Impact of Co-Fed Hydrogen on High Conversion Propylene Aromatization on H-ZSM-5 and Ga/H-ZSM-5
Abstract
:1. Introduction
2. Results
2.1. Product Distribution on H-ZSM-5 with and without H2
2.2. Characterization of Ga on ZSM-5
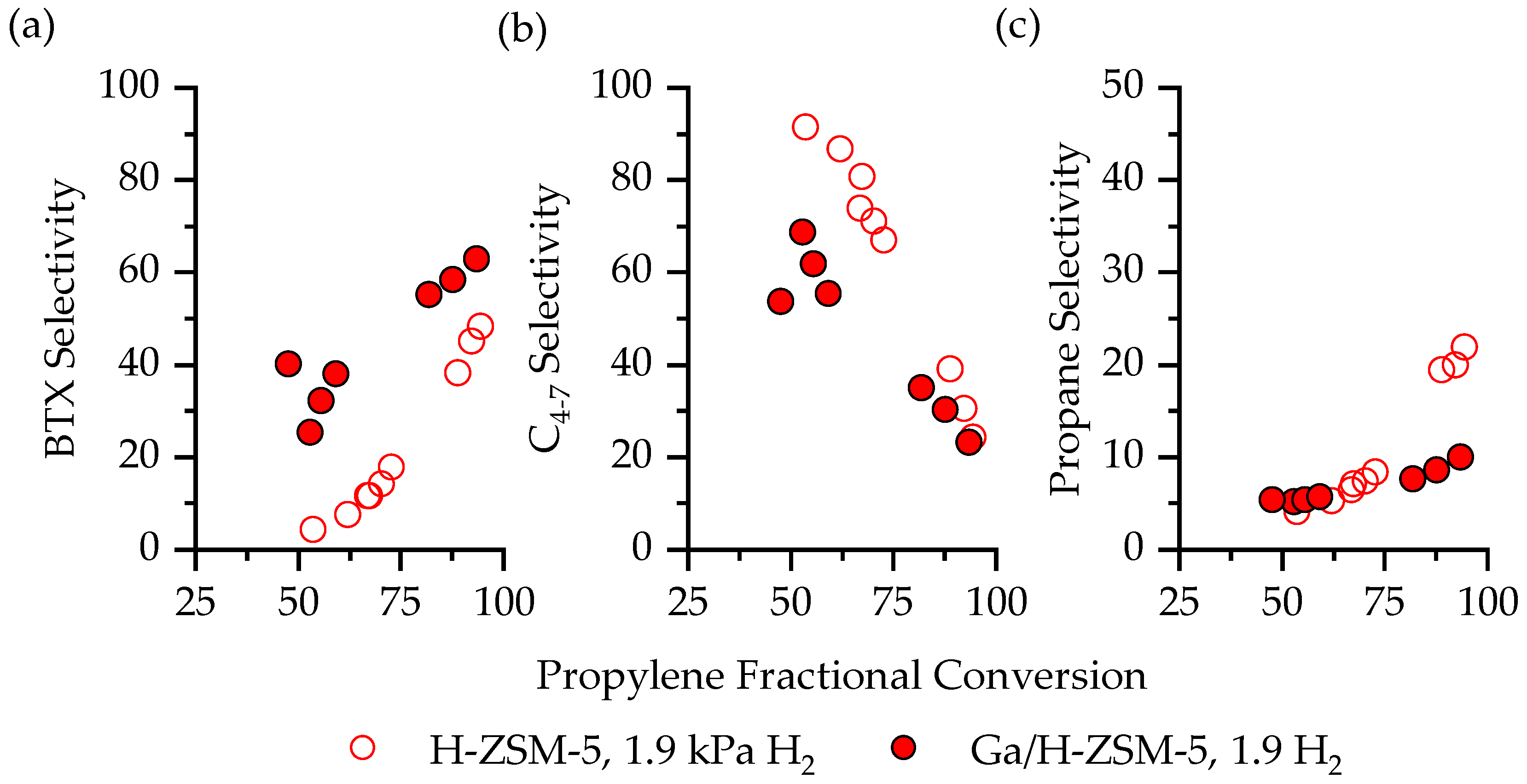
2.3. Product Distribution on Ga/H-ZSM-5 with and without H2
3. Discussion
3.1. The Impact of H2 on C3H6 Aromatization Product Distribution
3.2. The Impact of Ga on C3H6 Aromatization Product Distribution
3.3. The Impact of C3H6 Partial Pressure on Aromatization Product Distribution
4. Materials and Methods
4.1. Catalyst Synthesis
4.2. Reaction Performance Testing
4.3. X-ray Absorption Spectroscopy
4.4. Elemental Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ridha, T.; Li, Y.; Gençer, E.; Siirola, J.; Miller, J.; Ribeiro, F.; Agrawal, R. Valorization of Shale Gas Condensate to Liquid Hydrocarbons through Catalytic Dehydrogenation and Oligomerization. Processes 2018, 6, 139. [Google Scholar] [CrossRef]
- Krannila, H.; Haag, W.O.; Gates, B.C. Monomolecular and Bimolecular Mechanisms of Paraffin Cracking: N-Butane Cracking Catalyzed by HZSM-5. J. Catal. 1992, 135, 115–124. [Google Scholar] [CrossRef]
- Santilli, D.S. Mechanism of Hexane Cracking in ZSM-5. Appl. Catal. 1990, 60, 137–141. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Head-Gordon, M.; Bell, A.T. Mechanism and Kinetics of Light Alkane Dehydrogenation and Cracking over Isolated Ga Species in Ga/H-MFI. ACS Catal. 2021, 11, 2062–2075. [Google Scholar] [CrossRef]
- Nozik, D.; Bell, A.T. Role of Ga3+Sites in Ethene Oligomerization over Ga/H-MFI. ACS Catal. 2022, 12, 14173–14184. [Google Scholar] [CrossRef]
- Zhou, Y.; Thirumalai, H.; Smith, S.K.; Whitmire, K.H.; Liu, J.; Frenkel, A.I.; Grabow, L.C.; Rimer, J.D. Ethylene Dehydroaromatization over Ga-ZSM-5 Catalysts: Nature and Role of Gallium Speciation. Angew. Chem. 2020, 132, 19760–19769. [Google Scholar] [CrossRef]
- Qiu, P.; Lunsford, J.H.; Rosynek, M.P. Characterization of Ga/ZSM-5 for the Catalytic Aromatization of Dilute Ethylene Streams. Catal. Lett. 1998, 52, 37–42. [Google Scholar] [CrossRef]
- Lechert, H.; Bezouhanova, C.; Dimitrov, C.; Nenova, V. Intermeditaes in the Formation of Aromatics from Propene and 2-Propanol on H-ZSM-5 Zeolites. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989; pp. 91–98. [Google Scholar]
- Bhan, A.; Delgass, W.N. Propane Aromatization over HZSM-5 and Ga/HZSM-5 Catalysts. Catal. Rev.-Sci. Eng. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Ono, Y.; Kitagawa, H.; Sendoda, Y. Transformation of But-1-Ene into Aromatic Hydrocarbons Over ZSM-5 Zeolites. J. Chem. Soc. Faraday Trans. 1987, 83, 2913–2923. [Google Scholar] [CrossRef]
- Chen, N.Y.; Yan, T.Y. M2 Forming-A Process for Aromatization of Light Hydrocarbons. Ind. Eng. Chem. Process Des. Dev. 1986, 25, 151–155. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Panjala, D.; Banerjee, S. Aromatization of Propene and N-Butene over H-Galloaluminosilicate (ZSM-5 Type) Zeolite. Appl. Catal. A Gen. 2002, 231, 243–251. [Google Scholar] [CrossRef]
- Shibata, M.; Kitagawa, H.; Sendoda, Y.; Ono, Y. Transformation of Propene into Aromatic Hydrocarbons over ZSM-5 Zeolites. Stud. Surf. Sci. Catal. 1986, 28, 717–724. [Google Scholar] [CrossRef]
- Chang, C.W.; Pham, H.N.; Alcala, R.; Datye, A.K.; Miller, J.T. Dehydroaromatization Pathway of Propane on PtZn/SiO2+ ZSM-5 Bifunctional Catalyst. ACS Sustain. Chem. Eng. 2022, 10, 394–409. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, Y.; Wang, R.; Bao, L.; Zhang, Z.; Shi, D.; Zhang, A.; Zhang, Y.; Liu, Q.; Wu, Q.; et al. Unraveling the Structure-Activity-Stability Relationship over Gallium-Promoted HZSM-5 Nanocrystalline Aggregates for Propane Aromatization. Langmuir 2024, 40, 11998–12008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, L.; Sun, Y.; Liang, F.; Wang, C. Transformation of Metal Species and Catalytic Reaction Mechanism of Metal Modified ZSM-5 in Alkane Aromatization. Fuel Process. Technol. 2023, 245, 107739. [Google Scholar] [CrossRef]
- Inoue, R.; Miyake, K.; Hotta, Y.; Xinyu, L.; Yashiro, R.; Hirota, Y.; Uchida, Y.; Miyamoto, M.; Oumi, Y.; Yi Kong, C.; et al. Stable Dehydroaromatization of Ethane over Zn Ion Exchanged MFI Type Galloaluminosilicate Zeolite. Fuel 2021, 305, 121487. [Google Scholar] [CrossRef]
- Oseke, G.G.; Peter, E.E.; Atta, A.Y.; Mukhtar, B.; El-Yakubu, B.J.; Aderemi, B.O. Improved Aromatic Yield and Toluene Selectivity in Propane Aromatization over Zn–Co/ZSM-5: Effect of Metal Composition and Process Conditions. J. Porous Mater. 2023, 30, 999–1010. [Google Scholar] [CrossRef]
- Csicsery, S.M. Dehydrocyclodimerization. III. Dehydrocyclodimerization of Butanes over Transition Metal Oxide Catalysts. J. Catal. 1970, 17, 315–322. [Google Scholar] [CrossRef]
- Fadaeerayeni, S.; Chen, G.; Toghiani, H.; Xiang, Y. Mechanism and Kinetics of Ethane Aromatization According to the Chemical Transient Analysis. Top. Catal. 2020, 63, 1463–1473. [Google Scholar] [CrossRef]
- Inui, T.; Makino, Y.; Okazumi, F.; Nagano, S.; Mivamoto, A. Selective Aromatization of Light Paraffins on Platinum-Ion-Exchanged Gallium-Silicate Bifunctional Catalysts. Ind. Eng. Chem. Res. 1987, 26, 647–652. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Alario, F. Aromatization of Short Chain Alkanes on Zeolite Catalysts. Appl. Catal. A Gen. 1992, 89, 1–30. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Ling, L.; Zhang, Y.; Zhang, R.; Shen, X.; Li, X.; Wang, B. The Active Site for Dehydrogenation and Cyclization on Zn2+/HZSM-5 Catalyst Aiming at Long-Chain C6 Mono-Olefins Aromatization. Fuel 2024, 366, 131362. [Google Scholar] [CrossRef]
- Nagamori, Y.; Kawase, M. Converting Light Hydrocarbons Containing Olefins to Aromatics (Alpha Process). Microporous Mesoporous Mater. 1998, 21, 439–445. [Google Scholar] [CrossRef]
- Pidko, E.A.; Hensen, E.J.M.; Santen, R.A.V. Anionic Oligomerization of Ethylene over Ga/ZSM-5 Zeolite: A Theoretical Study. J. Phys. Chem. C 2008, 112, 19604–19611. [Google Scholar] [CrossRef]
- Iisa, K.; Kim, Y.; Orton, K.A.; Robichaud, D.J.; Katahira, R.; Watson, M.J.; Wegener, E.C.; Nimlos, M.R.; Schaidle, J.A.; Mukarakate, C.; et al. Ga/ZSM-5 Catalyst Improves Hydrocarbon Yields and Increases Alkene Selectivity during Catalytic Fast Pyrolysis of Biomass with Co-Fed Hydrogen. Green Chem. 2020, 22, 2403–2418. [Google Scholar] [CrossRef]
- Derouane, E.G.; Nagy, J.B.; Dejaifve, P.; van Hooff, J.H.C.; Spekman, B.P.; Védrine, J.C.; Naccache, C. Elucidation of the Mechanism of Conversion of Methanol and Ethanol to Hydrocarbons on a New Type of Synthetic Zeolite. J. Catal. 1978, 53, 40–55. [Google Scholar] [CrossRef]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent Trends and Fundamental Insights in the Methanol-to-Hydrocarbons Process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Liu, R.; Shao, X.; Wang, C.; Dai, W.; Guan, N. Reaction Mechanism of Methanol-to-Hydrocarbons Conversion: Fundamental and Application. Chin. J. Catal. 2023, 47, 67–92. [Google Scholar] [CrossRef]
- Kwak, B.S.; Sachtler, W.M.H.; Haag, W.O. Catalytic Conversion of Propane to Aromatics: Effects of Adding Ga and/or Pt to HZSM-5. J. Catal. 1994, 149, 465–473. [Google Scholar] [CrossRef]
- Buchanan, J.S.; Santiesteban, J.G.; Haag, W.O. Mechanistic Considerations in Acid-Catalyzed Cracking of Olefins. J. Catal. 1996, 158, 279–287. [Google Scholar] [CrossRef]
- Jessy, A.N.; Firth, D.; Bisiriyu, M.T.; Szeto, K.C.; Merle, N.; De Mallmann, A.; Gauvin, R.M.; Delevoye, L.; Olsbye, U.; Taoufik, M. Ga(IBu)3 Supported on Meso H-ZSM-5: Effect of Si/Al Ratio on the Activity and Selectivity in Propane Aromatization. Catal. Commun. 2024, 187, 106825. [Google Scholar] [CrossRef]
- Roh, J.; Lim, Y.H.; Hwang, Y.; Nam, K.; Ryu, H.W.; Kim, D.H. Controlling Catalytic and Reaction Factors for Regulating the Distribution of Aromatic Products in N-Butane Aromatization over Ga/HZSM-5. Fuel 2024, 372, 132186. [Google Scholar] [CrossRef]
- Samanta, A.; Bai, X.; Robinson, B.; Chen, H.; Hu, J. Conversion of Light Alkane to Value-Added Chemicals over ZSM-5/Metal Promoted Catalysts. Ind. Eng. Chem. Res. 2017, 56, 11006–11012. [Google Scholar] [CrossRef]
- Csicsery, S.M. Dehydrocyclodimerization. I. Dehydrocyclodimerization of Butanes over Supported Platinum Catalysts. J. Catal. 1970, 17, 207–215. [Google Scholar] [CrossRef]
- Chen, G.; Griffin, A.; Qiang, Z.; Toghiani, H.; Xiang, Y. Ethane and Propane Dehydroaromatization on Pt/HZSM-5 Catalyst: Influence of Pt Loading ≤ 500 Ppm. Appl. Catal. A Gen. 2023, 665, 119368. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Zhang, M.; Wang, W.; Zhang, X.H.; Lu, F.; Cheng, K.; Zhang, Q.; Wang, Y. Boosting Propane Dehydroaromatization by Confining PtZn Alloy Nanoparticles within H-ZSM-5 Crystals. Catal. Sci. Technol. 2022, 12, 7281–7292. [Google Scholar] [CrossRef]
- Chen, G.; Griffin, A.; Qiang, Z.; Toghiani, H.; Yizhi, X. Light Alkane Dehydroaromatization over Pt-Zn/HZSM-5 Catalyst with Ultralow Pt Loading. Top. Catal. 2024, 1–10. [Google Scholar] [CrossRef]
- Getsoian, A.B.; Das, U.; Camacho-Bunquin, J.; Zhang, G.; Gallagher, J.R.; Hu, B.; Cheah, S.; Schaidle, J.A.; Ruddy, D.A.; Hensley, J.E.; et al. Organometallic Model Complexes Elucidate the Active Gallium Species in Alkane Dehydrogenation Catalysts Based on Ligand Effects in Ga K-Edge XANES. Catal. Sci. Technol. 2016, 6, 6339–6353. [Google Scholar] [CrossRef]
- Higby, P.L.; Shelby, J.E.; Phillips, J.C.; Legrand, A.D. EXAFS Study of Alkali Galliosilicate Glasses. J. Non-Cryst. Solids 1988, 105, 139–148. [Google Scholar] [CrossRef]
- Behrens, P.; Kosslick, H.; Tuan, V.A.; Fröba, M.; Neissendorfer, F. X-Ray Absorption Spectroscopic Study on the Structure and Crystallization of Ga-Containing MFI-Type Zeolites. Microporous Mater. 1995, 3, 433–441. [Google Scholar] [CrossRef]
- Nishi, K.; Shimizu, K.I.; Takamatsu, M.; Yoshida, H.; Satsuma, A.; Tanaka, T.; Yoshida, S.; Hattori, T. Deconvolution Analysis of Ga K-Edge XANES for Quantification of Gallium Coordinations in Oxide Environments. J. Phys. Chem. B 1998, 102, 10190–10195. [Google Scholar] [CrossRef]
- LiBretto, N.J.; Xu, Y.; Quigley, A.; Edwards, E.; Nargund, R.; Vega-Vila, J.C.; Caulkins, R.; Saxena, A.; Gounder, R.; Greeley, J.; et al. Olefin Oligomerization by Main Group Ga3+ and Zn2+ Single Site Catalysts on SiO2. Nat. Commun. 2021, 12, 2322. [Google Scholar] [CrossRef] [PubMed]
- Phadke, N.M.; Van Der Mynsbrugge, J.; Mansoor, E.; Getsoian, A.B.; Head-Gordon, M.; Bell, A.T. Characterization of Isolated Ga3+ Cations in Ga/H-MFI Prepared by Vapor-Phase Exchange of H-MFI Zeolite with GaCl3. ACS Catal. 2018, 8, 6106–6126. [Google Scholar] [CrossRef]
- Xu, Y.; Libretto, N.J.; Zhang, G.; Miller, J.T.; Greeley, J. First-Principles Analysis of Ethylene Oligomerization on Single-Site Ga3+Catalysts Supported on Amorphous Silica. ACS Catal. 2022, 12, 5416–5424. [Google Scholar] [CrossRef]
- Cybulskis, V.J.; Pradhan, S.U.; Lovón-Quintana, J.J.; Hock, A.S.; Hu, B.; Zhang, G.; Delgass, W.N.; Ribeiro, F.H.; Miller, J.T. The Nature of the Isolated Gallium Active Center for Propane Dehydrogenation on Ga/SiO2. Catal. Lett. 2017, 147, 1252–1262. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]




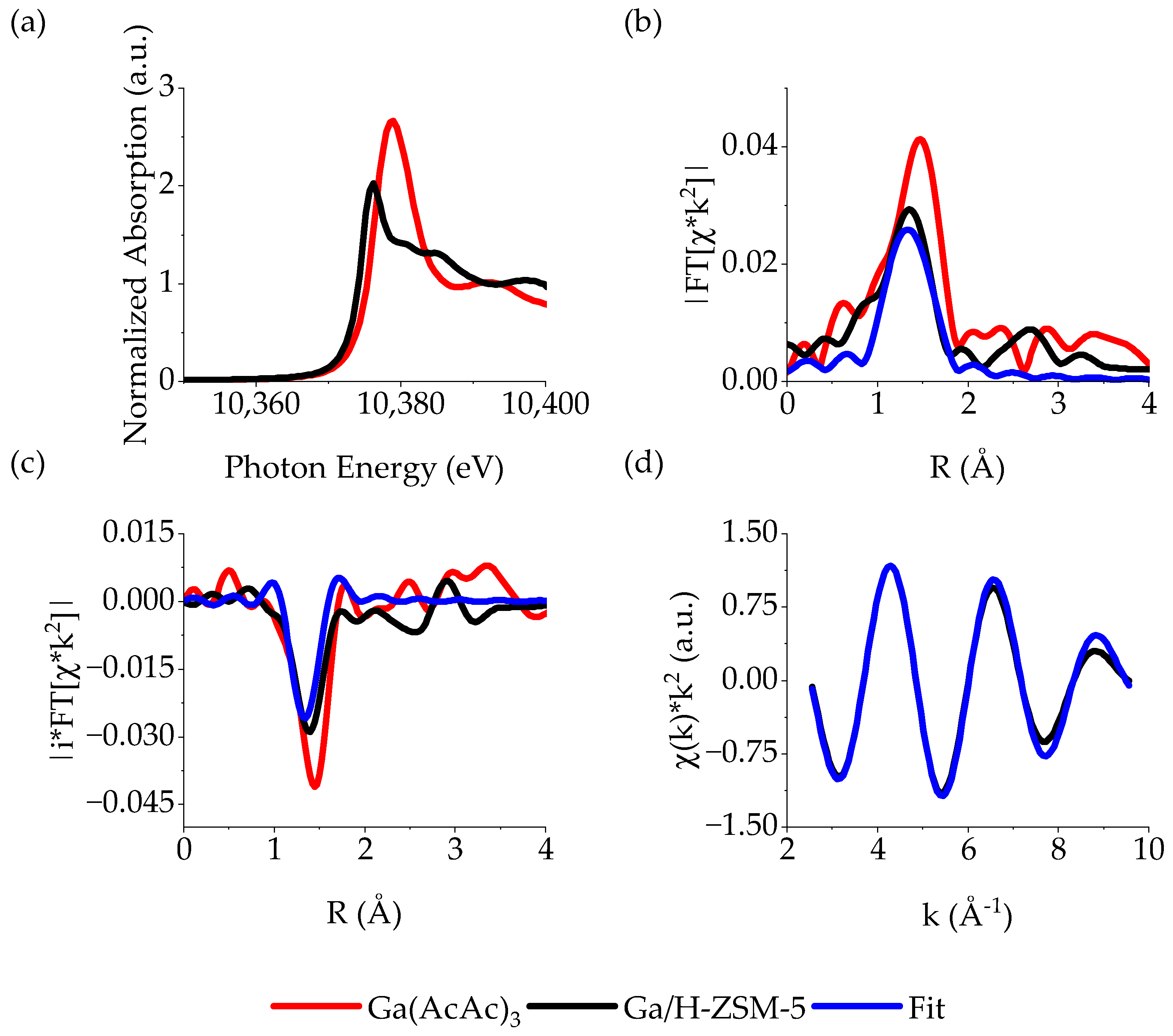






| Sample | Edge Energy (eV) | White Line Energy (eV) | Notable Shoulders (eV) | Coordination Geometry |
|---|---|---|---|---|
| Ga/H-ZSM-5 | 10,372.6 | 10,376.2 | 10,381 eV | Td |
| Ga(AcAc)3 | 10,376.5 | 10,379.0 | - | Oh |
| Sample | CN (±10%) | R (±0.02 Å) | σ2 | Eo |
|---|---|---|---|---|
| Ga(AcAc)3 | 6 * | 1.94 | 0.005 | −1.54 |
| Ga/H-ZSM-5 | 4.0 | 1.84 | 0.005 * | −1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, C.K.; Rockey, J.L.; Hanna, R.N.; Miller, J.T. Impact of Co-Fed Hydrogen on High Conversion Propylene Aromatization on H-ZSM-5 and Ga/H-ZSM-5. Catalysts 2024, 14, 405. https://doi.org/10.3390/catal14070405
Russell CK, Rockey JL, Hanna RN, Miller JT. Impact of Co-Fed Hydrogen on High Conversion Propylene Aromatization on H-ZSM-5 and Ga/H-ZSM-5. Catalysts. 2024; 14(7):405. https://doi.org/10.3390/catal14070405
Chicago/Turabian StyleRussell, Christopher K., Josiah L. Rockey, Rebecca N. Hanna, and Jeffrey T. Miller. 2024. "Impact of Co-Fed Hydrogen on High Conversion Propylene Aromatization on H-ZSM-5 and Ga/H-ZSM-5" Catalysts 14, no. 7: 405. https://doi.org/10.3390/catal14070405







