Engineering the Integration of Titanium and Nickel into Zinc Oxide Nanocomposites through Nanolayered Structures and Nanohybrids to Design Effective Photocatalysts for Purifying Water from Industrial Pollutants
Abstract
:1. Introduction
2. Results
2.1. Characterizations of the Ni–Ti–Zn Nanolayers and Ni–Ti–Zn–Organic Nanohybrids
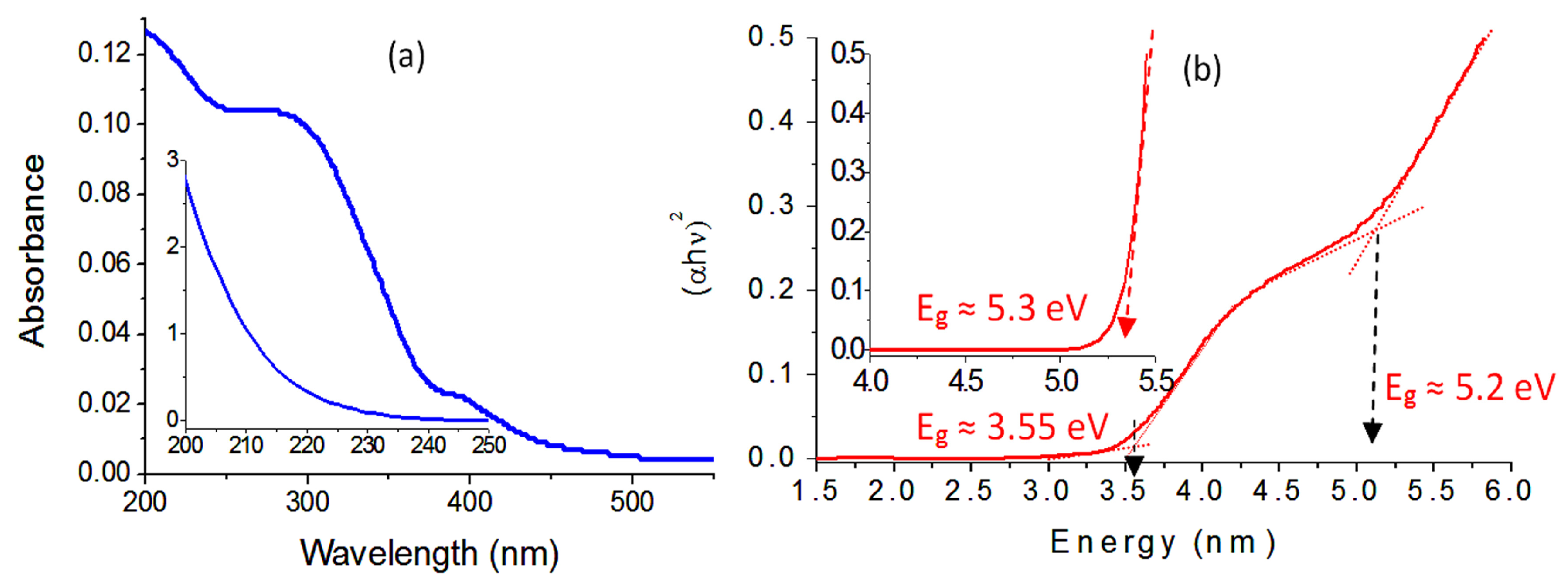
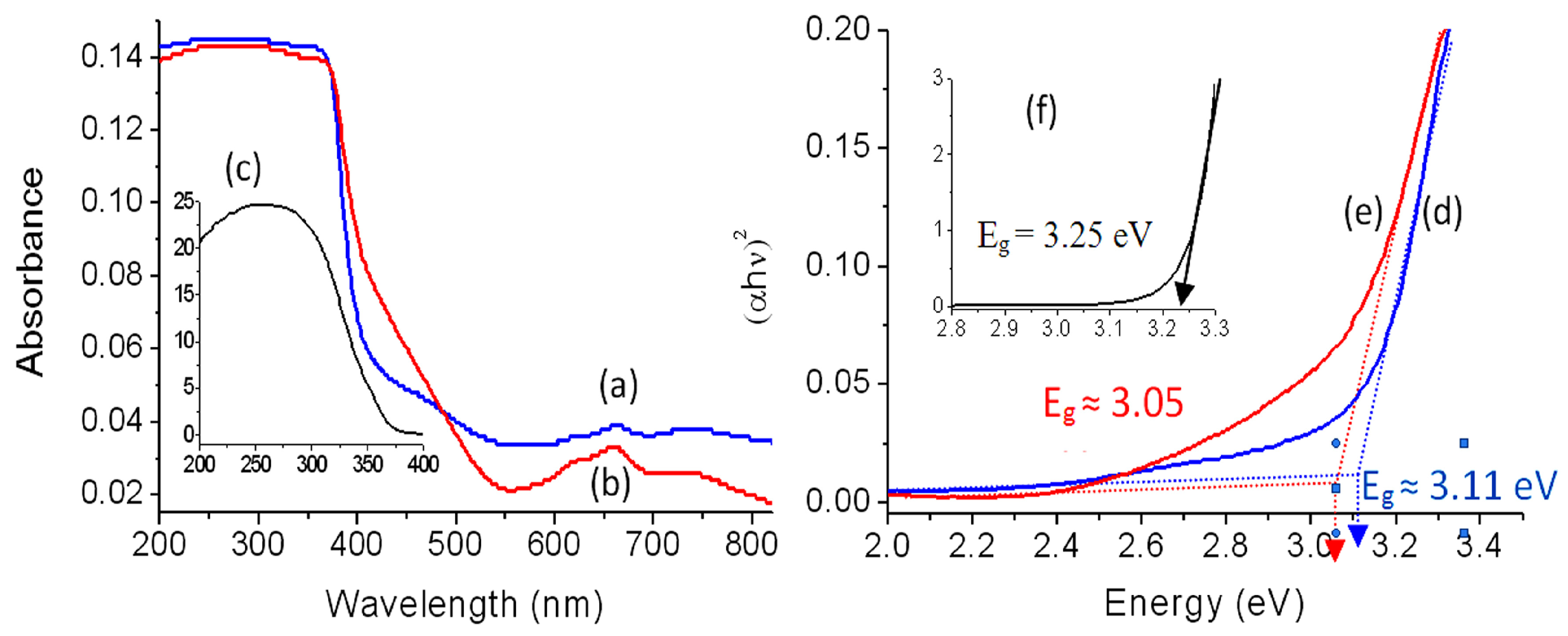
2.2. Optical Properties
2.3. Photocatalytic Degradation of Green Dyes
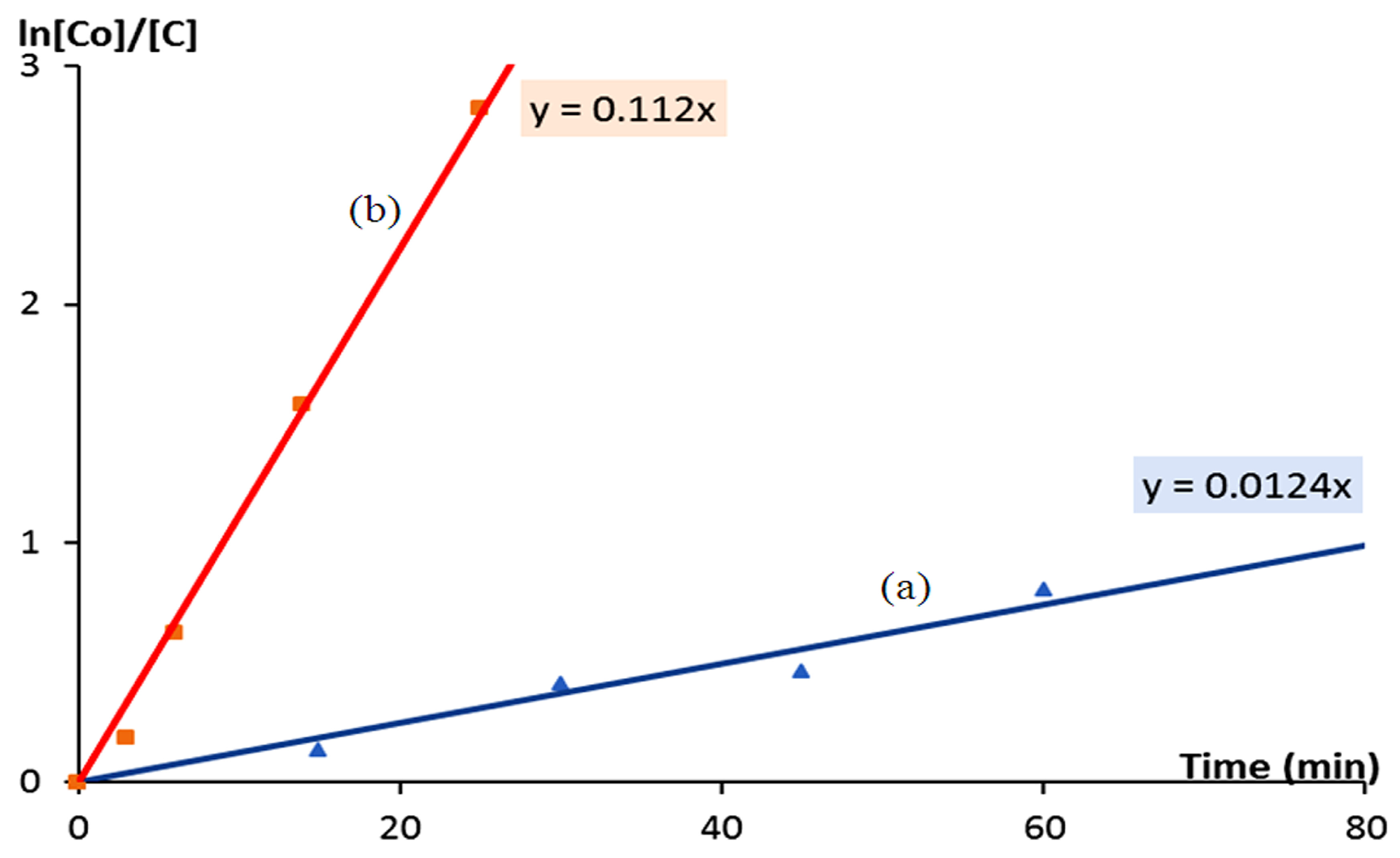
2.4. Kinetic Study of the Photocatalytic Degradation of Green Dyes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Design of a Tripartite System of Nanolayered Materials
5.2. Design of Ni–Zn–Ti–Organic Nanohybrids
5.3. Characterization of the Prepared Samples
5.4. Photocatalytic Processes
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saber, O.; Osama, A.; Alshoaibi, A.; Shaalan, N.M.; Osama, D. New Approach for Designing Zinc Oxide Nanohybrids to Be Effective Photocatalysts for Water Purification in Sunlight. Nanomaterials 2022, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Saber, O.; Osama, A.; Alshoaibi, A.; Shaalan, N.M.; Osama, D. Designing inorganic–magnetic–organic nanohybrids for producing effective photocatalysts for the purification of water. RSC Adv. 2022, 12, 18282–18295. [Google Scholar] [CrossRef] [PubMed]
- Ljubas, D.; Juretić, H.; Badrov, A.; Biošić, M.; Babić, S. Photocatalytic Degradation of Pharmaceutical Trimethoprim in Aqueous Solution over Nanostructured TiO2 Film Irradiated with Simulated Solar Radiation. Appl. Sci. 2023, 13, 5681. [Google Scholar] [CrossRef]
- Xu, S.M.; Pan, T.; Dou, Y.B.; Yan, H.; Zhang, S.T.; Ning, F.Y.; Shi, W.Y.; Wei, M. Theoretical and experimental study on (MMIII)-M-II-layered double hydroxides as efficient photocatalysts toward oxygen evolution from water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Soltani, R.D.C.; Dinpazhoh, L.; Bhatnagar, A. Photocatalytic degradation of gemifloxacin antibiotic using Zn-Co-LDH@biochar nanocomposite. J. Hazard. Mater. 2020, 382, 121070. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Y.; Zhao, Y.F.; Shi, R.; Yin, W.J.; Zhao, Y.X.; Waterhouse, G.I.N.; Zhang, T.R. Selective photocatalytic CO2 reduction over Zn-based layered double hydroxides containing tri or tetravalent metals. Sci. Bull. 2020, 65, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Parida, K.M.; Mohapatra, L. Carbonate intercalated Zn/Fe layered double hydroxide: A novel photocatalyst for the enhanced photo degradation of azo dyes. Chem. Eng. J. 2012, 179, 131–139. [Google Scholar] [CrossRef]
- Bouaziz, Z.; Soussan, L.; Janot, J.-M.; Jaber, M.; Amara, A.B.H.; Balme, S. Dual role of layered double hydroxide nanocomposites on antibacterial activity and degradation of tetracycline and oxytetracyline. Chemosphere 2018, 206, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, P.; Li, S.; Chen, M.; Cai, W.; Zou, D.; Zhu, N.; Dang, Z. Synergistic deep removal of As(III) and Cd(II) by a calcined multifunctional MgZnFe-CO3 layered double hydroxide: Photooxidation, precipitation and adsorption. Chemosphere 2019, 225, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, W. Graphitic-C3N4 modified ZnAl-layered double hydroxides for enhanced photocatalytic removal of organic dye. Appl. Clay Sci. 2017, 138, 107–113. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yan, T.; Zhu, R.; Yan, L.; Pei, Z. Adsorption and photocatalytic reduction of aqueous Cr(VI) by Fe3O4-ZnAl-layered double hydroxide/TiO2 composites. J. Colloid Interface Sci. 2020, 562, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Zheng, Z.H.; Yang, M.H.; Chen, J.F.; Li, C.; Zhang, C.H.; Zhang, X.D. In-situ fabrication of a spherical-shaped Zn-Al hydrotalcite with BiOCl and study on its enhanced photocatalytic mechanism for perfluorooctanoic acid removal performed with a response surface methodology. J. Hazard. Mater. 2020, 399, 123070. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Ding, Z.; Mahadi, A.H.; Zhao, Y.; Song, Y.-F. Photocatalytic selective oxidation of benzene to phenol in water over layered double hydroxide: A thermodynamic and kinetic perspective. Chem. Eng. J. 2020, 388, 124248. [Google Scholar] [CrossRef]
- Ye, J.; Liu, J.; Huang, Z.; Wu, S.; Dai, X.; Zhang, L.; Cui, L. Effect of reduced graphene oxide doping on photocatalytic reduction of Cr(VI) and photocatalytic oxidation of tetracycline by ZnAlTi layered double oxides under visible light. Chemosphere 2019, 227, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, Z.; Wang, F.; Qi, Y.; Zhang, W.; Wang, C. State-of-the-art and prospects of Zn-containing layered double hydroxides (Zn-LDH)-based materials for photocatalytic water remediation. Chemosphere 2021, 278, 130367. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Xu, M.; Bi, B.; Xu, B.; Sun, Z.; Xu, L. Polyoxometalate-intercalated ZnAlFelayered double hydroxides for adsorbing removal and photocatalytic degradation of cationic dye. Appl. Clay Sci. 2018, 157, 86–91. [Google Scholar] [CrossRef]
- Shao, M.; Han, J.; Wei, M.; Evans, G.D.; Duan, X. The synthesis of hierarchical Zn–Ti layered double hydroxide for efficient visible-light photocatalysis. Chem. Eng. J. 2011, 168, 519–524. [Google Scholar] [CrossRef]
- Xia, S.-J.; Liu, F.-X.; Ni, Z.M.; Xue, J.-L.; Qian, P.-P. Layered double hydroxides as efficient photocatalysts for visible-light degradation of Rhodamine B. J. Colloid Interface Sci. 2013, 405, 195–200. [Google Scholar] [CrossRef]
- Chowdhury, P.R.; Bhattacharyya, K.G. Ni/Ti layered double hydroxide: Synthesis, characterization and application as a photocatalyst for visible light degradation of aqueous methylene blue. Dalton Trans. 2015, 44, 6809–6824. [Google Scholar] [CrossRef]
- Parida, K.; Mohapatra, L. Recent progress in the development of carbonate-intercalated Zn/Cr LDH as a novel photocatalyst for hydrogen evolution aimed at the utilization of solar light. Dalton Trans. 2012, 41, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Li, B.; Yan, H.; He, S.; Tian, L.; Shi, W.; Ma, J.; Wei, M.; Evans, D.G.; et al. A Family of Visible-Light Responsive Photocatalysts Obtained by Dispersing CrO6 Octahedra into a Hydrotalcite Matrix. Chem.—Eur. J. 2011, 17, 13175–13181. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gou, H.; Zhu, P.; Jin, Z. Ingenious Design of CoAl-LDH p-n Heterojunction Based on CuI as Holes Receptor for Photocatalytic Hydrogen Evolution, Chinese Journal of Structural Chemistry. Chin. J. Struct. Chem. 2022, 41, 2206079. [Google Scholar]
- Han, G.; Xu, F.; Cheng, B.; Li, Y.; Yu, J.; Zhang, L. Enhanced Photocatalytic H2O2 Production over Inverse Opal ZnO@ Polydopamine S-Scheme Heterojunctions. Acta Phys.—Chim. Sin. 2022, 38, 2112037–2112047. [Google Scholar] [CrossRef]
- Long, X.; Meng, J.; Gu, J.; Ling, L.; Li, Q.; Liu, N.; Wang, K.; Li, Z. Interfacial Engineering of NiFeP/NiFe-LDH Heterojunction for Efficient Overall Water Splitting. Chin. J. Struct. Chem. 2022, 41, 2204046–2204053. [Google Scholar]
- Baran, T. Efficiency of volatile organic compound degradation in air using doped strontium titanate photocatalysts. Quenching experiments towards understanding of doping mechanisms. React. Kinet. Mech. Catal. 2023, 136, 3243–3256. [Google Scholar] [CrossRef]
- Constantino, V.R.L.; Pinnavaia, T. Basic properties of Mg2+1−xAl3+x layered double hydroxides intercalated by carbonate, hydroxide, chloride, and sulfate anions. Inorg. Chem. 1995, 34, 883. [Google Scholar] [CrossRef]
- Yun, S.; Pinnavaia, T. Water content and particle texture of synthetic hydrotalcite-like layered double hydroxides. Chem. Mater. 1995, 7, 348–354. [Google Scholar] [CrossRef]
- Saber, O.; Tagaya, H. New layered double hydroxide, Zn–Ti LDH: Preparation and intercalation reactions. J. Incl. Phenom. Macrocycl. Chem. 2003, 45, 109–116. [Google Scholar] [CrossRef]
- Saber, O.; Tagaya, H. Preparation and intercalation reactions of nano-structural materials, Zn–Al–Ti LDH. Mater. Chem. Phys. 2008, 108, 449–455. [Google Scholar] [CrossRef]
- Saber, O.; Tagaya, H. Preparation and intercalation reactions of Zn-Sn LDH and Zn-Al-Sn LDH. J. Porous Mater. 2003, 10, 83–91. [Google Scholar] [CrossRef]
- Saber, O.; Tagaya, H. Preparation of a new nano-layered materials and organic–inorganic nano-hybrid materials, Zn–Si LDH. J. Porous Mater. 2009, 16, 81–89. [Google Scholar] [CrossRef]
- Muramatsu, K.; Saber, O.; Tagaya, H. Preparation of new layered double hydroxide, Zn-Mo LDH. J. Porous Mater. 2007, 14, 481–484. [Google Scholar] [CrossRef]
- Saber, O.; Ansari, S.A.; Parveen, N.; Shaalan, N.M.; Osama, A.; Osama, M. Positive Influence of Oxalate and Cyanate on the Supercapacitance Performance of V/Co 2D-Nanolayered Structures. Inorganics 2023, 11, 458. [Google Scholar] [CrossRef]
- Nakamoto, N. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Mugumo, R.; Ichipi, E.; Tichapondwa, S.M.; Chirwa, E.M.N. Visible-Light-Induced Photocatalytic Degradation of Rhodamine B Dye Using a CuS/ZnS p-n Heterojunction Nanocomposite under Visible-Light irradiation. Catalysts 2023, 13, 1184. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhou, Y.; Wu, C.; Chen, Z.; Chen, G. Synergistic Promotion of Photocatalytic Degradation of Methyl Orange by Fluorine- and Silicon-Doped TiO2/AC Composite Material. Molecules 2023, 28, 5170. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.R.; Abdulkhair, B.Y.; Elamin, N.Y.; Ibnaouf, K.H.; Idriss, H.; Bakheit, R.; Modwi, A. Application of Synthesized Vanadium–Titanium Oxide Nanocomposite to Eliminate Rhodamine-B Dye from Aqueous Medium. Molecules 2023, 28, 176. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-J.; Huang, Y.-S.; Wu, Q.-Y.; Wang, W.-L.; Wu, J.J. Hollow-structured Pd/TiO2 as a dual functional photocatalyst for methyl orange oxidation and selective reduction of nitrate into nitrogen. J. Ind. Eng. Chem. 2023, 119, 386–394. [Google Scholar]
- Zhang, S.; Lei, C.; Song, L. Chiral induction enhanced photocatalytic degradation of methyl orange by Ag@Ag3PO4 and the reaction mechanism. Ceram. Int. 2023, 49, 26548–26557. [Google Scholar] [CrossRef]
- Bi, T.; Du, Z.; Chen, S.; He, H.; Shen, X.; Fu, Y. Preparation of flower-like ZnO photocatalyst with oxygen vacancy to enhance the photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2023, 614, 156240. [Google Scholar] [CrossRef]
- Khan, K.A.; Shah, A.; Nisar, J.; Haleem, A.; Shah, I. Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review. Molecules 2023, 28, 4600. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Shafiq, A.; Chen, S.Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Chen, S.Q.; Pan, J.M.; He, W.D. Gamma radiation induced synthesis of double network hydrophilic cryogels at low pH loaded with AuNPs for fast and efficient degradation of Congo red. J. Hazard. Mater. Adv. 2023, 10, 100299. [Google Scholar] [CrossRef]

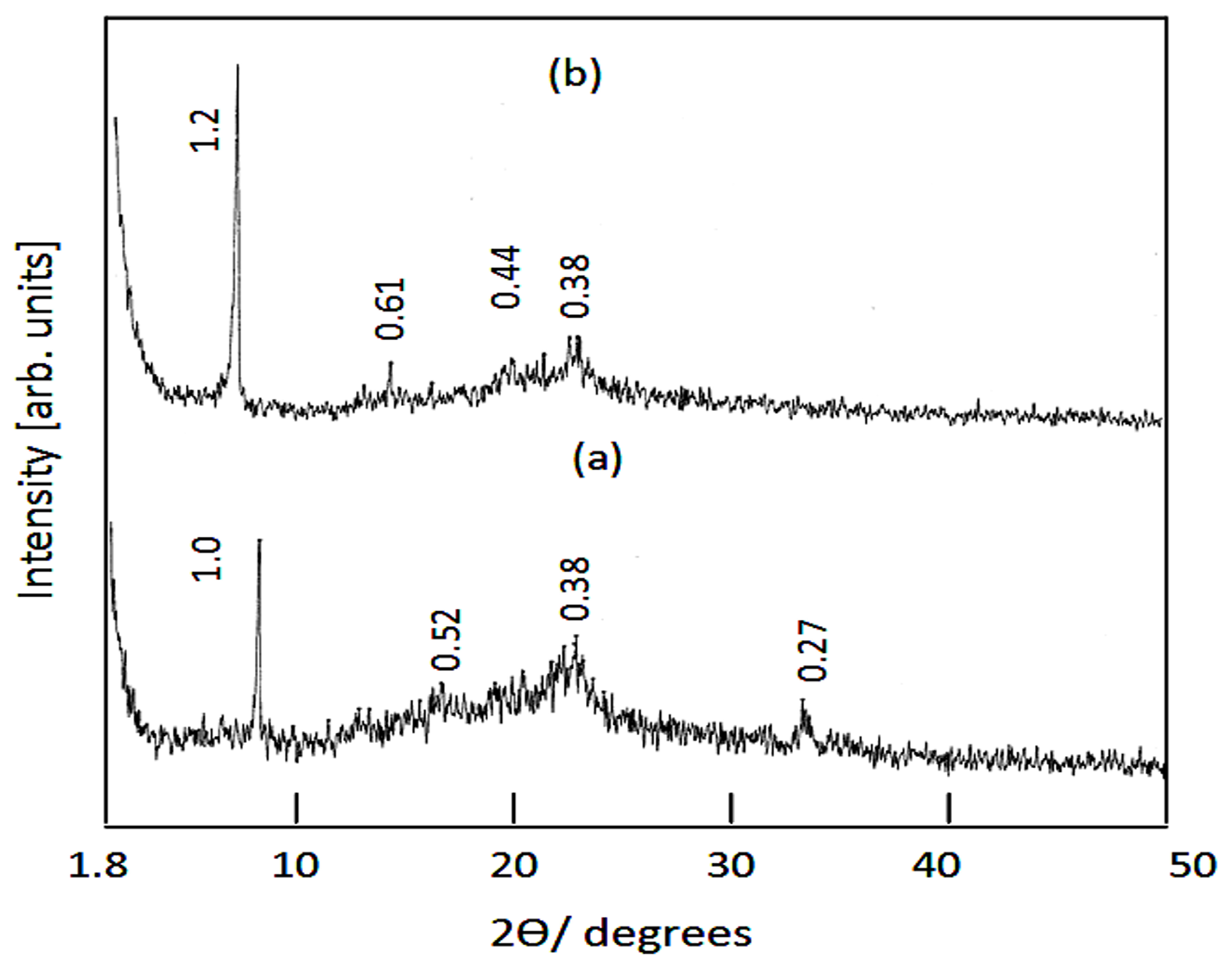
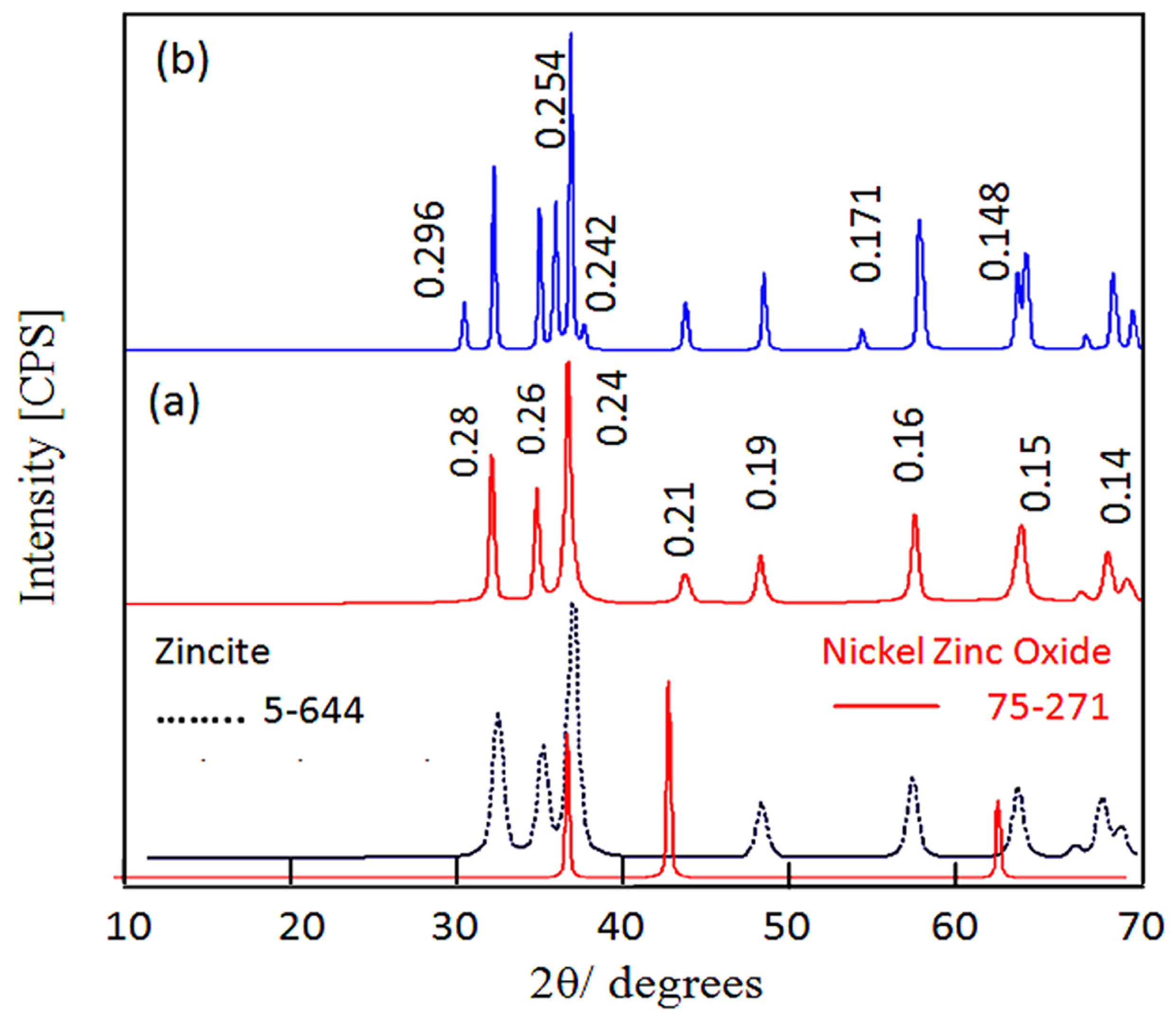

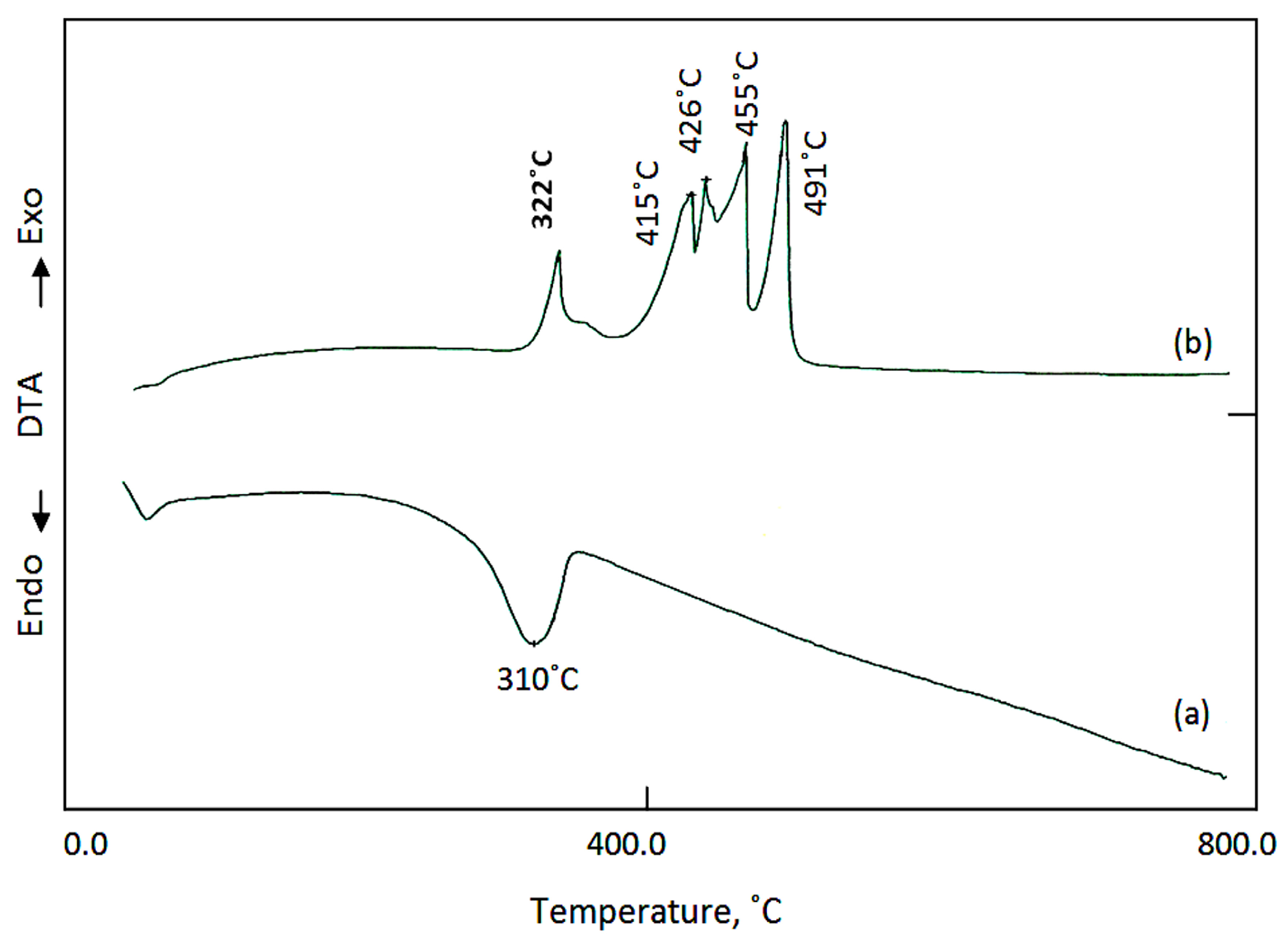
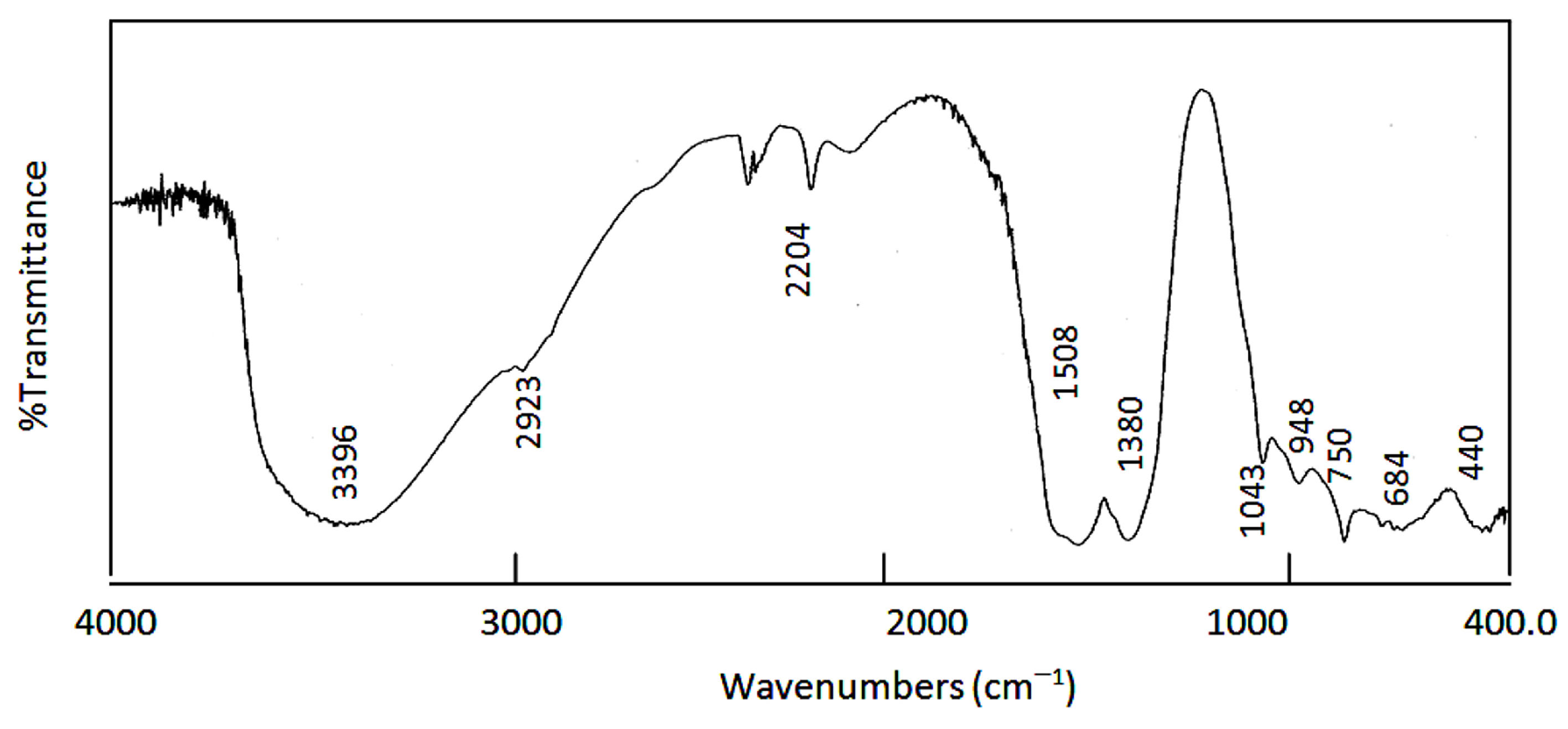

| The Nanolayered Structures | The Main Guest | Synthesis Temp. (°C) | d003 (nm) | Peaks in XRD (nm) | Peaks (2Ѳ) |
|---|---|---|---|---|---|
| Zn-Al LDH | Carbonate | 80 | 0.76 | 0.76, 0.37, 0.26 | 11.7, 24.3, 34.3 |
| Ni-Zn-Ti LDH | Carbonate | 80 | 0.67 | 0.67, 0.34, 0.26 | 13.3, 26.3, 34.3 |
| Ni-Zn-Ti-CB10 | n-capric acid CH3(CH2)8COOH | 60 | 2.6 | 2.6, 2.3, 1.3, 1.2, 0.82, 0.67 | 3.4, 3.8, 6.7, 7.3 10.8, 13.3 |
| Ni-Zn-Ti-CB14 | Myristic acid CH3(CH2)12COOH | 60 | 3.4 | 3.4, 1.8, 1.2 | 2.6, 4.9, 7.3 |
| Ni-Zn-Ti-CB18 | Stearic acid CH3(CH2)16COOH | 60 | 4.4 | 4.4, 2.2, 1.5 | 2.0, 4.0, 5.9 |
| Ni-Zn-Ti-CD6 | Suberic acid (COOHC6H12COOH) | 60 | 1.0 | 1.0, 0.52, 0.38, 0.27 | 8.8, 16.9, 23.6, 33.6 |
| Ni-Zn-Ti-CD8 | Sebacic acid (COOHC8H16 COOH) | 60 | 1.2 | 1.2, 0.61, 0.44, 0.38 | 7.3, 14.5, 20.4, 23.4 |
| Samples | Band Gap Energy (eV) |
|---|---|
| Zn–Al LDH | 5.3 |
| Zn–Al–500 | 3.25 |
| Ni–Zn–Ti LDH | 5.2 and 3.55 |
| Ni–Zn–Ti–500 | 3.11 |
| Ni–Zn–Ti–900 | 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saber, O.; Osama, A.; Shaalan, N.M.; Osama, M. Engineering the Integration of Titanium and Nickel into Zinc Oxide Nanocomposites through Nanolayered Structures and Nanohybrids to Design Effective Photocatalysts for Purifying Water from Industrial Pollutants. Catalysts 2024, 14, 340. https://doi.org/10.3390/catal14060340
Saber O, Osama A, Shaalan NM, Osama M. Engineering the Integration of Titanium and Nickel into Zinc Oxide Nanocomposites through Nanolayered Structures and Nanohybrids to Design Effective Photocatalysts for Purifying Water from Industrial Pollutants. Catalysts. 2024; 14(6):340. https://doi.org/10.3390/catal14060340
Chicago/Turabian StyleSaber, Osama, Aya Osama, Nagih M. Shaalan, and Mostafa Osama. 2024. "Engineering the Integration of Titanium and Nickel into Zinc Oxide Nanocomposites through Nanolayered Structures and Nanohybrids to Design Effective Photocatalysts for Purifying Water from Industrial Pollutants" Catalysts 14, no. 6: 340. https://doi.org/10.3390/catal14060340







