CO2 Reduction Performance with Double-Layered Cu/TiO2 and P4O10/TiO2 as Photocatalysts under Different Light Illumination Conditions
Abstract
1. Introduction
- Electron-hole pair generation process
- Oxidization reaction process
- Reduction reaction process
2. Results and Discussion
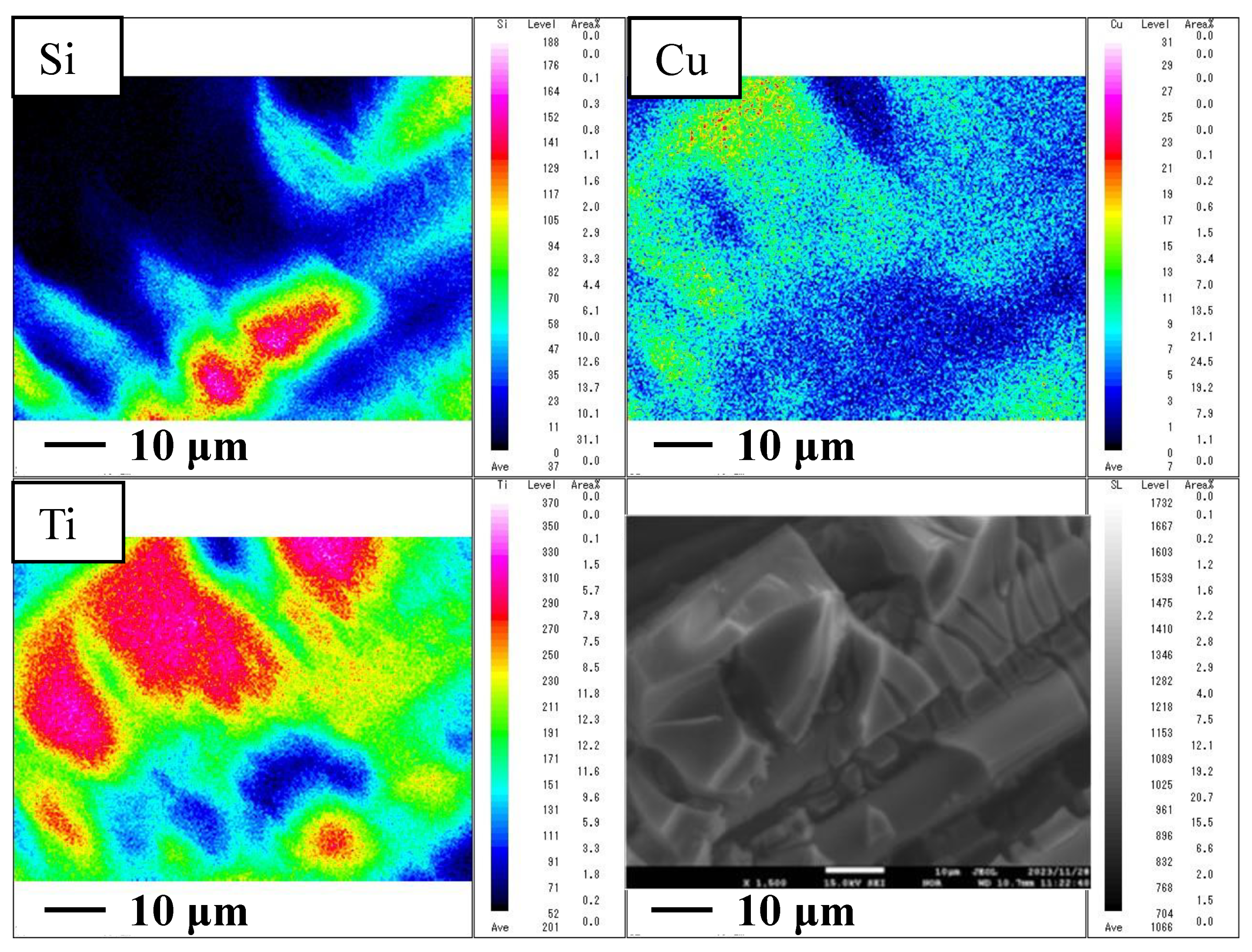
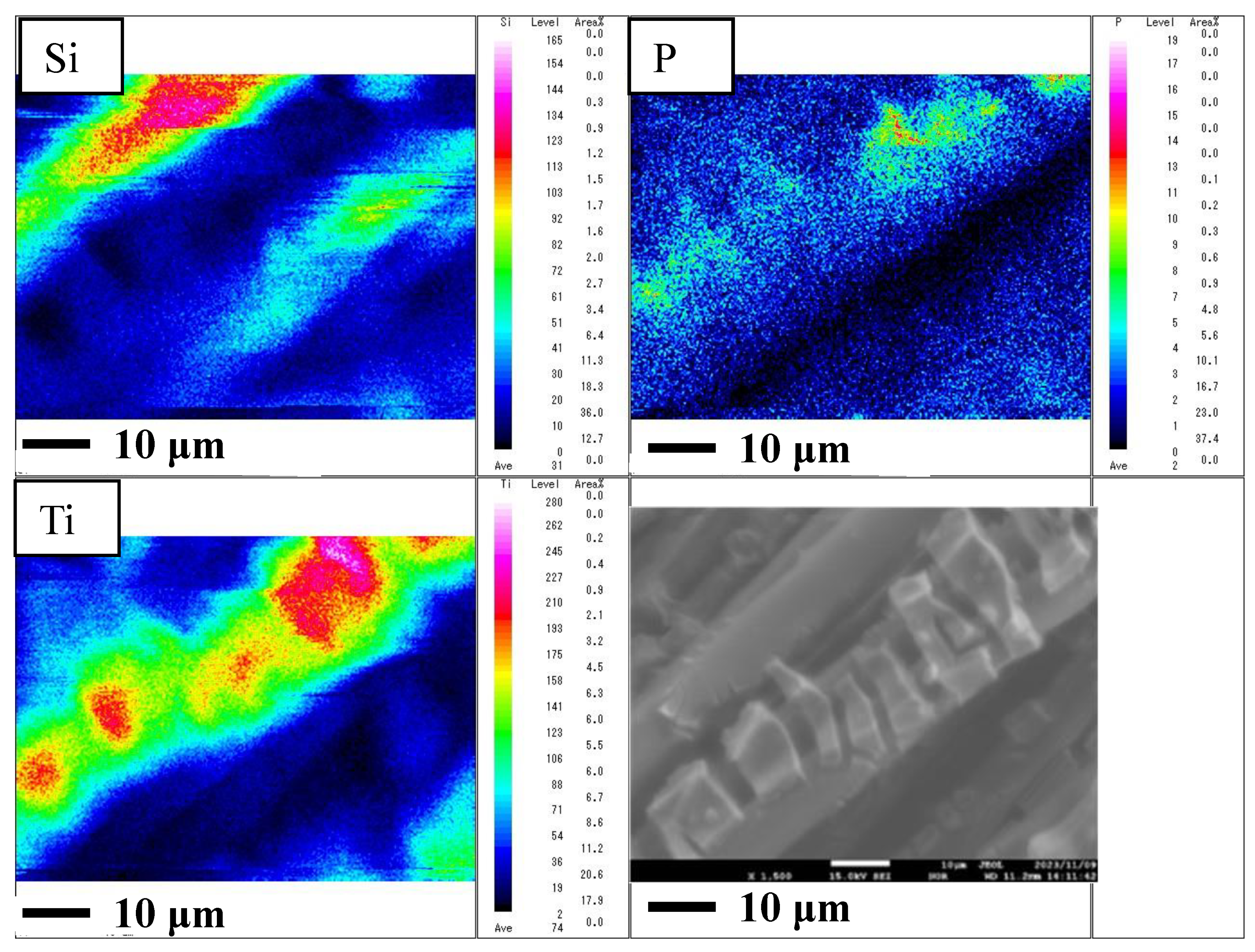
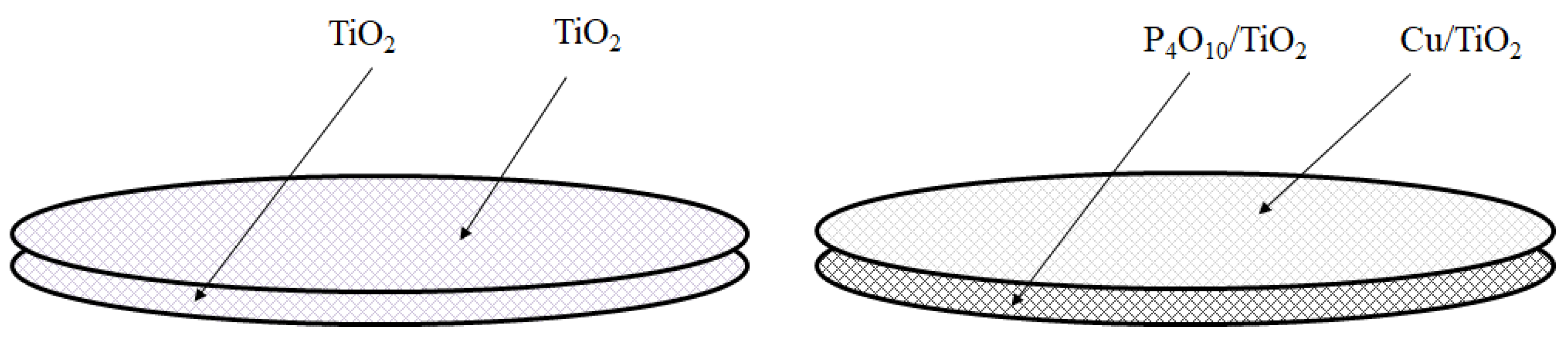
2.1. Characterization of Cu/TiO2 and P4O10/TiO2
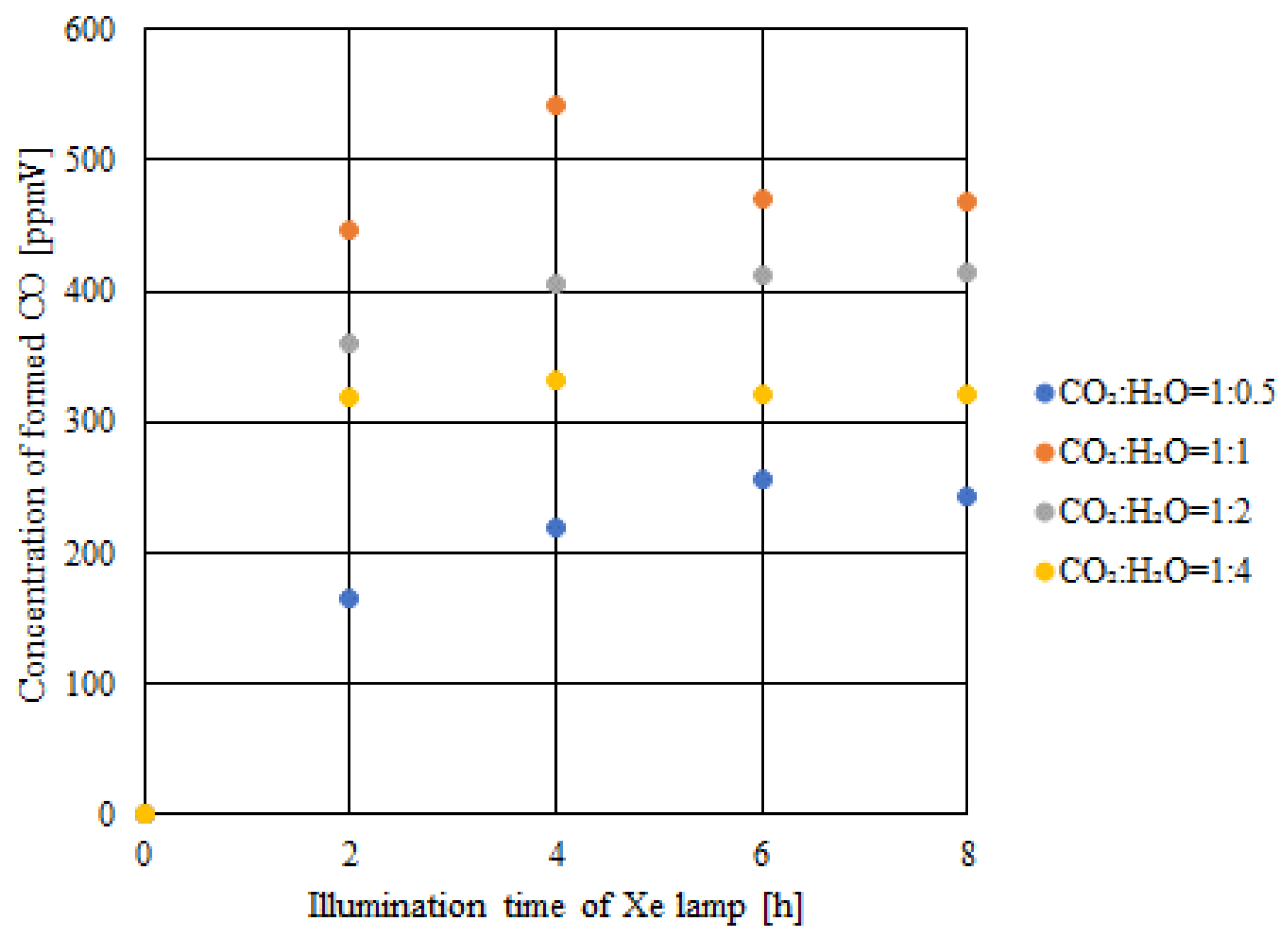
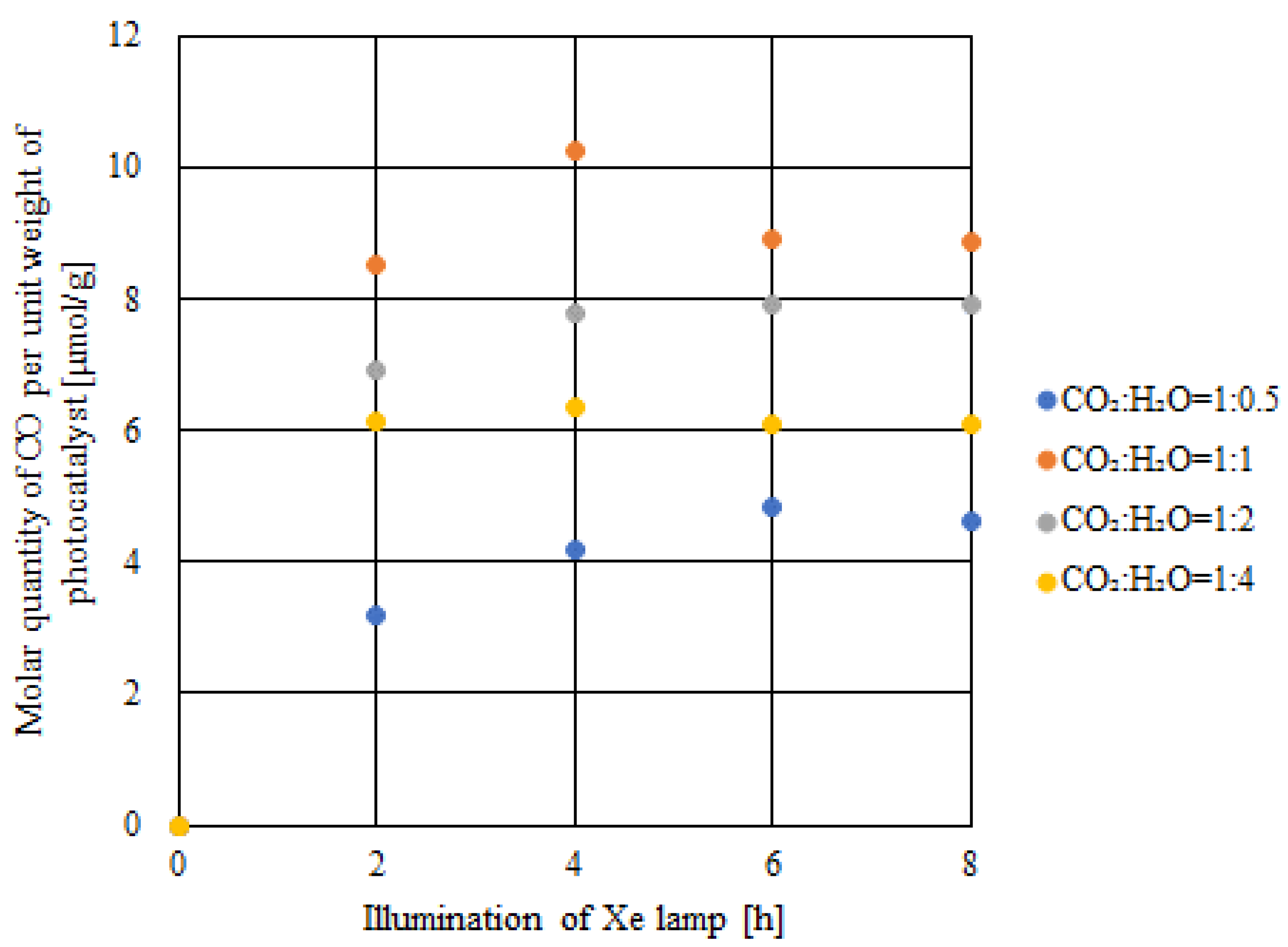
2.2. CO2 Reduction Characteristics of Double-Layered TiO2 under the Light Illumination Condition of Xe Lamp with UV + VIS + IR
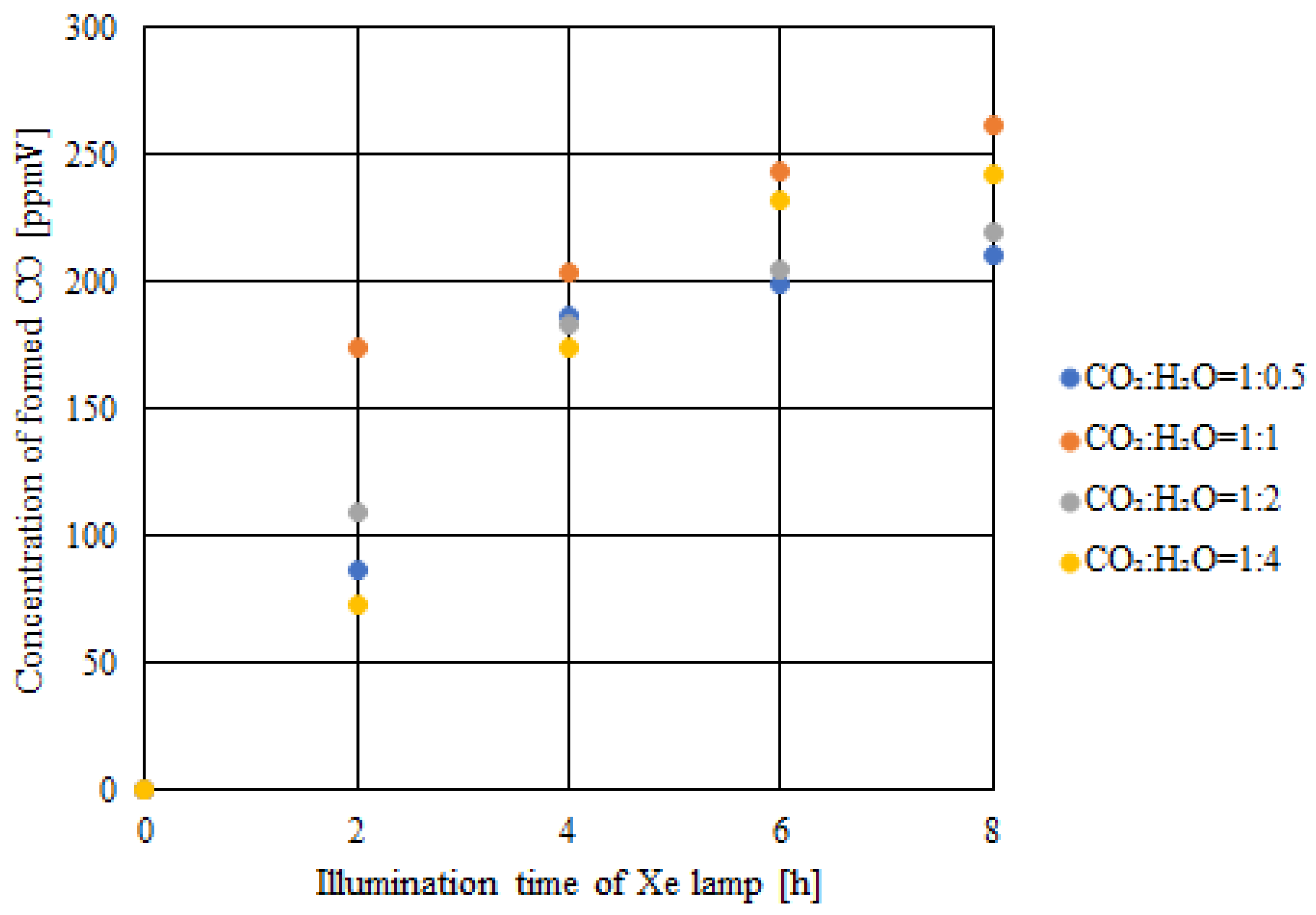
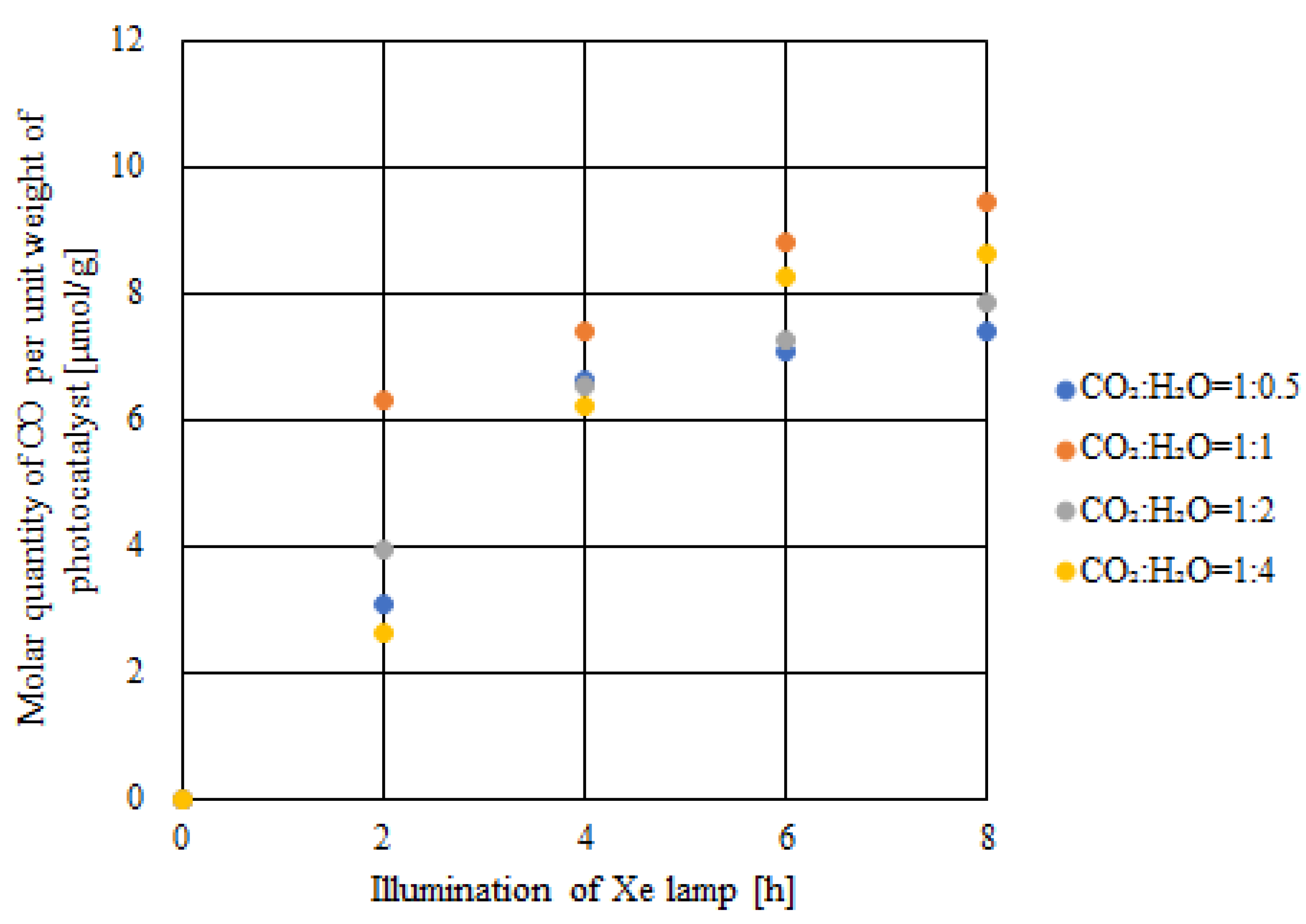
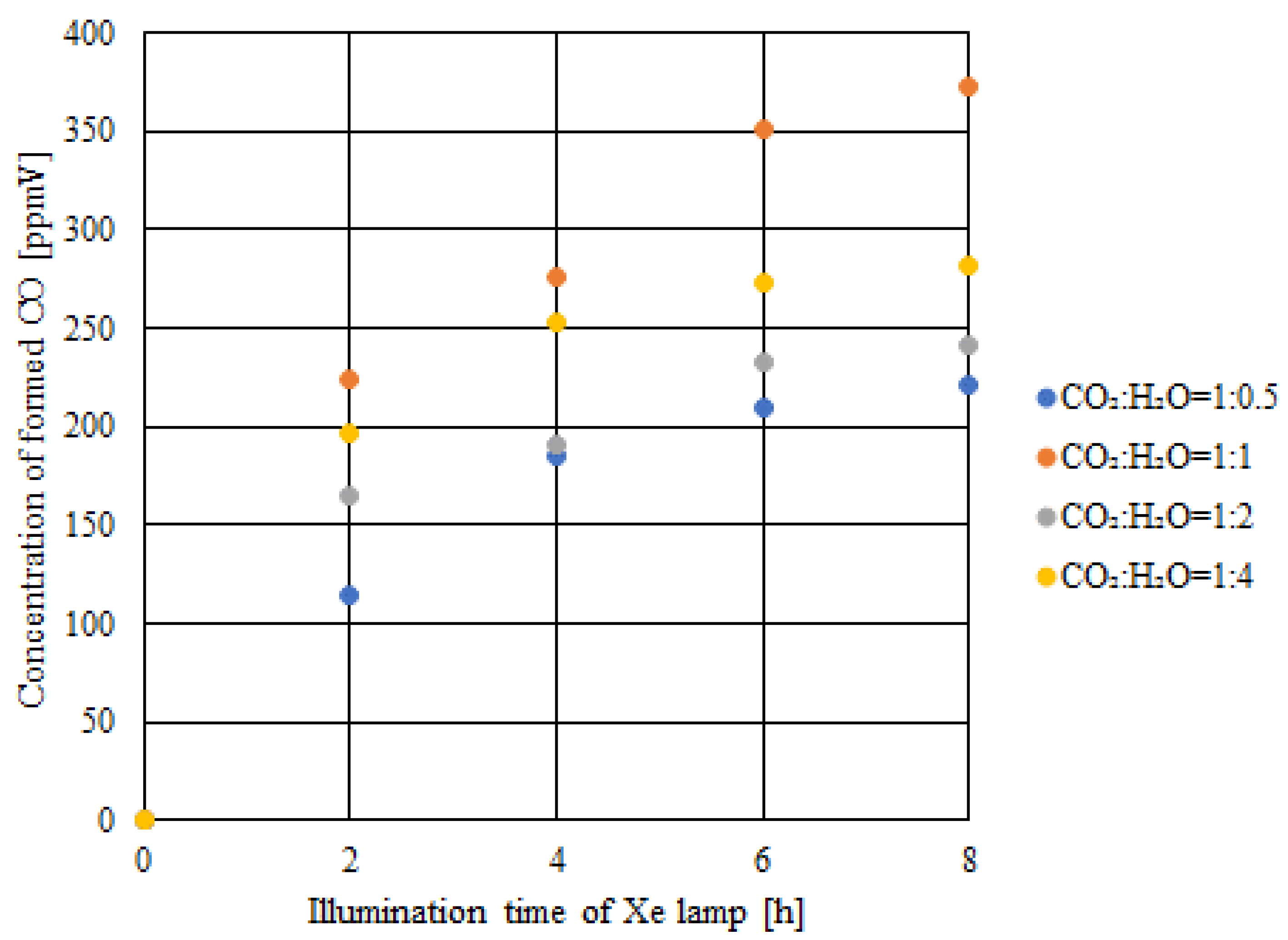
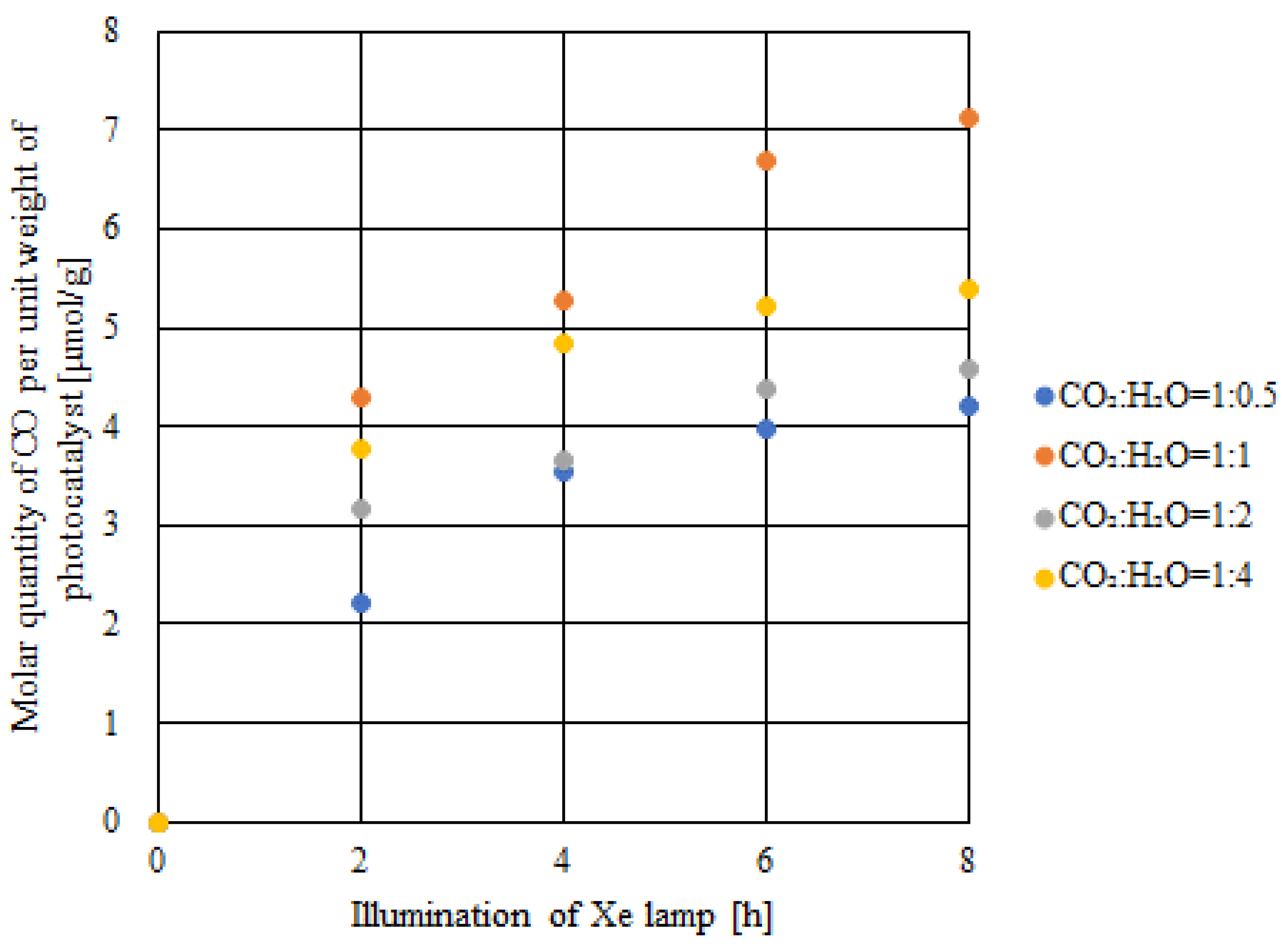
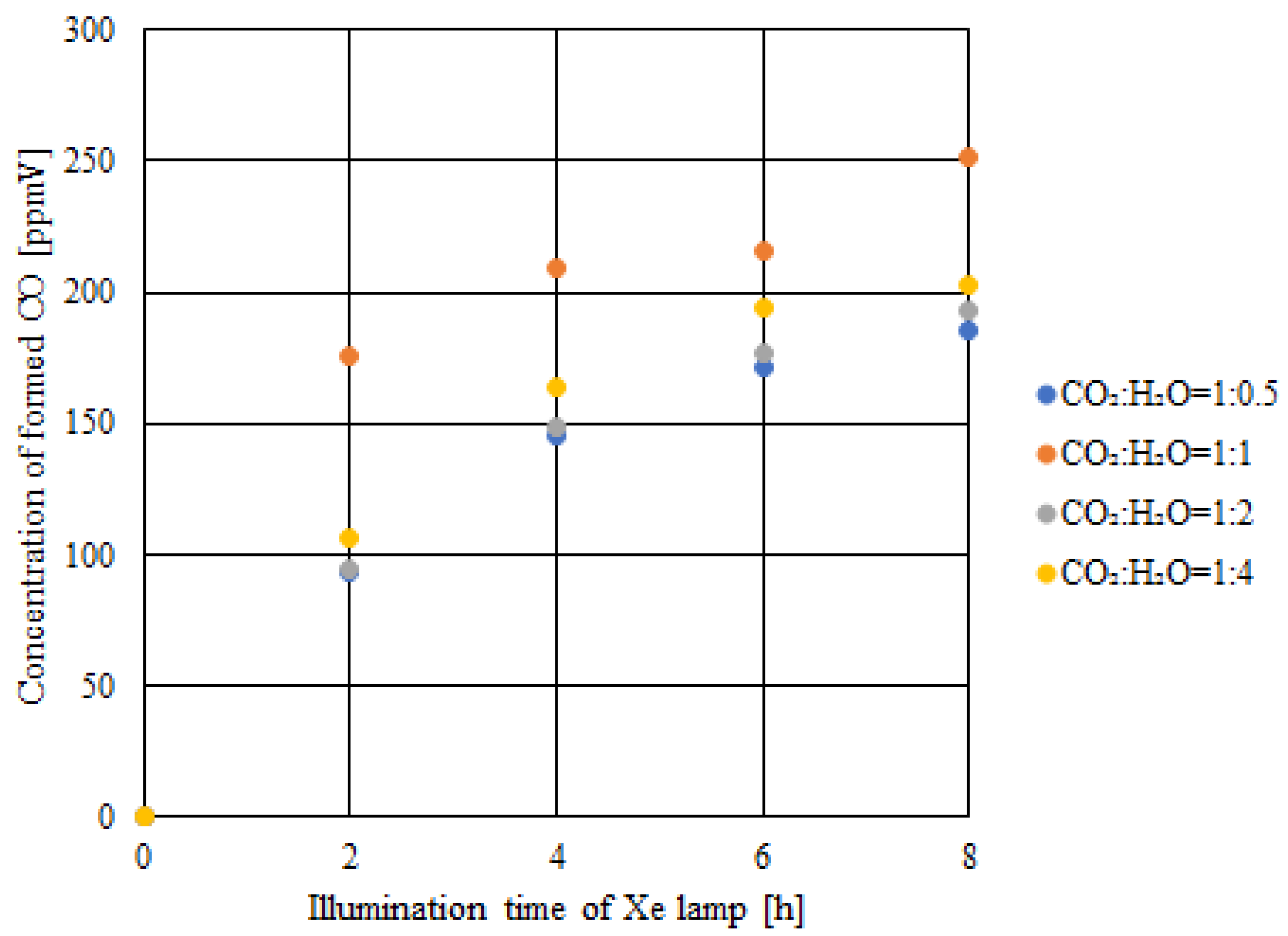
2.3. CO2 Reduction Characteristics of Double-Layered Cu/TiO2 and P4O10/TiO2 with the Illumination of UV + VIS + IR
2.4. CO2 Reduction Characteristics of Double-Layered Cu/TiO2 and P4O10/TiO2 with VIS + IR Illumination
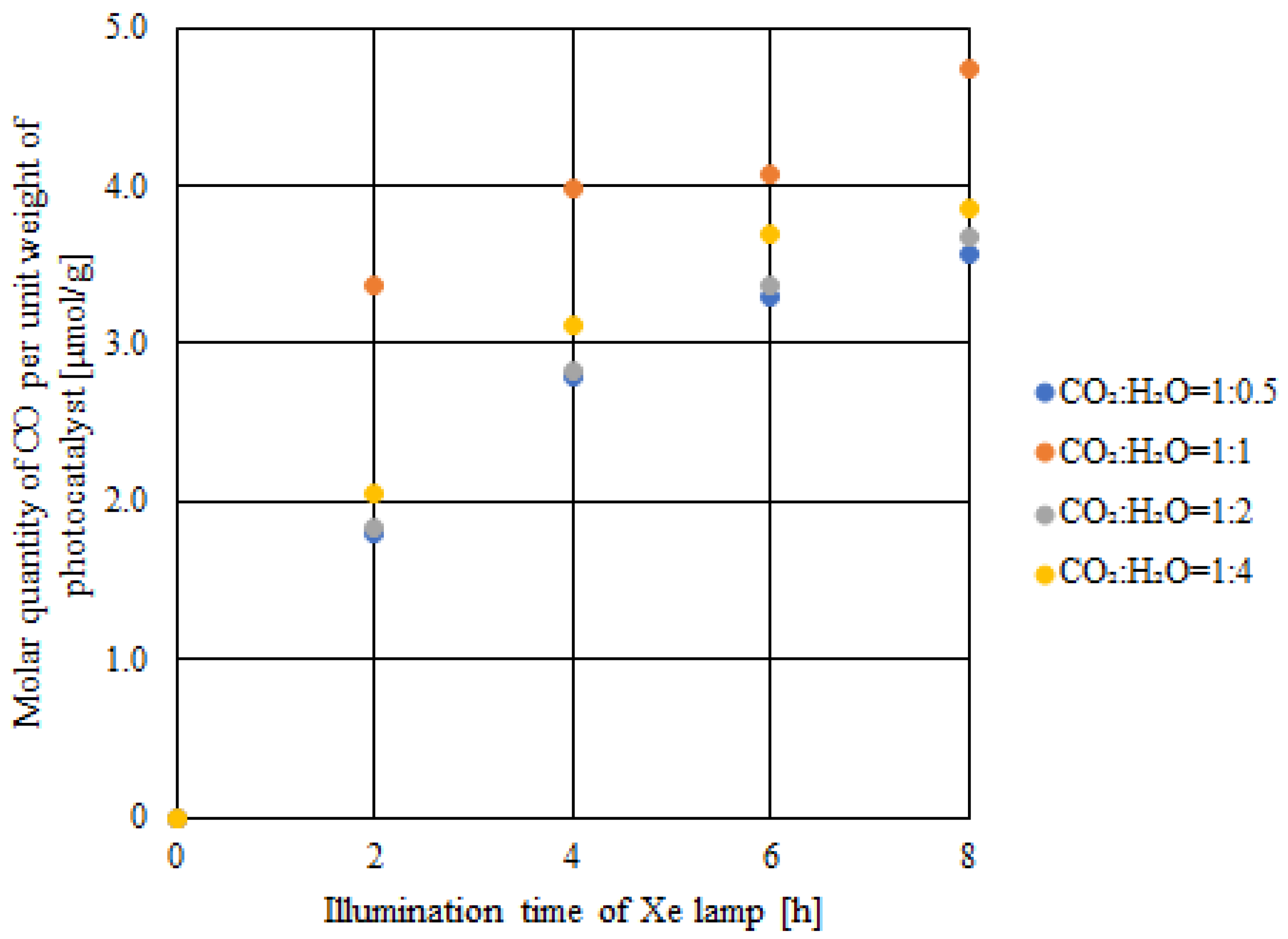
2.5. CO2 Reduction Characteristics of Double-Layered Cu/TiO2 and P4O10/TiO2 with IR Only Illumination
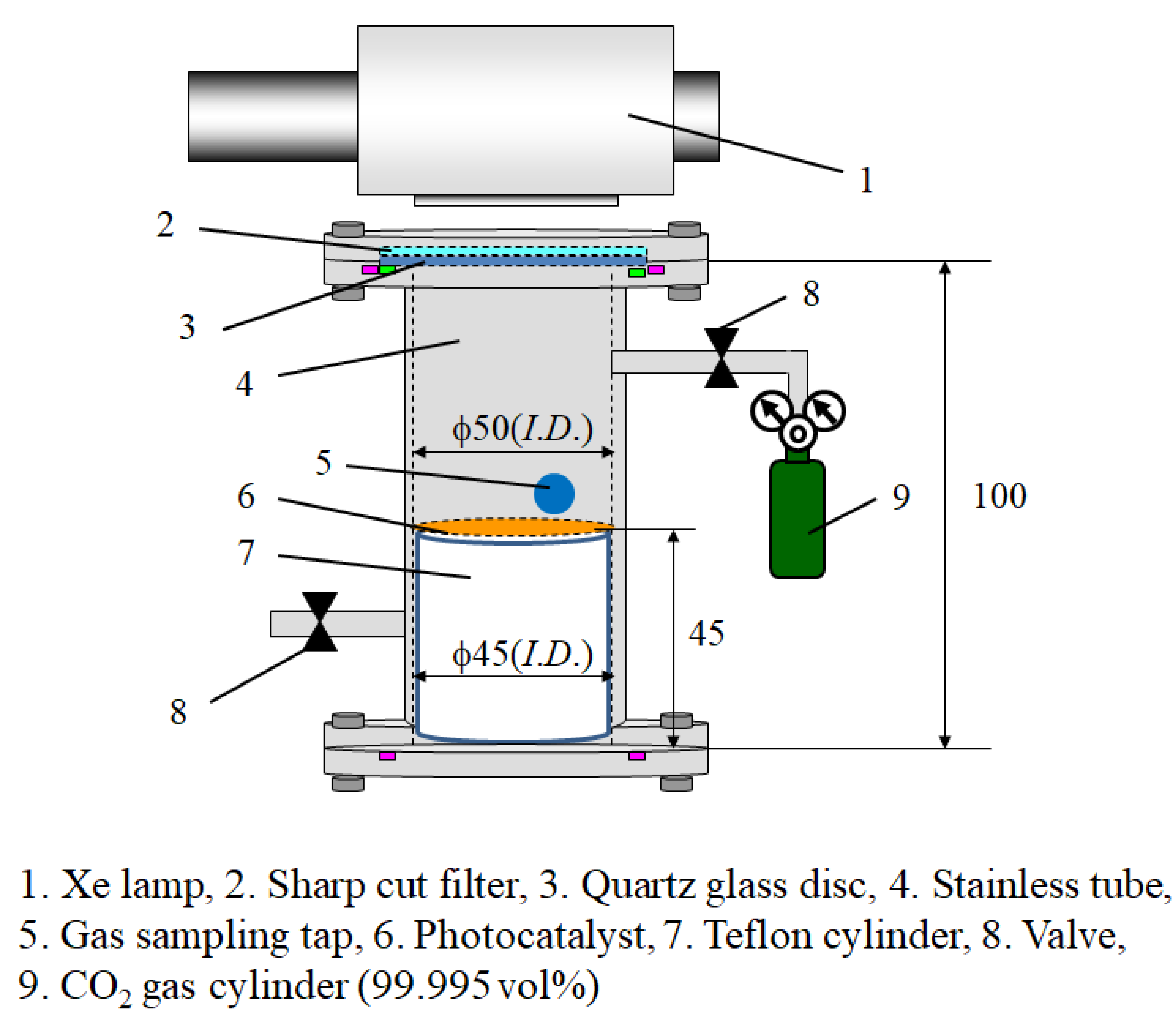
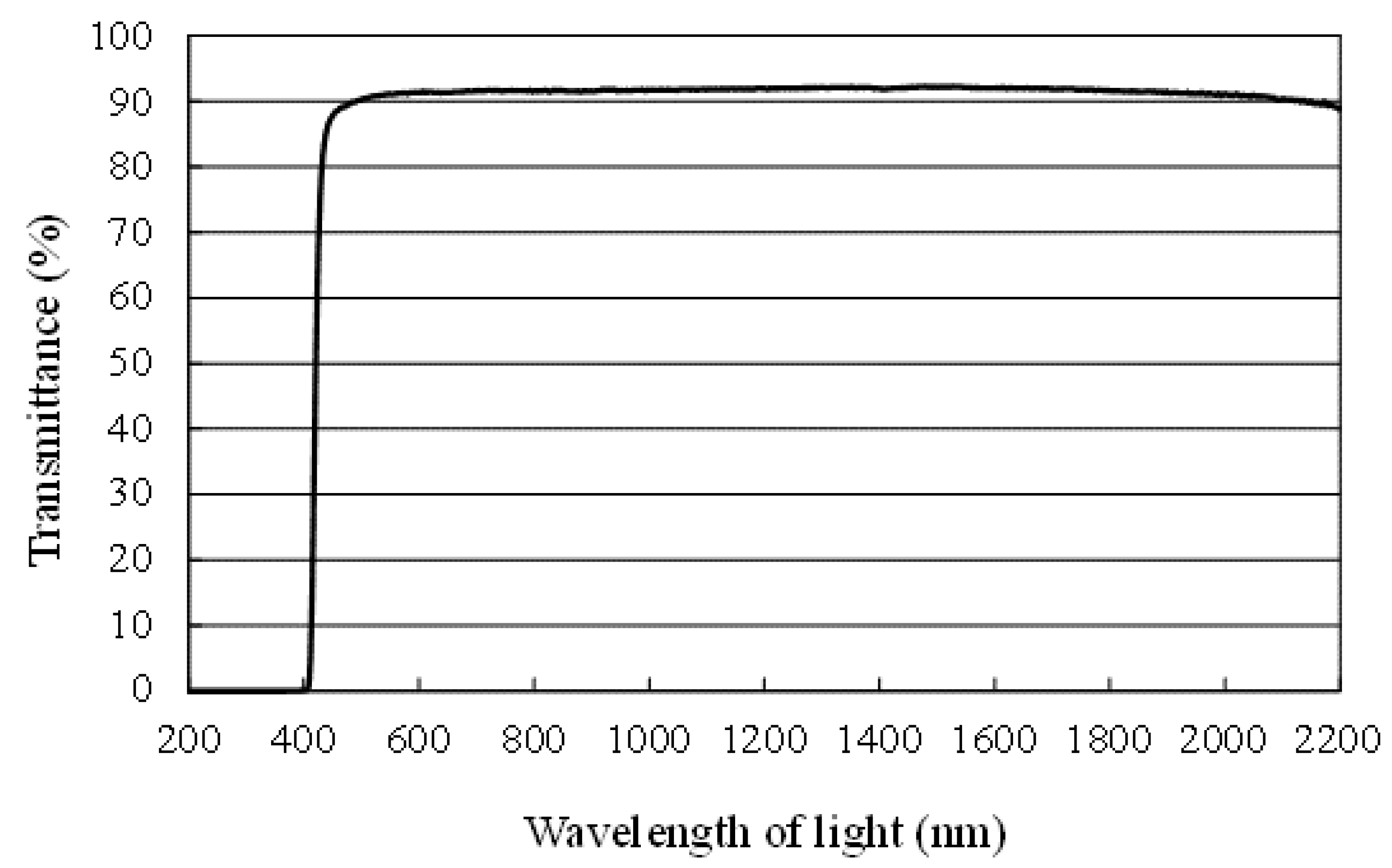
3. Experimental Procedure
3.1. Preparation Procedure of Cu/TiO2 and P4O10/TiO2
3.2. The Characterization Procedure of Cu/TiO2 and P4O10/TiO2
3.3. The Experimental Procedure of CO2 Reduction
4. Conclusions
- (i)
- The highest concentration of formed CO and the molar quantity of CO per unit weight of the photocatalyst were achieved when the molar ratio of CO2:H2O = 1:1, which matched the theoretical molar ratio to produce CO according to the reaction scheme of CO2 reduction with H2O, irrespective of the light illumination conditions.
- (ii)
- Comparing the results of double-layered Cu/TiO2 and P4O10/TiO2 with the results of double-layered TiO2 obtained under the UV + VIS + IR illumination, the highest concentration of formed CO and the molar quantity of CO per unit weight of the photocatalyst increased by 281 ppmV and 0.8 μmol/g, respectively, when CO2:H2O = 1:1.
- (iii)
- With VIS + IR illumination, the highest concentration of formed CO and the molar quantity of CO per unit weight of the photocatalyst reached 373 ppmV and 7.1 μmol/g, respectively. This proves that CO2 reduction could be activated with VIS + IR illumination if TiO2 loaded with Cu and P4O10 was used as the photocatalyst.
- (iv)
- With only IR illumination, the highest concentration of formed CO and the molar quantity of CO per unit weight of the photocatalyst was 251 ppmV and 4.7 μmol/g, respectively. This proves that CO2 reduction reaction could be activated with IR only illumination if TiO2 loaded with Cu and P4O10 was used as the photocatalyst.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jesic, D.; Jurkovic, L.D.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering Photocatalytic and Photoelectrocatalytic CO2 Reduction Reactions: Mechanisms, Intrinsic Kinetics, Mass Transfer Resistances, Reactors and Multi-scale Modeling Simulations. Chem. Eng. J. 2021, 407, 126799. [Google Scholar] [CrossRef]
- Kaushik, R.; Singh, P.K.; Halder, A. Modulation Strategies in Titania Photocatalyst for Energy Recovery and Environmental Remediation. Catal. Today 2022, 384–386, 45–69. [Google Scholar] [CrossRef]
- Wang, Z.W.; Shi, Y.Z.; Liu, C.; Kang, Y.Y.; Wu, L. Cu+-Ti3+ Interface Interaction Mediated CO2 Coordination model for Controlling the Selectivity of Photocatalytic Reduction CO2. Appl. Catal. B Environ. 2022, 301, 120803. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Yuan, Z.; Chen, J.; Huang, B.; Dionysiou, D.D.; Yang, G. Enhanced Photocatalytic CO2 Reduction via the Synergetic Effect between Ag and Activated Carbon in TiO2/AC-Ag Ternary Composite. Chem. Eng. J. 2018, 348, 592–598. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Borker, R.; Bansiwal, A.; Labhsetwar, N.; Jain, S.L. Metal-organic hybrid: Photoreduction of CO2 using graphitic carbon nitride supported heteroleptic iridium complex under visible light irradiation. Carbon 2017, 123, 371–379. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Hasan, F.A.; Hasan, M.R. Advances in Photocatalytic CO2 Reduction with Water: A Review. Materials 2017, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Ola, O.; Maroto-Valer, M.M. Review of Material Design and Reactor Engineering on TiO2 Photocatalysis for CO2 Reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Tasbihi, M.; Fresno, F.; Simon, U.; Villar-Garcia, I.J.; Perez-Dieste, V.; Escudero, C.; O’Shea, V.A.D.L.P. On the Selectivity of CO2 Photoreduction towards CH4 Using Pt/TiO2 Catalysts Supported on Mesoporous Silica. Appl. Catal. B Environ. 2018, 239, 68–76. [Google Scholar] [CrossRef]
- Remiro-Buenamanana, S.; Garcia, H. Photoassisted CO2 Conversion to Fuels. ChemCatChem 2019, 11, 342–536. [Google Scholar] [CrossRef]
- Yu, Y.; Lan, Z.; Guo, L.; Wang, E.; Yao, J.; Cao, Y. Synergetic Effects of Zn and Pd Species in TiO2 towards Efficient Photo-reduction of CO2 into CH4. R. Soc. Chem. 2018, 42, 483–488. [Google Scholar]
- Singhal, N.; Kumar, U. Noble Metal Modified TiO2: Selective Photoreduction of CO2 to Hydrocarbons. Mol. Catal. 2017, 439, 91–99. [Google Scholar] [CrossRef]
- Yui, T.; Kan, A.; Saitoh, C.; Koike, K.; Ibusuki, T.; Ishitani, O. Photochemical Reduction of CO2 Using TiO2: Effects of Organic Adsorbates on TiO2 and Deposition of Pd onto TiO2. ACS Appl. Mater. Interfaces 2011, 3, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Abdulah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 Photocatalyst for CO2 Photocatalytic Reduction: An Overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Camarillo, R.; Toston, S.; Martinez, F.; Jimenez, C.; Rincon, J. Improving the Photo-reduction of CO2 to Fuels with Catalysts Synthesized under High Pressure: Cu/TiO2. J. Chem. Technol. Biotechnol. 2018, 93, 1237–1248. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Engene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 Heterostructures for CO2 Reduction through a Direct Z-scheme: Protecting Cu2O from Photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Kulandaivalu, T.; Rashid, S.A.; Sabli, N.; Tan, T.L. Visible Light Assisted Photocatalytic Reduction of CO2 to Ethane Using CQDs/Cu2O Nanocomposite Photocatalyst. Diam. Relat. Mater. 2019, 91, 64–73. [Google Scholar] [CrossRef]
- Nishimura, A.; Sakakibara, Y.; Koshio, A.; Hu, E. The Impact of Amount of Cu on CO2 Reduction Performance of Cu/TiO2 with NH3 and H2O. Catalysts 2021, 11, 610. [Google Scholar] [CrossRef]
- Zhao, H.; Rao, G.; Wang, L.; Xu, L.; Liu, L.; Li, X. Synthesis of Novel MgAl Layered Double Oxide Grafted TiO2 Cuboids and their Photocatalytic Activity on CO2 Reduction with Water Vapor. Catal. Sci. Technol. 2015, 5, 3288–3295. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Z.; Li, C.; Zuo, Y.; Zhou, Y. Monolithic g-C3H4/Reduced Graphene Oxide Aerogel with in Situ Embedding of Pd Nanoparticles for Hydrogeneration of CO2 to CH4. Appl. Suf. Sci. 2019, 475, 953–960. [Google Scholar] [CrossRef]
- Yang, M.M.; Cao, J.M.; Qi, G.D.; Shen, X.Y.; Yan, G.Y.; Wang, Y.; Dong, W.W.; Zhao, J.; Li, D.S.; Zhang, Q. Construction of low-cost z-scheme heterojunction Cu2O/PCN-250 photocatalysts simultaneously for the enhanced photoreduction of CO2 to alcohols and photooxidation of water. Inorg. Chem. 2023, 62, 15963–15970. [Google Scholar] [CrossRef]
- Dong, W.W.; Jia, J.; Wang, Y.; An, J.R.; Yang, O.Y.; Gao, X.J.; Liu, Y.L.; Zhao, J.; Li, D.S. Visible-light-driven solvent-free photocatalytic CO2 reduction to CO by Co-MOF/Cu2O heterojunction with superior selectivity. Chem. Eng. J. 2022, 438, 135622. [Google Scholar] [CrossRef]
- Hong, L.F.; Guo, R.T.; Yuan, Y.; Ji, X.Y.; Lin, Z.D.; Gu, J.W.; Pan, W.G. Urchinlike W18O49/g-C3H4 Z-Scheme Heterojunction for Highly Efficient Photocatalytic Reduction of CO2 under Full Spectrum Light. Energy Fuels 2021, 35, 11468–11478. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Luo, S.; Hu, X.; Yang, L.; Zhang, S.; Li, B.; Luo, X.; Zou, J. WS2 Quantum Dots Seeding in Bi2S3 Nanotubes: A Novel Vis-NIR Light Sensitive Photocatalyst with Low-Resistance Junction Interface for CO2 Reduction. Chem. Eng. J. 2020, 389, 123430. [Google Scholar] [CrossRef]
- Gan, J.; Wang, H.; Hu, H.; Su, M.; Chen, F.; Xu, H. Efficient Synthesis of Tunable Band-Gap CuInZnS Decorated g-C3H4 Hybrids for Enhanced CO2 Photocatalytic Reduction and Near-Infrared- Triggered Photordegradation Performance. Appl. Surf. Sci. 2021, 564, 150396. [Google Scholar] [CrossRef]
- Yu, M.; Lv, X.; Idris, A.M.; Li, S.; Lin, J.; Lin, H.; Wang, J.; Li, Z. Upconversion Nanoparticles Coupled with Hierarchical ZnIn2S4 Nanorods as a Near-Infrared Responsive Photocatalyst for Photocatalytic CO2 Reduction. J. Colloid Interface Sci. 2022, 612, 782–791. [Google Scholar] [CrossRef]
- Nishimura, A.; Mae, H.; Kato, T.; Hu, E. Utilization from ultraviolet to infrared light for CO2 reduction with P4O10/TiO2 photocatalyst. Phys. Astron. Int. J. 2022, 6, 145–154. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Advances in Visible Light Responsive Titamium Oxide Based Photocatalysts for CO2 Conversion to Hydrocarbon Fuels. Energy Convers. Manag. 2013, 76, 194–214. [Google Scholar] [CrossRef]
- Goren, Z.; Willner, I.; Nelson, A.J. Selective Photoreduction of CO2/HCO3− to Formate by Aqueous Suspensions and Colloids of Pd-TiO2. J. Physic. Chem. 1990, 94, 3784–3790. [Google Scholar] [CrossRef]
- Tseng, I.H.; Chang, W.C.; Wu, J.C.S. Photoreduction of CO2 Using Sol-gel Derived Titania and Titania-supported Copper Catalysts. Appl. Catal. B 2002, 37, 37–38. [Google Scholar] [CrossRef]
- Izumi, Y. Recent Advances in the Photocatalytic Conversion of Carbon Dioxide to Fuels with Water and/or Hydrogen Using Solar Energy and Beyond. Coord. Chem. Rev. 2013, 257, 171–186. [Google Scholar] [CrossRef]
- Japan Society of Mechanical Engineering. Heat Transfer Hand Book, 1st ed.; Maruzen: Tokyo, Japan, 1993; pp. 367–369. [Google Scholar]
- Yokoi, Y.; Ando, N.; Yokoi, H.; Iwashita, H.; Suzuki, R. Application of sintered titanium dioxide to biomaterials: Sintering temperature of anatase-type TiO2 and cell proliferation of L929 cells. J. Jpn. Soc. Oral Implantol. 2012, 25, 262–270. Available online: https://www.jstage.jst.go.jp/article/jsoi/25/2/25_262/_pdf/-char/en (accessed on 14 April 2024).
- Kovalevskiy, N.S.; Lyulyukin, M.N.; Kozlov, D.V.; Selishchev, D.S. Cu-grafted TiO2 photocatalysts: Effect of Cu on the action spectrum of composite materials. Mendeleev Commun. 2021, 31, 644–646. [Google Scholar] [CrossRef]
- Nishimura, A.; Ishida, N.; Tatematsu, D.; Hirota, M.; Koshio, A.; Kokai, F.; Hu, E. Effect of Fe Loading Condition and Reductants on CO2 Reduction Performance with Fe/TiO2 Photocatalyst. Int. J. Photoenergy 2017, 2017, 1625274. [Google Scholar] [CrossRef]
- Licensable Patent Information Database. P2009-184861A. 2022. Available online: https://plidb.inpit.go.jp (accessed on 14 April 2024).
- Brochure of Hamamatsu Photonics Corp. 2000. Available online: https://www.hamamatsu.com/content/dam/hamamatsu-photonics/sites/documents/99_SALES_LIBRARY/etd/Xe-HgXe_TLS1016J.pdf (accessed on 14 April 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Senoue, H.; Mae, H.; Hanyu, R.; Hu, E. CO2 Reduction Performance with Double-Layered Cu/TiO2 and P4O10/TiO2 as Photocatalysts under Different Light Illumination Conditions. Catalysts 2024, 14, 270. https://doi.org/10.3390/catal14040270
Nishimura A, Senoue H, Mae H, Hanyu R, Hu E. CO2 Reduction Performance with Double-Layered Cu/TiO2 and P4O10/TiO2 as Photocatalysts under Different Light Illumination Conditions. Catalysts. 2024; 14(4):270. https://doi.org/10.3390/catal14040270
Chicago/Turabian StyleNishimura, Akira, Hiroki Senoue, Homare Mae, Ryo Hanyu, and Eric Hu. 2024. "CO2 Reduction Performance with Double-Layered Cu/TiO2 and P4O10/TiO2 as Photocatalysts under Different Light Illumination Conditions" Catalysts 14, no. 4: 270. https://doi.org/10.3390/catal14040270
APA StyleNishimura, A., Senoue, H., Mae, H., Hanyu, R., & Hu, E. (2024). CO2 Reduction Performance with Double-Layered Cu/TiO2 and P4O10/TiO2 as Photocatalysts under Different Light Illumination Conditions. Catalysts, 14(4), 270. https://doi.org/10.3390/catal14040270







