Abstract
Photocatalysis technology is an economical and effective new energy technology which depends on the conversion and storage of light energy through an energy transfer process or charge transfer process. Recently, organic semiconductor photocatalytic materials with the advantages of controllable structure, broad spectral response, designability, and flexibility have received wide attention. In particular, the organic polymeric materials containing poly-perylene diimides (PDI) show significant promise in the realm of photocatalysis due to their impressive catalytic capabilities and wide spectral reactivity. However, a poor charge separation and transportation (CST) process undermines their photocatalytic efficiency in most polymer photocatalysts, as well as in PDI photocatalysts. In this context, we propose a new strategy through regulating the monomer symmetry to construct highly efficient PDI photocatalysts. As proof-of-concept, a series of new PDI-based organic supramolecular photocatalytic materials with full visible spectral response from the perspectives of both the π-π conjugated structure and the symmetry of chain structure are successfully synthesized. Meanwhile, the structural compositions, morphology features, electrical properties, and photocatalytic performances of those obtained PDI photocatalysts were systematically studied. The results shown that the as-prepared PDI-1,5NDA exhibits 1.6-fold and 3.7-fold higher levels of photosynthesis of H2O2 activity than those of PDI-1,4NDA and PDI-PDA, respectively, which could be ascribe to its lower symmetry and large π-conjugate systems greatly enhances the separation of charge carriers.
1. Introduction
In the past hundred years, traditional fossil energy has brought great improvement to human material life, but it has also brought a series of inevitable energy and environmental problems [1,2]. The effective use of clean energy and protection of the living environment are necessary guarantees for the sustainable coexistence of all species on the earth [3]. In the current research, photocatalysis technology using clean solar energy has aroused great attention [4,5]. Among various solar photocatalytic technologies, photocatalytic H2O2 production is a viable solution for dealing with energy and environmental challenges, because of its minimal energy usage, security, and green credentials [6]. In addition, H2O2 has a significant market potential as an industrial effluent treatment involving sterilization and pulp bleaching [7]. Notably, the performance of photocatalysts determines the conversion and storage efficiency of photocatalytic technology, which is the key issue affecting the development of photocatalysis technology [8]. Since Fujishima and Honda discovered in 1972 that titanium dioxide as an electrode can decompose water to produce hydrogen under light [9], researchers have been working hard to improve the solar energy conversion efficiency of photocatalytic materials, and continue to explore the space configuration, electronic structure, interfacial reaction, and ultrafast charge dynamics theory of photocatalytic materials [10,11,12]. However, the recombination rate of photogenerated carriers of these materials is still very high, resulting a low light quantum efficiency [3,13,14,15,16]. Fortunately, organic semiconductors, as a new parallel substitute material for photocatalysis, have attracted great attention and the interest of researchers by the virtue of controllable structure, broad spectral response, designability, and flexibility [11,17,18,19]. However, a poor charge separation and transportation (CST) process has undermined photocatalytic efficiency in most organic semiconductor-based photocatalysts [2,17,18,19,20,21]. Therefore, there is an urgent need to explore the preparation of new high-efficiency organic semiconductor photocatalytic materials.
Perylene diimides (PDI) and their derivatives are one of the best n-type organic semiconductors [22,23]. They demonstrate very unique photoelectrochemical properties and excellent light and thermal stability [24,25,26]. In recent years, with the development of photocatalytic materials, PDI and their derivatives have gradually entered the attention of photocatalysis researchers because of their ultra-wide spectral response and excellent carrier transport properties [27]. PDI-composite catalytic materials with different central catalysts have shown wide application prospects in the field of environment and energy [28]. These include PDI-Ni, PDI-TiO2, PDI-C3N4, and other photocatalytic catalysts used for hydrogen production [29,30,31,32,33]. Although PDI and their derivatives have been reported in the field of photocatalysis, while PDI exist in the forms of phototrapping/photosensitizer in these catalyst systems, they are not the main catalytic material. As far as we know, there are few studies on PDI as a main photocatalyst, and many problems still need to be solved, such as the effects of conjugation degree and symmetry on the photocatalytic performance of perylene imide, and its regulation mechanism is not very clear.
Herein, we propose a new strategy aiming to construct efficient perylene imide-based photocatalysts by regulation of its monomer symmetry. Accordingly, a novel polymer photocatalyst, named PDI-1,5NDA, was successfully constructed through a simple polymerization method. Furthermore, through a series of experiments, we adjusted the conjugation degree and symmetry of perylene imide by changing the linker and measured the photocatalytic performance data under different conditions. The structural characteristics and photocatalytic performance of the obtained perylene imide are discussed in detail, and the mechanisms of influence of conjugation degree and symmetry on its photocatalytic performance are proposed.
2. Results and Discussion
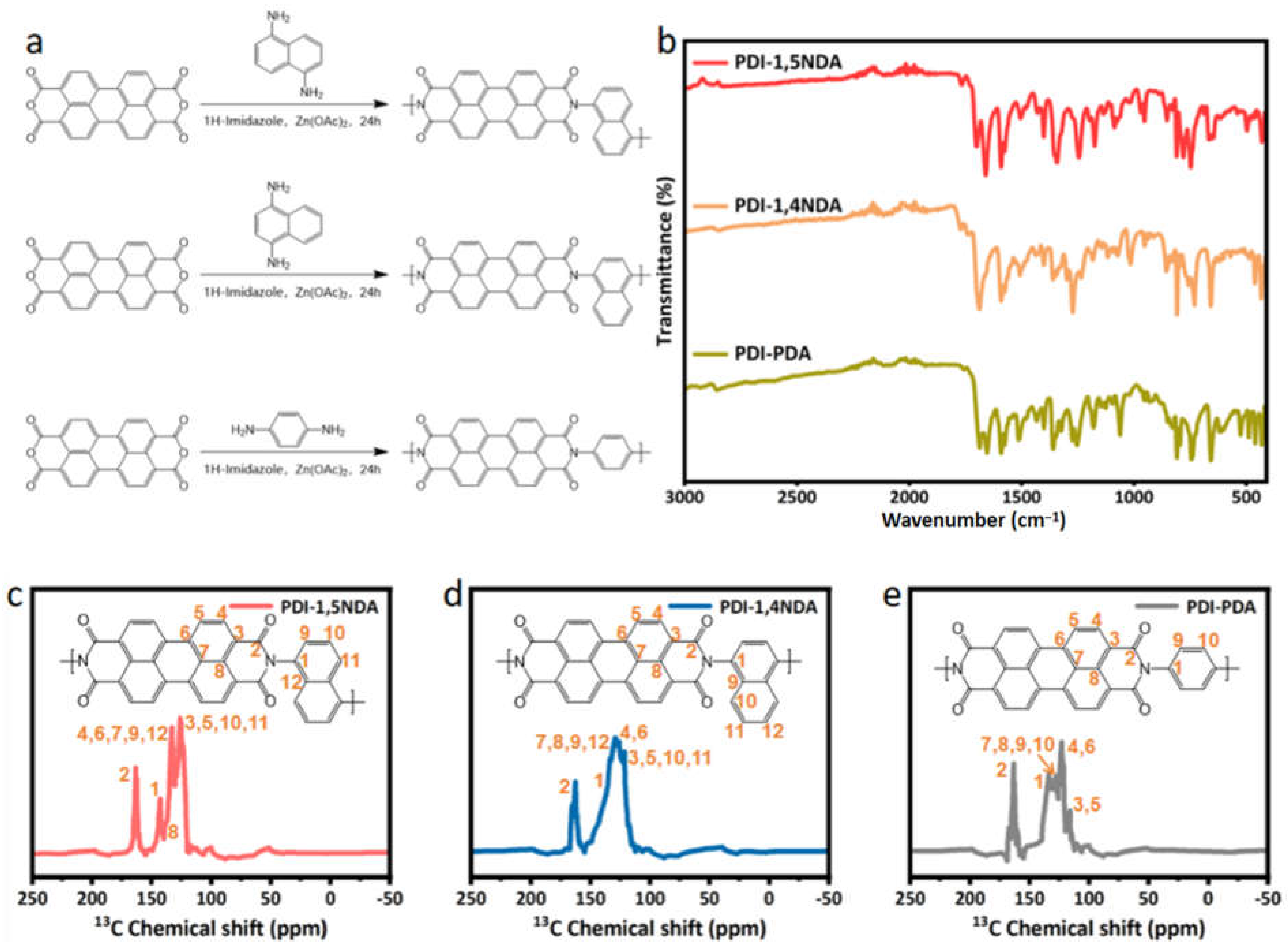
During the synthesis, 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) was selected as the monomer with perylene groups, while the 1,5-diaminonaphthalene was used as the linker to construct the polymer photocatalyst, and the prepared polymer was named PDI-1,5NDA (Figure 1a). For purposes of comparison, p-phenylenediamine was used to explore the effect of conjugation, and 1,4-diaminonaphthalene was used to compare the influence of monomer symmetry. The PTCDA was reacted with the diamine monomer through imide condensation reaction under the catalysis of a typical Lewis acid, zinc acetate. In order to verify the chemical structure of the synthesized products, two kinds of samples were measured by Fourier transform infrared spectroscopy (FT-IR). As shown in Figure 1b, the stretching vibration peak of the -C=O bond appears at around 1640 cm−1 in all three kinds of PDI samples, while the stretching vibration signals of -O-H/-N-H disappear. Additionally, the absorption peaks at around 1020–1350 cm−1 verified the generation of a -C-N bond in three PDI. These absorption peaks clearly indicated that the carbonyl bond of PDI is formed by polycondensation. The peaks in the range of 1600 to 1450 cm−1 come from the carbon atoms in the central nucleus of conjugated PDI of the prepared samples. The FT-IR results show that the synthesized PDI have been imidized completely; there is no unreacted monomer residue, and all of them are consistent with the target products.
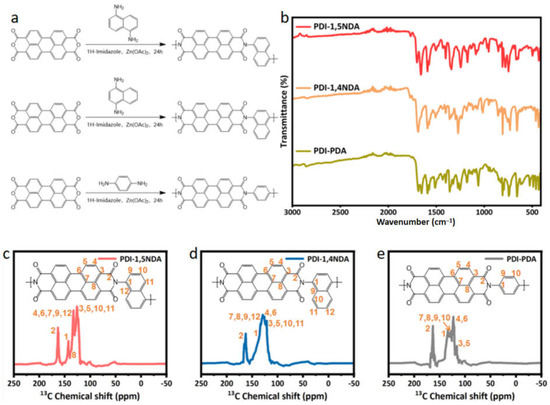
Figure 1.
Structural characterization of the PDI samples: (a) The synthesis route of the three PDI. (b) FT-IR spectra of PDI. (c–e) Solid-state 13C-NMR spectra of PDI-1,5NDA; PDI-1,4NDA; and PDI-PDA samples.
Additionally, solid-state Cross-Polarization Magic Angle Spinning Carbon-13 Nuclear Magnetic Resonance (CP/MAS-NMR) was used to determine the chemical environments. In order to exclude the effects of sidebands, cross polarization/total sideband suppression (CP-TOSS) NMR spectra were used to analyze the chemical structure of PDI samples. As shown in Figure 1c–e, the strong peak at 164 ppm in the low field is typical imine carbons, and various peaks at 110–150 ppm were assigned to carbons on the aromatic motif of PDI and benzene/naphthalene moieties. The above-mentioned information confirmed the formation of the targeted PDI unambiguously.
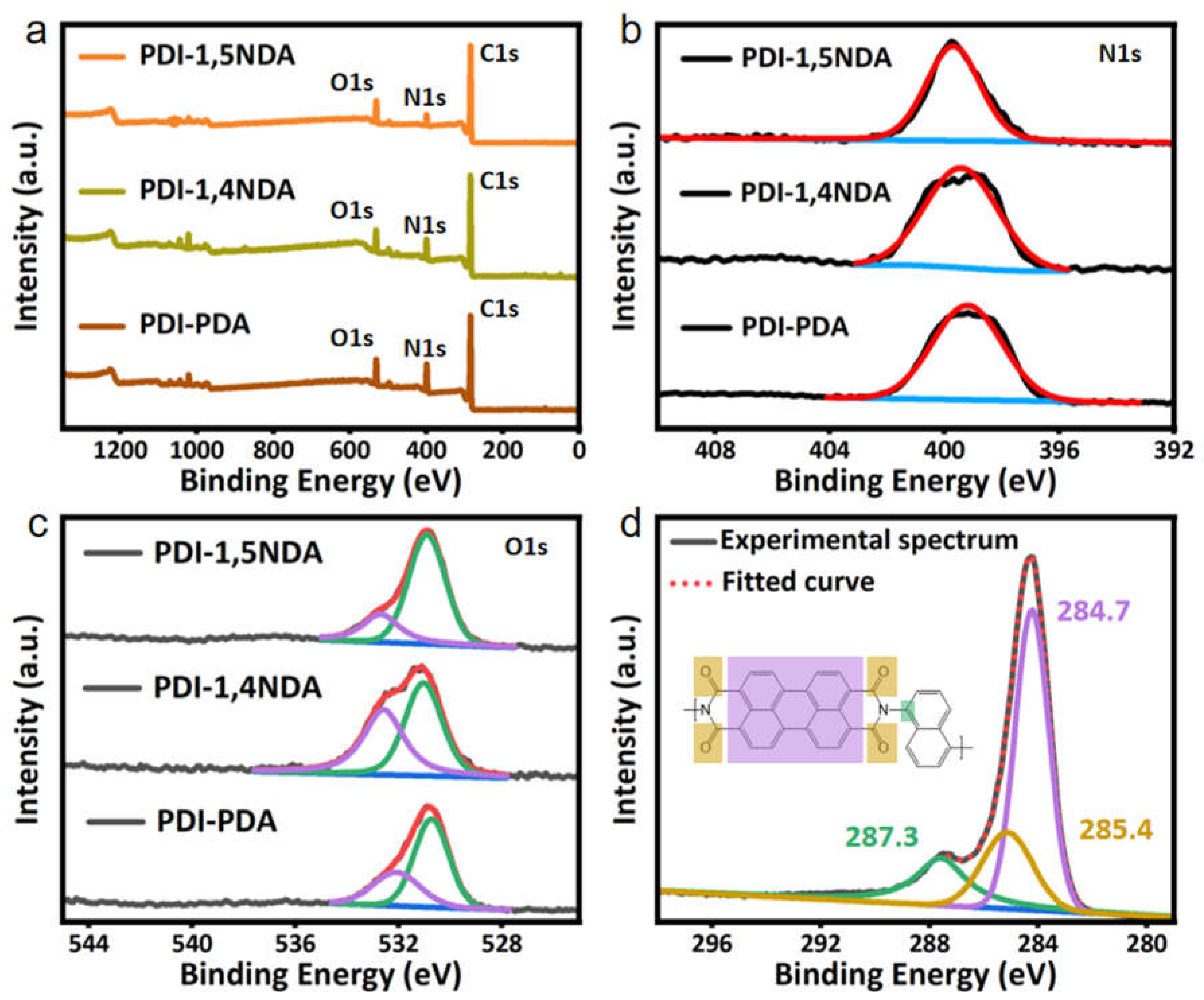
The chemical structures of PDI were analyzed by XPS technologies. XPS survey spectra show that all PDI are composed of C, N, and O, and no zinc residue was detected (Figure 2a), which indicated that the PDI are completely metal-free polymers and the Zn salts are completely removed. The solo peak around 399.0 eV in high-resolution scans of the N1s in all the spectra further confirmed the formation of the imine bonds during the Lewis acid-catalyzed poly-condensation reaction (Figure 2b). High-resolution scans of the O1s peak could be deconvoluted into the main peak at 530.5 eV and a smaller peak at 532.1 eV, which corresponds to C=O bonds in PDI moieties and oxygen-containing species adsorbed on the surface, respectively (Figure 2c). As shown in Figure 3d, the key C1s of carbonyl carbon in 1,5-diaminonaphthalene was detected at around 287.3 eV, while that in PDI was at 285.4 eV, and the analyses of another two kinds of PDI were approximately the same as PDI-1,5NDA. The above-mentioned information about valence bond confirmed the accuracy of the chemical structures of PDI unambiguously.
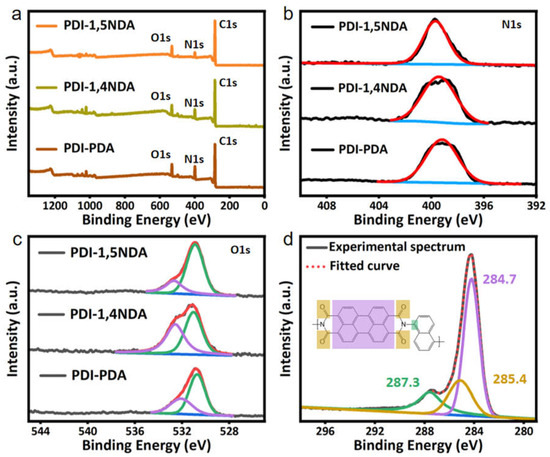
Figure 2.
XPS spectra of the PDI samples: (a) XPS survey profile. (b) N1s high-resolution XPS profile. Black lines are the experimental spectra of PDI samples. Red lines are fitted curve. Blue lines are the background. (c) O1s high-resolution XPS profile. Black lines are the experimental spectra of PDI samples. Red lines are fitted curve. Green lines and purple lines are fitted peak. Blue lines are the background. (d) C1s high-resolution XPS profile.
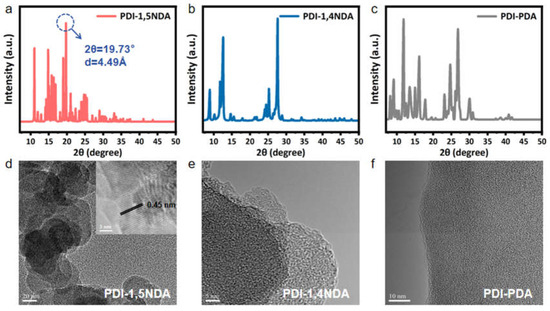
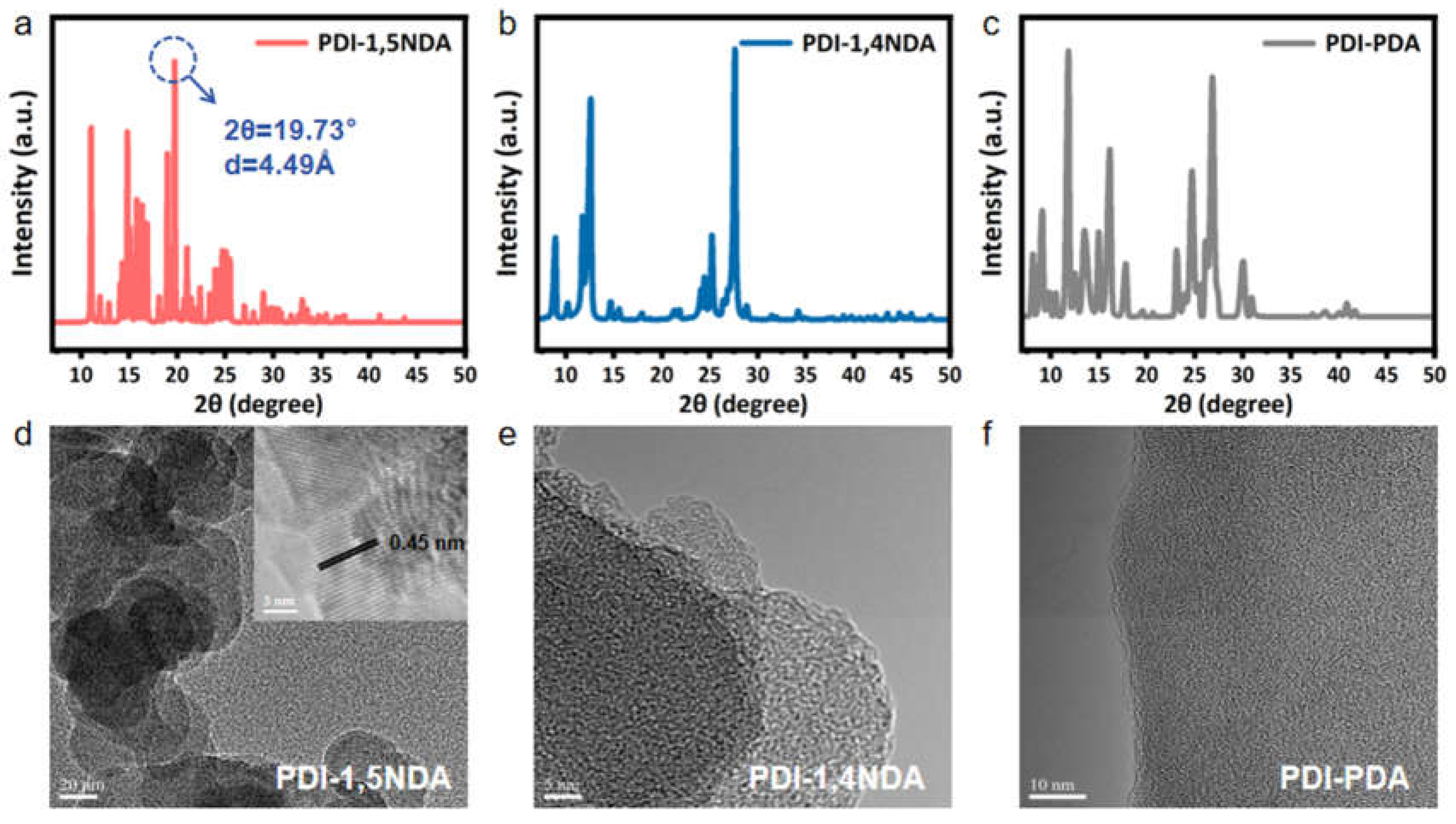
Figure 3.
Crystal structure and morphology of PDI: (a–c) PXRD patterns of PDI-1,5NDA; PDI-1,4NDA; and PDI-PDA samples. (d–f) TEM image of three PDI and HR-TEM image (inset) of PDI-1,5NDA.
In order to distinguish the crystal structures of three PDI, the powder X-ray diffractometer (PXRD) was used to characterize the samples. From the X-ray diffraction spectra, it can be inferred that all three kinds of materials have a certain degree of crystallinity, and the crystallinity of PDI-1,5NDA is the best among them, followed by those of the other two materials (Figure 3a–c). Le Bail fitting of the PXRD patterns of PDI-1,5NDA (Figure 3a) indicated that PDI-1,5NDA had a monoclinic structure with lattice parameters of a = 9.395 Å, b = 7.007 Å, c = 29.134 Å, and β = 124.4° (space group: Pm). The results indicated that the achieved PDI products remain as highly crystalline materials, which can be attributed to the high level of conjugation. Meanwhile, the lower symmetry of the monomer could not destroy the high level of crystallinity of the PDI polymeric materials.
The morphologies of the PDI were subsequently characterized by transmission electronic microscopy (TEM). As presented in Figure 3d, the highly crystalline PDI-1,5NDA shows the typical nanosheet morphology. The high-resolution transmission electronic microscopy (HRTEM) results for PDI-1,5NDA present clear lattice fringes with a lattice spacing of 0.449 nm, which perfectly matches the peak at 19.73° in the PXRD pattern (Figure 3a). This result further proves the high level of crystallinity of PDI-1,5NDA. Theoretically, the high level of crystallinity and good π-π stacking would lead to the regular overlap of delocalized π electrons in multiple layers, and the inter-layer electron transfer of PDI-1,5NDA can be greatly promoted through it, by means of which the sample would obtain relatively good catalytic activity. PDI-1,4NDA and PDI-PDA also show multi-layer nanosheet morphology in the images from TEM, but it is difficult to observe their lattice fringes (Figure 3e,f), indicating that their levels of crystallinity might be lower than that of the PDI-1,5NDA.
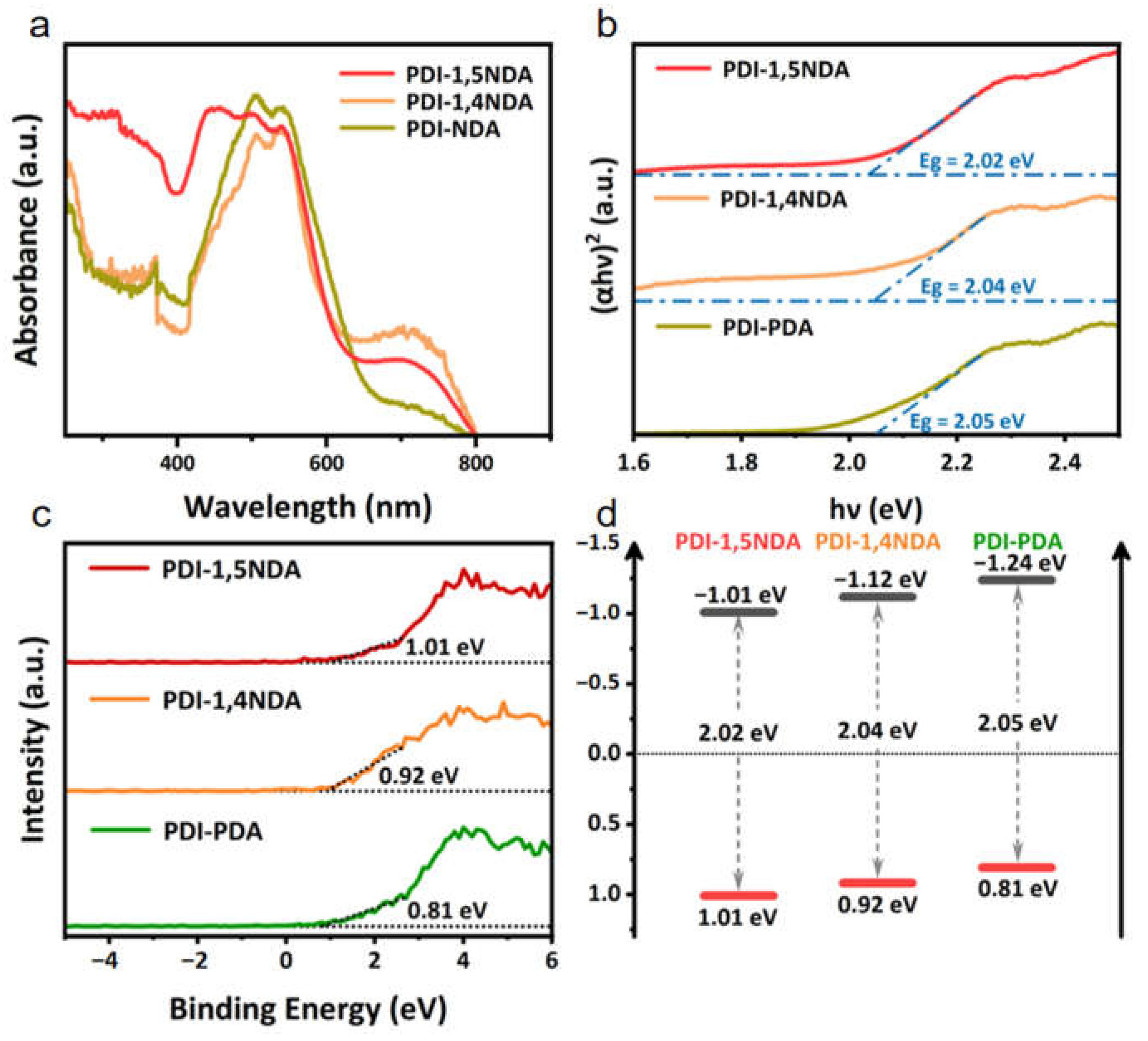
In general, the photocatalytic activity of a semiconductor photocatalyst is largely determined by the photon absorption process and the efficient separation and transportation process of the photogenerated charge. The light harvest capacity and band position of the photocatalyst determine the thermodynamically feasible photocatalytic reaction. The light-absorption of the three PDI samples was tested by UV-Vis diffuse reflectance spectroscopy (UV-DRS). Three PDI with different crystallinity showed similar good visible-light capture abilities (Figure 4a). All of the prepared materials were able to achieve full spectral absorption in the visible region. Notably, both the PDI-1,5NDA sample and the PDI-1,4NDA sample exhibited significant light-absorption in the range of 600–800 nm, which could be ascribed to the large conjugate systems. The band gaps of PDI with different levels of crystallinity are calculated by constructing Tauc Plots (Figure 4b). Meanwhile, combining the results of XPS valence band spectra and Mott–Schottky measurements, the energy band positions of three kinds of PDI are determined. As shown in the XPS valence band spectra (Figure 4c), the maximum valence band values (VB max) of PDI-1,5NDA; PDI-1,4NDA; and PDI-PDA are found to be 1.01eV, 0.92 eV, and 0.81 eV, respectively. Thereafter, the energy band structures can be obtained, a process which is shown schematically in Figure 4d. By using the band gap energy, the conduction band (CB) positions were calculated to be −1.01 eV, −1.12 eV, and −1.24 eV, respectively.
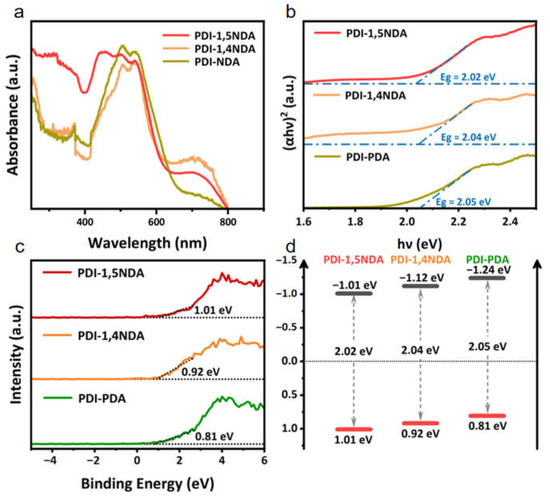
Figure 4.
Light harvest capacity and band structure of the PDI samples: (a) UV-vis diffuse reflectance spectra. (b) Tauc Plots. (c) XPS valence band spectra. (d) The scheme of band positions.
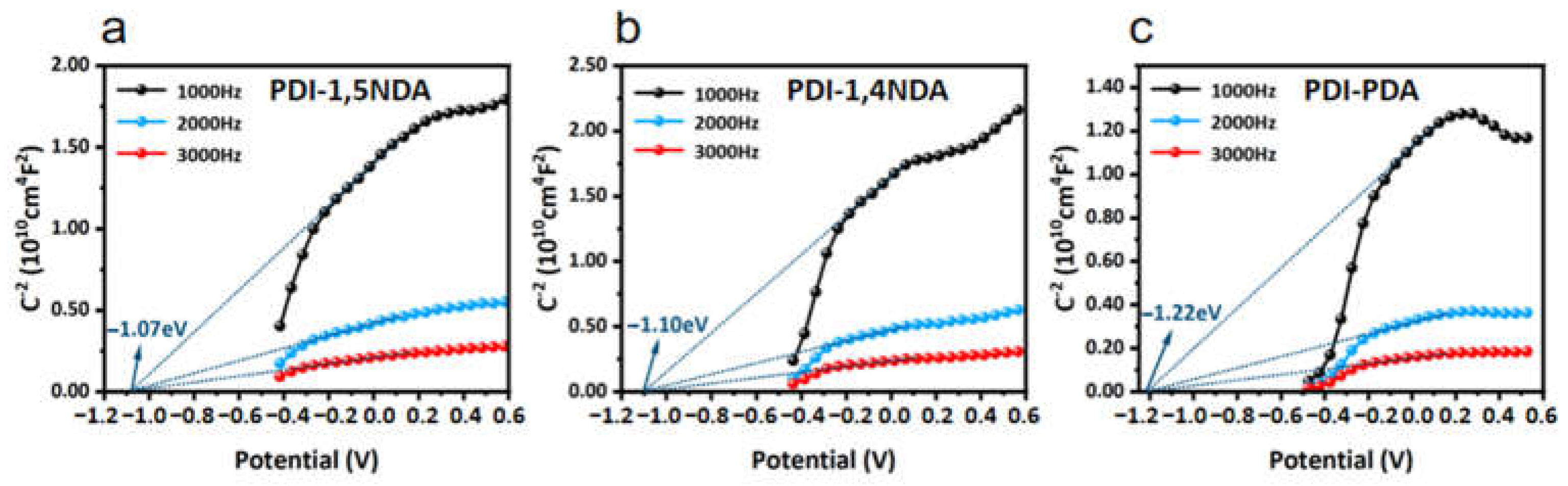
The positive slopes of the Mott–Schottky curves demonstrate that all three PDI are typical n-type semiconductors, and Mott–Schottky tests at three different frequencies pointed to the same flat band potential, which confirmed the accuracy of the experiments (Figure 5). The flat band potentials of the three kinds of PDI match well with the CB potentials calculated from XPS valence spectra and Tauc Plots. As a reference, the generation potentials of typical reactive oxygen species in photocatalytic water disinfection are −0.33 V (O2/•O2−), 0.28 V (O2/H2O2), 0.82 V (H2O/O2), and 1.1–1.99 V (H2O/•OH, depending on pH values). Compared with the CB potentials and the VB potentials obtained by the experiments, it can be inferred that the electronic band structures of the PDI are beneficial to the production of reactive oxygen species (ROS) in photocatalytic water experiments, and the kinetic factors of PDI will play an important role in their photocatalytic performance, since the band structures of PDI are quite similar.
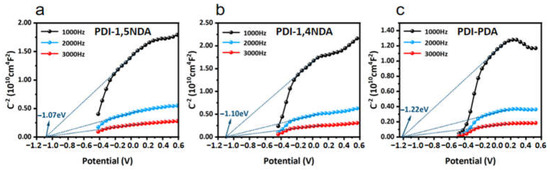
Figure 5.
Mott–Schottky(MS) plots of PDI samples at different frequencies: (a) MS plots of PDI-1,5NDA. (b) MS plots of PDI-1,4NDA. (c) MS plots of PDI-PDA.
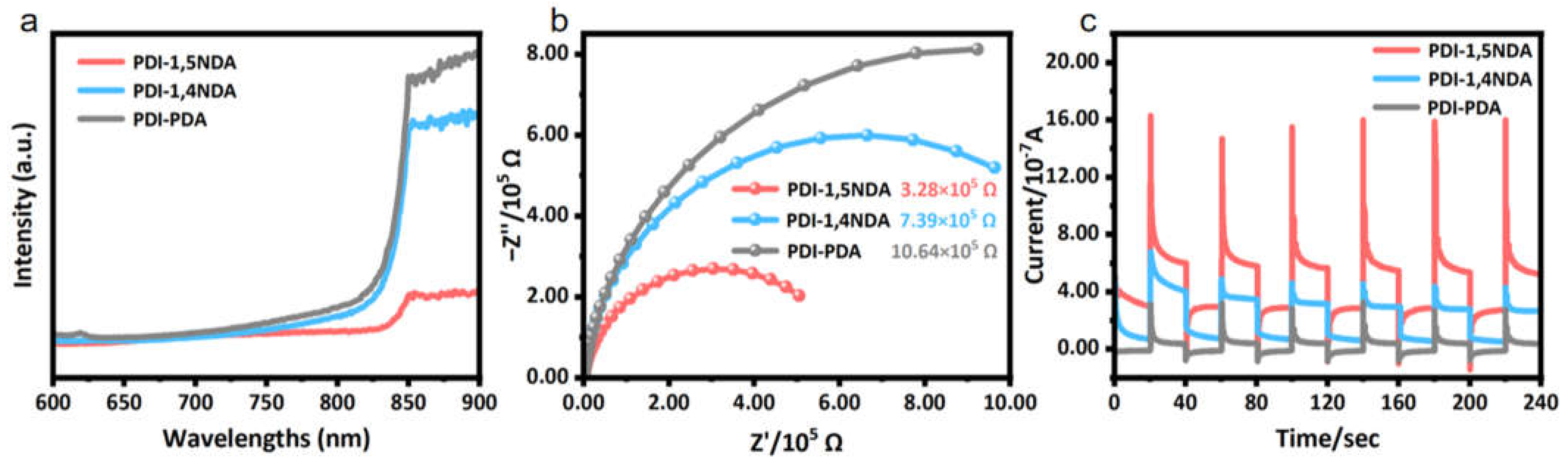
The separation and migration of photogenerated carriers are the decisive kinetic factors for the photocatalytic reaction, and the strong internal electric field provides a driving force for the process of charge separation and transportation. The photoluminescence spectra at 570 nm excitation wavelength (Figure 6a) showed that, compared to the PDI-1,4NDA and PDI-PDA samples with lower crystallinity, the PDI-1,5NDA, with a high level of crystallinity, tends to have lower photoluminescence intensity and a lower fluorescence response, which confirms that the recombination degree of its photogenerated carriers is lower; these experimental results indicated that PDI-1,5NDA has the highest photoquantum efficiency among the three materials.
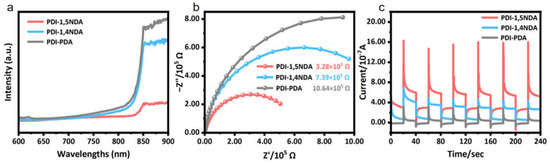
Figure 6.
Photoelectric properties of the PDI samples: (a) Steady-state PL spectra. (b) EIS profile under light irradiation. (c) Transient photocurrent response.
Electrochemical impedance spectroscopy (EIS) shows that PDI-1, 5NDA appears to have a smaller semicircular Nyquist curve radius than other PDI under the visible light illumination (Figure 6b), which indicates that the charge transfer resistance of PDI-1, 5NDA is lower. In the photocurrent test, the PDI-1,5NDA, with a high level of crystallinity, also showed higher instantaneous photocurrent (Figure 6c), indicating that the photogenerated electron-hole separation efficiency of PDI-1,5NDA was the highest, which further proved that the strong internal electric field greatly promoted the separation and transmission efficiency of photogenerated carriers in PDI-1,5NDA.
Since the highly crystalline PDI-1,5NDA has a stronger internal electric field, which promotes the separation and transport of photogenerated carriers, we boldly predict that the visible light-driven photocatalytic performance of PDI-1,5NDA will be stronger than those of the other two PDI samples. We carried out the photocatalytic hydrogen peroxide experiment to verify our prediction.
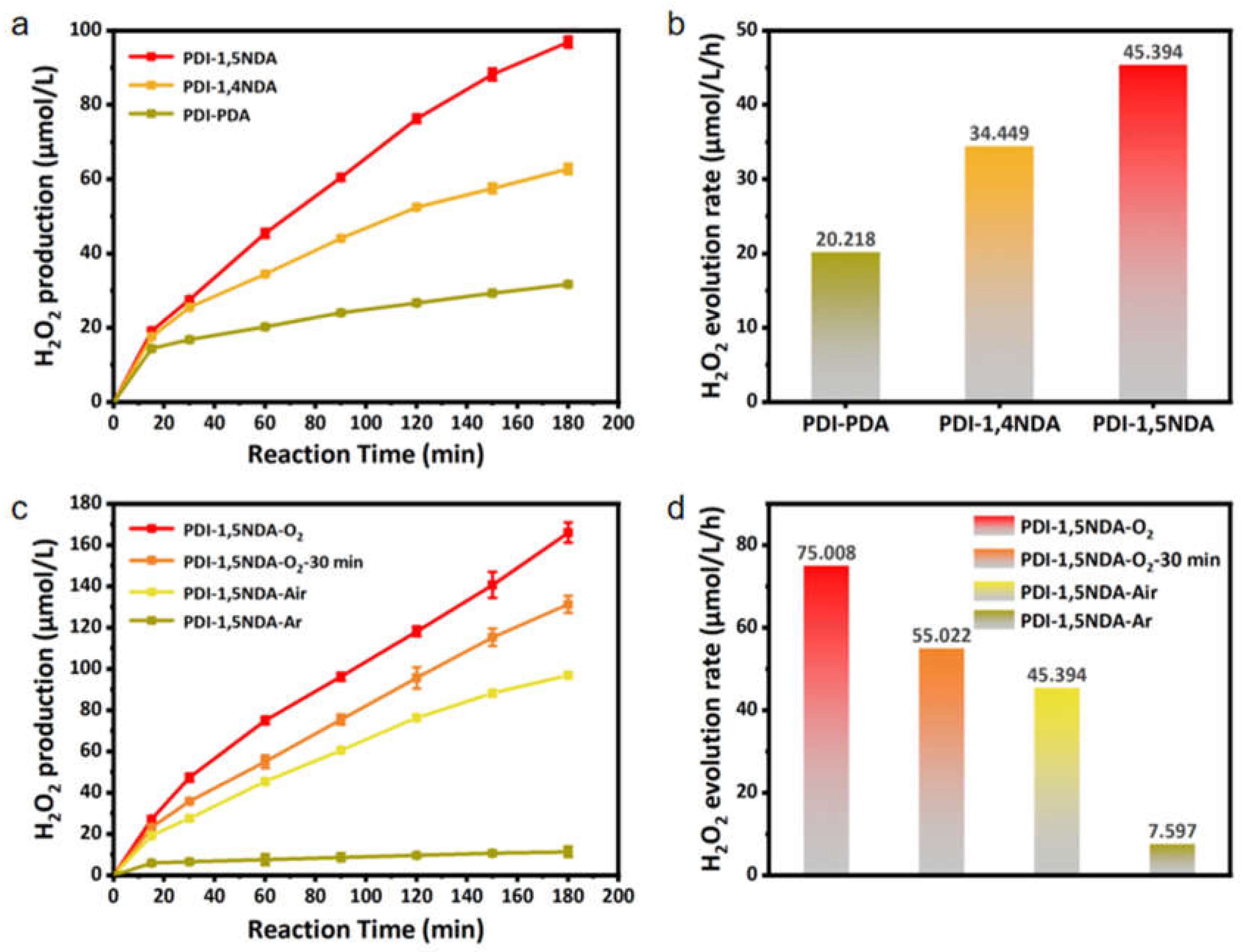
The experiment used 10% isopropanol solution as a sacrificial agent. Meanwhile, the 300W xenon lamp and the 420 nm cut-off filter were used for visible light irradiation. The photocatalytic activity levels of the three kinds of PDI samples were evaluated with the yield of hydrogen peroxide as the index. The concentration of hydrogen peroxide was determined by iodometry. From the test results (Figure 7), it can be seen that the photocatalytic activity of PDI-PDA is definitely poor, as only 20.218 μmol·L−1 H2O2 has been produced in one hour. The catalytic activity of perylene imide increased with the addition of a benzene ring to the connecting molecule, and the rate at which hydrogen peroxide was produced by 1 mol PDI-1,5NDA reached 45.394 μmol·L−1 per hour. These results also confirm that among the three PDI samples, PDI-1,5NDA can produce higher electron concentration and charge mobility. The charge separation ability of PDI-1,5NDA is more efficient than those of the other two PDI. Also, PDI-1,5NDA has the possibility of inhibiting carrier recombination.
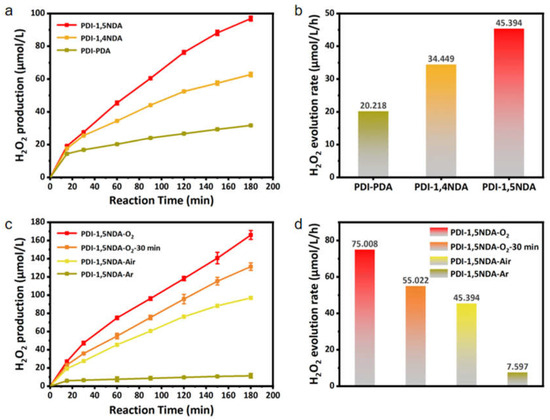
Figure 7.
Photocatalytic efficiency of the PDI samples: (a) Efficiency of photocatalytic decomposition of water to produce H2O2. (b) Production rate of H2O2 of the three PDI. (c) Efficiency of photocatalytic decomposition of water to produce H2O2 by PDI-1,5NDA under different experimental conditions. (d) Production rate of H2O2 of PDI-1,5NDA under different experimental conditions.
In order to further understand the photocatalytic reaction mechanism of PDI-1,5NDA, we explored the time process and rate of photocatalytic hydrogen peroxide production of PDI-1,5NDA under different concentrations of dissolved oxygen by controlling the concentration of dissolved oxygen in 10% isopropanol solution. The results clearly showed that in oxygen-saturated isopropanol solution, the photocatalytic rate of hydrogen peroxide catalyzed by PDI-1,5NDA is the highest, reaching 75.008 μmol·L−1·h−1, while the yield of H2O2 in argon is extremely low, at only 7.597 μmol·L−1·h−1. It can be inferred that the oxygen reduction reaction (ORR) is dominant in the photocatalytic process of hydrogen peroxide production by PDI-1,5NDA, and when ORR is inhibited due to hypoxia, another pathway, which is known as the water oxidation reaction (WOR), encounters difficulty in producing hydrogen peroxide. The asymmetric structural features of PDI-1,5NDA enhance the polarity of the polymerization unit, which facilitates oxygen adsorption. Based on the above analysis, we speculate that the efficient photocatalytic hydrogen peroxide production activity of PDI-1,5NDA is attributed to the asymmetric structural features that enhance the polarity of the polymerization unit, which is conducive to oxygen adsorption. Then, the absorbed O2 is reduced by the light-generated electron to produce H2O2. In addition, the structural unit of the imide bond is very conducive to the generation of hydrogen peroxide, which is also confirmed by previous reports in the literature [34,35]. Furthermore, the obtained H2O2 could be an excellent broad-spectrum bactericide without risk of increased drug resistance [36].
3. Experimental Procedures
3.1. Preparation of PDI-1,5NDA Polymer Photocatalyst
The mixture of 780 mg (1 eq, 2.0 mmol) 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA), 306 mg (1 eq, 2.0 mmol) 1,5-diaminonaphthalene, 439 mg (1 eq, 2.0 mmol) zinc acetate dihydrate, and 30 g 1H-imidazole was stirred in an oil bath at 140 °C for 24 h. After the reaction, the mixture was cooled down to 80 °C, 100 mL deionized water was added, and then the solvent was removed by centrifugation. The obtained dark red solid was washed thoroughly with ethanol to remove the residual monomer, then washed with 0.1 mol/L sodium bicarbonate solution to remove excess zinc acetate, and finally, the solvent residue was removed by freeze-drying.
3.2. Preparation of PDI-PDA Polymer Photocatalyst
The preparation of PDI-PDA is the same as the procedures described for the preparation of PDI-1,5NDA. The mixture of 780 mg (1 eq, 2.0 mmol) 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA), 216 mg (1 eq, 2.0 mmol) p-phenylenediamine (PDA), 439 mg (1 eq, 2.0 mmol) zinc acetate dihydrate, and 30 g 1H-imidazole was stirred in an oil bath at 140 °C for 24 h. After the reaction the mixture was cooled down to 80 °C, 100 mL deionized water was added and then the solvent was removed by centrifugation. The obtained dark red solid was washed thoroughly with ethanol to remove the residual monomer, then washed with 0.1 mol/L sodium bicarbonate solution to remove excess zinc acetate, and finally, the solvent residue was removed by freeze-drying.
3.3. Preparation of PDI-1,4NDA Polymer Photocatalyst
Firstly, the 1,4-diaminonaphthalene was prepared as follows: 1 g 4-nitro-1-naphthylamine was dissolved in 50 mL ethanol, and then 10 mg commercial Pd/C was mixed with the solution as catalyst. The NaBH4 powder was added slowly into the solution under stirring. More than 500 mg NaBH4 was added, for a total of 4 additions, until no bubbles came out, resulting in the 4-nitro-1-naphthylamine being completely reduced. The reaction process was tested by Liquid Chromatogram (LC). After the reaction was completed, the catalysts were removed by centrifugation and the solution was concentrated to 5 mL by vacuum distillation. A quantity of 20 mL deionized water was added into the concentrated solution to precipitate the 1,4-diaminonaphthalene out as the product. The achieved 1,4-diaminonaphthalene was further cleaned by deionized water 5 times to remove the salt, and dried in vacuum at 40 °C. Finally, 570 mg 1,4-diaminonaphthalene was prepared.
Secondly, the experimental procedures of the preparation of PDI-1,5NDA were repeated. The 780 mg (1 eq, 2.0 mmol) 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) and 306 mg (1 eq, 2.0 mmo1) 1,4-diaminonaphthalene were mixed with 439 mg (1 eq, 2.0 mmol) zinc acetate dihydrate, and then the mixture was added into 30 g 1H-imidazole and stirred for 24 h in an oil bath at 140 °C. After the reaction, the mixture was cooled down to 80 °C, 100 mL deionized water was added, and then the solvent was removed by centrifugation. The obtained dark red solid was washed thoroughly with ethanol to remove the residual monomer, then washed with 0.1 mol/L sodium bicarbonate solution to remove excess zinc acetate, and finally, the solvent residue was removed by freeze-drying.
3.4. Physicochemical Characterization
FT-IR analysis was carried out on an Agilent (Santa Clara, CA, USA) Cary 630 Fourier Transform Infrared Spectrophotometer, in the spectral range of 4000–400 cm−1. X-ray photoelectron spectra (XPS) were obtained on an ESCALAB250 X-ray photoelectron spectrometer with Al Kα radiation. The binding energy of C1s was used to calibrate the charge effect. The 13C NMR spectra of the solid state were acquired on a Bruker (Billerica, MA, USA) Avance 400 NMR. The powder X-ray diffraction spectra (PXRD) were analyzed by using Cu Kα radiation in the range of 2θ = 5–70 degrees on a Rigaku (Cedar Park, TX, USA) D/MAX-2500PC. The images from a transmission electron microscope (TEM) operating at 200 kV were recorded on an F20 G2 transmission electron microscope (FEI Tecnai). Scanning electron microscope images (SEM) were obtained on an Apreo field emission scanning electron microscope (FEI, Hillsboro, OR, USA). The UV-visible diffuse reflectance spectra (DRS) were recorded by using a Shimadzu (Tokyo, Japan) UV-2600 UV-Vis spectrophotometer and BaSO4 as the reflection standard. The steady-state photoluminescence (PL) spectra of the samples were collected by a Shimadzu RF 6000 fluorescence spectrophotometer. The transient fluorescence (PL) spectra were obtained at room temperature with an excitation laser at 525 nm on a fluorescence spectrophotometer (FLSP-920, Edinburgh instrument, Edinburgh, UK).
3.5. Photocatalytic H2O2 Evolution
The 20 mg photocatalyst was dissolved in 50 mL of 10% isopropyl alcohol (IPA) solution. Then, a 300W xenon lamp (Beijing Perfectlight Technology Co., Ltd., Beijing, China, Microsolar300) equipped with a 420 nm cut-off filter was used to provide visible light irradiation. The effective irradiation area was about 12.6 cm2. Samples comprising 1.5 mL of the reaction solution were taken at regular intervals and centrifuge to get the supernatant. The concentration of H2O2 was analyzed by iodometry. A quantity of 1.0 mL of 0.1 mol∙L−1 potassium hydrogen phthalate (KHC8H4O4) aqueous solution and 1.0 mL of 0.4 mol∙L−1 potassium iodide (KI) aqueous solution was added to the obtained solution, which was then kept for 30 min. The H2O2 molecules reacted with iodide anions (I−) under acidic conditions (H2O2 + 3I− + 2H+ → I3− + 2H2O) to produce triiodide anions (I3−), which can possess a strong absorption at around 350 nm. The concentration of I3− was determined by using a Shimadzu UV-2600 UV-Vis spectrophotometer on the basis of an absorbance of 350 nm, so that the concentration of H2O2 produced during each reaction and at each time period could be estimated by this method. All of the experiments were carried out at room temperature.
3.6. Electrochemical Analysis
Electrochemical and photoelectrochemical measurements were performed on an electrochemical workstation (CHI-660B, Shanghai, China) by using a standard three-electrode cell at room temperature. The working electrode is the PDI-1,5NDA sample loaded on the fluorine-doped tin oxide (FTO) transparent glass, and a platinum wire was used as the counter electrode, while the standard calomel electrode (SCE) was used as the reference electrode. The electrolyte is 0.2 M Na2SO4. The potentials were given with reference to SCE. The transient photocurrent response of the photocatalysts was measured at 0.0 V by turning the light on and off.
The preparation method for the working electrode is as follows: 8 mg catalyst was dispersed in 1.0 mL PVDF standard solution by ultrasonic means. Then, 20 μL of the mixed suspension was dripped onto the surface of the fluorine-doped tin oxide (FTO) transparent glass and dried in an oven at 60 °C.
4. Conclusions
In conclusion, this work affords a new strategy for facile and efficient fabrication of metal-free PDI-based conjugated polymer photocatalysts via modulation of the symmetry and degree of conjugation of the monomers. By using this strategy, three kinds of novel polymer photocatalysts with perylene imide as their principal part were successfully constructed through a simple polymerization method, using 1,5-diaminonaphthalene, 1,4-diaminonaphthalene and p-phenylenediamine as the intermediate materials. The obtained PDI-1,5NDA photocatalysts exhibit superior photocatalytic activity for photocatalytic H2O2 production under visible light irradiation, at levels 1.6-fold and 3.7-fold higher than those of PDI-1,4NDA and PDI-PDA, respectively. The enhanced performance is due to the synergic advantages of asymmetric structural features and large π-conjugate systems, which facilitate the photogenerated carrier separation and transportation process, thus boosting the H2O2 generation process. The results obtained here not only provide essential structural elements for the design of photocatalysts, but also could be used as part of a broader strategy for constructing catalytic materials in the fields of water treatment and environmental remediation.
Author Contributions
M.Z., methodology and writing—original draft preparation; Y.Y., conceptualization, data curation and formal analysis; J.Z., supervision and project administration; J.X., formal analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the Liaoning Revitalization Talents Program (Grant No. 1808013), and the Shenyang National Laboratory for Materials Science (Grant No. E01SL917).
Data Availability Statement
Data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, Z.; Yin, Z.; Zhang, L.; Yan, Y.; Jiang, Z.; Wu, H.; Wang, W. Synthesis of proton conductive copolymers of inorganic polyacid cluster polyelectrolytes and PEO bottlebrush polymers. Macromolecules 2022, 55, 3301–3310. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, M.; Liu, Y.; Wang, J.; Zhang, G.; Li, L.; Du, L.; Wang, G.; Yang, S.; Wang, X. Single atoms meet metal–organic frameworks: Collaborative efforts for efficient photocatalysis. Energy Environ. Sci. 2022, 15, 3722–3749. [Google Scholar] [CrossRef]
- Zheng, X.; You, J.; Zhu, Y.; Li, Y. Applications of scanning electron microscopy in polymer characterization. Acta Polym. Sin. 2022, 53, 539–560. [Google Scholar]
- Nurani, L.H.; Edityaningrum, C.A.; Irnawati, I.; Putri, A.R.; Windarsih, A.; Guntarti, A.; Rohman, A. Chemometrics-assisted UV-vis spectrophotometry for quality control of pharmaceuticals: A review. Indones. J. Chem. 2023, 23, 542–567. [Google Scholar] [CrossRef]
- Reza, M.S.; Ahmad, N.B.H.; Afroze, S.; Taweekun, J.; Sharifpur, M.; Azad, A.K. Hydrogen production from water splitting through photocatalytic activity of carbon-based materials. Chem. Eng. Technol. 2023, 46, 420–434. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, D.; Zhu, B.; Cheng, B.; Yu, J.; Yu, H. Enhancing photocatalytic H2O2 production with Au co-catalysts through electronic structure modification. Nat. Commun. 2024, 15, 3212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jin, Q.; Mohd, A.K.; Steve, L.; Samira, S.; Kibria, G.; Hu, J. Rational design of carbon nitride for remarkable photocatalytic H2O2 production. Chem. Catal. 2022, 2, 1720–1733. [Google Scholar] [CrossRef]
- Wang, H.; Cao, C.; Li, D.; Ge, Y.; Chen, R.; Song, R.; Gao, W.; Wang, X.; Deng, X.; Zhang, H.; et al. Achieving high selectivity in photocatalytic oxidation of toluene on amorphous BiOCl nanosheets coupled with TiO2. J. Am. Chem. Soc. 2023, 145, 16852–16861. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrocgemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xin, X.; Wang, Y.; Guo, P.; Wang, R.; Wang, B.; Huang, W.; Sobrido, A.J.; Li, X. Internal quantum efficiency higher than 100% achieved by combining doping and quantum effects for photocatalytic overall water splitting. Nat. Energy 2023, 8, 504–514. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, Y.; Zhu, Y. Photocatalytic sacrificial H2 evolution dominated by micropore-confined exciton transfer in hydrogen-bonded organic frameworks. Nat. Catal. 2023, 6, 574–584. [Google Scholar] [CrossRef]
- Marcoux Pierre, R.; Hasenknopf, B.; Vaissermann, J.; Gouzerh, P. Developing Remote Metal Binding Sites in Heteropolymolybdates. Eur. J. Inorg. Chem. 2003, 2003, 2406–2412. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, S.L.; Shao, W.; Zhang, X.D.; Chen, S.C.; Sun, X.S.; Zhang, Q.; Luo, Y.; Xie, Y. Optically switchable photocatalysis in ultrathin black phosphorus nanosheets. J. Am. Chem. Soc. 2018, 140, 3474–3480. [Google Scholar] [CrossRef] [PubMed]
- Kranz, C.; Wächtler, M. Characterizing photocatalysts for water splitting: From atoms to bulk and from slow to ultrafast processes. Chem. Soc. Rev. 2021, 50, 1407–1437. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, L.; Cohen, S.M. Photocatalytic metal–organic frameworks for organic transformations. CrystEngComm 2017, 19, 4126–4136. [Google Scholar] [CrossRef]
- Merkel, M.P.; Anson, C.E.; Kostakis, G.E.; Powell, A.K. Taking the third route for construction of POMOFs: The first use of carboxylate-functionalized mnIII anderson–evans POM-hybrid linkers and lanthanide nodes. Cryst. Growth Des. 2021, 21, 3179–3190. [Google Scholar] [CrossRef]
- Ding, H.; Wang, R.; Wang, X.; Ji, W. Molecularly imprinted covalent organic polymers for the selective extraction of benzoxazole fluorescent whitening agents from food samples. J. Sep. Sci. 2018, 41, 3294–3301. [Google Scholar] [CrossRef] [PubMed]
- Kosco, J.; Bidwell, M.; Cha, H.; Martin, T.; Howells, C.T.; Sachs, M.; Anjum, D.H.; Gonzalez Lopez, S.; Zou, L.; Wadsworth, A.; et al. Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles. Nat. Mater. 2020, 19, 559–565. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wang, Z.; Tang, L.; Zeng, G.; Xu, P.; Chen, M.; Xiong, T.; Zhou, C.; Li, X.; et al. Covalent organic framework photocatalysts: Structures and applications. Chem. Soc. Rev. 2020, 49, 4135–4165. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, Y.; Jiang, J.; Yan, W.; Feng, Y.; Ma, J. Research progress on hybrid organic-inorganic perovskites for photo-applications. Chin. Chem. Lett. 2020, 31, 3055–3064. [Google Scholar] [CrossRef]
- Ferguson, C.; Zhang, K. Classical polymers as highly tunable and designable heterogeneous photocatalysts. ACS Catal. 2021, 11, 9547–9560. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Zhang, H.; Liu, W.; Zhu, W.; Zhu, Y. A highly crystalline perylene imide polymer with the robust built-in electric field for efficient photocatalytic water oxidation. Adv. Mater. 2020, 32, 1907746. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wonneberger, H. Perylene imides for organic photovoltaics: Yesterday, today, and tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3, 4, 9, 10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef] [PubMed]
- Wuerthner, F.; Bauer, C.; Stepanenko, V.; Yagai, S. A black perylene bisimide super gelator with an unexpected J-type absorption band. Adv. Mater. 2008, 20, 1695–1698. [Google Scholar] [CrossRef]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Hu, J.; Sun, C.; Zhao, Y.; Yan, H. Advances in photocatalytic lignin depolymerization: Photocatalytic materials and mechanisms. J. Wood Chem. Technol. 2024, 44, 65–87. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.; Bunes, B.R.; Li, Y.; Wang, C.; Zang, L. Enhancement of visible-light-driven photocatalytic H2 evolution from water over g-C3N4 through combination with perylene diimide aggregates. Appl. Catal. A-Gen. 2015, 498, 63–68. [Google Scholar] [CrossRef]
- Diesen, V.; Jonsson, M. Formation of H2O2 in TiO2 photocatalysis of oxygenated and deoxygenated aqueous systems: A probe for photocatalytically produced hydroxyl radicals. J. Phys. Chem. C 2014, 118, 10083–10087. [Google Scholar] [CrossRef]
- Weingarten, A.S.; Kazantsev, R.V.; Palmer, L.C.; Mcclendon, M.; Koltonow, A.R.; Samuel, A.P.S.; Kiebala, D.J.; Wasielewski, M.R.; Stupp, S.I. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 2014, 6, 964–970. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, W.; Liu, D.; Zhang, Z.; Zhu, Y.; Wang, D. Supramolecular organic nanofibers with highly efficient and stable visible light photooxidation performance. Appl. Catal. B-Environ. 2017, 202, 289–297. [Google Scholar] [CrossRef]
- Ueda, M.; Aoki, T.; Akiyama, T.; Nakamuro, T.; Yamashita, K.; Yanagisawa, H.; Nureki, O.; Kikkawa, M.; Nakamura, E.; Aida, T.; et al. Alternating heterochiral supramolecular copolymerization. J. Am. Chem. Soc. 2021, 143, 5121–5126. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Li, X.; Zhang, S.; Zheng, Q.; Zhang, Y.; Lv, S.W. Embedding carbon quantum dots into crystalline polyimide covalent organic frameworks to enhance water oxidation for achieving dual-channel photocatalytic H2O2 Generation in a Wide pH Range. ACS Appl. Mater. Interfaces 2023, 15, 43799–43809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, C.; Wang, H.; Yan, Y.; Wang, Z.; Li, X.; Liang, Y.; Zhang, J.; Xiao, J. Regulating crystallinity in linear conjugated polymer to boost the internal electric field for remarkable visible-light-driven disinfection. J. Mater. Sci. Technol. 2023, 137, 26–35. [Google Scholar] [CrossRef]
- Chu, C.; Li, Q.; Miao, W.; Qin, H.; Liu, X.; Yao, D.; Mao, S. Photocatalytic H2O2 production driven by cyclodextrin-pyrimidine polymer in a wide pH range without electron donor or oxygen aeration. Appl. Catal. B-Environ. 2022, 314, 10–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).