Influence of Power Fluctuation on Ni-Based Electrode Degradation and Hydrogen Evolution Reaction Performance in Alkaline Water Splitting: Probing the Effect of Renewable Energy on Water Electrolysis
Abstract
:Highlights
- Square-wave power has the greatest influence on Ni cathode HER compared to step- and triangle-power.
- Increasing the amplitude of square-wave power enhances coated cathode’s degradation.
- Guidance for industrializing hydrogen production via renewable energy-coupled electrolytic water.
- Evaluation theory of cathode materials in electrolytic water.
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Voltage Fluctuation Shape on Electrode Degradation and HER Performance
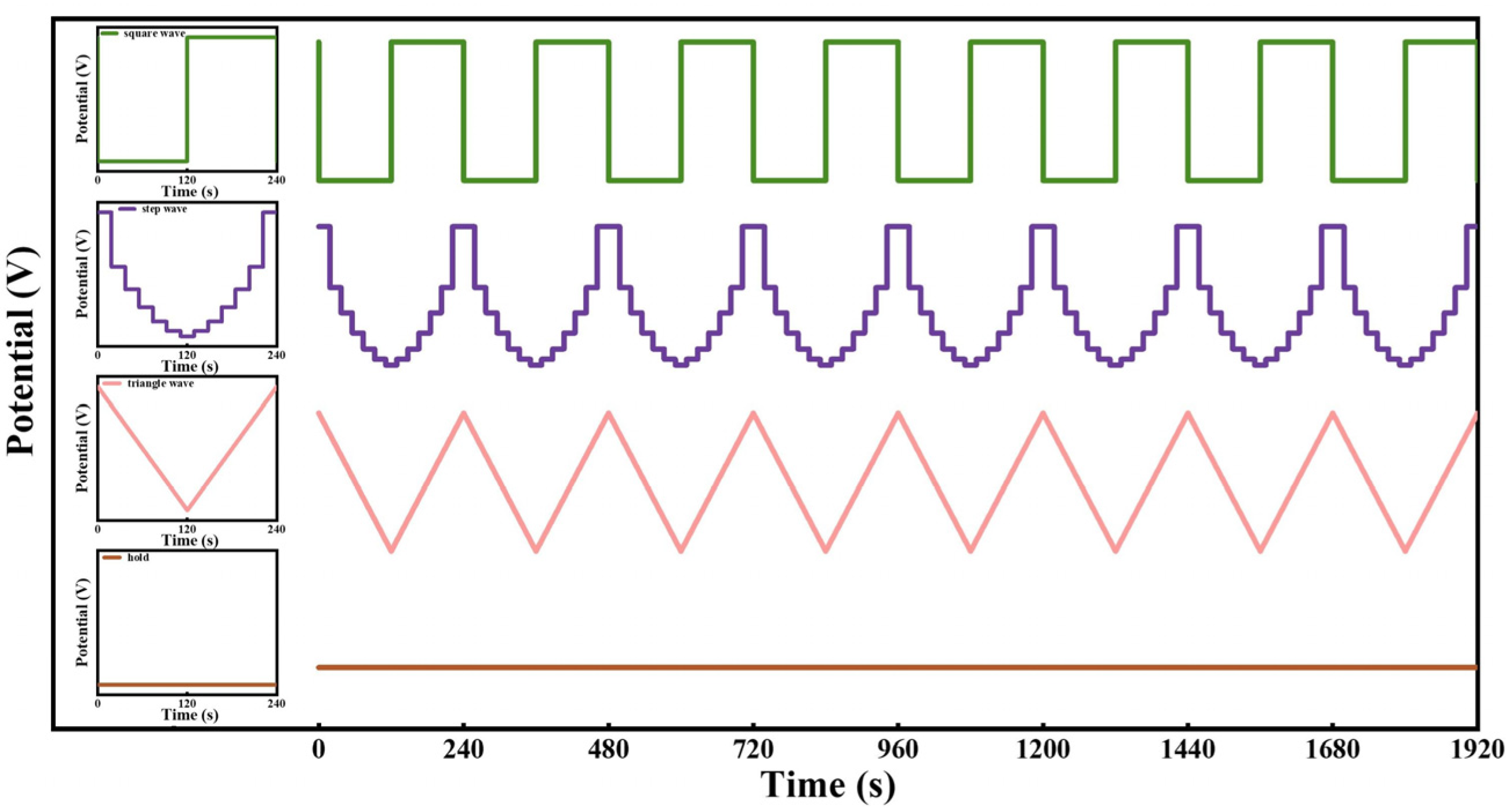
2.1.1. Time–Current Density Curves of Plate Ni Cathode with Fluctuating Power for HER
2.1.2. HER Performance Test
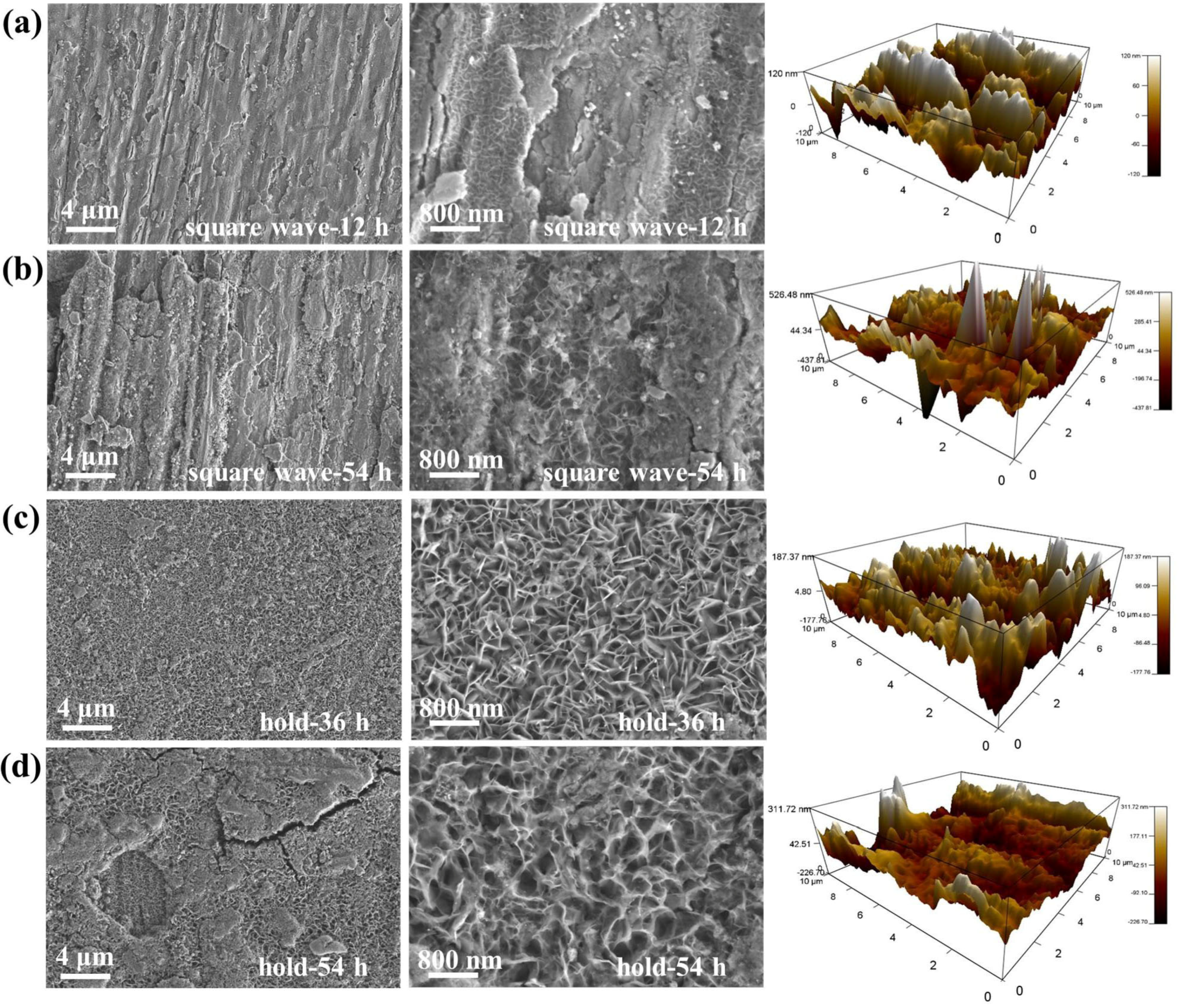
2.1.3. Surface Characterization of Plate Ni Cathode with Fluctuating Power
2.2. Effect of Voltage Fluctuation Amplitude on Electrode Degradation and HER Performance
2.2.1. Time–Current Density Curves of FeNiMo-LDH@NiMo/SS with Fluctuating Power for HER
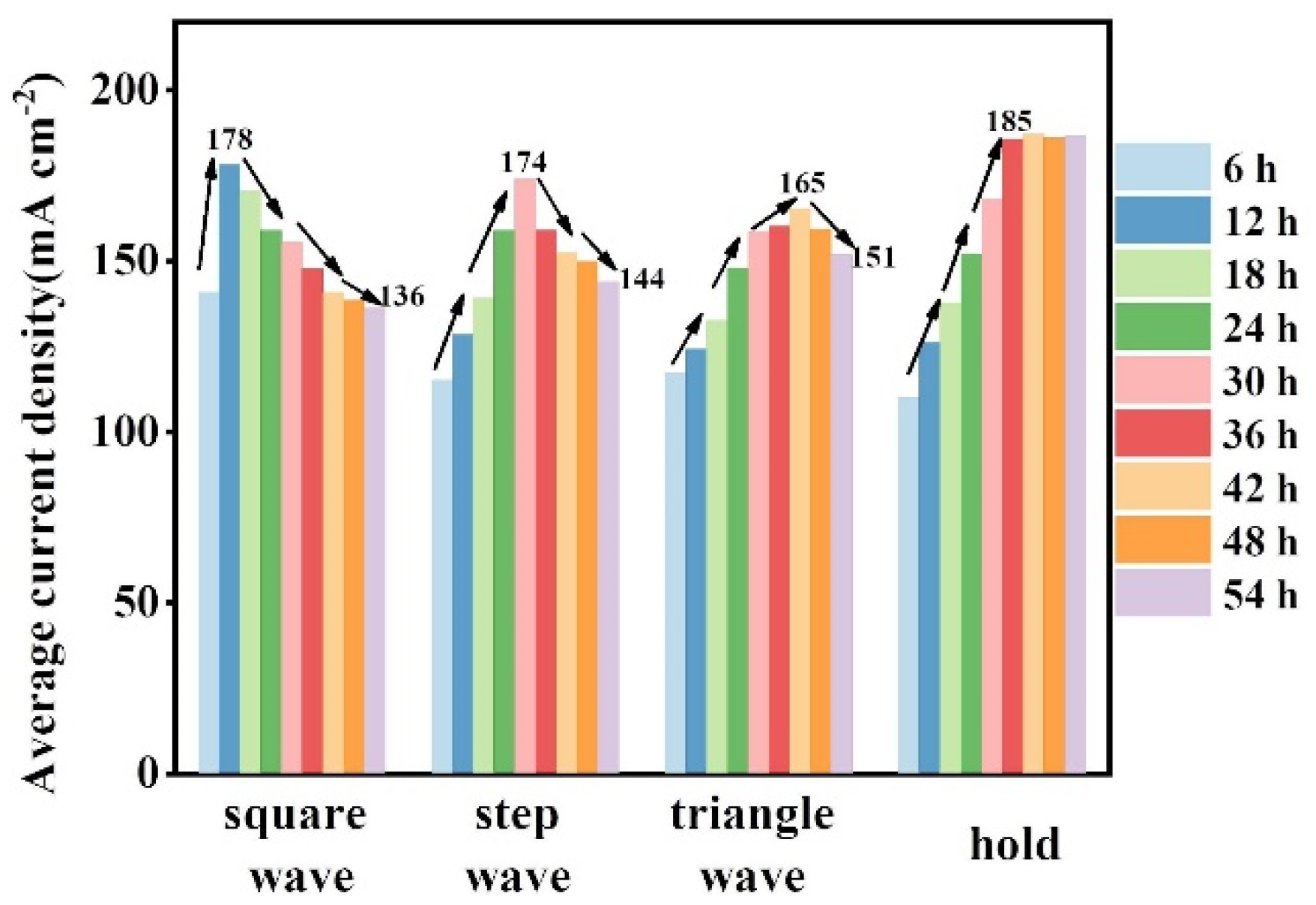
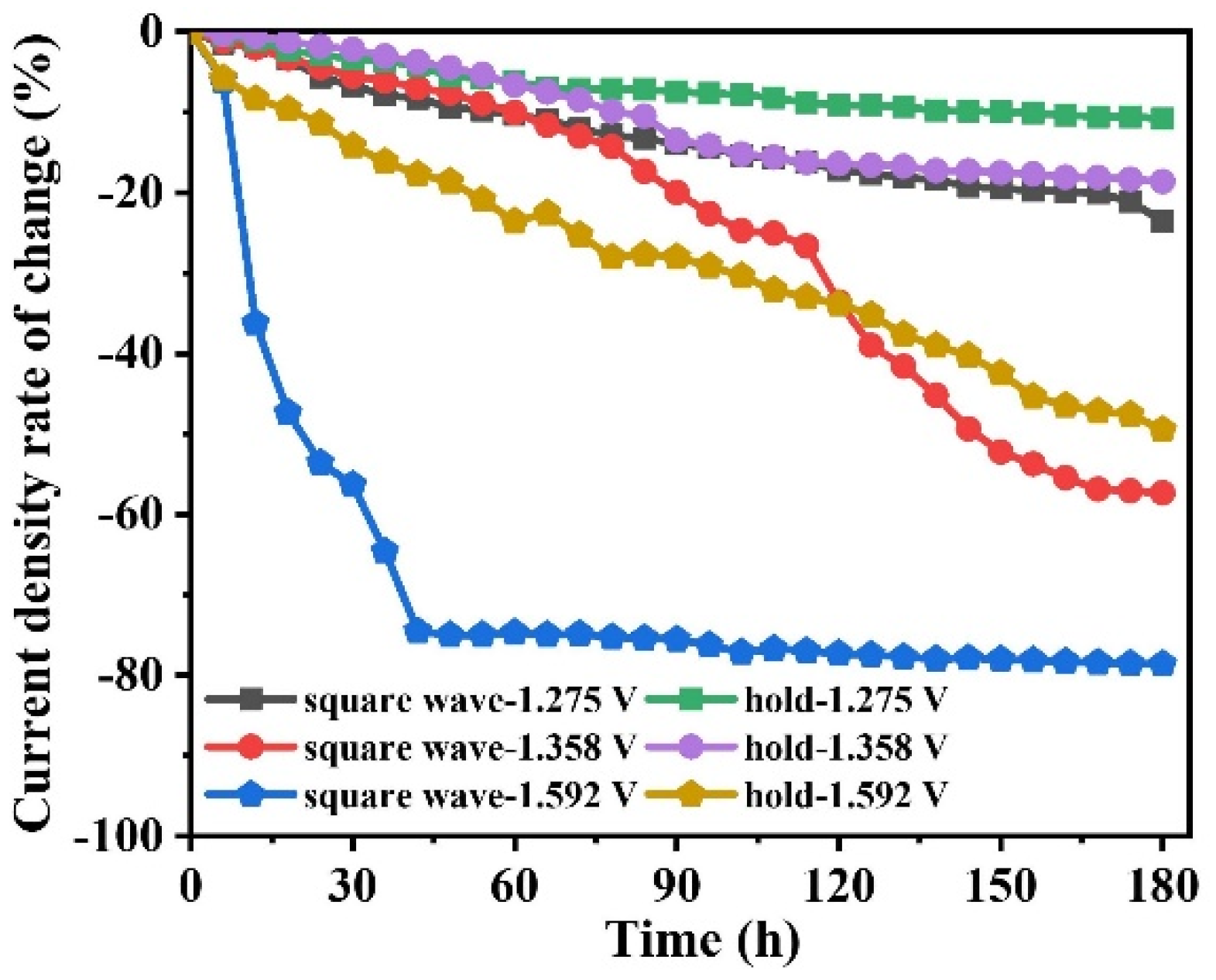
2.2.2. HER Performance Test
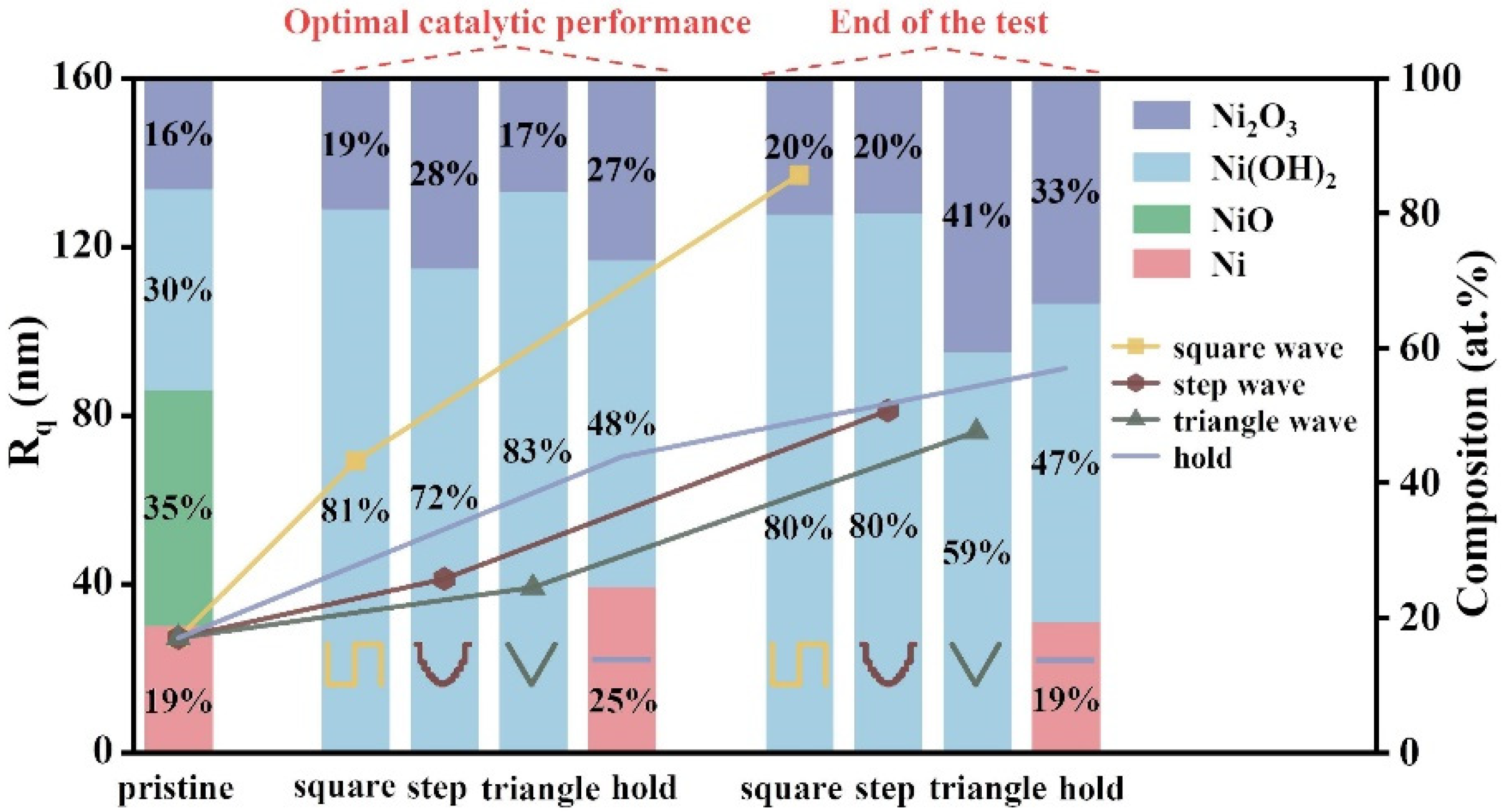
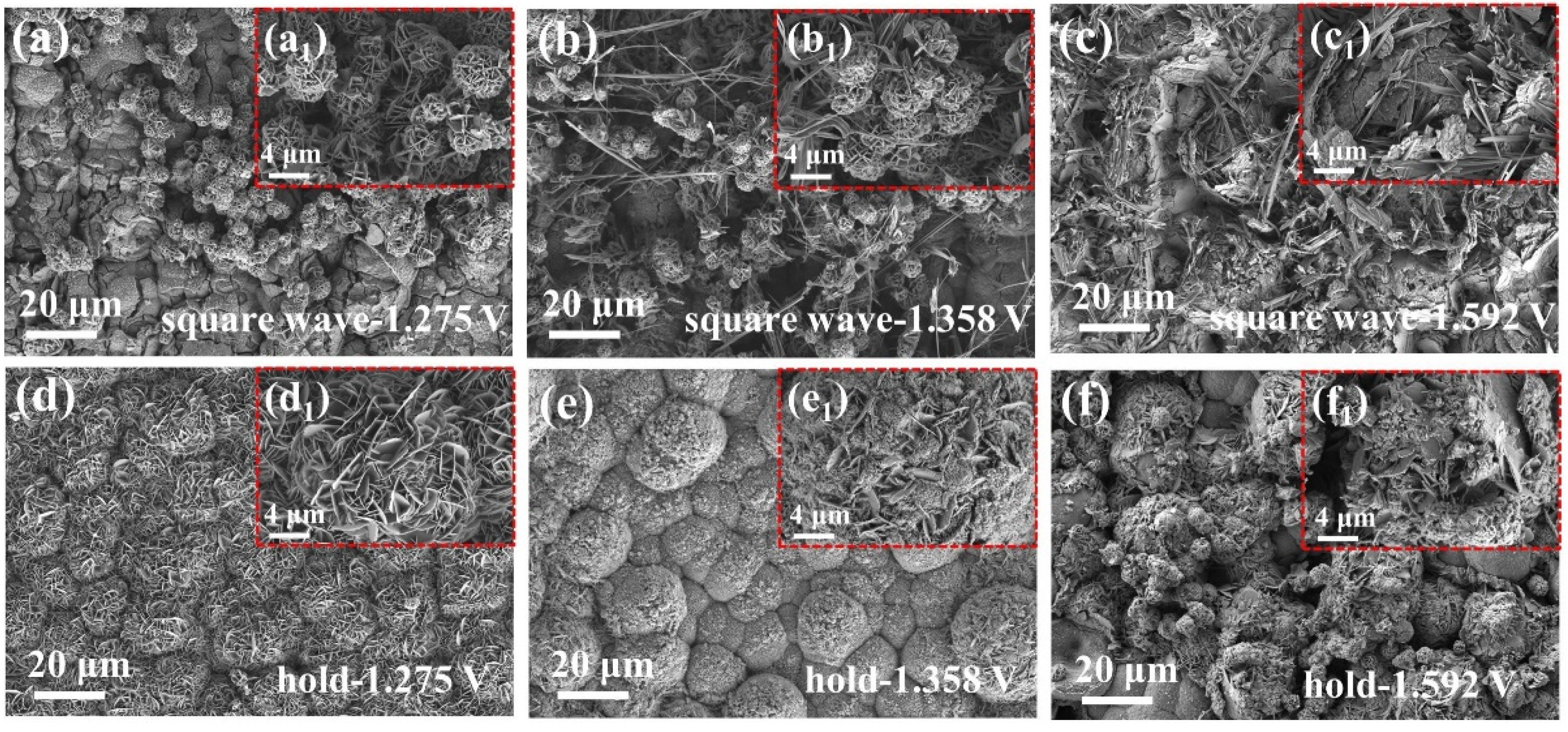
2.2.3. Surface Characterizations of FeNiMo-LDH@NiMo/SS with Fluctuating Power
3. Experimental Section
3.1. Materials
3.2. HER Electrode Preparation
3.3. HER Performance and Durability Test for Electrode under Fluctuating Power
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Ampah, J.D.; Zhao, Y.; Sun, X.; Xu, L.; Jiang, X.; Wang, S. A Perspective on the Overarching Role of Hydrogen, Ammonia, and Methanol Carbon-Neutral Fuels towards Net Zero Emission in the Next Three Decades. Energies 2023, 16, 280. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of renewable energy power fluctuations on water electrolysis for green hydrogen production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Caldeira, K.; Jain, A.K.; Hoffert, M.I. Climate Sensitivity Uncertainty and the Need for Energy Without CO2 Emission. Science 2003, 299, 2052–2054. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Acar, C.; Dincer, I. The potential role of hydrogen as a sustainable transportation fuel to combat global warming. Int. J. Hydrogen Energy 2020, 45, 3396–3406. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef] [PubMed]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Lin, B.; Wang, Y.; Song, K.; Zhang, H.; Li, Y.; Li, J.; Zheng, H.; Tang, J.; et al. Properties, mechanisms and advantages of metallic glass for electrocatalysis and HER in water splitting: A review. Int. J. Hydrogen Energy 2023, 48, 27182–27200. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: A mini review. Chem. Eng. J. 2020, 393, 124726. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef]
- Thoi, V.S.; Sun, Y.; Long, J.R.; Chang, C.J. Complexes of earth-abundant metals for catalytic electrochemical hydrogen generation under aqueous conditions. Chem. Soc. Rev. 2013, 42, 2388–2400. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Mohana, P.; Isacfranklin, M.; Yuvakkumar, R.; Ravi, G.; Kungumadevi, L.; Arunmetha, S.; Han, J.H.; Hong, S.I. Facile Synthesis of Ni-MgO/CNT Nanocomposite for Hydrogen Evolution Reaction. Nanomaterials 2024, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Shamskhou, K.; Awada, H.; Yari, F.; Aljabour, A.; Schöfberger, W. A Molecular Binuclear Nickel (II) Schiff Base Complex for Efficient HER Electrocatalysis. Catalysts 2023, 13, 1348. [Google Scholar] [CrossRef]
- Ash, P.A.; Vincent, K.A. Spectroscopic analysis of immobilised redox enzymes under direct electrochemical control. Chem. Commun. 2012, 48, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Tang, P.; Dai, L.; Yang, Y.; Li, L.; Liu, J.; Yang, M.; Deng, G. Highly Effective Electrochemical Water Splitting with Enhanced Electron Transfer between Ni2Co Layered Double Hydroxide Nanosheets Dispersed on Carbon Substrate. C 2023, 9, 94. [Google Scholar] [CrossRef]
- Wu, T.; Sun, M.; Wong, H.H.; Chan, C.H.; Lu, L.; Lu, Q.; Chen, B.; Huang, B. Recent advances and strategies of electrocatalysts for large current density industrial hydrogen evolution reaction. Inorg. Chem. Front. 2023, 10, 4632–4649. [Google Scholar] [CrossRef]
- Che, Q.; Li, Q.; Tan, Y.; Chen, X.; Xu, X.; Chen, Y. One-step controllable synthesis of amorphous (Ni-Fe)Sx/NiFe(OH)y hollow microtube/sphere films as superior bifunctional electrocatalysts for quasi-industrial water splitting at large-current-density. Appl. Catal. B Environ. 2019, 246, 337–348. [Google Scholar] [CrossRef]
- Liang, H.; Xu, M.; Asselin, E. Corrosion of monometallic iron- and nickel-based electrocatalysts for the alkaline oxygen evolution reaction: A review. J. Power Sources 2021, 510, 230387. [Google Scholar] [CrossRef]
- Wu, Z.P.; Lu, X.F.; Zang, S.Q.; Lou, X.W. Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 1910274. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Lu, W.; Cao, F. Amorphous Ni–Fe–Mo Suboxides Coupled with Ni Network as Porous Nanoplate Array on Nickel Foam: A Highly Efficient and Durable Bifunctional Electrode for Overall Water Splitting. Adv. Sci. 2020, 7, 1902034. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Y.; Tang, J.; Song, K.; Zhang, H.; Lin, B.; Zheng, H. FeNi-LDH@Ni film modified stainless-steel as self-supported electrodes for efficient and stable overall water splitting in alkaline environments. Surf. Coat. Technol. 2023, 464, 129502. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Jin, L.; Xu, H.; Du, Y. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Ma, Y.; Chen, D.; Ho, J.C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMat 2022, 4, e12357. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent Trends and Perspectives in Electrochemical Water Splitting with an Emphasis on Sulfide, Selenide, and Phosphide Catalysts of Fe, Co, and Ni: A Review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiong, D.; Petrovykh, D.Y.; Liu, L. Bifunctional Catalysts: Bifunctional Nickel Phosphide Nanocatalysts Supported on Carbon Fiber Paper for Highly Efficient and Stable Overall Water Splitting (Adv. Funct. Mater. 23/2016). Adv. Funct. Mater. 2016, 26, 4066. [Google Scholar] [CrossRef]
- Kitiphatpiboon, N.; Chen, M.; Li, X.; Liu, C.; Li, S.; Wang, J.; Peng, S.; Abudula, A.; Guan, G. Heterointerface engineering of Ni3S2@NiCo-LDH core-shell structure for efficient oxygen evolution reaction under intermittent conditions. Electrochim. Acta 2022, 435, 141438. [Google Scholar] [CrossRef]
- Kuroda, Y.; Nishimoto, T.; Mitsushima, S. Self-repairing hybrid nanosheet anode catalysts for alkaline water electrolysis connected with fluctuating renewable energy. Electrochim. Acta 2019, 323, 134812. [Google Scholar] [CrossRef]
- Alia, S.M.; Stariha, S.; Borup, R.L. Electrolyzer durability at low catalyst loading and with dynamic operation. J. Electrochem. Soc. 2019, 166, F1164. [Google Scholar] [CrossRef]
- Song, F.; Bai, L.; Moysiadou, A.; Lee, S.; Hu, C.; Liardet, L.; Hu, X. Transition Metal Oxides as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Solutions: An Application-Inspired Renaissance. J. Am. Chem. Soc. 2018, 140, 7748–7759. [Google Scholar] [CrossRef] [PubMed]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Stocks, A.; Rasimick, B.; More, K.; Xu, H. Highly Active, Durable Dispersed Iridium Nanocatalysts for PEM Water Electrolyzers. J. Electrochem. Soc. 2018, 165, F82–F89. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, F.; Ji, X.; Cui, L.; Li, C.; Liu, J. Facile generation of CeO2 nanoparticles on multiphased NiSx nanoplatelet arrays as a free-standing electrode for efficient overall water splitting. J. Colloid Interface Sci. 2024, 653, 308–315. [Google Scholar] [CrossRef]
- Niaz, A.K.; Akhtar, A.; Park, J.-Y.; Lim, H.-T. Effects of the operation mode on the degradation behavior of anion exchange membrane water electrolyzers. J. Power Sources 2021, 481, 229093. [Google Scholar] [CrossRef]
- Chen, Y.; Mojica, F.; Li, G.; Chuang, P.-Y.A. Experimental study and analytical modeling of an alkaline water electrolysis cell. Int. J. Energy Res. 2017, 41, 2365–2373. [Google Scholar] [CrossRef]
- Aykut, Y.; Yurtcan, A.B. Nanostructured electrocatalysts for low-temperature water splitting: A review. Electrochim. Acta 2023, 471, 143335. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, C.; Cai, X.; An, L.; Shen, S.; Yan, X.; Zhang, J. Bubble evolution and transport in PEM water electrolysis: Mechanism, impact, and management. Prog. Energy Combust. Sci. 2023, 96, 101075. [Google Scholar] [CrossRef]
- Uchino, Y.; Kobayashi, T.; Hasegawa, S.; Nagashima, I.; Sunada, Y.; Manabe, A.; Nishiki, Y.; Mitsushima, S. Relationship Between the Redox Reactions on a Bipolar Plate and Reverse Current after Alkaline Water Electrolysis. Electrocatalysis 2018, 9, 67–74. [Google Scholar] [CrossRef]
- Uchino, Y.; Kobayashi, T.; Hasegawa, S.; Nagashima, I.; Sunada, Y.; Manabe, A.; Nishiki, Y.; Mitsushima, S. Dependence of the Reverse Current on the Surface of Electrode Placed on a Bipolar Plate in an Alkaline Water Electrolyzer. Electrochemistry 2018, 86, 138–144. [Google Scholar] [CrossRef]
- Haleem, A.A.; Huyan, J.; Nagasawa, K.; Kuroda, Y.; Nishiki, Y.; Kato, A.; Nakai, T.; Araki, T.; Mitsushima, S. Effects of operation and shutdown parameters and electrode materials on the reverse current phenomenon in alkaline water analyzers. J. Power Sources 2022, 535, 231454. [Google Scholar] [CrossRef]
- Haleem, A.A.; Nagasawa, K.; Kuroda, Y.; Nishiki, Y.; Zaenal, A.; Mitsushima, S. A New Accelerated Durability Test Protocol for Water Oxidation Electrocatalysts of Renewable Energy Powered Alkaline Water Electrolyzers. Electrochemistry 2021, 89, 186–191. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Q.; Ng, Y.H.; Zhao, C. Reversible ternary nickel-cobalt-iron catalysts for intermittent water electrolysis. EcoMat 2019, 2, e12012. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Wang, B.; Yang, T.; Yang, S. Electrochemical formation of PtRu bimetallic nanoparticles for highly efficient and pH-universal hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 2090–2098. [Google Scholar] [CrossRef]
- Hou, Z.-Q.; Liu, R.-W.; Feng, X.-N.; Jia, X.-L.; Huang, K.-J. Durability over 11 days in electrocatalytic hydrogen evolution reaction via designing a 3D magnetic electrode and regulating the electronic structure. Fuel 2024, 357, 130054. [Google Scholar] [CrossRef]
- Babic, U.; Schmidt, T.J.; Gubler, L. Communication—Contribution of Catalyst Layer Proton Transport Resistance to Voltage Loss in Polymer Electrolyte Water Electrolyzers. J. Electrochem. Soc. 2018, 165, J3016–J3018. [Google Scholar] [CrossRef]
- Parache, F.; Schneider, H.; Turpin, C.; Richet, N.; Debellemanière, O.; Bru, É.; Thieu, A.T.; Bertail, C.; Marot, C. Impact of Power Converter Current Ripple on the Degradation of PEM Electrolyzer Performances. Membranes 2022, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Voronova, A.; Kim, H.; Jang, J.H.; Park, H.; Seo, B. Effect of low voltage limit on degradation mechanism during high-frequency dynamic load in proton exchange membrane water electrolysis. Int. J. Energy Res. 2022, 46, 11867–11878. [Google Scholar] [CrossRef]
- Morrison, A.; Leitch, J.; Szymanski, G.; Moula, G.; Barlow, B.; Burgess, I.; Shobeir, B.; Huang, H.; Lipkowski, J. Mechanism of Electrochemical Dissolution of Nickel Grown by Carbonyl Method. Electrochim. Acta 2017, 248, 112–122. [Google Scholar] [CrossRef]
- Morrison, A.; Leitch, J.; Szymanski, G.; Moula, G.; Barlow, B.; Burgess, I.J.; Shobeir, B.; Huang, H.; Lipkowski, J. Electrochemical dissolution of nickel produced by the Mond method under alternating temperatures and nickel carbonyl gas pressures. Electrochim. Acta 2018, 260, 684–694. [Google Scholar] [CrossRef]
- Iida, M.; Ohtsuka, T. Ellipsometry of passive oxide films on nickel in acidic sulfate solution. Corros. Sci. 2007, 49, 1408–1419. [Google Scholar] [CrossRef]
- Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-abundant transition-metal-based electrocatalysts for water electrolysis to produce renewable hydrogen. Chem. A Eur. J. 2018, 24, 18334–18355. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, Z.; Yang, F.; Li, J.; Liu, Z.; Ren, W.; Zhang, S.; Liu, B. Stabilized hydroxide-mediated nickel-based electrocatalysts for high-current-density hydrogen evolution in alkaline media. Energy Environ. Sci. 2021, 14, 4610–4619. [Google Scholar] [CrossRef]
- Amireh, S.F.; Heineman, N.N.; Vermeulen, P.; Barros, R.L.G.; Yang, D.; van der Schaaf, J.; de Groot, M.T. Impact of power supply fluctuation and part load operation on the efficiency of alkaline water electrolysis. J. Power Sources 2023, 560, 232629. [Google Scholar] [CrossRef]
- Liu, X.; Pei, Y.; Huang, L.; Lei, W.; Li, F.; Li, Y.; Zhang, H.; Jia, Q.; Zhang, S. Rational design of ultrahigh porosity Co foam supported flower-like FeNiP-LDH electrocatalysts towards hydrogen evolution reaction. Catal. Today 2022, 400–401, 6–13. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Dong, L.; Bin Wu, H.; Yu, X.-Y. Activating the hydrogen evolution and overall water splitting performance of NiFe LDH by cation doping and plasma reduction. Appl. Catal. B Environ. 2020, 266, 118627. [Google Scholar] [CrossRef]
- El Haleem, S.M.A.; El Wanees, S.A. Passivation of Nickel in NaOH Solutions. Prot. Met. Phys. Chem. Surfaces 2018, 54, 859–865. [Google Scholar] [CrossRef]
- He, D.; Cao, L.; Huang, J.; Kajiyoshi, K.; Wu, J.; Wang, C.; Liu, Q.; Yang, D.; Feng, L. In-situ optimizing the valence configuration of vanadium sites in NiV-LDH nanosheet arrays for enhanced hydrogen evolution reaction. J. Energy Chem. 2020, 47, 263–271. [Google Scholar] [CrossRef]
- Kulkarni, R.; Lingamdinne, L.P.; Karri, R.R.; Momin, Z.H.; Koduru, J.R.; Chang, Y.-Y. Catalytic efficiency of LDH@carbonaceous hybrid nanocomposites towards water splitting mechanism: Impact of plasma and its significance on HER and OER activity. Coord. Chem. Rev. 2023, 497, 215460. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Huang, Y. Electron-rich NiFe layered double hydroxides via interface engineering for boosting electrocatalytic oxygen evolution. Appl. Catal. B Environ. 2021, 297, 120453. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, L.; Wu, Z.; Wang, L.; Zhang, J.; Tang, Y.; Xu, L.; Xie, A.; Chen, Y.; Zhang, H.; et al. Nitrogen doped carbon encapsulated hierarchical NiMoN as highly active and durable HER electrode for repeated ON/OFF water electrolysis. Chem. Eng. J. 2022, 436, 134931. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, L.; Xiong, H.; Shao, M.; Xu, L.; Xie, A.; Zhuang, S.; Tang, Y.; Yang, X.; Chen, Y.; et al. 3D self-supported Ni nanoparticle@N-doped carbon nanotubes anchored on NiMoN pillars for the hydrogen evolution reaction with high activity and anti-oxidation ability. J. Mater. Chem. A 2019, 7, 13671–13678. [Google Scholar] [CrossRef]
- Yonas, S.; Gicha, B.B.; Adhikari, S.; Sabir, F.K.; Tran, V.T.; Nwaji, N.; Gonfa, B.A.; Tufa, L.T. Electric-Field-Assisted Synthesis of Cu/MoS2 Nanostructures for Efficient Hydrogen Evolution Reaction. Micromachines 2024, 15, 495. [Google Scholar] [CrossRef]
- Tian, Z.; Yang, L.; Wang, Z.; Xu, C.; Li, D. Cost-effective polymetallic selenides derived from stainless steel foam (SSF) for overall water splitting. Res. Chem. Intermed. 2021, 47, 4779–4787. [Google Scholar] [CrossRef]
- Anantharaj, S.; Chatterjee, S.; Swaathini, K.C.; Amarnath, T.S.; Subhashini, E.; Pattanayak, D.K.; Kundu, S. Stainless Steel Scrubber: A Cost Efficient Catalytic Electrode for Full Water Splitting in Alkaline Medium. ACS Sustain. Chem. Eng. 2018, 6, 2498–2509. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, T.; Li, Y.; Huang, Y.; Li, Y.; Balogun, M.-S.J.T. A Simple and Scalable Approach To Remarkably Boost the Overall Water Splitting Activity of Stainless Steel Electrocatalysts. ACS Omega 2019, 4, 16130–16138. [Google Scholar] [CrossRef]
- Alhakemy, A.Z.; Nassr, A.B.A.A.; Kashyout, A.E.-H.; Wen, Z. Modifying the 316L stainless steel surface by an electrodeposition technique: Towards high-performance electrodes for alkaline water electrolysis. Sustain. Energy Fuels 2022, 6, 1382–1397. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Wang, K.; Yu, J.; Liu, Q.; Liu, J.; Chen, R.; Zhu, J. Loading of Single Atoms of Iron, Cobalt, or Nickel to Enhance the Electrocatalytic Hydrogen Evolution Reaction of Two-Dimensional Titanium Carbide. Int. J. Mol. Sci. 2024, 25, 4034. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, W.; Weng, Y.; Jiang, J.; Mao, H.; Zhang, W.; Lu, T.; Long, D.; Jiang, F. Intercalated PtCo Electrocatalyst of Vanadium Metal Oxide Increases Charge Density to Facilitate Hydrogen Evolution. Molecules 2024, 29, 1518. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, J.; Hu, Y.; Wang, L.; Zhang, X.; Zhao, B. Ni Doped Co-MOF-74 Synergized with 2D Ti3C2Tx MXene as an Efficient Electrocatalyst for Overall Water-Splitting. Catalysts 2024, 14, 184. [Google Scholar] [CrossRef]
- Gultom, N.S.; Chen, T.-S.; Silitonga, M.Z.; Kuo, D.-H. Overall water splitting realized by overall sputtering thin-film technology for a bifunctional MoNiFe electrode: A green technology for green hydrogen. Appl. Catal. B Environ. 2023, 322, 122103. [Google Scholar] [CrossRef]
- Li, J.; Lin, B.; Zheng, H.; Wang, Y.; Zhang, H.; Zhang, Y.; Nie, Z.; Tang, J. Study on pitting corrosion behavior and semi in-situ pitting corrosion growth model of 304 L SS with elastic stress in NaCl corrosion environment. Corros. Sci. 2023, 211, 110862. [Google Scholar] [CrossRef]





| HER Catalyst | Overpotential at 10 mA cm−2 (mV) | Ref. |
|---|---|---|
| FeNiMo-LDH@NiMo/SS | 245 | This work |
| Plate Ni cathode | 430 | This work |
| NF | 378 | [63] |
| SSFSe | 237 | [64] |
| SS Scrubber | 418 | [65] |
| SSM-Ni | 346 | [66] |
| NiP@SS | 268 | [67] |
| FeNi LDH/NF | 315 | [68] |
| TiC/NiSA | 202 | [69] |
| 6-CMS | 249 | [63] |
| PtCoV/g-C3N4 | 346 | [70] |
| CoNi0.04-MOF-74/NF | 203 | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Lin, B.; Zhang, H.; Wang, Y.; Wang, H.; Tang, J.; Zou, C. Influence of Power Fluctuation on Ni-Based Electrode Degradation and Hydrogen Evolution Reaction Performance in Alkaline Water Splitting: Probing the Effect of Renewable Energy on Water Electrolysis. Catalysts 2024, 14, 307. https://doi.org/10.3390/catal14050307
Liu C, Lin B, Zhang H, Wang Y, Wang H, Tang J, Zou C. Influence of Power Fluctuation on Ni-Based Electrode Degradation and Hydrogen Evolution Reaction Performance in Alkaline Water Splitting: Probing the Effect of Renewable Energy on Water Electrolysis. Catalysts. 2024; 14(5):307. https://doi.org/10.3390/catal14050307
Chicago/Turabian StyleLiu, Congying, Bing Lin, Hailong Zhang, Yingying Wang, Hangzhou Wang, Junlei Tang, and Caineng Zou. 2024. "Influence of Power Fluctuation on Ni-Based Electrode Degradation and Hydrogen Evolution Reaction Performance in Alkaline Water Splitting: Probing the Effect of Renewable Energy on Water Electrolysis" Catalysts 14, no. 5: 307. https://doi.org/10.3390/catal14050307
APA StyleLiu, C., Lin, B., Zhang, H., Wang, Y., Wang, H., Tang, J., & Zou, C. (2024). Influence of Power Fluctuation on Ni-Based Electrode Degradation and Hydrogen Evolution Reaction Performance in Alkaline Water Splitting: Probing the Effect of Renewable Energy on Water Electrolysis. Catalysts, 14(5), 307. https://doi.org/10.3390/catal14050307







