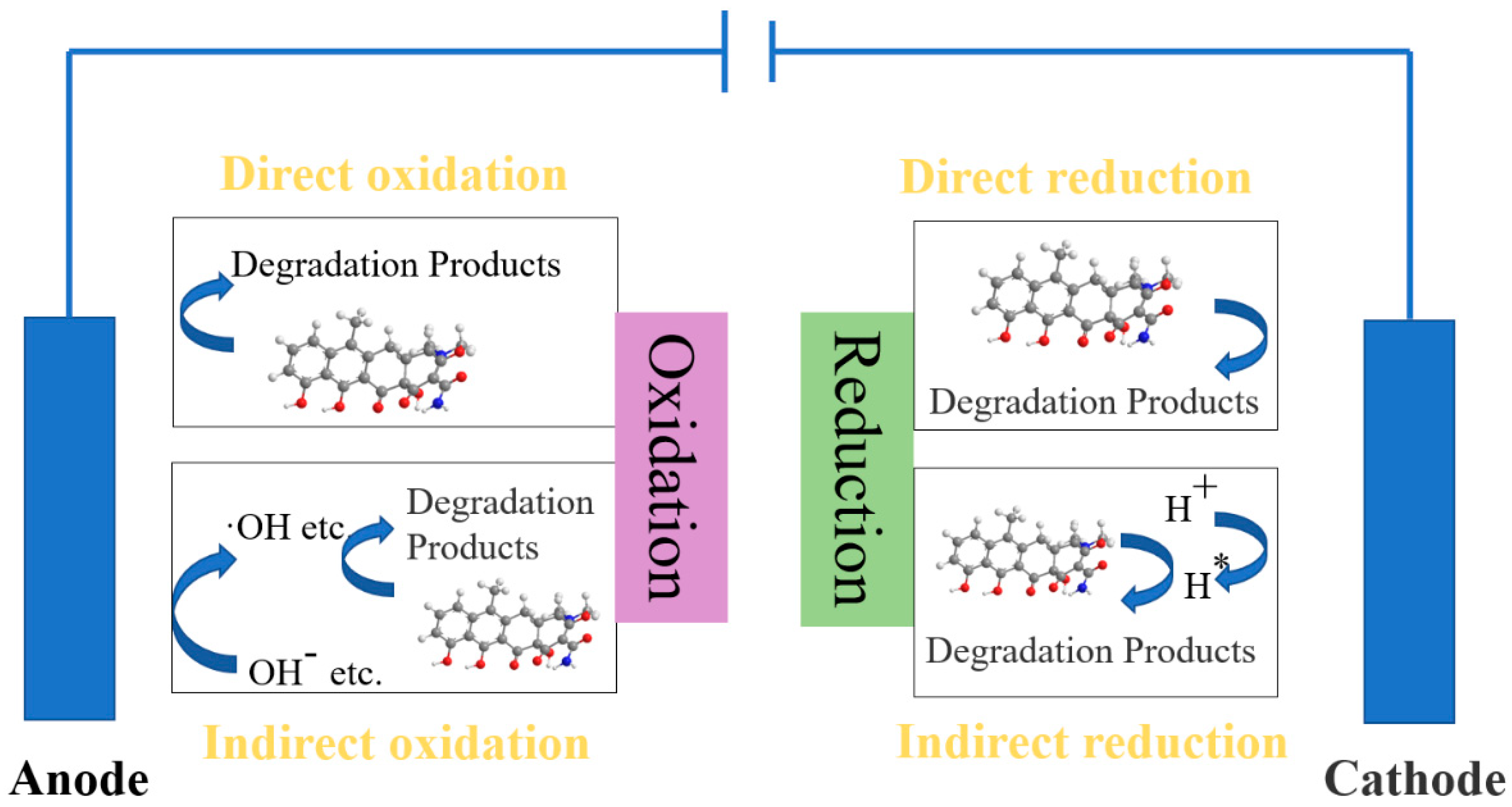
4.4.4. Electrocatalytic Method
4.5. Comprehensive Treatment Technology of Sewage Plant
5. Conclusions and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Research Progress on Distribution, Migration, Transformation of Antibiotics and Antibiotic Resistance Genes (ARGs) in Aquatic Environment. Crit. Rev. Biotechnol. 2018, 38, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Boeckel, T.V.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Jandira, L.; Yasmin, V.; Nicoly, W.; Siara, S.; Guilherme, L.D.; Elvis, C. A Review of the Occurrence, Disposal, Determination, Toxicity and Remediation Technologies of the Tetracycline Antibiotic. Process Saf. Environ. Prot. 2022, 160, 25–40. [Google Scholar]
- Haiyin, Z.; Qixing, Z. Research Progress on Treatment Technology of Tetracycline Antibiotics Pollution in the Environment. J. Environ. Eng. Technol. 2021, 11, 571–581. [Google Scholar]
- Chopra, I.; Roberts, M.C. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I. New Developments in Tetracycline Antibiotics: Glycylcyclines and Tetracycline Efflux Pump Inhibitors. Med. Resist. Updates 2002, 5, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.N. Antibiotics Traces in the Aquatic Environment: Persistence and Adverse Environmental Impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Xinyan, G.; Zheng, Y.; Yi, Z.; Weili, X.; Deyang, K.; Zhengjun, S.; Na, W. Behavior of Antibiotic Resistance Genes under Extremely High-Level Antibiotic Selection Pressures in Pharmaceutical Wastewater Treatment Plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar]
- Krakkó, D.; Heieren, B.T.; Illés, Á.; Kvamme, K.; Dóbé, S.; Záray, G. (V) UV Degradation of the Antibiotic Tetracycline: Kinetics, Transformation Products and Pathway. Process Saf. Environ. Prot. 2022, 163, 395–404. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, J.; Yang, C.; Hu, Z.; Liu, F.; Zhang, C. Removal of Tetracycline from Livestock Wastewater by Positive Single Pulse Current Electrocoagulation: Mechanism, Toxicity Assessment and Cost Evaluation. Sci. Total Environ. 2022, 810, 151955. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhang, X.L.; Li, W.; Lu, X.F.; Liu, B.; Wang, J. The Residues and Environmental Risks of Multiple Veterinary Antibiotics in Animal Faeces. Environ. Monit. Assess. 2013, 185, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, R.H.; Björklund, K.; Rendahl, P.; Johansson, M.I.; Tysklind, M.; Andersson, B.V. Environmental Risk Assessment of Antibiotics in the Swedish Environment with Emphasis on Sewage Treatment Plants. Water Res. 2007, 41, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and Fate of Antibiotics, Antibiotic Resistant Genes (ARGs) and Antibiotic Resistant Bacteria (ARB) in Municipal Wastewater Treatment Plant: An Overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef] [PubMed]
- Beyene, T. Veterinary Medicines Residues in Food-Animal Products: Its Risk Factors and Potential Effects on Public Health. J. Vet. Sci. Technol. 2016, 7, 285. [Google Scholar]
- Klimek, L.; Aderhold, C.; Sperl, A.; Trautmann, A. Allergic Reactions to Antibiotics—Two Sides of the Same Coin: Clearly Diagnose or Reliably Rule Out. Allergo J. Int. 2017, 26, 212–218. [Google Scholar] [CrossRef]
- Tadesse, T. Public Health Impacts of Antibiotic Residues in Foods of Animal Origin: A Review. Public Policy Adm. Res. 2017, 7, 6–11. [Google Scholar]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and Distribution of Antibiotics in Coastal Water of the Bohai Bay, China: Impacts of River Discharge and Aquaculture Activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Raheleh, K.; Fatemeh, P.; Mohammad, S.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.; Asgari, G.; Ramavandi, B. Occurrence, Distribution, and Potential Sources of Antibiotics Pollution in the Water-Sediment of the Northern Coastline of the Persian Gulf, Iran. Sci. Total Environ. 2018, 627, 703–712. [Google Scholar]
- Spongberg, A.L.; Witter, J.D.; Acuña, J.; Vargas, J.; Murillo, M.; Umaña, G.; Gómez, E.; Perez, G. Reconnaissance of Selected PPCP Compounds in Costa Rican Surface Waters. Water Res. 2011, 45, 6709–6717. [Google Scholar] [CrossRef]
- Li, J.L.; Wang, M.; Hu, F.W.; Dong, Y.H.; Sun, Z.X.; Wang, Y. Antibiotic Pollution Characteristics and Ecological Risk Assessment in Jinjiang River Basin, Jiangxi Province. Environ. Sci. 2022, 43, 4064–4073. [Google Scholar]
- Li, H.; Chen, Y.; Feng, M.J.; Wang, B.; Bu, Y.Q.; Zhang, S.H. Pollution characteristics and risk assessment of antibiotics in Nanjing drinking water sources. Acta Sci. Circumstantiae 2020, 40, 1269–1277. [Google Scholar]
- Yin, C.N.; Xie, P.; Jiao, M.; Guo, B.Y.; Yu, C.H. Distribution characteristics of 54 kinds of PPCPs in surface water in Tianjin. Environ. Chem. 2021, 40, 2820–2831. [Google Scholar]
- Lei, J.; Hu, X.; Yin, D.; Zhang, H.; Yu, Z. Occurrence, Distribution and Seasonal Variation of Antibiotics in the Huangpu River, Shanghai, China. Chemosphere 2011, 82, 822–828. [Google Scholar]
- Ma, J.S.; Wang, Z.; Zhang, Z.Y.; Liu, Q.; Li, L.J. Distribution Characteristics of 29 Antibiotics in Groundwater in Harbin. Rock Miner. Anal. 2021, 40, 944–953. [Google Scholar]
- Zhou, P.P. Investigation of Pollution and Health Risk Assessment on Tetracyclines in Drinking Water and Animal Derived Food of Anhui. Master’s Thesis, Anhui Medical University, Hefei, China, 2019. [Google Scholar]
- Turan, B.; Sarigol, G.; Demircivi, P. Adsorption of Tetracycline Antibiotics Using Metal and Clay Embedded Cross-Linked Chitosan. Mater. Chem. Phys. 2022, 279, 125781. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, Y.; Huo, Y.; Zhao, C.; Sun, L.; Han, B.; Cao, X.; Wang, X. Insights into the Adsorption Mechanism and Dynamic Behavior of Tetracycline Antibiotics on Reduced Graphene Oxide (RGO) and Graphene Oxide (GO) Materials. Environ. Sci. Nano 2019, 6, 3336–3348. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Z.; Yu, Q.; Cheng, Y.; Liu, Z.; Zhang, T.; Zhou, S. Removal of Tetracycline from an Aqueous Solution Using Manganese Dioxide Modified Biochar Derived from Chinese Herbal Medicines Residues. Environ. Res. Lett. 2020, 183, 109195. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Yoo, S.; Choi, Y.-K.; Park, S.; Kan, E. Adsorption Isotherm, Kinetic Modeling and Mechanism of Tetracycline on Pinus Taeda-Derived Activated Biochar. Bioresour. Technol. 2018, 259, 24–31. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.; Berendsen, H.C. Gromacs: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Rostamian, R.; Amiri, N.; Behnejad, H. How Does Graphene Nanosheet Affect the Pharmaceutical Adsorption? A Comprehensive Insight from Molecular Dynamics Simulation, Quantum Mechanics and Experimental Study. J. Mol. Liq. 2018, 269, 29–37. [Google Scholar] [CrossRef]
- Ninwiwek, N.; Hongsawat, P.; Punyapalakul, P.; Prarat, P. Removal of the Antibiotic Sulfamethoxazole from Environmental Water by Mesoporous Silica-Magnetic Graphene Oxide Nanocomposite Technology: Adsorption Characteristics, Coadsorption and Uptake Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123716. [Google Scholar] [CrossRef]
- Prarat, P.; Hongsawat, P.; Punyapalakul, P. Amino-Functionalized Mesoporous Silica-Magnetic Graphene Oxide Nanocomposites as Water-Dispersible Adsorbents for the Removal of the Oxytetracycline Antibiotic from Aqueous Solutions: Adsorption Performance, Effects of Coexisting Ions, and Natural Organic Matter. Environ. Sci. Pollut. Res. 2020, 27, 6560–6576. [Google Scholar]
- Yu, K.; Sun, C.; Zhang, B.; Hassan, M.; He, Y. Size-Dependent Adsorption of Antibiotics onto Nanoparticles in a Field-Scale Wastewater Treatment Plant. Environ. Pollut. 2019, 248, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, C.; Yang, L.; Cui, J.; Hao, Q.; Sun, D. Handy Purifier Based on Bacterial Cellulose and Ca-Montmorillonite Composites for Efficient Removal of Dyes and Antibiotics. Carbohydr. Polym. 2019, 222, 115017. [Google Scholar] [CrossRef]
- Yu, L.-L.; Cao, W.; Wu, S.-C.; Yang, C.; Cheng, J.-H. Removal of Tetracycline from Aqueous Solution by MOF/Graphite Oxide Pellets: Preparation, Characteristic, Adsorption Performance and Mechanism. Ecotoxicol. Environ. Saf. 2018, 164, 289–296. [Google Scholar] [CrossRef]
- Huang, B.; Liu, Y.; Li, B.; Liu, S.; Zeng, G.; Zeng, Z.; Wang, X.; Ning, Q.; Zheng, B.; Yang, C. Effect of Cu (Ⅱ) Ions on the Enhancement of Tetracycline Adsorption by Fe3O4@ SiO2-Chitosan/Graphene Oxide Nanocomposite. Carbohydr. Polym. 2017, 157, 576–585. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, X.; Liao, P.; Zhang, C.; Liu, C. Enhanced Sequestration of Tetracycline by Mn(Ⅱ) Encapsulated Mesoporous Silica Nanoparticles: Synergistic Sorption and Mechanism. Chemosphere 2021, 284, 131334. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, H.; Dai, H.; Wan, X.; Zhu, F.; Xu, Q.; Ji, W. Efficient, rapid and simple adsorption method by polydopamine polystyrene nanofibers mat for removal of multi-class antibiotic residues in environmental water. Chemosphere 2022, 288, 132616. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pan, X.; Ben, W.; Wang, J.; Hou, P.; Qiang, Z. Adsorptive Removal of Antibiotics from Water Using Magneticion Exchange Resin. J. Environ. Sci. 2017, 52, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wu, X. Microbial Degradation of Tetracycline in the Aquatic Environment: A Review. Crit. Rev. Biotechnol. 2020, 40, 1010–1018. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.; Nidheesh, P. Tetracyclines in the Environment: An Overview on the Occurrence, Fate, Toxicity, Detection, Removal Methods, and Sludge Management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Liu, C.-X.; Xu, Q.-M.; Yu, S.-C.; Cheng, J.-S.; Yuan, Y.-J. Bio-Removal of Tetracycline Antibiotics under the Consortium with Probiotics Bacillus Clausii T and Bacillus Amyloliquefaciens Producing Biosurfactants. Sci. Total Environ. 2020, 710, 136329. [Google Scholar] [CrossRef]
- De Cazes, M.; Belleville, M.P.; Petit, E.; Llorca, M.; Rodríguez-Mozaz, S.; De Gunzburg, J. Design and Optimization of an Enzymatic Membrane Reactor for Tetracycline Degradation. Catal. Today 2014, 236, 146–152. [Google Scholar] [CrossRef]
- Zhang, X.; Yajuan, W.; Hong, T.; Zhang, R.; Ma, Z. Screened and Degradation Characteristics of a Four Tetracycline Antibiotics Degrading Bacterium. Environ. Chem. 2022, 41, 2761–2770. [Google Scholar]
- Liao, Q.; Rong, H.; Zhao, M.; Luo, H.; Chu, Z.; Wang, R. Interaction between Tetracycline and Microorganisms During Wastewater Treatment: A Review. Sci. Total Environ. 2021, 757, 143981. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Bao, J.; Xiao, H.; Song, D.; Du, J.; Mohapatra, S.; Werner, D.; Wang, J. Transformation Mechanisms of Tetracycline by Horseradish Peroxidase with/without Redox Mediator Abts for Variable Water Chemistry. Chemosphere 2020, 258, 127306. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Biodegradation Mechanism of Tetracycline (TEC) by Strain Klebsiella sp. SQY5 as Revealed through Products Analysis and Genomics. Ecotoxicol. Environ. Saf. 2019, 185, 109676. [Google Scholar] [CrossRef]
- Qi, W.-N. Microbial Degradation Mechanism of Tetracycline Antibiotics. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2019. [Google Scholar]
- Gujarathi, N.P.; Haney, B.J.; Park, H.J.; Wickramasinghe, S.R.; Linden, J.C. Hairy Roots of Helianthus Annuus: A Model System to Study Phytoremediation of Tetracycline and Oxytetracycline. Biotechnol. Prog. 2010, 21, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Li, F.Y.; Hao, Y.B. The Preliminary Exploration of Remediation the Antibiotics Polluted Water by Two Hydrophytes. Subtrop. Plant Sci. 2012, 41, 1–7. [Google Scholar]
- Wojnárovits, L.; Wang, J.-L.; Chu, L.-B.; Tóth, T.; Kovács, K.; Bezsenyi, A.; Szabó, L.; Homlok, R.; Takács, E. Matrix effect in the hydroxyl radical induced degradation of β-lactam and tetracycline type antibiotics. Radiat. Phys. Chem. 2022, 193, 109980. [Google Scholar] [CrossRef]
- Zhang, J.; Giorno, L.; Drioli, E. Study of a Hybrid Process Combining PACs and Membrane Operations for Antibiotic Wastewater Treatment. Desalination 2005, 194, 101–107. [Google Scholar] [CrossRef]
- Safari, G.H.; Nasseri, S.; Mahvi, A.H.; Yaghmaeian, K.; Nabizadeh, R.; Alimohammadi, M. Optimization of sonochemical degradation of tetracycline in aqueous solution using sono-activated persulfate process. J. Environ. Health Sci. Eng. 2015, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Shibata, K.; Fujimori, K.; Ohtani, Y. Rapid Removal of Tetracycline Antibiotics from Water by Coagulation-Flotation of Sodium Dodecyl Sulfate and Poly(Allylamine Hydrochloride) in the Presence of Al(Ⅲ) Ions. Sep. Purif. Technol. 2017, 187, 76–83. [Google Scholar] [CrossRef]
- Liang, C.; Wei, D.; Zhang, S.; Ren, Q.; Shi, J.; Liu, L. Removal of Antibiotic Resistance Genes from Swine Wastewater by Membrane Filtration Treatment. Ecotoxicol. Environ. Saf. 2021, 210, 111885. [Google Scholar] [CrossRef] [PubMed]
- Xujian, Q.I.; Fanhao, W.E.I.; Jiawei, F.A.N. Research progress on treatment of antibiotics and their resistance genes by advanced oxidation technologies. Ind. Water Treat. 2022, 42, 55–64. [Google Scholar]
- Lopez Penalver, J.J.; Gomez Pacheco, C.V.; Sanchez Polo, M.; Rivera Utrilla, J. Degradation of Tetracyclines in Different Water Matrices by Advanced Oxidation/Reduction Processes Based on Gamma Radiation. J. Chem. Technol. Biotechnol. Adv. 2013, 88, 1096–1108. [Google Scholar] [CrossRef]
- Wang, J.-S.; Yi, X.-H.; Xu, X.; Ji, H.; Alanazi, A.M.; Wang, C.-C.; Zhao, C.; Kaneti, Y.V.; Wang, P.; Liu, W.; et al. Eliminating Tetracycline Antibiotics Matrix Via Photoactivated Sulfate Radical-Based Advanced Oxidation Process over the Immobilized MIL-88A: Batch and Continuous Experiments. Chem. Eng. J. 2022, 431, 133213. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, J.; Zhou, Y.; Luo, Z.; Sun, Y.; Wan, Z.; Luo, L.; Tsang, D.W.; Dionysiou, D. Development of Ozonation and Reactive Electrochemical Membrane Coupled Process: Enhanced Tetracycline Mineralization and Toxicity Reduction. Chem. Eng. J. 2020, 383, 123149. [Google Scholar] [CrossRef]
- Luu, H.T.; Lee, K. Degradation and Changes in Toxicity and Biodegradability of Tetracycline During Ozone/Ultraviolet-Based Advanced Oxidation. Water Sci. Technol. 2014, 70, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, H.; Li, K.; Zhang, L. Combined Process of Biofiltration and Ozone Oxidation as an Advanced Treatment Process for Wastewater Reuse. Front. Environ. Sci. Eng. 2015, 9, 1076–1083. [Google Scholar] [CrossRef]
- Wu, X.F.; Yang, K. Experimental study on advanced treatment of antibiotic wastewater by Fenton process. Straits Sci. 2017, 1, 19–21. [Google Scholar]
- Bishop, D.F.; Stern, G.; Fleischman, M.; Marshall, L. Hydrogen Peroxide Catalytic Oxidation of Refractory Organics in Municipal Waste Waters. Ind. Eng. Chem. Process Des. Dev. 1968, 7, 110–117. [Google Scholar] [CrossRef]
- Bai, J.L. Study on the Application of Iron-carbon Micro-electrolysis and Fenton Oxidation in Antibiotic Wastewate Treatment. Sichuan Nonferrous Met. 2019, 2, 51–54. [Google Scholar]
- Shao, C.; Zhang, J.; Wang, Z.; Zhang, L.; Wang, B.; Ren, J.; Zhang, X.; He, W. Photo-Fenton degradation of tetracycline on nitrogen vacancy and potassium-doped Z-scheme FeOCl/NvCN heterojunction with low H2O2 consumption: Activity and mechanism. J. Alloys Compd. 2023, 970, 172532. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, B.; Qi, G.H.; Yan, L.; Li, C.; Zhang, Z. Research Progress of Photocatalytic Oxidation Process to Antibiotic Wastewater Treatment with TiO2. Shanghai Chem. Ind. 2014, 39, 7–10. [Google Scholar]
- Xiao, M.W. A New Technology for Treatment of Antibiotic Wastewater by Photocatalytic Oxidation. Master’s Thesis, Guangdong University of Technology, Guangzhou, China, 2005. [Google Scholar]
- Reyes, C.; Fernandez, J.; Freer, J.; Mondaca, M.A.; Zaror, C.; Malato, S.; Mansilla, H.D. Degradation and Inactivation of Tetracycline by TiO2 Photocatalysis. J. Photochem. Photobiol. A Chem. 2006, 184, 141–146. [Google Scholar] [CrossRef]
- Das, S.; Ahn, Y.H. Synthesis and application of CdS nanorods for LED-based photocatalytic degradation of tetracycline antibiotic. Chemosphere 2022, 291, 132870. [Google Scholar] [CrossRef]
- Nagamine, M.; Osial, M.; Jackowska, K.; Krysinski, P.; Widera-Kalinowska, J. Tetracycline Photocatalytic Degradation under CdS Treatment. J. Mar. Sci. Eng. 2020, 8, 483. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, C.; Zhen, L.; Li, Y.; Bai, H.; Zhang, L.; Ren, G.; Wang, X. Treatment of antibiotics in water by SO3H-modified Ti3C2 Mxene photocatalytic collaboration with g-C3N4. J. Mater. Sci. Technol. 2024, 194, 124–137. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Qi, P.; Meng, S.; Zuo, N.; Mominou, N.; Li, S.Z.; Jing, C.Y.; Wang, L. Photocatalytic oxidation degradation of tetracycline over La/Co@TiO2 nanospheres under visible light. Environ. Res. 2022, 215, 114297. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Yin, Z.-L.; Chen, J.-R.; Zhang, S.; Sheng, W.-C.; Wei, W.-X.; Xiao, Y.-G.; Shi, Q.-Y.; Cao, S.-S. Novel p-n heterojunction Bi2O3/Ti3+-TiO2 photocatalyst enables the complete removal of tetracyclines under visible light. Chem. Eng. J. 2020, 417, 128058. [Google Scholar] [CrossRef]
- Li, Y.-C.; Yu, B.; Hu, Z.-Q.; Wang, H. Construction of direct Z-scheme SnS2@ZnIn2S4@kaolinite heterostructure photocatalyst for efficient photocatalytic degradation of tetracycline hydrochloride. Chem. Eng. J. 2022, 429, 132105. [Google Scholar] [CrossRef]
- Zong, Z.-Y.; Gilbert, E.; Wong, C.Y.; Usadi, L.; Qin, Y.; Huang, Y.-H.; Raymond, J.; Hankins, N.; Kwan, J. Efficient sonochemical catalytic degradation of tetracycline using TiO2 fractured nanoshells. Ultrason. Sonochem. 2023, 101, 106669. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, M.; Wang, Z.; Wang, H.; Deng, C.; Li, K. Effective Degradation of Aqueous Tetracycline Using a Nano-TiO2/Carbon Electrocatalytic Membrane. Materials 2016, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Feng, H.; Tang, J.; Wang, J.; Yu, J.; Yi, Y.; Wu, Y.; Guo, Y.; Tang, L. A Novel Electrocatalytic System with High Reactive Chlorine Species Utilization Capacity to Degrade Tetracycline in Marine Aquaculture Wastewater. Chemosphere 2022, 300, 134449. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Shi, Y.; Wang, Y.; Zheng, Y.; Liu, H.; Hu, Q.; Wei, S.-Y.; Gu, H.-B.; Guo, Z.-H. Electrospun Iron/Cobalt Alloy Nanoparticles on Carbon Nanofibers Towards Exhaustive Electrocatalytic Degradation of Tetracycline in Wastewater. Chem. Eng. J. 2021, 405, 126585. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.H. A Comprehensive Review on Catalysts for Electrocatalytic and Photoelectrocatalytic Degradation of Antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic Destruction of the Antibiotic Tetracycline in Aqueous Medium by Electrochemical Advanced Oxidation Processes: Effect of Electrode Materials. Appl. Catal. B Environ. 2013, 140–141, 92–97. [Google Scholar] [CrossRef]
- Li, Z.P.; Xu, G.H.; Cao, H.; Dai, X. On the Anaerobic Treatment Technology of Antibiotic Wastewater by Three Dimensional Electro Catalytic Coupling. Value Eng. 2014, 33, 305–306. [Google Scholar]
- Sun, W.; Sun, Y.; Shah, K.J.; Chiang, P.-C.; Zheng, H. Electrocatalytic Oxidation of Tetracycline by Bi-Sn-Sb/γ-Al2O3 Three-Dimensional Particle Electrode. J. Hazard. Mater. 2019, 370, 24–32. [Google Scholar] [CrossRef]
- Lu, M.; Sun, J.; Cui, B.; Zhang, J.; Ren, J.; Li, R. Pd, Ru/2D MXene nanosheets/3D self-supporting nickel foam composite electrode and its electrocatalytic synergistic degradation of antibiotics. Sep. Purif. Technol. 2024, 340, 126736. [Google Scholar] [CrossRef]
- Shao, C.; Zhang, J.; Liu, Y.; Jiang, Y.T.; Jia, Y.P.; Li, G.M.; Sun, Z. Effective degradation of tetracycline by Pd/AG/ITO electrode: Electrode preparation, characterization, kinetics, degradation mechanism and toxicity assessment. J. Environ. Chem. Eng. 2023, 11, 110344. [Google Scholar] [CrossRef]
- Jing, H.; Juan, Z.; Pan, W.; Tsang, Y.F. Occurrence and Fate of Antibiotics in a Wastewater Treatment Plant and Their Biological Effects on Receiving Waters in Guizhou. Process Saf. Environ. Prot. 2018, 113, 483–490. [Google Scholar]
- Chen, J.; Liang, J.; Li, C.; Dai, J.; Mai, W.; Wei, Y. An Enriched Ammonia-Oxidizing Microbiota Enables High Removal Efficiency of Ammonia in Antibiotic Production Wastewater. Chemosphere 2023, 310, 136854. [Google Scholar] [CrossRef] [PubMed]
- Pei, M.; Zhang, B.; He, Y.; Su, J.; Gin, K.; Lev, O.; Shen, G.; Hu, S. State of the Art of Tertiary Treatment Technologies for Controlling Antibiotic Resistance in Wastewater Treatment Plants. Environ. Int. 2019, 131, 105026. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Deng, J.; Cai, A.; Ma, X.; Li, J.; Li, Q.; Li, X. Comparison of UVC and UVC/Persulfate Processes for Tetracycline Removal in Water. Chem. Eng. J. 2020, 384, 123320. [Google Scholar] [CrossRef]


| Name | Formula | Acute Toxicity (mg/L) | Chronic Toxicity (mg/L) | ||||
|---|---|---|---|---|---|---|---|
| Fish (96 h-LC50) | Daphnia (48 h-LC50) | Green Alga (96 h-EC50) | Fish (ChV) | Daphnia (ChV) | Green Alga (ChV) | ||
| Aureomycin | 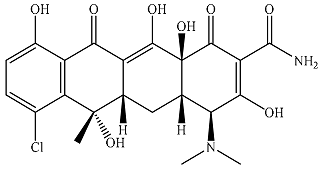 | 4.58 × 103 | 590 | 5.22 × 103 | 345 | 40.4 | 408 |
| Oxytetracycline |  | 1.39 × 105 | 6.70 × 103 | 1.47 × 105 | 8.41 × 103 | 314 | 6.80 × 103 |
| Doxycycline | 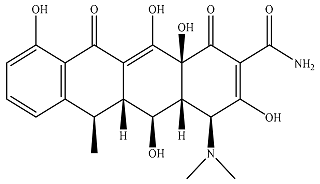 | 1.25 × 104 | 1.18 × 103 | 1.39 × 104 | 877 | 71.9 | 921 |
| Minocycline | 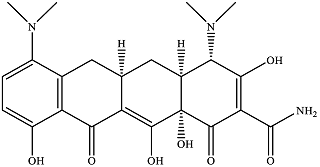 | 2.86 × 103 | 416 | 3.30 × 103 | 222 | 29.9 | 275 |
| Tetracycline | 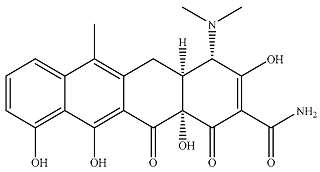 | 1.18 × 104 | 1.13 × 103 | 1.31 × 104 | 831 | 69.5 | 879 |
| P1 |  | 151 | 49.9 | 187 | 14.1 | 4.91 | 23.9 |
| P2 |  | 3.07 × 103 | 421 | 3.52 × 103 | 234 | 29.5 | 284 |
| P3 | 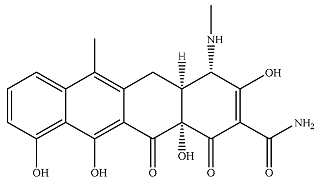 | 36.2 | 2.22 | 5.34 | 0.301 | 0.477 | 0.861 |
| P4 | 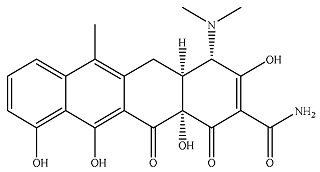 | 149 | 2.01 × 103 | 25.9 | 101 | 817 | 2.83 |
| P5 | 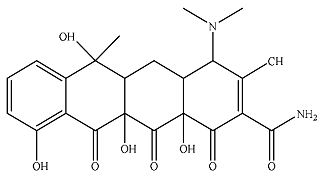 | 587 | 134 | 703 | 50.4 | 11.5 | 74.3 |
| P6 | 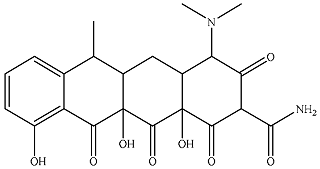 | 28.6 | 15.4 | 36.8 | 2.98 | 1.83 | 6.10 |
| P7 | 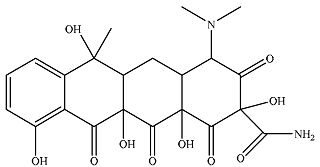 | 39.4 | 19.7 | 50.3 | 4.03 | 2.27 | 8.03 |
| P8 | 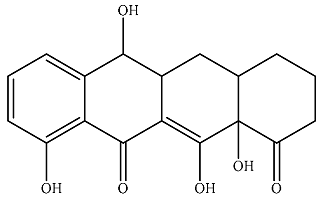 | 274 | 70.8 | 331 | 24.1 | 6.36 | 37.3 |
| P9 |  | 7.89 × 103 | 821 | 8.84 × 103 | 566 | 51.8 | 617 |
| P10 | 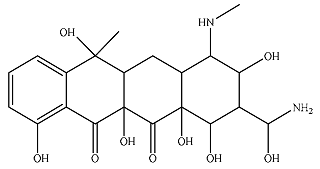 | 5.14 × 104 | 3.27 × 103 | 5.53 × 104 | 3.30 × 103 | 170 | 2.97 × 103 |
| P11 | 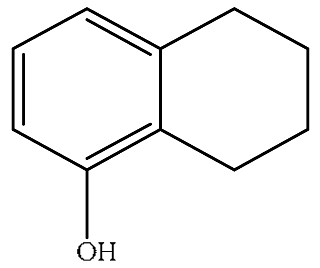 | 1.95 | 1.64 | 2.60 | 0.224 | 0.233 | 0.547 |
| Extremely toxic | Poisonous | Harmful | Non-poisonous | ||||
| Antibiotic Source | Region | Mass Concentration 1) | Reference |
|---|---|---|---|
| Ocean | Bohai Gulf | 16 ng·L−1 TC, 93 ng·L−1 OTC | [18] |
| Iran Persian Gulf | 4.0~71 ng·L−1 TET | [19] | |
| Costa Rica | 74~73,722 ng·L−1 DOX | [20] | |
| Surface water | Jiangxi Jinjiang | ND~86.1 ng·L−1 OTC, ND~5.92 ng·L−1 DOC, ND~5.92 ng·L−1 TC | [21] |
| Nanjing | ND~160 ng·L−1 DOX | [22] | |
| Tianjin | 0~9.74 ng·L−1 TET, 0~34.5 ng·L−1 OTC 0~3.74 ng·L−1 DOX CTC | [23] | |
| Huangpu River | 15.07~113.89 ng·L−1 TC | [24] | |
| Underground water | Jiangxi Jinjiang | ND~4.18 ng·L−1 CTC, ND~2.65 ng·L−1 OTC, ND~1.56 ng·L−1 DOC | [21] |
| Harbin | 0.35~3.91 ng·L−1 DOX | [25] | |
| waste water | Jiangxi Jinjiang | ND~58.6 ng·L−1 CTC, 54.0~2.72 × 103 ng·L−1 OTC, 10.9~49.3 ng·L−1 DOC, ND~27.4 ng·L−1 TC | [21] |
| Drinking Water | Huaihe river basin (wet season) | Mengxian 3.38 ng·L−1 TCs, Yingdong 4.73 ng·L−1 TCs, Yongqiao 5.99 ng·L−1 TCs, Lingbi 8.60 ng·L−1 TCs, Shouxian 2.02 ng·L−1 TCs, Hexian 3.28 ng·L−1 TCs | [26] |
| Technology | Treatment Efficiency | Cost | Advantage | Disadvantage |
|---|---|---|---|---|
| Adsorption method | High | Low | Multifunctional, low energy consumption, easy operation and no by-products | Easily causes secondary pollution |
| Biological method | Low | Low | Low cost and mature technology | Unsafe |
| Materialization method | Low | High | Simple operation | General effect |
| Advanced oxidation processes | High | High | High degree of mineralization of pollutants and stable effects | Pharmaceutical intermediate |
| Comprehensive treatment technology used in sewage plants | High | Low | Mature technology, simple operation, etc. | Sludge treatment is difficult |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Wang, Z.; Zhao, Y.; Zhang, J. Performance of Traditional and Emerging Water-Treatment Technologies in the Removal of Tetracycline Antibiotics. Catalysts 2024, 14, 269. https://doi.org/10.3390/catal14040269
Wang F, Wang Z, Zhao Y, Zhang J. Performance of Traditional and Emerging Water-Treatment Technologies in the Removal of Tetracycline Antibiotics. Catalysts. 2024; 14(4):269. https://doi.org/10.3390/catal14040269
Chicago/Turabian StyleWang, Fanjin, Ziyi Wang, Yue Zhao, and Jian Zhang. 2024. "Performance of Traditional and Emerging Water-Treatment Technologies in the Removal of Tetracycline Antibiotics" Catalysts 14, no. 4: 269. https://doi.org/10.3390/catal14040269
APA StyleWang, F., Wang, Z., Zhao, Y., & Zhang, J. (2024). Performance of Traditional and Emerging Water-Treatment Technologies in the Removal of Tetracycline Antibiotics. Catalysts, 14(4), 269. https://doi.org/10.3390/catal14040269