Abstract
The main groups of catalytic materials used in the conversion of methanol to dimethyl ether (the MTD process) were presented with respect to their advantages, disadvantages, and the methods of their modifications, resulting in catalysts with improved activity, selectivity, and stability. In particular, the effects of strength, surface concentration, and the type of acid sites, the porous structure and morphology of the catalytic materials, the role of catalyst activators, and others, were considered. The prosed mechanisms of the MTD process over various types of catalysts are presented. Moreover, the advantages of membrane reactors for the MTD process are presented and analysed. The perspectives in the development of effective catalysts for the dehydration of methanol to dimethyl ether are presented and discussed.
1. Introduction
Dimethyl ether (DME, methoxymethane) is the simplest ether, with the formula CH3OCH3. DME is a colourless and odourless gas which was first synthesised by Jean-Baptiste Dumas and Eugene Péligot in 1835 by the distillation of methanol and sulphuric acid [1]. Currently, dimethyl ether is produced from various raw materials such as natural gas, methanol, biomass, and coal. The low boiling point and zero sulphur content are the main properties of DME, which results in its continuous interest as a solvent in the chemical and petrochemical industries [2], as well as for the production of a variety of chemicals, such as diethyl sulphate, oxygenates, and olefins [3,4,5]. DME has been used as propellant and, interestingly, was the propellant used in the first aerosol package produced by its inventor, Erik Andreas Rotheim, in 1928 [6]. DME is also considered a promising raw material in fuel cells because it can be efficiently converted to hydrogen at low temperatures [7]. Another important area of DME application, with its increasing prognostic role in the future, is its blending with liquified petroleum gas (LPG) and the use of such DME-LPG blends in transportation and power generation [8]. The mixing of LPG and DME is a cost-effective way to reduce emissions from the existing LPG infrastructure, also making it an attractive option for household cooking and heating [8]. The combustion of DME-LPG blends was reported to reduce CO2 emissions by 30–80% and NOx emissions by 5–15% compared with the combustion of LPG [9]. Moreover, the growth of the DME market is expected due to the growing demand for aerosol propellants to replace the banned chlorofluorocarbons which are destroying the ozone layer [9]. DME production is also stimulated by an increasing interest in the use of dimethyl ether as a cleaner alternative to traditional fuels, such as diesel and gasoline [10]. Dimethyl ether has a higher cetane number than diesel, which improves the combustion efficiency. Moreover, the leakage of the C–C bound in the DME molecule reduces the formation and emission of particulate matter. In addition, the emission of other pollutants, such as NOx, is significantly reduced [11]. Furthermore, the replacement of diesel fuel for DME does not require significant changes in exiting engines [12]. DME was reported to have total compliance with the highly strict California ultra-low-emission vehicle (ULEV) regulations for medium-duty vehicles and has the highest efficiency of all synthetic liquid fuels, such as F-T diesel and methanol [13]. The growth of the role of dimethyl ether in the automotive segment is expected to continue in the coming years, mainly due to the increasing demand for cleaner fuels, rising energy prices, and stricter environmental regulations. The global DME market will possibly increase from USD 4.8 billion in 2022 to USD 12.8 billion in 2032 [9]. Today, more than 65% of the DME produced globally is blended with LPG and used for transportation, as well as domestic cooking and heating [14].
DME can be produced by methanol dehydration (methanol to DME, the MTD process, which is an indirect synthesis) in the presence of acid catalysts according to the following Equation (1):
2 CH3OH → CH3OCH3 + H2O
This is an exothermic reaction that occurs without variation in the mole number, and which is not thermodynamically affected by reaction pressure, whereas its thermodynamics is favoured at a low temperature [9]. Thermodynamic limitations in this case could be overcome by the selective removal of water from the reaction system, owing to the Le Chatelier principle, thus enhancing the conversion and selectivity of methanol dehydration to DME [15]. Various attempts, including the use of hydrophilic membranes for in situ water removal during methanol dehydration, were proposed [16,17,18,19].
Another method uses synthetic gas (CO and H2) which, in the first step, is converted to methanol in the presence of the Cu-ZnO-Al2O3 redox catalyst in the temperature range of 240–280 °C and a pressure between 30 and 70 bar, followed by the methanol dehydration reaction (Equation (1)) over the acid catalyst to obtain DME. In this case, the reaction (syngas to DME, direct synthesis) can be summarised by the following equation:
2 CO + 4 H2 → CH3OCH3 + H2O
The conversion of synthetic gas to DME takes place in the same reactor under process conditions similar to those of methanol synthesis, but in the presence of two catalyst beds [20] or, alternatively, a bifunctional catalyst with redox and acidic functions [21,22]. The additional benefit of this process is the shifting of the equilibrium of methanol synthesis by means of the alcohol dehydration to DME and increasing the conversion of the synthesis gas [23]. A very promising alternative to the direct synthesis of dimethyl ether from syngas is the direct synthesis of DME from the mixture containing CO2 and H2 [24], as follows:
2 CO2 + 6 H2 → CH3OCH3 + 3 H2O
This reaction, due to carbon dioxide utilization and the higher intrinsic reaction rate of CO2 hydrogenation compared to CO hydrogenation [25,26], is a very promising synthesis route and is one of the core technologies for carbon dioxide utilization [27]. CO2 hydrogenation can be limited by several factors, including the thermodynamic inertness of carbon dioxide, the generation of water as a by-product, and the formation of coke deposits [28]. Thus, there are still some limitations and problems related to direct and indirect DME synthesis that need to be solved, considering the significant prognosed increase in its production. Although the direct production of DME appears to be more efficient, the presence of unreacted syngas and CO2 in the product streams makes the next purification steps difficult [29]. On the other hand, the indirect DME synthesis method results in a higher purity of the product, and a higher conversion of methanol makes some profits for the associated industries [29]. Therefore, the indirect method has its advantages and cannot be completely abandoned.
The paper reviews recent achievements, trends, and perspectives in the research and technologies of dimethyl ether production by methanol dehydration. The most important and promising trends, as well as problems, are presented and analysed to predict the future direction of the development of DME technologies.
2. Methanol to DME (MTD Process)
The indirect DME synthesis method consists of two separate steps. In the first step, methanol is produced and purified, while, in the second step, methanol is dehydrated into DME, according to Equation (1), in a separate reactor. The dehydration of methanol to DME is an exothermic reaction, and, therefore, DME production is favoured at a low temperature.
Akarmazyan et al. [30] presented a thermodynamic analysis of the methanol dehydration reaction to DME (Equation (1)) carried out using Outokumpu HSC Chemistry® software (Espoo, Finland). The results of this analysis are shown in Figure 1, where the profiles of the Gibbs free energy (ΔG0T), entropy (ΔS0T), and enthalpy (ΔH0T) changes in the reaction are plotted as functions of the reaction temperature. The reaction is exothermic, and enthalpy (ΔH0T) and entropy (ΔS0T) decrease with the increasing temperature. Therefore, the Gibbs free energy (ΔG0T) increases with the temperature, and at temperatures higher than 525 °C, where ΔG0T takes positive values, the reaction is not favoured thermodynamically.
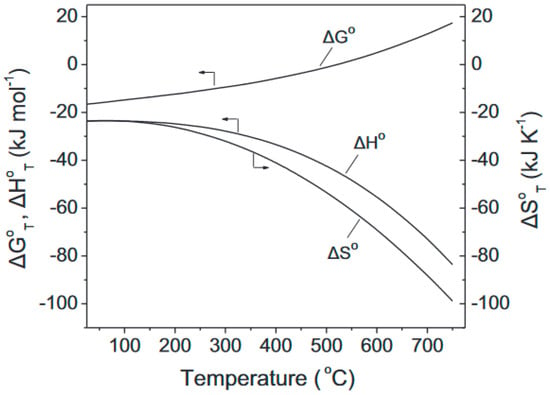
Figure 1.
Entropy (ΔS), Gibbs free energy (ΔG), and enthalpy (ΔH) changes from methanol dehydration to the DME reaction as functions of the reaction temperature. Reprinted from [30], copyright (2014), with permission from Elsevier.
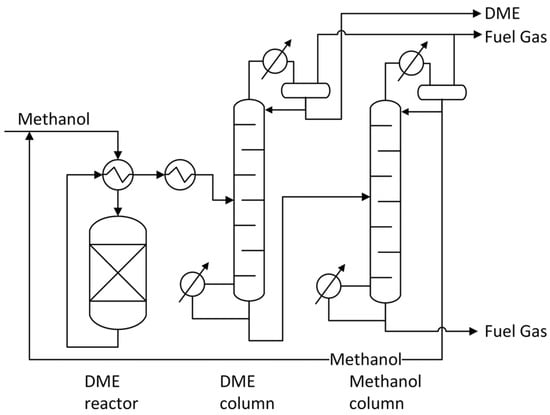
The flow diagram presenting the MTD process is shown in Scheme 1. Pure methanol is fed to the flow catalytic reactor, where methanol is dehydrated to DME (Equation (1)). The outlet stream from the reactor contains methanol, water, and DME. Moreover, some other chemicals, such as CH4, CO, CO2, and H2, can be present in the outlet stream [31]. The product mixture from the reactor is fed to the DME column, where dimethyl ether is separated from the other components of the outlet stream. The water and methanol mixture is striped from the bottom of this column and fed into the Methanol column, with depressurisation by the valve, where methanol and water are separated. Recovered methanol is recycled and reintroduced into the reactor [32]. In one pass, the conversion of methanol is in the range of 70–85% at typical operating conditions of 220 to 400 °C and a low pressure of 1–30 bar. Methanol consumption is approximately 1.4 tMethanol/tDME [31].
Scheme 1.
Flow diagram of the DME synthesis process. Reprinted from [31], copyright (2020), with permission from the Royal Society of Chemistry.
2.1. Al2O3-Based Catalysts
The conversion of methanol to DME requires acidic catalysts [33]; however, the efficiency of this reaction depends on the chemical nature of the acid sites, their strength, and location [34]. Al2O3, zeolites, and surface-modified silicas belong to the most intensively studied and most effective catalytic systems for the methanol-to-DME dehydration reported in the scientific literature [35,36].
Sung et al. [37] reported that the most catalytically active forms of alumina are γ-Al2O3 and η-Al2O3, while α-Al2O3 and κ-Al2O3 present a significantly lower catalytic potential for the process studied. γ-Al2O3 is characterised by a high surface area, excellent thermal stability, and mechanical resistance, as well as surface acidity, and was therefore intensively studied as a catalyst for the conversion of methanol to DME [38,39]. It is assumed that the active centres of aluminium oxide in the MTD reaction are Lewis-type acid sites (only this type of acid site was identified by the FT-IR analysis of the samples pre-adsorbed with pyridine). The highest concentrations of acid sites, determined by the NH3-TPD method, were found for γ-Al2O3 (36.4 μmol/g) and η-Al2O3, (24.4 μmol/g), while, for other alumina, including α-Al2O3 and κ-Al2O3, the surface concentration of the acid sites was below 21 μmol/g. Surprisingly, η-Al2O3 presented better catalytic activity than γ-Al2O3, indicating that not only concentration of acid sites but also other parameters influence the catalytic properties of alumna. The advantage of γ-Al2O3 is its high selectivity to the DME, possibly due to the lower contribution of strong acid sites compared to α-Al2O3. Akarmazyan et al. [30] reported 100% DME selectivity to DME for γ-Al2O3 catalysts in the temperature range of 150–325 °C. Above this temperature, by-products such as CO and CH4 are formed. The presence of water vapour in the system reduces the activity of γ-Al2O3 catalysts in methanol dehydration by the adsorption of water molecules at active sites, thereby blocking methanol molecules. Research by Akarmazyan et al. shows [30] that the deactivation of the γ-Al2O3 catalyst by water is a reversible process. Furthermore, the authors note that the activity of the γ-Al2O3 catalyst in the MTD process depends on the textural properties, such as the specific surface area, shape, distribution, and volume of pores, the pore size, the degree of crystallinity, and the surface concentration of acid sites [30]. Seo et al. [40] indicate that one of the key factors determining the activity of an MTD catalyst is the surface density of the acid sites. Two types of alumina, γ-Al2O3 and η-Al2O3, calcined at different temperatures were studied as catalysts of methanol-to-DME dehydration. The FT-IR analysis of the samples pre-adsorbed with pyridine showed that only the Lewis-type acid sites were present on the alumina surface; however, the surface concentration of the acid sites in γ-Al2O3 was slightly higher (67.7 μmol/g) compared to η-Al2O3 (61.6 μmol/g; in both cases, the results for the samples calcined at 500 °C). The dehydration process of methanol to dimethyl ether was catalysed by acid centres of both weak and medium strength with the contributions 45.6 and 45.7% of the total acid site concentrations in γ-Al2O3 and η-Al2O3 calcined at 500 °C. However, the presence of strong acid centres favoured the formation of by-products and carbon deposits on the surface of the catalyst, which led to a decrease in its activity [41,42].
Alumina can be obtained by various methods. One of them is precipitation followed by appropriate thermal treatment. The synthesis conditions, such as the type of precursor used and the calcination temperature, may significantly affect the physicochemical properties, and therefore their activity and stability [42,43]. Rahmanpour et al. [44] used an ultrasonic-assisted precipitation method, which resulted in nano-crystalline γ-Al2O3 (1–2 nm) active in methanol to the dehydration of DME. On the other hand, Keshavarz et al. [45] synthesised γ-Al2O3 by the modified sol–gel method using cationic surfactants. In this way, nano-crystalline, mesoporous γ-Al2O3 was obtained. Probably, the presence of surfactants prevented the aggregation of particles. The increased catalytic activity of such alumina is related to the better availability of active sites [45]. Hosseini et al. [46] compared the catalytic performance of γ-Al2O3 obtained by various methods in the MTD process. The catalysts obtained by the sol–gel method (both in aqueous and anhydrous media), characterised by a nano-crystalline structure, was found to be more active than those obtained by the precipitation method [46].
A disadvantage of γ-Al2O3 is its hydrophilic nature, and therefore the tendency to adsorb water more strongly than methanol, resulting in a partial loss of its activity in the MTD process [39,47]. Akarmazyan et al. [30] studied a large number of different γ-Al2O3 samples, including commercial alumina supplied by various producers and samples prepared in their laboratories. They formulated a general trend that catalytic performance is significantly improved with an increase in the specific surface area of the γ-Al2O3 samples, which was evidenced by a change in the methanol conversion profile towards lower reaction temperatures. The authors postulated that the enhanced catalytic activity of the high-surface-area samples was attributed not only to the higher concentration of the surface acid sites, active in methanol conversion, but also to other parameters, such as textural properties and the degree of crystallinity. Furthermore, it was shown that methanol conversion was generally higher for materials with a cylindrical pore, a high total porosity of 0.60–0.80 cm3g−1, and a crystallite size of about 7–9 nm [30]. Probably, these parameters are related with the high contribution of the crystalline alumina, which, in contrast to its highly amorphous form, is catalytically active in methanol-to-DME dehydration. The apparent activation energy of the reaction, determined for all the studied γ-Al2O3 catalysts, is nearly the same (24 ± 4 kcal mol−1). As mentioned, the presence of water, which is the reaction by-product (Equation (1)), limits the efficiency of the DME production by competitive adsorption with methanol. For the alumina catalysts, it was shown that the presence of water vapour in the reaction mixture did not influence the selectivity to the reaction products, but resulted in an increase in the apparent activation energy and a reversible shift of the methanol conversion profile into higher temperatures [30]. Sahebdelfar et al. [33] studied the effect of γ-Al2O3 deactivation induced by the presence of water vapour. It was postulated that the predominant deactivation mechanism was related to the reversible exothermic partial hydration of the active γ-Al2O3 phase to the less catalytically active boehmite (Equation (4)), as follows:
γ-Al2O3 + H2O → 2 γ-AlO(OH)
However, at higher reaction temperatures, this mechanism ceased at the expense of the irreversible sintering of the alumina crystallites [48]. Raoof et al. [49] showed that catalyst deactivation occurred very slowly when pure methanol was used as the process feed; however, adding water to methanol resulted in a rapid deactivation of γ-alumina. It was shown that an increase in the water vapour content to 20 wt.% caused a catalyst activity loss of more than 10-fold compared to the process with pure methanol. Thus, γ-alumina is not a suitable catalyst for the dehydration of crude methanol obtained without purification and containing 10–20 mol% water due to the partial loss of its catalytic activity [50]. Another deactivation pathway could be related to the formation of carbon deposits on the catalyst surface, which are intensified by stronger acid sites. On the surface of γ-Al2O3, mainly weak and moderate acid sites of the Lewis type are present, and therefore the catalyst coking in this case is limited [30].
The catalytic activity of γ-alumina in methanol-to-DME dehydration can be increased by using γ-alumina in the nano-size form. Rahmanpour et al. [44] successfully synthesised nano-size γ-Al2O3 (crystallite sizes of 1–2 nm) using the precipitation method under ultrasonic vibration. The catalyst obtained was characterised by the increased concentration of surface acid sites (88.3 μmol/g), mainly of weak and medium strength (with a contribution of approximately 90%), and therefore a relatively high catalytic activity in the reaction studied. Yaripour et al. [51] reported the promising catalytic performance of a series of γ-Al2O3 nano-catalysts prepared by the precipitation method and modified with various amounts of silica (0–15 wt.%). It was shown that the γ-alumina nano-catalyst, containing 2 wt.% SiO2, exhibited the highest activity in the studied series of the Al2O3-SiO2 catalysts. The results of the catalytic studies were related to the number of acidic sites in the modified nano-catalysts, which were increased by Al2O3 modification with silica up to 2 wt.% of its content, and afterward decreased. This effect could be explained by the possible formation of Brønsted-type sites, ≡Si–O(H)–Al≡, after the deposition of small amounts of silica. On the other side, the deposition of larger amounts of silica resulted in covering part of the alumina surface by SiO2 and blocking the access to acid centres located on its surface.
Apart from γ-Al2O3, η-Al2O3 was also reported to present interesting catalytic properties in methanol-to-DME dehydration [42,52,53]. η-alumina, characterised by a cubic structure, can be obtained in the form of fine particles, and therefore a material with a high surface area and relatively high stability [52]. Osman et al. [42] showed that γ-Al2O3 and η-Al2O3 can be prepared from different precursors, such as aluminium chloride and nitrate, respectively. The obtained alumina samples presented different surface morphologies and acidity. η-Al2O3 showed a higher catalytic activity in the methanol-to-DME dehydration process than commercial zeolite at reaction temperatures above 275 °C. The synthesis, which started with an aluminium nitrate solution precipitated by an ammonia solution, followed by drying at 120 °C and calcination at 550 °C, resulted in nano-size η-Al2O3 (with a crystallite size of about 5.5 nm) [53]. The η-Al2O3 catalyst showed the presence of mainly weak and medium-strength acid sites of the Lewis type, which were postulated to be active in methanol-to-DME conversion as well as inactive in the formation of carbon deposits. Kinetic studies have shown that methanol conversion significantly increased with the increasing catalyst weight, while it decreased dramatically with the increasing methanol or water content in the reaction feed at reaction temperatures below 250 °C [53].
Al2O3, which is the most widely used catalyst for the reaction of methanol to DME, was modified with various transition metals, such as Cu or Ag, introduced by the wet-impregnation method, to improve the Lewis acidity, as they act as electron acceptors [54,55]. The surface concentration of the Lewis acid sites in η-Al2O3 increased by about 2.3% after the deposition of 1 wt.% Ag and nearly by 15% after the introduction of 15 wt.% Ag. Osman et al. [42] showed the increased catalytic activity of the Ag/η-Al2O3 catalyst compared to pure η-Al2O3 (Figure 2a). Optimal catalytic properties were obtained for the catalyst containing 10 wt.% silver deposited on the alumina. The activating role of silver deposition on the alumina was attributed to the generation of additional Lewis-type acid sites, active in methanol dehydration, as well as changing the surface from superhydrophilic to hydrophilic, therefore limiting the adsorption of water on the catalyst surface, which resulted in the improvement of the catalyst stability under reaction conditions (Figure 2b). A similar effect was observed for γ-Al2O3 modified with copper [55]. It was reported that the deposition of copper on γ-Al2O3 significantly improved its catalytic performance in methanol-to-DME conversion. It was shown that the catalysts calcined at 550 °C exhibited higher activity than those calcined at 350 °C, which was explained by the thermally induced phase transformation of γ-AlOOH (boehmite) to γ-Al2O3, which occurred only for the samples calcined at higher temperatures. Deposition of 1 wt.% Cu into γ-Al2O3 increased the acid site concentrations by about 6%, while the introduction of 6 wt.% Cu increased the concentrations by nearly 34%. Deposition or larger amounts of copper resulted in the decrease in the surface acidity of the samples. The optimal copper loading was found to be 6 wt.% Cu/γ-Al2O3, resulting in improved stability under steady-state conditions. This effect was attributed to the enhancement in the surface acidity and hydrophobicity.
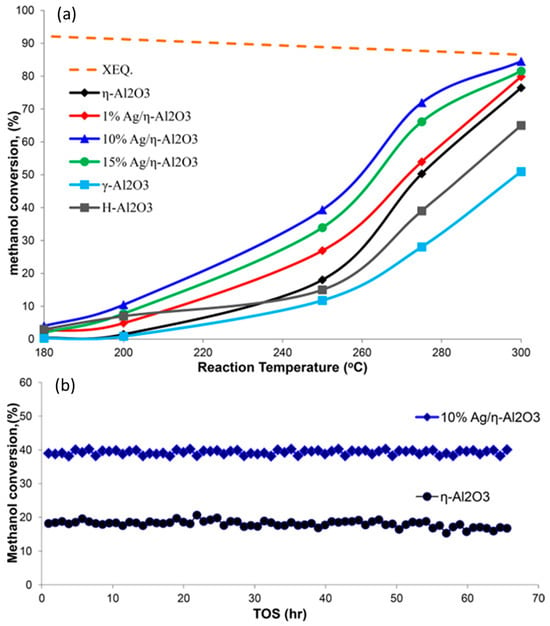
Figure 2.
Catalytic activity profiles: (a) The effect of reaction temperature on methanol dehydration to DME with different Ag loading, along with the superhydrophobic alumina (H-Al2O3); reaction conditions: T = 180–300 °C; catalyst weight = 50 mg; He flow rate = 80 mL/min; WHSV: 48.4 h−1. (b) Time-on-stream test at 250 °C for the 10% Ag/η-Al2O3 and pure η-Al2O3 catalysts. Reprinted from [54], copyright (2017), with permission from the American Chemical Society.
Another interesting attempt to activate alumina in methanol-to-DME dehydration was the study of Al2O3-SiO2 systems. Jo et al. [56] deposited silica on η-Al2O3 by the impregnation method. It was shown that, depending on the silica–alumina ratio, the surface concentration of acid sites can be increased or decreased. An increase in the Si content to 0.5 wt.% resulted in an increase in the surface concentration of the acid sites in the samples (from 429.6 μmol/g measured for η-Al2O3 μmol/g to 576.8 μmol/g for η-Al2O3 doped with 0.5 wt.% silica), while a higher loading decreased the surface concentration of the acid sites (228.8 μmol/g for η-Al2O3 doped with 5 wt.% silica). It was shown that the deposition of small amounts of silica resulted in the formation of stronger acid sites (the strength of the acid sites was compared by the positions of the ammonia desorption peaks in the NH3-TPD profiles). Possibly, such sites were created by the formation of Brønsted-type sites, ≡Si–O(H)–Al≡. On the other hand, the deposition of larger amounts of silica resulted in covering part of the alumina surface by SiO2, which finally blocked access to the acid centres located on its surface. The concentration of surface acid sites was very well correlated with the activity of the studied catalysts. It appears possible that the improved catalytic performance of the SiO2/η-Al2O3 system is also associated with the limitation of η-Al2O3 superhydrophilicity by the deposited silica, and therefore the increase in the adsorption of methanol on the catalyst surface. Of course, this hypothesis should be proved by future studies. Similar studies were conducted by Yaripour et al. [51], who verified the catalytic properties of the γ-Al2O3/SiO2 nano-aggregates in methanol-to-DME dehydration. They showed an increase in the DME yield for systems with a relatively low silica content compared to pure γ-Al2O3. The maximum yield of dimethyl ether was obtained in the presence of the catalyst containing 2 wt.% SiO2 deposited into γ-Al2O3.
Another attempt to improve the catalytic efficiency of γ-Al2O3 was its doping with titanium. Khaleel [57], who analysed the catalytic properties of γ-Al2O3 doped with titanium (3 to 20 wt.%), showed that catalysts containing small amounts of titanium were more active and selective toward DME compared to non-modified γ-Al2O3. The best catalytic activity was found for alumina modified with 3 wt.% titanium. This activating effect was assigned to the presence of well-dispersed Ti4+ cations on the alumina matrix, which possibly played the role of additional, relatively weak acid sites active in the conversion of methanol to DME. On the other hand, the deposition of higher amounts of titanium resulted in the formation of TiO2 phases, which were less active in the MTD reaction [57].
Liu et al. [58] tried to intensify the conversion of methanol to DME at lower temperatures (240–260 °C) by the modification of γ-Al2O3 with niobium oxide. The samples prepared using the impregnation method contained 1, 5, or 10 wt.% of Nb2O5. Among these samples, the most promising results of the catalytic tests were obtained for the catalyst with the highest loading of Nb2O5. The activating role of the introduction of niobium oxide to γ-Al2O3 was assigned to the generation of additional relatively weak acid sites, active in the dehydration of methanol. Furthermore, it was shown that Nb2O5 deposition shifted the reaction into lower temperatures with the maintenance of the high selectivity to DME [58].
Acid sites, as already mentioned, are necessary to activate methanol molecules to be converted into DME. It is postulated [30] that the interaction of methanol with the Al2O3 surface results in the formation of two kinds of methoxy groups of different adsorption strengths. Methoxy species that are weakly adsorbed on the Al2O3 surface are converted to DME, while more strongly held methoxy species decompose to yield formate and, eventually, CH4 and CO in the gas phase. Thus, it clearly shows that the appropriate strength of acid sites, modulated by the methods mentioned above, is very important to effectively convert methanol to DME.
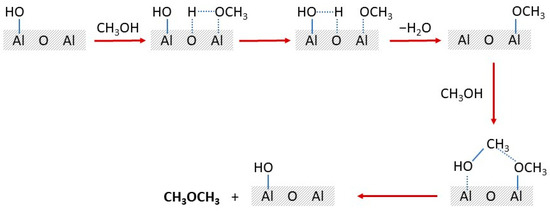
The proposed general mechanism of methanol-to-DME dehydration over the alumina surface is shown in Scheme 2 [59]. The methanol molecule interacts with the surface alumina oxygen anion and aluminium cation. In the next step, the hydrogen of the methanol hydroxy group reacts with the surface –OH with the formation of the water molecule. Finally, the surface methoxy group reacts with the next methanol molecule, resulting in DME.
Scheme 2.
Proposed reaction mechanism of methanol dehydration to DME over alumina catalysts.
2.2. Zeolite-Based Catalysts
Zeolites, in addition to alumina, are important catalysts for methanol-to-DME dehydration. The great potential of zeolites in the MTD process is related to their high surface area, uniform porous structure, and high thermal and hydrothermal stability (higher resistance to water compared to γ-Al2O3), but the most important appears to be their surface acidity. The number of acid sites (both the Lewis and Brønsted types) can be regulated by the Si/Al ratio [9,43]. Typically, acid sites in zeolites are stronger than in alumina, which results in the faster formation of carbon deposits on the surface of zeolite catalysts, and ultimately leads to a decrease in their activity and selectivity to DME. The causes of by-product formation and catalyst deactivation are believed to be acid centres that are too strong and which are in a microporous structure, hindering the diffusion of reagents by formed coke particles [5,9,43].
ZSM-5 zeolite was reported to be an effective catalyst for the MTD reaction. Vishwanathan et al. [60] compared the catalytic properties of ZSM-5 and γ-Al2O3. ZSM-5 had a higher methanol conversion (approximately 80%) than γ-Al2O3 at 230 °C. The same level of methanol conversion was achieved in the presence of γ-Al2O3 at approximately 320 °C [60]. However, ZSM-5 showed a lower selectivity to DME above 270 °C and a limited stability due to the formation of carbon deposits [60]. Moreover, it was shown that the introduction of sodium into ZSM-5 resulted in a decrease in strong acid site concentrations (by nearly 80% for the sample with 80 mol% Na) and an increase in weak acid sites (by about 56% for the sample with 80 mol% Na). The acid sides were classified as weak, medium, and strong by the temperature range of ammonia desorption in the NH3-TPD measurement—weak acid sites: 120–220 °C, medium sites: 220–390 °C, and strong sites: 390–570 °C. The samples modified with sodium presented high stability in the MTD process (15 h test), indicating the positive role of sodium in limiting of carbon deposit formation. Hassanpour et al. [61] studied the catalytic performance of ZSM-5 catalysts with different Si/Al ratios. It was shown that the Si/Al ratio significantly affected the acidity, and therefore also the activity, of the catalysts (Figure 3). The authors reported that the surface acidity of ZSM-5 increased with an increase in the Si/Al molar ratio from 25 to 125 (186 and 577 μmol/g, respectively), and decreased with a further increase in the Si/Al ratio from 125 to 250 (577 and 298 μmol/g, respectively). This surprising effect could be explained by the formation of the extra-framework alumina species in addition to the incorporation of aluminium into the zeolite structure in the case of the samples with a high intended aluminium content. Such extra-framework alumina species are characterised by a lower content of acid sites. The ZSM-5 sample with the Si/Al molar ratio of 125 showed the best catalytic properties for the MTD process. Moreover, it was shown that zeolites with the largest number of acid centres were characterised by the largest contribution of medium-strength sites effective in methanol-to-DME dehydration. According to Rownaghi et al. [62], another parameter that influences the activity of ZSM-5 is the size of the crystallites. The sample with the smallest ZSM-5 crystallites (with a size of about 120 nm) presented higher catalytic activity than the samples composed of larger crystallites. It was postulated that smaller crystallites allowed easier access of the reagents to the catalytic centres. However, the efficiency of ZSM-5 catalysts may also be influenced by the number of acid sites on the outer surfaces of crystallites [62].
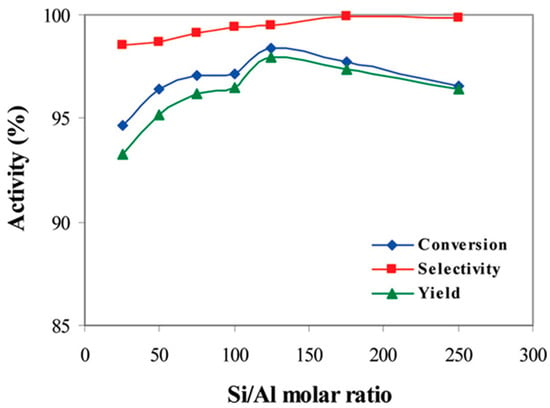
Figure 3.
Variation in the methanol activity of H-ZSM-5 catalysts as a function of the Si/Al molar ratio at T = 573 K, P = 16 bar, and WHSV = 26.5 h−1. Reprinted from [61], copyright (2010), with permission from the American Chemical Society.
The effect of acid sites that are too strong, which are responsible for the decreased selectivity to DME and the increased deactivation due to the formation of carbon deposits, can be improved by the deposition of basic elements. Vishwanathan et al. [60] reported that the deposition of sodium on the surface of ZSM-5 significantly improved the selectivity to DME in the temperature range of 230–340 °C. The introduction of sodium into ZSM-5 reduced its surface acidity mainly by eliminating strong acid sites [60]. Hassanpour et al. [63] tested ZSM-5 zeolites modified with sodium introduced by the impregnation method. It was postulated that mainly strong acid sites were neutralised by sodium. The zeolitic catalysts modified with sodium presented increased resistance for the formation of carbon deposits compared to non-modified ZSM-5 [63]. On the other hand, Kim et al. [64] decreased the surface acidity of H-ZSM-5 (Si/Al = 100) by its modification with a KNO3 solution using the wet-impregnation method to obtain the K/Al molar ratio of 0.6 in the K-H-ZSM-5 sample. The deposition of potassium decreased the contribution of strong acid sites (ammonia desorption above 250 °C in the NH3-TPD profile) in the zeolite sample. Such a modification of H-ZSM-5 resulted in the decrease in the total concentration of acid sites from 797 to 251 μmol/g. The introduction of water vapour into the feed stream decreased the methanol conversion, but a DME selectivity of 100% was achieved at high reaction temperatures without the hydrocarbon side-product formation. As the flow rate of the feed stream increased, the methanol conversion decreased at low temperature, and the DME selectivity increased at high temperature. The deactivation of the K-H-ZSM-5 catalyst resulted in coke formation or dealumination under specific reaction conditions. The deactivation due to dealumination process depended on the water vapour content in the feed stream. Simple-structured oxygenates were produced when water-containing methanol was used as the feed. However, aromatic-structured coke was formed on the catalyst surface with pure methanol as the feed. Therefore, it was postulated that the formation of aromatic-structured coke decreased the catalytic performance of K-H-ZSM-5 to a greater extent than dealumination [64].
Other very important information about coke formation under reaction conditions was provided by the studies of Chaudhary et al. [65], who analysed the selectivity to the side-reaction products for the MTD reaction performed over beta zeolite (Si/Al = 14) in the temperature range of 280–450 °C. It was shown that, at increased temperatures, strong acid sites present in beta zeolite promote higher hydrocarbon formation following the olefin-based cycle. The increase in the reaction temperature resulted in increased methanol conversion and decreased DME selectivity. Hydrocarbons between the C1 to C4 range and hexamethyl benzene (HMB) were also identified as the reaction product. The lower-molecular-weight C2 hydrocarbons were more selective at lower reaction temperatures, while C4 enrichment occurred at elevated temperatures. HMB was found to be the single component of the solid product, along with the deposition of poly-methylbenzene on the zeolite surface. Thus, it could be postulated that HMB is an intermediate product in the formation of carbon deposits. Furthermore, it was shown that the regeneration of the spent catalyst (calcination at 550 °C for 5 h) resulted only in a small loss of the structural properties of beta zeolite, which appears to be a promising candidate for the catalyst of the methanol dehydration reaction [65].
Rutkowska et al. [66] studied the zeolites beta (Si/Al = 21), Y (Si/Al = 16), and ZSM-5 (Si/Al = 16.5) desilicated with alkalic medium, and, additionally, mesoporous silicas doped with aluminium, Al-SBA-15 and Al-MCF, in the role of catalysts for the dehydration of methanol to DME. The preliminary catalytic tests showed an activation effect of the alkaline treatment of zeolites on their catalytic performance, including resistance to coke deposition. ZSM-5 was chosen for further, more detailed studies, including the generation of mesoporosity by alkali treatment. Treatment with NaOH solution partially leached silicon from the ZSM-5 structure, and therefore significantly increased the contribution of mesoporosity and modified the surface acidity of the zeolite. The treatment of ZSM-5 with 0.1 M NaOH solution for 2 h resulted in an increase in the mesopore volume from 0.011 to 0.160 cm3/g and increase in the surface concentration of the acid sites from 537 to 615 μmol/g, mainly by the formation of stronger acid sites (with a maximum of NH3 description of about 400 °C). The increase in the weak acid site concentrations (with a maximum of NH3 description of about 220 °C) was observed only for the sample treated with NaOH for 4 h. The zeolite modified in such way presented improved catalytic properties compared to classical microporous ZSM-5 zeolites [66]. Dalena et al. [67] studied the nature of the acid sites in desilicated H-ZSM-5 zeolites and their role in the conversion of methanol to DME. The nature and surface concentration of the acid sites were determined by the FT-IR analysis of the deuterated acetonitrile (CD3CN) pre-adsorbed samples. The desilicated zeolite samples were shown to have an increased content of Brønsted and Lewis acid sites, but only the increase in the Brønsted sites resulted in increased catalytic activity. Such an effect was not observed for the sample with the increased concentration of Lewis-type acid sites. The TOF (Turnover Frequency), which refers to the amount of Brønsted acid sites, was very similar for the zeolite samples desilicated under various conditions. It was postulated that methanol dehydration in zeolites occurs preferentially over Brønsted acid sites. Studies by Aloise et al. [68] presented increased catalytic activity and resistance to the formation of carbon deposits of the ZSM-5 zeolite (the parent zeolite with the Si/Al ratio of 25) desilicated with NaOH solution. It was shown that the treatment of zeolite with NaOH solution resulted in changes in the properties of acidic sites and the mesoporous volume, which increased after 1 h of modification, from 489 to 689 μmol/g and from 0.089 to 0.185 cm3/g, respectively. FT-IR studies showed that the contribution of Brønsted acid sites increased from 404 to 510 μmol/g, while the Lewis acid sites increased from 85 to 179 μmol/g, as a result of zeolite treatment with NaOH solution for 1 h. Thus, it was shown that the contact time of the alkaline solution treatment determined the final mesoporous structure of the zeolite sample. For the short contact time (30 min), the main effect was an increase in the mesopore volume. Treatment with NaOH solution for 60 min favourably impacted on the catalyst’s activity and stability against deactivation in terms of the methanol conversion and DME yield. The analysis of the coke formed confirmed that the most promising catalyst showed the lowest tendency for coke formation as the main consequence of the acid treatment.
The zeolite Y has also been reported as a potential catalyst for the MTD process. However, compared to ZSM-5, zeolite Y is characterised by lower stability under reaction conditions due to faster deactivation. Jin et al. [69] and Fei et al. [70] reported a dramatic drop in methanol conversion from 88 to 19% under isothermal catalytic test conditions at 245 °C for 14 h. The stability of Y zeolite can be significantly improved by the introduction of Zr and Ni (1.8–2.0 wt.%) by the ion-exchange method [70]. In this case, the methanol conversion decreased only by a few percent during the 14 h of the catalytic test. The amounts of carbon deposits formed on Zr–Y and Na–Y were much smaller compared to those of unmodified zeolite Y. On the other hand, zeolite Y modified with Fe, Co, or Cr presented an increased contribution of strong acid centres, which were responsible for rapid catalyst deactivation due to carbon deposits (even faster than for unmodified zeolite Y) [70]. The studies were extended for the deposition of Ce, Pr, Nd, Sm, and Eu into zeolite Y [69]. It was shown that deposition of La, Ce, and Pr resulted in the generation of mainly medium-strength acid centres active in methanol dehydration and with a limited tendency for the formation of carbon deposits. The deposition of Nd and Sm resulted mainly in strong acid centres with limited activity in the DME synthesis and active in coke formation. All modified Y zeolites presented higher activity and stability than the non-modified Y zeolite [69].
Mordenite is another zeolite presenting interesting catalytic properties in the MTD process. Moradi et al. [71] verified the catalytic activity of mordenites with different Si/Al ratios. Mordenites in protonic form presented better catalytic activity than mordenites in sodium form. Furthermore, it was shown that mordenite-based catalysts cannot be fully regenerated, indicating the irreversible formation of carbon deposits or the destruction of the zeolite structure under conditions of catalyst regeneration [71]. Catizzone et al. [72] and Migliori et al. [72] reported the high activity of mordenite in the MTD process, but also the rapid decrease in the methanol conversion (from the initial over 80% to less than 10% after 60 h). In this work, the catalytic performance of mordenite and ferrierite was compared. Both zeolites presented similar catalytic activity at a relatively low temperature (220 °C); however, ferrierite was significantly more stable, and any noticeable decrease in the methanol conversion was observed during the 60 h of the catalytic test [72,73].
Another way to improve the properties of zeolite-based catalysts is the preparation and the use of the composite materials. Zheng et al. [74] studied composite materials consisting of beta zeolite and mordenite, which were found to be more catalytically active and more selective toward dimethyl ether than a mechanical mixture of beta zeolite and mordenite. In the temperature range of 200–275 °C, the conversion of methanol in the presence of the composite catalyst was over 90%, with almost 100% selectivity to DME. Furthermore, stability tests showed that the high methanol conversion was stable at 275 °C for 72 h [74]. On the other hand, Tang et al. [75] studied ZSM-5/MCM-41, which combines the advantages of microporous zeolite ZSM-5 with ordered mesoporous silica of the MCM-41 type. The composite catalyst presented a methanol conversion similar to that obtained for ZSM-5 zeolite (in the temperature range of 170–310 °C). However, the advantage of the composite material over zeolite ZSM-5 is the lack of the decrease in the selectivity to DME, with an increase in the reaction temperature as well as improved stability. Methanol conversion in 30 day tests obtained in the presence of the composite catalysts was above 85%, while, for ZSM-5, it remained at the level of 85% for only 15 days. Later, it dropped significantly to 45% after 30 days. The improved stability was related to the sufficient acidity of the composite catalyst, as well as the mesoporosity, which ensures the easier diffusion of the reactants and contributes to fewer carbon deposits [75]. Ulfah et al. [76] prepared composite materials consisting of γ–Al2O3 and zeolite A or X as catalysts for the MTD process. Such material was characterised by a significant contribution of acid sites with medium strength (325 μmol/g) in the case of γ–Al2O3/A composites, and medium (308 μmol/g) and high (519 μmol/g) strength in the case of γ–Al2O3/X composites.
The size of the crystallites is another important property of zeolites influencing their catalytic performance in methanol-to-DME dehydration. Catizzone et al. [77] compared the catalytic activity of two series of zeolites, MFI (Si/Al = 23–27) and ferrierite (FER, Si/Al = 11). Each series consisted of nano-crystalline zeolites (crystallite size of 0.1–0.3 µm) and microcrystalline zeolites (crystallite size of 3–10 µm). It was shown that FER- and MFI-type zeolites are stable catalysts of the DME synthesis. The results of catalytic studies performed in the range of 180–240 °C indicate that the crystal size plays a crucial role in terms of the DME production rate, DME selectivity, and coke deposition. Nano-sized crystals exhibited superior performance than micro-sized crystals. In particular, nano-sized MFI presented a similar DME selectivity (above 96%) and conversion of micro-sized FER, but with a higher DME production rate and lower coke deposition. In the case of ferrierites, nano-sized FER showed the highest DME selectivity (above 99%). The authors postulate that zeolites may be considered as nano-scale reactors and, by controlling the residence time, it is possible to have a control of the product distribution in the case of consecutive reactions. Furthermore, the improvement of the internal diffusion rate by reducing the size of the crystallites improves the overall reaction efficiency [77]. Rutkowska et al. [78] reported the study focused on the synthesis of ZSM-5 composed of loosely adhered zeolite nano-crystals (with sizes in the range 10–20 nm) with the enhanced internal diffusion of reactants in spaces between such nano-crystals. Such materials, characterised by a bimodal porous structure, micropores in zeolite nano-crystals and larger pores between nano-crystals, were synthetised by the acidification of the zeolite seed slurry using HCl solution, followed by hydrothermal treatment, enabling the aggregation of zeolite nano-seeds with the formation of an interparticle mesoporous structure. It was shown that the ageing conditions of the parent zeolite before and after acidification influenced the porous and acidic properties of the final product. Moreover, it was reported that the high catalytic efficiency of the DME synthesis was correlated with the presence of strong acid sites of the Brønsted type. The as-synthesised micro-mesoporous samples showed high catalytic activity, which was similar to that of conventional microporous ZSM-5. However, the presence of weaker acid sites in their structure, connected with the generated mesoporosity, resulted in an improved reaction selectivity to DME. On the other hand, Abbasian and Taghizadeh [79] synthesised H-ZSM-5 nano-zeolites by the hydrothermal method using tetrapropylammonium hydroxide (TPAOH) as a template in the presence of various concentrations of tetrapropylammonium bromide (TPABr). The effect of different TPABr/TPAOH molar ratios on the catalytic performance of methanol-to-DME dehydration was studied. The H-ZSM-5 nano-zeolites (Si/Al = 125) were successfully synthesised by the hydrothermal crystallization method. It was demonstrated that the sample with the smaller crystallite size possessed a higher concentration of weak and medium acidic sites, and consequently presented higher catalytic activity in the DME synthesis. Zeolite with an average crystallite size of 27 nm was characterised by an acid site concentration of 660 μmol/g, while H-ZSM-5, with an average crystallite size of 81 nm, was significantly less acidic at 200 μmol/g.
Aboul-Fotouh et al. [80] modified the surface acidity of zeolites (H-ZSM-5, H-MOR, and H-Y) by their impregnation with ammonium chloride or ammonium fluoride and ultrasonic treatment. It was reported that the chlorination and fluorination of zeolites significantly improved their catalytic activity in methanol-to-DME conversion. In the case of fluorinated H-ZSM-5 and H-MOR, the catalytic activation effect was better than for chlorinated zeolites. The opposite halogenation effect was found for H-Y, which, in chlorinated form, presented higher catalytic activity in the MTD process compared to fluorinated zeolite. The ultrasonic treatment of all chlorinated zeolites additionally improved their catalytic activity, while the opposite effect was observed for fluorinated zeolites, which were deactivated by the ultrasonic treatment. These interesting effects were explained by the formation of additional acid sites under the conditions of zeolite halogenation, which was more effective in the case of the fluorinated samples. Ultrasonic radiation additionally increased the surface concentration of the acid sites for the chlorinated zeolites, e.g., the chlorination of H-MOR increased the acid site concentrations by about 10%, while the ultrasonic treatment increased the concentrations by 30%, mainly by the generation of weak acid sites (with a maximum of ammonia desorption in the NH3-TPD profile at about 170–180 °C). Moreover, the ultrasonic treatment of the chlorinated and fluorinated zeolites significantly improved the reaction selectivity toward DME. This is possibly related to the increased contribution of relatively weak Brønsted acid sites, active in the dehydration of methanol to DME.
Very interesting results of methanol-to-DME conversion were presented by Dennis-Smither et al. [81], who compared the catalytic activity of various zeolites with the similar molar Si/Al ratios of 20–25. It was shown that the size of the micropores in zeolites play an important role in the efficiency of the DME formation. The narrow-pore zeolite H-SSZ-13 (CHA) provided higher productivity to the DME than the medium-pore H-ZSM-5 (MFI), and the wide-pore zeolites H-beta (BEA) and H-mordenite (MOR). Thus, it was assumed that tighter confines in the zeolite pores provide higher activity for the DME formation. The high efficiency of narrow-pore zeolites, such as H-SSZ-13, for methanol dehydration to DME was also reported by Masih et al. [82].
Catizzone et al. [72,83] analysed the catalytic performance of zeolites, such as mordenite, ZSM-12, ZSM-22, EU-1, ferrierite, ZSM-5, beta, and SAPO-34, with different channel systems, pore dimensions, and framework structures in methanol-to-DME dehydration. It was postulated that zeolites with the 2D small and medium pore structures of ferrierite are suitable catalytic environments for the selective conversion of methanol to DME, which also inhibits coke formation. On the contrary, the 1D structures with large voids lead to the formation of by-products, such as olefins, and fast coke deposition. Large openings and side pockets in the one-dimensional pore structures increase carbon deposition, leading to catalyst deactivation, while the ferrierite structure, with a two-dimensional small pore channel system, showed high resistance to the deactivation by inhibiting coke deposition. The 3D structure promotes the deposition of heavier molecules that form coke and affect the catalytic performances. It was shown that the carbon deposit phase consists mainly of poly-substituted benzenes, with a substitution level depending on the channel size and topology. The most promising results of the catalytic tests in terms of methanol conversion, DME selectivity, and deactivation were obtained for ferrierite (with the Si/Al molar ratio of 8.4).
The effect of zeolite porosity and acidity was studied by Marosz et al. [84], who prepared a series of MCM-22 zeolites with various molar Si/Al ratios of 15, 25, and 50, as well as their delaminated (ITQ-2) and silica intercalated forms (MCM-36). It was shown that the catalytic activity of the studied samples in the MTD process mainly depended on the surface concentration of the acid sites, related to the content of alumina in the zeolitic samples. Aluminium present in the zeolite framework generated mainly Brønsted acid sites (≡Si–O(H)–Al≡). The surface concentration of the acid sites in MCM-22 decreased after their delamination and intercalation, e.g., in the case of MCM-22 with the Si/Al ratio of 15, the surface concentration of the acid sites decreased after delamination (ITQ-2) from 1202 to 943 μmol/g and after interaction with silica pillars (MCM-36) to 869 μmol/g. The dominating role of Brønsted acid sites in methanol dehydration was postulated.
Two mechanisms—dissociative and associative—were proposed for the conversion of methanol to DME in the presence of H-ZSM-5 (Scheme 3) [85,86]. It should be noted that, in both cases, the key role was played by the Brønsted acid sites. These mechanisms were postulated for H-ZSM-5, but they are probably also valid for other zeolite catalysts for the MTD process.
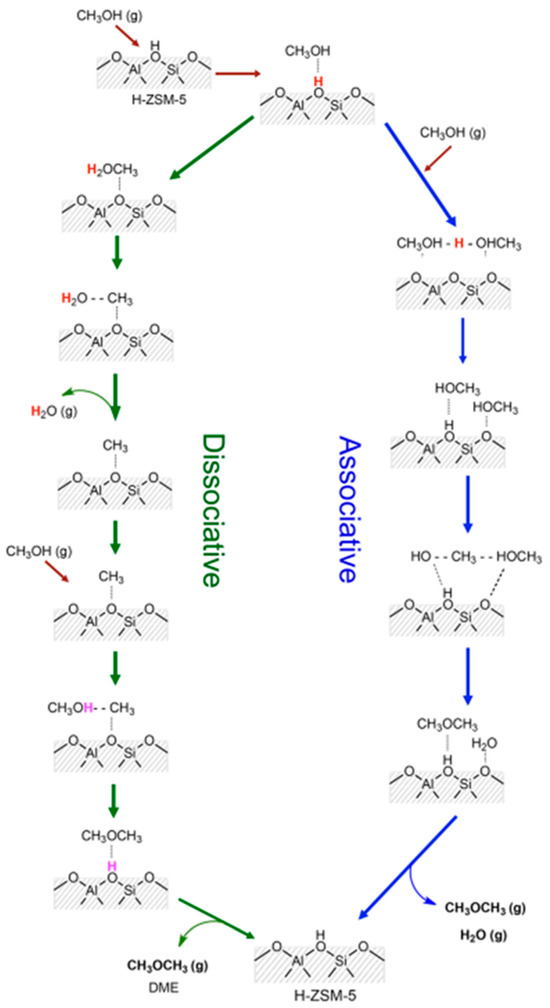
Scheme 3.
Proposed reaction mechanism of methanol dehydration to DME. Adapted from [85], copyright (2016), with permission from the American Chemical Society.
ITQ-2 and MCM-36, with the open interlayer structure, were less affected by coking deactivation compared to microporous MCM-22. This effect was related to the improved internal diffusion of the reactant molecules in the interlayer mesopores only partially blocked by carbon deposits. In contrast, the fully microporous MCM-22 structure can be plugged very quickly by the coke. The type of zeolite porosity only slightly influenced the overall efficiency of methanol to DME, as well as ethanol to diethyl ether (DEE) and ethylene conversion [84]. Similar conclusions were proposed by Święs et al. [87], who verified the catalytic activity of ferrierites and their delaminated (ITQ-6) and silica intercalated (ITQ-36) forms in methanol-to-DME, ethanol-to-DEE, and ethylene dehydration. It was reported that the surface acidity of the zeolite materials is crucial for their catalytic performance in alcohol dehydration, while their porous structure is significantly less important. The ferrierite sample with the higher content of aluminium (Si/Al = 30) in the zeolite framework, and therefore the higher concentration of acid sites (276 μmol/g), presented much better catalytic performance than high-silica zeolite (Si/Al = 50, surface acidity = 47 μmol/g). Similar results were obtained for delaminated ITQ-6 (Si/Al = 30, surface acidity = 314 μmol/g; Si/Al = 50, surface acidity = 39 μmol/g) and silica intercalated ITQ-36 (Si/Al = 30, surface acidity = 382 μmol/g; Si/Al = 50, surface acidity = 116 μmol/g). It was postulated that Brønsted acid sites, which dominate in ferrierite (for the sample with Si/Al = 30, the ratio of BAS/LAS = 5.4) and their delaminated ITQ-6 form (for the sample with Si/Al = 30, the ratio of BAS/LAS = 6.3) are more active in alcohol dehydration. This hypothesis was proved by the catalytic activity of ferrierite with the Si/Al molar ratio of 30, which contained only Brønsted acid sites (with no Lewis-type acidity) and was catalytically active in methanol-to-DME conversion.
Another important pathway is the use of organic activators capable of increasing the conversion of methanol to dimethyl ether (DME) over zeolite catalysts. Dennis-Smither et al. [81] reported that mono- and di-carboxylate esters, used as additives, can effectively promote the production of DME from methanol over zeolite catalysts. It was shown that the concentrations of such carboxylate esters as low as 10 ppm in the relation to methanol can significantly improve DME production. Molecular modelling methods applied to study the role of carboxylate ester, specifically n-hexanoate, in methanol-to-DME conversion over H-ZSM-5 resulted in the formulation of the following mechanism presented in Figure 4: (1) the direct reaction of methanol with methyl n-hexanoate H-bonded to a Brønsted acid site, involving the SN2 nucleophilic attack of methanol on the methoxy group of the adsorbed methyl ester; (2) the reaction of the methoxy group of such an ester with a methanol molecule, resulting in DME and a carboxylate ester, which closed the catalytic cycle.
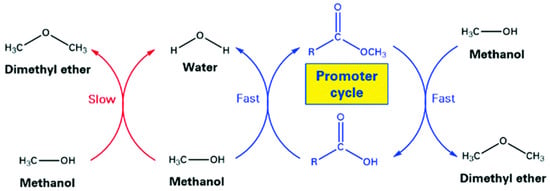
Figure 4.
Catalytic cycles for DME formation from methanol showing the role of methyl carboxylate promoters. Reprinted from [81], copyright (2019), with permission from the Royal Society of Chemistry.
2.3. Clay Mineral-Based Catalysts
Clay minerals and their modifications were studied as potential catalysts for the MTD process. Clay minerals are easily available and, therefore, relatively cheap. Moreover, various methods of their catalytic activation, including acid treatment [88], delamination [89], and intercalation with inorganic pillars [90,91], have been reported in the scientific literature.
Marosz et al. [92] treated natural vermiculite with a solution of nitric acid (0.8 M) at 95 °C for 2, 8, and 24 h. Such modification of clay mineral resulted in an increase in the specific surface area from 8 m2/g for the parent vermiculite to 159 m2/g, and surface concentrations of acid sites from about 8 to 108 μmol/g for the vermiculite treated with acid solution for 24 h. Moreover, the acid treatment of vermiculite resulted in a partial leaching of aluminium and iron cations from its layers. For the vermiculite samples treated with a nitric acid solution for 2 and 8 h, a significant increase in catalytic conversion of methanol to DME was observed, while, for the sample activated for 24 h, catalytic activity was significantly lower. The acid treatment of vermiculite resulted in two opposite effects. The first is related to the development of the clay mineral surface area, resulting in its catalytic activation. The second effect is attributed to the leaching of aluminium and iron cations from the vermiculite layers, resulting in a decrease in the acid site concentrations and finally the lower catalytic activity in the MTD process. The acid treatment of vermiculite for 24 h resulted in the most significant increase in its surface area, but, on the other hand, in the more effective extraction of aluminium and iron cations from the vermiculite layers, resulting in the reduced number of acid sites active in the conversion of methanol to DME. A similar acid treatment procedure (0.8 M HNO3; 95 °C; 2, 8, or 24 h) was used for the modification of allophane, palygorskite, and sepiolite [93]. The mineral samples were not purified prior to the acid treatment. The surface area determined for palygorskite increased after 24 h of acid treatment from 144 to 213 m2/g and for sepiolite from 99 to 247 m2/g. In the case of allophane, the acid treatment resulted in a decrease in the surface area. Moreover, aluminium and iron cations were partially leached from allophane and palygorskite by the acid treatment. In the case of sepiolite, such an effect was not observed, possibly due to the much stronger stabilisation of these cations in the sepiolite structure. In a group of the studied minerals, the best catalytic activity in the MTD process presented allophane; however, the acid treatment slightly decreased its catalytic activity. The opposite effect was reported for palygorskite and sepiolite, which were catalytically activated by the acid treatment. Thus, the acid treatment of the mineral materials may result in the development of their surface area and porosity, which is beneficial for catalysis but, on the other hand, the extraction of cations generates acid sites, which has an adverse impact on the catalytic operation in the MTD process.
Clay layered minerals, such as montmorillonites, vermiculites, or saponites, can be effectively intercalated with various inorganic pillars [90,91,94], resulting in the opening of the interlayer space for catalysis. The deposition of inorganic stable pillars into the interlayer space of the clay minerals results in the permanent separation of the mineral layers and the formation of high-surface-area materials. In contrast to acid-treated minerals, such pillared clays are characterised by a more uniform porous structure and preserved surface acidity, which can be increased by the deposition of the interlayer pillars containing elements generating acid sites, such as aluminium or titanium. Chmielarz et al. [95] studied porous clay heterostructures (PCHs) obtained by the intercalation of montmorillonite with silica, silica–alumina, silica–titania, and silica–zirconia pillars in the role of catalysts for methanol-to-DME conversion. The catalysts were prepared by the surfactant-directed method, including the deposition of cationic alkylammonium surfactants and neutral alkylammonium co-surfactants into the interlayer space of the montmorillonite. Surfactants and co-surfactants formed the ordered micellar structures in the interlayer space of mineral. In the next step, tetraethyl orthosilicate (TEOS) was hydrolysed by water present in the interlayer space of the clay mineral, which condensate around the micellar structure with the formation of silica pillars, permanently increasing the interlayer distance in the montmorillonite. In the final step, organic surfactants were removed from the porous structure of the PCHs by calcination. In the case of montmorillonite intercalation with silica–alumina, silica–titania, and silica–zirconia pillars, TEOS was mixed with the aluminium (aluminium isopropoxide), titanium (titanium isopropoxide), or zirconium (zirconium isopropoxide) source, respectively. The incorporation of these metals into the silica pillars resulted in the generation of additional acid sites. The specific surface area increased from 77 m2/g for the parent montmorillonite to 318–642 m2/g for the pillared samples. The PCH samples presented catalytic activity in the MTD process, which depended on the strength and concentration of the acid sites in the modified clay minerals. In this series of catalysts, as it is presented in Figure 5, the best results were obtained for the PCH sample intercalated with the silica–alumina pillars (PCH-Al), which were more catalytically active than the silica-intercalated montmorillonite (PCH-Si). In the latter case, the acid sites are located only on the montmorillonite layers, while, in PCH-Al, the acid sites are located both on the montmorillonite layers and on the silica–alumina pillars.
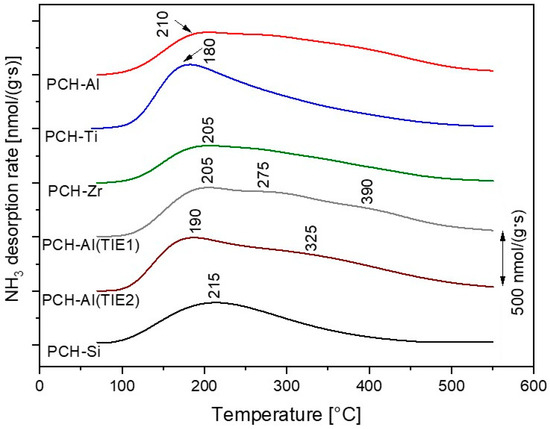
Figure 5.
Results of the catalytic tests in the process of methanol-to-dimethyl ether dehydration over the PCH-based catalysts. Reprinted from [95], copyright (2018), with permission from Elsevier.
Analysis of the NH3-TPD profiles, presented in Figure 6, shows that the alumina incorporated into the silica pillars resulted in stronger acid sites compared to sites located on the montmorillonite layers. Such stronger acid sites are postulated to be more effective in methanol-to-DME conversion. PCH-Ti and PCH-Zr presented a significant contribution of acid sites associated with the metal incorporated into the silica pillars, but were characterised by lower acid strength (Figure 6), and therefore were found to be less catalytically active in the MTD process (Figure 5). The continuation of these studies was the deposition of aluminium into PCH-Si by the template ion-exchange method, which is based on the exchange of the cationic surfactants for Al3+ cations in a freshly prepared sample [95]. The catalysts obtained by this method, PCH-Al (TIE1) and PCH-Al (TIE2), presented better catalytic activity than the sample with aluminium incorporated into the silica pillars, PCH-Al (Figure 5).
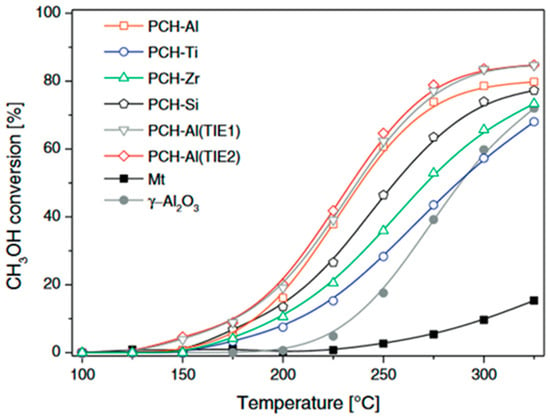
Figure 6.
NH3-TPD profiles obtained for the PCH samples. Reprinted from [95], copyright (2018), with permission from Elsevier.
Marosz et al. [92] studied acid-treated vermiculites intercalated with Al2O3 pillars as catalysts for the MTD process. Vermiculites, in contrast to montmorillonites, are characterised by the relatively high stabilisation of the interlayer cations, and therefore the direct exchange of such cations in parent vermiculite is difficult or even, in many cases, impossible. To decrease the stabilisation forces of the interlayer cations, vermiculite was treated with a nitric acid solution to leach part of Al3+ and Fe3+ cations from the vermiculite layers. In the next step, the leached cations were complexed in solution by oxalic acid (O) or citric acid (C). Vermiculite modified in this way was intercalated with alumina Keggin oligocations by the ion-exchange method and finally calcined, resulting in pillared interlayered clay (PILC). Intercalation of vermiculite with alumina pillars resulted in an increase in the surface area from 8 to 129 m2/g and surface acidity from 8 to 223 μmol/g for the sample without the pretreatment with complexing agents. The modification of the acid-treated vermiculite with oxalic or citric acid resulted in a more effective pillaring process, producing samples, PILC-O-Al and PILC-C-Al, with a larger surface area of 203 and 172 m2/g, and a surface acidity of 403 and 321 μmol/g, respectively. The PILC-C-Al catalyst presented significantly better catalytic properties with respect to methanol conversion (above 80% at 275 °C) and selectivity to DME (about 98% at 275 °C) than the other pillared vermiculites, indicating that the treatment of the acid-modified vermiculite with complexing agents, prior to the pillaring, is a very important step in the mineral clay activation for the MTD process.
2.4. Membrane-Based Catalytic Systems
The efficiency of methanol-to-DME dehydration can be significantly improved by the application of the membrane reactors. The application of such reactors attracted much attention in the last decade of the 20th century [96,97]. The operation of the membrane reactors is based on the simultaneous occurrence of the chemical reaction and separation of the reaction products using the membrane, resulting in a shift of the thermodynamic equilibrium towards higher reactant conversion. Membrane separation has been proposed to improve the performance of the conventional fixed-bed reactors used, for example, in the steam reforming process [98,99], the production of syngas methanol [100], or Fischer–Tropsch synthesis [101]. The application of membrane reactors for the MTD process is a very promising option. Farsi and Jahanmiri [17] analysed the possibility of the application of the membrane fixed-bed reactor for the large-scale DME production by methanol dehydration. In the proposed reactor, water vapour, produced as a side product of methanol dehydration, was selectively removed from the reaction zone by using the selective membrane (Figure 7), and therefore the equilibrium conversion of methanol increased owing to Le Chatelier’s principle. The simulation results indicated that the conversion of methanol can be increased by about 6.2% compared to the conventional industrial reactor. It was postulated that the application of the membrane reactor should lead to a higher catalyst lifetime and lower the cost of the final product purification. Zhou et al. [15] applied a sandwich FAU–LTA zeolite dual-layer membrane in the catalytic membrane reactor for the synthesis of dimethyl ether. In the top of such a membrane, the H-FAU layer with mild acidity for methanol dehydrated to DME was placed. Water formed as the side product was removed in situ through the hydrophilic Na-LTA layer, which is located between the porous alumina support and the H-FAU top layer. This combination of the zeolitic layers and alumina resulted in the continuous removal of water and an increase in the conversion of methanol (90.9% at 310 °C) with almost 100% selectivity to DME. Moreover, because of the selective and continuous removal of water through the Na-LTA membrane, the catalyst deactivation was effectively suppressed. Brunetti et al. [10] compared the catalytic operation of two membranes, ZSM-5-γ-Al2O3 and ZSM-5-TiO2, in the synthesis of DME through MeOH dehydration. The performance of the two membrane reactors was analysed as a function of the temperature and feed pressure, space velocity (WHSV), and feed composition. The ZSM-5-γ-Al2O3 membrane (Si/Al = 200; porosity of the zeolite layer = 0.2; thickness = 50 μm; membrane area = 50.6 cm2) always presented a greater methanol conversion than ZSM-5-TiO2 (Si/Al = 200; porosity of the zeolite layer = 0.2; thickness = 63 μm; membrane area = 18.8 cm2), which was attributed to the higher catalytic activity of the alumina than titania. It was reported that an increase in the feed pressure led to lower methanol conversions, most likely ascribable to a negative effect on the desorption of the reactants and products from catalytic sites. Moreover, it was shown that the conversion of methanol was significantly affected by the presence of nitrogen in the feed, which induced not only variation in the WHSV but also the depletion of the reaction rate due to the dilution of the methanol concentration. The best catalytic results, in terms of 86.6% of methanol conversion with 100% DME selectivity, were obtained for the ZSM-5-γ-Al2O3 membrane system at 200 °C and the WHSV of 1 h−1. In general, it was shown that the methanol conversion and DME selectivity obtained with both membrane reactors were better than that achieved with a traditional reactor. This effect was assigned to the positive role of the continuous exposition of the catalytic layer to a gas flow, which favours the removal of water from active sites and, therefore, limits catalyst deactivation and depletes the formation of possible secondary products. Mohammed et al. [102] modelled and simulated methanol-to-DME dehydration in an adiabatic tubular fixed-bed reactor with and without a membrane, and analysed the effect of in situ water removal on the overall methanol conversion. An optimisation approach was implemented to determine the best feed conditions to obtain the maximum conversion of methanol. In the study, a steady-state simulation of adiabatic single-tube reactors was performed using γ-Al2O3 pellets with strictly defended specifications as catalyst. The hydrophilic membrane (CSP2) developed by the Energy Research Center of the Netherlands (ECN), composed of four layers on a commercially available macroporous tube coated with two layers of macroporous α-Al2O3 (40 μm thick), a thin layer of γ-Al2O3 (2 μm thick), and the functional polymeric layer P84® (1 μm thick), was used. The obtained results showed that the methanol conversion exceeded the thermodynamic equilibrium limits when a membrane fixed-bed reactor was used instead of a traditional fixed-bed reactor. The conversion reached 96% at optimum feed conditions in the fixed-bed reactor with a membrane.
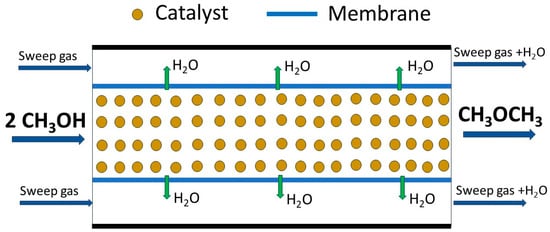
Figure 7.
Schematic presentation of the concept of a membrane reactor for the MTD process.
3. Summary and Perspectives
Dimethyl ether, due to its unique properties, has great potential for various applications, including its use as a solvent in chemical and petrochemical industries, as well as to produce different important chemicals, such as diethyl sulphate, oxygenates, and olefins. However, the prognosed increase in DME production is associated mainly with the possibility of its mixing with liquified petroleum gas (LPG) to obtain more efficient and environmentally friendly fuels for transportation and power generation. To intensify the DME production, more effective processes and catalysts are needed. Alumina and zeolites are studied as the catalysts for the conversion of methanol-to-DME dehydration. Alumina-based catalysts are hydrophilic, so water vapour, which is a side-reaction product, is accumulated on the surface and hinders the access of methanol molecules to acid sites. Moreover, the phase transition of alumina to boehmite, which is inactive in the MTD process, may occur at elevated temperatures in the presence of water vapour. Alumina is characterised by the presence of relatively weak and medium-strength acid sites, which are active in the conversion of methanol to DME and less active in the formation of carbon deposits. Furthermore, the catalyst’s coking is significantly limited by the amount of water accumulated on the alumina surface, and therefore such catalysts are characterised by high selectivity to DME. The hydrophilic character of alumina can be reduced in the composite Al2O3-SiO2 systems, which presented very promising catalytic properties in the MTD process. The number of acid sites, which play a role of active centres, can be increased, and therefore the efficiency of methanol-to-DME dehydration can be improved by using nano-crystalline alumina. Various methods of such a catalyst’s synthesis have been reported in the scientific literature. The surface acidity of the alumina-based catalysts can be adjusted by the deposition of various transition-metal cations that play the role of Lewis-type acid sites.
Zeolites are characterised by stronger acid sites, which are catalytically active in the MTD process, but are also more susceptible to the formation of carbon deposits. Due to the microporous structure of zeolites, their small pores can be effectively plugged by the formed deposits, and, consequently, the access to the acid sites located inside micropores is blocked. There are two main strategies focused on the solving of this problem, reducing the strength of acid sites to limit the formation rate of carbon deposits and using zeolite materials with the micro-mesoporous structure to improve the internal diffusion of reagents. The main methods focused on decreasing the acid site strength are based on the deposition of alkali metals or various transition metals, such as Ni, Co, Cr, Fe, or Zr, into zeolites, mainly by the ion-exchange method. The surface concentration of acid sites can be regulated by the synthesis of zeolites with the various Si/Al ratios or composite zeolite–silica materials. Different strategies of micro-mesoporous zeolite synthesis have been reported in the scientific literature. Among them, the most important are desilication of zeolites using alkali solutions, the synthesis of zeolites in the nano-crystalline form (with the intensified internal diffusion of reactants in the inter-crystalline spaces), as well as the delamination and intercalation of layered zeolites to create additional interlayer porosity.
Clay minerals, because of their relatively high availability, presence of weak and medium-strength acid sites, as well as various possible methods of their catalytic activation, are a very interesting alternative to alumina and zeolites. The acid treatment of such minerals in many cases result in a very significant increase in their surface area and porosity, which increases their catalytic activity in the MTD process, but, on the other hand, results in a partial leaching of elements creating acid sites, such as aluminium or iron, and therefore decreases their catalytic activity. Thus, the development of the optimal conditions of the acid treatment procedure is very important for the synthesis of effective catalysts for the MTD process. The other methods are based on the intercalation and delamination of layered clay minerals, resulting in the opening of the interlayer spaces for the catalytic process without the elimination of acid sites.
The dehydration of methanol to DME is a thermodynamically restricted process, and the equilibrium DME formation decreases with increasing temperature. Thus, from the low-temperature side, there is a kinetic limitation resulting in the low reaction rate, while, at higher temperatures, the efficiency of the MTD process is limited because of thermodynamical reasons. This clearly shows the crucial role of effective catalysts operating in the low-temperature range. The overall efficiency of the MTD process could be increased above the thermodynamical equilibrium level by the in situ removal of water (the side-product of the MTD process) from the catalytic reactor. There are some promising results presenting the use of the catalytic membrane reactors, in which water vapour can be selectively in situ removed from the reaction zone. It seems that the application of such catalytic membrane reactors will be the main future direction of MTD technology development. There are some problems that have to be solved to effectively implement such technologies. First, highly effective and stable membrane systems that can operate at the industrial scale must be developed. Secondly, effective catalysts cooperating with membranes, with the optimal strength and density of acid sites, as well as being resistant to poisoning by water vapour and carbon deposits, have to be developed. Another important problem is the coking of the catalysts under the conditions of the MTD process. Thus, a lot of attention should be paid to the effective regeneration of the catalysts, as well as those operating in the catalytic membrane reactors. Such regeneration, focused on carbon deposit removal, should not result in the destruction of the catalyst structure or the acidic properties. Much work has already been performed in this area, and it seems that further research directions have also been indicated, as demonstrated in this paper. A list of the catalysts studied as potential catalysts for the MTD process is summarised in Table 1.

Table 1.
List of various catalysts for the MTD process.
Funding
This research received no external funding.
Data Availability Statement
No new data were created.
Conflicts of Interest
The author declares no conflicts of interest.
List of Abbreviations
| BAS | Brønsted acid sites |
| DME | dimethyl ether |
| DEE | diethyl ether |
| FT-IR | Fourier transform infrared spectroscopy |
| F-T | Fischer–Tropsch process |
| HMB | hexamethyl benzene |
| LAS | Lewis acid sites |
| LPG | liquefied petroleum gas |
| MFI | mordenite framework inverted (zeolites) |
| Mt | montmorillonite |
| MTD | methanol to dimethyl ether conversion |
| NH3-TPD | temperature-programmed desorption of ammonia |
| PCH | porous clay heterostructure |
| PILCs | pillared interlayered clays |
| TIE | template ion-exchange method, one cycle (TIE1) and two cycles (TIE2) |
| ULEV | ultra-low-emission vehicle |
| WHSV | weight hourly space velocity |
References
- Dumas, J.B.; Péligot, E.M. Mémoire sur l’esprit-de-bois et les divers composés éthéres qui en proviennent. Ann. Chim. Phys. 1835, 58, 5–74. [Google Scholar]
- Chai, M.; Chen, Z.; Nourozieh, H.; Yang, M.; Chai, B. Introduce dimethyl ether (DME) as a solvent for steam-assisted gravity drainage (SAGD) co-injection: An effective and environmental application. Fuel 2023, 341, 127639. [Google Scholar] [CrossRef]
- Naik, S.P.; Ryu, T.; Bui, V.; Miller, J.D.; Drinnan, N.B.; Zmierczak, W. Synthesis of DME from CO2/H2 gas mixture. Chem. Eng. J. 2011, 167, 362–368. [Google Scholar] [CrossRef]
- Herring, H. Energy Efficiency—A Critical Review. Energy 2006, 31, 10–20. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Catalysis chemistry of dimethyl ether synthesis. ACS Catal. 2014, 4, 3346–3356. [Google Scholar] [CrossRef]
- Erik Rotheim—Famous Inventor. Available online: http://www.edubilla.com/inventor/erik-rotheim/ (accessed on 6 May 2024).
- Chen, W.H.; Lin, B.J.; Lee, H.M.; Huang, M.H. One-step synthesis of dimethyl ether from the gas mixture containing CO2 with high space velocity. Appl. Energy 2012, 98, 92–101. [Google Scholar]
- Dimethyl Ether Market Size—Global Industry, Share, Analysis, Trends and Forecast 2023–2032. Available online: https://www.acumenresearchandconsulting.com/dimethyl-ether-market (accessed on 6 May 2024).
- Mondal, U.; Yadav, G.D. Perspective of dimethyl ether as fuel: Part I. Catalysis. J. CO2 Util. 2019, 32, 299–320. [Google Scholar] [CrossRef]
- Brunetti, A.; Migliori, M.; Cozza, D.; Catizzone, E.; Giordano, G.; Barbieri, G. Methanol Conversion to Dimethyl Ether in Catalytic Zeolite Membrane Reactors. ACS Sustain. Chem. Eng. 2020, 8, 10471–10479. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2022, 7, 32–37. [Google Scholar] [CrossRef]
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Dimethyl Ether Market Size, Share, Industry, Forecast and Outlook (2023–2030). Available online: https://www.datamintelligence.com/research-report/dimethyl-ether-market (accessed on 6 May 2024).
- Zhou, C.; Wang, N.; Qian, Y.; Liu, X.; Caro, J.; Huang, A. Efficient Synthesis of Dimethyl Ether from Methanol in a Bifunctional Zeolite Membrane Reactor. Angew. Chem. Int. Ed. 2016, 55, 12678–12682. [Google Scholar] [CrossRef]
- Fedosov, D.; Smirnov, A.; Shkirskiy, V.; Voskoboynikov, T.; Ivanova, I. Methanol dehydration in NaA zeolite membrane reactor. J. Membr. Sci. 2015, 486, 189–194. [Google Scholar] [CrossRef]
- Farsi, M.; Jahanmiri, A. Enhancement of DME Production in an Optimized Membrane Isothermal Fixed-Bed Reactor. Int. J. Chem. React. Eng. 2011, 9, A74. [Google Scholar] [CrossRef]
- Volkov, V.; Novitskii, E.; Dibrov, G.; Samokhin, P.; Kipnis, M.; Yaroslavtsev, A. Catalytic conversion of methanol to dimethyl ether on polymer/ceramic composite membranes. Catal. Today 2012, 193, 31–36. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F.; Fongarland, P. Dimethyl Ether Synthesis with in situ H2O Removal in Fixed-Bed Membrane Reactor: Model and Simulations. Ind. Eng. Chem. Res. 2010, 49, 6870–6877. [Google Scholar] [CrossRef]
- Baracchini, G.; Klumpp, M.; Arnold, P.; Dittmeyer, R. Direct synthesis of dimethyl ether: A simulation study on the influence of the catalyst configuration. Chem. Eng. J. 2020, 396, 125155. [Google Scholar] [CrossRef]
- Moghaddam, A.L.; Ghavipour, M.; Kopyscinski, J.; Hazlett, M.J. Methanol dehydration to dimethyl ether over KFI zeolites. Effect of template concentration and crystallization time on catalyst properties and activity. Appl. Catal. A Gen. 2024, 158, 119594. [Google Scholar] [CrossRef]
- Luque-Álvarez, L.A.; González-Arias, J.; Romero-Sarria, F.; Reina, T.R.; Bobadilla, L.F.; Odriozola, J.A. Mechanistic insights into methanol carbonylation to methyl acetate over an efficient organic template-free Cu-exchanged mordenite. Catal. Sci. Technol. 2024, 15, 128–136. [Google Scholar] [CrossRef]
- Leonzio, G. State of art and perspectives about the production of methanol, dimethyl ether and syngas by carbon dioxide hydrogenation. J. CO2 Util. 2018, 27, 326–354. [Google Scholar] [CrossRef]
- Li, H.; Ren, S.; Zhang, S.; Padinjarekutt, S.; Sengupta, B.; Liang, X.; Lic, S.; Yu, M. The high-yield direct synthesis of dimethyl ether from CO2 and H2 in a dry reaction environment. J. Mater. Chem. A 2021, 9, 2678–2682. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G.O.; Perez-Ramırez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar]
- Waugh, K.C. Methanol Synthesis. Catal. Lett. 2012, 142, 1153–1166. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar]
- Peinado, C.; Liuzzi, D.; Retuerto, M.; Boon, J.; Peña, M.A.; Rojas, S. Study of catalyst bed composition for the direct synthesis of dimethyl ether from CO2-rich syngas. Chem. Eng. J. Adv. 2020, 4, 100039. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Parhoudeh, M.; Rahimpour, M.R. Optimal conditions in converting methanol to dimethyl ether, methyl formate, and hydrogen utilizing a double membrane heat exchanger reactor. J. Nat. Gas Sci. Eng. 2016, 28, 31–45. [Google Scholar] [CrossRef]
- Akarmazyan, S.S.; Panagiotopoulou, P.; Kambolis, A.; Papadopoulou, C.; Kondarides, D.I. Methanol dehydration to dimethylether over Al2O3 catalysts. Appl. Catal. B Environ. 2014, 145, 136–148. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Kansha, Y.; Ishizuka, M.; Song, C.; Tsutsumi, A. An innovative dimethyl ether (DME) production using self-heat recuperation. Chem. Eng. Trans. 2014, 39, 109–114. [Google Scholar]
- Banivaheb, S.; Pitter, S.; Delgado, K.H.; Rubin, M.; Sauer, J.; Dittmeyer, R. Recent Progress in Direct DME Synthesis and Potential of Bifunctional Catalysts. Chemie Ingenieur Technik 2022, 94, 240–255. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Jiao, W.-H.; Zuo, Z.-J.; Gao, Z.-H.; Huang, W. DME synthesis from methanol over hydrated γ-Al2O3(110) surface in slurry bed using continuum and atomistic models. J. Theor. Comp. Chem. 2017, 16, 1750029. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Ying, W.; Fang, D. Dehydration of methanol to dimethyl ether over γ-Al2O3 catalyst: Intrinsic kinetics and effectiveness factor. Can. J. Chem. Eng. 2013, 91, 1538–1546. [Google Scholar] [CrossRef]
- Ardy, A.; Rizkiana, J.; Gunawan, M.L.; Susanto, H. Characterization and Catalytic Activity of γ-Al2O3-ITB on Methanol Dehydration. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012054. [Google Scholar] [CrossRef]
- Sung, D.M.; Kim, Y.H.; Park, E.D.; Yie, J.E. Correlation between acidity and catalytic activity for the methanol dehydration over various aluminum oxides. Res. Chem. Intermed. 2010, 36, 653–660. [Google Scholar] [CrossRef]
- Keshavarz, A.R.; Rezaei, M.; Yaripour, F. Preparation of nanocrystalline γ-Al2O3 catalyst using different procedures for methanol dehydration to dimethyl ether. J. Nat. Gas Chem. 2011, 20, 334–338. [Google Scholar] [CrossRef]
- Lertjiamratn, K.; Praserthdam, P.; Arai, M.; Panpranot, J. Modification of acid properties and catalytic properties of AlPO4 by hydrothermal pretreatment for methanol dehydration to dimethyl ether. Appl. Catal. A Gen. 2010, 378, 119–123. [Google Scholar] [CrossRef]
- Seo, C.W.; Jung, K.D.; Lee, K.Y.; Yoo, K.S. Influence of Structure Type of Al2O3 on Dehydration of Methanol for Dimethyl Ether Synthesis. Ind. Eng. Chem. Res. 2008, 47, 6573–6578. [Google Scholar] [CrossRef]
- Xu, M.; Lunsford, J.H.; Goodman, D.W.; Bhattacharyya, A. Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts. Appl. Catal. A Gen. 1997, 149, 289–301. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Halawy, S.A.; Mohamed, M.A.; Abdelkader, A. Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2012, 127, 307–315. [Google Scholar] [CrossRef]
- Bateni, H.; Able, C. Development of Heterogeneous Catalysts for Dehydration of Methanol to Dimethyl Ether: A Review. Catal. Ind. 2019, 11, 7–33. [Google Scholar] [CrossRef]
- Rahmanpour, O.; Shariati, A.; Nikou, M.R.K. New Method for Synthesis Nano Size γ-Al2O3 Catalyst for Dehydration of Methanol to Dimethyl Ether. Int. J. Chem. Eng. Appl. 2012, 3, 125–128. [Google Scholar] [CrossRef]
- Keshavarz, A.R.; Rezaei, M.; Yaripour, F. Nanocrystalline gamma-alumina: A highly active catalyst for dimethyl ether synthesis. Powder Technol. 2010, 199, 176–179. [Google Scholar] [CrossRef]
- Hosseini, Z.; Taghizadeh, M.; Yaripour, F. Synthesis of nanocrystalline γ-Al2O3 by sol-gel and precipitation methods for methanol dehydration to dimethyl ether. J. Nat. Gas Chem. 2011, 20, 128–134. [Google Scholar] [CrossRef]
- Mollavali, M.; Yaripour, F.; Atashi, H.; Sahebdelfar, S. Intrinsic Kinetics Study of Dimethyl Ether Synthesis from Methanol on γ-Al2O3 Catalysts. Ind. Eng. Chem. Res. 2008, 47, 3265–3273. [Google Scholar] [CrossRef]
- Sahebdelfar, S.; Bijani, P.M.; Yaripour, F. Deactivation kinetics of γ-Al2O3 catalyst in methanol dehydration to dimethyl ether. Fuel 2022, 310, 122443. [Google Scholar] [CrossRef]
- Raoof, F.; Taghizadeh, M.; Eliassi, A.; Yaripour, F. Effects of temperature and feed composition on catalytic dehydration of methanol to dimethyl ether over γ-alumina. Fuel 2008, 87, 2967–2971. [Google Scholar] [CrossRef]
- Boon, J.; Kampen, J.; Hoogendoorn, R.; Tanase, S.; Berkel, F.P.F.; Sint Annaland, M. Reversible deactivation of γ-alumina by steam in the gas-phase dehydration of methanol to dimethyl ether. Catal. Commun. 2019, 119, 22–27. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. The effects of synthesis operation conditions on the properties of modified γ-alumina nanocatalysts in methanol dehydration to dimethyl ether using factorial experimental design. Fuel 2015, 139, 40–50. [Google Scholar] [CrossRef]
- Nogueira, D.J.; Arl, M.; Köerich, J.S.; Simioni, C.; Ouriques, L.C.; Vicentini, D.S.; Matias, W.G. Comparison of cytotoxicity of α-Al2O3 and η-Al2O3 nanoparticles toward neuronal and bronchial cells. Toxicol. In Vitro 2019, 61, 104596. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K. Kinetic Investigation of η-Al2O3 Catalyst for Dimethyl Ether Production. Catal. Lett. 2018, 148, 1236–1245. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Abdelkader, A.; Hassan, N.M.; Laffir, F.; McLaren, M.; Rooney, D. Silver-Modified η-Al2O3 Catalyst for DME Production. J. Phys. Chem. C 2017, 121, 25018–25032. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Thompson, J.; Halawy, S.A.; Mohamed, M.A. Surface hydrophobicity and acidity effect on alumina catalyst in catalytic methanol dehydration reaction. J. Chem. Technol. Biotechnol. 2017, 92, 2952–2962. [Google Scholar] [CrossRef]
- Jo, H.; Jung, H.; Park, J.; Jung, K.-D. Surface Modification of η–Al2O3 by SiO2 Impregnation to Enhance Methanol Dehydration Activity. Bull. Korean Chem. Soc. 2017, 38, 307–312. [Google Scholar] [CrossRef]
- Khaleel, A. Titanium-doped alumina for catalytic dehydration of methanol to dimethyl ether at relatively low temperatures. Fuel 2011, 90, 2422–2427. [Google Scholar] [CrossRef]
- Liu, D.; Yao, C.; Zhang, J.; Fang, D.; Chen, D. Catalytic dehydration of methanol to dimethyl ether over modified γ-Al2O3 catalyst. Fuel 2011, 90, 1738–1742. [Google Scholar] [CrossRef]
- Khaleel, A.; Ahmed, M.; Sowaid, S.B. Ti-doped γ-Al2O3 versus ZSM5 zeolites for methanol to dimethyl ether conversion: In-situ DRIFTS investigation of surface interactions and reaction mechanism. Colloid. Surf. A Physicochem. Eng. Asp. 2019, 571, 174–181. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Jun, K.W.; Kim, J.W.; Roh, H.S. Vapour phase dehydration of crude methanol to dimethyl ether over Na-modified H-ZSM-5 catalysts. Appl. Catal. A Gen. 2004, 276, 251–255. [Google Scholar] [CrossRef]
- Hassanpour, S.; Taghizadeh, M.; Yaripour, F. Preparation, Characterization, and Activity Evaluation of H-ZSM-5 Catalysts in Vapor-Phase Methanol Dehydration to Dimethyl Ether. Ind. Eng. Chem. Res. 2010, 49, 4063–4069. [Google Scholar] [CrossRef]
- Rownaghi, A.A.; Rezaei, F.; Stante, M.; Hedlund, J. Selective dehydration of methanol to dimethyl ether on ZSM-5 nanocrystals. Appl. Catal. B Environ. 2012, 119–120, 56–61. [Google Scholar] [CrossRef]
- Hassanpour, S.; Yaripour, F.; Taghizadeh, M. Performance of modified H-ZSM-5 zeolite for dehydration of methanol to dimethyl ether. Fuel Process. Technol. 2010, 91, 1212–1221. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.T.; Zhang, C.; Kwak, G.; Jun, K.-W. Effect of Reaction Conditions on the Catalytic Dehydration of Methanol to Dimethyl Ether Over a K-modified HZSM-5 Catalyst. Catal. Lett. 2017, 147, 792–801. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Arundhathi, R.; Kasture, M.W.; Samanta, C.; Vankayala, R.; Thota, C. Temperature-dependent synthesis of dimethyl ether (DME) from methanol over beta zeolite: A novel approach to a sustainable fuel. R. Soc. Open Sci. 2023, 10, 230524. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Macina, D.; Mirocha-Kubień, N.; Piwowarska, Z.; Chmielarz, L. Hierarchically structured ZSM-5 obtained by desilication as new catalyst for DME synthesis from methanol. Appl. Catal. B Environ. 2015, 174–175, 336–343. [Google Scholar]
- Dalena, F.; Giglio, E.; Giorgianni, G.; Cozza, D.; Marino, A.; Aloise, A. DME Production via Methanol Dehydration with H form and Desilicated ZSM-5 Type Zeolitic Catalysts: Study on the Correlation Between Acid Sites and Conversion. Chem. Eng. Trans. 2021, 84, 211–216. [Google Scholar]
- Aloise, A.; Marino, A.; Dalena, F.; Giorgianni, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Bonura, G.; Frusteri, F.; Giordano, G. Desilicated ZSM-5 zeolite: Catalytic performances assessment in methanol to DME dehydration. Micropor. Mesopor. Mater. 2020, 302, 110198. [Google Scholar] [CrossRef]
- Jin, D.; Zhu, B.; Hou, Z.; Fei, J.; Lou, H.; Zheng, X. Dimethyl ether synthesis via methanol and syngas over rare earth metals modified zeolite Y and dual Cu–Mn–Zn catalysts. Fuel 2007, 86, 2707–2713. [Google Scholar] [CrossRef]
- Fei, J.; Hou, Z.; Zhu, B.; Lou, H.; Zheng, X. Synthesis of dimethyl ether (DME) on modified HY zeolite and modified HY zeolite-supported Cu–Mn–Zn catalysts. Appl. Catal. A Gen. 2006, 304, 49–54. [Google Scholar] [CrossRef]
- Moradi, G.R.; Yaripour, F.; Vale-Sheyda, P. Catalytic dehydration of methanol to dimethyl ether over mordenite catalysts. Fuel Process. Technol. 2010, 91, 461–468. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. From 1-D to 3-D zeolite structures: Performance assessment in catalysis of vapour-phase methanol dehydration to DME. Micropor. Mesopor. Mater. 2017, 243, 102–111. [Google Scholar] [CrossRef]
- Migliori, M.; Catizzone, E.; Aloise, A.; Bonura, G.; Gómez-Hortigüela, L.; Frusteri, L.; Cannilla, C.; Frusteri, F.; Giordano, G. New insights about coke deposition in methanol-to-DME reaction over MOR-, MFI- and FER-type zeolites. J. Ind. Eng. Chem. 2018, 68, 196–208. [Google Scholar] [CrossRef]
- Zheng, J.; Ma, J.; Wang, Y.; Bai, Y.; Zhang, X.; Li, R. Synthesis and Catalytic Property of a Zeolite Composite for Preparation of Dimethyl Ether from Methanol Dehydration. Catal. Lett. 2009, 130, 672–678. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, H.; Zheng, Y.; Wang, J.; Li, H.; Zhang, J. Catalytic dehydration of methanol to dimethyl ether over micro–mesoporous ZSM-5/MCM-41 composite molecular sieves. Appl. Catal. A Gen. 2012, 413–414, 36–42. [Google Scholar] [CrossRef]
- Ulfah, M.; Octavia, S.; Suherman, H.; Laniwati, M.; Makerthiharta, I.G.B.N.; Subagjo. Synthesis and characterization of modified γ-Alumina-NaA and γ-Alumina-NaX zeolite composites as methanol dehydration catalysts in synthesis Dimethyl Ether (DME). IOP Conf. Ser. Mater. Sci. Eng. 2020, 823, 012010. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Giglio, E.; Ferrarelli, G.; Bianco, M.; Migliori, M.; Giordano, G. MFI vs. FER zeolite during methanol dehydration to dimethyl ether: The crystal size plays a key role. Catal. Commun. 2021, 149, 106214. [Google Scholar] [CrossRef]
- Rutkowska, M.; Macina, D.; Piwowarska, Z.; Gajewska, M.; Díaz, U.; Chmielarz, L. Hierarchically structured ZSM-5 obtained by optimized mesotemplate-free method as active catalyst for methanol to DME conversion. Catal. Sci. Technol. 2016, 6, 4849–4862. [Google Scholar] [CrossRef]
- Abbasian, S.; Taghizadeh, M. Preparation of H-ZSM-5 Nano-Zeolite Using Mixed Template Method and its Activity Evaluation for ethanol to DME Reaction. Int. J. Nanosci. Nanotechnol. 2014, 10, 171–180. [Google Scholar]
- Aboul-Fotouh, S.M.K.; Aboul-Gheit, N.A.K.; Naghmash, M.A. Dimethylether production on zeolite catalysts activated by Cl-, F- and/or ultrasonication. J. Fuel Chem. Technol. 2016, 44, 428–436. [Google Scholar] [CrossRef]
- Dennis-Smither, B.J.; Yang, Z.; Buda, C.; Liu, X.; Sainty, N.; Tan, X.; Sunley, G.J. Getting zeolite catalysts to play your tune: Methyl carboxylate esters as switchable promoters for methanol dehydration to DME. Chem. Commun. 2019, 55, 13804–13807. [Google Scholar] [CrossRef]
- Masih, D.; Rohani, D.; Kondo, J.N.; Tatsumi, T. Low-temperature methanol dehydration to dimethyl ether over various small-pore zeolites. Appl. Catal. B Environ. 2017, 217, 247–255. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. The effect of FER zeolite acid sites in methanol-to-dimethyl-ether catalytic dehydration. J. Energy Chem. 2016, 26, 406–415. [Google Scholar] [CrossRef]
- Marosz, M.; Samojeden, B.; Kowalczyk, A.; Rutkowska, M.; Motak, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. MCM-22, MCM-36, and ITQ-2 Zeolites with Different Si/Al Molar Ratios as Effective Catalysts of Methanol and Ethanol Dehydration. Materials 2020, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour, A.; Rimer, J.D.; Grabow, L.C. Computational Assessment of the Dominant Factors Governing the Mechanism of Methanol Dehydration over H-ZSM-5 with Heterogeneous Aluminum Distribution. ACS Catal. 2016, 6, 2287–2298. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Święs, A.; Kowalczyk, A.; Gil, B.; Chmielarz, L. Dehydration of methanol and ethanol over ferrierite originated layered zeolites—The role of acidity and porous structure. RSC Adv. 2022, 12, 9395–9403. [Google Scholar] [CrossRef]
- Chmielarz, L.; Dziembaj, R. Modified Layered Silicas as Catalysts for Conversion of Nitrogen Pollutants in Flue Gases—A Review. Catalysts 2021, 11, 644. [Google Scholar] [CrossRef]
- Silva, A.C.; Ciuffi, K.J.; Reis, M.J.; Calefi, P.S. Emerson Henrique de Faria, Influence of physical/chemical treatments to delamination of nanohybrid kaolinite-dipicolinate. Appl. Clay Sci. 2016, 126, 251–258. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Wojciechowska, M.; Boroń, P.; Dudek, M.; Michalik, M. Montmorillonite Intercalated with SiO2, SiO2-Al2O3 or SiO2-TiO2 Pillars by Surfactant-Directed Method as Catalytic Supports for DeNOx Process. Chem. Pap. 2014, 68, 1219–1227. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Kuśtrowski, P.; Węgrzyn, A.; Gil, B.; Kowalczyk, A.; Dudek, B.; Dziembaj, R.; Michalik, M. Comparison Study of Titania Pillared Interlayered Clays and Porous Clay Heterostructures Modified with Copper and Iron as Catalysts of the DeNOx Process. Appl. Clay Sci. 2011, 53, 164–173. [Google Scholar] [CrossRef]
- Marosz, M.; Kowalczyk, A.; Chmielarz, L. Modified vermiculites as effective catalysts for dehydration of methanol and ethanol. Catal. Today 2020, 355, 466–475. [Google Scholar] [CrossRef]
- Marosz, M.; Kowalczyk, A.; Gil, B.; Chmielarz, L. Acid-treated Clay Minerals as Catalysts for Dehydration of Methanol and Ethanol. Clays Clay Miner. 2020, 68, 23–371. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Piwowarska, Z.; Dudek, B.; Gil, B.; Michalik, M. Montmorillonite, vermiculite and saponite based porous clay heterostructures modified with transition metals as catalysts for the DeNOx process. Appl. Catal. B Environ. 2009, 88, 331–340. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Skoczek, M.; Rutkowska, M.; Gil, B.; Natkański, P.; Radko, M.; Motak, M.; Dębek, R.; Ryczkowski, J. Porous clay heterostructures intercalated with multicomponent pillars as catalysts for dehydration of alcohols. Appl. Clay Sci. 2018, 160, 116–125. [Google Scholar] [CrossRef]
- Kikuchi, E. Palladium/ceramic membranes for selective hydrogen permeation and their application to membrane reactor. Catal. Today 1995, 25, 333–337. [Google Scholar] [CrossRef]
- Lina, Y.M.; Leeb, G.L.; Rei, M.H. An integrated purification and production of hydrogen with a palladium membrane–catalytic reactor. Catal. Today 1998, 44, 343–349. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; Kaliaguine, S. Methane steam reforming in asymmetric Pd–Ag and Pd–Ag/porous SS membrane reactors. Appl. Catal. 1994, 119, 305–325. [Google Scholar] [CrossRef]
- Uemiya, S.; Sato, N.; Ando, H.; Matsuda, T.; Kikuchi, E. Steam reforming of methane in a hydrogen-permeable membrane reactor. Appl. Catal. 1991, 67, 223–230. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Lotfinejad, M. Enhancement of Methanol Production in a Membrane Dual-Type Reactor. Chem. Eng. Technol. 2007, 30, 1062–1076. [Google Scholar] [CrossRef]
- Khassin, A.A.; Sipatrov, A.G.; Yurieva, T.M.; Chermashentseva, G.K.; Rudina, N.A.; Parmon, V.N. Performance of a catalytic membrane reactor for the Fischer–Tropsch synthesis. Catal. Today 2005, 105, 362–366. [Google Scholar] [CrossRef]
- Mohammed, M.B.A.; Wagialla, K.M.E. Effect of In-situ Membrane Removal of H2O on Methanol Conversion during Dimethyl Ether Synthesis Reaction. Int. J. Sci. Basic Appl. Res. 2016, 27, 42–52. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).